Abstract
There is substantial evidence that the prevalence of vitamin D deficiency is unacceptably high in the population, and this requires action from a public health perspective. Circulating 25-hydroxyvitamin D [25(OH)D] is a robust and reliable marker of vitamin D status and has been used by numerous agencies in the establishment of vitamin D dietary requirements and for population surveillance of vitamin D deficiency or inadequacy. In a wider context, modeling of serum 25(OH)D data and its contributory sources, namely dietary vitamin D supply and UVB availability, can inform our understanding of population vitamin D status. The aim of this review is to provide the current status of knowledge in relation to modeling of such vitamin D–relevant data. We begin by highlighting the importance of the measurement of 25(OH)D and its standardization, both of which have led to new key data on the prevalence of vitamin D deficiency and inadequacy in North America and Europe. We then overview how state-of-the-art modeling can be used to inform our understanding of the potential effect of ergocalciferol and 25(OH)D on vitamin D intake estimates and how meteorological data on UVB availability, when coupled with other key data, can help predict population serum 25(OH)D concentration, even accounting for seasonal fluctuations, and lastly, how these in silico approaches can help inform policymakers on strategic options on addressing low vitamin D status through food-based approaches and supplementation. The potential of exemplar food-based solutions will be highlighted, as will the possibility of synergies between vitamin D and other dairy food–based micronutrients, in relation to vitamin D status and bone health. Lastly, we will briefly consider the interactions between season and vitamin D supplements on vitamin D status and health.
Keywords: vitamin D deficiency, 25(OH)D assessment, mathematical modeling, vitamin D food fortification, UVB availability
Introduction
The major source of vitamin D in humans is the UVB component of sunlight; UVB radiation stimulates cutaneous synthesis of vitamin D3 (cholecalciferol), which undergoes hydroxylation in the liver to 25-hydroxyvitamin D [25(OH)D] (1). Several environmental factors, such as latitude and prevailing weather conditions, determine whether UVB of sufficient strength is available to stimulate the conversion of 7-dehydrocholesterol in the skin to precholecalciferol (2). Personal attributes, such as skin pigmentation, age, clothing, working environment, physical activity, sunscreen use, and sun exposure behavior, can also much reduce, if not prevent, cholecalciferol synthesis (2). Vitamin D also occurs in the diet, both naturally and as a fortificant, as cholecalciferol and vitamin D2 (ergocalciferol) and in nutritional supplements.
The well-known late-winter nadir in circulating 25(OH)D concentrations means that substantial portions of the population resident at latitudes greater than ∼40° rely on body stores and vitamin D in the diet to maintain healthy vitamin D status all year. Because body stores are dependent on sun exposure, the importance of the diet in maintaining vitamin D status above the level of deficiency is a corollary of UVB sunlight deficit (3). There is increasing evidence that the dietary supply is currently unable to offset the seasonal sunlight deficit, which increases with latitude and the duration of winter (4). There are very few rich natural sources of vitamin D; these are oily fish and cod liver oil (which are consumed sporadically), egg yolk, fortified foods, and UV-exposed mushrooms or UV-irradiated yeast, in which ergocalciferol is found. Food consumption surveys throughout Europe, Canada, the United States, and beyond have all consistently reported low vitamin D intake and widespread dietary inadequacy (4).
This review is based on 4 vitamin D–related presentations from Session V of the 4th International Vitamin Conference held in Copenhagen, Denmark, 25-27 May 2016. We begin the review by highlighting the importance of measurement of circulating 25(OH)D and its standardization, both of which have led to new key data on the prevalence of vitamin D deficiency and inadequacy. We then provide an overview on how state-of-the-art modeling can be used to inform our understanding of the potential effect of the minor vitamer and metabolite of vitamin D in the food chain [i.e., ergocalciferol and 25(OH)D, respectively] on vitamin D intake estimates and of how changes in estimates in overall vitamin D intake, in the presence and absence of UVB availability, effects population serum 25(OH)D concentrations. This is important in terms of informing food-based approaches toward improving vitamin D status and preventing vitamin D deficiency. The potential of exemplar food-based solutions for addressing low vitamin D status, such as vitamin D–biofortified foods as well as traditional vitamin D fortification of more novel dairy-based foods, will be highlighted, and the possibility of synergies between vitamin D and other naturally present micronutrients present in dairy foods will be explored. Lastly, we briefly consider interactions between seasons and vitamin D supplements on vitamin D status and health.
Current Status of Knowledge
25(OH)D as a marker of vitamin D status and its assessment
There is consensus that serum or plasma 25(OH)D concentration should be used to assess vitamin D status because it reflects the contributions from both diet and synthesis in the skin (1, 5). A systematic review of existing and potentially novel functional markers of vitamin D status reported that serum 25(OH)D concentration increased in response to supplemental vitamin D intake in all the included randomized controlled trials (RCTs), irrespective of whether ergocalciferol or cholecalciferol was used, differing analytical techniques, study duration (6 wk to >2 y), or age group of the participants (6). Serum or plasma 25(OH)D concentration was used as an indicator of vitamin D status by the Institute of Medicine (IOM) DRI committee on calcium and vitamin D in North America (1) as well as the UK and European Union authorities (5, 7–9) to establish dietary reference values for vitamin D.
Several reports have shown that available 25(OH)D assays can yield markedly differing results (10–12), and this has confounded international efforts to develop evidenced-based guidelines (12). Importantly, the issue of international standardization of serum 25(OH)D measurement has been progressed by the Vitamin D Standardization Program (VDSP), a collaborative initiative between the Office of Dietary Supplements of the NIH, the CDC, the National Institute of Standards and Technology, and a number of the national health surveys around the world (12, 13). The VDSP has developed protocols for standardization of existing serum 25(OH)D data from national nutrition and health surveys and cohort studies (14–17), which allow for more valid between-country or -region comparisons of vitamin D status and prevalence of vitamin D deficiency (see below).
Serum 25(OH)D thresholds underpinning international vitamin D recommendations and some associated population surveillance estimates
The IOM DRI committee in the United States, with the use of bone health as the basis for developing DRI for vitamin D, suggested that people are at risk of deficiency at serum 25(OH)D concentrations <30 nmol/L; some, but not all, people are potentially at risk of inadequacy at serum 25(OH)D concentrations from 30 to ≤50 nmol/L; and that practically all people are vitamin D sufficient at concentrations >50 nmol/L (1). In contrast, the Endocrine Society Task Force on Vitamin D in the United States (18) suggests that individuals should be identified as vitamin-D-deficient at a cut-off level of 50 nmol/L serum 25(OH)D, and to maximize the effect of vitamin D on calcium, bone, and muscle metabolism, serum 25(OH)D concentration should exceed 75 nmol/L. A serum 25(OH)D threshold of 50 nmol/L in terms of adequacy of vitamin D has been adopted by several European agencies, including the European Food Safety Authority (9) [for recent reviews, see Cashman (19) and Hayes and Cashman (20)]. However, the Scientific Advisory Committee on Nutrition in the United Kingdom, after considering the evidence, suggested that the risk of poor musculoskeletal health was increased at serum 25(OH)D concentrations <25 nmol/L (5). There is universal agreement that we do not wish to have individuals in the populations with circulating 25(OH)D concentrations <25–30 nmol/L.
