Abstract
Purpose
Cognitive dysfunction is reported in people with cancer. Therefore, we evaluated longitudinal changes in cognitive function and underlying mechanisms in people with colorectal cancer (CRC) and healthy controls (HCs).
Patients and Methods
Participants completed cognitive assessments and questionnaires reporting cognitive symptoms, fatigue, quality of life, and anxiety/depression at baseline (before chemotherapy, if given) and 6, 12, and 24 months. Blood tests included cytokines, clotting factors, apolipoprotein E genotype, and sex hormones. Primary end point was overall cognitive function measured by the Global Deficit Score at 12 months.
Results
We recruited 289 patients with localized CRC (173 received chemotherapy; median age, 59 years; 63% male), 73 patients with limited metastatic/recurrent CRC, and 72 HCs. Cognitive impairment was more frequent in patients with localized CRC than HCs at baseline (43% v 15%, respectively; P < .001) and 12 months (46% v 13%, respectively; P < .001), with no significant effect of chemotherapy. Attention/working memory, verbal learning/memory, and complex processing speed were most affected. Cognitive impairment was similar in patients with localized and metastatic CRC. Cytokine levels were elevated in patients with CRC compared with HCs. There was no association between overall cognitive function and fatigue, quality of life, anxiety/depression, or any blood test. Cognitive symptoms at 12 months were reported in 25% of patients with localized CRC versus 17% of HCs (P = .19). More participants who received chemotherapy had cognitive symptoms at 6 months (32%) versus those who did not (16%; P = .007), with no significant difference at 12 months (29% v 21%, respectively; P = .19). Objective cognitive function was only weakly associated with cognitive symptoms.
Conclusion
Patients with CRC had substantially more cognitive impairment at every assessment than HCs, with no significant added effect of chemotherapy. Mechanisms of cognitive impairment remain unknown.
INTRODUCTION
At diagnosis, approximately 30% of women with breast cancer have impairment on formal neuropsychological testing.1–3 In some women, this may be exacerbated by adjuvant chemotherapy, but most women have subsequent improvement, although for 15% to 45%, the effects are sustained.2,4,5 Less is known about cognitive function in people with colorectal cancer (CRC). Two single-arm studies have evaluated cognitive function in patients with CRC after oxaliplatin/fluorouracil chemotherapy. One reported no cognitive impairment but was limited by small sample size, lack of a comparator group, use of a brief cognitive battery, and failure to account for practice effect.6 The other study reported 37% to 39% cognitive impairment before and 6 months after chemotherapy, with a decline from baseline in 52% of participants, particularly in verbal memory.7
We recently published baseline data from a prospective longitudinal study evaluating cognitive function in patients with CRC, which showed that 43% had objective cognitive impairment shortly after diagnosis, compared with 15% in healthy controls (HCs) without cancer.8 There was no significant difference in probability of cognitive impairment based on disease stage or whether cognitive assessments were performed before or after surgery. Neuropsychological performance was not associated with perceived cognitive function, fatigue, anxiety/depression, or quality of life (QOL), but these variables were associated with each other. Here, we present the results of longitudinal cognitive assessments at 6, 12, and 24 months, with evaluation of candidate mechanisms by which cancer and chemotherapy might lead to cognitive impairment.
PATIENTS AND METHODS
Patient characteristics and methods are described in Vardy et al.8 The main study compared cognitive function and fatigue in the following three groups: patients with stage III or high-risk stage II CRC, treated with surgery and adjuvant or neoadjuvant chemotherapy; patients who underwent surgery but did not receive chemotherapy, most of whom had stage I or II CRC; and HCs. A substudy comprised patients with limited metastatic or locally recurrent CRC who received first-line chemotherapy for metastatic disease.
Participants were ≤ 75 years old at baseline with no prior malignancy or comorbidities that might impact on cognitive performance and at least year 8 English fluency.8 Participants with localized CRC were chemotherapy naïve, and recurrence of disease resulted in their withdrawal from the study. Participants with metastatic/recurrent disease could have received (neo)adjuvant chemotherapy more than 12 months previously.
Participants with CRC were recruited from hospitals in Toronto, Canada (n = 235), and Sydney, Australia (n = 127). HCs were from Sydney and were generally family or friends of patients with cancer. The study had research ethics board approval at each institution, and all participants provided written informed consent.
Assessments
Cognitive testing and patient-reported outcome (PRO) questionnaires were completed and blood collected at baseline (before chemotherapy, if given) and 6 and 12 months for all participants, except blood tests were not required for participants with recurrent/metastatic CRC. Patients with localized CRC also completed assessments at 24 months.
Cognitive function was evaluated by clinical neuropsychological tests, the computerized Cambridge Neuropsychological Test Automated Battery (CANTAB), and the modified Six Elements Test.8 PROs included the Functional Assessment of Cancer Therapy–Fatigue (FACT-F), which incorporates the FACT-General9,10 evaluating general QOL, the FACT–Cognitive Function version 211 evaluating cognitive symptoms, and the General Health Questionnaire-12 assessing anxiety and depression.12 Blood tests included CBC, creatinine, liver function tests, carcinoembryonic antigen, sex hormones, selected cytokines, markers of blood clotting, and apolipoprotein genotyping.8
Analysis of Cognitive Function
Raw scores from the clinical neuropsychological tests and CANTAB were converted to demographically corrected T or Z scores (based on age, education, and sex),13 and a deficit score ranging from 0 (no impairment, T score > 39) to 5 (severe impairment, T score < 20) was derived. Deficit scores were averaged to determine Global Deficit Scores (GDS) to reflect overall cognitive performance for the clinical neuropsychological tests and CANTAB.14 Cognitive impairment was defined in the following two ways8: GDS more than 0.514; and using International Cancer and Cognition Task Force criteria of ≥ 2 standard deviations below HCs on at least one cognitive test or ≥ 1.5 standard deviations below HCs on two or more tests.15
Primary end points were cognitive function (GDS for clinical neuropsychological tests) and fatigue (FACT-F subscale). Detailed information about fatigue will be presented elsewhere. Secondary end points included other aspects of cognitive function and PROs; candidate mechanisms and associations between primary and secondary end points were explored.
