Abstract
In order to gain a better understanding of aphasia one must consider the complex combinations of language impairments along with the pattern of paraphasias. Despite the fact that both deficits and paraphasias feature in diagnostic criteria, most research has focused only on the lesion correlates of language deficits, with minimal attention on the pattern of patients' paraphasias. In this study, we used a data-driven approach (principal component analysis - PCA) to fuse patient impairments and their pattern of errors into one unified model of chronic post-stroke aphasia. This model was subsequently mapped onto the patients' lesion profiles to generate the triangulation of language-cognitive impairments, naming errors and their neural correlates. Specifically, we established the pattern of co-occurrence between fifteen error types, which avoids focussing on a subset of errors or the use of experimenter-derived methods to combine across error types. We obtained five principal components underlying the patients' errors: omission errors; semantically-related responses; phonologically-related responses; dysfluent responses; and a combination of circumlocutions with mixed errors. In the second step, we aligned these paraphasia-related principal components with the patients' performance on a detailed language and cognitive assessment battery, utilising an additional PCA. This omnibus PCA revealed seven unique fused impairment-paraphasia factors: output phonology; semantics; phonological working memory; speech quanta; executive-cognitive skill; phonological (input) discrimination; and the production of circumlocution errors. In doing so we were able to resolve the complex relationships between error types and impairments. Some are relatively straightforward: circumlocution errors formed their own independent factor; there was a one-to-one mapping for phonological errors with expressive phonological abilities and for dysfluent errors with speech fluency. In contrast, omission-type errors loaded across both semantic and phonological working memory factors, whilst semantically-related errors had the most complex relationship by loading across four factors (phonological ability, speech quanta, executive-cognitive skills and circumlocution-type errors). Three components had unique lesion correlates: phonological working memory with the primary auditory region; semantics with the anterior temporal region; and fluency with the pre-central gyrus, converging with existing literature. In conclusion, the data-driven approach allowed derivation of the triangulation of deficits, error types and lesion correlates in post-stroke aphasia.
Keywords: Naming errors, Semantic, Phonology, Speech production, Principal component analysis, Lesion-mapping
Highlights
-
•
Using principal component analysis to identify structure in naming errors.
-
•
Determining the relationship between language impairments and naming errors.
-
•
Identifying neural correlates of behavioural deficits in performance and errors.
-
•
Seven independent factors identified to describe performance and error pattern.
-
•
Phonological working memory, semantic skill and speech quanta had lesion correlates.
1. Introduction
The most common cause of aphasia is stroke, with approximately 30% of cases suffering from language problems in the acute phase and 20% chronically (Berthier, 2005, Engelter et al., 2006). Clinical diagnosis and management of aphasia is founded on establishing the pattern of language deficits and preserved skills. In addition, perhaps more than in any other disorder of higher cognition, aphasiology also heavily emphasises the types of speech errors made by patients, with both deficits and paraphasias featuring in diagnostic criteria. In recent years, considerable advances in knowledge and analysis techniques have been made by large-scale studies that have mapped language deficits and the underlying principal computational components to the patients' lesion distributions (e.g. Bates et al., 2003, Butler et al., 2014, Corbetta et al., 2015, Halai et al., 2017, Lacey et al., 2017, Mirman et al., 2015a, Mirman et al., 2015b). Given their importance in aphasiology, the key purpose of the current study was to assimilate the patterns of patients' paraphasias thereby generating the much broader lesion-symptom-error mapping for post-stroke aphasia, for the first time.
In order to resolve the triangulation of lesions, symptoms and error types, each of the pairwise combinations is required. For the link between language impairments and error types, we might start with the most straightforward hypothesis that there is a one-to-one mapping (for example, phonological impairments with phonological paraphasias). Studies of individual error types or specific patient groups, however, have shown that this simple hypothesis is incorrect. For example, seminal studies of semantic errors (cf. Morton and Patterson, 1980) noted that these could arise from multiple different underlying impairments, and conversely most patients generate a collection of different paraphasias (Schwartz et al., 2006). Whilst a variety of paraphasias can reflect multiple co-occurring deficits in each patient, multiple error types are generated even in disorders such as semantic dementia (omissions, superordinate and coordinate semantic errors, and partial descriptions: Lambon Ralph et al., 2001, Woollams et al., 2008), which is characterised by a selective semantic impairment and atrophy consistently centred on the anterior temporal region (Mummery et al., 2000, Warrington, 1975). Taken together, these results suggest that (i) different error types can co-occur because they are generated by the same underlying impairment and also (ii) that the same error type can be caused by more than one type of deficit. In order to unpick the ‘many-to-many’ relationships between error types and impairments in post-stroke aphasia, we utilised principal component analysis with varimax rotation on a large patient dataset in order to extract: (a) how different error types cluster and differentiate across patients; and (b) the underlying principal ways in which impairments and error types co-occur and dissociate.
In comparison to the relative paucity of aphasiological studies linking error types and impairment patterns, there have been many explorations of the relationship between different language impairments and their associated lesions, over the long history of aphasiology. In addition, this mass of research activity continues to spawn ever more sophisticated methods to relate impairments and lesions (e.g., lesion mapping: (Damasio and Damasio, 1980); voxel-based lesion-symptom mapping: (Bates et al., 2003); multivariate symptom decomposition: (Lambon Ralph et al., 2003); and multivariate lesion mapping: (i.e. Hope et al., 2013, Yourganov et al., 2015, Zhang et al., 2014). Numerous studies have conducted voxel-lesion mapping for individual language tasks (e.g., repetition, naming, comprehension, etc. (i.e. Baldo et al., 2013, Baldo et al., 2012, Dronkers et al., 2004) or features of aphasic performance (e.g., fluency: Borovsky et al., 2007). Extracting an exact understanding of the cognitive and neural bases for aphasic performance, from such analyses, is challenging for a number of reasons. First, any given cognitive-language task relies on multiple different processes/computations (e.g., naming requires visual decoding, semantic activation, phonological processing and articulation) and thus poor performance on the same task can arise for different reasons, each with different neural bases. Secondly, patients with more severe deficits perform poorly across many different tasks, albeit potentially for different reasons. This is because, thirdly, lesions correspond to the vascular rather than functional structure, and thus infarcts often disrupt multiple processes. In order to isolate the key cognitive dimensions underlying aphasic performance and their associated neural substrates, we recently applied principal component analysis (PCA) with varimax rotation on a substantial and detailed behavioural dataset to yield cognitively-interpretable, statistically-independent factors, which are ideal for use in voxel-based lesion-symptom mapping (Butler et al., 2014, Halai et al., 2017). Accordingly, in the current study we utilised the same approach on a larger patient database containing both performance across a detailed neuropsychological battery and coding of all the naming errors generated by the same patients.
With regard to the final part of the triangulation, error-lesion mapping, again, there is a relatively limited number of studies in the current literature. Few, if any, studies have tackled all aphasic paraphasias simultaneously. Instead, a handful of previous studies have focussed on two of the most prominent error types: semantic and phonological errors. In a series of studies of chronic aphasia, Schwartz and colleagues found that semantic errors were associated with damage to the left anterior temporal lobe (ATL), prefrontal and posterior temporal areas though these two non-ATL correlates disappeared when performance variance on an executively-demanding semantic task was partialled out (Schwartz et al., 2012, Schwartz et al., 2009, Walker et al., 2011). In contrast, Cloutman et al. (2009) found an association between semantic errors and hypoperfusion of the left posterior temporal lobe (BA 37) in acute stroke cases, though it is hard to compare these studies directly as they varied: (i) in the types of patient included, (ii) the definition of error types, (iii) the measure of brain integrity (lesion vs. perfusion), and (iv) the formal of analysis (including covariates). There was much greater consistency across these studies, however, with regard to phonological errors, which were associated with damage to precentral gyrus (preCG: (Cloutman et al., 2009, Schwartz et al., 2012, Walker et al., 2011). Although aphasic patients generate many other types of paraphasia beyond semantic and phonological errors, few have explored their neural correlates and their relationship to the patients' impairments (for two important exceptions that explored semantic and phonological errors alongside background neuropsychological results, see Mirman et al., 2015a, Mirman et al., 2015b). This includes omission errors which, although very common, are often discarded from analyses (because this error type, by itself, provides no clues as to its source). To address these challenges in the present study, we (a) included all paraphasia types; (b) used a data-driven approach to cluster co-occurring error types (rather than using a user-defined set of criteria for collapsing errors); and explored the relationship of the grouped paraphasias to the patients' pattern of impairments and the lesion correlates.