Estimates of the prevalence of vitamin D deficiency [based on data of VDSP standardized serum 25(OH)D <30 nmol/L] in representative population samples in the United States (n = 15,652) (14), Canada (n = 11,336) (15), and Europe [n = 55,844; with the use of a collection of 14 nationally or regionally representative studies in the European Commission (EC)-funded ODIN (Food-Based Solutions for Optimal Vitamin D Nutrition and Health Through the Life Cycle) project; www.odin-vitd.eu] (16) have been reported recently as 5.9%, 7.4%, and 13%, respectively. As worrisome as these population estimates are, they do not capture the differences in prevalence arising from factors such as age, seasonality, geographical location, and ethnicity in these regions. For example, the prevalence of serum 25(OH)D concentrations <30 nmol/L increases from 8.2% in summer to 17.7% in winter in the European sample (16) and from 3.3% in summer to 9.3% in winter in the United States (14). Across ethnic groups, the prevalence of serum 25(OH)D concentrations <30 nmol/L in non-Hispanic white, Hispanic, and non-Hispanic black populations in the United States is 2.3%, 6.4%, and 24%, respectively (14). Young adults are at high risk: in the United States, the prevalence of serum 25(OH)D concentrations <30 nmol/L in those aged 1–11 y is only 0.7%, whereas it is 8.2% in those aged 20–39 y (14). The average yearly population prevalence of standardized serum 25(OH)D concentrations <50 nmol/L in Europe, the United States, and Canada is 40.4%, 24.0%, and 36.8%, respectively (14–16). Clearly, regardless of which threshold is used, strategies for vitamin D deficiency prevention are required (19). The typical average intake for populations within the EU and the United States are generally ∼3–8 μg/d on average, depending on the country (21). There is a significant gap between the typical intake in both European and North American populations and the estimated average requirement (EAR) for vitamin D intake, which was set by the IOM at 10 μg/d for those aged ≥1 y (1).
Modeling the potential effect of ergocalciferol and 25(OH)D in the food chain on vitamin D intake estimates
Although much of the vitamin D in the diet (including in fortified foods and supplements) is in the form of cholecalciferol, ergocalciferol and 25(OH)D are also present and may be underestimated contributors to vitamin D nutritional status.
25(OH)D in food and effect on vitamin D nutriture.
25(OH)D is present in certain foods of animal origin, such as meat, offal, eggs, and, to a lesser extent, fish [for reviews, see Cashman (22) and Ovesen et al. (23)]. The total vitamin D activity of these animal foods in food compositional tables in the United Kingdom (and those in Denmark and Switzerland) accounts not only for the vitamin D content of the food, but also for the content of the 25(OH)D multiplied by a factor of 5 (24–26). However, the US food composition database does not account for the 25(OH)D content of food or apply an efficacy factor (27). Importantly, Taylor et al. (28) performed some modeling to include overall food-derived 25(OH)D content in intake estimates for US adults, which showed that there was a potentially meaningful increase (1.7–2.9 μg or 15–30% of the EAR) in vitamin D intake estimates. However, there is some debate around the use of the factor of 5, with alternate suggested factors ranging from 1 to 9 [for reviews, see Cashman (22), Ovesen et al. (23), and Heaney et al. (29)]. In a specifically designed RCT aimed at addressing this question, consumption of orally consumed synthetic 25-hydroxycholecalciferol [25(OH)D3] was shown to be 5 times more effective than an equivalent amount of cholecalciferol at improving the serum 25(OH)D concentrations of older adults in winter (30).
Although the use of synthetic 25(OH)D as a food fortificant has not been approved yet, its use in animal feeds for certain species is permitted in Europe and the United States (31, 32), and this can increase the content of 25(OH)D3 in the human food chain. For example, the addition of commercially available 25(OH)D3 to the diet of hens has been shown to increase egg 25(OH)D3 content (33–35). This approach of increasing the total vitamin D content of foods of animal origin by increasing the dietary vitamin D or 25(OH)D amount in animal feed has been referred to as biofortification (19). Importantly, a recent winter-based RCT of older adults (n = 55) showed that weekly consumption of 7 vitamin D–biofortified eggs, produced by hens provided with feed containing 25(OH)D3 (or cholecalciferol) at the allowable maximum content, prevented the typical decline in serum 25(OH)D concentration during winter and any incidence of vitamin D deficiency (36). The control group in the study, who were requested to consume up to a maximum of 2 commercially available eggs/wk, had a significant decline in serum 25(OH)D over the 8 wk of winter, and 22% had vitamin D deficiency [serum 25(OH)D <25 nmol/L] at the endpoint (36).
Vitamin D2 in food and effect vitamin D nutriture.
It has been suggested that ergocalciferol is not very prevalent in the human food chain. However, data from a number of recent intervention studies as well from the National Adult Nutrition Survey (NANS) in Ireland, a nationally representative sample of Irish adults, suggest that the majority of subjects had measurable serum 25-hydroxyergocalciferol [25(OH)D2] concentrations (37). Serum 25(OH)D2, unlike 25(OH)D3, is not directly influenced by skin exposure to UVB sunlight and thus has only dietary origins; however, quantifying dietary ergocalciferol is difficult because of the limitations of food composition data. Cashman et al. (37) used serum 25(OH)D2 concentrations in the NANS participants to estimate the intake of ergocalciferol with the use of a mathematical modeling approach. This approach used recently published RCT data on the relation between ergocalciferol intake and the responses of serum 25(OH)D2 concentrations in combination with data on serum 25(OH)D2 concentration distribution in NANS. The projected median intake of ergocalciferol ranged from 1.7 to 2.3 μg/d, suggesting that it may have an effect on nutritional adequacy at a population level and thus warrants further investigation (37).
Modeling of serum 25(OH)D concentration to inform approaches toward improving vitamin D status
Modeling UVB and dietary vitamin D data to predict serum 25(OH)D concentrations in the population and changes arising from vitamin D fortification or supplementation.
Even accounting for potentially underestimated contributions of dietary-derived 25(OH)D3 and ergocalciferol to overall vitamin D intake in some populations, it is unrealistic to expect the habitual Western-style diet to supply vitamin D at 10 μg/d [i.e., the EAR (1)] across the population. For example, Roman Viñas et al. (38) showed that, of European national nutrition surveys reporting vitamin D intake data from 2000 on, 77–100% and 55–100% of adults (19–64 y of age) and the elderly people (>64 y of age), respectively, had intakes below the EAR. Consequently, there have been calls for the use of vitamin D supplements as a means of correcting low vitamin D intake and status in European populations, and, in fact, vitamin D supplement use has been recommended as national policy in certain countries, particularly for at-risk population groups (39). Although vitamin D supplementation has been shown to significantly improve vitamin D intake across a variety of age and sex groups, with dose-dependent increases in serum 25(OH)D concentrations (6), relying on supplements is not an appropriate public health strategy to increase intake across the population because supplements are only effective for those who consume them, and uptake within the population is generally too low to provide widespread population protection, as is outlined elsewhere (40). Based on the collective evidence from food-based RCTs, novel food fortification approaches may represent the best opportunity to increase the vitamin D supply to the population (41, 42).
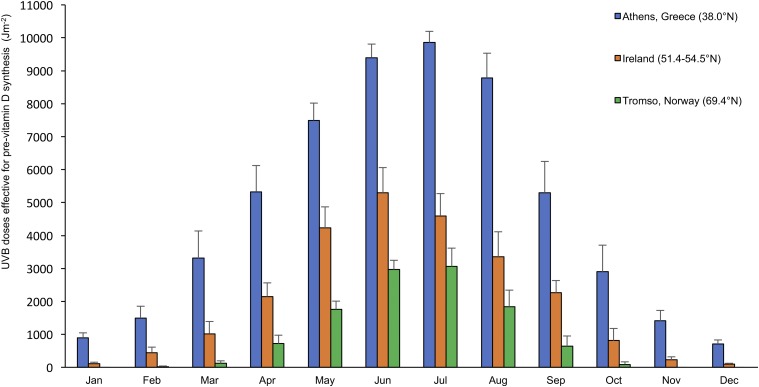
To enable food fortification strategies to be evidence-based, mathematical models can be developed and used to identify the appropriate amounts of food fortification as well as potential vehicles to ensure adequacy of vitamin D intake in population groups. Such mathematical models can provide underpinning supportive data and complement intervention-based trials (43). The models relate vitamin D intake, arising from habitual food consumption as well as from various food fortification scenarios, to serum 25(OH)D concentrations, while accounting for the contribution of UVB-induced synthesis in the skin to the distribution of serum 25(OH)D concentrations within the population. The UVB data that underpin these mathematical models of population serum 25(OH)D concentrations can be from direct ground-based measurements or can themselves be modeled with the use of data from various satellites (44), and on an annual basis, these data show clear and consistent seasonal variation as well as striking latitudinal variation in vitamin D–effective UVB availability (Figure 1). Such modeled vitamin D–effective UVB availability over a typical 12 mo period has recently been mapped for several locations in Europe, ranging from 35°N to 69°N, and clearly highlights the considerable variability across the region as one moves from southerly to northerly latitudes (45).
