Abstract
Background
The hippocampus has recently been identified to play a key role in the pathophysiology of adult obsessive-compulsive disorder (OCD). Surprisingly, there is only limited evidence regarding the potential relationships with symptom dimensions. Due to the heterogeneity of symptoms in OCD, we aimed at further examining, whether hippocampal volume differences might be related to symptom profiles instead of single symptom dimensions.
Methods
In order to find out more about the potential association between clinical symptom profiles and alterations in hippocampal volume we categorized a large sample of OCD patients (N = 66) into distinct symptom profile groups using K-means clustering. In addition, hippocampal volumes of the different symptom profile groups were compared with hippocampal volumes in a sample of 66 healthy controls.
Results
We found significant differences in hippocampal volume between the different symptom profile groups which remained significant after correcting for age, sex, total intracranial volume, OCI-total score, depression, medication, disease duration and scanner. The patient group characterized by overall lower symptom scores and without high symptom severity in any specific domain showed the highest hippocampal volume. Finally, the comparison with healthy controls demonstrated significantly lower hippocampal volumes in those patients whose symptom profile was characterized by a high severity of ordering and checking symptoms.
Conclusions
Present results provide further confirmation for alterations in hippocampus structure in OCD and suggest that symptom profiles which take into account the multi-symptomatic character of the disorder should be given greater attention in this context.
Keywords: Hippocampus, Obsessive-compulsive, OCD, Symptom dimension, Freesurfer, MRI
Highlights
-
•
Different symptom profiles are associated with differences in hippocampus volume.
-
•
This effect seems to be independent of other clinical parameters.
-
•
Symptom interrelations seem to link structural alterations and psychopathology.
1. Introduction
Despite increasing evidence for structural brain alterations in obsessive-compulsive disorder (OCD) the overall picture has to be considered as rather heterogeneous with findings reporting both increases and decreases in gray matter volume, thickness, surface area or gyrification (Fan et al., 2013, Kuhn et al., 2013, Nakamae et al., 2012, Piras et al., 2015, Rus et al., 2016, Shaw et al., 2015, Shin et al., 2007, Venkatasubramanian et al., 2012, Wobrock et al., 2010). In an attempt to reduce overall result heterogeneity and to filter out the most meaningful alterations, an increasing number of meta-analyses pooling data from multiple OCD sites worldwide are emerging in the OCD research community (Boedhoe et al., 2017, De Wit et al., 2014, Fouche et al., 2017). The ENIGMA consortium analysis constitutes the largest meta-analysis on structural alterations in OCD to date. Employing a coordinated and standardized analysis approach, meta- and mega-analysis of data from 1830 OCD patients (N = 335 children, N = 1495 adults) and 1759 controls was conducted to identify alterations in subcortical brain volumes in OCD patients compared to healthy controls (Boedhoe et al., 2017). As one of the main findings the analysis revealed the adult patient sample to have significantly increased pallidum and significantly smaller hippocampus volumes compared to healthy controls. The pallidum is regarded as one of the core regions within the frequently discussed cortico-striato-thalamo-cortical (CSTC) circuit. A dysbalance within this circuit is assumed to represent a central psychopathological mechanism underlying obsessions and compulsions in OCD. In contrast, the hippocampus has not been the focus of OCD psychopathophysiology up to now. Its volume, however, is frequently found to be decreased in other psychiatric disorders such as depression (Frodl and O'Keane, 2013, Malykhin and Coupland, 2015) and PTSD (Ahmed-Leitao et al., 2016, O'Doherty et al., 2015). One potential mechanism underlying volumetric changes in the hippocampus seems to be uncontrollable stress (i.e., stress perceived as distress) which is one of the main characteristics of many psychiatric disorders such as PTSD. Distress has been demonstrated to change neuronal morphology, suppress neuronal proliferation, and reduce hippocampal volume (Kim et al., 2015). According to ICD-10, OCD is classified as a stress-related disorder and patients with OCD tend to report high levels of stress and anxiety independent of their specific symptoms or symptom profiles (Stein et al., 2010). Therefore, there is strong reason to assume that hippocampal volume differences may be clinically relevant in OCD as well. Of note, the ENIGMA meta-analysis identified hippocampal volume differences to be larger in medicated patients, however, no relationship with symptoms was found. The ENIGMA study related volume differences to specific symptoms as assessed by the Y-BOCS checklist. However, it should be noted that the majority of all OCD patients are multi-symptomatic and the individual symptom profiles of OCD patients are heterogeneous to the extent that two patients may display different overlapping or even non-overlapping symptom patterns (Mataix-Cols et al., 2005). Hence, instead of correlating outcome measures with specific symptoms one at a time, it may be reasonable to adopt an approach that accounts for possible interrelations of different symptom dimensions in patients. The fact that Boedhoe et al. (2017) found no significant correlations between symptom dimensions and hippocampus volumes is striking given the clear involvement of volume differences in patients found in their study. One possible explanation might be that symptom dimensions were related to structural alterations while controlling for the effects of other symptom dimensions, therefore effectively treating each symptom in isolation. To find out more about the clinical relevance of the recently reported differences in hippocampal structure, the present study employs a cluster analysis approach on dimensional symptoms to reach a differentiation into distinct symptom composition profiles, comparing hippocampal volumes between the different symptom profile groups. Thus, we aimed at exploring whether taking into account the interrelation between different symptoms, i.e., patients´ symptom composition profile, would be a valuable approach to relate structural alterations to clinically relevant features. We assumed that if the hippocampus would indeed be differentially affected in dependence on specific symptom composition profiles volume differences should be related to different symptom profiles. If hippocampus volumes would not be related to symptom profiles, this would rather speak in favor of a clinically unspecific hippocampal involvement in the disease.