2. Materials and methods
2.1. Participants
Fifty-one chronic stroke patients (either ischaemic or haemorrhagic) were recruited into the current study, who had impairment in producing and/or understanding spoken language. No restrictions were placed according to aphasia type or severity (spanning global to minimal aphasia). Given the emphasis on a full range of error types, in this study we excluded five patients who did not attempt at least 50% of items in each naming test. All patients were at least 12 months post-stroke at the time of scanning and assessment, were native English speakers with normal or corrected-to-normal hearing and vision (see Supplementary Table 1 for demographic details). In brief, there were 33 males and 13 females with a mean age of 65.46 years (SD = 11.49). The mean years of education were 12.07 years (SD = 1.97) and mean months post-stroke were 54.65 (SD = 43.28). Participants were pre-morbidly right-handed, had one stroke and did not have any other significant neurological conditions. Informed consent was obtained from all participants prior to participation under approval from the local NHS ethics committee. MRI data from a healthy age and education matched control group (8 female, 11 male) was used in the lesion identification procedure for each patient (Seghier et al., 2008).
2.2. Neuropsychological assessments and analysis
Explicit naming responses to the 64-item naming test from the Cambridge Semantic Battery (CNT) (Bozeat et al., 2000) and 60-item Boston Naming Test (BNT) (Kaplan et al., 1983) were recorded and coded for error type. The CNT contains 64 items (spanning three living and non-living categories: animals, bids, fruits, household items, tools and vehicles). The BNT is relatively harder as it is graded in difficulty; it consists of 60 black and white line drawings. In both cases, the patient was shown each item and asked to provide the name. The first complete (i.e., non-fragment) response for each item was scored. Fragmented responses were taken into account in the case of initial phoneme errors (INITIAL) where a fragmented response was given without any further response (‘ca’ for cat). The criteria to code each error is as follows (modified from Budd et al., 2010, Crisp et al., 2011, Hodges et al., 1991, Woollams et al., 2008). Semantic errors (SEM) included responses that were co-ordinate (‘dog’ for cat), super-ordinate (‘animal’ for cat) and associative (‘milk’ for cat) to the target; phonemic errors (PHON) were non-word responses that had at least 30% phoneme overlap with the target name in any position (‘cag’ for cat); neologism errors (NEO) were phonologically unrelated nonwords (‘coj’ for cat); formal errors (FORM) were real words that were phonologically related (> 30% phoneme overlap) but not semantically related (‘cap’ for cat); mixed errors (MIX) were semantically and phonologically related; dysfluency errors (DYS) were correct repaired responses (‘ca cat’ for cat); morphological errors (MORPH) shared a morpheme with the target (‘toast’ for toaster); unrelated errors (UNREL) were real words that were neither semantically or phonologically related (‘pen’ for cat); perseveration errors (PERS) were repetitions of a previous response; circumlocution errors (CIRC) were a description of a target, either informative or uninformative, without producing a name (‘oh, yes, we have a ginger stripy one’ for cat); ‘not a’ errors where instances when the patient was unsure about their response or if the response was posed as a question, these were marked as correct (NOTACOR) (‘is it a cat?’ for cat) or incorrect (NOTAINCOR) (‘is it a pen?’ for cat); visual errors (VIS) were incorrect visual identification of the target (rope for pretzel); omission errors (OM) included responses where no attempt was made to produce the target, including ‘don’t know’ response, filler (‘umm’, ‘err’, ‘ahh’, etc.), gestures or no response. If the response did not fall into these categories they were marked as other errors (OTHER). We found that six categories contributed less than 2% of the total errors (formal, morphological, perseveration, unrelated, visual and other) and the variance within each of these error types were less than 1.5%. Accordingly, these were likely to add noise and therefore were collapsed into OTHER, leaving 10 categories. To generate the dependent variable, we divided the number of errors by the total number of items each participant attempted and converted this into a percentage based on the max value in the group for each error type (see Supplementary Table 2).
In addition to the naming errors, we utilised an extensive battery of neuropsychological tests to assess participants’ language and cognitive abilities (described in Butler et al., 2014, Halai et al., 2017), enabling us to understand how naming errors relate to the patients' input and output phonological, semantic, speech fluency and general executive demand abilities (see Supplementary Table 3). These included subtests from the Psycholinguistic Assessments of Language Processing in Aphasia (PALPA) battery (Kay et al., 1992): auditory discrimination using non-word (PALPA 1) and word minimal pairs (PALPA 2); and immediate and delayed repetition of non-words (PALPA 8) and words (PALPA 9). Tests from the 64-item Cambridge Semantic Battery (Bozeat et al., 2000) were included: spoken and written versions of the word-to-picture matching task and Camel and Cactus Test (picture). To increase the sensitivity to mild semantic deficits we used a written 96-trial synonym judgement test (Jefferies et al., 2009). The spoken sentence comprehension task from the Comprehensive Aphasia Test (CAT) (Swinburn et al., 2005) was used to observe syntax level deficits. The cognitive tests included forward and backward digit span (Wechsler, 1987), the Brixton Spatial Rule Anticipation Task (Burgess and Shallice, 1997), and Raven's Coloured Progressive Matrices (Raven, 1962). Speech quanta (the amount of speech generated: cf. Halai et al., 2017) was extracted from the Cookie Theft Description. These included words per minute (WPM), number of speech tokens (TOK), mean length of utterances in morphemes (MLU) and type/token ratio (TTR). All scores were converted into percentage. Assessments were conducted with participants over several testing sessions, with the pace and number determined by the participant.
2.3. Principal component analysis
First, to understand the co-occurrence structure underlying different error responses, the naming errors were entered into a PCA with varimax rotation (SPSS 20.0). All error types were included except the OTHER category, as this contained a mixture of different rare error types and undifferentiated errors. Factors with an eigenvalue exceeding 1.0 were extracted and then rotated to allow a clear behavioural interpretation of each factor. The target of the second PCA was to understand the relationships between the patients' error types and their language-cognitive impairments. In order to achieve this aim, a second PCA included the clustered error types (by extracting the patients' factor scores from the first PCA, containing paraphasias only) plus the patients' results on the large battery of background neuropsychological measures (see above).
For both PCAs, individual participants’ scores on each factor were extracted and then used as (orthogonal) behavioural covariates in the neuroimaging analysis.
2.4. Acquisition of neuroimaging data
High resolution structural T1-weighted Magnetic Resonance Imaging (MRI) scans were acquired on a 3.0 Tesla Philips Achieva scanner (Philips Healthcare, Best, The Netherlands) using an 8-element SENSE head coil. A T1-weighted inversion recovery sequence with 3D acquisition was employed, with the following parameters: TR (repetition time) = 9.0 ms, TE (echo time) = 3.93 ms, flip angle = 8°, 150 contiguous slices, slice thickness = 1 mm, acquired voxel size 1.0 × 1.0 × 1.0 mm3, matrix size 256 × 256, FOV = 256 mm, TI (inversion time) = 1150 ms, SENSE acceleration factor 2.5, total scan acquisition time = 575 s.