FIGURE 1.
Monthly modeled UVB doses effective for precholecalciferol synthesis (Jm−2) in Tromsø, Norway (latitude 69°N), Republic of Ireland (latitude 51–54°N), and Athens, Greece (latitude 38°N). Values are means (2003–2012 data) ± SDs, n = 28–31. Data are from O’Neill et al. (45).
With the use of stepwise approaches, models based on UVB availability data, hours of sunlight, and a key component, namely, the dose-response of serum 25(OH)D to UVB in adults, have been used to predict changes in population serum 25(OH)D concentrations throughout the year in the United Kingdom (46–48), Ireland (43), and Germany (49, 50), some of which have been validated against VDSP standardized serum 25(OH)D concentration data from nationally representative nutrition surveys. By inclusion of one additional key component into a model, namely the dose-response of 25(OH)D to increased vitamin D intake, one can use the resulting integrated model to predict changes in the population concentrations of 25(OH)D that may arise from various dietary fortification approaches, while accounting for the seasonal variation in serum 25(OH)D concentrations due to UVB availability. This was illustrated recently when published estimates of the effect of 3 hypothetical vitamin D food fortification scenarios on vitamin D intake in a representative sample of Irish adults (51) were used in the Irish model as a test and showed how mathematical models can inform how vitamin D food fortification in various constructs may affect population serum 25(OH)D concentrations and the prevalence of vitamin D deficiency (43).
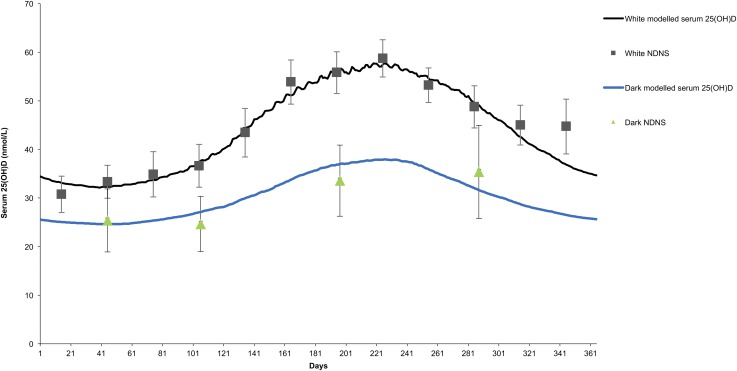
Although the majority of the abovementioned models have all been exclusively based on white populations, recently, a model has been developed to account for the more complex and relevant proportion of European populations at increased risk of vitamin D deficiency due to skin color and ethnicity (48) (Figure 2). This is important because within Europe, North America, and other continents, dark-skinned ethnic groups are worryingly at a much increased risk of vitamin D deficiency than their white counterparts (Table 1). Although many of the environmental factors that contribute to this elevated risk, such as latitude (52), skin color (2), and cultural clothing practices (53) are not modifiable, in contrast, an important modifiable factor is the vitamin D supply in the diet and the obvious, albeit complex, solution is to develop strategies to address the low vitamin D intake in ethnic subgroups, thereby preventing deficiency safely throughout the population (48). Such a tailored, dark-skinned ethnic group mathematical model presents a viable approach to estimating changes in the population concentrations of 25(OH)D that may arise from various dietary fortification approaches and that are cognizant of appropriate food vehicles for these ethnic subgroups.
FIGURE 2.
Modeled changes in serum 25(OH)D concentration over a typical year for white adults (white) and adults of Asian or black ethnic background (dark) in the United Kingdom with the use of daily mean UVB availability estimates [modified from O’Neill et al. (48)]. Squares (and associated error bars) are monthly geometric means ± 95% CIs of measured serum 25(OH)D concentrations for white adults (18–70 y of age) in the NDNS (n = 35–101/mo) in the United Kingdom. Triangles (and associated error bars) are geometric means ± 95% CIs of measured serum 25(OH)D concentrations for each season in the adults (18–70 y of age) of Asian or black ethnic background in the NDNS (n = 9–27/season). Seasons were used in place of monthly geometric means because of the much lower sample number for Asian or black ethnic adults and were defined as follows: winter (December, January, and February), spring (March, April, and May), summer (June, July, and August), and autumn (September, October, and November), with each triangle marker placed at the midpoint of each season. NDNS, National Diet and Nutrition Survey; 25(OH)D, 25-hydroxyvitamin D.
TABLE 1.
Prevalence of vitamin D deficiency in European dark-skinned ethnic groups and their white counterparts1
| Country, population subgroup | Study (reference) | Prevalence of serum 25(OH)D concentration <30 nmol/L, % |
| Finland | ||
| Dark-skinned ethnic | Finnish Migrant Health and Wellbeing Study (16) | 28.0 Somali (n = 364) |
| 50.4 Kurdish (n = 500) | ||
| White | Health 2011 (17) | 0.4 (n = 4102) |
| United Kingdom | National Diet and Nutrition Survey [4-y rolling program (16)] | |
| United Kingdom dark-skinned ethnic | 35.7 Black (n = 28) | |
| 59.6 Asian (n = 52) | ||
| White | 19.6 (n = 1359) | |
| Norway (Oslo) | The Oslo Health Study (17) | |
| South Asian (Pakistani) immigrant | 64.8 (n = 176) | |
| White native, adult | 1.3 (n = 866) | |
| United States | Nutrition and Health Examination Survey 2007–2010 (≥1 y of age) (14) | |
| Non-Hispanic white | 2.3 (n = 6711) | |
| Hispanic | 6.4 (n = 5138) | |
| Non-Hispanic black | 24.0 (n = 2997) |
Prevalence estimates are based on Vitamin D Standardization Program standardized serum 25(OH)D concentration data. n = total population sample. 25(OH)D, 25-hydroxyvitamin D.
Novel food-based solutions for addressing low vitamin D status
As outlined above, there is a need for sustainable food-based strategies to bridge the gap between current and recommended intakes of vitamin D to minimize the prevalence of vitamin D deficiency (4, 19–22). While acknowledging the valuable contribution that vitamin D–fortified milk makes to vitamin D intake among consumers, particularly children, and the continued need for fortification of milk and other dairy products (54), strategic approaches to fortification, including biofortification of a wider range of foods, has the potential to increase vitamin D intake in the population and minimize the prevalence of low serum 25(OH)D concentration without increasing the risk of excessive dosing (4, 40). In terms of traditional fortification of foods, bread and orange juice have also been shown to be effective in improving vitamin D status in a number of RCTs (55–58). Beyond these foods, eggs, beef, pig and sheep meat, poultry, milk, and cultured fish are potentially important targets for vitamin D–biofortified foods (20). In addition, the use of the vitamin D–fortified, reduced-fat cheese is worthy of consideration as an additional sustainable food-based strategy, beyond traditional dairy foods. For example, 2 systematic reviews examining the effectiveness of consuming vitamin D–fortified products in raising serum 25(OH)D concentrations in an RCT setting (41, 42) highlighted the fact that most studies have examined the effectiveness of vitamin D–fortified milk and yogurt, and there have been limited studies exploring the effectiveness of vitamin D–fortified cheese. Even with the studies that have been carried out with vitamin D–fortified cheese, the results have been quite mixed. This may relate to the quality of some of these studies, as suggested by Black et al. (41), as a limitation, but also to the fact that fortification of cheese with vitamin D has certain technological considerations, particularly for reduced-fat cheese varieties (59).
