Abstract
The aim of this systematic review and meta-analysis was to summarize the evidence on the relation of the intakes of 12 major food groups, including whole grains, refined grains, vegetables, fruits, nuts, legumes, eggs, dairy, fish, red meat, processed meat, and sugar-sweetened beverages (SSBs) with the risk of hypertension. PubMed, Scopus, and Web of Science were searched systematically until June 2017 for prospective studies having quantitatively investigated the above-mentioned foods. We conducted meta-analysis on the highest compared with the lowest intake categories and linear and nonlinear dose-response meta-analyses to analyze the association. Summary RRs and 95% CIs were estimated by using a random-effects model. Overall, 28 reports were included in the meta-analysis. An inverse association for the risk of hypertension was observed for 30 g whole grains/d (RR: 0.92; 95% CI: 0.87, 0.98), 100 g fruits/d (RR: 0.97; 95% CI: 0.96, 0.99), 28 g nuts/d (RR: 0.70; 95% CI: 0.45, 1.08), and 200 g dairy/d (RR: 0.95; 95% CI: 0.94, 0.97), whereas a positive association for 100 g red meat/d (RR: 1.14; 95% CI: 1.02, 1.28), 50 g processed meat/d (RR: 1.12; 95% CI: 1.00, 1.26), and 250 mL SSB/d (RR: 1.07; 95% CI: 1.04, 1.10) was seen in the linear dose-response meta-analysis. Indication for nonlinear relations of the intakes of whole grains, fruits, fish, and processed meats with the risk of hypertension was detected. In summary, this comprehensive dose-response meta-analysis of 28 reports identified optimal intakes of whole grains, fruits, nuts, legumes, dairy, red and processed meats, and SSBs related to the risk of hypertension. These findings need to be seen under the light of very-low to low quality of meta-evidence. However, the findings support the current dietary guidelines in the prevention of hypertension.
Keywords: food groups, diet, meta-analysis, dose-response, hypertension
Introduction
Approximately 40% of people aged >25 y worldwide have hypertension, amounting to 1 billion in 2015, and the prevalence keeps rising sharply (1, 2). Additionally, hypertension is the most important risk factor for premature cardiovascular disease and accounts globally for 50% of all ischemic heart diseases and stroke events (3).
Evidence indicates that dietary factors have an important impact on the primary and secondary prevention of hypertension (4). Nevertheless, in various countries, dietary recommendations for the prevention of hypertension are still vague. For instance, according to the 2016 Canadian Hypertension program, there is a moderate quality of evidence that a diet high in whole grains, fruits, vegetables, and low-fat dairy products is effective in the primary and secondary prevention of hypertension. However, the organization gives no recommendation regarding the specific quantities of these foods that should be consumed for the best preventive effect (5). In general, dietary guidelines to reduce the risk of hypertension in a primary prevention setting are mainly based on the results of the Dietary Approaches to Stop Hypertension Trial (DASH) (diet rich in fruits, vegetables, and low-fat dairy products and low in sodium and total and saturated fats) (4, 6–11). DASH showed an important reduction in diastolic and systolic blood pressure by 3 and 5.5 mm Hg, respectively, compared with the control group, and reductions between 1.9 and 2.7 mm Hg compared with the fruit-and-vegetable group (7). Among hypertensive patients systolic and diastolic blood pressure reductions ranged between 11 and 5.5 mm Hg, and larger blood pressure–reduction effects were observed in black subjects compared with those of other ethnicities (7). Changes of potential confounders, such as SSC, body weight, and alcohol, were similarly distributed across the intervention groups.
Recently, we showed that the optimal consumption of risk-decreasing foods (whole grains, vegetables, fruits, and dairy) resulted in a 42% reduction of type 2 diabetes, and a high intake of risk-increasing foods [red and processed meats, sugar-sweetened beverages (SSBs), and eggs] resulted in a 200% increase compared with nonconsumption of the respective foods (12). Similar results could be observed regarding all-cause mortality (13).
Because of the high prevalence of hypertension and the preventive effects of dietary factors, the following question has yet to be answered: What is the trustworthiness of meta-evidence regarding the association of food groups in relation to the risk of hypertension?
Therefore, this meta-analysis was conducted with the aim of investigating the associations of 12 previously used food groups, including whole grains, refined grains, vegetables, fruits, nuts, legumes, eggs, dairy, fish, red meat, processed meat, and SSBs, with the risk of hypertension by evaluating the available evidence. Special focuses in our analyses were the strength and the shape (dose-specific) of the relation to identify an optimal intake of food groups for the lowest risk of hypertension. By applying the NutriGrade scoring system, we aimed to evaluate the food groups’ trustworthiness of meta-evidence on their relation to hypertension risk.
Methods
The previously registered systematic review protocol was updated in PROSPERO (https://www.crd.york.ac.uk/prospero/, identifier CRD42016037069). Our strategy for the systematic review and meta-analysis was predefined in a published protocol (14) and has already been implemented for 2 recently published meta-analyses on all-cause mortality and type 2 diabetes, respectively (12, 13). This meta-analysis followed the guidelines for reporting proposed by the Meta-analyses Of Observational Studies in Epidemiology (15).
Search strategy
PubMed, Scopus, Web of Science, and Google Scholar, were searched until June 2017. The search was not restricted to calendar date or language. The full search strategy for PubMed is given in Supplemental Material 1.
Searches of reference lists from included prospective studies supplemented the electronic database searches. One author (LS) performed the literature search, while another author (HB) reviewed uncertain cases. Consensus was reached through discussion between both authors.
Study selection
We included studies with cohort, case-cohort, and nested case-control designs, as well as follow-ups of randomized controlled trials (RCTs). Included studies investigated the association for ≥1 of the 12 food groups (whole grains or cereals, refined grains or cereals, vegetables, fruits, nuts, legumes, eggs, dairy products, fish, red meat, processed meat, and SSBs) on the risk of hypertension; the incidence of hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg for the first time in any follow-up checkup or when taking antihypertensive medication also for the first time in the follow-up visits in adults (those ≥18 y old).
Data extraction
Information extracted from the included studies included the name of first author, year of the publication, country, cohort name, number of participants, number of incident hypertension cases, baseline age, sex, length of follow-up, specification of outcome assessment, method of dietary assessment, quantity of food intake, multivariable effect estimate (RRs, HRs, or ORs including the corresponding CIs), and adjusted covariates. When the risk estimates for participants were reported only separately for men and women in a study, the RRs were combined by using a fixed-effect model.
Statistical analysis
To derive summary RRs and 95% CIs we applied a random-effects model (16) to investigate the associations of the categories of highest compared with lowest intake and the dose-response estimate for each of the 12 a priori–defined food groups with the risk of hypertension. Using an inverse variance method, we calculated the SE for the logarithm RR of each study. This was in turn considered the estimated variance of the logarithm RR (16). Meta-analysis was based on the assumption that all measures are RRs. For the dose-response analysis we applied the method described by Greenland and Longnecker (17) and Orsini et al. (18). The distribution of cases and person-years or noncases, as well as the RRs with the 95% CIs, was required for ≥3 quantitative exposure categories for the application of this method. If directly reported in a study, a linear dose-response trend with a 95% CI or SE was directly used in our analyses.
As previously described, if studies reported only the total number of cases or person-years and the exposure was defined in categories, the number of person-years or cases in each category was obtained from the total number of person-years or cases divided by the number of reported categories. We assigned the median or mean intake by quantile to the corresponding risk estimate. If studies reported intakes only as a range by quantile, the midpoint was calculated. In the case of an open-ended intake range, we assumed that the width was the same as the contiguous category. If the exposure was expressed per given unit of energy intake, we used the provided mean energy intake to rescale it.
