Abstract
An improved understanding of the endocannabinoid system has provided new avenues of drug discovery and development toward the management of pain and other behavioral maladies. Exogenous cannabinoid type 1 (CB1) receptor agonists such as Δ9-tetrahydrocannabinol are increasingly used for their medicinal actions; however, their utility is constrained by concern regarding abuse-related subjective effects. This has led to growing interest in the clinical benefit of indirectly enhancing the activity of the highly labile endocannabinoids N-arachidonoylethanolamine [AEA (or anandamide)] and/or 2-arachidonoylglycerol (2-AG) via catabolic enzyme inhibition. The present studies were conducted to determine whether such actions can lead to CB1 agonist–like subjective effects, as reflected in CB1-related discriminative stimulus effects in laboratory subjects. Squirrel monkeys (n = 8) that discriminated the CB1 full agonist AM4054 (0.01 mg/kg) from vehicle were used to study, first, the inhibitors of fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase (MGL) alone or in combination [FAAH (URB597, AM4303); MGL (AM4301); FAAH/MGL (JZL195, AM4302)] and, second, the ability of the endocannabinoids AEA and 2-AG to produce CB1 agonist–like effects when administered alone or after enzyme inhibition. Results indicate that CB1-related discriminative stimulus effects were produced by combined, but not selective, inhibition of FAAH and MGL, and that these effects were nonsurmountably antagonized by low doses of rimonabant. Additionally, FAAH or MGL inhibition revealed CB1-like subjective effects produced by AEA but not by 2-AG. Taken together, the present data suggest that therapeutic effects of combined, but not selective, enhancement of AEA or 2-AG activity via enzyme inhibition may be accompanied by CB1 receptor–mediated subjective effects.
Introduction
Accumulating evidence confirming the medicinal effects of the cannabinoid receptor type 1 (CB1) partial agonist Δ9-tetrahydrocannabinol (Δ9-THC), the principal psychoactive constituent of marijuana, has led to the current availability of medicinal marijuana in the majority of states in the United States. Notwithstanding such growing popularity, concerns regarding its safety, especially during adolescence (Gruber et al., 2014), cloud the further development of Δ9-THC and other exogenous CB1 agonists as pharmacotherapies. An alternative approach to the use of exogenous CB1 agonists for medicinal purposes may lie in increasing the activity of endogenous CB1 receptor ligands, for example, the endocannabinoids N-arachidonoylethanolamine [AEA (anandamide)] and 2-arachidonoylglycerol (2-AG). Although AEA and 2-AG are highly labile, complicating their in vivo evaluation, each has been reported to produce CB1-mediated behavioral effects. For example, the infusion of 2-AG into the dorsolateral periaqueductal gray has been reported to produce CB1-mediated increases in anxiolytic-like behavior (Almeida-Santos et al., 2013; Gobira et al., 2016). Furthermore, both AEA and 2-AG, like Δ9-THC, maintain intravenous self-administration behavior in, respectively, monkeys and rats, presumably due to rapid delivery to relevant brain regions permitted by intravenous administration (Justinova et al., 2005, 2011; DeLuca et al., 2014). Findings such as these suggest that AEA and 2-AG can independently produce CB1 receptor–mediated behavioral effects.
In addition to the CB1-mediated actions of exogenous AEA and 2-AG, their effects also have been examined indirectly by inhibiting their rapid enzymatic degradation in vivo. Compounds that inhibit the activity of fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MGL) can increase the concentrations of, respectively, AEA and 2-AG at CB1 receptors and, after single or combined administration, have been shown to produce varying levels of effect in the CB1 tetrad test (Little et al., 1988; Schlosburg et.al., 2014; Ghosh et al., 2015; Wilkerson et al., 2017). Thus, dual FAAH/MGL inhibition was found to produce full effects on all measures (antinociception, hypomotility, hypothermia, catalepsy), whereas selective inhibition of either FAAH or MGL has been reported to produce only antinociception in rodent models of acute and/or chronic pain (Ahn et al., 2011; Ignatowska-Jankowska et al., 2014). FAAH inhibitors additionally have been reported to modulate addiction-related behavior (Justinova et al., 2015; Wilkerson et al., 2017) and, in assays of emotional control, mood (Varvel et al., 2007; Rossi et al., 2010) and post-traumatic stress (Zer-Aviv and Akirav, 2016), have produced results supporting their further development as anxiolytic and antidepressant drugs (Gaetani et al., 2009).
Although the potential medicinal benefits of modulating endocannabinoid activity have received considerable attention, there is relatively little information on possible adverse effects of such actions, including CB1-related abuse liability. Along these lines, drug discrimination procedures have been used to study CB1 receptor–mediated discriminative stimulus effects, which can serve as a valid behavioral marker of subjective effects of CB1 agonists (e.g., Δ9-THC) that are linked to abuse liability. The discriminative stimulus effects of directly acting CB1 receptor agonists have been extensively evaluated in both rodent and nonhuman primate species (e.g., McMahon, 2006; Vann et al., 2009; Kangas et al., 2013; Järbe et al., 2014). However, there have been few comparable studies of endocannabinoids to date. Solinas et al. (2007) initially reported that the metabolically stable AEA analog methanandamide or coadministration of the FAAH inhibitor URB597 and AEA fully substituted for Δ9-THC in Δ9-THC–trained rats—findings that have been supported in subsequent studies in mice and nonhuman primates (Long et al., 2009; Stewart and McMahon, 2011; Wiley et al., 2014). The discriminative stimulus effects of 2-AG, on the other hand, are even less understood. Wiley et al. (2014) found that 2-AG, in the presence or absence of an MGL inhibitor, failed to substitute for Δ9-THC, whereas partial substitution was produced by an MGL inhibitor (JZL184) in mice and by coadministration of FAAH (URB597) and MGL (JZL184) inhibitors in rats. Owens et al. (2016) further reported that the dual FAAH-MGL inhibitor SA-57 can be readily trained as a discriminative stimulus in mice and substitutes for synthetic cannabinoid agonists in an antagonist-sensitive manner. Of interest, selective MGL, but not FAAH, inhibitors fully substituted for the training stimulus, suggesting that the CB1 receptor–mediated effects of AEA and 2-AG differ in a fundamental, though as yet unclear, manner.
The present research was conducted to further examine the ability of endocannabinoid action to produce CB1 receptor–mediated discriminative stimulus effects. Monkeys trained to discriminate the CB1 full agonist AM4054 from vehicle were used to evaluate the agonist-like effects of nonselective enzyme inhibitors, selective FAAH and MGL inhibitors alone and in combination, and to compare the ability of the endocannabinoids AEA and 2-AG to produce CB1 agonist–like effects alone or after enzyme inhibition.
Materials and Methods
Subjects
Eight adult male squirrel monkeys (Saimiri sciureus) served in the present studies. Subjects were housed individually in a temperature- and humidity-controlled vivarium with a 12-hour light/dark cycle (lights on at 7:00 AM). This facility is licensed by the US Department of Agriculture and complies with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (http://www.ncbi.nlm.nih.gov/books/n/nap12910/pdf). All procedures in the present studies were approved by the McLean Hospital Institutional Animal Care and Use Committee. Throughout the present studies, all subjects were maintained at approximate free-feeding weights by postsession access to a nutritionally balanced diet of high-protein banana-flavored biscuits (Purina Monkey Chow, St. Louis, MO) and had unlimited access to water in their home cage. In addition, they were provided with fresh fruit and environmental enrichment daily. Four subjects (31, 101, 103, and 115) had served in cannabinoid drug discrimination studies prior to the present experiments; the remaining subjects (2, 88, 113, and 134) had previously served in experiments examining other psychoactive drugs (e.g., opioids, monoaminergic stimulants) but, prior to the present studies, had not received drug treatments for at least 2 months. Additionally, three subjects (88, 113, and 134) were prepared with intravenous catheters (Herd et al., 1969) for studies of the endocannabinoids AEA and 2-AG.
Apparatus
Subjects were seated in a Plexiglas chair (Spealman et al., 1977; Kangas et al., 2013) within a ventilated light- and sound-attenuating enclosure facing an intelligence panel equipped with two response levers, 6 cm left and right of center. Each lever press with a force of at least 0.25 N closed a microswitch, produced an audible relay click, and was recorded as a response. Red stimulus lights were positioned 10 cm above each lever. Prior to each session, a shaved portion of the tail of each subject was coated with electrode paste and placed under brass electrodes for the delivery of brief, low-intensity shock stimuli (see below). All experimental events and data collection were controlled via Med Associates (St. Albans, VT) operating software and interfacing hardware.
