Abstract
Prader-Willi syndrome (PWS) is a rare genetic disorder associated with excessive weight gain. Hyperphagia associated with PWS may result in higher energy intake, but alterations in energy expenditure may also contribute to energy imbalance. The purpose of this critical literature review is to determine the presence of alterations in energy expenditure in individuals with PWS. Ten studies that measured total energy expenditure (TEE), resting energy expenditure (REE), sleep energy expenditure (SEE), activity energy expenditure (AEE), and diet induced thermogenesis (DIT) were included in this review. The studies provided evidence that absolute TEE, REE, SEE, and AEE are lower in individuals with PWS than in age-, sex-, and body mass index–matched individuals without the syndrome. Alterations in lean body mass and lower physical activity amounts appear to be responsible for the lower energy expenditure in PWS rather than metabolic differences. Regardless of the underlying mechanism for lower TEE, the estimation of energy requirements with the use of equations derived for the general population would result in weight gain in individuals with PWS. The determination of energy requirements for weight management in individuals with PWS requires a more comprehensive understanding of energy metabolism. Future studies should aim to comprehensively profile all specific components of energy expenditure in individuals with PWS with the use of appropriately matched controls and gold standard methods to measure energy metabolism and body composition. One component of energy expenditure that is yet to be explored in detail in PWS is DIT. A reduced DIT (despite differences in fat free mass), secondary to hormonal dysregulation, may be present in PWS individuals, leading to a reduced overall energy expenditure. Further research exploring DIT in PWS needs to be conducted. Dietary energy recommendations for weight management in PWS have not yet been clearly established.
Keywords: Prader-Willi syndrome, energy metabolism, resting energy expenditure, diet-induced thermogenesis, activity energy expenditure
Introduction
Prader-Willi syndrome (PWS), first described in 1956 and formerly known as Prader-Labhart-Willi syndrome, is a rare genetic disorder in which multiple genes on the paternal chromosome 15 (q 11–13) are deleted or unexpressed (1). PWS occurs in 1 in 10,000–16,000 live-born infants, and is characterized by dysmorphic features, muscular hypotonia, short stature, low fat-free mass (FFM), cognitive delay, and behavioral abnormalities (2). PWS is also associated with an insatiable hunger drive, which develops insidiously and is often the catalyst for the development of obesity in this population (3). Excessive weight gain can be observed by the age of 2 y, and at ∼3–5 y, obesity becomes conspicuous (4). Furthermore, as a result of profound obesity (many individuals weigh >200% of their ideal body weight), obesity-related morbidity and mortality are high in this population (5, 6).
The excessive weight gain associated with PWS is of concern to health care professionals and caregivers who acknowledge that weight management is an essential but challenging aspect of care for individuals with PWS. To improve the effectiveness of treatments to curb the development of obesity in PWS, a more complete understanding of the underlying mechanisms associated with altered energy balance in these individuals is needed. Excess energy intake associated with insatiable hyperphagia could contribute to energy imbalance. However, the reported lower energy requirements of individuals with PWS to prevent excessive weight gain (7) suggests that their energy expenditure is lower than predicted. Therefore, the aim of this article was to critically review published studies that measured total energy expenditure (TEE) and its components [resting energy expenditure (REE), activity energy expenditure (AEE), sleeping energy expenditure (SEE), and diet-induced thermogenesis (DIT)] in individuals with PWS compared with matched controls to determine the presence of alterations in energy expenditure. Understanding which components of energy expenditure are altered will enable a more targeted approach for dietary and pharmacologic therapies to manage weight, as well as assist in the development of evidence-based dietary guidelines.
Literature Search Methods
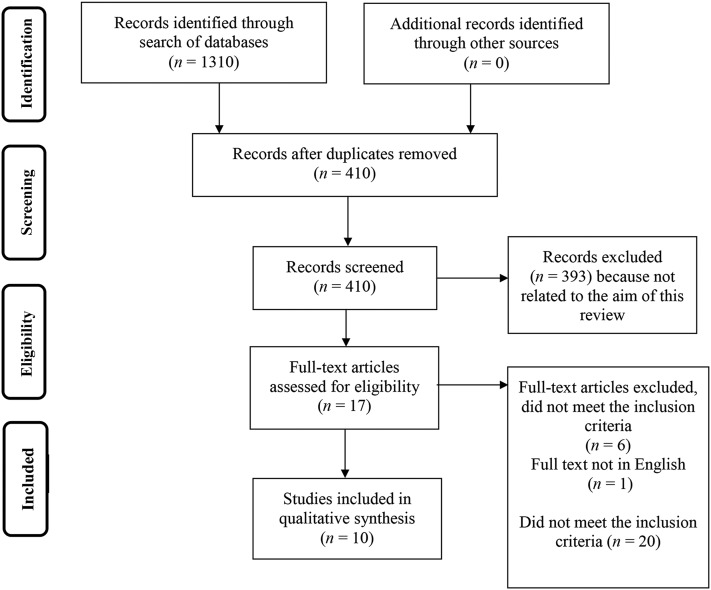
Literature searches were performed on PubMed, Web of Science, SCOPUS, and Medline with the use of the following keywords: “energy metabolism,” “energy expenditure,” “resting energy expenditure,” “resting metabolic rate,” “basal metabolic rate,” “basal energy expenditure,” “activity energy expenditure,” “total energy expenditure,” “daily energy expenditure,” “diet-induced thermogenesis,” “thermic effect of food,” “postprandial thermogenesis,” “indirect calorimetry,” “doubly labeled water,” and “Prader-Willi syndrome.” Studies published between inception and February 2017 were included in this review. Studies were included in this review if energy expenditure in individuals with PWS was compared with matched controls and energy expenditure was measured in both groups with the use of indirect calorimetry (IC) or doubly labeled water (DLW). A total of 1310 articles were found. After excluding duplicates (n = 899), irrelevant articles [not related to the aim of this review (n = 393)] and articles that did not meet the inclusion criteria (n = 8), 10 articles were included in this review.
A flowchart of the literature review process is given in Figure 1 and relevant terms related to energy metabolism are defined in Table 1. As explained by Carneiro et al. (12), multiple terms to describe specific components of energy expenditure are used interchangeably in the literature [i.e., REE and basal energy expenditure (BEE)]. However, differences in the specific measurement conditions for obtaining REE and BEE exist and should be used to clarify which component (REE or BEE) is actually being assessed (Table 1). For this review, these components were classified as either BEE or REE according to their measurement conditions as described in the methodology sections of the reviewed paper, even when they were different from terms chosen by the study authors. Additionally, to ensure consistency in reporting and ease of comparison, all energy expenditure values were expressed as kcal/d. Study details, including the population investigated and methods used to assess the specific components of TEE, are presented in Table 2.
FIGURE 1.
A flowchart of the literature review process.
TABLE 1.