2.5. Analysis of neuroimaging data
Structural MRI scans were pre-processed with Statistical Parametric Mapping software (SPM8: Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/). The images were normalised into standard Montreal Neurological Institute (MNI) space using a modified unified segmentation-normalisation procedure optimised for focal lesioned brains (Seghier et al., 2008). Data from all participants with stroke aphasia and all healthy controls were entered into the segmentation-normalisation. Images were then smoothed with an 8 mm full-width-half-maximum (FWHM) Gaussian kernel and used in the lesion analyses described below. The lesion of each patient was automatically identified using an outlier detection algorithm, compared to healthy controls, based on fuzzy clustering. The default parameters were used apart from the lesion definition ‘U-threshold’, which was set to 0.5 to create a binary lesion image. We modified the U-threshold from 0.3 to 0.5 after comparing the results obtained from a sample of patients to what would be nominated as lesioned tissue by an expert neurologist. The images generated were used to create the lesion overlap map in Fig. IA. We selected the Seghier et al. (2008) method as it is objective and efficient for a large sample of patients (Wilke et al., 2011), in comparison to a labour intensive hand-traced lesion mask. We should note here, explicitly, that although commonly referred to as an automated ‘lesion’ segmentation method, the technique detects areas of unexpected tissue class – and, thus, identifies missing grey and white matter but also areas of augmented CSF space. These images were for lesion visualisation and the smoothed T1 images were used in the main analyses.
The normalised and bias-corrected T1-weighted images were used to determine the brain regions where tissue concentration correlated with individual measures or PCA factor scores using a voxel-based correlational methodology (VBCM) (Tyler et al., 2005), a variant of voxel-lesion symptom mapping (VLSM) (Bates et al., 2003) in which both the behaviour and signal intensity measures are treated as continuous variables (conducted in SPM8). For the neural correlate analysis, we are assuming that lower T1-weighted intensity is related to tissue damage or atrophy. There were three stages to the analysis. First, as a baseline/initial analysis we explored the relationship between T1-weighted signal intensity and the rate of each error category, entered separately. Secondly, instead of entering the error types separately, we used the results from the first paraphasia-only PCA, which grouped the errors in a data driven manner. The patients' factor scores from this PCA were entered simultaneously into the second VBCM analysis. Finally, the participants’ factor scores from the omnibus PCA (fusing the entire neuropsychological test battery and naming errors) were entered simultaneously into a VBCM analysis. In order to ensure that the results were not merely attributable to lesion size, each participants’ lesion volume was calculated from the lesion identified by the automated lesion identification method (Seghier et al., 2008). The participants’ lesion volume, age and years of education were entered as covariates in each VBCM.
3. Results
3.1. Neuropsychological and lesion profiles
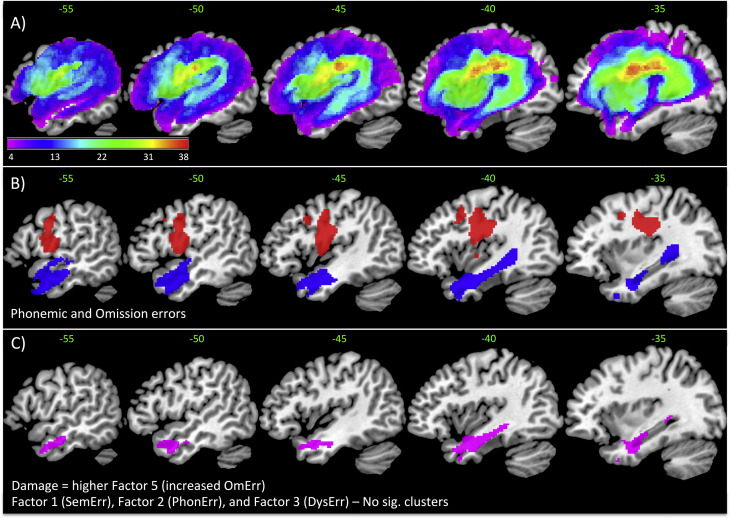
A lesion overlap map for all stroke participants is provided in Fig. 1A, and primarily covers the left hemisphere area supplied by the middle cerebral artery (Phan et al., 2005). The maximum number of participants who had a lesion in any one voxel was 38 (MNI coordinate − 23 − 9 26, located in the left cortico-spinal tract). We note that all significant neural correlates reported are confined to the left hemisphere.
Fig. 1.
A) Lesion overlap map across for 46 patients in MNI standard space (colour bar represents frequency between 4 and 38) (green values represent MNI sagittal value). B) VBCM analysis using each error category separately: Phonological (red) and omission (blue). No other error categories were significant. C) All error types were subjected to a principal component analysis and the resulting factors were entered simultaneously into a VBCM analysis. A negative correlation was observed for Factor 5 (omission-related errors; violet) (lower tissue concentration is related to higher Factor 5). Factor 1 (semantic-related errors), 2 (phonological-related errors), 3 (dysfluency-related errors) and 4 (circumlocution errors) did not reveal any significant clusters. [B) and C) were thresholded at p < 0.005 voxel height, cluster corrected at familywise error of p < 0.05]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Neural correlates of each error type
The first set of analyses investigated how each error type related to lesion location. Fig. 1B shows the neural correlates for the categories that survived the threshold p < 0.005 voxel height, FWE-cluster corrected p < 0.05. Increased phonological errors correlated with damage to the preCG and increased omission errors correlated with damage to the ATL extending posteriorly through the white matter. No other categories produced significant clusters, even at a reduced threshold of p < 0.01 voxel height, FWE-cluster corrected p < 0.05. Finally, we created a model that included all errors simultaneously and the results showed a significant cluster for omission errors in the same position (anterior temporal region) as the previous result, with no significant clusters for the remaining errors. This outcome probably reflects the level of inter-correlations between the paraphasia types (Table 1) and thus motivates the use of a PCA approach to extract the clustering of the error types.
Table 1.
Inter-correlations between the naming errors present during picture naming.
| Correlations | Semantic | ‘Not a’ Incorrect | Initial | Neologism | Phonemic | Dys-fluency | ‘Not a’ Correct | Circum-locution | Mixed | Omission |
|---|---|---|---|---|---|---|---|---|---|---|
| Semantic | 1 | .534⁎⁎ | − 0.035 | − 0.199 | − 0.242 | − 0.037 | − 0.149 | 0.155 | .420⁎⁎ | 0.032 |
| ‘Not a’ incorrect | 1 | − 0.001 | − 0.245 | − 0.196 | 0.171 | 0.064 | 0.043 | 0.209 | − 0.06 | |
| Initial | 1 | .425⁎⁎ | 0.221 | − 0.078 | − 0.139 | − 0.079 | 0.084 | 0.137 | ||
| Neologism | 1 | .450⁎⁎ | − 0.198 | − 0.246 | − 0.131 | − 0.165 | 0.113 | |||
| Phonemic | 1 | − 0.164 | − 0.23 | − 0.199 | − 0.234 | − 0.141 | ||||
| Dysfluency | 1 | .389⁎ | 0.02 | − 0.06 | − 0.196 | |||||
| ‘Not a’ Correct | 1 | 0.257 | − 0.041 | − 0.252 | ||||||
| Circumlocution | 1 | 0.239 | 0.021 | |||||||
| Mixed | 1 | − 0.199 | ||||||||
| Omission | 1 | |||||||||
| Age | − 0.107 | − 0.231 | 0.049 | 0.266 | 0.109 | 0.059 | − 0.108 | − 0.175 | − 0.096 | − 0.022 |
| Education | − 0.192 | 0.061 | 0.145 | − 0.121 | − 0.030 | 0.153 | 0.593⁎⁎ | 0.180 | 0.140 | − 0.139 |
| Months post onset | 0.184 | 0.030 | − 0.133 | − 0.081 | − 0.169 | 0.061 | − 0.094 | − 0.017 | 0.279 | − 0.156 |
| Lesion volume | 0.205 | 0.134 | 0.126 | 0.002 | − 0.089 | − 0.091 | − 0.309⁎ | − 0.252 | 0.105 | 0.397⁎⁎ |
Indicates p < 0.01.