The dose-response was expressed in the quantities as previously described (12–14). If studies reported exposure in serving size but did not specify the amount, recommended conversions were used (Supplemental Table 1).
Restricted cubic splines for each study with >3 quantiles of exposure were calculated to explore possible nonlinear associations. We used 3 fixed knots through the total range of the reported intake at 10%, 50%, and 90% and combined these using multivariate meta-analysis (19).
Moreover, the potential of foods to reduce the risk of hypertension was calculated by multiplying the RR by selecting an optimal consumption of risk-reducing foods (calculated by  ) and risk-increasing foods noted as (
) and risk-increasing foods noted as (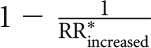 ). The optimal consumption of single food groups was defined as the serving category with the strongest association for hypertension risk.
). The optimal consumption of single food groups was defined as the serving category with the strongest association for hypertension risk.
Heterogeneity between studies was evaluated by using the Q test and the I2 statistic. A value >50% for the I2 statistic was regarded as potentially important statistical heterogeneity (20). If >5 studies were available for a food group in the linear dose-response analysis, subgroup analyses were performed to identify potential sources of heterogeneity by the following characteristics: sex, length of follow-up (mean or median ≥5 compared with <5 y), geographic location (by continent), number of cases (≥1000 compared with <1000), and validated or nonvalidated dietary assessment. Moreover, sensitivity analysis was carried out for a low risk of bias studies (>5 studies available).
As recommended by the Cochrane Handbook, if ≥10 studies were available (21), we explored potential small-study effects, such as publication bias by using Egger´s test and funnel plots (22). Stata version/SE 14.2 software (StataCorp) and Review Manager 5.3 (Nordic Cochrane Centre) were used to conduct statistical analyses.
Risk of bias assessment
The risk of bias of the included prospective studies was assessed by considering 4 categories (23): 1) exposure assessment [low risk of bias: validated, calibrated FFQ or 24-h recall, diet history, or diet records (multiple days)], 2) assessment of outcome (low risk of bias: accepted clinical criteria, record linkage (International Classification of Diseases codes), self-reported, and validated], 3) adequacy of follow-up length (low risk of bias: ≥5 y), and 4) adjusted basic model (low risk of bias, ≥2 factors: e.g., sex, education, and ethnicity; if only one sex included, then ≥1 factor) and outcome-relevant adjustments [low risk of bias, ≥3 factors: e.g., BMI (in kg/m2), smoking, energy intake, and physical activity].
As previously described, included reports were considered to be at low risk of bias if the rating did not correspond to high or unclear risk of bias in any of the 4 domains (13).
Quality of meta-evidence
Evidence-based dietary recommendations should be based on the completeness of the available evidence. However, many meta-analyses have not evaluated the quality of such evidence, which decreases our confidence in the observed effect.
To evaluate the trustworthiness (credibility) of meta-evidence for the association between 12 predefined food groups and the risk of hypertension we applied our recently developed NutriGrade scoring system (maximum of 10 points). NutriGrade should be applied to summarize the meta-evidence of diet-disease relations in meta-analyses, umbrella reviews, and meta-epidemiological studies (23).
Compared with the well-established Gradings of Recommendations Assessment, Development and Evaluation approach, NutriGrade differs in the following aspects: it gives more weight to the evaluation of prospective observational study designs, because such design is important for the investigation of diet-disease relations; it assesses nutrition-specific aspects, such as dietary assessment methods and their validation, calibration of FFQs, or the assessment of diet associated biomarkers; and finally it also considers the conflict of interest and funding bias as a separate item.
This tool is based on the following 8 items for cohort studies: 1) risk of bias, study quality, study limitations (maximum 2 points); 2) precision (maximum 1 point); 3) heterogeneity (maximum 1 point); 4) directness (maximum 1 point); 5) publication bias (maximum 1 point); 6) funding bias (maximum 1 point); 7) effect size (maximum 2 points); and 8) dose-response (maximum 1 point) (23). To evaluate and interpret the meta-evidence, we recommend 4 categories based on this scoring system: high (≥8 points), moderate (6 to <8 points), low (4 to <6 points), and very low (0 to <4 points).
Results
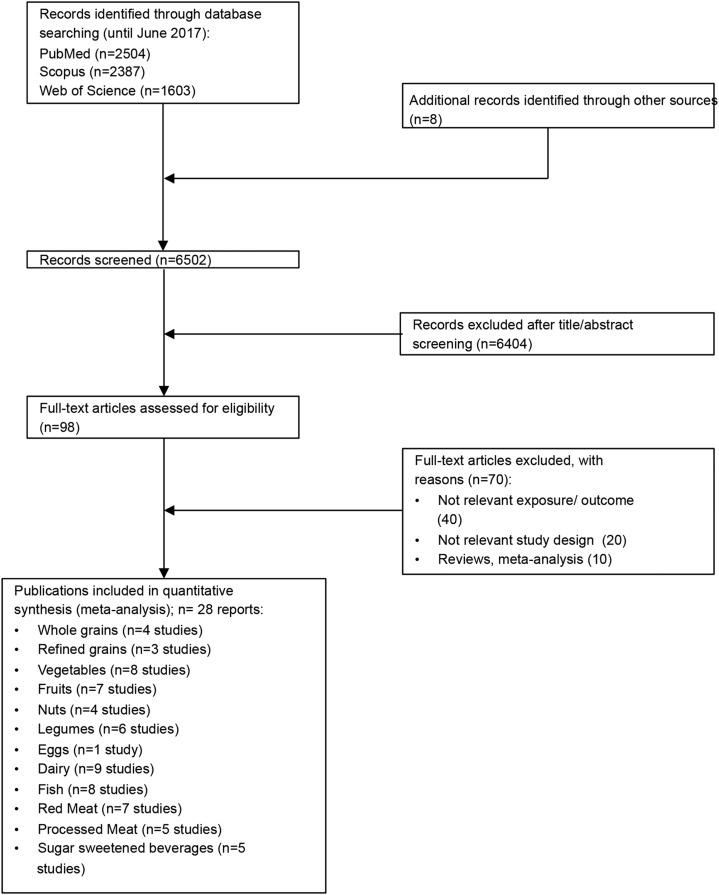
Of the 6502 records that were identified by the literature search, 98 full text articles were assessed in detail because they reported on ≥1 of the targeted 12 foods groups and risk of hypertension (Figure 1, Supplemental Material 2).
FIGURE 1.
Flow diagram illustrating the identification and selection of studies.
Four prospective studies were included in the meta-analysis for consumption of whole grains (24–27), 3 studies for refined grains (25–27), 8 for vegetables (6 reports) (27–32), 7 for fruits (5 reports) (27, 28, 30–32), 4 for nuts (27, 33–35), 6 for legumes (4 reports) (27, 28, 34, 36), 1 for eggs (34), 9 for dairy products (27, 37–44), 8 for fish (6 reports) (27, 34, 38, 45–47), 7 for red meat (5 reports) (34, 38, 45, 48, 49), 5 for processed meat (3 reports) (45, 48, 49), and 5 for SSBs (3 reports) (27, 50, 51) (Supplemental Tables 2–13).
Whole grains
Four studies with 28,069 incident hypertension cases were included in the highest compared with the lowest intake category meta-analysis (overall intake range: 0−92 g/d). An inverse association between the risk of hypertension and whole-grain intake was observed (RR: 0.86; 95% CI: 0.79, 0.93; I2 = 72%; P-heterogeneity = 0.01) when comparing extreme categories (Supplemental Figure 1). An increase in whole-grain intake by 30 g/d was inversely associated with the risk of hypertension by 8% (RR: 0.92; 95% CI: 0.87, 0.98; I2 = 88%; P-heterogeneity < 0.0001; n = 4) (Supplemental Figure 2).