Experimental Procedures
CB1 Discrimination Training Procedure.
Experimental sessions were conducted daily (Monday to Friday). Initially, subjects were trained to respond on either lever to terminate visual stimuli associated with the delivery of a brief, low-intensity shock stimulus (200 ms; 3 mA; see above). Next, subjects were trained to discriminate between the injection of the CB1 agonist AM4054 (0.01 mg/kg, i.m.; Kangas et al., 2013; Järbe et al., 2016) and vehicle using two-lever drug discrimination procedures. Briefly, one response lever was designated as the drug (AM4054) lever and the other as the vehicle lever. Assignment remained the same for each subject throughout the study but was counterbalanced across subjects. AM4054 or vehicle was administered intramuscularly 50 minutes prior to each training session. Training sessions began with a 10-minute time-out period during which all lights were extinguished and responding had no programmed consequences. After the time-out period, two red stimulus lights above each lever were illuminated and completion of 10 consecutive responses (FR10) on the injection-associated (correct) lever extinguished all stimulus lights and initiated a 50-second time out. Responses on the other (incorrect) lever reset the FR requirement. Shock delivery was scheduled to occur every 10 seconds until either the FR10 was completed on the correct lever or 30 seconds elapsed, whichever came first. Each presentation of the FR schedule constituted a trial, and sessions were composed of 20 trials. A double-alternation injection schedule (i.e., drug-drug-vehicle-vehicle) was used throughout training, with a third drug or vehicle training session arranged intermittently to avoid associations based on the periodicity of the double alternation schedule.
CB1 Discrimination Testing Procedures.
Drug tests for substitution were conducted only when the first FR10 was completed on the injection-appropriate lever and overall discrimination performance was at least 90% accurate for four of the last five sessions and in the immediately preceding session. Test sessions differed procedurally from training sessions in three ways. First, 10 consecutive responses on either the drug or vehicle lever extinguished the stimulus lights and initiated the 50-second time-out. Second, cumulative dosing procedures were used to study the effects of a range of doses of each drug, permitting the determination of dose-response relationships for the discriminative stimulus effects of drugs that substituted for the training drug. Test sessions consisted of four components of 10 trials, with each component beginning with a 30-minute time-out period, and generally were completed within 2.5 hours. This procedure permitted the study of up to four incremental doses of a drug delivered at the onset of sequential time-out periods of a single test session (Spealman, 1985; Lamb et al., 2000; Kangas et al., 2013). The effects of five or more doses were determined by administering overlapping ranges of cumulative doses over different test sessions. For each drug except AM4301, doses ranged up to those that fully substituted for the training drug or produced a ≥50% decrease in response rate from control values. (AM4301 did not appreciably decrease response rates at the highest doses studied.) Third, no shock deliveries were scheduled during test sessions so as to preclude their possible influences on performance. All other experimental contingencies were identical.
Discriminative Stimulus Effects of Endocannabinoids.
Five sets of experiments were conducted in the present study. First, the effects of intramuscular injections of AEA (0.3–32 mg/kg) and 2-AG (0.3–18 mg/kg) were studied in a group of four subjects; the effects of cumulative doses of 2-AG (0.3–18 mg/kg, i.v.) also were evaluated in three subjects. Inasmuch as both compounds are rapidly metabolized in vivo (Willoughby et al., 1997; Savinainen et al., 2001; Stewart and McMahon, 2011), the drug pretreatment time preceding test components was shortened from 30 to 10 minutes. Experiments to determine the effects of intravenous AEA also were initiated; however, as described below in Results, these experiments were discontinued due to adverse effects of the highest dose of AEA (32 mg/kg) in the absence of CB1-related discrimination in the first subject studied.
Discriminative Stimulus Effects of FAAH and MGL Inhibitors.
Next, studies were conducted to examine the effects of five enzyme inhibitors previously characterized in in vitro studies of their CB1 receptor affinity and their potency in inhibiting both FAAH and MGL (Table 1) (Kathuria et al., 2003; Long et al., 2009). These included enzyme inhibitors that were designated as follows: 1) FAAH selective [URB597 (0.3–5.6 mg/kg); AM4303 (0.3–10 mg/kg)]; 2) MGL selective [AM4301 (0.3–10 mg/kg)]; and 3) FAAH/MGL [JZL195 (0.1–5.6 mg/kg); AM4302 (0.1–5.6 mg/kg)].
TABLE 1.
IC50 values, selectivity ratio, and CB1 receptor affinity (Ki) for each compound studied
Values were derived for URB597 from Kathuria et al. (2003), and for JZL195 from Long et al. (2009). Values were obtained for AM4303, AM4301, and AM4302 from S.O.A., L.J., V.G.S., Y.L., S.P.N., and A.M. from in vitro data using rat and/or human enzyme assays following previously described procedures (Alapafuja et al., 2012; see also Makriyannis et al., 2016; Parker et al., 2016).
| Compound | rFAAH IC50 (nM) | hMGL IC50 (nM) | FAAH/MGL Selectivity Ratio | CB1 Ki |
|---|---|---|---|---|
| URB597 | 4.6 ± 1.6 | >30,000 | >100,000 | >300,000 |
| AM4303 | 1.92 ± 0.24 | >10,000 | >5208 | >1000 |
| AM4301 | 4920 ± 1600 | 10.65 ± 2.5 | <0.01 | >1000 |
| JZL195 | 2 | 4 | 0.5 | >20,000 |
| AM4302 | 31.2 ± 3.8 | 41.9 ± 3.3 | 0.74 | 940 |
Data are the mean ± S.E.M., unless otherwise indicated. hMGL, human MGL; Ki, inhibition constant; rFAAH, rat FAAH.
Discriminative Stimulus Effects of Endocannabinoids After Enzyme Inhibition.
In a third set of experiments, the effects of the endocannabinoids AEA and 2-AG were redetermined after pretreatment with selective (URB597, AM4303, AM4301) and nonselective enzyme inhibitors (JZL195 and AM4302). Based upon previous studies of the time course of FAAH inhibition in rats and monkeys (Fegley et al., 2005; Justinova et al., 2008, Long et al., 2009), all enzyme inhibitors were administered 60 minutes prior to treatment with AEA or 2-AG. Cumulative doses of the endocannabinoids were administered 10 minutes prior to each test component.
Discriminative Stimulus and Time Course Effects of Selective Enzyme Inhibitor Combinations.
Additional studies with URB597, AM4303, and AM4301 were conducted to determine the lowest doses of FAAH- and MGL-selective inhibitors that, when combined, would fully mimic the discriminative stimulus effects of AM4054. Thus, in the presence of 1.0 mg/kg URB597 administered 60 minutes prior to the test session, the effects of cumulative doses of AM4301 (0.03–1.0 mg/kg) delivered 30 minutes prior to each test component were studied to determine the lowest effective dose of AM4301. Next, the lowest effective dose of AM4301 (1.0 mg/kg) was administered 60 minutes before sessions in which cumulative doses of either AM4303 (0.003–0.1 mg/kg) or URB597 (0.01–0.3 mg/kg) were administered 30 minutes prior to each test component. Finally, a time course function for the duration of action of the dose combination of 1.0 mg/kg AM4301 and 0.3 mg/kg URB597 was determined by conducting test sessions 15, 60, 240, and 480 minutes after their intramuscular administration.
Antagonism of CB1 Discriminative Stimulus Effects of Selective Enzyme Inhibitor Combinations.
A last set of experiments was conducted to examine antagonism of the CB1-related discriminative stimulus effects resulting from combined FAAH (URB597) and MGL (AM4301) enzyme inhibition. In these experiments, doses of the selective CB1 inverse agonist/antagonist SR141716A (rimonabant; 0.003–0.3 mg/kg) were administered 60 minutes prior to sessions in which either a fixed dose of AM4301 was given before cumulative dosing with URB597 (0.01–3.2 mg/kg) or, alternatively, a fixed dose of URB597 was given before cumulative dosing with AM4301 (0.03–3.2 mg/kg). Pretreatment times were the same as those described above.