A recent RCT within the EC-funded ODIN project showed how the daily consumption of 60 g of cholecalciferol-fortified, reduced-fat Gouda cheese could counterbalance the expected decrease in serum 25(OH)D concentrations during 8 wk of winter in postmenopausal women (60), a group at risk of low vitamin D status and the associated risks of osteoporosis and related fractures. The study showed that although the average serum 25(OH)D concentration increased significantly (by 5.1 nmol/L) in the cholecalciferol-fortified cheese group (receiving an additional dose of 5.7 μg of cholecalciferol), it decreased significantly (by 4.6 nmol/L) in the control group, as would be expected in winter in individuals not taking additional vitamin D (habitual intake ∼2 μg/d). None of the women in the cholecalciferol-fortified cheese group were vitamin D deficient [defined as serum 25(OH)D concentration <30 nmol/L] after the 8-wk study compared with 41% of women in the control group, a significant difference (P = 0.001) (60). Evidence of the effectiveness of food fortification approaches from RCTs that evaluate their effect on reducing the prevalence of vitamin D deficiency in the populations studied is a key priority (4), and the positive data from these recent RCTs provides a high level of evidence in relation to vitamin D–fortified, reduced-fat cheese (60) and vitamin D–biofortified eggs (36) among other potential food-based solutions to vitamin D deficiency. Data from these and other RCTs also underpin dietary vitamin D modeling analysis based on data from nationally representative dietary surveys, which in turn, when used in the mathematical models of population serum 25(OH)D concentration, can provide in silico projections of how these food interventions may affect the prevalence of vitamin D deficiency in the population. Such work is currently underway in the EC-funded ODIN project (61). Preliminary modeling of national food intake data from 4 European countries within the ODIN project has shown that consumption of vitamin D–biofortified foods together with traditional dairy fortification does not put the population at risk in terms of breaching the vitamin D tolerable upper intake level for adults [100 μg/d (1)], even for those taking vitamin D supplements containing 10 or 25 μg/d (M Kiely, ODIN, personal communication, 2017). This is of importance in light of the high percentage of some populations taking vitamin D–containing supplements (62). Increased food fortification together with high-dose vitamin D supplements (i.e., 50 μg/d) led to ∼20% of individual breaching the Tolerable Upper Intake Level (M Kiely, ODIN, personal communication, 2017).
Dairy-based foods are good vehicles for vitamin D fortification not only because dairy foods are a major part of the diet for a high proportion of individuals in many, but clearly not all, population subgroups, but also as the bioavailability of vitamin D from dairy-based foods has been shown to be good in RCTs (41, 42, 60, 63). In addition, vitamin D may work synergistically with dairy-based nutrients to improve bone health. For example, globally, an adequate supply of both calcium and vitamin D form part of the nutritional recommendations in relation to ensuring good bone health. The importance of both an appropriate dietary calcium intake and adequate serum 25(OH)D concentrations for skeletal health (64) has been confirmed by several meta-analyses (1, 65, 66).
Riboflavin and its flavoenzymes may play a role in the biosynthesis of vitamin D [for a review, see Pinto and Cooper (67)]. Deficits in riboflavin intake and metabolism as well as defects in flavoenzyme activity result in marked structural alterations within the skeletal and central nervous systems similar to those of disorders (inborn errors) in the biosynthetic pathways that lead to cholesterol, steroid hormones, vitamin D, and their metabolites (67). A high protein intake via the production of the osteotropic hormone, insulin-like growth factor-I (IGF-I), which is important for bone formation, has been shown to positively interact with vitamin D metabolism. High circulating IGF-I concentration may be a contributory factor for the enhanced renal production of 1,25-dihydroxyvitamin D (68). The synergy between protein and vitamin D was confirmed in a retrospective analysis with the use of data from a 3-y RCT with calcium and vitamin D supplementation (69). The 342 healthy people (aged ≥65 y) who completed the trial were stratified based on tertiles of protein intakes (as assessed by FFQ), and this analysis revealed an additional effect of high protein intake on top of the vitamin D and calcium intervention for bone mineral density (BMD) at the femoral neck and for total body BMD (69).
Finally, there may be synergy between vitamins D and K. In a case-control study, Torbergsen et al. (70) examined the possible synergistic effect of vitamins D and K. They found that circulating phylloquinone and 25(OH)D, independently and synergistically, were associated with a lower risk of hip fracture in elderly subjects (70). In addition, 2 RCTs showed that vitamin K in combination with vitamin D was better than either vitamin alone in terms of effects on BMD of the lumbar spine (71) and of the ultradistal radius (72). Vitamin D may also work synergistically with other micronutrients, such as vitamin A, but that is beyond the scope of the present review and has been discussed elsewhere (73).
Thus, at a mechanistic level, interactions between vitamin D and calcium, as well as other micronutrients, may have implications for the biosynthesis and regulation of circulating 25(OH)D, but also directly for bone health outcomes (1, 64, 70–75). Milk and dairy are known for their bone-augmenting qualities, but also contain the abovementioned nutrients capable of interacting with vitamin D. Therefore, we reviewed the literature in a systematic way with a view toward substantiating the synergy of vitamin D with dairy nutrients in relation to bone in a RCT setting and, in so doing, providing additional evidence that dairy foods are a good choice for vitamin D fortification. The systematic literature review on vitamin D and dairy nutrients in relation to bone (the details of the search terms used are available from 2 of the authors, EGHMvdH and RJWS, on request) identified 5, 2, 0, 1, and 2 RCTs in which vitamin D with at least calcium, vitamin K, protein, zinc, or phosphorus, respectively, were used as the intervention. Only 2 RCTs changed either the vitamin D or calcium intake without changing the intake of other nutrients. One study of Chinese adolescents compared an intervention with calcium-fortified dairy supplying 245 mg/d of calcium with or without additional vitamin D (3.3 μg/d). The vitamin D enrichment resulted in lower concentrations of bone-specific alkaline phosphatase (a marker of bone formation) (76) and more favorable changes in BMD and bone mineral content (77, 78). The effects were mainly on the lower limbs (79). Another RCT, which kept vitamin D constant at 5 μg/d and changed the calcium intake (1110 compared with 655 mg/d), reported an enhanced bone mineral gain at the hip sites in girls, but had no observable effect in boys (80). Three studies in elderly women, aged 61–99 y, increased the daily calcium concentrations [i.e., an extra 66 mg (81) to 240 mg/d (82, 83)] as well as vitamin D via fortified soft cheese or yogurt. The addition of 10 μg cholecalciferol to yogurt resulted in decreases in 2 markers of bone resorption in older women in a community-dwelling home (83) or elderly institutionalized women (82). A prospective, crossover RCT of institutionalized women showed that a soft, plain cheese fortified with 2.5 μg cholecalciferol, compared with a nonfortified equivalent cheese, led to an increase in serum IGF-I and significant decreases in markers of bone resorption (81). These studies confirm a synergistic role of vitamin D and calcium in terms of reducing the rate of bone resorption and turnover, at least in women with low intake of calcium or vitamin D status. An increased rate of bone turnover in adults may be a risk factor for fracture (84) because it exacerbates bone loss (85).
Two studies included vitamin K in addition to vitamin D in a dairy matrix (86). A short-term study (16 wk) of young women aged 20–35 y showed no additional benefit of phylloquinone added to milk, which was fortified with calcium and vitamin D, on the rate of bone turnover (86), whereas in a 12-mo RCT of postmenopausal women, daily consumption of dairy foods containing 800 mg calcium and 10 μg vitamin D plus 100 μg menaquinone or phylloquinone was favorable for lumbar spine BMD and a marker of bone resorption as compared with dairy fortified with calcium and vitamin D only (87).
There were 3 studies on dairy foods containing vitamin D with different amounts of phosphorus or zinc (88–90), but the findings were such that it was not possible to draw firm conclusions on the possible interaction of vitamin D with zinc or phosphorus in terms of bone health outcomes.