2. Methods and materials
2.1. Participants
Data from two samples were combined. Sample one (S1) comprised n = 42 patients and n = 46 healthy controls and sample two (S2) comprised n = 24 patients and n = 20 healthy controls resulting in a total size of n = 66 patients with OCD as the primary diagnosis according to DSM-IV criteria and n = 66 healthy controls (see Table 1 for demographic and clinical details). Patients and controls were matched for sex and age in both samples. All patients were recruited from the Windach Institute and Hospital of Neurobehavioural Research and Therapy, Germany, and diagnoses were made by an experienced psychiatrist. Exclusion criteria for all participants were a history of clinically important head injuries, seizures or neurological diseases. At time of the study, n = 48 patients were drug-naive or medication free for at least 3 weeks and n = 30 patients had one or more comorbid diagnoses. To assess clinical severity of obsessive-compulsive symptoms, patients were administered the self-rated version of the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) (Goodman et al., 1989, Hand and Büttner-Westphal, 1991). The Obsession-Compulsion Inventory revisited (OCI-R) (Foa et al., 2002, Gonner et al., 2008) was administered to more specifically assess different symptom dimensions. Additionally, depressive symptoms were evaluated based on the Beck Depression Inventory (BDI-II) (Beck et al., 1996, Hautzinger et al., 2009) in patients of sample S1 and the Hamilton Depression Scale (HAM-D) (Hamilton, 1960) in patients of sample S2. The study was approved by the local Ethics Committee of the Klinikum rechts der Isar, München and was conducted in accordance with the Declaration of Helsinki.
Table 1.
Demographic and clinical sample characteristics.
| Characteristics | OCD |
HC |
|---|---|---|
|
n |
n |
|
| Mean ± SD | Mean ± SD | |
| Sample size | 66 | 66 |
| Female | 46 (69.7%) | 46 (69.7%) |
| Age (years) | 32.4 ± 10.5 | 31.6 ± 10.3‡ |
| Disease duration | 16.0 ± 10.8 | |
| Y-BOCS total | 21.0 ± 6.2 | |
| Obsession | 11.0 ± 3.6 | |
| Compulsions | 9.9 ± 3.9 | |
| OCI-R total | 25.4 ± 10.0 | |
| Hoarding | 2.3 ± 2.6 | |
| Checking | 5.5 ± 3.6 | |
| Ordering | 3.9 ± 3.8 | |
| Neutralizing | 2.2 ± 2.9 | |
| Washing | 4.8 ± 3.9 | |
| Obsessing | 6.8 ± 3.6 | |
| BDI (S1) | 18.0 ± 11.5 | |
| HAM-D (S2) | 12.6 ± 4.9 | |
| Comorbidities | 30 (45.5%) | |
| Depression | 23 | |
| Anxiety disorder | 10 | |
| Personality disorder | 4 | |
| Eating disorder | 2 | |
| ADHD | 2 | |
| Medication | 48 (72.7%) | |
| SSRI | 35 | |
| SSRNI | 6 | |
| Neuroleptic | 5 | |
| TCA | 3 | |
| Methylphenidate | 1 | |
| Benzodiazepine | 1 | |
| NDRI | 1 | |
| NaSSA | 1 |
Note that multiple comorbid diagnoses as well as different medication types can be present in a single patient; abbreviations for medication: NaSSA, noradrenergic and specific serotonergic antidepressant; NDRI, norepinephrine-dopamine reuptake inhibitor; SSNRI, selective serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Two-sample t-test (t(130) = 0.442, p = 0.659).
2.2. Image acquisition
Magnetic resonance imaging was conducted on a 3T Philips Ingenia (Philips Healthcare, Best, The Netherlands) using a 12-channel (SENSE) head coil. For sample S1, structural imaging consisted of a T1-weighted 3D MPRAGE sequence with an isotropic resolution of 1 mm (170 slices, sagittal orientation, 240 × 240 matrix, TR = 9 ms, TE = 4 ms, flip angle = 8°) while for sample S2, imaging consisted of a T1-weighted 3D MPRAGE sequence with a resolution of 0.7 × 0.75 × 0.7 mm (230 slices, sagittal orientation, 368 × 340 matrix, TR = 11 ms, TE = 5.1 ms, flip angle = 8°). Prior to analysis the 24 submillimeter data sets of sample S2 were downsampled in order for all images to have a consistent resolution of 1 mm isotropic.