Relevant terms and definitions related to energy metabolism
| Term | Definition |
| Basal energy expenditure | Minimum energy required to maintain vital body functions. It represents ∼50–75% of total energy expenditure. It is measured under standard conditions, which include: a full night’s sleep in the metabolic unit, optimal fasting conditions for 12–14 h, laying down, remaining awake but motionless, abstaining from exercise for ≥12 h, and no physical exercise on the day of testing (8) |
| Resting energy expenditure | Energy required to support the body’s basic metabolic activities; resting energy expenditure can be 3–10% higher than basal energy expenditure |
| Activity energy expenditure | Energy expended to support physical activity (exercise and nonexercise) (9) |
| Diet-induced thermogenesis | Energy required after food intake for digestion, absorption, usage, and storage of nutrients (10) |
| Sleeping energy expenditure | Energy required to maintain vital body functions during sleep; it is 5–10% lower than basal energy expenditure (11) |
TABLE 2.
Summary of studies investigating differences in the components of energy expenditure in individuals with or without PWS1
| Author, year (ref.) | Study design and purpose | Study population (mean ± SD) | Measurements | Outcomes |
| Schoeller et al., 1988 (5) | Cross-sectional study comparing TEE, BMR, and BC | PWS, n = 10, 5 F/5 M | TEE: DLW, 7 d | TEE compared with controls: |
| Age: 16 ± 4 y | BEE: IC ventilated hood, 30 min | 47% lower in PWS subjects | ||
| BMI, kg/m2: 28.9 ± 8.1 (PWS lower than controls) | AEE: estimated based on predictive equation that includes FFM | BEE compared with predictive equations: | ||
| FFM, kg: 29.8 ± 9.6 (PWS lower than controls) | BC: total body water | |||
| FM, %: 48 ± 7 | Measured 3–12% lower than predictive equations. Predictive equation that included FFM not different from measured BMR | |||
| GH treatment: no information | Estimated AEE compared with controls: | |||
| PWS subtype: 5 DEL/5 not reported | 66% lower in PWS subjects; no difference per kg BW | |||
| PWS for BMR, n = 6, 3 F/3 M | ||||
| Age: 24 ± 4 y | ||||
| BMI: 22.7 ± 1.8 | ||||
| PWS subtype: not reported | ||||
| Controls, n = 10; 5 F/5 M | ||||
| Age: 15 ± 3.6 y | ||||
| BMI: 36.3 ± 7.5 | ||||
| FFM, kg: 56.4 ± 13.8 | ||||
| FM, %: 45 ± 6 | ||||
| Davies and Joughin, 1993 (13) | Cross-sectional study to assess TEE and REE | PWS, n = 10, 5 F/5 M | TEE: DLW, details not provided | TEE compared with controls: |
| Age, y: 11.65 ± 3.5 | REE: IC hood, 20 min | 28% lower in PWS subjects, unadjusted; no difference with adjustment for age, FFM, and sex | ||
| BMI: not reported | AEE: TEE/REE | REE compared with controls: | ||
| FFM, kg: 23.9 ± 10.3 | BC: total body water | 18% lower in PWS subjects, unadjusted; no difference with adjustment for age, FFM, and sex | ||
| FM, %: 43 ± 10.5 | Estimated AEE compared with controls: | |||
| GH treatment: no information | 13% lower in PWS subjects, unadjusted; lower with adjustment for age | |||
| PWS subtype: 7 DEL/8 not reported | ||||
| Controls, n = 60, M/F: no information | ||||
| Age, y: 12.56 ± 4.2 | ||||
| BMI: not reported | ||||
| FFM, kg: 34.2 ± 15.1 kg | ||||
| FM, %: 23.8 ± 9 (controls lower than PWS) | ||||
| van Mil et al., 2000 (14) | Cross-sectional study to measure AEE, corrected for body size | PWS, n = 17, 10 F/7 M | TEE: DLW, 14 d | Absolute TEE compared with controls: |
| Age, y: 11.9 ± 3.4 | BEE: ventilated hood, 10 min | 28% lower in PWS subjects, unadjusted | ||
| BMI: 23.5 ± 6 | AEE: 0.9 × (TEE – BEE), correcting for 10% DIT | TEE/FFM (per kg FFM): | ||
| FFM, kg: 27.5 ± 9.9 (PWS lower than controls) | BC: total body water | 42% and 38% lower in PWS subjects with BW and BW covariates, respectively | ||
| FM, kg: 22.4 ± 11.7 | BEE compared with controls: | |||
| FM, %: 43.7 ± 7.9 | 16% lower in PWS subjects, unadjusted; 21% lower with BW as a covariate; no difference with FFM as a covariate | |||
| Bone age, y: 12.7 ± 2.9 | AEE compared with controls: | |||
| PWS subtype: DEL or UPD | 58% lower for PWS subjects unadjusted; 50% lower with BW as a covariate for PWS subjects adjusting for BW/d | |||
| GH: none ever received GH | ||||
| Controls, n = 17; 10 F/7 M | ||||
| Age, y: 11.3 ± 2.6 | ||||
| BMI: 26 ± 6.5 | ||||
| FFM, kg: 35.9 ± 13.4 | ||||
| FM, kg: 25.6 ± 12.7 | ||||
| FM, %: 39.1 ± 8.8 | ||||
| Bone age, y: 12.7 ± 3.2 | ||||
| Bekx et al., 2003 (1) | Cross-sectional study to evaluate BC in relation to energy expenditure in infants and toddlers with PWS | PWS, n = 16, 8 F/8 M | TEE: DLW, 7 d | Absolute TEE compared with published normative data: |
| Age, mo: 12.4 ± 6 | BC: DXA, DLW | 24% lower in PWS | ||
| DXA FFM, kg: 5.7 ± 1.4 | TEE adjusted as linear regression for FFM (kg FFM): | |||
| FM, %: 28.8 ± 6 | Rates for PWS subjects comparable with published normative data | |||
| DLW FM, %: 34.9 ± 7 | ||||
| PWS subtype: 10 DEL/5 UPD/ 1 ID | ||||
| GH: 14 current GH | ||||
| Butler et al., 2007 (15) | Cross-sectional study to determine the relations among body composition, activity amounts, and metabolic rates | PWS, n = 48; 27 F/21 M | TEE: whole body IC, 8 h | TEE compared with controls: 20% lower in PWS subjects, kcal/8 h, unadjusted; lower with adjustment for FM; no difference with adjustment for LBM |
| Age, y: 23 ± 9 | REE: whole body IC, 30 min | REE compared with controls: | ||
| BMI: 34 ± 9 (PWS lower than controls) | AEE: energy expended during the periods when the participants were instructed and encouraged to do exercises | 16% lower in PWS subjects, kcal/min; lower with adjustment for FM; no difference with adjustments for LBM or BW | ||
| LBM, kg: 35 ± 7 (PWS lower than controls) | BC: DXA | AEE compared with controls: | ||