Data Analysis
In the present studies, the two primary dependent measures were the allocation of responding to the AM4054-associated lever, expressed as the percentage responding on the CB1-associated lever, and overall response rate. The percentage responding on the CB1-associated lever was calculated by dividing the number of responses on the AM4054-associated lever by the total number of responses. Response rate was calculated by dividing the total number of responses on both levers by the total session time (excluding all time-out periods). Doses of drugs were considered to substitute fully when the subject responded on the AM4054-associated lever >90% and response rates were >0.2 responses/s.
Drugs
2-AG, AEA, AM4054, AM4301, AM4302, AM4303, JZL195, and URB597 were synthesized for these studies by S.O.A., L.J., V.G.S., Y.L., S.P.N., and A.M. in the Center for Drug Discovery at Northeastern University (Boston, MA). Rimonabant was provided by the National Institute on Drug Abuse Drug Supply Program (Rockville, MD). All drugs usually were prepared for administration in a 20:20:60 mixture of 95% ethanol, Tween-80, and saline. When concentrations >10 mg/ml were necessary, drugs were dissolved in dimethylsulfoxide. Drug solutions were refrigerated and protected from light. Injections of drug or vehicle were prepared in volumes of 0.3 ml/kg body weight or less and administered intramuscularly in calf or thigh muscle and intravenously through the venous access ports.
Results
CB1 Discrimination.
All subjects acquired the drug discrimination in approximately 30–60 sessions. Throughout subsequent control sessions, injections of the training dose of 0.01 mg/kg AM4054 produced an average of >99% responding on its associated lever. Injections of vehicle produced <1% responding on the AM4054-associated lever. Response rates after intramuscular training doses of AM4054 were somewhat lower than after vehicle administration in all subjects, with group averages of 3.1 ± 0.6 and 3.6 ± 0.7 responses/s, respectively (mean ± S.E.M.). This small (<20%) difference in response rate was evident at the outset of training and remained constant throughout the present studies.
Discriminative Stimulus Effects of Endocannabinoids.
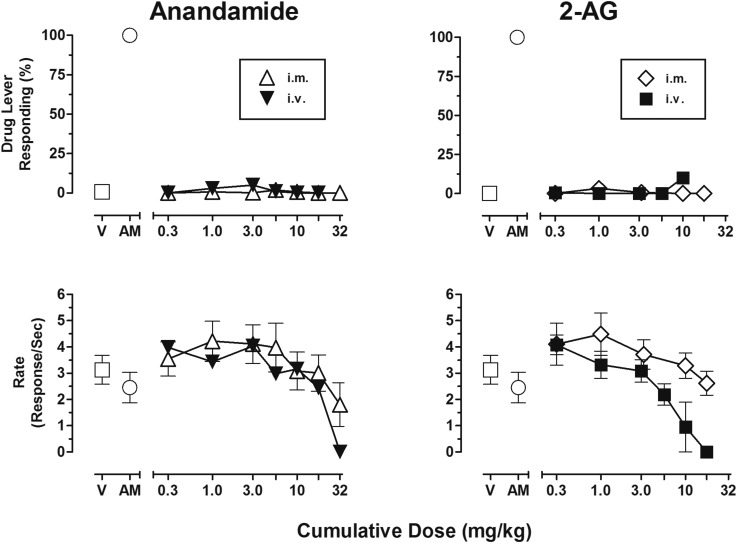
As shown in the top panels of Fig. 1, neither cumulative intramuscular doses of the endocannabinoids 2-AG or AEA up to 18 and 32 mg/kg, respectively, nor cumulative intravenous doses of 2-AG up to 18 mg/kg engendered responding on the AM4054-associated lever. The effects of cumulative intravenous doses of AEA up to 32 mg/kg were studied in one subject and, similarly, produced no responding on the CB1-associated lever. However, the subject collapsed immediately after the cumulative intravenous dose of 32 mg/kg AEA, most likely due to non-CB1 mechanisms—perhaps vanniloid-1 receptor activation (Panlilio et al., 2009)—and further studies were not conducted in other subjects to avoid similar adverse events. As shown in the bottom panels of Fig. 1, the effects of intramuscular AEA on response rate were dose dependent: cumulative doses below 32 mg/kg were within the range of control values or produced increases in response rate, whereas the highest cumulative dose of 32 mg/kg produced, on average, an approximately 25% decrease in responding after intramuscular administration and abolished responding after intravenous injection. As with AEA, response rates after most cumulative doses of 2-AG (0.3–18 mg/kg, i.m. or i.v.) were either within the range of control values or only increased; however, the intravenous injection of 18 mg/kg 2-AG completely abolished responding and produced a short-lived episode of syncope in at least one subject.
Fig. 1.
(Left panels) Dose-effect functions for AEA administered either intramuscularly (open triangle, n = 4) or intravenously (closed inverted-triangle, n = 1) in subjects trained to discriminate 0.01 mg/kg AM4054 from vehicle. Abscissae, cumulative dose, log scale; ordinate, percentage of responses on the AM4054-associated lever (top left panel), response rate (bottom left panel). Symbols left of the abscissae break indicate performance during vehicle (V) and AM4054 (AM) control sessions. Points represent averages (±S.E.M.) for the groups of subjects. (Right panels) Dose-effect functions for 2-AG, administered either intramuscularly (open diamond, n = 4) or intravenously (closed square, n = 3) in subjects trained to discriminate 0.01 mg/kg AM4054 from vehicle. Abscissae, cumulative dose, log scale; ordinate, percentage of responses on the AM4054-associated lever (top left panel), response rate (bottom left panel). Symbols left of the abscissae break indicate performance during V and AM control sessions. Points represent averages (±S.E.M.) for the groups of subjects.
Discriminative Stimulus Effects of FAAH and MGL Inhibitors.
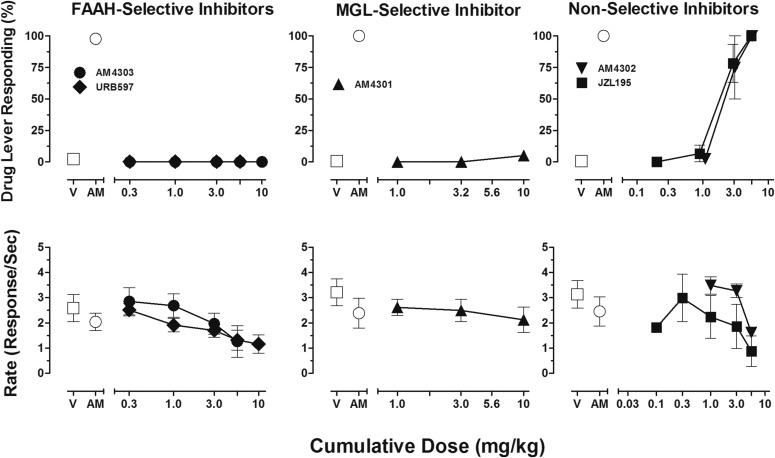
Figure 2 presents mean data for responding on the CB1-associated lever (Fig. 2, top panels) and response rate (Fig. 2, bottom panels) after cumulative doses of selective and nonselective enzyme inhibitors. Selective inhibitors including the FAAH inhibitors AM4303 and URB597 (Fig. 2, left panels) and the MGL inhibitor AM4301 (Fig. 2, middle panels) did not substitute for AM4054 but, excepting AM4301, produced a >50% decrease in response rate after the highest cumulative dose of 10 mg/kg. The MGL inhibitor AM4301 did not alter responding but was available in limited supply, precluding the evaluation of cumulative doses >10 mg/kg. In contrast, both of the FAAH/MGL inhibitors AM4302 and JZL195 (Fig. 2, right panels) produced dose-dependent AM4054-related discriminative stimulus effects and fully substituted after the cumulative dose of 5.6 mg/kg. Additionally, both AM4302 and JZL195 produced dose-related decreases in responding, with an approximately 34% and 64% reduction in response rates, respectively, after the cumulative dose of 5.6 mg/kg.
Fig. 2.