Indicates p < 05.
3.3. PCA of naming errors
The paraphasia-only PCA produced five factors exceeding an eigenvalue of 1, explaining 75.33% of the original data. A breakdown of how each error type loaded across each factor is shown in Table 2. Factor 1 loaded on semantic and “not a [correct]” errors (26.61% variance explained), and thus is likely to reflect semantically-related errors in general (termed SemErr). Factor 2 loaded on initial fragments, neologisms and phonological errors (17.26% variance explained) and thus reflects phonologically-related errors in general (termed PhonErr). Factor 3 loaded on dysfluent and “not a [correct]” responses (12.27% variance explained), reflecting difficulty in fluent speech output (and thus termed DysErr). Factor 4 loaded on circumlocution errors and to a weaker extent with mixed errors (10.96% variance explained). Given the previous (statistically independent) factors, this component may reflect the ability to produce lots of connected speech (termed CircErr). Finally, Factor 5 loaded on omission errors only (10.25% variance explained) and therefore we interpret this factor as the inability to produce a response (termed OmErr).
Table 2.
Principal factors identified from a wide variety of naming errors during picture naming. Naming errors that load strongly (> 0.5) with each factor are marked in bold, allowing for behavioural interpretation. In addition, the correlation between age, education, months post onset and lesion volume are shown.
| Semantic errors | Phonological errors | Dysfluency errors | Circumlocution errors | Omission errors | |
|---|---|---|---|---|---|
| Semantic | 0.834 | − 0.099 | − 0.177 | 0.147 | 0.032 |
| ‘Not a’ incorrect | 0.822 | − 0.044 | 0.242 | − 0.114 | 0.016 |
| Initial | 0.131 | 0.867 | 0.035 | 0.062 | 0.123 |
| Neologism | − 0.255 | 0.766 | − 0.200 | − 0.104 | 0.011 |
| Phonemic | − 0.296 | 0.503 | − 0.228 | − 0.342 | − 0.394 |
| Dysfluency | 0.118 | − 0.051 | 0.832 | − 0.120 | − 0.066 |
| ‘Not a’ correct | − 0.163 | − 0.163 | 0.755 | 0.325 | − 0.130 |
| Circumlocution | − 0.011 | − 0.051 | 0.102 | 0.862 | 0.069 |
| Mixed | 0.523 | 0.007 | − 0.215 | 0.544 | − 0.344 |
| Omission | − 0.022 | 0.100 | − 0.206 | − 0.003 | 0.921 |
| Age | − 0.184 | 0.230 | − 0.020 | − 0.181 | − 0.024 |
| Education | − 0.088 | − 0.055 | 0.421⁎ | 0.191 | − 0.135 |
| Months post onset | 0.133 | − 0.158 | − 0.008 | 0.062 | − 0.149 |
| Lesion volume | 0.199 | − 0.001 | − 0.224 | − 0.198 | 0.421⁎ |
Denotes p < 0.05.
When these paraphasia-PCA factors were entered into a simultaneous VBCM, only OmErr revealed a significant cluster at the threshold p < 0.005 voxel height, FWE-cluster corrected p < 0.05. Fig. 1C shows that these omission-related errors (OmErr; violet) negatively correlated with the integrity of the anterior lateral middle temporal gyrus (MTG) extending medially towards parahippocampal gyrus (PHG) (i.e. more damage leads to increased omission related errors).
3.4. Omnibus PCA of language and cognitive assessments plus naming errors
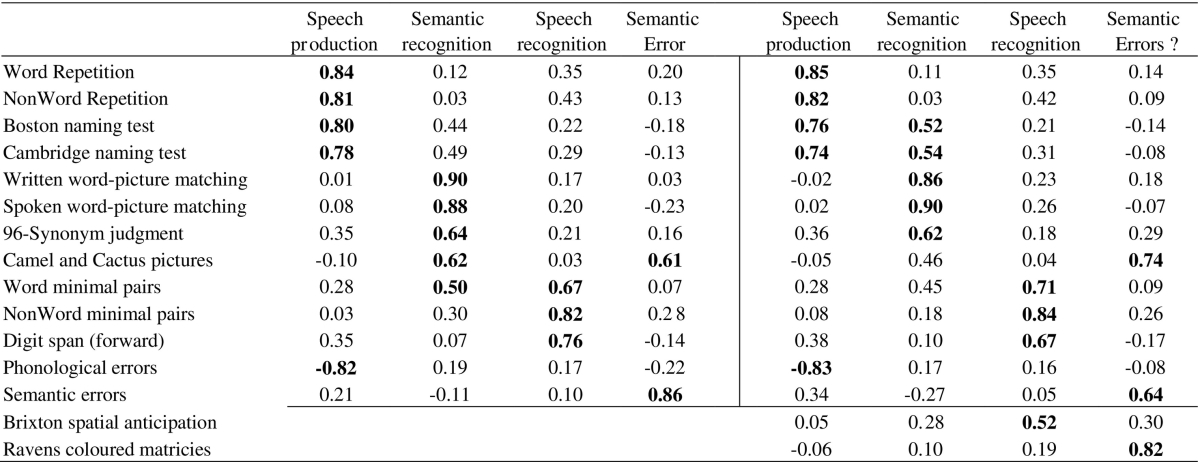
The omnibus PCA revealed seven independent factors exceeding an eigenvalue of 1 and explained a total of 80.94% of the total data. A summary of the loadings across these seven factors is shown in Table 3, and demonstrates how the clustered paraphasia types align with the patients' cognitive-language profiles. The patients' scores for each factor are provided in Supplementary Table 4. The first factor loaded positively with expressive phonological ability and negatively with PhonErr (− 0.85) (38.48% variance explained) – i.e., patients with good phonological abilities were less likely to make phonological errors. The second factor reflected the patients' semantic ability (naming, word-picture matching and synonym judgements) (11.45% variance explained). The third factor assimilated measures of the patients' phonological working memory (digit span, auditory sentence comprehension and delayed repetition) (9.55% variance explained). Omission-type errors had negative loading with these semantic (− 0.68) and phonological working memory factors (− 0.46). The fourth factor unified fluency related abilities and error types, loading on speech quanta measures (words-per-minute, tokens and mean length per utterance), and also loaded positively with the dysfluency-related errors (0.66) (6.53% variance explained). The fifth factor reflected the patients' ability on executively-demanding tests (Raven's, Brixton, camel and cactus, spoken sentence comprehension test) (5.71% variance explained). The sixth factor loaded with auditory discrimination tasks (word and nonword minimal pairs) (5.36% variance explained) and the seventh factor loaded with circumlocutory-type errors (0.85) (3.97% variance explained). Finally, we found that the rate of semantically-related errors (SemErr) had the most complex relationship with the extracted factors, loading across phonological ability (0.42), speech quanta (− 0.30), executive-demand (0.45) and circumlocutions (0.31).
Table 3.