| FM, kg: 39 ± 14 (PWS lower than controls) | 38% lower in PWS subjects, kcal/min, with a 35% reduction in mechanical work >8 h | |||
| FM, %: 51 ± 8 | ||||
| PWS subtype: 27 DEL/21 UPD | ||||
| GH: none receiving GH | ||||
| Controls, n = 24; 15 F/9 M | ||||
| Age, y: 27 ± 13 | ||||
| BMI: 41 ± 8 | ||||
| LBM, kg: 51 ± 12 | ||||
| FM, kg: 50 ± 14 | ||||
| FM, %: 50 ± 7 | ||||
| Hill et al., 1990 (16) | Cross-sectional study to determine whether the relation between REE and BW or BC is different in individuals with PWS | PWS, n = 22; 13 F/9 M | REE: IC hood, 15–20 min | REE compared with controls: |
| Age, y: 13 ± 1 | BC: BIA | 37% lower in PWS subjects than in obese controls and 20% lower than in lean controls, unadjusted, no difference with adjustment for FFM | ||
| BMI: 24 ± 1 | ||||
| PWS subtype and GH: no information | ||||
| Obese controls, n = 11; 7 F/4 M | ||||
| Age, y: 10 ± 1 | ||||
| BMI: 28 ± 1 | ||||
| Lean controls n = 20; 9 F/11 M | ||||
| Age 10 ± 1 y | ||||
| BMI 19 ± 1 | ||||
| FM and FFM data not reported. | ||||
| van Mil et al., 2000 (17) | Cross-sectional study to measure SEE, adjusted for FFM | Same participants as van Mil et al. (14) | SEE: respiratory camper, overnight | Absolute SEE compared with controls: |
| 17% lower in PWS subjects, unadjusted; lower with adjustment for BW; no difference with adjustment for FFM | ||||
| Goldstone et al., 2002 (18) | Cross-sectional study to assess REE | PWS, n = 8 F | REE: whole body IC, ≥20 min | Absolute REE compared with controls: |
| Age, y: 25 ± 2 | BC: MRI | 8% lower in PWS subjects than in obese controls, unadjusted; lower after adjustment for age, height, and weight and after adjustment for age and FM; higher after adjustment for age and FFM; no difference after adjustment for age, FFM, and FM | ||
| BMI: 42.1 ± 3 (higher than obese and lean controls) | ||||
| FFM, kg: 46.1 ± 3.7 (lower than obese controls) | ||||
| FM, kg: 49.6 ± 6.3 (higher than obese and lean controls) | ||||
| FM, %: 50.8 ± 2 | ||||
| PWS subtype: not reported | ||||
| GH: none receiving GH | ||||
| Obese controls, n = 13 F | ||||
| Age, y: 26 ± 3 | ||||
| BMI: 37.1 ± 1.6 | ||||
| FFM, kg: 58.5 ± 1.8 | ||||
| FM, kg: 43.2 ± 2.9 | ||||
| FM, %: 42.1 ± 1.2 | ||||
| Lean controls, n = 28 F | ||||
| Age, y: 31 ± 1 | ||||
| BMI: 23.7 ± 0.5 | ||||
| FFM, kg: 46.1 ± 0.9 | ||||
| FM, kg: 19.5 ± 0.9 | ||||
| FM, %: 29.1 ± 0.8 | ||||
| Lloret-Linares et al., 2013 (19) | Cross-sectional study to compare REE of adults with PWS or lesional genetic hypothalamic obesity with obese controls | PWS, n = 16 F | REE: IC hood, details not provided | Absolute REE compared with controls: |
| Age, y: 25.8 ± 4.9 | BC: DXA | Female PWS subjects had rates similar to controls and subjects with HD, unadjusted; when adjusted for age, PWS subjects had 13% higher rates than controls and similar rates to HD subjects when adjusted for LBM | ||
| BMI: 46.6 ± 8.5 | Male PWS subjects had 19% lower rates than controls and similar rates to HD subjects, unadjusted; no differences when adjusted for age and LBM | |||
| LBM, kg: 46.7 ± 10 (*lower than females with HD) | ||||
| FM, kg: 53 ± 13.2 | ||||
| FM, %: 52.5 ± 4 | ||||
| PWS, n = 11 M | ||||
| Age, y: 25.5 ± 10.4 | ||||
| BMI: 46.6 ± 9.1 | ||||
| LBM, kg: 58.6 ± 12.6 (*lower than male HD and control subjects) | ||||
| FM, kg: 58.6 ± 19.2 | ||||
| FM, %: 48.9 ± 7.3 | ||||
| PWS subtype (all subjects): 18 DEL/8 UPD/1 ID | ||||
| GH (all subjects): 1 current, 5 in childhood | ||||
| HD, n = 5 F | ||||
| Age, y: 42.2 ± 16.1 | ||||
| BMI: 49 ± 7.6 | ||||
| LBM, kg: 61.7 ± 11.7 | ||||
| FM, kg: 57.9 ± 13.4 | ||||
| FM, %: 47 ± 1.6 | ||||
| HD, n = 10 M | ||||
| Age, y: 33.8 ± 13.2 | ||||
| BMI: 47.2 ± 7.7 | ||||
| LBM, kg: 75 ± 12.3 | ||||
| FM, kg: 58 ± 14 | ||||
| FM, %: 40 ± 7.4 | ||||
| GH (all HD subjects): 1 current, 5 in childhood | ||||
| Controls, n = 176 F | ||||
| Age, y: 36 ± 8.4 | ||||
| BMI: 46.8 ± 6.3 | ||||
| LBM, kg: 61.7 ± 8.3 | ||||
| FM, kg: 60.5 ± 12.3 | ||||
| FM, %: 47.5 ± 4.5 | ||||
| Controls, n = 30 M | ||||
| Age, y: 41.7 ± 7.9 | ||||
| BMI: 46.3 ± 5.7 | ||||
| LBM, kg: 78.8 ± 8.3 | ||||
| FM, kg: 58.5 ± 14 FM, %: 40.4 ± 4.6 | ||||
| Purtell et al., 2015 (20) | Cross-sectional study to measure changes in energy expenditure in response to meals | PWS, n = 11, 4 F/7 M | REE: IC hood, 30 min | Absolute REE compared with controls: |
| Age, y: 27.5 ± 2.7 | Postprandial REE: IC hood, 240 min | Rates for PWS subjects were comparable with those of obese and lean controls, unadjusted; no differences when adjusted for FFM and FM | ||
| BMI: 37.35 ± 2.9 (*higher than controls) | BC: DXA | Absolute postprandial REE compared with controls | ||
| LBM, kg: 43.21 (*lower than obese controls) | Rates for PWS subjects were comparable with those of obese and lean controls, unadjusted; no differences when adjusted for FFM and FM | |||
| FM, kg: 3.06 | ||||
| FM, %: 47.68 | ||||
| PWS subtype: 6 DEL/5 UPD | ||||
| GH: none ever received GH Obese controls, n = 12; 5 F/7 M Age, y: 32.25 ± 2.5 BMI: 34.21 ± 1.2 LBM, kg: 52.67 FM, kg: 40.33 FM, %: 46.25 |
||||
| Lean controls, n = 10; 5 F/5 M | ||||
| Age, y: 28.8 ± 1.1 | ||||
| BMI: 21.4 ± 0.4 | ||||
| LBM, kg: 71.54 | ||||
| FM, kg: 14.63 FM, %: 24.32 |
AEE, BEE, REE, and TEE values are expressed as kcal/d unless otherwise indicated. AEE, activity energy expenditure; BC, body composition; BEE, basal energy expenditure; BIA, bioelectrical impedance analysis; BMR, basal metabolic rate; BW, body weight; DEL, deletion; DLW, doubly labeled water; F, female; FFM, fat-free mass; FM, fat mass; GH, growth hormone; HD, hypothalamic damage; IC, indirect calorimetry; ID, imprinting center defects, LBM, lean body mass; M, male; PWS, Prader-Willi syndrome; REE, resting energy expenditure; ref., reference; SEE, sleeping energy expenditure; TEE, total energy expenditure; UPD, uniparental disomy.