Interactions between seasons and supplements on vitamin D status and health
As mentioned above, vitamin D can be provided by UVB sunlight and dietary sources, including fortified foods and vitamin D supplements. The main differences between the 2 sources are summarized in Table 2, and the relative contribution of each source on an individual’s vitamin D status depends very much on their lifestyle (91).
TABLE 2.
Key differences in sunlight and dietary supply as contributors to vitamin D nutritional status
| Sunlight | Diet |
| UVB is only available seasonally at higher latitude | Available year-round |
| UVB is only available at higher latitude from 1–6 mo of the year at latitudes ranging from 37–60°N, respectively: no vitamin D can be made in the skin1 | Few foods naturally contain vitamin D |
| To make vitamin D, a person needs to be outside and expose the skin to sunlight | Very little vitamin D is obtained from most normal diets |
| A person cannot overdose on sunlight-derived vitamin D (but cancer risk is increased) | Vitamin D supplements make a significant contribution to vitamin D status |
| A person can overdose if they take large amounts orally (high-dose vitamin D supplements for long periods) |
Data are from O’Neill et al. (45).
As summer UVB-rich sunlight is the major contributor to vitamin D status for most of the population who do not cover up when outside, the associations between vitamin D status and a reduced risk of chronic disease (e.g., cancer, cardiovascular, and autoimmune diseases) are confounded by being outside and being exposed to sunlight. Not only are those who are unwell more likely to spend time indoors, but there may be health benefits of light that are independent of vitamin D. To tease out the effect of vitamin D from sunlight, one needs a study specifically designed to account for the effect of season. The Vitamin D and Cardiovascular risk study was a 1-y RCT in Scotland (latitude 57°N) (92). All 305 female participants (aged 60–70 y) started at the same time between January and March, the point in the year when serum 25(OH)D is at its lowest (mean ± SD: 34 ± 15 nmol/L) and were assessed every 2 mo. The study found that serum 25(OH)D in the placebo group went up in the summer to a mean peak of 55 ± 18 nmol/L and returned to 32 ± 15 nmol/L by the following winter, whereas serum 25(OH)D reached plateaus for the groups receiving daily vitamin D (68 ± 16 nmol/L for 400 IU/d; 77 ± 18 nmol/L for 1000 IU/d) (data are means ± SDs). The small incremental differences between the 3 groups in summer compared with the large gap between placebo and both treatment groups in winter shows that there is interaction between light-derived vitamin D and oral vitamin D, i.e., the 2 sources are not additive.
The study found no change in markers of cardiovascular risk (total, HDL, and LDL cholesterol, TGs, apoA-1, and vitamin B-100), insulin resistance (homeostatic model assessment), or inflammation (high-sensitivity C-reactive protein, IL-6, and soluble intracellular adhesion molecule-1) either between the treatment groups or during the year, with one exception (92). Blood pressure went down in summer (mean ± SD systolic blood pressure decreased by 6.6 ± 10.8 mm Hg) and went back up in winter for all 3 groups, indicating that there are vascular effects of seasons that are independent of circulating 25(OH)D. BMD was a secondary outcome (93). Only the group taking the 1000-IU/d dose of vitamin D showed no hip bone loss compared with the mean 0.6% loss seen in both the 400-IU/d and placebo groups. In this case, the bone benefits do not appear to be directly linked to change in 25(OH)D concentration.
There is evidence to suggest that skin autoimmune conditions may be affected by UVB exposure through additional mechanisms, independent from the effects of light-derived vitamin D. A small study of outpatients undergoing UVB therapy, during which the UVB dose differed throughout treatment according to resistance to erythema, showed that the increase in the number of regulatory T cells was related to the change in circulating 25(OH)D, whereas the decrease in cytokine IL-10 was associated with the dose of UVB the patient received (94).
Conclusions
Although circulating 25(OH)D is a robust and reliable marker of vitamin D status, standardization of serum 25(OH)D data are extremely important in terms of within- and between-country comparisons of the prevalence of vitamin D deficiency. By using standardized serum 25(OH)D data and depending on the 25(OH)D threshold selected (30 or 50 nmol/L), vitamin D deficiency in Europe and North America can be classified as a mild (5–19.9%) or severe (>40%) public health problem based on WHO criteria (95). Regardless of which threshold is used, strategies for vitamin D deficiency prevention are required. Fortification, including biofortification, of a wider range of foods is likely to have the potential to increase vitamin D intakes across the population distribution and, in so doing, minimize the prevalence of vitamin D deficiency. Recent RCTs provide high-level evidence in relation to vitamin D–fortified, reduced-fat cheese and vitamin D–biofortifed eggs (36, 60). Evidence of the effectiveness of other food fortification approaches from RCTs that evaluate their effect on reducing the prevalence of vitamin D deficiency in the populations studied is undoubtedly a key priority. The interactions between vitamin D and other micronutrients in dairy-based foods in relation to beneficial effects on bone underscore their importance as vitamin D–fortified foods. Dietary modeling analysis based on data from nationally representative dietary surveys can provide in silico projections of how these food-based vitamin D interventions may affect the degree of vitamin D intake inadequacy in the population. Furthermore, although we acknowledge their simplicity and limitations, computational models can inform vitamin D food fortification strategies by assessing their potential effect on population serum 25(OH)D concentrations and the prevalence of vitamin D deficiency in the absence and presence of sufficient UVB availability. Those computational models, which can account for ethnic dark-skinned subpopulation groups, are particularly important because these subpopulation groups are at a much higher risk of vitamin D deficiency than their white counterparts. Such modeling of nationally representative vitamin D intake estimates, UVB availability, and ultimately, population serum 25(OH)D concentration can contribute to our understanding of population vitamin D status and means of improving such status. Finally, there is emerging evidence that there may be interactions between sunlight and oral supply of vitamin D, such that seasonal health benefits could be independent of vitamin D status, an area deserving of future research.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: BMD, bone mineral density; EAR, Estimated Average Requirement; EC, European Commission; IGF-1, insulin-like growth factor-I; IOM, Institute of Medicine; NANS, National Adult Nutrition Survey; ODIN, Food-Based Solutions for Optimal Vitamin D Nutrition and Health Through the Life Cycle; RCT, randomized controlled trial; VDSP, Vitamin D Standardization Program; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D2, 25-hydroxyergocalciferol; 25(OH)D3, 25-hydroxycholecalciferol.
References
- 1.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington (DC): The National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.Webb AR. Who, what, where and when-influences on cutaneous vitamin D synthesis. Prog Biophys Mol Biol 2006;92:17–25. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Deficiency of sunlight and vitamin D. BMJ 2008;336:1318–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cashman KD, Kiely M. Towards prevention of vitamin D deficiency and beyond: knowledge gaps and research needs in vitamin D nutrition and public health. Br J Nutr 2011;106:1617–27. [DOI] [PubMed] [Google Scholar]
- 5.Scientific Advisory Committee on Nutrition. Report on vitamin D and health [Internet]. 2016 [cited 2016 Jul 1]. Available from: http://www.sacn.gov.uk/pdfs/sacn_vitamin D_and_health_report_web.pdf.
- 6.Seamans KM, Cashman KD. Existing and potentially novel functional markers of vitamin D status: a systematic review. Am J Clin Nutr 2009;89:1997S–2008S. [DOI] [PubMed] [Google Scholar]
- 7.German Nutrition Society. New reference values for vitamin D. Ann Nutr Metab 2012;60:241–6. [DOI] [PubMed] [Google Scholar]
- 8.Nordic Cooperation. Nordic Nutrition Recommendations. 5th ed. Vitamin D [Internet]. 2013. [cited 2017 Aug 20]. Available from: https://www.norden.org/en/theme/former-themes/themes-2016/nordic-nutrition-recommendation/nordic-nutrition-recommendations-2012.