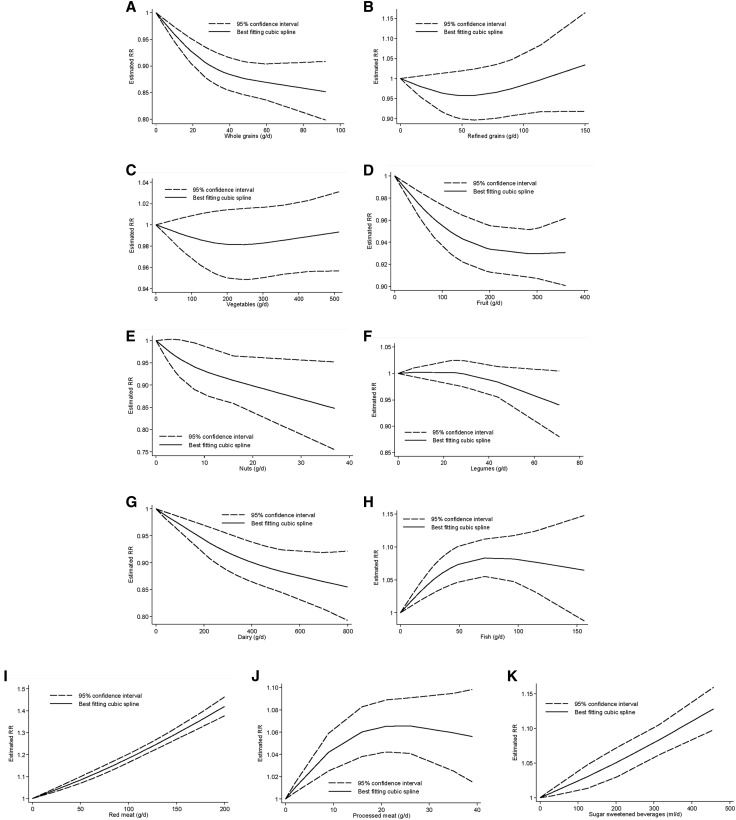
Furthermore, we detected that with an increase in the intake of whole grains, the risk of hypertension decreased by 15%. Despite a significant test for nonlinearity (P-nonlinearity < 0.01; n = 4 studies), an inverse relation was seen for an intake of whole grains as high as ∼90 g/d (Figure 2).
FIGURE 2.
Nonlinear dose-response relation between daily intakes of whole grains (A) (P-nonlinearity < 0.01; n = 4 studies), refined grains (B) (P-nonlinearity = 0.11; n = 3 studies), vegetables (C) (P-nonlinearity = 0.25; n = 7 studies), fruits (D) (P-nonlinearity = 0.06; n = 6 studies), nuts (E) (P-nonlinearity = 0.40; n = 4 studies), legumes (F) (P-nonlinearity = 0.17; n = 5 studies), dairy (G) (P-nonlinearity = 0.22; n = 8 studies), fish (H) (P-nonlinearity < 0.01; n = 7 studies), red meat (I) (P-nonlinearity = 0.30; n = 7 studies), processed meat (J) (P-nonlinearity < 0.001; n = 4 studies), and sugar-sweetened beverages (K) (P-nonlinearity = 0.73; n = 4 studies) and the risk of hypertension.
Refined grains
Three studies with 18,842 incident hypertension cases were included in the highest compared with lowest intake category meta-analysis (range of intake: 0−122 g/d). Comparing categories of highest and lowest intake of refined grains, we observed no association with the risk of hypertension (RR: 0.95; 95% CI: 0.88, 1.03; I2 = 17%; P-heterogeneity = 0.30) (Supplemental Figure 3). Similarly, an increase in refined-grain intake by 30 g/d was not associated with the risk of hypertension (RR: 0.99; 95% CI: 0.96, 1.02; I2 = 37%; P-heterogeneity = 0.20; n = 3) (Supplemental Figure 4). No evidence of a nonlinear dose-response association was detected (P-nonlinearity = 0.11; n = 3 studies) (Figure 2).
Vegetables
Eight studies including 94,772 incident hypertension cases were included in the meta-analysis comparing extreme intake categories (range of intake: 0−512 g/d). In this analysis we observed an inverse association between the risk of hypertension and vegetable intake (RR: 0.96; 95% CI: 0.91, 1.01; I2 = 0%; P-heterogeneity = 0.46) (Supplemental Figure 5) but not in the dose-response analysis (RR per 100 g/d: 1.00; 95% CI: 0.98, 1.01; I2 = 58%; P-heterogeneity = 0.03; n = 7) (Supplemental Figure 6).
The subgroup analyses showed an inverse association in studies conducted in Asia and Australia but not in Europe and America, in studies with a follow-up term <5 y, and in studies with <1000 cases (Supplemental Table 14). The stratified analyses showed evidence of heterogeneity between subgroups. There was no evidence of a nonlinear dose-response association (P-nonlinearity = 0.25; n = 7 studies) (Figure 2).
Fruits
Seven studies with 94,507 incident cases were included in the meta-analysis comparing extreme intake categories (range of intake: 0−360 g/d). In this analysis we observed an inverse association between the risk of hypertension and fruit intake (RR: 0.93; 95% CI: 0.87, 1.00; I2 = 55%; P-heterogeneity = 0.04) (Supplemental Figure 7). An increase in fruit intake by 100 g/d was inversely associated with the risk of hypertension (RR: 0.97; 95% CI: 0.96, 0.99; I2 = 64%; P-heterogeneity = 0.02; n = 6) (Supplemental Figure 8).
The heterogeneity persisted largely in stratified analyses (Supplemental Table 15). The subgroup analyses showed no inverse association in shorter-term studies, in European studies, and in studies with <1000 cases. Some evidence of heterogeneity was detected between subgroups in stratified analyses for country.
There was evidence of a nonlinear dose-response trend (P-nonlinearity = 0.06; n = 6 studies) with the strongest risk reduction at lower amounts of fruit intake. The risk of hypertension decreased by ~7% with increasing the intake of fruits ≤∼300 g/d (Figure 2).
Nuts
Four studies with 11,962 incident hypertension cases were included in the meta-analysis comparing extreme intake categories (range of intake: 0−37 g/d). An inverse association between the risk of hypertension and nut intake observed (RR: 0.85; 95% CI: 0.78, 0.92; I2 = 0%; P-heterogeneity = 0.92) when comparing extreme categories (Supplemental Figure 9). An increase in nut intake by 28 g/d was inversely associated with the risk of hypertension (RR: 0.70; 95% CI: 0.45, 1.08; I2 = 69%; P-heterogeneity = 0.02; n = 4) (Supplemental Figure 10).
No evidence of a nonlinear dose-response association was observed (P-nonlinearity = 0.40; n = 4 studies). The risk of hypertension decreased by ~15% with increasing the intake of nuts ≤∼40 g/d (Figure 2).
Legumes
Six studies with 80,871 incident hypertension cases were included in the meta-analysis comparing extreme intake categories (range of intake: 0−71 g/d). In this analysis we observed an inverse association between the risk of hypertension and legume intake (RR: 0.92; 95% CI: 0.86, 0.98; I2 = 0%; P-heterogeneity = 0.49) (Supplemental Figure 11), but there was no association for each additional daily 50-g intake (RR: 0.98; 95% CI: 0.95, 1.01; I2 = 0%; P-heterogeneity = 0.43; n = 5) (Supplemental Figure 12).