Factor loadings from an omnibus principal component analysis with naming errors. Tests that loaded strongly (> 0.5) with each factor are marked in bold.
| Phonology | Semantic | Working phonological memory | Speech quanta | Executive | Auditory discrimination | Circumlocution | |
|---|---|---|---|---|---|---|---|
| NonWord repetition Imm | 0.81 | 0.01 | 0.34 | 0.15 | 0.07 | 0.17 | − 0.01 |
| NonWord repetition Del | 0.67 | 0 | 0.56 | 0.18 | 0.02 | 0.21 | − 0.03 |
| Word repetition Imm | 0.91 | 0.10 | 0.20 | 0.10 | 0.17 | 0.07 | 0.04 |
| Word repetition Del | 0.85 | 0.14 | 0.29 | 0.18 | 0.08 | 0.17 | 0.05 |
| Cambridge naming test | 0.76 | 0.51 | 0.24 | 0.14 | − 0.03 | 0.03 | − 0.16 |
| Boston naming test | 0.73 | 0.47 | 0.31 | 0.15 | − 0.10 | − 0.11 | − 0.18 |
| Phonological errors | − 0.85 | − 0.01 | 0.12 | − 0.19 | 0.07 | − 0.08 | − 0.14 |
| Spoken word-picture matching | 0.08 | 0.90 | 0.10 | 0.03 | − 0.01 | 0.21 | − 0.16 |
| Written word-picture matching | 0.05 | 0.87 | − 0.02 | 0.06 | 0.18 | 0.25 | 0 |
| 96 Synonym judgment | 0.23 | 0.59 | 0.46 | 0.27 | 0.25 | − 0.16 | 0.15 |
| Omission errors | − 0.23 | − 0.68 | − 0.46 | − 0.04 | − 0.09 | 0.06 | 0 |
| Spoken sentence comprehension | 0.28 | 0.32 | 0.63 | 0 | 0.48 | − 0.02 | − 0.06 |
| Forward digit span | 0.35 | 0.11 | 0.82 | − 0.05 | -0.02 | 0.18 | − 0.01 |
| Backward digit span | 0.15 | 0.13 | 0.79 | 0.27 | − 0.04 | 0.27 | − 0.12 |
| Token | 0.12 | − 0.02 | − 0.06 | 0.82 | 0.18 | 0.04 | 0.21 |
| Dysfluency errors | 0.23 | 0.17 | 0.11 | 0.66 | 0.12 | 0.08 | − 0.14 |
| Mean length of utterance | 0.48 | 0.21 | 0.13 | 0.62 | 0.14 | − 0.09 | 0.34 |
| Words per minute | 0.37 | 0.09 | 0.35 | 0.60 | 0.05 | − 0.04 | 0.23 |
| Camel and cactus pictures | − 0.04 | 0.47 | − 0.03 | 0.14 | 0.63 | 0.05 | 0.29 |
| Brixton spatial anticipation | 0.20 | 0.20 | 0.10 | 0.24 | 0.58 | 0.26 | -0.49 |
| Ravens coloured matrices | − 0.06 | 0.04 | 0.08 | 0.21 | 0.84 | 0.08 | 0.03 |
| NonWord minimal pairs | 0.23 | 0.25 | 0.29 | − 0.03 | 0.25 | 0.80 | 0.08 |
| Word minimal pairs | 0.39 | 0.48 | 0.23 | 0.17 | 0.07 | 0.62 | − 0.01 |
| Circumlocution errors | 0.06 | − 0.05 | − 0.04 | 0.27 | 0.11 | 0.07 | 0.85 |
| Type/token ratio | 0.09 | 0.38 | 0.48 | − 0.47 | 0.19 | − 0.34 | 0.20 |
| Semantic errors | 0.42 | − 0.25 | − 0.07 | − 0.30 | 0.45 | 0.08 | 0.31 |
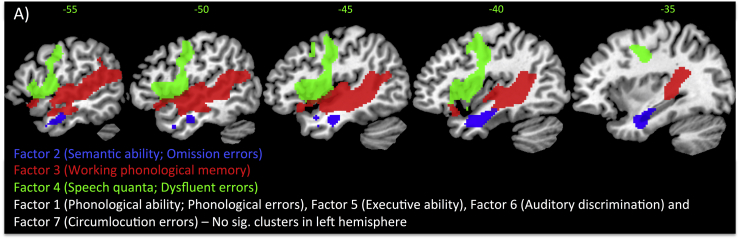
The omnibus PCA components were simultaneously entered for lesion analysis which included lesion volume, age and years in education as nuisance regressors. Fig. 2 shows the significant clusters thresholded at p < 0.005 voxel height, FWE-cluster corrected p < 0.05 (with the exception of the blue cluster which is FDR-cluster corrected p < 0.05). There were significant lesion correlates for three factors: phonological working memory, semantic ability and speech quanta. We employed a reduced threshold for the semantic factor, as this cluster has consistently been found in previous reports in the same location (Butler et al., 2014, Halai et al., 2017). The phonological working memory factor correlated positively with the superior temporal gyrus (STG)/MTG extending anteriorly into temporal pole (TP), posteriorly into Heschl's gyrus (HG), and supramarginal gyrus (SMG) and medially into the insula. The speech quanta factor correlated positively with preCG extending into the central and frontal opercular cortex. The semantic ability cluster was located in the anterior MTG bordering between TP, inferior temporal gyrus (ITG), temporal fusiform gyrus (TFG) and PHG. The remaining factors did not correlate significantly with tissue concentration.
Fig. 2.
Positive VBCM correlations for the omnibus PCA including cognitive-language assessments and naming errors. Significant clusters are [t-map (scale 2.7) thresholded at p < 0.005 voxel height, cluster corrected at family-wise error of p < 0.05, except Factor 2, which is cluster corrected at false discovery rate p < 0.05]. As omission errors has a negative loading with Factor 2, damage to this area (blue) results in more omissions. As dysfluency errors has a positive loading with Factor 4, intact tissue within this area (green) results in more dysfluency errors. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. Relationship to previous studies of naming errors
As noted in the Introduction few, if any, investigations have considered the full range of paraphasia types nor have they combined these results with detailed neuropsychological assessment. Two previous studies explored semantic and phonological errors, resulting in a different pattern of lesion correlates to those reported above (Mirman et al., 2015a, Mirman et al., 2015b, Walker et al., 2011). We tested (and confirmed) our hypotheses that these alternative outcomes reflected (a) the simpler error classification used by Walker et al.; and (b) the somewhat more limited range of background test employed by Mirman and colleagues. By way of prologue, we were able to replicate both sets of results in our independent sample of patients by restricting the error classification used and the breadth of neuropsychological assessment.
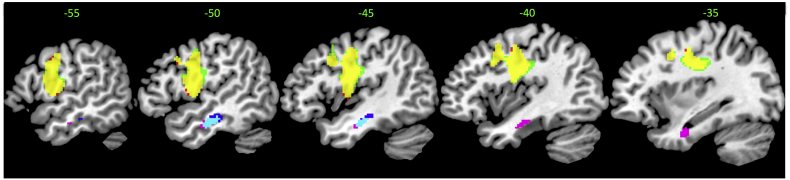
First, as described above, in the present study we included all paraphasias and utilised a data-driven method (PCA) to differentiate different clusters of error type. In contrast, Walker and colleagues used experimenter-derived definitions for phonologically-related (including sub categories of phonemic and formal errors) and semantically-related errors (consisting of sub categories of semantic, mixed and circumlocution errors). When we restricted our analyses to the same subset of paraphasias, we directly replicated the Walker et al. results: ‘phonological’ errors were found to correlate with preCG and central opercular cortex (p < 0.005 voxel height, FWE-cluster corrected p < 0.05); ‘semantic’ errors were correlated with MTG, with the edge of the cluster extending into the ITG, TFG and PHG (p = 0.005 voxel height, uncorrected voxel extent > 130) (see Supplementary Fig. 1 for details). Walker et al. also controlled for receptive semantic ability in a subsequent analysis. Accordingly, following their method, we also added a composite score of a synonym judgement (verbal) and Camel and Cactus Task (nonverbal) to the semantic error model. Again our results were almost identical, showing correlations within the MTG. Following Walker et al., we also added a control for receptive phonology (a composite score of word and nonword minimal pairs; PALPA 1 and 2) to the phonological error model. Again, like Walker et al., we found that adding this measure made little difference to the results, with ‘phonological’ errors still correlating with the integrity of preCG.