Change in cognitive function with time was based on summary regression-based change scores of raw neuropsychological summary scores, with adjustment for practice effect based on time-dependent changes in cognitive scores for the HCs.16 Regression-based change scores were determined for each individual from their baseline performance with adjustments for demographics (age, sex, and education) and time between assessments. Multiple linear regression was used to predict test scores. The 5% and 95% cutoffs of the summary regression-based change scores among HCs were used to classify patients with CRC as improvers, stable, or decliners (ie, those greater than, within, or less than the 90% CI for HCs).17
Statistical Analysis
The Kruskal-Wallis test was used for continuous variables, the Cochran-Armitage test for ordinal variables, and χ2 tests for categorical variables, to compare between groups at baseline. Linear mixed models assessed longitudinal association between CRC group and global T score as a result of skewedness of GDS. An interaction term between CRC group and time (group-time) was added into regression models to examine its influence on time-dependent neurocognitive performance. Kaplan-Meier methods compared time to decline in cognitive function (decliners v stable/improved) for participants with CRC and HCs, whereas risk ratios for earlier cognitive decline were estimated using Cox proportional hazards regression. Associations between outcomes were derived using Spearman rank sum correlation coefficients. P values are two-sided and reported as unadjusted values. Analyses were performed in SAS version 9.0 (SAS Institute, Cary, NC).
As described previously,8 a sample size of 170 patients who received chemotherapy and 120 patients who did not gave 80% power to detect a difference of 8% in incidence of cognitive impairment at 12 months based on expected rates of 20% and 12%, respectively. The HCs (n = 72) provided power to detect a ≥ 20% difference in baseline cognitive impairment between patients with localized CRC and HCs. Sample size for participants with metastatic/recurrent disease was set arbitrarily at 75 patients.
RESULTS
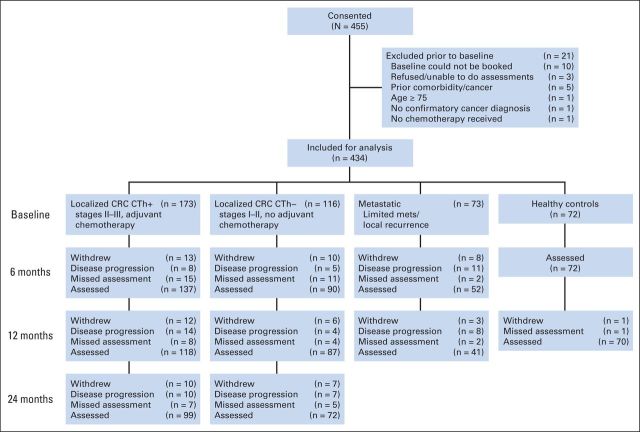
There were 434 participants, including 289 patients with localized CRC, of whom 173 received chemotherapy and 116 did not, 73 patients in the metastatic/recurrent group, and 72 HCs. Figure 1 details the number of participants tested at each assessment and reasons for attrition. Patients with CRC who completed more assessments had higher levels of education (P = .025) and were more likely to be primary English speakers (P < .001; Data Supplement).
Fig 1.
CONSORT diagram. CRC, colorectal cancer; CTh+, received adjuvant chemotherapy; CTh–, did not receive adjuvant chemotherapy.
Demographics and baseline characteristics of disease and treatment are listed in Table 1. Median age of patients with localized CRC was 59 years (range, 23 to 75 years), and 63% were men. Groups were relatively well balanced except that baseline assessment in patients who received chemotherapy occurred at a median 6.8 weeks after surgery, compared with 9.2 weeks in patients who did not receive chemotherapy, and HCs included more women than men and more HCs had English as their primary language than patients with CRC.
Table 1.