No evidence of a nonlinear dose-response association was detected (P-nonlinearity = 0.17; n = 5 studies). The risk of hypertension decreased by ~5% with increasing the intake of legumes ≤∼70 g/d (Figure 2).
Eggs
One study with 144 incident hypertension cases was detected (range of intake: 0−23 g/d). This study showed an inverse association between the highest and lowest egg-intake category (RR: 0.54; 95% CI: 0.32, 0.91) (Supplemental Figure 13) and for each additional daily 50-g intake (RR: 0.25; 95% CI: 0.08, 0.74) (Supplemental Figure 14).
Dairy
Nine studies with 31,509 incident hypertension cases were included in the meta-analysis comparing extreme intake categories (range of intake: 0−800 g/d). An inverse association between the risk of hypertension and dairy intake was observed (RR: 0.89; 95% CI: 0.86, 0.93; I2 = 0%; P-heterogeneity = 0.65) when comparing extreme categories (Supplemental Figure 15). An increase in dairy intake by 200 g/d was inversely associated with the risk of hypertension by 5% (RR: 0.95; 95% CI: 0.94, 0.97; I2 = 0%; P-heterogeneity = 0.50; n = 9) (Supplemental Figure 16).
No significant differences were observed when comparing low- and high-fat dairy products. No inverse association was observed in studies with a shorter-term follow-up or in studies with nonvalidated dietary assessment (Supplemental Table 16). No evidence of a nonlinear dose-response association between dairy products and the risk of hypertension was detected (P-nonlinearity = 0.22; n = 8 studies). The risk of hypertension decreased by ~15% with increasing the intake of dairy ≤∼800 g/d (Figure 2).
Fish
Eight studies with 83,612 incident hypertension cases were included in the meta-analysis comparing extreme intake categories (range of intake: 0−156 g/d). Comparing categories of highest and lowest intake of fish, we observed no association with the risk of hypertension (RR: 1.01; 95% CI: 0.92, 1.10; I2 = 57%; P-heterogeneity = 0.02) (Supplemental Figure 17). Similarly, an increase in fish intake by 100 g/d was not associated with the risk of hypertension (RR: 1.07; 95% CI: 0.98, 1.16; I2 = 74%; P-heterogeneity < 0.0001; n = 7) (Supplemental Figure 18).
None of the subgroup analyses showed an association between fish intake and the risk of hypertension (Supplemental Table 17). Evidence of a nonlinear dose-response association was observed (P-nonlinearity < 0.01; n = 7 studies). The risk increased by 8% with increasing the intake of fish ≤100 g/d. No additional risk increasing association is apparent above this value (Figure 2).
Red meat
Seven studies with 97,745 incident hypertension cases were included in the meta-analysis comparing extreme intake categories (range of intake: 0−121 g/d). A positive association between the risk of hypertension and red meat intake was observed (RR: 1.15; 95% CI: 1.02, 1.28; I2 = 84%; P-heterogeneity < 0.001) when comparing extreme categories (Supplemental Figure 19). Each additional daily 100-g red-meat intake was associated with a 14% increased risk of hypertension (RR: 1.14; 95% CI: 1.02, 1.28; I2 = 88%; P-heterogeneity < 0.001; n = 7) (Supplemental Figure 20). The observed heterogeneity persisted in the additional subgroup analyses. The positive associations were present in studies with a longer-term follow-up, studies including women and larger number of cases, and in studies conducted in the United States (Supplemental Table 18).
No evidence of a nonlinear dose-response association was detected (P-nonlinearity = 0.30; n = 7 studies). The risk increased by 40% with increasing the intake of red meat ≤200 g/d (Figure 2).
Processed meat
Five studies with 97,441 incident hypertension cases were included in the meta-analysis comparing extreme intake categories (range of intake: 0−39 g/d). A positive association between the risk of hypertension and processed-meat intake was observed (RR: 1.12; 95% CI: 1.02, 1.23; I2 = 81%; P-heterogeneity < 0.001) when comparing extreme categories (Supplemental Figure 21), as well as for each additional daily 50-g processed-meat consumption (RR: 1.12; 95% CI: 1.00, 1.26; I2 = 82%; P-heterogeneity < 0.001; n = 4) (Supplemental Figure 22).
The test for nonlinearity showed a significant relation (P-nonlinearity < 0.001; n = 4 studies). The risk of hypertension increased by ~7% with increasing the intake of processed meat ≤∼30 g/d. No additional risk-increasing association is apparent above this value (Figure 2).
SSBs
Five studies including 81,495 incident hypertension cases were included in the meta-analysis comparing extreme intake categories (range of intake: 0−457 mL/d). A positive association between the risk of hypertension and SSB intake was observed (RR: 1.12; 95% CI: 1.06, 1.18; I2 = 59%; P-heterogeneity = 0.04) when comparing extreme categories (Supplemental Figure 23), as well as for each additional daily 250-mL SSB consumption (RR: 1.07; 95% CI: 1.04, 1.10; I2 = 64%; P-heterogeneity = 0.04; n = 4) (Supplemental Figure 24). There was no evidence for a nonlinear relation (P-nonlinearity = 0.73; n = 4 studies); the risk of hypertension increased by ~13% with increasing the intake of SSB ≤∼450 mL/d (Figure 2).
Summary across food groups
Table 1 shows the RR for hypertension from the nonlinear dose-response analysis of the 12 predefined food groups according to servings per day. Optimal consumption (lowest serving with significant results and no further substantial change in risk or no further data for higher amounts) of risk-decreasing foods (3 servings/d = 90 g whole grains/d, RR: 0.85; 2 servings/d = 160 g fruits/d, RR: 0.93; 1 serving/d = 28 g nuts/d, RR: 0.88; three-fourths serving/d = 75 g legumes/d, RR: 0.94; 4 servings/d = 800 g dairy/d, RR: 0.85) results in a 44% reduction of hypertension (calculated by 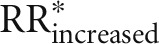 ) when compared with nonconsumption of these foods.
) when compared with nonconsumption of these foods.
TABLE 1.
RRs from nonlinear dose-response analysis of 12 pre-defined food groups and risk of hypertension according to intakes of servings per day1
| Servings/d |
|||||||
| Food group and daily serving size | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Inverse association | |||||||
| Whole grains (30 g) | 1.00 | 0.90 (0.87, 0.93) | 0.87 (0.84, 0.90) | 0.85 (0.80, 0.91) | NA | NA | NA |
| Fruits (80 g) | 1.00 | 0.96 (0.95, 0.98) | 0.93 (0.91, 0.96) | 0.93 (0.91, 0.95) | 0.93 (0.91, 0.95) | 0.93 (0.90, 0.96) | NA |
| Dairy (200 g) | 1.00 | 0.94 (0.91, 0.97) | 0.90 (0.86, 0.94) | 0.87 (0.82, 0.92) | 0.85 (0.79, 0.92) | NA | NA |
| Nuts (28 g) | 1.00 | 0.88 (0.80, 0.96) | NA | NA | NA | NA | NA |
| Legumes (100 g) | 1.00 | 0.94 (0.88, 1.00)2 | NA | NA | NA | NA | NA |
| Positive association | |||||||
| Fish (100 g) | 1.00 | 1.08 (1.05, 1.12) | NA | NA | NA | NA | NA |
| Red meat (85 g) | 1.00 | 1.16 (1.14, 1.18) | 1.35 (1.32, 1.38) | NA | NA | NA | NA |
| Processed meat (30 g) | 1.00 | 1.07 (1.04, 1.09) | NA | NA | NA | NA | NA |
| SSB (250 mL) | 1.00 | 1.06 (1.04, 1.08) | 1.14 (1.11, 1.17) | NA | NA | NA | NA |
| No association | |||||||
| Refined grains (30 g) | 1.00 | 0.96 (0.92, 1.01) | 0.96 (0.90, 1.02) | 0.97 (0.91, 1.05) | 1.00 (0.92, 1.08) | 1.03 (0.92, 1.16) | NA |
| Vegetables (80 g) | 1.00 | 0.99 (0.97, 1.01) | 0.98 (0.95, 1.01) | 0.98 (0.95, 1.02) | 0.98 (0.95, 1.02) | 0.99 (0.96, 1.02) | 1.00 (0.96, 1.03) |
| Not applicable | |||||||
| Eggs (55 g) | 1.00 | NA | NA | NA | NA | NA | NA |
Values are RRs (95% CIs). NA, not applicable; SSB, sugar-sweetened beverage.