(Left panels) Dose-effect functions for the selective FAAH inhibitors URB597 (closed diamond, n = 3) and AM4303 (closed circle, n = 3) in subjects trained to discriminate 0.01 mg/kg AM4054 from vehicle. Abscissae, cumulative dose, log scale; ordinate, percentage of responses on the AM4054-associated lever (top panel), response rate (bottom panel). Symbols left of abscissae break indicate performance during vehicle (V) and AM4054 (AM) control sessions. Points represent averages (±S.E.M.) for the groups of subjects. (Middle panels) Dose-effect functions for the selective MGL-inhibitor AM4301 (closed triangle, n = 4) in subjects trained to discriminate 0.01 mg/kg AM4054 from vehicle. Abscissae, cumulative dose, log scale; ordinate, percentage of responses on the AM4054-associated lever (top panel), response rate (bottom panel). Symbols left of abscissae break indicate performance during V and AM control sessions. Points represent averages (±S.E.M.) for the groups of subjects. (Right panels) Dose-effect functions for nonselective FAAH/MGL inhibitors AM4302 (closed inverted triangle, n = 4) and JZL195 (closed square, n = 3) in subjects trained to discriminate 0.01 mg/kg AM4054 from vehicle. Abscissae, cumulative dose, log scale; ordinate, percentage of responses on the AM4054-associated lever (top right panel), response rate (bottom right panel). Symbols left of abscissae break indicate performance during V and AM control sessions. Points represent averages (±S.E.M.) for the groups of subjects.
Discriminative Stimulus Effects of Endocannabinoids After Enzyme Inhibition.
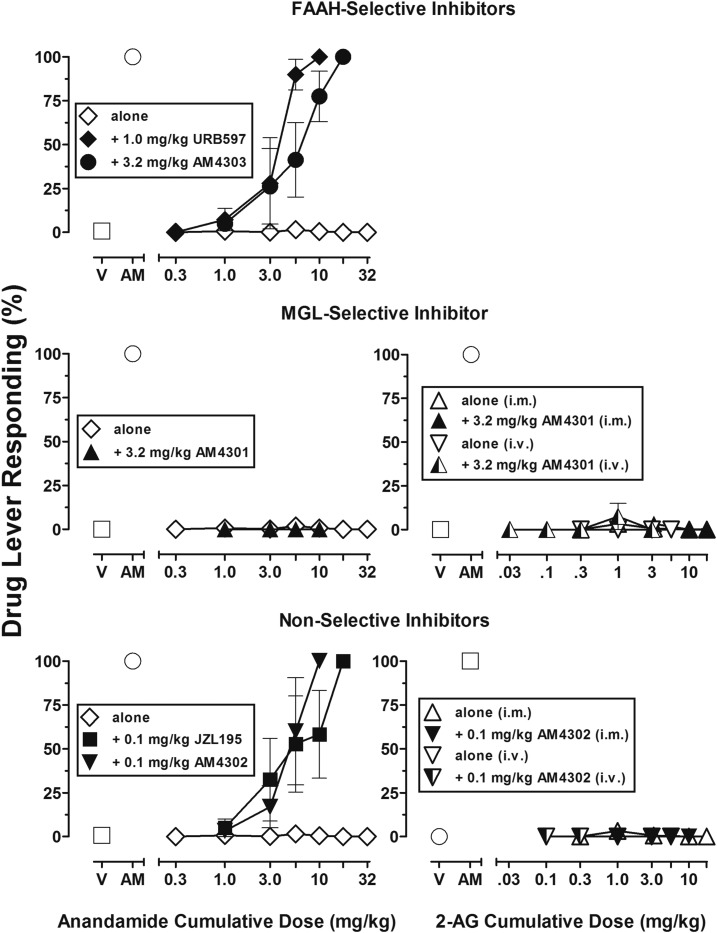
The left panels of Fig. 3 present mean data for responding on the CB1-associated lever after cumulative intramuscular doses of AEA alone (Fig. 3, open diamonds) and after intramuscular treatment with either FAAH, MGL, or FAAH/MGL enzyme inhibitors. Exogenously administered AEA after treatment with the FAAH-selective inhibitors (URB597 and AM4303) (Fig. 3, top panel) produced dose-dependent cannabimimetic discriminative stimulus effects. Full substitution was observed in all subjects after a cumulative dose of 10 mg/kg AEA when pretreated with a dose of 1.0 mg/kg URB597, and 18 mg/kg AEA when pretreated with a dose of 3.2 mg/kg AM4303. Likewise, both nonselective FAAH/MGL inhibitors (AM4302 and JZL195) (Fig. 3, bottom left panel) also disclosed dose-related cannabimimetic discriminative stimulus effects of exogenously administered AEA. Thus, after treatment with 0.1 mg/kg AM4302 and JZL195, full substitution for AM4054 occurred in all subjects after cumulative doses of, respectively, 10 and 18 mg/kg AEA. In contrast, pretreatment with the MGL-selective inhibitor AM4301 (3.2 mg/kg) failed to alter the effects of AEA up to a cumulative dose of 10 mg/kg, which, after pretreatment, produced an approximately 50% decrease in response rate (data not shown).
Fig. 3.
(Top panel) Dose-effect functions for AEA alone (open diamonds, n = 4) and after pretreatment with the selective FAAH inhibitors URB597 (1.0 mg/kg; closed diamond, n = 4) and AM4303 (3.2 mg/kg; closed circle, n = 4) in subjects trained to discriminate 0.01 mg/kg AM4054 from vehicle. Abscissae, cumulative dose, log scale; ordinate, percentage of responses on the AM4054-associated lever. Symbols left of abscissae break indicate performance during Vehicle (V) and AM4054 (AM) control sessions. Points represent averages (±S.E.M.) for the groups of subjects. (Middle panels) Dose-effect functions for AEA (left middle panel) and 2-AG (right panel) administered alone intramuscularly (AEA; open diamond, n = 4; 2-AG; open triangle; n = 4), alone intravenously (2-AG; open inverted-diamond, n = 3), or after pretreatment with the selective MGL inhibitor AM4301 (3.2 mg/kg) in subjects trained to discriminate 0.01 mg/kg AM4054 from V. After pretreatment with AM4301, AEA was administered intramuscularly (left middle panel; closed triangle, n = 3) and 2-AG was administered either intramuscularly (right middle panel; closed triangle, n = 3) or intravenously (right middle panel; semi-filled triangle, n = 2). Abscissae, cumulative dose, log scale; ordinate, percentage of responses on the AM4054-associated lever (left middle and right middle panels). Symbols left of abscissae break indicate performance during V and AM control sessions. Points represent averages (±S.E.M.) for the groups of subjects. (Bottom panels) Dose-effect functions for AEA (left panel) and 2-AG (right panel) after pretreatment with nonselective FAAH/MGL inhibitors AM4302 (0.1 mg/kg) and JZL195 (0.1 mg/kg) in subjects trained to discriminate 0.01 mg/kg AM4054 from vehicle. AEA was administered intramuscularly alone (open diamond, n = 4) after pretreatment with JZL195 (closed square, n = 4) and after pretreatment with AM4302 (closed inverted triangle, n = 4). 2-AG was administered intramuscularly alone (open triangle, n = 4) and after AM4302 (closed inverted triangle, n = 3) as well as intravenously alone (open inverted-triangle, n = 3) and after AM4302 (semi-filled inverted triangle, n = 2). Abscissae, cumulative dose, log scale; ordinate, percentage of responses on the AM4054-associated lever (bottom left and bottom right panels). Symbols left of abscissae break indicate performance during V and AM control sessions. Points represent averages (±S.E.M.) for the groups of subjects.
The right panels of Fig. 3 present mean data for responding on the CB1-associated lever after cumulative intramuscular or intravenous doses of 2-AG alone (Fig. 3, open triangles) and after intramuscular treatment with the MGL-selective inhibitor AM4301 (Fig. 3, middle right panel), and the FAAH/MGL inhibitors AM4302 and JZL195 (Fig. 3, bottom right panels). Cumulative doses of 2-AG up to 18 mg/kg administered intramuscularly or intravenously failed to produce AM4054-associated responding alone or after treatment with any of the three enzyme inhibitors. As in initial studies of its effects alone, the highest intravenous dose of 2-AG (18 mg/kg) produced >75% decreases in response rate in the two monkeys in which it was studied after treatment with AM4301 (data not shown).
Discriminative Stimulus and Time Course Effects of Selective Enzyme Inhibitor Combinations.