Comparison of Study Sample by Study Group Status
| Characteristic | No. of Participants (%) |
||||
|---|---|---|---|---|---|
| Localized CRC (n = 289) |
Metastatic CRC (n = 73) | Healthy Controls (n = 72) | |||
| CTh+ (n = 173) | CTh– (n = 116) | Total | |||
| Median age, years (range) | 57.0 (23-75) | 60.5 (23-75) | 59.0 (23-75) | 55.5 (28-75) | 58.5 (27-75) |
| Sex | |||||
| Male | 117 (68) | 66 (57) | 183 (63) | 40 (55) | 31 (43) |
| Female | 56 (32) | 50 (43) | 106 (37) | 33 (44) | 41 (57) |
| Country of residence | |||||
| Canada | 123 (71) | 60 (52) | 183 (63) | 60 (82) | 0 (0) |
| Australia | 50 (29) | 56 (48) | 106 (37) | 13 (18) | 72 (100) |
| Years of education | |||||
| Mean (standard deviation) | 13.8 (3.3) | 13.7 (3.5) | 13.8 (3.4) | 13.7 (3.4) | 13.6 (2.9) |
| Median (range) | 14 (5-20) | 14 (4-21) | 14 (4-21) | 14 (5-20) | 15 (6-20) |
| Non-English primary language | 48 (27.8) | 21 (18.1) | 69 (23.9) | 19 (26) | 5 (6.9) |
| Tumor stage | |||||
| I | 2 (1.2) | 48 (41.4) | 50 (17.3) | 0 (0.0) | 0 (0.0) |
| II | 46 (26.6) | 60 (51.7) | 106 (36.7) | 0 (0.0) | 0 (0.0) |
| III | 125 (72.3) | 3 (2.6) | 128 (44.3) | 4 (5.5) | 0 (0.0) |
| IV | 0 (0.0) | 0 (0.0) | 0 (0.0) | 69 (94.5) | 0 (0.0) |
| Cancer site | NA | ||||
| Colon | 104 (61) | 89 (77) | 193 (67) | 54 (77) | |
| Rectum | 66 (39) | 27 (23) | 93 (33) | 16 (23) | |
| Chemotherapy | NA | NA | NA | ||
| Adjuvant | 123 (71) | 123 (71) | |||
| Neoadjuvant | 46 (27) | 46 (27) | |||
| UNK | 4 (2) | 4 (2) | |||
| Chemotherapy regimen | |||||
| No chemotherapy | 0 (0) | 116 (100) | 116 (40.1) | 1 (1.4) | 72 (100) |
| FU | 54 (31.2) | 54 (18.7) | 4 (5.5) | ||
| Oxaliplatin | 72 (41.6) | 72 (24.9) | 36 (49.3) | ||
| Chemoradiation | 44 (25.4) | 44 (15.2) | 2 (2.7) | ||
| Irinotecan | 0 (0) | 0 (0) | 20 (27.4) | ||
| Other | 0 (0) | 0 (0) | 2 (2.7) | ||
| Missing | 3 (1.7) | 3 (1.0) | 4 (5.5) | ||
| Marital status | |||||
| Married/common law | 115 (66.5) | 73 (62.9) | 188 (65.1) | 53 (72.6) | 47 (65.3) |
| Separated/divorced | 24 (13.9) | 19 (16.4) | 43 (14.9) | 6 (8.2) | 8 (11.1) |
| Single | 24 (13.9) | 13 (11.2) | 37 (12.8) | 9 (12.3) | 12 (16.7) |
| Widowed | 2 (1.2) | 6 (5.2) | 8 (2.8) | 2 (2.7) | 5 (6.9) |
| Unknown | 8 (4.6) | 5 (4.3) | 13 (4.5) | 3 (4.1) | 0 (0.0) |
| Alcohol (glasses per day) | |||||
| 0-1 | 84 (48.6) | 44 (37.9) | 128 (44.3) | 43 (58.9) | 24 (33.3) |
| 2-4 | 49 (28.3) | 45 (38.8) | 94 (32.5) | 20 (27.4) | 41 (56.9) |
| 5+ | 18 (10.4) | 11 (9.5) | 29 (10.0) | 2 (2.7) | 5 (6.9) |
| Unknown | 22 (12.7) | 16 (13.8) | 38 (13.1) | 8 (11.0) | 2 (2.8) |
| Smoking status | |||||
| Nonsmoker | 78 (45.1) | 62 (53.5) | 140 (48.4) | 46 (63.0) | 39 (54.2) |
| Ex-smoker | 18 (10.4) | 6 (5.2) | 24 (8.3) | 2 (2.7) | 7 (9.7) |
| Smoker | 69 (39.9) | 43 (37.1) | 112 (38.8) | 23 (31.5) | 26 (36.1) |
| Unknown | 8 (4.6) | 5 (4.3) | 13 (4.5) | 2 (2.7) | 0 (0.0) |
Abbreviations: CRC, colorectal cancer; CTh+, received adjuvant chemotherapy, CTh–, did not receive adjuvant chemotherapy; FU, fluorouracil or capecitabine; NA, not applicable; UNK, unknown.
Cognitive Function
At baseline, 43% of participants with localized CRC had cognitive impairment based on GDS for the clinical neuropsychological tests, compared with 15% of HCs (P < .001). There was no significant difference between participants evaluated before surgery who received subsequent neoadjuvant chemotherapy and those evaluated after surgery. Adjusting for practice effect, rates of cognitive impairment were 39%, 46%, and 36% at 6, 12, and 24 months, respectively, in the localized CRC group, compared with 6% and 13% in HCs at 6 and 12 months, respectively (all P < .001; Fig 2A and Table 2; Data Supplement). Using International Cancer and Cognition Task Force criteria, there was cognitive impairment in 48% to 52% of patients with localized CRC at all time points, compared with 13% to 19% of HCs (all P < .001; Fig 2B). There were no significant differences at any assessment in clinical neuropsychological test results between those who received chemotherapy and those who did not or between those with localized and those with recurrent/metastatic cancer. Patients with CRC had impaired processing speed, verbal learning and memory, and attention and working memory domains, with higher rates of cognitive impairment than HCs at every time point (Table 3; Data Supplement). Participants who completed more assessments had less cognitive impairment than those who dropped out earlier (P = .012; Data Supplement).
Fig 2.