This value refers to a three-fourths serving (75 g).
Risk-increasing foods were red meat, processed meat, fish, and SSBs. Compared with nonconsumption, an intake of 2 servings red meat/d (170 g, RR: 1.35), 1 serving processed meat/d (35 g, RR: 1.07), 1 serving fish/d (100 g, RR: 1.08), and 2 servings SSBs/d (500 mL, RR: 1.14) was associated with a 78% increased risk (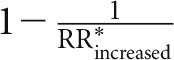 ). On the other hand, a risk reduction by ∼44% would be achieved by not consuming these foods (calculated by
). On the other hand, a risk reduction by ∼44% would be achieved by not consuming these foods (calculated by 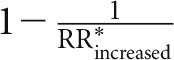 ).
).
Risk of bias and quality of meta-evidence
Because of the overall low number of included studies for each food group, it was not possible to conduct low-risk-of-bias sensitivity analysis for all food groups, except in the case of vegetable, fruit, dairy, fish, and red-meat intake (Supplemental Table 16). Findings including studies with a low risk of bias confirmed the results of the primary meta-analysis for these food groups. Overall, the quality of meta-evidence for the association between the 12 a priori defined food groups and the risk of hypertension was rated very low (refined grains, vegetables, legumes, and eggs) to low (whole grains, fruits, nuts, dairy, fish, red meat, processed meat, and SSBs) (Supplemental Table 19).
Discussion
This meta-analysis systematically evaluated the associations between 12 food groups (defined a priori) and the risk of hypertension by comparing extreme intake quantiles (highest compared with lowest) and by analyzing linear and nonlinear dose-responses.
In the linear dose-response meta-analysis an inverse association was present for whole grains, fruits, nuts, eggs, and dairy. On the other side, red meat, processed meat, and SSBs were associated with a higher risk of hypertension. We observed some evidence of nonlinearity between the intakes of whole grains, fruits, fish, and processed meat with the risk of hypertension. The trustworthiness of meta-evidence was rated very-low to low by applying the NutriGrade scoring system, suggesting that further high-quality research would provide important evidence on the confidence for the association between the 12 food groups and the risk of hypertension.
To the best of our knowledge, there has not been another meta-analytical synthesis of any association of the food groups whole grains, refined grains, legumes, eggs, red meat, and processed meat with the risk of hypertension. For fruits, vegetables, dairy, fish, nuts, and SSBs, findings similar to ours have been shown in previous meta-analyses. These publications, like our results, reported an inverse association of hypertension with the consumption of nuts (52, 53), fruits (54, 55), and dairy (56, 57), a positive association with the consumption of SSBs (58–61), no association with fish (62), and conflicting associations for vegetable intake (54, 55). All these meta-analyses were based on a single food group (52–62), several publications combined different types of observational studies (prospective cohort studies, cross-sectional studies) (55, 62), and none of them differentiated between incident hypertension and the risk of elevated blood pressure (>130 mm Hg systolic blood pressure or >85 mm Hg diastolic blood pressure) (52–62). Moreover, most of these meta-analyses did not explore nonlinear dose-response relations and the trustworthiness of meta-evidence.
The observed inverse association between whole-grain intake and hypertension in the linear and nonlinear dose-response analysis is remarkably identical to results from meta-analyses that have linked whole-grain consumption with the reduced risk of weight gain, type 2 diabetes, cardiovascular diseases, cancer, and overall mortality (12, 13, 63, 64). Two RCTs have shown important reductions in blood pressure by comparing a whole-grain diet with a diet high in refined grains (65, 66). Antihypertensive effects of whole-grain intake are biologically plausible because of a lower risk of adiposity and the fact that ~75% of the incidence of hypertension is related directly to obesity (67). Furthermore, whole-grain constituents, such as phytochemicals, and nutrients, such as magnesium, potassium, selenium, zinc, and fiber, have been shown to lower blood pressure (4, 68).
A recent umbrella review of 14 systematic reviews summarized the strength of the evidence for the association between the consumption of nuts and cardiovascular disease. Authors concluded that to clarify the mechanism behind the inverse association between the consumption of nuts and the risk of hypertension, more clinical and experimental studies are needed; meta-analyses of RCTs showed no effect of nut consumption on blood pressure in the normal range (69), despite the recently highly perceived findings from the PREDIMED (Prevención con Dieta Mediterránea) trial (70). Nuts are rich in MUFAs, PUFAs, magnesium, potassium, fiber, antioxidants, and vitamins that are linked to lower blood pressure (71–74).
Many meta-analyses have consistently found evidence of an inverse association between the intake of fruits and vegetables and the risk of weight gain, cardiovascular disease, cancer, type 2 diabetes, and all-cause mortality (12, 13, 75, 76). Moreover, a Cochrane review of 10 intervention trials including 1730 participants observed a reduction in blood pressure after fruit- and vegetable-based interventions (77). Although 2 servings fruits/d was associated with a 7% reduced risk of hypertension, no significant association was detected for vegetable consumption in general in our dose-response meta-analysis. One explanation could be the potential role of BMI as mediator for this association (67). Wang et al. (32) observed an elimination of the inverse associations after adjustment for BMI (potential overadjustment), suggesting that body-weight maintenance could be one important pathway through which vegetable consumption may contribute to blood pressure regulation (76). Further intervention studies are warranted to clarify the association of different types of vegetables and different cooking and processing methods with the risk of hypertension. In line with our findings, a clinical trial incorporating legumes as part of a low–glycemic index diet resulted in a significant blood pressure reduction in 121 participants (78).
The present meta-analysis shows a 13% lower risk of hypertension for 3 servings dairy/d. Several beneficial components of dairy products may contribute to the antihypertensive effect, including calcium and potassium (79), or lactotripeptides (80). Potential mechanistic evidence suggests that these peptides may inhibit the blood vessel–constrictive effect of angiotensin I–converting enzyme (81). Moreover, some evidence suggests that dairy products, especially fermented ones, are associated with a reduced risk of adiposity (82), one of the main causes of hypertension. Contradictory to our findings, a meta-analysis of the published clinical trials showed that a higher dairy intake has no significant effect on change in systolic blood pressure (83).
A detrimental effect of SSBs on hypertension is biologically plausible because of the convincing evidence that the consumption of SSBs is associated with weight gain and obesity in adults (84). Moreover, previous dose-response meta-analyses have shown a moderate to high quality of meta-evidence for associations between SSBs and the increased risk of type 2 diabetes, coronary heart disease, stroke, heart failure, and all-cause mortality (12, 13). Our findings are supported by evidence from an RCT in which overweight participants consuming high quantities of SSBs (1 L/d for 6 mo) had significantly higher blood pressure values (85).