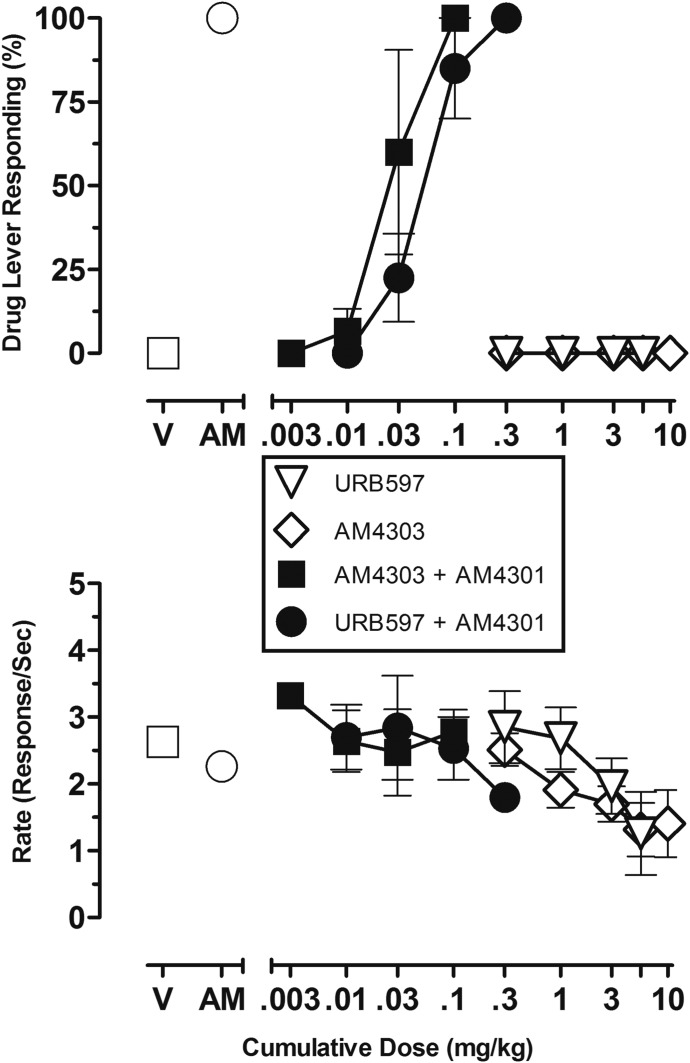
Figure 4 presents the mean data for CB1-associated responding (Fig. 4, top panel) and response rate (Fig. 4, bottom panel) after administration of the FAAH-selective inhibitors URB597 or AM4303 alone and after treatment with the selective MGL inhibitor AM4301 (1.0 mg/kg). Although URB597 or AM4303 alone failed to produce AM4054-associated responding up to doses that decreased the response rate by >50% (Fig. 4, open symbols; see also Fig. 2), cumulative doses of both URB597 and AM4303 engendered dose-related increases in AM4054-associated responding after presession administration of AM4301. Full substitution for the training dose of AM4054 was observed in the latter experiments after cumulative doses of 0.3 and 0.1 mg/kg, respectively, of URB597 and AM4303.
Fig. 4.
Dose-effect functions for selective FAAH inhibitors alone (URB597, open inverted triangle, n = 3; AM4303, open diamond, n = 3) and in combination with 1.0 mg/kg selective MGL inhibitor AM4301 (URB597, closed circle, n = 3; AM4303, closed square, n = 3), administered in subjects trained to discriminate 0.01 mg/kg AM4054 from vehicle (V). Abscissae, cumulative dose, log scale; ordinate, percentage of responses on the AM4054-associated lever (top panel), response rate (bottom panel). Symbols left of abscissae break indicate performance during V and AM4054 (AM) control sessions. Points represent averages (±S.E.M.) for the groups of subjects.
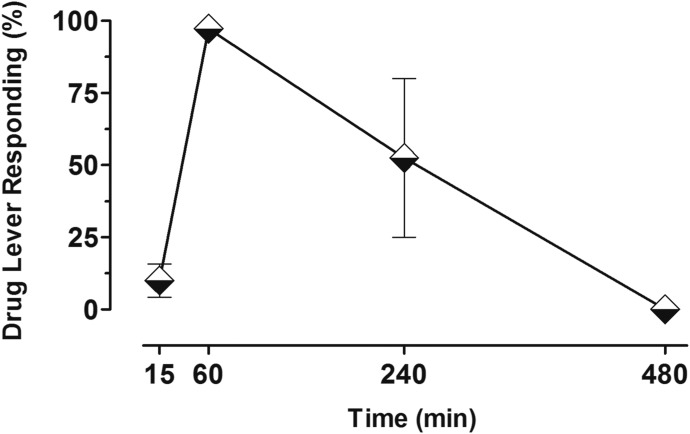
Figure 5 presents the time course of CB1-associated discriminative stimulus effects after treatment with the dose combination of 0.3 mg/kg URB597 and 1.0 mg/kg AM4301. The full time course of action for the AM4054-related discriminative stimulus effects of this dose combination was captured within 8 hours (i.e., all subjects responded ≤20% on the AM4054-associated lever 15 minutes after treatment, ≥90% after 60 minutes, and 0% at 8 hours post-treatment). At 4 hours after treatment, the response distribution varied among individual subjects, yielding a mean value of approximately 50% responding on both levers. Inspection of individual data shows that two subjects responded exclusively on the AM4054-associated lever at the 4-hour post-treatment time, whereas ≥80% responding occurred on the vehicle-associated lever for the remaining two subjects.
Fig. 5.
Time course of 0.3 mg/kg URB597 in combination with 1.0 mg/kg AM4301 (n = 4) in subjects trained to discriminate 0.01 mg/kg AM4054 from vehicle. Abscissae, time interval after injection in which discrimination session occurred; ordinate, percentage of responses on the AM4054-associated lever. Points represent averages (±S.E.M.) for the groups of subjects.
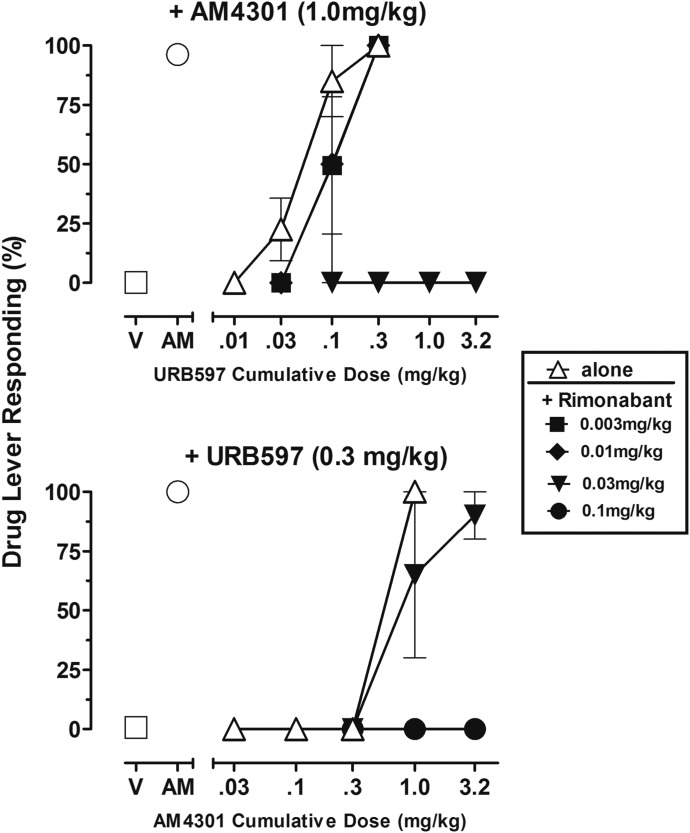
Antagonism of CB1 Discriminative Stimulus Effects of Selective Enzyme Inhibitor Combinations.
Figure 6 presents the effects of rimonabant on the cannabinomimetic discriminative stimulus effects produced by the combination of selective enzyme inhibitors. The top panel of Fig. 6 shows modification by rimonabant of the cannabinomimetic effects of cumulative doses of URB597 administered in the presence of 1.0 mg/kg AM4301. As shown, the doses of 0.003 and 0.01 mg/kg rimonabant slightly attenuated the effects of lower doses of URB597 without altering the effects of the highest cumulative dose of URB597 (0.32 mg/kg) in the presence of AM4301. However, 0.03 mg/kg rimonabant completely blocked CB1 discriminative stimulus effects of URB597/AM4301 up to the highest cumulative dose of URB597 that could be studied (3.2 mg/kg). The bottom panel of Fig. 6 shows the effects of rimonabant on the effects of cumulative doses of AM4301 after pretreatment with 0.3 mg/kg URB597. Pretreatment with 0.03 mg/kg rimonabant produced a small (<3-fold) rightward movement of the dose-effect function, whereas a dose of 0.1 mg/kg rimonabant completely blocked the CB1 discriminative stimulus effects of AM4301 up to the cumulative dose of 3.2 mg/kg after pretreatment with 0.3 mg/kg URB597.