Supplementary Fig. 1.
Showing neural correlates for phonological and semantic errors with and without partial covariates as defined by Walker et al. (2011). The first result shows the overlap (yellow) between the neural correlates of phonological errors (red) and phonological errors with a partial covariate of receptive phonology (green). These results are thresholded at p < 0.005, cluster corrected at familywise error of p < 0.05. The second result shows the overlap (cyan) between the neural correlates of semantic errors (blue) and semantic errors with a partial covariate of comprehension (purple). These results are thresholded at p < 0.005, uncorrected voxel extent > 130.
More recently, Mirman et al. (2015a) and Mirman et al. (2015b), used PCA to align semantic and phonological errors with the patients' background language tests (but not the additional non-language cognitive assessments included in our broader background battery). Again, when we restricted our omnibus PCA only to the language assessments, phonological and semantic errors (leaving out the remaining assessments and paraphasia types), we replicated the same four factor solution reported by Mirman and colleagues (speech production, speech recognition, semantic recognition and semantic error components: see Table 4). Interestingly, when we re-integrated the cognitive measures from our assessment battery (but retained only phonological and semantic errors), the four factor solution that emerged remained similar, but the semantic errors were subsumed into an executive-cognitive factor (implying that these types of non-language assessment are important to include in aphasiological studies: cf. Brownsett et al., 2014, Geranmayeh et al., 2014, Lambon Ralph et al., 2010).
Table 4.
Showing the effect of including executive assessments on factors obtained in principal component analysis of language tests and naming errors performed in Mirman et al., 2015a, Mirman et al., 2015b. Tests that loaded strongly (> 0.5) with each factor are marked in bold.
To be explicit, these additional replication analyses are not a criticism of the results from Walker et al. and Mirman et al. Rather in a time when researchers are concerned about lack of replication, these analyses show that (a) the previous results can be directly replicated in an independent patient sample, tested on similar (though not identical) language assessments; and (b) that a potentially more detailed outcome can be found by (i) utilising a data-driven clustering of a wider range of paraphasias and (ii) an assessment battery that includes both language and non-language cognitive assessments.
4. Discussion
Both research and clinical practice for disorders of higher cognition rely on careful exploration of behavioural deficits and their lesion correlates. Ever since the seminal studies of the 19th century neurologists, diagnosing aphasia has relied on combining information on patients' language impairments with the pattern of paraphasias. Despite the fact that both deficits and paraphasias feature in diagnostic criteria, contemporary clinical neuroscience research has tended to focus only on the lesion correlates of language deficits, with minimal attention made to the pattern of patients' paraphasias. Utilising a data-driven approach, the present study was able to assimilate deficits, paraphasias and lesions into a single, unified model of chronic, post stroke aphasia.
To achieve a comprehensive model, we avoided selecting only a subset of paraphasia types and utilising experimenter-designed combinations of error. Instead, we included a large range of fifteen different error types and used principal component analysis (PCA) to derive a data-driven extraction of the underlying pattern of error coalescences. Specifically, we obtained five principal components underlying error patterns: omission errors; semantically-related responses; phonologically-related word and nonword responses (including partial fragments); dysfluent responses; and a combination of circumlocutions with mixed errors. In the second step, we aligned these errors with the patients' performance on a detailed language and cognitive assessment battery, utilising an additional PCA. This omnibus PCA revealed seven unique fused deficit + paraphasia factors: output phonology; semantics; phonological working memory; speech quanta; executive-cognitive skill; phonological (input) discrimination; and the production of circumlocution errors. As noted in the Introduction, there is a complex relationship between error types and language-cognitive deficits which can be mapped out using this data-driven approach. Specifically, circumlocution errors formed their own factor, independent of other error types and all underlying deficits. Phonological errors aligned simply with expressive phonological abilities. There was a similar one-to-one mapping between speech fluency and the generation of dysfluent naming errors. Other error types had a more complex relationship with the background neuropsychological results. For example, omission-type errors had a double loading with both semantic and phonological working memory factors, whilst the most complex relationship was found for semantically-related errors which loaded across four factors (phonological ability, speech quanta, executive-cognitive skills and circumlocution-type errors).
Three of these seven deficit + paraphasia components had unique lesion correlates: phonological working memory with the primary auditory region extending to inferior parietal areas; semantics with a cluster centred on the white matter in the anterior temporal region; and fluency with pre-central gyrus. These results fit very closely with previous symptom-lesion mapping studies that have focussed entirely on deficits alone (Butler et al., 2014, Halai et al., 2017) as well as other convergent neuroscience data on these primary language components. Specifically, the anterior temporal region is firmly associated with a key role in semantic representation (Lambon Ralph et al., 2017), with convergent data from PET (Mion et al., 2010), fMRI (Binder et al., 2009, Price, 2010, Vigneau et al., 2006, Visser et al., 2009), brain stimulation studies (Pobric et al., 2007, Pobric et al., 2010) and neuroanatomical-constrained dual-pathway models of language (Ueno et al., 2011). Omission errors loaded heavily into this same factor and were associated with ATL damage even when explored individually (see Fig. 1B), which is striking given that omissions are the most prevalent paraphasia in semantic dementia (Lambon Ralph et al., 2001, Woollams et al., 2008). Similarly with regards to phonological working memory, the left mid to posterior STG, MTG, STS and HG, as well as the underlying white matter (arcuate fasciculus portion of the dorsal language pathway) have been identified in various previous reviews of phonological processing (Hickok and Poeppel, 2007, Price, 2012, Vigneau et al., 2006, Wise et al., 2001). Finally, the neural structures related to the fluency factor were located within preCG, pars opercularis and superior insula dissecting the anterior part of the superior longitudinal fasciculus and lateral part of the frontal aslant tract (FAT). This finding is consistent with our previous investigation (Halai et al., 2017), coincides with a study showing that stimulation of the FAT resulted in speech inhibition (Kinoshita et al., 2014) and has also been implicated in reduced speech production in primary progressive aphasia (Catani et al., 2013).
In contrast to the simple relationships of phonological and dysfluency errors to their corresponding underlying language components, the relationship was most complex for semantic errors. Seminal cognitive neuropsychological studies noted that semantic errors might have multiple sources even within the language system (Morton and Patterson, 1980). This might explain why no alignment of semantic errors to patient deficits was found in two previous studies (Mirman et al., 2015a, Mirman et al., 2015b), and is directly compatible with the fact that we found semantic errors to load across multiple computational processes, suggesting that these errors may arise due to dysfunction of multiple core computations, including executive-cognitive processing. One additional piece of evidence in favour of the hypothesis that semantic errors can reflect damage to multiple language systems is the co-loading of semantic errors with mixed errors in the first PCA of the naming errors. This result suggested that mixed errors and semantic errors share some variance and therefore loaded into the same factor. The very nature of mixed errors indicates that this error type reflects contributions from, or dysfunction, across multiple language systems. Consistent with Morton and Patterson's observation, the full PCA likewise showed that semantic errors load across multiple factors – and thus it is logical that semantic and mixed errors load together in the PCA of errors.
We note that we only detected neural correlates for phonemic and omission errors when using the raw naming error values, which reduced to only the omission error factor when investigating the neural correlates of the factor scores of the error types (Fig. 1B and C). It is difficult to pin-point the cause for the lack of effect for other errors or factor scores but we suggest that, as the PCA model includes the effect of all errors simultaneously (as a multiple regression), it is much harder to detect effects unique to a given factor. For example, the fact that a cluster in the anterior temporal lobe for the omissions factor survives correction indicates that the integrity of this region is uniquely related to omissions over and above any other error component. As the region related to phonemic errors was no longer detected, we suggest that either one of the other factors shared some variance in the preCG (i.e. one could imagine that circumlocution errors share some features of articulation with is region) or we lacked power with the current dataset.