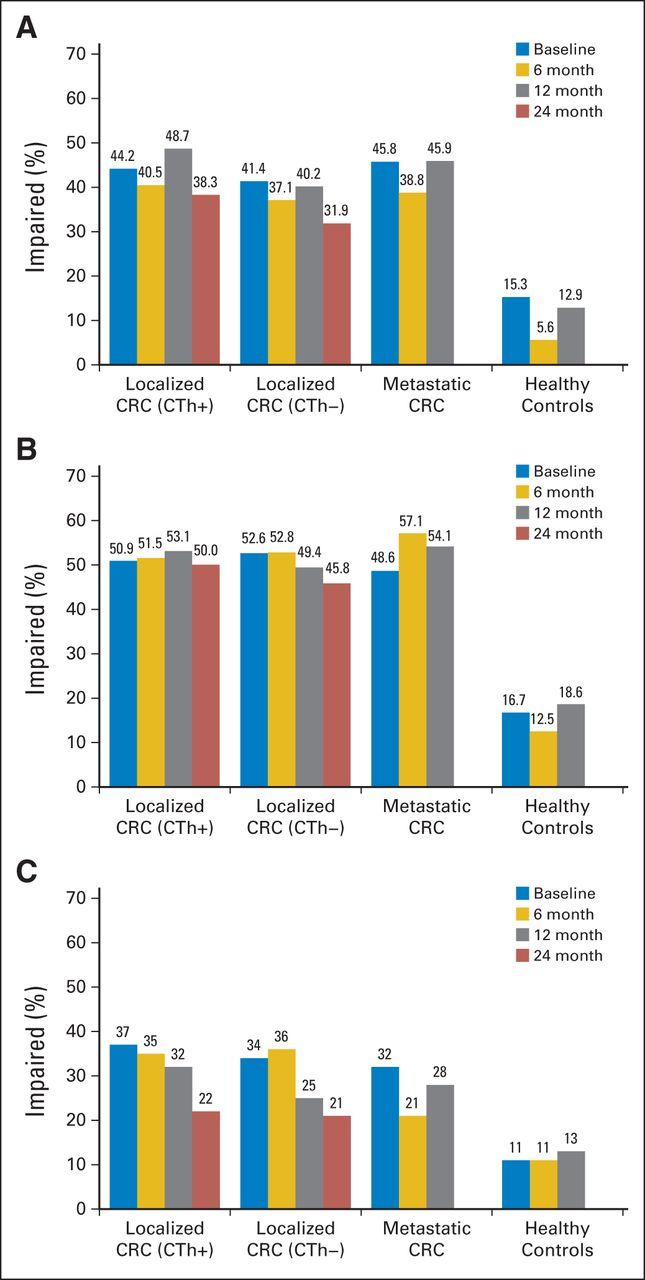
Prevalence of overall neuropsychological impairment (NPI) by study group and visit. (A) Global Deficit Score (GDS) on clinical neuropsychological tests (the primary end point). (B) International Cancer and Cognition Task Force (ICCTF) criteria on clinical neuropsychological tests. (C) GDS on Cambridge Neuropsychological Test Automated Battery tests. NPI is defined as GDS greater than 0.5, and GDS is adjusted for practice effect at 6-, 12-, and 24-month visits. ICCTF criteria are defined as T score less than 1.5 standard deviations (SDs) below the mean (T < 35) on two tests or T score less than 2 SDs (T < 30) on one test. CRC, colorectal cancer; CTh+, received adjuvant chemotherapy; CTh–, did not receive adjuvant chemotherapy.
Table 2.
Participants Classified as Impaired on NP Clinical Tests and CANTAB Tests by Study Group and Visit
| NP Test, Impairment Criteria, and Time of Visit | Localized CRC |
Metastatic CRC |
Healthy Controls |
P† | P‡ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTh+ |
CTh– |
P* | Total |
||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | ||||
| Clinical NP tests | |||||||||||||
| Overall cognitive impairment (GDS criteria)§ | |||||||||||||
| Baseline | 77 | 44 | 48 | 41 | .637 | 125 | 43 | 35 | 46 | 11 | 15 | .671 | < .001 |
| 6 months | 53 | 41 | 33 | 37 | .614 | 86 | 39 | 19 | 39 | 4 | 6 | .967 | < .001 |
| 12 months | 55 | 49 | 35 | 40 | .234 | 90 | 46 | 17 | 46 | 9 | 13 | .915 | < .001 |
| 24 months | 36 | 38 | 23 | 32 | .397 | 59 | 36 | — | — | — | — | — | — |
| Overall cognitive impairment (ICCTF criteria)‖ | |||||||||||||
| Baseline | 88 | 51 | 61 | 53 | .774 | 149 | 52 | 32 | 46 | 12 | 17 | .655 | < .001 |
| 6 months | 68 | 52 | 47 | 53 | .850 | 115 | 51 | 28 | 57 | 9 | 13 | .517 | < .001 |
| 12 months | 60 | 54 | 43 | 49 | .606 | 103 | 52 | 20 | 54 | 13 | 19 | .775 | < .001 |
| 24 months | 47 | 50 | 33 | 46 | .594 | 80 | 48 | — | — | — | — | — | — |
| CANTAB tests | |||||||||||||
| Overall cognitive impairment (GDS criteria)§ | |||||||||||||
| Baseline | 47 | 37 | 34 | 33 | .49 | 81 | 35 | 19 | 33 | 8 | 11 | .76 | < .001 |
| 6 months | 42 | 35 | 31 | 35 | 1.00 | 73 | 35 | 8 | 19 | 8 | 11 | .048 | < .001 |
| 12 months | 34 | 32 | 20 | 24 | .26 | 54 | 29 | 10 | 28 | 9 | 13 | 1.00 | .009 |
| 24 months | 20 | 22 | 12 | 17 | .55 | 32 | 20 | — | — | — | — | — | — |
| Overall cognitive impairment (ICCTF criteria)‖ | |||||||||||||
| Baseline | 67 | 53 | 60 | 58 | .51 | 127 | 55 | 38 | 66 | 13 | 18 | .18 | < .001 |
| 6 months | 60 | 50 | 51 | 57 | .33 | 111 | 53 | 15 | 36 | 20 | 29 | .062 | < .001 |
| 12 months | 60 | 57 | 33 | 40 | .027 | 93 | 50 | 20 | 56 | 18 | 26 | .59 | < .001 |
| 24 months | 31 | 34 | 27 | 39 | .51 | 58 | 36 | — | — | — | — | — | — |
Abbreviations: CANTAB, Cambridge Neuropsychological Test Automated Battery; CRC, colorectal cancer; CTh+, received adjuvant chemotherapy, CTh–, did not receive adjuvant chemotherapy; GDS, Global Deficit Score; ICCTF, International Cognition and Cancer Task Force; NP, neuropsychological.