Fig. 6.
(Top panel) Dose-effect function of URB597 after a pretreatment with 1.0 mg/kg AM4301 in subjects trained to discriminate 0.01 mg/kg AM4054 from vehicle, administered alone (open triangle, n = 4) or after various pretreatment doses of SR141716A (closed symbols). Abscissae, cumulative dose, log scale; ordinate, percentage of responses on the AM4054-associated lever. Open symbols left of abscissae break indicate performance during Vehicle (V) and AM4054 (AM) control sessions. Points represent averages (±S.E.M.) for the groups of subjects. (Bottom panel) Dose-effect function of AM4301 after pretreatment with 0.3 mg/kg URB597 in subjects trained to discriminate 0.01 mg/kg AM4054 from V, administered alone (open triangle, n = 2) or after various pretreatment doses of SR141716A (closed symbols). Abscissae, cumulative dose, log scale; ordinate, percentage of responses on the AM4054-associated lever. Open symbols left of abscissae break indicate performance during V and AM control sessions. Points represent averages (±S.E.M.) for the groups of subjects.
Discussion
In the present studies, ligands that selectively inhibit the metabolic deactivation of the endocannabinoids AEA by FAAH (URB597 and AM4303) and 2-AG by MGL (AM4301) failed to produce CB1 discriminative stimulus effects when administered alone. The present results with FAAH inhibitors agree well with data from previous studies of URB597 in monkeys that were trained to discriminate ∆9-THC from vehicle (Solinas et al., 2007; Long et al., 2009; Stewart and McMahon, 2011) and extend those observations to the novel and selective FAAH inhibitor AM4303. The highest doses of URB597 in the present studies have been shown previously to produce up to a 10-fold enhancement of brain AEA levels in squirrel monkeys (Justinova et al., 2008), indicating that such high levels of endogenous AEA alone do not sufficiently activate CB1 receptors to engender CB1-mediated discriminative stimulus effects.
Administration of exogenous AEA or 2-AG also failed to produce CB1-mediated discriminative stimulus effects in the present experiments. The inability of AEA to produce CB1-related discriminative stimulus effects likely reflects its rapid enzymatic degradation in vivo (Willoughby et al., 1997; Savinainen et al., 2001; Stewart and McMahon, 2011). Supporting this view, the relatively stable analog of AEA, methanandamide, has been shown to reproduce CB1-mediated discriminative stimulus effects in both rats and monkeys (Kangas et al., 2013; Järbe et al., 2014), despite having lesser efficacy than associated with Δ9-THC or CB1 full agonists like WIN55,212-2 (Kangas et al., 2016). Moreover, the behavioral effects of AEA could be revealed in the present studies after inhibition of its metabolism by either FAAH-selective or dual FAAH/MGL ligands. The full and dose-dependent substitutions observed with AEA under these conditions are consistent with previous reports that either pharmacological (Solinas et al., 2007; Stewart and McMahon, 2011) or genetic (Walentiny et al., 2011) inactivation of FAAH can potentiate CB1-mediated discriminative stimulus effects.
The absence of CB1-like effects of both URB597 and intravenous AEA in the present studies stands in contrast to the results of previous intravenous self-administration studies. URB597 is not readily self-administered, perhaps reflecting a lack of rewarding effect of URB597-increased levels of AEA or the kinetics of FAAH inhibition that are not conducive to the demonstration of reinforcing effects (i.e., a too gradual accumulation of AEA). On the other hand, intravenous AEA, which has fast onset but is rapidly degraded, has been shown to have reinforcing effects in squirrel monkeys (Justinova et al., 2005, 2008). It is important to note, however, that the pretreatment times for AEA and URB597 in the present studies were 10 and 60 minutes, respectively, whereas intravenous infusions were delivered immediately in the self-administration studies. Whether this can be attributed to differences in the temporal requirements for discriminative and reinforcing stimuli to achieve behavioral efficacy remains to be determined.
The ability of dual FAAH/MGL inhibition, but not FAAH inhibition alone, to substitute for AM4054 in the present experiments suggests a functional role for 2-AG in CB1-mediated discriminative stimulus effects. However, in contrast to results with AEA after FAAH inhibition, 2-AG displayed no capacity for producing such behavioral effects alone, even after selective MGL or dual FAAH/MGL inhibition. The reasons for this are unclear. Endocannabinoids are thought to modulate brain permeability, and it may be that, unlike AEA, exogenously administered 2-AG itself does not adequately penetrate the blood-brain barrier to sufficiently activate CB1 receptors in the central nervous system (CNS) (Pellkofer et al., 2013; Hind et al., 2015). Notwithstanding this caveat, the highest intravenous dose of 2-AG in the present studies produced clear behavioral effects in all monkeys, which is consistent with CNS activity. It is possible that these effects of 2-AG were mediated by its activation of peroxisome proliferator–activated receptors or through the prostanoid system consequent to its oxygenation (Pertwee et al., 2010; Alhouayek et al., 2013; Sticht et al., 2015). However, 2-AG, like AEA, has previously been reported to maintain rimonabant-sensitive intravenous self-administration in squirrel monkeys, which is thought to reflect CB1 receptor–mediated actions in the CNS (Justinova et al., 2005, 2011). Alternatively, and presuming that 2-AG does adequately penetrate the CNS to bind CB1 receptors, it may be that 2-AG alone cannot trigger CB1 discriminative stimulus effects in the absence of elevated levels of AEA. Mounting evidence suggests a functional interaction between AEA and 2-AG, and they may play differential and, perhaps, cooperative roles in mediating CB1-related discriminative stimulus effects. For example, dual FAAH/MGL inhibition has been shown to produce a distinctly different behavioral profile in the rodent tetrad test than selective blockade of either enzyme alone (Long et al., 2009). Similarly, the effects of dual FAAH/MGL inhibition in the present study strengthen the idea that complementary, but not individual, actions of the AEA and 2-AG are required to produce CB1-related discriminative stimulus effects. However, this explanation for the absence of CB1-like effects of 2-AG, although plausible, does not easily comport with the ability of intravenous 2-AG to produce reinforcing effects in squirrel monkeys, and must be viewed with caution. Additional work is necessary to more clearly understand the role of both CB1 and non-CB1 mechanisms that may mediate the behavioral effects of 2-AG.
Both FAAH-selective and dual FAAH/MGL inhibitors, but not the MGL-selective inhibitor, produced dose-related decreases in response rate in squirrel monkeys—differences that also have been observed in both rats and mice (Järbe et al., 2001; Wiley et al., 2014). The rate-decreasing effects of the enzyme inhibitors studied may result from actions of AEA metabolites at off-site targets (e.g., vanilloid receptors) (Wiley et al., 2006; Panlilio et al., 2009). Similar non-CB1 mechanisms may also explain the profound behavioral effects of intravenous AEA observed at high doses. Importantly, neither AEA nor 2-AG produced CB1-related discriminative stimulus effects when administered intravenously up to doses that substantially decreased response rate, supporting the idea that the rate-decreasing effects of cannabinoids like ∆9-THC, which typically are produced by discriminable doses and have been attributed to CB1-mediated actions, can be distinguished from those of the endocannabinoids. Unfortunately, the adverse effects observed with rate-decreasing doses of AEA and 2-AG in the present studies precluded further antagonism experiments to confirm this possibility.
Although neither selective FAAH nor MGL inhibitors produced AM4054-related discriminative stimulus effects, the dual FAAH/MGL inhibitors JZL195 and AM4302 consistently substituted for the CB1 agonist. Moreover, the combined administration of selective FAAH and MGL inhibitors similarly produced AM4054-like effects. These findings provide the first example of CB1-mediated discriminative stimulus effects of the combination of FAAH and MGL inhibition in nonhuman primates and are consistent with previous findings in drug discrimination studies in which neither maximal FAAH nor MGL inhibition alone sufficed to reproduce CB1 discriminative stimulus effects in rodents or monkeys (Solinas et al., 2007; Long et al., 2009; Stewart and McMahon, 2011). They also agree with reports of CB1 discriminative stimulus effects in mice that result from dual MGL/FAAH inactivation via genetic and/or pharmacological means (Long et al., 2009; see also Ignatowska-Jankowska et al., 2014). Taken together, these previous and present findings indicate that endocannabinergic production of CB1 discriminative stimulus requires dual FAAH and MGL inhibition, presumably reflecting an integrated signaling mechanism resulting from elevated levels of both AEA and 2-AG in brain.