- 9.European Food Safety Authority Panel on Dietetic Products, Nutrition, and Allergies. Scientific opinion on Dietary Reference Values for vitamin D. EFSA J 2016;14:e04547. [Google Scholar]
- 10.Cashman KD, Hayes A, Galvin K, Merkel J, Jones G, Kaufmann M, Hoofnagle AN, Carter GD, Durazo-Arvizu RA, Sempos CT. Significance of serum 24,25-dihydroxyvitamin D in the assessment of vitamin D status: a double-edged sword? Clin Chem 2015;61:636–45. [DOI] [PubMed] [Google Scholar]
- 11.Carter GD, Berry JL, Gunter E, Jones G, Jones JC, Makin HL, Sufi S, Wheeler MJ. Proficiency testing of 25-Hydroxyvitamin D (25-OHD) assays. J Steroid Biochem Mol Biol 2010;121:176–9. [DOI] [PubMed] [Google Scholar]
- 12.Binkley N, Sempos CT; Vitamin D Standardization Program (VDSP). Standardizing vitamin D assays: the way forward. J Bone Miner Res 2014;29:1709–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Notice of vitamin D standardization program. 76 Federal Register 41 (March 2, 2011). p. 11502.
- 14.Schleicher RL, Sternberg MR, Looker AC, Yetley EA, Lacher DA, Sempos CT, Taylor CL, Durazo-Arvizu RA, Maw KL, Chaudhary-Webb M, et al. National estimates of serum total 25-hydroxyvitamin D and metabolite concentrations measured by liquid chromatography-tandem mass spectrometry in the US Population during 2007–2010. J Nutr 2016;146:1051–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarafin K, Durazo-Arvizu R, Tian L, Phinney KW, Tai S, Camara JE, Merkel J, Green E, Sempos CT, Brooks SP. Standardizing 25-hydroxyvitamin D values from the Canadian Health Measures Survey. Am J Clin Nutr 2015;102:1044–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cashman KD, Dowling KG, Škrabáková Z, Gonzalez-Gross M, Valtueña J, De Henauw S, Moreno L, Damsgaard CT, Michaelsen KF, Mølgaard C, et al. Vitamin D deficiency in Europe – Pandemic? Am J Clin Nutr 2016;103:1033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cashman KD, Dowling KG, Škrabáková Z, Kiely M, Lamberg-Allardt C, Durazo-Arvizu RA, Sempos CT, Koskinen S, Lundqvist A, Sundvall J, et al. Standardizing serum 25-hydroxyvitamin D data from four Nordic population samples using the vitamin D standardization program protocols: shedding new light on vitamin D status in Nordic individuals. Scand J Clin Lab Invest 2015;75:549–61. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM; Endocrine Society. Evaluation, treatment, and prevention of vitamin d deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- 19.Cashman KD. Vitamin D: dietary requirements and food fortification as a means of helping achieve adequate vitamin D status. J Steroid Biochem Mol Biol 2015;148:19–26. [DOI] [PubMed] [Google Scholar]
- 20.Hayes A, Cashman KD. Food-based solutions for vitamin D deficiency: putting policy into practice and the key role for research. Proc Nutr Soc 2017;76:54–63. [DOI] [PubMed] [Google Scholar]
- 21.Kiely M, Black LJ. Dietary strategies to maintain adequacy of circulating 25-hydroxyvitamin D concentrations. Scand J Clin Lab Invest Suppl 2012;243:14–23. [DOI] [PubMed] [Google Scholar]
- 22.Cashman KD. The role of vitamers and dietary-based metabolites of vitamin D in prevention of vitamin D deficiency. Food Nutr Res 2012;56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ovesen L, Brot C, Jakobsen J. Food contents and biological activity of 25-hydroxyvitamin D: a vitamin D metabolite to be reckoned with? Ann Nutr Metab 2003;47:107–13. [DOI] [PubMed] [Google Scholar]
- 24.Food Standards Agency UK. McCance and Widdowson’s composition of foods integrated dataset [Internet]. 2008. [cited 2011 Jun 1]. Available from: http://www.food.gov.uk/science/dietarysurveys/dietsurveys/.
- 25.Saxholt E, Christensen AT, Møller A, Hartkopp HB, Hess Ygil K, Hels OH. Danish food composition databank, revision 2 [Internet]. Søborg (Denmark): Department of Nutrition, National Food Institute, Technical University of Denmark; c2008 [updated 2017 Jun 6; cited 11 Jun 1]. Available from: http://frida.fooddata.dk/.
- 26.Federal Department of Home Affairs, Federal Food Safety and Veterinary Office. Swiss food composition database. Version 5.2 [Internet]. c2015 [updated 2017 Mar 30; cited 2016 Aug 1]. Available from: http://naehrwertdaten.ch/request?xml=MessageData&xml=MetaData&xsl=Start&lan=en&pageKey=Start.
- 27.USDA, Agricultural Research Service. USDA national nutrient database for standard reference, release 28 [Internet]. c2016. [updated 2016 May; cited 2016 Jan 24]. Available from: http://ndb.nal.usda.gov/ndb/foods/show/112.
- 28.Taylor CL, Patterson KY, Roseland JM, Wise SA, Merkel JM, Pehrsson PR, Yetley EA. Including food 25-hydroxyvitamin D in intake estimates may reduce the discrepancy between dietary and serum measures of vitamin D status. J Nutr 2014;144:654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heaney RP, Armas LA, French C. All-source basal vitamin D inputs are greater than previously thought and cutaneous inputs are smaller. J Nutr 2013;143:571–5. [DOI] [PubMed] [Google Scholar]
- 30.Cashman KD, Seamans KM, Lucey AJ, Stöcklin E, Weber P, Kiely M, Hill TR. Relative effectiveness of oral 25-hydroxyvitamin D3 and vitamin D3 in raising wintertime serum 25-hydroxyvitamin D in older adults. Am J Clin Nutr 2012;95:1350–6. [DOI] [PubMed] [Google Scholar]
- 31.European Food Safety Authority Panel on Additives and Products or Substances used in Animal Feed. Opinion of the Scientific Panel on Additives and Products or Substances Used in Animal Feed on a request from the Commission on the evaluation of safety and efficacy of “HyD” (calcifediol), based on 25-hydroxylcholecalciferol/25-hydroxy-pre-cholecalciferol, as feed additive in accordance with Council Directive 70/524/EEC. EFSA J 2005;224:1–35. [Google Scholar]
- 32. Food and Drug Administration. Food substances affirmed as generally recognized as safe in feed and drinking water of animals: 25-hydroxyvitamin D3. 21 CFR Part 584 (2007).