AEE in Individuals with PWS
TEE
Despite the obvious concerns for weight management in the PWS population, there is a paucity of studies investigating the energy expenditure of individuals with PWS. To date, only a handful of studies have examined TEE and its components in this cohort (1, 5, 13–16, 18–20), and few have investigated differences between PWS and normal weight subjects or individuals with obesity to understand the propensity for the development of obesity in persons with PWS.
In the 1980s, evidence that suggested that there was a reduction in energy expenditure in PWS first emerged with studies showing that the TEE expenditure in PWS was ∼30% lower than control subjects. To date, 5 studies measured TEE in individuals with PWS. All studies found a reduction in TEE (kilocalories per day) in individuals with PWS compared with matched controls [for either age or BMI (in kg/m2)] ranging from 20% to 46%. Differences in the magnitude of the reduction may be related to the methods employed or the control group used for comparison.
The majority of studies assessed free-living energy expenditure with the use of the gold standard method, DLW (1, 5, 13, 14). The use of DLW allows all components of energy expenditure (REE or BEE, AEE, SEE, and DIT) to be captured over a 7–14 d period. The highest reduction in TEE was reported by Schoeller and colleagues (5) in the late 1980s, who used DLW (under free-living conditions) for 7 d in adolescents with PWS (1980 ± 580 kcal/d). Energy expenditure in PWS was 47% lower than age- and sex-matched controls (3700 ± 820 kcal/d, P < 0.05). However, mean BMI was higher in the control group, for which the measured BMI was 36 compared with a mean BMI of 29 for individuals with PWS. This would lead one to expect a higher TEE based on the higher body weights of the controls, assuming a higher amount of FFM; thus, the reported differences between the cohorts might have been exaggerated.
Another study conducted by van Mil et al. (14) measured TEE with the use of DLW for 14 d and reported a 28% lower TEE rate in PWS children and adolescents (1705 ± 411 kcal/d) compared with the control group, which was matched for age, sex, bone age, and BMI (2374 ± 631 kcal/d; P < 0.01). A similar reduction of 28% in TEE (1758 ± 569 kcal/d) in PWS children and adolescents was reported by Davies and Joughin (13), who used DLW (duration not provided), compared with age- and sex-matched controls (2474 ± 724 kcal/d; P < 0.01). Interestingly, Bekx et al. (1) measured TEE in infants with PWS by using DLW for 7 d and reported that TEE was 24% lower in PWS infants (587 ± 189 kcal/d) compared with normative data for age and sex (775 ± 150 kcal/d; P < 0.001). These latter findings suggest that changes in the energy metabolism profile in PWS originate early on in development.
To date, to our knowledge, only one study has measured TEE through the use of whole-body indirect calorimetry (WBIC). TEE was measured for only 8 h, and the results extrapolated to a full day (15). Under these controlled environment conditions, the authors also reported that PWS adults had a 20% lower TEE (2346 ± 465 kcal/d) than age-matched adults (2973 ± 708 kcal/d; P < 0.001); however, BMI was not matched.
The overall impact of lower TEE in individuals with PWS compared with both healthy age-matched and BMI-matched individuals is that they are expected to have reduced energy requirements. Available energy recommendations and published predictive equations are not specific for PWS and therefore overestimate the energy requirement needs of individuals with PWS. It is well known that a 30–40% reduction in overall energy requirements is recommended for individuals with PWS (21); however, the lack of precise energy recommendations for individuals with PWS makes it difficult to establish baseline values from which adjustments can be made. The current energy recommendation for individuals with PWS is to lower energy intake to maintain a healthy body weight. However, this strategy does not take into consideration hyperphagia, dysfunction in satiety, and food-seeking behaviors that are inherent in PWS (22). Considering these additional factors is crucial when deriving energy needs and assessing satiety to facilitate the development of optimal diets for weight maintenance in children with PWS.
REE and BEE
The majority of studies captured in this review examined differences in REE with the use of indirect calorimetry (16) via a hood canopy system (13, 16, 19, 20) or via a WBIC system (15, 18), with measurement periods ranging from 10 to 30 min. All studies found lower REE or BEE rates (kilocalories per day) in individuals with PWS compared with controls, ranging from 3% to 37%. Differences in the magnitude of reduction may be related to the varied methods of measuring REE compared with BEE (which is measured under standard conditions), length of the measurement, and the characteristics of the control group with which it was compared.
Two studies used the IC hood system to determine REE. Hill et al. (16) measured REE for 15–20 min in PWS children and adolescents (1104 kcal/d) and found that REE was 37% lower than individuals with obesity (1752 kcal/d) and 20% lower than healthy-weight individuals (1392 kcal/d; P < 0.05). Davies and Joughin (13) also assessed REE for 20 min and reported an 18% difference between PWS children and adolescents (1324 ± 408 kcal/d) compared with age- and weight-matched controls (1615 ± 376 kcal/d; P < 0.05).
However, studies measuring REE with the use of IC hood systems in adults reported different findings. Lloret-Linares et al. (19) measured REE in adults with PWS compared with lesional, genetic hypothalamic obesity (HO) and healthy obese individuals. Measured REE in PWS women (1758 ± 360 kcal/d) was comparable with that seen in HO- and BMI-matched control individuals; however, REE in PWS men (1946 ± 428 kcal/d) was reported to be 19% lower than BMI-matched individuals (2405 ± 423 kcal/d; P < 0.05), yet similar to the HO group (19). Lean mass in PWS individuals was 25% lower than BMI-matched individuals, but was comparable to lean mass in the HO group. Purtel et al. (20) measured REE for 30 min in adults with PWS compared with obese- and normal-weight individuals and also reported that REE was comparable to age-matched individuals.