Administration of the selective CB1 receptor antagonist rimonabant verified that the discriminative stimulus effects produced by FAAH/MGL inhibitors were mediated by the CB1 receptor. Interestingly, unlike direct CB1 agonists, escalating doses of FAAH/MGL inhibitors failed to surmount CB1 receptor blockade by a modest dose of rimonabant. For example, the relatively small pretreatment dose of 0.1 mg/kg rimonabant fully reversed the effects of FAAH/MGL inhibition but, in earlier studies, produced only a 3-fold shift in the AM4054 dose-response curve (Kangas et al., 2013). These findings disclose the limited extent to which enzyme inhibition can elevate AEA and 2-AG levels to exert CB1 receptor–mediated actions. These data also suggest that a nearly complete inhibition of the two enzymes may be required to produce discriminative stimulus effects because the dose-response curve for coadministered FAAH and MGL inhibitors was not shifted rightward (i.e., the antagonistic effects of rimonabant could not be surmounted). These observations are consistent with previous findings showing that approximately 85% of FAAH must be inactivated by URB597 to maintain an elevated AEA tone (Fegley et al., 2005).
In conclusion, the present studies suggest that discriminative stimulus, and perhaps subjective, effects associated with CB1 agonists also can be provoked through endocannabinoid mechanisms involving both AEA and 2-AG. Although AEA can mediate such cannabimimetic effects directly when administered exogenously in the presence of an FAAH inhibitor, exogenous 2-AG administered alone or after enzyme inhibition failed to produce similar effects up to doses that dramatically reduced the response rate. The discriminative stimulus effects elicited by dual FAAH/MGL inhibition, but not by either type of inhibition alone, further suggest a functionally cooperative interaction between the endocannabinoids that remain to be clearly defined. Finally, the insurmountable antagonism by rimonabant of the CB1-related effects of combined FAAH and MGL inhibition strongly suggests that the magnitude of tolerance and dependence produced by directly acting CB1 agonists (e.g., ∆9-THC) and cannabinomimetic enzyme inhibitors may differ qualitatively. From the perspective of medication development, such findings encourage the idea that endocannabinoid enzyme inhibitors may display less abuse liability than is associated with currently available CB1-related drugs.
Abbreviations
- AEA
N-arachidonoylethanolamine
- 2-AG
2-arachidonoylglycerol
- AM4054
9β-(hydroxymethyl)-3-(1-adamantyl)-hexahydrocannabinol
- AM4301
aryl 4-benzhydrylpiperidine-1-carboxylate
- AM4302
aryl 4-benzylpiperidine-1-carboxylate
- AM4303
aryl 4-phenylpiperidine-1-carboxylate
- CB1
cannabinoid receptor type 1
- CNS
central nervous system
- FAAH
fatty acid amide hydrolase
- FR
fixed ratio
- JZL 184
4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate
- JZL195
4-nitrophenyl 4-(3-phenoxybenzyl)piperazine-1-carboxylate
- MGL
monoacylglycerol lipase
- SA-57
4-[2-(4-chlorophenyl)ethyl]-1-piperidinecarboxylic acid 2-(methylamino)-2-oxoethyl ester
- SR141716A
5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1Hpyrazole-3-carboxamide
- Δ9-THC
Δ9-tetrahydrocannabinol
- URB597
(3′-(aminocarbonyl)[1,1′-biphenyl]-3-yl)-cyclohexylcarbamate
- WIN 55,212
(R)-(+)-[2,3-Dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate
Authorship Contributions
Participated in research design: Bergman, Leonard, and Kangas.
Conducted experiments: Leonard.
Contributed new reagents or analytic tools: Alapafuja, Ji, Shukla, Liu, Nikas, and Makriyannis.
Performed data analysis: Leonard and Kangas.
Wrote or contributed to the writing of the manuscript: Leonard, Bergman, and Kangas.
Footnotes
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grants K01-DA035974 (to B.D.K.) and R01-DA035857 (J.B.)].
References
- Ahn K, Smith SE, Liimatta MB, Beidler D, Sadagopan N, Dudley DT, Young T, Wren P, Zhang Y, Swaney S, et al. (2011) Mechanistic and pharmacological characterization of PF-04457845: a highly potent and selective fatty acid amide hydrolase inhibitor that reduces inflammatory and noninflammatory pain. J Pharmacol Exp Ther 338:114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alapafuja SO, Nikas SP, Bharathan IT, Shukla VG, Nasr ML, Bowman AL, Zvonok N, Li J, Shi X, Engen JR, et al. (2012) Sulfonyl fluoride inhibitors of fatty acid amide hydrolase. J Med Chem 55:10074–10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhouayek M, Masquelier J, Cani PD, Lambert DM, Muccioli GG. (2013) Implication of the anti-inflammatory bioactive lipid prostaglandin D2-glycerol ester in the control of macrophage activation and inflammation by ABHD6. Proc Natl Acad Sci USA 110:17558–17563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida-Santos AF, Gobira PH, Rosa LC, Guimaraes FS, Moreira FA, Aguiar DC. (2013) Modulation of anxiety-like behavior by the endocannabinoid 2-arachidonoylglycerol (2-AG) in the dorsolateral periaqueductal gray. Behav Brain Res 252:10–17. [DOI] [PubMed] [Google Scholar]
- De Luca MA, Valentini V, Bimpisidis Z, Cacciapaglia F, Caboni P, Di Chiara G. (2014) Endocannabinoid 2-arachidonoylglycerol self-administration by Sprague-Dawley rats and stimulation of in vivo dopamine transmission in the nucleus accumbens shell. Front Psychiatry 5:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. (2005) Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther 313:352–358. [DOI] [PubMed] [Google Scholar]
- Gaetani S, Dipasquale P, Romano A, Righetti L, Cassano T, Piomelli D, Cuomo V. (2009) The endocannabinoid system as a target for novel anxiolytic and antidepressant drugs. Int Rev Neurobiol 85:57–72. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Kinsey SG, Liu QS, Hruba L, McMahon LR, Grim TW, Merritt CR, Wise LE, Abdullah RA, Selley DE, et al. (2015) Full fatty acid amide hydrolase inhibition combined with partial monoacylglycerol lipase inhibition: augmented and sustained antinociceptive effects with reduced cannabimimetic side effects in mice. J Pharmacol Exp Ther 354:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobira PH, Almeida-Santos AF, Guimaraes FS, Moreira FA, Aguiar DC. (2016) Role of the endocannabinoid 2-arachidonoylglycerol in aversive responses mediated by the dorsolateral periaqueductal grey. Eur Neuropsychopharmacol 26:15–22. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gönenç A, Lukas SE. (2014) Worth the wait: effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology (Berl) 231:1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd JA, Morse WH, Kelleher RT, Jones LG. (1969) Arterial hypertension in the squirrel monkey during behavioral experiments. Am J Physiol 217:24–29. [DOI] [PubMed] [Google Scholar]
- Hind WH, Tufarelli C, Neophytou M, Anderson SI, England TJ, O’Sullivan SE. (2015) Endocannabinoids modulate human blood-brain barrier permeability in vitro. Br J Pharmacol 172:3015–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Ghosh S, Crowe MS, Kinsey SG, Niphakis MJ, Abdullah RA, Tao Q, O’ Neal ST, Walentiny DM, Wiley JL, et al. (2014) In vivo characterization of the highly selective monoacylglycerol lipase inhibitor KML29: antinociceptive activity without cannabimimetic side effects. Br J Pharmacol 171:1392–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe, TU, Lamb, RJ, Lin, S, Makriyannis, A (2001) (R)-Methanandamide and Δ9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology (Berl) 156:369–380. [DOI] [PubMed] [Google Scholar]