By employing a data driven methodology using principal component analysis, this study has achieved multiple aims including our key goal of fusing patient deficits and paraphasias into one unified model of chronic aphasia. In addition, we note that it also allowed us to include all types of naming errors rather than alternative approaches which (a) focus solely upon a subset of paraphasia types and (b) use experimenter-derived methods to combine across error types. In contrast, the PCA approach allows us to extract the underlying latent structure within all naming errors without pre-assigning errors to broad categories. One striking example of the benefits of this PCA approach is omission errors. Although one of the most prevalent error types in many different aphasic patient groups, this paraphasia type are commonly removed from analyses. This is understandable because, when dealing with omission errors in isolation, this error type – by definition – provides no information. By considering this error type alongside all the others, however, we have been able to establish when these errors coincide, their relationship to underlying language impairments (semantic and phonological skills) and their neural correlates (see above). In conclusion, the PCA data-driven approach has allowed us to triangulate language deficits, error types and lesion correlates in a single model for post-stroke aphasia. Given its success in this patient group, it seems likely that the same methodology could be used to investigate the nature and neural correlates of the many other multi-faceted disorders of high cognition and behaviour.
The following are the supplementary data related to this article.
Supplementary tables
Participant scores on the behavioural assessment battery. The values have been converted to % correct, if there is no upper limit, the max value within the group is used.
Funding
This research was supported by funding from the Rosetrees Trust, an ERC advanced grant (GAP: 670428 - BRAIN2MIND_NEUROCOMP) and an MRC (UK) programme grant to MALR (MR/J004146/1).
Acknowledgments
Acknowledgements
We are grateful to all the patients and carers for their continued support of our research programme. We thank Dr. Rebecca Butler for collecting part of the naming data analysed in this study and Dr Lauren Cloutman for her assistance in error classification.
Contributor Information
Ajay D. Halai, Email: ajay.halai@manchester.ac.uk.
Matthew A. Lambon Ralph, Email: matt.lambon-ralph@manchester.ac.uk.
References
- Baldo J.V., Katseff S., Dronkers N.F. Brain regions underlying repetition and auditory-verbal short-term memory deficits in aphasia: evidence from voxel-based lesion symptom mapping. Aphasiology. 2012;26(3–4):338–354. doi: 10.1080/02687038.2011.602391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo J.V., Arévalo A., Patterson J.P., Dronkers N.F. Grey and white matter correlates of picture naming: evidence from a voxel-based lesion analysis of the Boston Naming Test. Cortex. 2013;49(3):658–667. doi: 10.1016/j.cortex.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E., Wilson S.M., Saygin A.P., Dick F., Sereno M.I., Knight R.T., Dronkers N.F. Voxel-based lesion-symptom mapping. Nature Neuroscience. 2003;6(5):448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Berthier M.L. Poststroke aphasia: epidemiology, pathophysiology, and treatment. Drugs & Aging. 2005;22(2):163–182. doi: 10.2165/00002512-200522020-00006. [DOI] [PubMed] [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovsky A., Saygin A.P., Bates E., Dronkers N. Lesion correlates of conversational speech production deficits. Neuropsychologia. 2007;45(11):2525–2533. doi: 10.1016/j.neuropsychologia.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozeat S., Lambon Ralph M.A., Patterson K., Garrard P., Hodges J.R. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38(9):1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Brownsett S.L.E., Warren J.E., Geranmayeh F., Woodhead Z., Leech R., Wise R.J.S. Cognitive control and its impact on recovery from aphasic stroke. Brain. 2014;137(1):242–254. doi: 10.1093/brain/awt289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd M.A., Kortte K., Cloutman L., Newhart M., Gottesman R.F., Davis C.…Hillis A.E. The nature of naming errors in primary progressive aphasia versus acute post-stroke aphasia. Neuropsychology. 2010;24(5):581–589. doi: 10.1037/a0020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P.W., Shallice T. Thames Valley Test Company; Thurston, Suffolk: 1997. The Hayling and Brixton Tests. [Google Scholar]
- Butler R.A., Lambon Ralph M.A., Woollams A.M. Capturing multidimensionality in stroke aphasia: mapping principal behavioural components to neural structures. [Journal Article] Brain. 2014;137(12):3248–3266. doi: 10.1093/brain/awu286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Mesulam M.M., Jakobsen E., Malik F., Martersteck A., Wieneke C.…Rogalski E. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. [Journal Article] Brain. 2013;136(8):2619–2628. doi: 10.1093/brain/awt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutman L., Gottesman R., Chaudhry P., Davis C., Kleinman J.T., Pawlak M.…Hillis A.E. Where (in the brain) do semantic errors come from? Cortex; a journal devoted to the study of the nervous system and behavior. 2009;45(5):641–649. doi: 10.1016/j.cortex.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Ramsey L., Callejas A., Baldassarre A., Hacker Carl D., Siegel Joshua S.…Shulman Gordon L. Common behavioral clusters and subcortical anatomy in stroke. Neuron. 2015;85(5):927–941. doi: 10.1016/j.neuron.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp J., Howard D., Lambon Ralph M.A. More evidence for a continuum between phonological and deep dyslexia: novel data from three measures of direct orthography-to-phonology translation. Aphasiology. 2011;25(5):615–641. [Google Scholar]
- Damasio H., Damasio A. The anatomical basis of conduction aphasia. Brain. 1980;103(2):337–350. doi: 10.1093/brain/103.2.337. [DOI] [PubMed] [Google Scholar]
- Dronkers N.F., Wilkins D.P., Van Valin Jr R.D., Redfern B.B., Jaeger J.J. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92(1–2):145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Engelter S.T., Gostynski M., Papa S., Frei M., Born C., Ajdacic-Gross V.…Lyrer P.A. Epidemiology of aphasia attributable to first ischemic stroke: incidence, severity, fluency, etiology, and thrombolysis. Stroke. 2006;37(6):1379–1384. doi: 10.1161/01.STR.0000221815.64093.8c. [DOI] [PubMed] [Google Scholar]
- Geranmayeh F., Wise R.J.S., Mehta A., Leech R. Overlapping networks engaged during spoken language production and its cognitive control. The Journal of Neuroscience. 2014;34(26):8728–8740. doi: 10.1523/JNEUROSCI.0428-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halai A.D., Woollams A.M., Lambon Ralph M.A. Using principal component analysis to capture individual differences within a unified neuropsychological model of chronic post-stroke aphasia: revealing the unique neural correlates of speech fluency, phonology and semantics. Cortex. 2017;86:275–289. doi: 10.1016/j.cortex.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hodges J.R., Salmon D.P., Butters N. The nature of the naming deficit in Alzheimer's and Huntington's disease. Brain. 1991;114:1547–1558. doi: 10.1093/brain/114.4.1547. [DOI] [PubMed] [Google Scholar]
- Hope T.M.H., Seghier M.L., Leff A.P., Price C.J. Predicting outcome and recovery after stroke with lesions extracted from MRI images. NeuroImage: Clinical. 2013;2:424–433. doi: 10.1016/j.nicl.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies E., Patterson K., Jones R.W., Lambon Ralph M.A. Comprehension of concrete and abstract words in semantic dementia. Neuropsychology. 2009;23(4):492–499. doi: 10.1037/a0015452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E., Goodglass H., Weintraub S. Lea & Febiger; Philadelphia: 1983. Boston Naming Test. [Google Scholar]
- Kay J., Lesser R., Coltheart M. Lawrence Erlbaum Associates Ltd.; Hove, UK: 1992. Psycholinguistic Assessments of Language Processing in Aphasia. [Google Scholar]