In contrast to studies that used an IC hood system, studies that measured REE with the use of a WBIC system reported a reduced REE in adults with PWS (15, 18). Goldstone et al. (18) measured REE and reported an 8% reduction in REE in PWS individuals (1584 ± 108 kcal/d) compared with BMI-matched controls (1716 ± 69 kcal/d; P < 0.01). Butler et al. (15) reported a 16% lower REE in PWS individuals (2074 ± 360 kcal/d) compared with age-matched controls (2448 ± 475 kcal/d; P < 0.05). It is important to highlight that Butler et al. (15) measured REE when participants were seated (due to feasibility issues for PWS patients). Therefore, this approach may have incorrectly elevated the reported readings, because standardized REE protocols require the patient to be supine, with minimal body motion, and to remain awake during testing. It is also not clear if this adapted, seated REE protocol was applied to individuals without PWS as well. Finally, differences in REE might exist between children with PWS as compared with adults with PWS. Two studies have assessed BEE with the use of an IC hood system. One study by van Mil et al. (14) measured BEE with the use of an IC hood system. They reported results for only 10 min of measurement and concluded that BEE was reduced by ≤16% in PWS children and adolescents (1280 ± 282 kcal/d) compared with age-, sex-, and BMI-matched controls (1524 ± 370 kcal/d; P < 0.05). In another study, Schoeller et al. (5) measured BEE in PWS and found that measured BEE values in PWS adolescence and adults (1160 ± 95 kcal/d) were 3–12% lower than values obtained from various predictive equations: Harris-Benedict (1310 ± 82 kcal/d); Passmore (1400 ± 120 kcal/d); and Cunningham (1200 ± 78 kcal/d; P < 0.05). However, Schoeller et al. (5) did not compare measured BEE in PWS individuals with matched controls.
SEE
Excessive sleepiness, daytime hypersomnolence, and sleep apnea (both central and obstructive) have been reported in individuals with PWS, possibly leading to alterations in the sleeping metabolic rate of individuals with PWS. Only one study has measured SEE in PWS through the use of WBIC. Van Mil et al. (17) reported that SEE was significantly lower in individuals with PWS than in controls (1103 ± 257 kcal/d compared with 1337 ± 63 kcal/d; P < 0.05). This is in agreement with TEE and REE findings in TEE and REE sections, which are reported to be lower for individuals with PWS than those of their counterparts. In summary, absolute values of REE, BEE, and SEE in PWS individuals were found to be lower than those of obese individuals. This finding might be explained by their abnormal body composition (reduction in FFM), which could impact energy expenditure values. Appropriately adjusting for variability in FFM between PWS and controls and the impact this has on the inferences drawn from these studies is discussed in detail in the Contribution of Body Composition to Lower Energy Expenditure section.
AEE
Four studies have measured AEE in individuals with PWS and all reported a lower AEE (13–66%) in PWS than that of controls. Three of these studies assess TEE in free-living conditions with the use of DLW and derived AEE. Mil et al. (14) estimated AEE by subtracting BEE and DIT from TEE; Davies and Joughin (13) estimated AEE by dividing TEE by REE; and Schoeller et al. (5) estimated AEE based on a predictive equation that included FFM based on the methods of Ravussin et al. (23), with daily energy expenditure calculated as (667 + 20.5 FFM).
The greatest difference in AEE was reported by Schoeller et al. (5). In individuals with PWS, AEE (650 ± 310 kcal/d) was 66% less than the AEE measured in age- and sex-matched controls (1940 ± 640 kcal/d; P < 0.05). A 58% difference in AEE when comparing individuals with PWS (256 ±165 kcal/d) to age-, sex-, and BMI-matched controls (611 ± 246 kcal/d; P < 0.001) was also reported by van Mil et al. (14). Furthermore, Davies and Joughin (13) reported daily activity to be 13% lower in individuals with PWS than that of age- and weight-matched individuals (P < 0.05). Physical activity amounts were reported to be lower (1.33 ± 0.21) in individuals with PWS than in age- and weight-matched individuals (1.53 ± 0.23, P < 0.05). The data for AEE was not given and was estimated based on the total means given for TEE and REE. The estimated AEE was found to be lower (526 kcal/d) in individuals with PWS than in age- and weight-matched individuals (859 kcal/d).
Only one study measured AEE directly through the use of a whole-body calorimetry unit (15); the energy expended on physical activity during the 8 h was 38% lower in individuals with PWS (1.9 ± 0.61 kcal/min compared with 3 ± 0.99 kcal/min for the controls; P < 0.001). In addition, standing energy expenditure was reported to be lower in PWS individuals (1.5 ± 0.3 kcal/min) than in age-matched control subjects (1.8 ± 0.4 kcal/min; P < 0.001). In summary, the literature to date suggests that adults and children with PWS tend to have lower absolute AEE values. Again, these differences may be explained by muscle hypotonia and less skeletal muscle mass, leading to lower values of FFM (15).
DIT
Unfortunately, only one study has investigated DIT in individuals with PWS. Purtell et al. (20) found no difference in DIT between PWS and control groups after consumption of a breakfast meal high in carbohydrate and fat (600 kcal: 50% carbohydrate, 35% fat, and 15% protein). Because examination of DIT was not the primary objective of the Purtell et al. (20) study, details concerning the measurement protocol for DIT were not reported. Therefore, it is unclear how these differences were assessed and whether baseline adjustments for body composition were made.
Exploring the impact of DIT on PWS is important because dietary macronutrient manipulation may have an impact on energy metabolism in PWS. It is well known that meals of similar energy content but different macronutrient composition will impact DIT, which could influence the overall estimates of TEE. Protein has the greatest impact on DIT in both normal-weight and obese cohorts (24, 25). Studies have now demonstrated that higher-protein diets are able to increase satiety hormones, decrease hunger, and increase DIT, and thereby promote maintenance of FFM in the setting of low energy intake (26). We speculate that DIT is lower in individuals with PWS, which also contributes to their lower REE. The hierarchy of macronutrients with respect to their impact on DIT is protein, then carbohydrate, and, lastly, fat. It has been documented that protein plays an important role in body weight regulation through satiety related to DIT (27). In conclusion, macronutrient composition should be taken into consideration when estimating the energy needs of individuals with PWS. Although DIT contributes to only 5–15% of daily energy expenditure, alterations in DIT could translate into energy imbalance, leading to significant excessive weight gain over the longer term. Future research should clarify additional means to favorably manipulate DIT to improve overall energy balance.
Contribution of Body Composition to Lower Energy Expenditure
A unique altered body composition consisting of higher fat mass (FM) and a lower FFM for body weight is described in PWS infants, even before hyperphagia manifests (1). This suggests a possible genetic or developmental origin to the altered body composition phenotype documented in PWS. As mentioned previously, a major determinant of energy expenditure is body composition, specifically, the metabolically active tissues that make up the FFM compartment. The amount of FFM (in kilograms) can vary considerably between PWS, obese, and nonobese individuals (28). Overall, individuals with PWS tend to have lower FFM. Therefore, the absolute value of REE tends to be lower in PWS individuals due to their reduced FFM; however, once FFM is taken into consideration, these differences between PWS and controls mainly disappear (1, 5, 13, 14, 16, 17, 19, 20), suggesting that there might not be a major disruption to energy metabolism at a cellular level in PWS.