2.3. Image processing
Based on these T1-weighted images, cortical and subcortical structures were initially segmented and labeled using Freesurfer (Version 6.0, http://surfer.nmr.mgh.harvard.edu/) (Dale et al., 1999, Fischl et al., 2002, Fischl et al., 1999). Processing included automatic segmentation into gray and white matter tissue compartments followed by parcellation of the gray matter mask into distinct brain regions and reconstruction of brain surfaces. These results were subsequently used to initialize the labeling of hippocampi using the recently released hippocampal subfield segmentation algorithm implemented in the Freesurfer package. Compared to previous versions, the labeling rests on an atlas which was built based on ex vivo MRI data of postmortem brain tissue acquired at 7T with sub-millimeter resolution and results have been shown to be in better agreement with histological studies (Iglesias et al., 2015). Hippocampus segmentations were visually inspected and volumes were quantitatively checked for outliers.
2.4. Symptom composition analysis (SCA)
In order to partition the patients according to their symptom composition, all OCI-R items were entered into a K-means cluster analysis in SPSS (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp). This type of analysis allows to derive subgroups whose members are characterized by being rather similar in symptom composition within each subgroup while being as different as possible in symptom composition to members of other subgroups. The number of clusters (k) to be extracted was predefined to k = 3. This number was chosen in order to extract a number of clusters that allows for sufficient differentiability of patients while preserving a relatively large number of subjects per clusters (see Supplementary Fig. 1 for further details).
2.5. Statistical analysis
Demographic and clinical characteristics of subjects forming the three different clusters were compared using one-way ANOVAs with the respective demographic or clinical variable as dependent variable and cluster membership as factor with three factor levels. In line with Boedhoe et al. (2017) hippocampus volumes of the left and right hemisphere were averaged to yield a single hippocampus volume for each subject. For patients only, an ANCOVA model was fit to assess cluster-related differences in hippocampus volume while controlling for the following covariates: age, sex, total intracranial volume, OCI-total score, depression, medication, disease duration, and scanner. Controlling for OCI-total scores allows the assessment of potential effects of cluster membership irrespective of cluster-specific differences in global OCI symptom severity. Medication was entered as a dichotomous variable indicating whether patients were medication naïve or medication free for at least three weeks prior to scanning. HAM-D and BDI scores were transformed into a dichotomous variable and used as a proxy to indicate the absence or presence of clinically relevant depressive symptoms. HAM-D scores ≥ 9 and BDI scores ≥ 13 were considered to indicate the presence of relevant depressive symptoms according to the German National Disease Management Guideline Depression (DGPPN and KBV, 2015). In a second analysis, potential cluster-related differences in hippocampus volumes between patients and healthy controls were assessed. To this end, an ANCOVA model was fit treating all healthy controls as belonging to one synthetic cluster of their own resulting in the factor cluster with four levels. Additionally, the analysis was controlled for the following covariates: age, sex, and total intracranial volume.
3. Results
3.1. Symptom composition analysis (SCA)
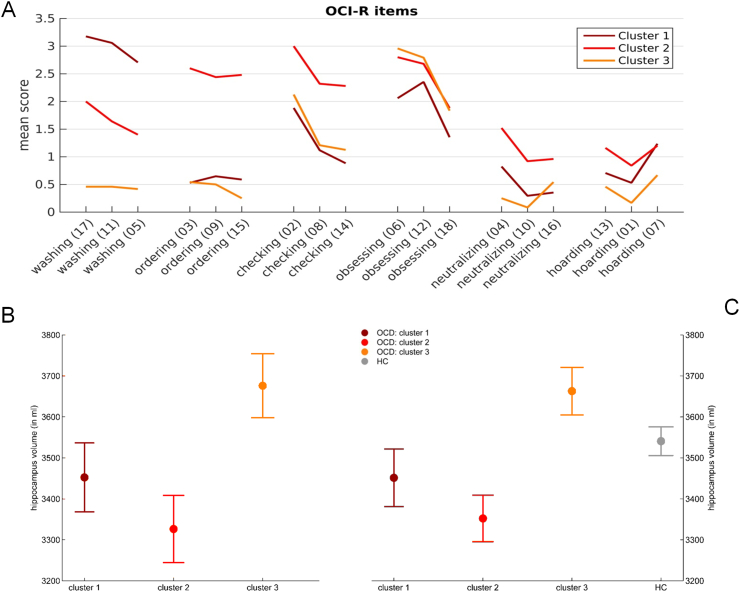
The mean scores of each OCI-R item according to cluster membership are depicted in Fig. 1A. Items are grouped together according to OCI symptom scales. ANOVA analyses revealed a significant main effect of cluster on OCI total score (F(2,63) = 34.924, p < 0.001, corrected). Information regarding demographic characteristics and statistical differences between each patient cluster are depicted in Table 2.
Fig. 1.
Symptom profile composition and hippocampal volumes. Symptom composition analysis (A): Mean scores of each OCI-R item according to cluster membership, grouped by symptom dimensions. Numbers in parentheses indicate the number of each item in the questionnaire. Within patients analysis (B): Marginal means ± standard error. There was a significant main effect of cluster membership on global hippocampal volume while controlling for age, sex, total intracranial volume, total OCI-R score, clinically relevant depression, medication, disease duration and scanner (F(2,55) = 3.301, p = 0.044). Between groups analysis (C): Marginal means ± standard error. There was a significant main effect of cluster on global hippocampal volume while controlling for age, sex, and total intracranial volume (F(3,125) = 4.752, p = 0.004).
Table 2.