- Kinoshita M., de Champfleur N., Deverdun J., Moritz-Gasser S., Herbet G., Duffau H. Role of fronto-striatal tract and frontal aslant tract in movement and speech: an axonal mapping study. Brain Structure and Function. 2014:1–14. doi: 10.1007/s00429-014-0863-0. [DOI] [PubMed] [Google Scholar]
- Lacey E.H., Skipper-Kallal L.M., Xing S., Fama M.E., Turkeltaub P.E. Mapping common aphasia assessments to underlying cognitive processes and their neural substrates. Neurorehabilitation and Neural Repair. 2017;31(5):442–450. doi: 10.1177/1545968316688797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph M.A., McClelland J.L., Patterson K., Galton C.J., Hodges J.R. No right to speak? The relationship between object naming and semantic impairment: neuropsychological evidence and a computational model. Journal of Cognitive Neuroscience. 2001;13:341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph M.A., Patterson K., Graham N., Dawson K., Hodges J.R. Homogeneity and heterogeneity in mild cognitive impairment and Alzheimer's disease: a cross-sectional and longitudinal study of 55 cases. Brain. 2003;126(11):2350–2362. doi: 10.1093/brain/awg236. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph M.A., Snell C., Fillingham J.K., Conroy P., Sage K. Predicting the outcome of anomia therapy for people with aphasia post CVA: both language and cognitive status are key predictors. Neuropsychological Rehabilitation. 2010;20(2):289–305. doi: 10.1080/09602010903237875. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph M.A., Jefferies E., Patterson K., Rogers T.T. The neural and computational bases of semantic cognition. Nat Rev Neurosci. 2017;18(1):42–55. doi: 10.1038/nrn.2016.150. [DOI] [PubMed] [Google Scholar]
- Mion M., Patterson K., Acosta-Cabronero J., Pengas G., Izquierdo-Garcia D., Hong Y.T.…Nestor P.J. Vol. 133. 2010. What the left and right anterior fusiform gyri tell us about semantic memory. [DOI] [PubMed] [Google Scholar]
- Mirman D., Chen Q., Zhang Y., Wang Z., Faseyitan O.K., Coslett H.B., Schwartz M.F. Neural organization of spoken language revealed by lesion-symptom mapping. [Article] Nat. Commun. 2015;6 doi: 10.1038/ncomms7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D., Zhang Y., Wang Z., Coslett H.B., Schwartz M.F. The ins and outs of meaning: Behavioral and neuroanatomical dissociation of semantically-driven word retrieval and multimodal semantic recognition in aphasia. Neuropsychologia. 2015;0 doi: 10.1016/j.neuropsychologia.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J., Patterson K. A new attempt at an interpretation, or, an attempt at a new interpretation. In: Coltheart M., editor. Deep dyslexia. Routledge & Kegan Paul; London: 1980. pp. 91–118. [Google Scholar]
- Mummery C.J., Patterson K., Price C.J., Ashburner J., Frackowiak R.S.J., Hodges J.R. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Annals of Neurology. 2000;47(1):36–45. [PubMed] [Google Scholar]
- Phan T.G., Donnan G.A., Wright P.M., Reutens D.C. A digital map of middle cerebral artery infarcts associated with middle cerebral artery trunk and branch occlusion. Stroke. 2005;36(5):986–991. doi: 10.1161/01.STR.0000163087.66828.e9. [DOI] [PubMed] [Google Scholar]
- Pobric G., Jefferies E., Lambon Ralph M.A. Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proceedings of the National Academy of Sciences. 2007;104(50):20137–20141. doi: 10.1073/pnas.0707383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G., Jefferies E., Lambon Ralph M.A. Amodal semantic representations depend on both anterior temporal lobes: evidence from repetitive transcranial magnetic stimulation. Neuropsychologia. 2010;48(5):1336–1342. doi: 10.1016/j.neuropsychologia.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Price C.J. The anatomy of language: a review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences. 2010;1191(1):62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Price C.J. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J.C. H. K. Lewis; London: 1962. Advanced Progressive Matrices, Set II. [Google Scholar]
- Schwartz M.F., Dell G.S., Martin N., Gahl S., Sobel P. A case-series test of the interactive two-step model of lexical access: evidence from picture naming. Journal of Memory and Language. 2006;54(2):228–264. doi: 10.1016/j.jml.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.F., Kimberg D.Y., Walker G.M., Faseyitan O., Brecher A., Dell G.S., Coslett H.B. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. 2009;Vol. 132 doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.F., Faseyitan O., Kim J., Coslett H.B. The dorsal stream contribution to phonological retrieval in object naming. Brain. 2012;135(12):3799–3814. doi: 10.1093/brain/aws300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L., Ramlackhansingh A., Crinion J.T., Leff A.P., Price C.J. Lesion identification using unified segmentation-normalisation models and fuzzy clustering. NeuroImage. 2008;41:1253–1266. doi: 10.1016/j.neuroimage.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinburn K., Baker G., Howard D. Psychology Press; New York: 2005. CAT: The Comprehensive Aphasia Test. [Google Scholar]
- Tyler L.K., Marslen-Wilson W., Stamatakis E.A. Dissociating neuro-cognitive component processes: voxel-based correlational methodology. Neuropsychologia. 2005;43(5):771–778. doi: 10.1016/j.neuropsychologia.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Ueno T., Saito S., Rogers Timothy T., Ralph Lambon, Matthew A. Lichtheim 2: synthesizing aphasia and the neural basis of language in a neurocomputational model of the dual dorsal-ventral language pathways. Neuron. 2011;72(2):385–396. doi: 10.1016/j.neuron.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Vigneau M., Beaucousin V., Hervé P.Y., Duffau H., Crivello F., Houdé O.…Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30(4):1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Visser M., Jefferies E., Lambon Ralph M.A. Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. Journal of Cognitive Neuroscience. 2009;22(6):1083–1094. doi: 10.1162/jocn.2009.21309. [DOI] [PubMed] [Google Scholar]
- Walker G.M., Schwartz M.F., Kimberg D.Y., Faseyitan O., Brecher A., Dell G.S., Coslett H.B. Support for anterior temporal involvement in semantic error production in aphasia: new evidence from VLSM. Brain and Language. 2011;117(3):110–122. doi: 10.1016/j.bandl.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington E.K. The selective impairment of semantic memory. Quarterly Journal of Experimental Psychology. 1975;27(4):635–657. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- Wechsler D.A. Psychological Corporation; New York: 1987. Wechsler Memory Scale—Revised Manual. [Google Scholar]
- Wilke M., de Haan B., Juenger H., Karnath H.-O. Manual, semi-automated, and automated delineation of chronic brain lesions: a comparison of methods. NeuroImage. 2011;56(4):2038–2046. doi: 10.1016/j.neuroimage.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Wise R.J.S., Scott S.K., Blank C., Mummery C.J., Murphy K., Warburton E.A. Separate neural subsystems within ‘Wernicke's area’. Brain. 2001;124(1):83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
- Woollams A.M., Cooper-Pye E., Hodges J.R., Patterson K. Anomia: a doubly typical signature of semantic dementia. Neuropsychologia. 2008;46(10):2503–2514. doi: 10.1016/j.neuropsychologia.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Yourganov G., Smith K.G., Fridriksson J., Rorden C. Predicting aphasia type from brain damage measured with structural MRI. Cortex. 2015;73:203–215. doi: 10.1016/j.cortex.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Kimberg D.Y., Coslett H.B., Schwartz M.F., Wang Z. Multivariate lesion-symptom mapping using support vector regression. Human brain mapping. 2014;35(12):5861–5876. doi: 10.1002/hbm.22590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables
Participant scores on the behavioural assessment battery. The values have been converted to % correct, if there is no upper limit, the max value within the group is used.