Red meat and processed meat were associated with an increase in risk of hypertension of 12–14% in the linear dose-response meta-analysis. The mechanism by which these types of meat increase hypertension risk seems to be complex and controversial. Some evidence indicates that Maillard reaction products in cooked meat, such as heterocyclic amines, advanced glycosylation end-products, and acrylamides, may induce hypertension because of inflammatory and oxidation pathways (86, 87). However, our findings regarding red meat and the risk of hypertension are only partly supported by RCT evidence. A recent meta-analysis of 24 RCTs did not find detrimental effects on blood pressure comparing ≥0.5 servings/d with <0.5 serving of total red-meat intake (88). Additionally, in the Beef in an Optimal Lean Diet study, a moderate-protein DASH-like diet including lean beef, decreased systolic blood pressure in normotensive individuals (89).
In our study, fish consumption was associated with a slight increase in hypertension risk in the nonlinear dose-response analysis. No other association was observed in the high-compared with low-intake categories and dose-response meta-analysis. Because of these inconsistencies further research will provide important evidence.
Large clinical trials have shown that the DASH dietary pattern substantially lowers blood pressure among normotensive and hypertensive patients (6, 7). The DASH dietary pattern is based on several food groups, which are largely included in the present meta-analysis and comprise higher amounts of whole grains, fruits, vegetables, dairy products (mainly low-fat), and nuts, and lower intakes of red meat, processed meat, fats, oils, and SSBs (7). All the food groups recommended as part of the DASH dietary pattern (90) showed an inverse association with hypertension risk in the present meta-analysis via the linear and nonlinear dose-response analyses with the exception of vegetables. Moreover, food groups that should be avoided or consumed in lower amounts, such as red meat, processed meat, and SSBs, were consistently associated with hypertension risk.
With our operational definition, the optimal consumption of foods inversely associated with incident hypertension was associated with 44% lower risk, whereas avoidance of foods associated with higher risk was also associated with 44% lower incidence of hypertension.
Some strengths of the present meta-analysis are the large number of investigated food groups, the multiple types of meta-analyses performed (highest compared with lowest intake category, as well as linear and nonlinear dose-response), calculation of the optimal intake of combined food groups, the risk of bias assessment, and the assessment of the trustworthiness of the quality of the meta-evidence.
Some limitations should be considered when interpreting the results of the present meta-analytical synthesis. First, people with higher intakes of whole grains, fruits, vegetables, dairy, and nuts might have a different socioeconomic status or lifestyle than those with a lower intake, thus confounding by lifestyle factors should be considered a potential bias (28, 35, 44, 91). Second, because of the overall low number of included studies, subgroup and sensitivity analyses could be performed only for 5 (fruits, vegetables, dairy, fish, and red meat) of the 12 food groups. In line with this, it was also not possible to assess publication bias and small study effects. The results for several food groups should be cautiously interpreted because of the small number of studies, especially for whole grains, refined grains, nuts, and eggs (<5 prospective studies available).
In summary, this comprehensive meta-analysis of 28 studies identified the optimal intakes of whole grains, fruits, nuts, legumes, dairy, red meat, processed meat, and SSBs regarding the risk of hypertension. These findings need to be seen under the light of very-low to low credibility of meta-evidence. However, the findings support the current dietary recommendations in the primary prevention of hypertension.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: DASH, Dietary Approaches to Stop Hypertension Trial; RCT, randomized controlled trial; SSB, sugar-sweetened beverage.
References
- 1.WHO. Raised blood pressure: situation and trends. Global health observatory [Internet]. c2012 [cited 2017 Feb 4]. Available from: http://www.who.int/gho/ncd/risk_factors/blood_pressure_prevalence/en/.
- 2.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017;389:37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet 2008;371:1513–8. [DOI] [PubMed] [Google Scholar]
- 4.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 2006;47:296–308. [DOI] [PubMed] [Google Scholar]
- 5.Leung AA, Nerenberg K, Daskalopoulou SS, McBrien K, Zarnke KB, Dasgupta K, Cloutier L, Gelfer M, Lamarre-Cliche M, Milot A, et al. Hypertension Canada's 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol 2016;32:569–88. [DOI] [PubMed] [Google Scholar]
- 6.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER III, Simons-Morton DG, et al. ; DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 7.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- 8.Obarzanek E, Sacks FM, Vollmer WM, Bray GA, Miller ER III, Lin PH, Karanja NM, Most-Windhauser MM, Moore TJ, Swain JF, et al. ; DASH Research Group. Effects on blood lipids of a blood pressure-lowering diet: the Dietary Approaches to Stop Hypertension (DASH) Trial. Am J Clin Nutr 2001;74:80–9. [DOI] [PubMed] [Google Scholar]
- 9.Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi T, Azizi F. Beneficial effects of a dietary approaches to stop hypertension eating plan on features of the metabolic syndrome. Diabetes Care 2005;28:2823–31. [DOI] [PubMed] [Google Scholar]
- 10.Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, Caccia C, Johnson J, Waugh R, Sherwood A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med 2010;170:126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the healthy eating index, the alternate healthy eating index, the dietary approaches to stop hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 2017. [DOI] [PubMed] [Google Scholar]
- 12.Schwingshackl L, Hoffmann G, Lampousi AM, Knuppel S, Iqbal K, Schwedhelm C, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol 2017;32:363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwingshackl L, Schwedhelm C, Hoffmann G, Lampousi A-M, Knüppel S, Iqbal K, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr 2017;105:1462–73. [DOI] [PubMed] [Google Scholar]
- 14.Schwingshackl L, Chaimani A, Bechthold A, Iqbal K, Stelmach-Mardas M, Hoffmann G, Schwedhelm C, Schlesinger S, Boeing H. Food groups and risk of chronic disease: a protocol for a systematic review and network meta-analysis of cohort studies. Syst Rev 2016;5:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 17.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- 18.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J 2006;6:40–57. [Google Scholar]
- 19.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. Oxford (United Kingdom): The Cochrane Collaboration; 2011. [updated 2011 Mar; cited 2017 May 20]. Available from: http://handbook-5-1.cochrane.org/. [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwingshackl L, Knüppel S, Schwedhelm C, Hoffmann G, Missbach B, Stelmach-Mardas M, Dietrich S, Eichelmann F, Kontopanteils E, Iqbal K, et al. Perspective: nutriGrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr 2016;7:994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flint AJ, Hu FB, Glynn RJ, Jensen MK, Franz M, Sampson L, Rimm EB. Whole grains and incident hypertension in men. Am J Clin Nutr 2009;90:493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kochar J, Gaziano JM, Djousse L. Breakfast cereals and risk of hypertension in the Physicians’ Health Study I. Clin Nutr 2012;31:89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Gaziano JM, Liu S, Manson JE, Buring JE, Sesso HD. Whole- and refined-grain intakes and the risk of hypertension in women. Am J Clin Nutr 2007;86:472–9. [DOI] [PubMed] [Google Scholar]