Demographic and clinical sample characteristics in the three patient groups.
| Characteristics | Cluster 1 |
Cluster 2 |
Cluster 3 |
F-statistic | p-Value |
|---|---|---|---|---|---|
| Mean ± SD |
Mean ± SD |
Mean ± SD |
|||
| n(%) | n(%) | n(%) | |||
| OCI total | 23.47 ± 8.65 | 34.12 ± 6.46 | 17.67 ± 6.13 | 34.924 | < 0.001⁎ |
| Age | 31.38 ± 11.20 | 33.63 ± 11.06 | 31.90 ± 9.72 | 0.266 | 0.767 |
| Disease duration | 12.88 ± 6.61 | 18.92 ± 13.59 | 15.04 ± 9.58 | 1.742 | 0.184 |
| Male | 1 (5.9%) | 9 (36.0%) | 10 (41.7%) | 3.531 | 0.035 |
| Depression | 9 (52.9%) | 21 (84%) | 17 (70.8%) | 2.451 | 0.094 |
| Medication | 15 (88.2%) | 20 (80.0%) | 14 (58.3%) | 2.779 | 0.070 |
p < 0.05, Bonferroni-corrected for the total number of ANOVAs computed.
3.2. Hippocampus volume
3.2.1. Within patients analysis
There was a significant main effect of cluster on global hippocampus volume while controlling for age, sex, total intracranial volume, total OCI-R score, clinically relevant depression, medication, disease duration, and scanner (F(2,55) = 3.301, p = 0.044, η2 = 0.057). Additionally, there was a significant main effect of sex (F(1,55) = 6.429, p = 0.014, η2 = 0.055), total intracranial volume (F(1,55) = 7.291, p = 0.009, η2 = 0.063), presence or absence of clinically relevant depression (F(1,55) = 5.613, p = 0.021, η2 = 0.048) and scanner (F(1,55) = 5.354, p = 0.024, η2 = 0.017) (see Fig. 1B as well as Table 3). Post-hoc tests indicated that hippocampus volume was significantly different between cluster 2 and 3 (p = 0.020, 95% CI [− 562.77, − 49.47]) while there was a trend significant difference between cluster 1 and 3 (p = 0.056, 95% CI [− 432.59, 5.75]) and no significant difference between clusters 1 and 2 (p = 0.456, 95% CI [− 154.82, 340.21]). For exploratory analyses of hippocampus subfield volumes see Supplementary Table I.
Table 3.
ANCOVA model details for within patients analysis.
| Sum of squares (Typ III) | df | Mean squares | F | Significance | η2 | Partial η2 | |
|---|---|---|---|---|---|---|---|
| Corrected model | 5,342,274.95 | 10 | 534,227.50 | 6.107 | < 0.001 | 0.526 | |
| Constant term | 3,484,000.10 | 1 | 3,484,000.10 | 39.829 | < 0.001 | 0.420 | |
| Cluster | 577,495.26 | 2 | 288,747.63 | 3.301 | 0.044⁎ | 0.057 | 0.107 |
| Age | 27,210.95 | 1 | 27,210.95 | 0.311 | 0.579 | 0.003 | 0.006 |
| Sex | 562,325.53 | 1 | 562,325.53 | 6.429 | 0.014⁎ | 0.055 | 0.105 |
| ICV | 637,788.24 | 1 | 637,788.24 | 7.291 | 0.009⁎ | 0.063 | 0.117 |
| OCI total | 11,522.83 | 1 | 11,522.83 | 0.132 | 0.718 | 0.001 | 0.002 |
| Depression | 490,991.26 | 1 | 490,991.26 | 5.613 | 0.021⁎ | 0.048 | 0.093 |
| Medication | 16,746.94 | 1 | 16,746.94 | 0.191 | 0.663 | 0.002 | 0.003 |
| Disease duration | 66,142.75 | 1 | 66,142.75 | 0.756 | 0.388 | 0.007 | 0.014 |
| Scanner | 168,298.30 | 1 | 168,298.30 | 5.354 | 0.024⁎ | 0.017 | 0.089 |
| Error | 4,811,035.05 | 55 | 87,473.37 | ||||
| Total | 812,213,858.70 | 66 | |||||
| Corrected total variation | 10,153,310.00 | 65 |
= p < 0.05;
ANCOVA model formulation:
hippovol = 2697.20 − 213.42∗ clust1 − 306.12∗ clust2 − 3.18∗ age + 275.47∗ sex + 0.001∗ ICV + 2.09∗ OCItotal − 204.19∗ depression + 38.93∗ medication + 5.14∗ disease duration + 231.78∗ scanner.
3.2.2. Between groups analysis
There was a significant main effect of cluster on global hippocampus volume while controlling for age, sex, total intracranial volume and scanner (F(3,125) = 4.752, p = 0.004, η2 = 0.071). Additionally, there was a significant main effect of sex (F(1,125) = 10.914 p = 0.001, η2 = 0.081), total intracranial volume (F(1,125) = 17.758 p < 0.001, η2 = 0.088) and scanner (F(1,125) = 6.797 p = 0.010, η2 = 0.034) (see Fig. 1C as well as Table 4). Post-hoc tests were conducted to compare each patient cluster with healthy controls (c1 vs. HC, c2 vs. HC, c3 vs. HC). For this comparison alpha was Bonferroni-corrected to be α = 0.05/3 or α = 0.017. Cluster 2 was found to be significantly different from HC (p = 0.012, 95% CI [− 297.39, − 37.47]). Differences between cluster 3 and healthy controls (p = 0.063, 95% CI [− 255.23, 6.75]) as well as between cluster 1 and HC were not significant (p = 0.366, 95% CI [− 82.68, 222.46]).