Localized CRC: adjuvant chemotherapy v no adjuvant chemotherapy.
Localized CRC v metastatic CRC.
Localized CRC v healthy controls.
Impairment defined as GDS > 0.5.
Impairment defined as T score < 35 on two tests or T score < 30 on one test.
Table 3.
Global Deficit Scores, Global T Score, or sRCS by Study Group and Visit
| NP Test, Impairment Criteria, and Time of Visit | Localized CRC |
Metastatic CRC |
Healthy Controls |
P† | P‡ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTh+ |
CTh– |
P* | Total |
||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Clinical NP tests | |||||||||||||
| GDS | |||||||||||||
| Baseline | 0.62 | 0.59 | 0.63 | 0.61 | .913 | 0.62 | 0.60 | 0.59 | 0.55 | 0.25 | 0.39 | .700 | < .001 |
| 6 months | 0.61 | 0.59 | 0.53 | 0.48 | .310 | 0.57 | 0.55 | 0.59 | 0.56 | 0.19 | 0.37 | .820 | < .001 |
| 12 months | 0.68 | 0.66 | 0.57 | 0.57 | .256 | 0.63 | 0.62 | 0.70 | 0.72 | 0.25 | 0.38 | .533 | < .001 |
| 24 months | 0.58 | 0.66 | 0.52 | 0.51 | .511 | 0.56 | 0.60 | — | — | — | — | — | — |
| Global T score§ | |||||||||||||
| Baseline | 45.6 | 7.7 | 45.5 | 6.8 | .874 | 45.6 | 7.4 | 45.9 | 6.6 | 49.9 | 6.0 | .742 | < .001 |
| 6 months | 46.3 | 7.5 | 46.9 | 6.5 | .541 | 46.5 | 7.1 | 46.7 | 6.2 | 50.9 | 6.2 | .901 | < .001 |
| 12 months | 45.6 | 7.6 | 46.3 | 6.5 | .493 | 45.9 | 7.2 | 45.6 | 6.9 | 50.9 | 6.6 | .681 | < .001 |
| 24 months | 46.9 | 8.2 | 47.1 | 7.0 | .925 | 47.0 | 7.7 | — | — | — | — | — | — |
| sRCS‖ | |||||||||||||
| Baseline | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 6 months | −0.14 | 0.48 | −0.06 | 0.39 | .161 | −0.11 | 0.45 | −0.16 | 0.42 | 0.00 | 0.38 | .475 | .069 |
| 12 months | −0.37 | 0.45 | −0.20 | 0.45 | .010 | −0.30 | 0.46 | −0.31 | 0.39 | 0.00 | 0.47 | .800 | < .001 |
| 24 months | −0.23 | 0.51 | −0.17 | 0.45 | .424 | −0.20 | 0.48 | — | — | — | — | — | — |
| CANTAB tests | |||||||||||||
| GDS | |||||||||||||
| Baseline | 0.54 | 0.51 | 0.48 | 0.38 | .85 | 0.52 | 0.46 | 0.50 | 0.37 | 0.25 | 0.28 | .82 | < .001 |
| 6 months | 0.49 | 0.47 | 0.44 | 0.34 | .81 | 0.47 | 0.42 | 0.38 | 0.42 | 0.21 | 0.23 | .17 | < .001 |
| 12 months | 0.46 | 0.41 | 0.39 | 0.38 | .22 | 0.43 | 0.39 | 0.45 | 0.41 | 0.25 | 0.34 | .90 | < .001 |
| 24 months | 0.34 | 0.39 | 0.28 | 0.29 | .77 | 0.31 | 0.35 | — | — | — | — | — | — |
| Global T score§ | |||||||||||||
| Baseline | 47. | 6.3 | 47.3 | 4.9 | .77 | 47.3 | 5.7 | 47.0 | 5.3 | 50.0 | 4.6 | .50 | < .001 |
| 6 months | 48.1 | 6.1 | 48.7 | 5.4 | .64 | 48.3 | 5.8 | 49.2 | 5.6 | 51.2 | 4.6 | .18 | .001 |
| 12 months | 48.5 | 5.5 | 49.1 | 5.6 | .47 | 48.8 | 5.5 | 48.7 | 6.2 | 50.8 | 4.7 | .64 | .003 |
| 24 months | 51.0 | 6.1 | 51.2 | 5.6 | .93 | 51.1 | 5.9 | — | — | — | — | — | — |
NOTE. Because the health controls only had three assessments, the 24-month data were analyzed by assuming that practice effect was similar from 12-24 months as from 6-12 months.
Abbreviations: CRC, colorectal cancer; CTh+, received adjuvant chemotherapy, CTh–, did not receive adjuvant chemotherapy; GDS, Global Deficit Score; NP, neuropsychological; SD, standard deviation; sRCS, summary regression-based change score (negative score indicates decline in cognitive function).
Localized CRC: adjuvant chemotherapy v no adjuvant chemotherapy.
Localized CRC v metastatic CRC.
Localized CRC v healthy controls.
Computed by averaging the T scores of individual clinical NP tests.
Computed using regression-based equations developed from the healthy controls group.
Consistent results were obtained using the computer-based CANTAB; patients with localized CRC had significantly more impairment than HCs at each assessment (Fig 2C), with no significant differences between patients who did and did not receive chemotherapy. Deficits were observed particularly in the domains of attention and complex reaction time and spatial working memory (Data Supplement). Moderate association was seen between the GDS for clinical neuropsychological tests and the CANTAB at each time point (r = 0.46 to 0.51; P < .001). No differences by group were found on the Six Elements Test.
Preplanned subgroup analysis by chemotherapy regimen found no difference in rates of cognitive impairment in those who received adjuvant fluorouracil or capecitabine at baseline or 6 months compared with those who received an oxaliplatin regimen, but impairment was more common at 12 and 24 months in those who received fluorouracil (P = .017).