- Järbe TU, LeMay BJ, Halikhedkar A, Wood J, Vadivel SK, Zvonok A, Makriyannis A. (2014) Differentiation between low- and high-efficacy CB1 receptor agonists using a drug discrimination protocol for rats. Psychopharmacology (Berl) 231:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TU, LeMay BJ, Thakur GA, Makriyannis A. (2016) A high efficacy cannabinergic ligand (AM4054) used as a discriminative stimulus: generalization to other adamantyl analogs and Δ(9)-THC in rats. Pharmacol Biochem Behav 148:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, King AR, Redhi GH, Yasar S, Piomelli D, et al. (2008) Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry 64:930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Panlilio LV, Moreno-Sanz G, Redhi GH, Auber A, Secci ME, Mascia P, Bandiera T, Armirotti A, Bertorelli R, et al. (2015) Effects of fatty acid amide hydrolase (FAAH) inhibitors in non-human primate models of nicotine reward and relapse. Neuropsychopharmacology 40:2185–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Solinas M, Tanda G, Redhi GH, Goldberg SR. (2005) The endogenous cannabinoid anandamide and its synthetic analog R(+)-methanandamide are intravenously self-administered by squirrel monkeys. J Neurosci 25:5645–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinová Z, Yasar S, Redhi GH, Goldberg SR. (2011) The endogenous cannabinoid 2-arachidonoylglycerol is intravenously self-administered by squirrel monkeys. J Neurosci 31:7043–7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Delatte MS, Vemuri VK, Thakur GA, Nikas SP, Subramanian KV, Shukla VG, Makriyannis A, Bergman J. (2013) Cannabinoid discrimination and antagonism by CB(1) neutral and inverse agonist antagonists. J Pharmacol Exp Ther 344:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Leonard MZ, Shukla VG, Alapafuja SO, Nikas SP, Makriyannis A, Bergman J. (2016) Comparisons of Δ9-tetrahydrocannabinol and anandamide on a battery of cognition-related behavior in nonhuman primates. J Pharmacol Exp Ther 357:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, et al. (2003) Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med 9:76–81. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Järbe TUC, Makriyannis A, Lin S, Goutopoulos A. (2000) Effects of Δ 9-tetrahydrocannabinol, (R)-methanandamide, SR 141716,and d-amphetamine before and during daily Δ 9-tetrahydrocannabinol dosing. Eur J Pharmacol 398:251–258. [DOI] [PubMed] [Google Scholar]
- Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR. (1988) Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther 247:1046–1051. [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, et al. (2009) Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci USA 106:20270–20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makriyannis A, Shukla VG, and Alapafuja SO (2016) inventors, Northeastern University, assignee. Urea/carbamates FAAH, MAGL or dual FAAH/MAGL inhibitors and uses thereof. WO patent WO2016014975A2. Mar 17 2016.
- McMahon LR. (2006) Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Delta9-tetrahydrocannabinol. J Pharmacol Exp Ther 319:1211–1218. [DOI] [PubMed] [Google Scholar]
- Owens RA, Ignatowska-Jankowska B, Mustafa M, Beardsley PM, Wiley JL, Jali A, Selley DE, Niphakis MJ, Cravatt BF, Lichtman AH. (2016) Discriminative stimulus properties of the endocannabinoid catabolic enzyme inhibitor SA-57 in mice. J Pharmacol Exp Ther 358:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Mazzola C, Medalie J, Hahn B, Justinova Z, Drago F, Cadet JL, Yasar S, Goldberg SR. (2009) Anandamide-induced behavioral disruption through a vanilloid-dependent mechanism in rats. Psychopharmacology (Berl) 203:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Limebeer CL, Rock EM, Sticht MA, Ward J, Turvey G, Benchama O, Rajarshi G, Wood JT, Alapafuja SO, et al. (2016) A comparison of novel, selective fatty acid amide hydrolase (FAAH), monoacyglycerol lipase (MAGL) or dual FAAH/MAGL inhibitors to suppress acute and anticipatory nausea in rat models. Psychopharmacology (Berl) 233:2265–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellkofer HL, Havla J, Hauer D, Schelling G, Azad SC, Kuempfel T, Magerl W, Huge V. (2013) The major brain endocannabinoid 2-AG controls neuropathic pain and mechanical hyperalgesia in patients with neuromyelitis optica. PLoS One 8:e71500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, et al. (2010) International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev 62:588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, De Chiara V, Musella A, Sacchetti L, Cantarella C, Castelli M, Cavasinni F, Motta C, Studer V, Bernardi G, et al. (2010) Preservation of striatal cannabinoid CB1 receptor function correlates with the antianxiety effects of fatty acid amide hydrolase inhibition. Mol Pharmacol 78:260–268. [DOI] [PubMed] [Google Scholar]
- Savinainen JR, Järvinen T, Laine K, Laitinen JT. (2001) Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB(1) receptor-dependent G-protein activation in rat cerebellar membranes. Br J Pharmacol 134:664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Kinsey SG, Ignatowska-Jankowska B, Ramesh D, Abdullah RA, Tao Q, Booker L, Long JZ, Selley DE, Cravatt BF, et al. (2014) Prolonged monoacylglycerol lipase blockade causes equivalent cannabinoid receptor type 1 receptor-mediated adaptations in fatty acid amide hydrolase wild-type and knockout mice. J Pharmacol Exp Ther 350:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, Goldberg SR. (2007) The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther 321:370–380. [DOI] [PubMed] [Google Scholar]
- Spealman RD. (1985) Discriminative-stimulus effects of midazolam in squirrel monkeys: comparison with other drugs and antagonism by Ro 15-1788. J Pharmacol Exp Ther 235:456–462. [PubMed] [Google Scholar]
- Spealman RD, Goldberg SR, Kelleher RT, Goldberg DM, Charlton JP. (1977) Some effects of cocaine and two cocaine analogs on schedule-controlled behavior of squirrel monkeys. J Pharmacol Exp Ther 202:500–509. [PubMed] [Google Scholar]
- Stewart JL, McMahon LR. (2011) The fatty acid amide hydrolase inhibitor URB 597: interactions with anandamide in rhesus monkeys. Br J Pharmacol 164:655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sticht MA, Limebeer CL, Rafla BR, Parker LA. (2015) Intra-visceral insular cortex 2-arachidonoylglycerol, but not N-arachidonoylethanolamide, suppresses acute nausea-induced conditioned gaping in rats. Neuroscience 286:338–344. [DOI] [PubMed] [Google Scholar]
- Vann RE, Warner JA, Bushell K, Huffman JW, Martin BR, Wiley JL. (2009) Discriminative stimulus properties of delta9-tetrahydrocannabinol (THC) in C57Bl/6J mice. Eur J Pharmacol 615:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, Wise LE, Niyuhire F, Cravatt BF, Lichtman AH. (2007) Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology 32:1032–1041. [DOI] [PubMed] [Google Scholar]
- Walentiny DM, Gamage TF, Warner JA, Nguyen TK, Grainger DB, Wiley JL, Vann RE. (2011) The endogenous cannabinoid anandamide shares discriminative stimulus effects with ∆(9)-tetrahydrocannabinol in fatty acid amide hydrolase knockout mice. Eur J Pharmacol 656:63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Razdan RK, Martin BR. (2006) Evaluation of the role of the arachidonic acid cascade in anandamide’s in vivo effects in mice. Life Sci 80:24–35. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Walentiny DM, Wright MJ, Jr, Beardsley PM, Burston JJ, Poklis JL, Lichtman AH, Vann RE. (2014) Endocannabinoid contribution to Δ9-tetrahydrocannabinol discrimination in rodents. Eur J Pharmacol 737:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JL, Ghosh S, Mustafa M, Abdullah RA, Niphakis MJ, Cabrera R, Maldonado RL, Cravatt BF, Lichtman AH. (2017) The endocannabinoid hydrolysis inhibitor SA-57: Intrinsic antinociceptive effects, augmented morphine-induced antinociception, and attenuated heroin seeking behavior in mice. Neuropharmacology 114:156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby KA, Moore SF, Martin BR, Ellis EF. (1997) The biodisposition and metabolism of anandamide in mice. J Pharmacol Exp Ther 282:243–247. [PubMed] [Google Scholar]
- Zer-Aviv TM, Akirav I. (2016) Sex differences in hippocampal response to endocannabinoids after exposure to severe stress. Hippocampus 26:947–957. [DOI] [PubMed] [Google Scholar]