Table 4.
ANCOVA model details for between groups analysis.
| Sum of squares (Typ III) | df | Mean squares | F | Significance | η2 | Partial η2 | |
|---|---|---|---|---|---|---|---|
| Corrected model | 5,882,377.33 | 7 | 840,339.618 | 11.003 | < 0.001 | 0.383 | |
| Constant term | 9,327,886.03 | 1 | 9,327,886.03 | 122.130 | < 0.001 | 0.496 | |
| Cluster | 1,088,735.90 | 3 | 362,911.97 | 4.752 | 0.004⁎ | 0.071 | 0.103 |
| Age | 6681.42 | 1 | 6681.42 | 0.087 | 0.768 | < 0.001 | 0.001 |
| Sex | 833,600.24 | 1 | 833,600.24 | 10.914 | 0.001⁎ | 0.054 | 0.081 |
| ICV | 1,356,312.68 | 1 | 1,356,312.68 | 17.758 | < 0.001⁎ | 0.088 | 0.125 |
| scanner | 519,145.23 | 1 | 519,145.23 | 6.797 | 0.010⁎ | 0.034 | 0.052 |
| Error | 9,470,732.19 | 124 | 76,376.87 | ||||
| Total | 1,647,001,109.00 | 132 | |||||
| Corrected total variation | 15,353,109.51 | 131 |
= p < 0.05;
ANCOVA model formulation:
hippovol = 2534.54 − 69.89∗ clust1 − 167.43∗ clust2 + 124.29∗ clust3 + 0.70∗ age + 200.56∗ sex + 0.001∗ ICV + 159.60∗ scanner.
4. Discussion
To find out more about the clinical relevance of hippocampal volume changes in OCD, in the present study we categorized a large sample of OCD patients into three distinct symptom profiles and compared alterations in hippocampal volume between the resulting groups. We further compared the resulting clusters with healthy participants. With this procedure we aimed at further elucidating the clinical significance of hippocampal volume alterations by better accounting for the clinical heterogeneity of the disorder. The cluster analysis showed that the relatively large patient sample could be subdivided most adequately into three symptom profile groups. Common to all clusters was the moderate to high level of obsessing symptoms. This feature thus does not seem be the major driving factor regarding hippocampus volume differences. Similar, but less pronounced are the dimensions neutralizing and hoarding. Here, the overall symptom strength is low to moderate with slight differences between clusters. The main differences between clusters could be found for the dimensions washing, ordering, and checking. Here cluster 1 revealed by far the highest washing scores while being on par with cluster 3 on ordering and checking symptoms. Cluster 2 revealed intermediate washing symptoms while scoring the highest on ordering as well as checking symptoms. On a side note, cluster 1, characterized by the highest washing symptoms, contained only a single male patient and 16 female patients. This is in line with earlier studies reporting washing symptoms predominantly in female patients (Labad et al., 2008, Mathis et al., 2011, Torresan et al., 2013). As a main finding the present analysis demonstrated that hippocampus volume differed significantly between the three groups with post-hoc tests indicating that cluster 2 had significantly smaller hippocampal volumes than cluster 3. Importantly, this result was corrected for the influence of overall symptom severity (i.e., OCI-R total score) which indicates that the respective symptom profiles account for variation in hippocampal volume independent of overall symptom severity. Hence, present findings clearly demonstrate that the classification into different OCD symptom profiles – an approach which has been recommended already years ago (Mataix-Cols et al., 2005) – significantly accounts for variation in hippocampal volume reduction. Additionally, there was a difference between hippocampal volumes when including a group of healthy subjects, with post-hoc tests indicating significant differences between cluster 2 and healthy controls. Present findings moreover extend recent results from the currently largest meta-analysis on structural alterations in OCD (i.e., the ENIGMA consortium meta-analysis) which revealed significantly smaller hippocampal volumes in adult OCD patients compared to healthy controls (Boedhoe et al., 2017). The meta-analysis showed the effect to be stronger in medicated patients compared to controls but not significantly related to clinical symptoms. However, unlike in the present study, in this meta-analysis symptom spectra or the interrelation between different symptoms was not taken into account but symptoms were assessed independently for each Y-BOCS checklist symptom dimension. Present findings not only corroborate the clinical relevance of hippocampal volume alterations in OCD as reported before (Honda et al., 2017) but strongly suggest that the interrelation of symptom dimensions should be taken into account in this regard. As also shown in Fig. 1A, it seems that a high severity of mainly ordering and checking symptoms (i.e., cluster 2) may be predominantly indicative of a reduction in hippocampus volume. The hippocampus is a highly stress-sensitive structure (Kim et al., 2015) and is often found to be reduced in volume in other stress-related disorders such as depression (MacQueen, 2009) and PTSD (Ahmed-Leitao et al., 2016). Hence, there is reason to assume that