Predictors of Cognitive Impairment
For all participants, group, time, group-time interaction, sex, and primary language were significant predictors of cognitive impairment on global T scores, but age and education were not. On multivariable analysis, all variables except group-time interaction for localized CRC remained significant (Data Supplement). Our model found that patients with localized CRC (P < .001) and metastatic CRC (P = .003) had significantly lower global T scores compared with HCs. Multivariable analysis of the localized CRC group only found no significant difference between those who did and did not receive chemotherapy, but women had lower global T scores than men (P < .001). There was no difference by country of residence. Follow-up time was positively associated with improvement in neurocognitive function (P = .016), except for those with recurrent/metastatic disease who experienced a decline in neurocognitive performance relative to HCs (P = .002). Associations between CANTAB and CRC study group were similar to the clinical neuropsychological results.
We examined the effects of CRC disease status and key demographic characteristics on neurocognitive performance over the study period. Female sex and non-English primary language were negatively associated with cognitive performance. In multivariable analysis, CRC disease status, duration of follow-up, female sex, and non-English primary language remained significant (P < .05) predictors of impaired neurocognitive performance. Multivariable analysis excluding non-English primary language participants found similar results.
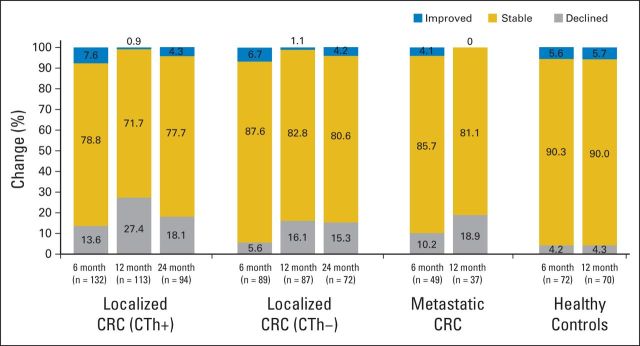
Longitudinal Changes in Cognitive Function
After adjusting for practice effect, 20% of patients with localized CRC had significant decline in cognitive function from baseline to 12 months compared with 4% of HCs (P = .001; Fig 3). Men (P < .001) with CRC and those with English as a second language (P = .019) were more likely to have cognitive decline with time, but there was no significant difference in age or education between those who did and did not decline (Data Supplement). On multivariable analysis, only male sex remained significant (P < .001). Results were similar after excluding non-English primary language participants. Baseline cognitive impairment was not predictive for subsequent decline (Data Supplement). Patients who received chemotherapy had more cognitive decline than those who did not, but this was not significant after adjusting for age, education, sex, language, and country. Findings were similar for interaction of chemotherapy and sex; the main effect for chemotherapy (hazard ratio, 1.51; 95% CI, 0.43 to 5.37; P = .521) and the interactive effect were not significant (P = .823), but sex remained significant (hazard ratio, 3.95; 95% CI, 1.34 to 11.67; P = .013). Analysis of summary regression-based change scores confirmed more cognitive impairment in patients with CRC than HCs (P < .001) in all cognitive domains except visual memory, and cognitive impairment was greatest in women (P < .001; Data Supplement).
Fig 3.
Change in neuropsychological functioning from baseline using 5% (top and bottom) of regression-based summary scores. CRC, colorectal cancer; CTh+, received adjuvant chemotherapy; CTh–, did not receive adjuvant chemotherapy.
Self-Reported Cognitive Impairment
Perceived cognitive impairment was more common at 6 months in participants who received chemotherapy (32%) than in those who did not (16%; P = .007) or in HCs (12.5%), but there were no significant differences between groups at 12 months. At each time point, there was only weak association between objective neuropsychological performance on both of the clinical tests and CANTAB and a summary score of self-reported cognitive function (12 months: clinical, r = 0.20; CANTAB, r = −0.15). A moderate association was found at each assessment between cognitive symptoms and fatigue (r = 0.37 to 0.55), QOL (r = 0.40 to 0.54), and anxiety/depression (r = 0.36 to 0.43), but these variables were not associated with objective neuropsychological performance.
Blood Results
Mean hemoglobin levels were within the normal range but were lower at baseline in patients with CRC than HCs and lower at 6 months in those who received chemotherapy. Estradiol levels were higher in female HCs than women with CRC, with a trend for higher testosterone levels in men with CRC. Patients with CRC had significantly higher levels of most cytokines compared with HCs, with levels highest in those receiving chemotherapy (who had higher stage disease). Several cytokines remained elevated long term despite no evidence of recurrent disease (Data Supplement). Other blood tests remained in or close to the normal range.
Apolipoprotein E genotyping was available in 243 patients with localized CRC, with 57 (22%) having at least one E4 allele, and 16 (23%) of 70 HCs had at least one E4 allele. Two patients with CRC and three HCs were homozygotes (E4/4).
No significant association was found between global cognitive function (clinical GDS, CANTAB GDS) and hemoglobin, liver function tests, clotting factors, or cytokines. In women, low estradiol was associated with global cognitive impairment (ρ = 0.29; P < .001) and reduced information-processing speed and verbal and visual memory (ρ = 0.2 to 0.38; P < .001). Some cytokines (especially interleukin [IL] -6, IL-8, IL-10, and IL-12) were associated with reduced information-processing speed (ρ = 0.18 to 0.40). No association was found between the presence of E4 alleles and any measure of cognitive function, receipt of chemotherapy, or smoking status.
DISCUSSION
This large prospective study compared longitudinal cognitive changes in individuals after diagnosis of CRC with a group of HCs and compared patients with CRC who did and did not receive chemotherapy. The HCs allowed evaluation of the effect of the cancer itself on cognitive function. The substudy of patients with recurrent/metastatic disease provided preliminary information about the influence of more advanced disease on cognitive function.