- 27.Weng LC, Steffen LM, Szklo M, Nettleton J, Chambless L, Folsom AR. A diet pattern with more dairy and nuts, but less meat is related to lower risk of developing hypertension in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Nutrients 2013;5:1719–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borgi L, Muraki I, Satija A, Willett WC, Rimm EB, Forman JP. Fruit and vegetable consumption and the incidence of hypertension in three prospective cohort studies. Hypertension 2016;67:288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golzarand M, Bahadoran Z, Mirmiran P, Zadeh-Vakili A, Azizi F. Consumption of nitrate-containing vegetables is inversely associated with hypertension in adults: a prospective investigation from the Tehran Lipid and Glucose Study. J Nephrol 2016;29:377–84. [DOI] [PubMed] [Google Scholar]
- 30.Nuñez-Cordoba JM, Alonso A, Beunza JJ, Palma S, Gomez-Gracia E, Martinez-Gonzalez MA. Role of vegetables and fruits in Mediterranean diets to prevent hypertension. Eur J Clin Nutr 2009;63:605–12. [DOI] [PubMed] [Google Scholar]
- 31.Tsubota-Utsugi M, Ohkubo T, Kikuya M, Metoki H, Kurimoto A, Suzuki K, Fukushima N, Hara A, Asayama K, Satoh H, et al. High fruit intake is associated with a lower risk of future hypertension determined by home blood pressure measurement: the OHASAMA study. J Hum Hypertens 2011;25:164–71. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Manson JE, Gaziano JM, Buring JE, Sesso HD. Fruit and vegetable intake and the risk of hypertension in middle-aged and older women. Am J Hypertens 2012;25:180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Djoussé L, Rudich T, Gaziano JM. Nut consumption and risk of hypertension in US male physicians. Clin Nutr 2009;28:10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golzarand M, Bahadoran Z, Mirmiran P, Azizi F.. Protein foods group and 3-year incidence of hypertension: a prospective study from Tehran Lipid and Glucose Study. J Ren Nutr 2016;26:219–25. [DOI] [PubMed] [Google Scholar]
- 35.Martínez-Lapiscina EH, Pimenta AM, Beunza JJ, Bes-Rastrollo M, Martínez JA, Martínez-González MA. Nut consumption and incidence of hypertension: the SUN prospective cohort. Nutr Metab Cardiovasc Dis 2010;20:359–65. [DOI] [PubMed] [Google Scholar]
- 36.Núñez-Córdoba JM, Valencia-Serrano F, Toledo E, Alonso A, Martínez-González MA. The Mediterranean diet and incidence of hypertension: the Seguimiento Universidad de Navarra (SUN) study. Am J Epidemiol 2009;169:339–46. [DOI] [PubMed] [Google Scholar]
- 37.Alonso A, Beunza JJ, Delgado-Rodriguez M, Martinez JA, Martinez-Gonzalez MA. Low-fat dairy consumption and reduced risk of hypertension: the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr 2005;82:972–9. [DOI] [PubMed] [Google Scholar]
- 38.Camões M, Oliveira A, Pereira M, Severo M, Lopes C. Role of physical activity and diet in incidence of hypertension: a population-based study in Portuguese adults. Eur J Clin Nutr 2010;64:1441–9. [DOI] [PubMed] [Google Scholar]
- 39.Engberink MF, Hendriksen MA, Schouten EG, van Rooij FJ, Hofman A, Witteman JC, Geleijnse JM. Inverse association between dairy intake and hypertension: the Rotterdam Study. Am J Clin Nutr 2009;89:1877–83. [DOI] [PubMed] [Google Scholar]
- 40.Engberink MF, Geleijnse JM, de Jong N, Smit HA, Kok FJ, Verschuren WM. Dairy intake, blood pressure, and incident hypertension in a general Dutch population. J Nutr 2009;139:582–7. [DOI] [PubMed] [Google Scholar]
- 41.Heraclides A, Mishra GD, Hardy RJ, Geleijnse JM, Black S, Prynne CJ, Kuh D, Soedamah-Muthu SS. Dairy intake, blood pressure and incident hypertension in a general British population: the 1946 birth cohort. Eur J Nutr 2012;51:583–91. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Manson JE, Buring JE, Lee IM, Sesso HD. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension 2008;51:1073–9. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Fox CS, Troy LM, McKeown NM, Jacques PF. Longitudinal association of dairy consumption with the changes in blood pressure and the risk of incident hypertension: the Framingham Heart Study. Br J Nutr 2015;114:1887–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talaei M, Pan A, Yuan JM, Koh WP. Dairy food intake is inversely associated with risk of hypertension: the Singapore Chinese Health Study. J Nutr 2017;147:235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borgi L, Curhan GC, Willett WC, Hu FB, Satija A, Forman JP. Long-term intake of animal flesh and risk of developing hypertension in three prospective cohort studies. J Hypertens 2015;33:2231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gillum RF, Mussolino ME, Madans JH. Fish consumption and hypertension incidence in African Americans and whites: the NHANES I Epidemiologic Follow-up Study. J Natl Med Assoc 2001;93:124–8. [PMC free article] [PubMed] [Google Scholar]
- 47.Xun P, Hou N, Daviglus M, Liu K, Morris JS, Shikany JM, Sidney S, Jacobs DR, He K. Fish oil, selenium and mercury in relation to incidence of hypertension: a 20-year follow-up study. J Intern Med 2011;270:175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lajous M, Bijon A, Fagherazzi G, Rossignol E, Boutron-Ruault MC, Clavel-Chapelon F. Processed and unprocessed red meat consumption and hypertension in women. Am J Clin Nutr 2014;100:948–52. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Manson JE, Buring JE, Sesso HD. Meat intake and the risk of hypertension in middle-aged and older women. J Hypertens 2008;26:215–22. [DOI] [PubMed] [Google Scholar]
- 50.Cohen L, Curhan G, Forman J. Association of sweetened beverage intake with incident hypertension. J Gen Intern Med 2012;27:1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sayon-Orea C, Martinez-Gonzalez MA, Gea A, Alonso A, Pimenta AM, Bes-Rastrollo M. Baseline consumption and changes in sugar-sweetened beverage consumption and the incidence of hypertension: the SUN project. Clin Nutr 2015;34:1133–40. [DOI] [PubMed] [Google Scholar]
- 52.Guo K, Zhou Z, Jiang Y, Li W, Li Y. Meta-analysis of prospective studies on the effects of nut consumption on hypertension and type 2 diabetes mellitus. J Diabetes 2015;7:202–12. [DOI] [PubMed] [Google Scholar]
- 53.Zhou D, Yu H, He F, Reilly KH, Zhang J, Li S, Zhang T, Wang B, Ding Y, Xi B. Nut consumption in relation to cardiovascular disease risk and type 2 diabetes: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr 2014;100:270–7. [DOI] [PubMed] [Google Scholar]
- 54.Wu L, Sun D, He Y. Fruit and vegetables consumption and incident hypertension: dose-response meta-analysis of prospective cohort studies. J Hum Hypertens 2016;30:573–80. [DOI] [PubMed] [Google Scholar]
- 55.Li B, Li F, Wang L, Zhang D. Fruit and vegetables consumption and risk of hypertension: a meta-analysis. J Clin Hypertens (Greenwich) 2016;18:468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soedamah-Muthu SS, Verberne LD, Ding EL, Engberink MF, Geleijnse JM. Dairy consumption and incidence of hypertension: a dose-response meta-analysis of prospective cohort studies. Hypertension 2012;60:1131–7. [DOI] [PubMed] [Google Scholar]
- 57.Ralston RA, Lee JH, Truby H, Palermo CE, Walker KZ. A systematic review and meta-analysis of elevated blood pressure and consumption of dairy foods. J Hum Hypertens 2012;26:3–13. [DOI] [PubMed] [Google Scholar]