- 33.Mattila P, Lehikoinen K, Kiiskinen T, Piironen V. Cholecalciferol and 25-hydroxycholecalciferol content of chicken egg yolk as affected by the cholecalciferol content of feed. J Agric Food Chem 1999;47:4089–92. [DOI] [PubMed] [Google Scholar]
- 34.Mattila PH, Valkonen E, Valaja J. Effect of different vitamin D supplementations in poultry feed on vitamin D content of eggs and chicken meat. J Agric Food Chem 2011;59:8298–303. [DOI] [PubMed] [Google Scholar]
- 35.Browning LC, Cowieson AJ. Vitamin D fortification of eggs for human health. J Sci Food Agric 2014;94:1389–96. [DOI] [PubMed] [Google Scholar]
- 36.Hayes A, Duffy S, O’Grady M, Jakobsen J, Galvin K, Teahan-Dillon J, Kerry J, Kelly A, O’Doherty J, Higgins S, et al. Vitamin D-enhanced eggs are protective of wintertime serum 25-hydroxyvitamin D in a randomized controlled trial of adults. Am J Clin Nutr 2016;104:629–37. [DOI] [PubMed] [Google Scholar]
- 37.Cashman KD, Kinsella M, McNulty BA, Walton J, Gibney MJ, Flynn A, Kiely M. Dietary vitamin D2 - a potentially underestimated contributor to vitamin D nutritional status of adults? Br J Nutr 2014;112:193–202. [DOI] [PubMed] [Google Scholar]
- 38.Roman Viñas B, Ribas Barba L, Ngo J, Gurinovic M, Novakovic R, Cavelaars A, de Groot LC, van’t Veer P, Matthys C, Serra Majem L. Projected prevalence of inadequate nutrient intakes in Europe. Ann Nutr Metab 2011;59:84–95. [DOI] [PubMed] [Google Scholar]
- 39.Dietary reference values for food energy and nutrients for the United Kingdom. Report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy. Rep Health Soc Subj (London) 1991;41:1–210. [PubMed] [Google Scholar]
- 40.Cashman KD, Kiely M. Recommended dietary intakes for vitamin D: where do they come from, what do they achieve and how can we meet them? J Hum Nutr Diet 2014;27:434–42. [DOI] [PubMed] [Google Scholar]
- 41.Black LJ, Seamans KM, Cashman KD, Kiely M. An updated systematic review and meta-analysis of the efficacy of vitamin D food fortification. J Nutr 2012;142:1102–8. [DOI] [PubMed] [Google Scholar]
- 42.O’Donnell S, Cranney A, Horsley T, Weiler HA, Atkinson SA, Hanley DA, Ooi DS, Ward L, Barrowman N, Fang M, et al. Efficacy of food fortification on serum 25-hydroxyvitamin D concentrations: systematic review. Am J Clin Nutr 2008;88:1528–34. [DOI] [PubMed] [Google Scholar]
- 43.Cashman KD, Kazantzidis A, Webb AR, Kiely M. An integrated predictive model of population serum 25-hydroxyvitamin D for application in strategy development for vitamin D deficiency prevention. J Nutr 2015;145:2419–25. [DOI] [PubMed] [Google Scholar]
- 44.Kazantzidis A, Smedley A, Kift R, Rimmer J, Berry JL, Rhodes LE, Webb AR. A modeling approach to determine how much UV radiation is available across the UK and Ireland for health risk and benefit studies. Photochem Photobiol Sci 2015;14:1073–81. [DOI] [PubMed] [Google Scholar]
- 45.O’Neill CM, Kazantzidis A, Ryan MJ, Barber N, Sempos CT, Durazo-Arvizu RA, Jorde R, Grimnes G, Eiriksdottir G, Gudnason V, et al. Seasonal changes in vitamin D-effective UVB availability in Europe and associations with population serum 25-hydroxyvitamin D. Nutrients 2016;8:E533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diffey BL. Modelling the seasonal variation of vitamin D due to sun exposure. Br J Dermatol 2010;162:1342–8. [DOI] [PubMed] [Google Scholar]
- 47.Diffey BL. Modelling vitamin D status due to oral intake and sun exposure in an adult British population. Br J Nutr 2013;110:569–77. [DOI] [PubMed] [Google Scholar]
- 48.O’Neill CM, Kazantzidis A, Kiely M, Cox L, Meadows S, Goldberg G, Prentice A, Kift R, Webb AR, Cashman KD. A predictive model of serum 25-hydroxyvitamin D in UK white as well as black and Asian minority ethnic population groups for application in food fortification strategy development towards vitamin D deficiency prevention. J Steroid Biochem Mol Biol 2016 Sep 13 (Epub ahead of print; DOI: 10.1016/j.jsbmb.2016.09.010). [DOI] [PubMed] [Google Scholar]
- 49.Brown J, Ignatius A, Amling M, Barvencik F. New perspectives on vitamin D sources in Germany based on a novel mathematical bottom-up model of 25(OH)D serum concentrations. Eur J Nutr 2013;52:1733–42. [DOI] [PubMed] [Google Scholar]
- 50.Brown J, Sandmann A, Ignatius A, Amling M, Barvencik F. New perspectives on vitamin D food fortification based on a modeling of 25(OH)D concentrations. Nutr J 2013;12:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Black LJ, Walton J, Flynn A, Cashman KD, Kiely M. Small increments in vitamin D intake by Irish adults over a decade show that strategic initiatives to fortify the food supply are needed. J Nutr 2015;145:969–76. [DOI] [PubMed] [Google Scholar]
- 52.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 1988;67:373–8. [DOI] [PubMed] [Google Scholar]
- 53.Webb AR, Aseem S, Kift RC, Rhodes LE, Farrar MD. Target the message: a qualitative study exploring knowledge and cultural attitudes to sunlight and vitamin D in Greater Manchester, UK. Br J Dermatol 2016;175: 1401–3. [DOI] [PubMed] [Google Scholar]
- 54.Cashman KD, Kiely M. Tackling inadequate vitamin D intakes within the population: fortification of dairy products with vitamin D may not be enough. Endocrine 2016;51:38–46. [DOI] [PubMed] [Google Scholar]
- 55.Natri AM, Salo P, Vikstedt T, Palssa A, Huttunen M, Karkkainen MU, Salovaara H, Piironen V, Jakobsen J, Lamberg-Allardt CJ. Bread fortified with cholecalciferol increases the serum 25-hydroxyvitamin D concentration in women as effectively as a cholecalciferol supplement. J Nutr 2006;136:123–7. [DOI] [PubMed] [Google Scholar]
- 56.Mocanu V, Vieth R. Three-year follow-up of serum 25-hydroxyvitamin D, parathyroid hormone, and bone mineral density in nursing home residents who had received 12 months of daily bread fortification with 125 mug of vitamin D3. Nutr J 2013;12:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tangpricha V, Koutkia P, Rieke SM, Chen TC, Perez AA, Holick MF. Fortification of orange juice with vitamin D: a novel approach for enhancing vitamin D nutritional health. Am J Clin Nutr 2003;77:1478–83. [DOI] [PubMed] [Google Scholar]
- 58.Biancuzzo RM, Young A, Bibuld D, Cai MH, Winter MR, Klein EK, Ameri A, Reitz R, Salameh W, Chen TC, et al. Fortification of orange juice with vitamin D(2) or vitamin D(3) is as effective as an oral supplement in maintaining vitamin D status in adults. Am J Clin Nutr 2010;91:1621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wagner D, Sidhom G, Whiting SJ, Rousseau D, Vieth R. The bioavailability of vitamin D from fortified cheeses and supplements is equivalent in adults. J Nutr 2008;138:1365–71. [DOI] [PubMed] [Google Scholar]
- 60.Manios Y, Moschonis G, Mavrogianni C, van den Heuvel E, Singh-Povel CM, Kiely M, Cashman KD. Reduced-fat Gouda-type cheese enriched with vitamin D3 effectively prevents vitamin D deficiency during winter months in postmenopausal women in Greece. Eur J Nutr 2016. Jul 22 (Epub ahead of print; DOI: 10.1007/s00394-016-1277-y). [DOI] [PubMed] [Google Scholar]
- 61.Kiely M, Cashman K; on behalf of the ODIN Consortium. The ODIN project: development of food-based approaches for prevention of vitamin D deficiency throughout life. Nutr Bull 2015;40:235–46. [Google Scholar]
- 62.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults From 1999–2012. JAMA 2016;316:1464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knapen MH, Braam LA, Teunissen KJ, van ’t Hoofd CM, Zwijsen RM, Theuwissen E, Vermeer C. Yogurt drink fortified with menaquinone-7 improves vitamin K status in a healthy population. J Nutr Sci 2015;4:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carmeliet G, Dermauw V, Bouillon R. Vitamin D signaling in calcium and bone homeostasis: a delicate balance. Best Pract Res Clin Endocrinol Metab 2015;29:621–31. [DOI] [PubMed] [Google Scholar]