Our main finding is that diagnosis of CRC leads to substantial cognitive impairment compared with HCs, and this persists at 2 years. Depending on criteria used, rates of cognitive impairment for patients with localized CRC ranged from 36% to 52% between baseline and 24 months compared with 6% to 19% in HCs. Cognitive domains affected were processing speed, verbal memory, and attention/working memory. We found no significant difference in rates of objective cognitive impairment in those who received chemotherapy and those who did not, even at 6 months, soon after completing chemotherapy.
One difficulty in performing longitudinal studies is determining true change over time because cognitive testing is influenced by practice. We used a conservative measure of cognitive decline with repeated testing in the HCs to correct for practice effect. Our results suggest that 24% of patients with CRC had deterioration greater than expected from baseline to 12 months, compared with 7% of HCs. There was a nonsignificant trend for more cognitive decline in patients with localized CRC who received chemotherapy than in those that did not (32% v 23%, respectively; P = .14). Cognitive decline in patients with CRC might be an underestimate because those who dropped out early had lower baseline cognitive scores, less education, and higher rates of English as a second language; those who struggled initially may have been less likely to return for further assessments. Cognitive decline over time raises the question of whether patients with cancer, particularly those who receive chemotherapy, may be at risk for accelerated cognitive aging.18,19 Those with lower preexisting cognitive reserve are expected to be at greater risk20,21; although our study did not show such an effect, it is confounded because those with less cognitive reserve were more likely to drop out. Longer follow-up is required to address this issue.
The vast majority of cognitive studies in patients with cancer have been in women with breast cancer.4 The present large study allows the comparison of differences in cognitive function between men and women with cancer. Women with CRC had more cognitive impairment than men at each assessment, but men had a greater risk of cognitive decline over time.
The finding of more impairment at 12 and 24 months in patients receiving adjuvant fluorouracil alone compared with oxaliplatin combinations should be interpreted with caution because of the small numbers in the subgroups, particularly at 24 months. This may be a selection bias because patients with comorbidities, who are older or have poorer performance status, are less likely to be offered oxaliplatin.
Consistent with previous results, we found only weak association between neuropsychological performance and cognitive symptoms. Despite significant differences in objective cognitive function between patients with cancer and HCs, there was no difference in cognitive symptoms at baseline. There was a transient effect for patients who received chemotherapy to report more cognitive symptoms at 6 months than those who did not, without significant differences in rates of objective cognitive impairment.
As in other studies, we found no association of fatigue, anxiety/depression, or QOL with neuropsychological performance, but these variables were associated with cognitive symptoms. This highlights the importance of assessing fatigue and anxiety/depression, and treating them if present, in patients who self-report cognitive impairment.
The etiology of cognitive impairment in patients with cancer remains elusive. Our patients with CRC had persistently elevated cytokine levels without evidence of a recurrence, but there was no association of cognitive impairment with cytokine levels. In contrast to studies in women with breast cancer,22,23 we found no evidence of higher rates of cognitive impairment in those with an E4 allele on apolipoprotein genotyping.
Limitations of our study are that our HCs were not well balanced for sex or primary language and were not assessed at 24 months. Because women had more cognitive impairment than men, this could underestimate cognitive differences between patients with localized CRC and HCs. As in all longitudinal studies, data were missing for some patients with cancer. Withdrawal was most often secondary to disease progression, time constraints, and a desire not to be reminded of their cancer.
Strengths of our study include the large sample size in a cancer that affects men and women, the prospective longitudinal study design with two control groups (one that is disease specific and one of HCs), comprehensive cognitive assessments, and the mechanistic correlates. Our data show that the diagnosis of even localized cancer is associated with substantial rates of sustained cognitive impairment. Patients with a new diagnosis of cancer should be advised about possible cognitive effects of their disease.
Acknowledgment
We thank David Laurence, our data manager, and Anya Umlauf and Robert Heaton, from the University of California San Diego, for their invaluable assistance with the generation of normative adjustments for the clinical neuropsychological tests. Finally, we thank collaborators, study coordinators, and participants from the following hospitals: Toronto: Princess Margaret, Toronto General, Toronto Western, Mount Sinai, Sunnybrook, Credit Valley, Humber River, St Michael's, and Toronto East General; and Sydney: Concord Repatriation & General, Royal Prince Alfred, Bankstown, Royal North Shore, Prince of Wales, and Nepean.
Footnotes
Supported by a grant from the National Cancer Institute of Canada, a Young Investigator Award awarded by the American Society of Clinical Oncology, a National Health Medical Research Council Grant, Australia, and fellowships from the Cancer Institute New South Wales, Australia.