- 58.Jayalath VH, de Souza RJ, Ha V, Mirrahimi A, Blanco-Mejia S, Di Buono M, Jenkins AL, Leiter LA, Wolever TM, Beyene J, et al. Sugar-sweetened beverage consumption and incident hypertension: a systematic review and meta-analysis of prospective cohorts. Am J Clin Nutr 2015;102:914–21. [DOI] [PubMed] [Google Scholar]
- 59.Kim Y, Je Y. Prospective association of sugar-sweetened and artificially sweetened beverage intake with risk of hypertension. Arch Cardiovasc Dis 2016;109:242–53. [DOI] [PubMed] [Google Scholar]
- 60.Cheungpasitporn W, Thongprayoon C, Edmonds PJ, Srivali N, Ungprasert P, Kittanamongkolchai W, Erickson SB. Sugar and artificially sweetened soda consumption linked to hypertension: a systematic review and meta-analysis. Clin Exp Hypertens 2015;37:587–93. [DOI] [PubMed] [Google Scholar]
- 61.Xi B, Huang Y, Reilly KH, Li S, Zheng R, Barrio-Lopez MT, Martinez-Gonzalez MA, Zhou D. Sugar-sweetened beverages and risk of hypertension and CVD: a dose-response meta-analysis. Br J Nutr 2015;113:709–17. [DOI] [PubMed] [Google Scholar]
- 62.Yang B, Shi MQ, Li ZH, Yang JJ, Li D. Fish, long-chain n-3 PUFA and incidence of elevated blood pressure: a meta-analysis of prospective cohort studies. Nutrients 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aune D, Keum N, Giovannucci E. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose–response meta-analysis of prospective studies. BMJ 2016;353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr 2012;142:1304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kirwan JP, Malin SK, Scelsi AR, Kullman EL, Navaneethan SD, Pagadala MR, Haus JM, Filion J, Godin JP, Kochhar S, et al. A whole-grain diet reduces cardiovascular risk factors in overweight and obese adults: a randomized controlled trial. J Nutr 2016;146:2244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tighe P, Duthie G, Vaughan N, Brittenden J, Simpson WG, Duthie S, Mutch W, Wahle K, Horgan G, Thies F. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized controlled trial. Am J Clin Nutr 2010;92:733–40. [DOI] [PubMed] [Google Scholar]
- 67.Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, Sowers J. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of The Obesity Society and the American Society of Hypertension. J Clin Hypertens (Greenwich) 2013;15:14–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alonso A, Beunza JJ, Bes-Rastrollo M, Pajares RM, Martinez-Gonzalez MA. Vegetable protein and fiber from cereal are inversely associated with the risk of hypertension in a Spanish cohort. Arch Med Res 2006;37:778–86. [DOI] [PubMed] [Google Scholar]
- 69.Schwingshackl L, Hoffmann G, Missbach B, Stelmach-Mardas M, Boeing H. An umbrella review of nuts intake and risk of cardiovascular disease. Curr Pharm Des 2017;23:1016–27. [DOI] [PubMed] [Google Scholar]
- 70.Toledo E, Hu FB, Estruch R, Buil-Cosiales P, Corella D, Salas-Salvadó J, Covas MI, Arós F, Gómez-Gracia E, Fiol M, et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: results from a randomized controlled trial. BMC Med 2013;11:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwingshackl L, Strasser B, Hoffmann G. Effects of monounsaturated fatty acids on cardiovascular risk factors: a systematic review and meta-analysis. Ann Nutr Metab 2011;59:176–86. [DOI] [PubMed] [Google Scholar]
- 72.Han H, Fang X, Wei X, Liu Y, Jin Z, Chen Q, Fan Z, Aaseth J, Hiyoshi A, He J, et al. Dose-response relationship between dietary magnesium intake, serum magnesium concentration and risk of hypertension: a systematic review and meta-analysis of prospective cohort studies. Nutr J 2017;16:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Houston MC. The importance of potassium in managing hypertension. Curr Hypertens Rep 2011;13:309–17. [DOI] [PubMed] [Google Scholar]
- 74.Schwingshackl L, Hoffmann G. Monounsaturated fatty acids and risk of cardiovascular disease: synopsis of the evidence available from systematic reviews and meta-analyses. Nutrients 2012;4:1989–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, Greenwood DC, Riboli E, Vatten LJ, Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol 2017. Feb 22 (Epub ahead of print; DOI:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwingshackl L, Hoffmann G, Kalle-Uhlmann T, Arregui M, Buijsse B, Boeing H. Fruit and vegetable consumption and changes in anthropometric variables in adult populations: a systematic review and meta-analysis of prospective cohort studies. PLoS One 2015;10:e0140846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hartley L, Igbinedion E, Holmes J, Flowers N, Thorogood M, Clarke A, Stranges S, Hooper L, Rees K. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev 2013:CD009874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jenkins DJ, Kendall CW, Augustin LS, Mitchell S, Sahye-Pudaruth S, Blanco Mejia S, Chiavaroli L, Mirrahimi A, Ireland C, Bashyam B, et al. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med 2012;172:1653–60. [DOI] [PubMed] [Google Scholar]
- 79.Houston MC, Harper KJ. Potassium, magnesium, and calcium: their role in both the cause and treatment of hypertension. J Clin Hypertens (Greenwich) 2008;10(7 Suppl 2):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fekete ÁA, Givens DI, Lovegrove JA. Casein-derived lactotripeptides reduce systolic and diastolic blood pressure in a meta-analysis of randomised clinical trials. Nutrients 2015;7:659–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boelsma E, Kloek J. Lactotripeptides and antihypertensive effects: a critical review. Br J Nutr 2009;101:776–86. [DOI] [PubMed] [Google Scholar]
- 82.Schwingshackl L, Hoffmann G, Schwedhelm C, Kalle-Uhlmann T, Missbach B, Knuppel S, Boeing H. Consumption of dairy products in relation to changes in anthropometric variables in adult populations: a systematic review and meta-analysis of cohort studies. PLoS One 2016;11:e0157461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ding M, Huang T, Bergholdt HK, Nordestgaard BG, Ellervik C, Qi L. Dairy consumption, systolic blood pressure, and risk of hypertension: Mendelian randomization study. BMJ 2017;356:j1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr 2013;98:1084–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maersk M, Belza A, Stodkilde-Jorgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, Richelsen B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr 2012;95:283–9. [DOI] [PubMed] [Google Scholar]
- 86.Baumann M. Role of advanced glycation end products in hypertension and cardiovascular risk: human studies. J Am Soc Hypertens 2012;6:427–35. [DOI] [PubMed] [Google Scholar]
- 87.Uribarri J, Cai W, Sandu O, Peppa M, Goldberg T, Vlassara H. Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann N Y Acad Sci 2005;1043:461–6. [DOI] [PubMed] [Google Scholar]
- 88.O’Connor LE, Kim JE, Campbell WW. Total red meat intake of ≥0.5 servings/d does not negatively influence cardiovascular disease risk factors: a systemically searched meta-analysis of randomized controlled trials. Am J Clin Nutr 2017;105:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roussell MA, Hill AM, Gaugler TL, West SG, Ulbrecht JS, Vanden Heuvel JP, Gillies PJ, Kris-Etherton PM. Effects of a DASH-like diet containing lean beef on vascular health. J Hum Hypertens 2014;28:600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schwingshackl L, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 2015;115:780–800.e5. [DOI] [PubMed] [Google Scholar]
- 91.Kyrø C, Skeie G, Dragsted LO, Christensen J, Overvad K, Hallmans G, Johansson I, Lund E, Slimani N, Johnsen NF, et al. Intake of whole grains in Scandinavia is associated with healthy lifestyle, socio-economic and dietary factors. Public Health Nutr 2011;14:1787–95. [DOI] [PubMed] [Google Scholar]