the association between a high level of predominantly ordering/checking (cluster 2) and - to a somewhat lesser extent predominantly washing (cluster 1) symptoms - and reduced hippocampal volume may be mediated via stress and stress-related physiological processes going along with these symptom profiles and their associated behavior. In this context it is interesting to note that the association remained significant even after correcting for the comorbidity of depression. Moreover, the association between symptom profile and hippocampal volume also remained significant after correcting for the influence of disease duration. In this case, disease duration did not have a significant effect on hippocampal volume. This finding seems to contradict the above formulated assumption that stress going along with the disorder may play a relevant role in this context. However, findings from meta-analyses on hippocampal volumes in depression produced relatively conflicting results and suggested that disease duration may be a significant influencing factor mainly in elderly patients (Eker and Gonul, 2010) (i.e., hippocampal degenerative processes due to disorder-related stress may become manifest predominantly in elderly patients who had been suffering from depression for various years). Of note, the average disease duration between clusters was not significantly different, i.e., overall effects of disease duration had no significant influence on this type of analysis. This finding does therefore not rule out the possibility of disease duration related effects on hippocampal volumes in general. Apart from the above mentioned meta-analysis (Boedhoe et al., 2017) which showed a significantly decreased hippocampal volume in patients with OCD, a limited amount of previous studies already reported alterations in hippocampus structure and neurochemistry in patients with OCD. For instance, Honda et al. (2017) found a decreased hippocampal volume in OCD patients employing voxel-based analyses and Hong et al. (2007) observed a bilateral hippocampal shape deformity in OCD patients compared to healthy controls when performing a shape analysis of the hippocampus. Regarding hippocampal neurochemistry lower hippocampal ratio of N-acetyl-l-aspartate/choline (NAA/CHO) which is considered to indicate loss of neurons and axons has been reported in patients with OCD (Atmaca et al., 2009). Interestingly, follow-up studies found these alterations to partly normalize by effective treatment and clinical improvement (Atmaca et al., 2015). Hence, our finding that patients with a symptom profile characterized by a high level of predominantly checking/ordering symptoms (cluster 2) showed stronger hippocampal volume differences compared to patients without a high severity in any specific domain as well as an overall lower symptom severity (cluster 3) complements these results. Taken together, present and earlier findings suggest that alterations in hippocampal volume in terms of neuroplasticity or partial reversal of tissue loss may be an indicator of treatment-related clinical improvement whereas hippocampal volume in terms of volumetric loss may represent a state marker of disease severity if assessed dimensionally according to specific symptom spectra or the interrelation between specific symptom dimensions. Longitudinal study designs might further elucidate an interaction between attenuation of strength in symptom profiles due to therapy and associated hippocampus volume changes.
5. Limitations
In opposition to the results of the currently largest meta-analysis (Boedhoe et al., 2017) which found that hippocampal volume reductions were stronger in medicated patients compared to controls we only found a trend significant influence of medication on volumes. These partly conflicting findings may have mainly statistical reasons as it must be assumed that the meta-analysis based on a sample of 1495 adult OCD patients had considerably larger detection power than the present study. The definition of clinically relevant depression was based on two different questionnaires (self-rated and clinician-rated) resulting from the aggregation of two different samples. Therefore, the factor depression should be assessed in further studies using the same questionnaires for the definition of cut-offs.
Funding
This study was supported by German Research Foundation (DFG) grants to KK (DFG KO 3744/2-1 and DFG KO 3744/7-1) and GW (DFG WA 3001/3-1). Additionally, this work was supported by the German Research Foundation (DFG) and the Technical University of Munich (TUM) in the framework of the Open Access Publishing Program. The funding agency had no influence on the design and conduct of the study including collection, management, analysis and interpretation of data, as well as preparation, review and approval of the manuscript.
Declaration of interest
The authors report no biomedical financial interests or potential conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2017.11.006.