In most studies included in this review, the adjustment method was performed by simply dividing REE or BEE by FFM. However, this method is not optimal because FFM includes bone, skeletal mass, and highly metabolically active organs, such as the brain, heart, liver, kidney, and gastrointestinal tract (12, 29). When body weight instead of FFM is used to calculate BEE and SEE, these values remained significantly lower in individuals with PWS compared with age-, sex-, and BMI-matched controls (30). However, the use of body weight for adjustment might not be ideal due to unique differences in body composition in individuals with PWS. Finally, Butler et al. (15) reported that REE remained significantly (P < 0.001) lower when adjusting for FM between the 2 groups. The justification for expressing energy expenditure data adjusted for FM is questionable, and the authors offer no rationale for their choice of adjustment methods. Additionally, a major limitation of the above study is that individuals with PWS were not compared with a BMI-matched group.
It has been suggested that the adjustment of energy expenditure for FFM should be carried out by calculating residuals from regression models, including other covariates (e.g., sex, age, FM, and height), as described by Carneiro et al. (12). Such an adjustment method that uses multiple liner regression analysis was used in the study by Goldstone et al. (18), who reported that individuals with PWS had lower REE than normal-weight and BMI-matched controls, after adjustment for FM and age; REE was higher after adjustment for age and FFM. These findings highlight the importance of correctly accounting for differences in FFM when comparing groups with different body composition, because without proper adjustment, results may be misinterpreted. Another study conducted by Hill et al. (16) that used multiple linear regression analysis reported no difference in REE adjusted for FFM in individuals with PWS compared with controls. A limitation of this study was the use of bioelectrical impedance analysis (BIA) to measure FFM. BIA assesses total body water and then estimates FFM and FM with the use of algorithms. These algorithms may not be suitable for use in subjects with PWS because they have not been validated in this clinical population. Additionally, BIA assumes a constant FFM hydration factor (31), which varies considerably with age and clinical condition (32). Body composition is altered in PWS individuals, with specific alterations in body water distribution noted (33); thus, the underlying assumptions that the BIA technique relies on may be violated. This would result in an overestimation of the amount of FFM and an underestimation of FM in individuals with PWS (28, 31). Therefore, REE may be overestimated in individuals with PWS, which would lead to the erroneous conclusion that obese individuals have a lower REE than PWS individuals due to the inadequate normalization for FFM.
It is important to highlight the importance of the use of adequate body composition tools in PWS to better account for FFM in analysis. The use of total body potassium to estimate whole-body cell mass and metabolically active tissue (34) to examine body composition in PWS has not yet been utilized. However, using total body potassium would provide a more accurate measure of metabolically active lean tissue without the adverse effect of potential hydration changes.
Overall, these studies suggest that individuals with PWS have lower absolute REE values than obese individuals due to their lower FFM. Efforts to increase FFM in individuals with PWS may therefore be an effective strategy to increase their overall energy expenditure.
Contribution of Endocrine Dysfunction to Lower Energy Expenditure
The common hormonal abnormalities observed in PWS, such as growth hormone (GH) and thyroid-stimulating hormone deficiencies, as well as testosterone insufficiency, contribute to lower energy expenditure due to their effects on FFM (35). The altered body composition (i.e., lower FFM values) observed in individuals with PWS has been a point of interest for clinicians and has resulted in the increased use of GH therapy and thyroid and testosterone supplementation (36, 37). GH treatment during childhood and in adults with PWS has been shown to increase FFM (37–39). In addition, testosterone supplementation in adults with PWS has been shown to increase the amount of FFM (36). The resultant increased FFM likely increases energy expenditure and may help individuals with PWS obtain and maintain a healthy body weight. GH has potent protein anabolic and lipid and carbohydrate metabolic effects. In general, GH stimulates lipolysis, hyperinsulinemia, and stimulation of insulin-like growth factor 1 activity. This results in potent protein anabolic effects, increased amino acid uptake, increased protein synthesis, and decreased protein oxidation (40). Finally, controlled studies have reported significant increases in REE with the use of GH in PWS (37).
Of the previous studies reviewed, only 2 reported the use of GH (1, 19) in the assessed subjects with PWS. Lloret-Linares et al. (19) reported that 22% of individuals (6 out of 27 subjects) with PWS were receiving GH therapy. Bekx et al. (1) reported that 87.5% of individuals (14 out of 16 subjects) with PWS were receiving GH treatment. The use of GH in the remainder of the examined studies is unknown due to a lack of reporting. Additionally, details as to whether GH status was considered as a covariate in the final analysis are unknown, which may also explain the inconsistent findings between studies.
Summary and Gaps in Understanding of Energy Expenditure in PWS
From the literature to date, the findings suggest that PWS individuals have lower absolute energy expenditure than age-, sex-, and BMI-matched controls as a consequence of reduced REE, SEE, and AEE; these differences may be secondary to altered body composition and hormone dysfunction. However, due to the paucity of information on DIT in individuals with PWS, the relative contribution of specific components of TEE to the altered metabolism in PWS is not clear. Once body composition is taken into consideration, many differences disappear, suggesting that there might in fact be no overall disturbance in whole-body energy metabolism. However, these results should be interpreted with caution because of the inherent limitations in the use of adjustment methods and the discrepancies in the methods used to measure body composition in PWS. Additionally, DIT needs to be assessed in individuals with PWS and compared with healthy obese individuals, whereas differences in energy expenditure estimates according to genetic subtype and impact of hormonal therapies need further study.
The mechanism of action for the significant overall reduction in energy expenditure in individuals with PWS is likely multifactorial. Approximately one-third of children with PWS have hypothyroidism due to hypothalamic-pituitary dysfunction, resulting in low or low-normal concentrations of thyroid-stimulating hormone and low concentrations of total or free thyroxine. Therefore, central hypothyroidism may play a role in the reduced energy expenditure demonstrated in children with PWS (41). A PWS mouse model [mice lacking MAGE-like 2 (Magel2)] demonstrated normal leptin sensitivity in proopiomelanocortin neurons into the early postweaning period; however, thereafter, the mice demonstrated progressive leptin insensitivity, which is predicted to impair the release of the melanocortin receptor agonist α-melanocyte stimulating hormone, with downstream effects to reduce energy expenditure (42). It is speculated that a similar reduction in leptin responsiveness occurs over time in children with PWS; this would account for a reduction in energy expenditure in individuals with PWS, which would worsen with aging. Previous research has examined whether individuals with PWS exhibit reduced fat oxidation. One study by Purtell et al. (20) did not detect metabolic defect in respiratory quotient or fat oxidation in individuals with PWS after a mixed high-carbohydrate and high-fat meal. However, Rubin et al. (43) examined the effect of PWS on the hormonal and metabolic response to resistance exercise; individuals with PWS demonstrated earlier increases in FAs during recovery and exhibited higher glycerol and ketone concentrations than controls, suggesting incomplete fat oxidation (43). Previous longitudinal studies in Pima Indians report that low fat oxidizers (90th percentile for respiratory quotient) had a 2.5 times greater risk of gaining ≥5 kg of body weight than high fat oxidizers (44). Furthermore, those successful at maintaining weight loss tend to have higher fat oxidation rates than those experiencing weight relapse (45). Thus, lower fat oxidation in individuals with PWS might also contribute to the imbalance between intake and energy expenditure. Finally, lower spontaneous physical activity (15) and sympathetic activity (46) have been reported in individuals with PWS. Children with PWS demonstrated no exercise-induced increase in catecholamines and an ∼40% lower heart rate elevation in response to exercise compared with controls. These findings suggest a sympathetic autonomic deficit in PWS (47–49). In summary, altered endocrine function (thyroid and leptin deficits) and lower fat oxidation, sympathetic activity, and spontaneous physical activity are multiple factors that might play an etiologic role in the reduction in energy expenditure that is characteristic of individuals with PWS. Further longitudinal studies are required to determine if this defect in energy expenditure progressively worsens with age.