- 65.Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev 2014;4:CD000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lips P, Gielen E, van Schoor NM. Vitamin D supplements with or without calcium to prevent fractures. Bonekey Rep 2014;3:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinto JT, Cooper AJ. From cholesterogenesis to steroidogenesis: role of riboflavin and flavoenzymes in the biosynthesis of vitamin D. Adv Nutr 2014;5:144–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonjour J-P, Kraenzlin M, Levasseur R, Warren M, Whiting S. Dairy in adulthood: from foods to nutrient interactions on bone and skeletal muscle health. J Am Coll Nutr 2013;32:251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dawson-Hughes B, Harris SS. Calcium intake influences the association of protein intake with rates of bone loss in elderly men and women. Am J Clin Nutr 2002;75:773–9. [DOI] [PubMed] [Google Scholar]
- 70.Torbergsen AC, Watne LO, Wyller TB, Frihagen F, Strømsøe K, Bøhmer T, Mowe M. Vitamin K1 and 25(OH)D are independently and synergistically associated with a risk for hip fracture in an elderly population: a case control study. Clin Nutr 2015;34:101–6. [DOI] [PubMed] [Google Scholar]
- 71.Ushiroyama T, Ikeda A, Ueki M. Effect of continuous combined therapy with vitamin K(2) and vitamin D(3) on bone mineral density and coagulofibrinolysis function in postmenopausal women. Maturitas 2002;41:211–21. [DOI] [PubMed] [Google Scholar]
- 72.Bolton-Smith C, McMurdo ME, Paterson CR, Mole PA, Harvey JM, Fenton ST, Prynne CJ, Mishra GD, Shearer MJ. Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J Bone Miner Res 2007;22:509–19. [DOI] [PubMed] [Google Scholar]
- 73.Conaway HH, Henning P, Lerner UH. Vitamin A metabolism, action, and role in skeletal homeostasis. Endocr Rev 2013;34:766–97. [DOI] [PubMed] [Google Scholar]
- 74.Lips P. Interaction between vitamin D and calcium. Scand J Clin Lab Invest Suppl 2012;243:60–4. [DOI] [PubMed] [Google Scholar]
- 75.Cranney A, Horsley T, O’Donnell S, Weiler H, Puil L, Ooi D, Atkinson S, Ward L, Moher D, Hanley D, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007;158:1–235. [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu K, Du X, Cowell CT, Greenfield H, Blades B, Dobbins TA, Zhang Q, Fraser DR. Effects of school milk intervention on cortical bone accretion and indicators relevant to bone metabolism in Chinese girls aged 10–12 y in Beijing. Am J Clin Nutr 2005;81:1168–75. [DOI] [PubMed] [Google Scholar]
- 77.Du X, Zhu K, Trube A, Fraser DR, Greenfield AH, Zhang Q, Ma G, Hu X. Effects of school milk intervention on growth and bone mineral accretion in Chinese girls aged 10–12 years: accounting for cluster randomisation. Br J Nutr 2005;94:1038–9. [DOI] [PubMed] [Google Scholar]
- 78.Du X, Zhu K, Trube A, Zhang Q, Ma G, Hu X, Fraser DR, Greenfield H. School-milk intervention trial enhances growth and bone mineral accretion in Chinese girls aged 10–12 years in Beijing. Br J Nutr 2004;92:159–68. [DOI] [PubMed] [Google Scholar]
- 79.Zhu K, Greenfield H, Du X, Zhang Q, Ma G, Hu X, Cowell CT, Fraser DR. Effects of two years’ milk supplementation on size-corrected bone mineral density of Chinese girls. Asia Pac J Clin Nutr 2008;17:147–50. [PubMed] [Google Scholar]
- 80.Zhang ZQ, Ma XM, Huang ZW, Yang XG, Chen YM, Su YX. Effects of milk salt supplementation on bone mineral gain in pubertal Chinese adolescents: a 2-year randomized, double-blind, controlled, dose-response trial. Bone 2014;65:69–76. [DOI] [PubMed] [Google Scholar]
- 81.Bonjour JP, Benoit V, Pourchaire O, Rousseau B, Souberbielle JC. Nutritional approach for inhibiting bone resorption in institutionalized elderly women with vitamin D insufficiency and high prevalence of fracture. J Nutr Health Aging 2011;15:404–9. [DOI] [PubMed] [Google Scholar]
- 82.Bonjour JP, Benoit V, Payen F, Kraenzlin M. Consumption of yogurts fortified in vitamin D and calcium reduces serum parathyroid hormone and markers of bone resorption: a double-blind randomized controlled trial in institutionalized elderly women. J Clin Endocrinol Metab 2013;98:2915–21. [DOI] [PubMed] [Google Scholar]
- 83.Bonjour JP, Benoit V, Atkin S, Walrand S. Fortification of yogurts with vitamin D and calcium enhances the inhibition of serum parathyroid hormone and bone resorption markers: a double blind randomized controlled trial in women over 60 living in a community dwelling home. J Nutr Health Aging 2015;19:563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Riggs BL, Melton LJ III, O’Fallon WM. Drug therapy for vertebral fractures in osteoporosis: evidence that decreases in bone turnover and increases in bone mass both determine antifracture efficacy. Bone 1996;18:197S–201S. [DOI] [PubMed] [Google Scholar]
- 85.Hansen MA, Overgaard K, Riis BJ, Christiansen C. Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. BMJ 1991;303:961–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kruger MC, Booth CL, Coad J, Schollum LM, Kuhn-Sherlock B, Shearer MJ. Effect of calcium fortified milk supplementation with or without vitamin K on biochemical markers of bone turnover in premenopausal women. Nutrition 2006;22:1120–8. [DOI] [PubMed] [Google Scholar]
- 87.Kanellakis S, Moschonis G, Tenta R, Schaafsma A, van den Heuvel EG, Papaioannou N, Lyritis G, Manios Y. Changes in parameters of bone metabolism in postmenopausal women following a 12-month intervention period using dairy products enriched with calcium, vitamin D, and phylloquinone (vitamin K(1)) or menaquinone-7 (vitamin K (2)): the Postmenopausal Health Study II. Calcif Tissue Int 2012;90:251–62. [DOI] [PubMed] [Google Scholar]
- 88.Chan GM, McElligott K, McNaught T, Gill G. Effects of dietary calcium intervention on adolescent mothers and newborns: a randomized controlled trial. Obstet Gynecol 2006;108:565–71. [DOI] [PubMed] [Google Scholar]
- 89.Kruger MC, Schollum LM, Kuhn-Sherlock B, Hestiantoro A, Wijanto P, Li-Yu J, Agdeppa I, Todd JM, Eastell R. The effect of a fortified milk drink on vitamin D status and bone turnover in post-menopausal women from South East Asia. Bone 2010;46:759–67. [DOI] [PubMed] [Google Scholar]
- 90.Palacios S, Castelo-Branco C, Cifuentes I, von Helde S, Baró L, Tapia-Ruano C, Menéndez C, Rueda C. Changes in bone turnover markers after calcium-enriched milk supplementation in healthy postmenopausal women: a randomized, double-blind, prospective clinical trial. Menopause 2005;12:63–8. [DOI] [PubMed] [Google Scholar]
- 91.Macdonald HM. Contributions of sunlight and diet to vitamin D status. Calcif Tissue Int 2013;92:163–76. [DOI] [PubMed] [Google Scholar]
- 92.Wood AD, Secombes KR, Thies F, Aucott L, Black AJ, Mavroeidi A, Simpson WG, Fraser WD, Reid DM, Macdonald HM. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab 2012;97:3557–68. [DOI] [PubMed] [Google Scholar]
- 93.Macdonald HM, Wood AD, Aucott LS, Black AJ, Fraser WD, Mavroeidi A, Reid DM, Secombes KR, Simpson WG, Thies F. Hip bone loss is attenuated with 1000 IU but not 400 IU daily vitamin D3: a 1-year double-blind RCT in postmenopausal women. J Bone Miner Res 2013;28:2202–13. [DOI] [PubMed] [Google Scholar]
- 94.Milliken SV, Wassall H, Lewis BJ, Logie J, Barker RN, Macdonald H, Vickers MA, Ormerod AD. Effects of ultraviolet light on human serum 25-hydroxyvitamin D and systemic immune function. J Allergy Clin Immunol 2012;129:1554–61. [DOI] [PubMed] [Google Scholar]
- 95.de Benoist B, McLean E, Egli I, Cogswell M, editors. Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. Geneva (Switzerland): WHO; 2008.