Appendix A. Supplementary data
Supplementary material
References
- Ahmed-Leitao F., Spies G., van den Heuvel L., Seedat S. Hippocampal and amygdala volumes in adults with posttraumatic stress disorder secondary to childhood abuse or maltreatment: a systematic review. Psychiatry Res. Neuroimaging. 2016;256:33–43. doi: 10.1016/j.pscychresns.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Atmaca M., Yildirim H., Ozdemir H., Koc M., Ozler S., Tezcan E. Neurochemistry of the hippocampus in patients with obsessive–compulsive disorder. Psychiatry Clin. Neurosci. 2009;63:486–490. doi: 10.1111/j.1440-1819.2009.01993.x. [DOI] [PubMed] [Google Scholar]
- Atmaca M., Yildirim H., Yilmaz S., Caglar N., Mermi O., Gurok M.G., Kekilli Y., Turkcapar H. 1HMRS results of hippocampus in the patients with obsessive–compulsive disorder before and after cognitive behavioral therapy. Int. J. Psychiatry Clin. Pract. 2015;19:285–289. doi: 10.3109/13651501.2015.1072220. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G. Psychological Corporation; San Antonio, TX: 1996. Manual for the Beck Depression Inventory-II. [Google Scholar]
- Boedhoe P.S., Schmaal L., Abe Y., Ameis S.H., Arnold P.D., Batistuzzo M.C., Benedetti F., Beucke J.C., Bollettini I., Bose A., Brem S., Calvo A., Cheng Y., Cho K.I., Dallaspezia S., Denys D., Fitzgerald K.D., Fouche J.P., Gimenez M., Gruner P., Hanna G.L., Hibar D.P., Hoexter M.Q., Hu H., Huyser C., Ikari K., Jahanshad N., Kathmann N., Kaufmann C., Koch K., Kwon J.S., Lazaro L., Liu Y., Lochner C., Marsh R., Martinez-Zalacain I., Mataix-Cols D., Menchon J.M., Minuzzi L., Nakamae T., Nakao T., Narayanaswamy J.C., Piras F., Piras F., Pittenger C., Reddy Y.C., Sato J.R., Simpson H.B., Soreni N., Soriano-Mas C., Spalletta G., Stevens M.C., Szeszko P.R., Tolin D.F., Venkatasubramanian G., Walitza S., Wang Z., van Wingen G.A., Xu J., Xu X., Yun J.Y., Zhao Q., Group, E.O.W, Thompson P.M., Stein D.J., van den Heuvel O.A. Distinct subcortical volume alterations in pediatric and adult OCD: a worldwide meta- and mega-analysis. Am. J. Psychiatry. 2017;174:60–69. doi: 10.1176/appi.ajp.2016.16020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- De Wit S.J., Alonso P., Schweren L., Mataix-Cols D., Lochner C., Menchón J.M., Stein D.J., Fouche J.-P., Soriano-Mas C., Sato J.R. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am. J. Psychiatry. 2014;171:340–349. doi: 10.1176/appi.ajp.2013.13040574. [DOI] [PubMed] [Google Scholar]
- DGPPN B., KBV A. DGPPN, ÄZQ, AWMF–Berlin; Düsseldorf: 2015. S3-Leitlinie/Nationale Versorgungsleitlinie Unipolare Depression-Langfassung. [Google Scholar]
- Eker C., Gonul A.S. Volumetric MRI studies of the hippocampus in major depressive disorder: meanings of inconsistency and directions for future research. World J. Biol. Psychiatry. 2010;11:19–35. doi: 10.1080/15622970902737998. [DOI] [PubMed] [Google Scholar]
- Fan Q., Palaniyappan L., Tan L., Wang J., Wang X., Li C., Zhang T., Jiang K., Xiao Z., Liddle P. Surface anatomical profile of the cerebral cortex in obsessive–compulsive disorder: a study of cortical thickness, folding and surface area. Psychol. Med. 2013;43:1081–1091. doi: 10.1017/S0033291712001845. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Foa E.B., Huppert J.D., Leiberg S., Langner R., Kichic R., Hajcak G., Salkovskis P.M. The obsessive-compulsive inventory: development and validation of a short version. Psychol. Assess. 2002;14:485–496. [PubMed] [Google Scholar]
- Fouche J.P., du Plessis S., Hattingh C., Roos A., Lochner C., Soriano-Mas C., Sato J.R., Nakamae T., Nishida S., Kwon J.S., Jung W.H., Mataix-Cols D., Hoexter M.Q., Alonso P., Consortium O.C.D.B.I., de Wit S.J., Veltman D.J., Stein D.J., van den Heuvel O.A. Cortical thickness in obsessive-compulsive disorder: multisite mega-analysis of 780 brain scans from six centres. Br. J. Psychiatry. 2017;210:67–74. doi: 10.1192/bjp.bp.115.164020. [DOI] [PubMed] [Google Scholar]
- Frodl T., O'Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol. Dis. 2013;52:24–37. doi: 10.1016/j.nbd.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Gonner S., Leonhart R., Ecker W. The obsessive-compulsive inventory-revised (OCI-R): validation of the German version in a sample of patients with OCD, anxiety disorders, and depressive disorders. J. Anxiety Disord. 2008;22:734–749. doi: 10.1016/j.janxdis.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Goodman W.K., Price L.H., Rasmussen S.A., Mazure C., Fleischmann R.L., Hill C.L., Heninger G.R., Charney D.S. The Yale-Brown obsessive compulsive scale. I. Development, use, and reliability. Arch. Gen. Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand I., Büttner-Westphal H. Die Yale-Brown Obsessive Compulsive Scale (Y-BOCS): Ein halbstrukturiertes Interview zur Beurteilung des Schweregrades von Denk-und Handlungszwängen. Verhaltenstherapie. 1991;1:223–225. [Google Scholar]
- Hautzinger M., Keller F., Kühner C. Pearson Assessment; Frankfurt: 2009. BDI-II. Beck-Depressions-Inventar. Revision., 2. Auflage ed. [Google Scholar]