Finally, it is important to highlight that although no metabolic differences have been documented to date, accurate assessments of FM and FFM could certainly affect the calculation of energy requirements for those with PWS compared with healthy controls. Currently, energy requirements are based on predictive equations used to assess REE. These equations incorporate the use of body weight and assume a “healthy or reference” body composition, which is problematic in this clinical cohort in which disturbances in body composition have been clearly documented. The abnormal body composition in individuals with PWS (characterized by reduced FFM and increased FM) would lower energy expenditure and thus lower energy requirements compared with healthy individuals. Thus, assessing body composition accurately with the use of a valid techniques and determining energy requirements taking body composition into consideration are integral parts of dietary management in individuals with PWS. Increasing FFM and maximizing physical activity should be considered to increase energy expenditure in PWS.
Acknowledgments
All authors read and approved the final version of the paper.
Footnotes
Abbreviations used: AEE, activity energy expenditure; BEE, basal energy expenditure; BIA, bioelectrical impedance analysis; DIT, diet-induced thermogenesis; DLW, doubly labeled water; FFM, fat-free mass; FM, fat mass; GH, growth hormone; HO, hypothalamic obesity; IC, indirect calorimetry; PWS, Prader-Willi syndrome; SEE, sleeping energy expenditure; TBK; total body potassium; TEE, total energy expenditure; WBIC, whole-body indirect calorimetry.
References
- 1.Bekx MT, Carrel AL, Shriver TC, Li Z, Allen DB. Decreased energy expenditure is caused by abnormal body composition in infants with Prader-Willi Syndrome. J Pediatr 2003;143:372–6. [DOI] [PubMed] [Google Scholar]
- 2.Cassidy SB, Driscoll DJ. Prader-Willi syndrome. Eur J Hum Genet 2009;17:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burman P, Ritzén EM, Lindgren AC. Endocrine dysfunction in Prader-Willi Syndrome: a review with special reference to GH. Endocr Rev 2001;22:787–99. [DOI] [PubMed] [Google Scholar]
- 4.Carrel AL, Myers SE, Whitman BY, Allen DB. Prader-Willi syndrome: the effect of growth hormone on childhood body composition. Endocrinologist 2000;10(4 Suppl 1):43S–9S. [Google Scholar]
- 5.Schoeller DA, Levitsky LL, Bandini LG, Dietz WW, Walczak A. Energy expenditure and body composition in Prader-Willi syndrome. Metabolism 1988;37:115–20. [DOI] [PubMed] [Google Scholar]
- 6.Coplin SS, Hine J, Gormican A. Out-patient dietary management in the Prader-Willi syndrome. J Am Diet Assoc 1976;68:330–4. [PubMed] [Google Scholar]
- 7.Butler MG. Management of obesity in Prader-Willi syndrome. Nat Clin Pract Endocrinol Metab 2006;2:592–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinheiro Volp AC, Esteves de Oliveira FC, Duarte Moreira Alves R, Esteves EA, Bressan J. Energy expenditure: components and evaluation methods. Nutr Hosp 2011;26:430–40. [DOI] [PubMed] [Google Scholar]
- 9.Westerterp KR. Physical activity and physical activity induced energy expenditure in humans: measurement, determinants, and effects. Front Physiol 2013;4:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blasco Redondo R. Resting energy expenditure; assessment methods and applications. Nutr Hosp 2015;31 Suppl 3:245–54. [DOI] [PubMed] [Google Scholar]
- 11.Garby L, Kurzer MS, Lammert O, Nielsen E. Energy expenditure during sleep in men and women: evaporative and sensible heat losses. Hum Nutr Clin Nutr 1987;41:225–33. [PubMed] [Google Scholar]
- 12.Carneiro IP, Elliott SA, Siervo M, Padwal R, Bertoli S, Battezzati A, Prado CM. Is obesity associated with altered energy expenditure? Adv Nutr 2016;7:476–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies PS, Joughin C. Using stable isotopes to assess reduced physical activity of individuals with Prader-Willi syndrome. Am J Ment Retard 1993;98:349–53. [PubMed] [Google Scholar]
- 14.van Mil EG, Westerterp KR, Kester AD, Curfs LM, Gerver WJ, Schrander-Stumpel C, Saris WH. Activity related energy expenditure in children and adolescents with Prader-Willi syndrome. Int J Obes Relat Metab Disord 2000;24:429–34. [DOI] [PubMed] [Google Scholar]
- 15.Butler MG, Theodoro MF, Bittel DC, Donnelly JE. Energy expenditure and phvsical activity in Prader-Willi syndrome: comparison with obese subjects. Am J Med Genet A 2007;143A:449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill JO, Kaler M, Spetalnick B, Reed G, Butler MG. Resting metabolic rate in Prader-Willi Syndrome. Dysmorphol Clin Genet 1990;4:27–32. [PMC free article] [PubMed] [Google Scholar]
- 17.van Mil EA, Westerterp KR, Gerver WJ, Curfs LM, Schrander-Stumpel CT, Kester AD, Saris WH. Energy expenditure at rest and during sleep in children with Prader-Willi syndrome is explained by body composition. Am J Clin Nutr 2000;71:752–6. [DOI] [PubMed] [Google Scholar]
- 18.Goldstone AP, Brynes AE, Thomas EL, Bell JD, Frost G, Holland A, Ghatei MA, Bloom SR. Resting metabolic rate, plasma leptin concentrations, leptin receptor expression, and adipose tissue measured by whole-body magnetic resonance imaging in women with Prader-Willi syndrome. Am J Clin Nutr 2002;75:468–75. [DOI] [PubMed] [Google Scholar]
- 19.Lloret-Linares C, Faucher P, Coupaye M, Alili R, Green A, Basdevant A, Clément K, Poitou C. Comparison of body composition, basal metabolic rate and metabolic outcomes of adults with Prader Willi syndrome or lesional hypothalamic disease, with primary obesity. Int J Obes (Lond) 2013;37:1198–203. [DOI] [PubMed] [Google Scholar]
- 20.Purtell L, Viardot A, Sze L, Loughnan G, Steinbeck K, Sainsbury A, Herzog H, Smith A, Campbell LV. Postprandial metabolism in adults with Prader-Willi syndrome. Obesity (Silver Spring) 2015;23:1159–65. [DOI] [PubMed] [Google Scholar]
- 21.Miller JL, Lynn CH, Shuster J, Driscoll DJ. A reduced-energy intake, well-balanced diet improves weight control in children with Prader-Willi syndrome. J Hum Nutr Diet 2013;26:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martínez Michel L, Haqq AM, Wismer WV. A review of chemosensory perceptions, food preferences and food-related behaviours in subjects with Prader-Willi Syndrome. Appetite 2016;99:17–24. [DOI] [PubMed] [Google Scholar]