Presented in part at the 45th Annual Meeting of the American Society of Clinical Oncology, Orlando, FL, May 29-June 2, 2009; the 48th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2012; the 36th Annual Meeting of the Clinical Oncology Society of Australia, Gold Coast, Queensland, Australia, November 17-19, 2009; the International Cognition and Cancer Task Force Conference, Paris, France, March 15-17, 2012; the Annual Scientific Meeting of the Medical Oncology Group Australia, Brisbane, Queensland, Australia, August 8-10, 2012; and International Psycho-Oncology Society 14th World Congress, Brisbane, Queensland, Australia, November 13-15, 2012.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Janette L. Vardy, Ian F. Tannock
Financial support: Ian F. Tannock
Administrative support: Corrinne Renton, Anna Dodd
Provision of study materials or patients: Philip Beale, Stephen Clarke
Collection and assembly of data: Janette L. Vardy, Haryana M. Dhillon, Corrinne Renton, Anna Dodd, Haibo Zhang, Philip Beale, Stephen Clarke
Data analysis and interpretation: Janette L. Vardy, Haryana M. Dhillon, Gregory R. Pond, Sean B. Rourke, Tsegaye Bekele, Ian F. Tannock
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cognitive Function in Patients With Colorectal Cancer Who Do and Do Not Receive Chemotherapy: A Prospective, Longitudinal, Controlled Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Janette L. Vardy
No relationship to disclose
Haryana M. Dhillon
No relationship to disclose
Gregory R. Pond
No relationship to disclose
Sean B. Rourke
No relationship to disclose
Tsegaye Bekele
No relationship to disclose
Corrinne Renton
No relationship to disclose
Anna Dodd
No relationship to disclose
Haibo Zhang
No relationship to disclose
Philip Beale
Consulting or Advisory Role: Roche
Travel, Accommodations, Expenses: Roche, Ipsen
Stephen Clarke
Consulting or Advisory Role: Merck, Roche/Genentech
Expert Testimony: Merck
Travel, Accommodations, Expenses: AstraZeneca, Merck
Ian F. Tannock
Consulting or Advisory Role: Ipsen
Research Funding: Ipsen
REFERENCES
- 1.Ahles TA Saykin AJ McDonald BC, etal: Cognitive function in breast cancer patients prior to adjuvant treatment Breast Cancer Res Treat 110:143–152,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schagen SB Muller MJ Boogerd W, etal: Change in cognitive function after chemotherapy: A prospective longitudinal study in breast cancer patients J Natl Cancer Inst 98:1742–1745,2006 [DOI] [PubMed] [Google Scholar]
- 3.Hermelink K Untch M Lux MP, etal: Cognitive function during neoadjuvant chemotherapy for breast cancer: Results of a prospective, multicenter, longitudinal study Cancer 109:1905–1913,2007 [DOI] [PubMed] [Google Scholar]
- 4.Wefel JS, Schagen SB: Chemotherapy-related cognitive dysfunction Curr Neurol Neurosci Rep 12:267–275,2012 [DOI] [PubMed] [Google Scholar]
- 5.Vardy J, Rourke S, Tannock IF: Evaluation of cognitive function associated with chemotherapy: A review of published studies and recommendations for future research J Clin Oncol 25:2455–2463,2007 [DOI] [PubMed] [Google Scholar]
- 6.Andreis F Ferri M Mazzocchi M, etal: Lack of a chemobrain effect for adjuvant FOLFOX chemotherapy in colon cancer patients: A pilot study Support Care Cancer 21:583–590,2013 [DOI] [PubMed] [Google Scholar]
- 7.Cruzado JA López-Santiago S Martínez-Marín V, etal: Longitudinal study of cognitive dysfunctions induced by adjuvant chemotherapy in colon cancer patients Support Care Cancer 22:1815–1823,2014 [DOI] [PubMed] [Google Scholar]
- 8.Vardy J Dhillon HM Pond GR, etal: Cognitive function and fatigue after diagnosis of colorectal cancer Ann Oncol 25:2404–2412,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yellen SB Cella DF Webster K, etal: Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system J Pain Symptom Manage 13:63–74,1997 [DOI] [PubMed] [Google Scholar]
- 10.Cella D: The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: A new tool for the assessment of outcomes in cancer anemia and fatigue Semin Hematol 34:13–19,1997 [PubMed] [Google Scholar]
- 11.Wagner L Sweet J Butt Z, etal: Measuring patient self-reported cognitive function: Development of the Functional Assessment of Cancer Therapy–Cognitive Function instrument J Support Oncol 7:W32–W39,2009 [Google Scholar]
- 12.Goldberg D, Williams P: A User's Guide to the General Health Questionnaire 1991Windsor, United Kingdom: NFER-Nelson [Google Scholar]
- 13.Carey CL Woods SP Rippeth JD, etal: Initial validation of a screening battery for the detection of HIV-associated cognitive impairment Clin Neuropsychol 18:234–248,2004 [DOI] [PubMed] [Google Scholar]
- 14.Carey CL Woods SP Gonzalez R, etal: Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection J Clin Exp Neuropsychol 26:307–319,2004 [DOI] [PubMed] [Google Scholar]
- 15.Wefel JS Vardy J Ahles T, etal: International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer Lancet Oncol 12:703–708,2011 [DOI] [PubMed] [Google Scholar]
- 16.Cysique LA Franklin D Jr Abramson I, etal: Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change J Clin Exp Neuropsychol 33:505–522,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sauerbrei W Meier-Hirmer C Benner A, etal: Multivariable regression model building by using fractional polynomials: Description of SAS, STATA, and R programs Comput Stat Data An 50:3463–3485,2006 [Google Scholar]
- 18.Khan NF Mant D Carpenter L, etal: Long-term health outcomes in a British cohort of breast, colorectal and prostate cancer survivors: A database study Br J Cancer 105:S29–S37,2011suppl 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stouten-Kemperman MM de Ruiter MB Boogerd W, etal: Very late treatment-related alterations in brain function of breast cancer survivors J Int Neuropsychol Soc 21:50–61,2015 [DOI] [PubMed] [Google Scholar]
- 20.Ahles TA Saykin AJ McDonald BC, etal: Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve J Clin Oncol 28:4434–4440,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandelblatt JS Stern RA Luta G, etal: Cognitive impairment in older patients with breast cancer before systemic therapy: Is there an interaction between cancer and comorbidity? J Clin Oncol 32:1909–1918,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahles TA Saykin AJ Noll WW, etal: The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy Psychooncology 12:612–619,2003 [DOI] [PubMed] [Google Scholar]
- 23.Koleck TA Bender CM Sereika SM, etal: Apolipoprotein E genotype and cognitive function in postmenopausal women with early-stage breast cancer Oncol Nurs Forum 41:E313–E325,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]