- Honda S., Nakao T., Mitsuyasu H., Okada K., Gotoh L., Tomita M., Sanematsu H., Murayama K., Ikari K., Kuwano M. A pilot study exploring the association of morphological changes with 5-HTTLPR polymorphism in OCD patients. Ann. General Psychiatry. 2017;16:2. doi: 10.1186/s12991-017-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.B., Shin Y.-W., Kim S.H., Yoo S.Y., Lee J.-M., Kim I.Y., Kim S.I., Kwon J.S. Hippocampal shape deformity analysis in obsessive–compulsive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2007;257:185–190. doi: 10.1007/s00406-006-0655-5. [DOI] [PubMed] [Google Scholar]
- Iglesias J.E., Augustinack J.C., Nguyen K., Player C.M., Player A., Wright M., Roy N., Frosch M.P., McKee A.C., Wald L.L., Fischl B., Van Leemput K., Alzheimer's Disease Neuroimaging I. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. NeuroImage. 2015;115:117–137. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.J., Pellman B., Kim J.J. Stress effects on the hippocampus: a critical review. Learn. Mem. 2015;22:411–416. doi: 10.1101/lm.037291.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S., Kaufmann C., Simon D., Endrass T., Gallinat J., Kathmann N. Reduced thickness of anterior cingulate cortex in obsessive-compulsive disorder. Cortex. 2013;49:2178–2185. doi: 10.1016/j.cortex.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Labad J., Menchon J.M., Alonso P., Segalas C., Jimenez S., Jaurrieta N., Leckman J.F., Vallejo J. Gender differences in obsessive-compulsive symptom dimensions. Depress. Anxiety. 2008;25:832–838. doi: 10.1002/da.20332. [DOI] [PubMed] [Google Scholar]
- MacQueen G.M. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J. Psychiatry Neurosci. 2009;34:41. [PMC free article] [PubMed] [Google Scholar]
- Malykhin N., Coupland N. Hippocampal neuroplasticity in major depressive disorder. Neuroscience. 2015;309:200–213. doi: 10.1016/j.neuroscience.2015.04.047. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D., do Rosario-Campos M.C., Leckman J.F. A multidimensional model of obsessive-compulsive disorder. Am. J. Psychiatry. 2005;162:228–238. doi: 10.1176/appi.ajp.162.2.228. [DOI] [PubMed] [Google Scholar]
- Mathis M.A., Alvarenga P., Funaro G., Torresan R.C., Moraes I., Torres A.R., Zilberman M.L., Hounie A.G. Gender differences in obsessive-compulsive disorder: a literature review. Rev. Bras. Psiquiatr. 2011;33:390–399. doi: 10.1590/s1516-44462011000400014. [DOI] [PubMed] [Google Scholar]
- Nakamae T., Narumoto J., Sakai Y., Nishida S., Yamada K., Kubota M., Miyata J., Fukui K. Reduced cortical thickness in non-medicated patients with obsessive-compulsive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2012;37:90–95. doi: 10.1016/j.pnpbp.2012.01.001. [DOI] [PubMed] [Google Scholar]
- O'Doherty D.C., Chitty K.M., Saddiqui S., Bennett M.R., Lagopoulos J. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res. Neuroimaging. 2015;232:1–33. doi: 10.1016/j.pscychresns.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Piras F., Piras F., Chiapponi C., Girardi P., Caltagirone C., Spalletta G. Widespread structural brain changes in OCD: a systematic review of voxel-based morphometry studies. Cortex. 2015;62:89–108. doi: 10.1016/j.cortex.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Rus O., Reess T., Wagner G., Zaudig M., Zimmer C., Koch K. Hypogyrification in obsessive-compulsive disorder. Psychol. Med. 2016:1–9. doi: 10.1017/S0033291716003202. [DOI] [PubMed] [Google Scholar]
- Shaw P., Sharp W., Sudre G., Wharton A., Greenstein D., Raznahan A., Evans A., Chakravarty M., Lerch J., Rapoport J. Subcortical and cortical morphological anomalies as an endophenotype in obsessive-compulsive disorder. Mol. Psychiatry. 2015;20:224–231. doi: 10.1038/mp.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y.W., Yoo S.Y., Lee J.K., Ha T.H., Lee K.J., Lee J.M., Kim I.Y., Kim S.I., Kwon J.S. Cortical thinning in obsessive compulsive disorder. Hum. Brain Mapp. 2007;28:1128–1135. doi: 10.1002/hbm.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D.J., Fineberg N.A., Bienvenu O.J., Denys D., Lochner C., Nestadt G., Leckman J.F., Rauch S.L., Phillips K.A. Should OCD be classified as an anxiety disorder in DSM-V? Depress. Anxiety. 2010;27:495–506. doi: 10.1002/da.20699. [DOI] [PubMed] [Google Scholar]
- Torresan R.C., Ramos-Cerqueira A.T., Shavitt R.G., do Rosario M.C., de Mathis M.A., Miguel E.C., Torres A.R. Symptom dimensions, clinical course and comorbidity in men and women with obsessive-compulsive disorder. Psychiatry Res. 2013;209:186–195. doi: 10.1016/j.psychres.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian G., Zutshi A., Jindal S., Srikanth S.G., Kovoor J.M., Kumar J.K., Janardhan Reddy Y.C. Comprehensive evaluation of cortical structure abnormalities in drug-naive, adult patients with obsessive-compulsive disorder: a surface-based morphometry study. J. Psychiatr. Res. 2012;46:1161–1168. doi: 10.1016/j.jpsychires.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Wobrock T., Gruber O., McIntosh A.M., Kraft S., Klinghardt A., Scherk H., Reith W., Schneider-Axmann T., Lawrie S.M., Falkai P. Reduced prefrontal gyrification in obsessive–compulsive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2010;260:455–464. doi: 10.1007/s00406-009-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material