- 23.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest 1986;78:1568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maffeis C, Schutz Y, Grezzani A, Provera S, Piacentini G, Tato L. Meal-induced thermogenesis and obesity: is a fat meal a risk factor for fat gain in children? J Clin Endocrinol Metab 2001;86:214–9. [DOI] [PubMed] [Google Scholar]
- 25.Baum JI, Gray M, Binns A. Breakfasts higher in protein increase postprandial energy expenditure, increase fat oxidation, and reduce hunger in overweight children from 8 to 12 years of age. J Nutr 2015;145:2229–35. [DOI] [PubMed] [Google Scholar]
- 26.Pesta DH, Samuel VT. A high-protein diet for reducing body fat: mechanisms and possible caveats. Nutr Metab (Lond) 2014;11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westerterp KR. Diet induced thermogenesis. Nutr Metab (Lond) 2004;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bedogni G, Grugni G, Tringali G, Agosti F, Sartorio A. Assessment of fat-free mass from bioelectrical impedance analysis in obese women with Prader-Willi syndrome. Ann Hum Biol 2015;42:538–42. [DOI] [PubMed] [Google Scholar]
- 29.Heymsfield SB, Gallagher D, Kotler DP, Wang Z, Allison DB, Heshka S. Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am J Physiol Endocrinol Metab 2002;282:E132–8. [DOI] [PubMed] [Google Scholar]
- 30.Colditz GA. Overview of the epidemiology methods and applications: strengths and limitations of observational study designs. Crit Rev Food Sci Nutr 2010;50 Suppl 1:10–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deurenberg P. Limitations of the bioelectrical impedance method for the assessment of body fat in severe obesity. Am J Clin Nutr 1996;64(3 Suppl):449S–52S. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, Heymsfield SB. Hydration of fat-free body mass: review and critique of a classic body-composition constant. Am J Clin Nutr 1999;69:833–41. [DOI] [PubMed] [Google Scholar]
- 33.van Mil EG, Westerterp KR, Gerver WJ, Van Marken Lichtenbelt WD, Kester AD, Saris WH. Body composition in Prader-Willi syndrome compared with nonsyndromal obesity: relationship to physical activity and growth hormone function. J Pediatr 2001;139:708–14. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Heshka S, Pietrobelli A, Chen Z, Silva AM, Sardinha LB, Wang J, Gallager D, Heymsfield S. A new total body potassium method to estimate total body skeletal muscle mass in children. J Nutr 2007;137:1988–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aycan Z, Bas VN. Prader-Willi syndrome and growth hormone deficiency. J Clin Res Pediatr Endocrinol 2014;6:62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kido Y, Sakazume S, Abe Y, Oto Y, Itabashi H, Shiraishi M, Yoshino A, Tanaka Y, Obata K, Murakami N, et al. Testosterone replacement therapy to improve secondary sexual characteristics and body composition without adverse behavioral problems in adult male patients with Prader-Willi syndrome: an observational study. Am J Med Genet A 2013;161A:2167–73. [DOI] [PubMed] [Google Scholar]
- 37.Haqq AM, Stadler DD, Jackson RH, Rosenfeld RG, Purnell JQ, LaFranchi SH. Effects of growth hormone on pulmonary function, sleep quality, behavior, cognition, growth velocity, body composition, and resting energy expenditure in Prader-Willi syndrome. J Clin Endocrinol Metab 2003;88:2206–12. [DOI] [PubMed] [Google Scholar]
- 38.Carrel AL, Moerchen V, Myers SE, Bekx MT, Whitman BY, Allen DB. Growth hormone improves mobility and body composition in infants and toddlers with Prader-Willi syndrome. J Pediatr 2004;145:744–9. [DOI] [PubMed] [Google Scholar]
- 39.Höybye C. Endocrine and metabolic aspects of adult Prader-Willi syndrome with special emphasis on the effect of growth hormone treatment. Growth Horm IGF Res 2004;14:1–15. [DOI] [PubMed] [Google Scholar]
- 40.Møller N, Jørgensen JO, Møller J, Orskov L, Ovesen P, Schmitz O, Christiansen JS, Orskov H. Metabolic effects of growth hormone in humans. Metabolism 1995;44:33–6. [DOI] [PubMed] [Google Scholar]
- 41.Emerick JE, Vogt KS. Endocrine manifestations and management of Prader-Willi syndrome. Int J Pediatr Endocrinol 2013;2013:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pravdivyi I, Ballanyi K, Colmers WF, Wevrick R. Progressive postnatal decline in leptin sensitivity of arcuate hypothalamic neurons in the Magel2-null mouse model of Prader-Willi syndrome. Hum Mol Genet 2015;24:4276–83. [DOI] [PubMed] [Google Scholar]
- 43.Rubin DA, Clark SJ, Haqq AM, Castner DM, Ng J, Judelson DA. Hormonal and metabolic responses to a single bout of resistance exercise in Prader-Willi Syndrome. Horm Res Paediatr 2017;87:153–61. [DOI] [PubMed] [Google Scholar]
- 44.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, Swinburn BA, Knowler WC, Bogardus C, Ravussin E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol 1990;259:E650–7. [DOI] [PubMed] [Google Scholar]
- 45.Froidevaux F, Schutz Y, Christin L, Jequier E. Energy expenditure in obese women before and during weight loss, after refeeding, and in the weight-relapse period. Am J Clin Nutr 1993;57:35–42. [DOI] [PubMed] [Google Scholar]
- 46.Rubin DA, Clark SJ, Ng J, Castner DM, Haqq AM, Judelson DA. Hormonal and metabolic responses to endurance exercise in children with Prader-Willi syndrome and non-syndromic obesity. Metabolism 2015;64:391–5. [DOI] [PubMed] [Google Scholar]
- 47.Castner DM, Rubin DA, Judelson DA, Haqq AM. Effects of adiposity and Prader-Willi Syndrome on postexercise heart rate recovery. J Obes 2013;2013:384167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DiMario FJ Jr, Dunham B, Burleson JA, Moskovitz J, Cassidy SB. An evaluation of autonomic nervous system function in patients with Prader-Willi syndrome. Pediatrics 1994;93:76–81. [PubMed] [Google Scholar]
- 49.Richer LP, DeLorey DS, Sharma AM, Kreier F, Freemark M, Mackenzie ML, Haqq A. Autonomic nervous system (ANS) dysfunction in PWS and childhood obesity: preliminary findings. Proceedings of the 31st Prader-Willi Syndrome Association (USA) Annual National Conference, 2011; Orlando (FL).