Abstract
Manganese superoxide dismutase (MnSOD) is a mitochondrial resident enzyme that reduces superoxide to hydrogen peroxide (H2O2), which can be further reduced to water by glutathione peroxidase (GPX1). Data from human studies has indicated that common polymorphisms in both of these proteins are associated with the risk of several cancers, including breast cancer. Moreover, polymorphisms in MnSOD and GPX1 were shown to interact to increase the risk of breast cancer. To gain an understanding of the molecular mechanisms behind these observations, we engineered human MCF-7 breast cancer cells to exclusively express GPX1 and/or MnSOD alleles and investigated the consequences on the expression of several proteins associated with cancer etiology. Little or no effect was observed on the ectopic expression of these genes on the phosphorylation of Akt, although allele specific effects and interactions were observed for the impact on the levels of Bcl-2, E-cadherin and Sirt3. The patterns observed were not consistent with the steady state levels of H2O2 determined in the transfected cells. These results indicate plausible contributing factors to the effects of allelic variations on cancer risk observed in human epidemiological studies.
Keywords: GPX1, MnSOD, hydrogen peroxide, Bcl-2, E-cadherin, Sirt3
Introduction
The identification of breast cancer susceptibility genes becomes a daunting challenge when at-risk alleles exhibit low penetrance. Unlike the effects of mutations in BRCA1/2 that result in a very high likelihood of a woman developing breast cancer, susceptibility genes with relatively low penetrance are much harder to identify and this can be confounded even more if the expression of the at-risk phenotype is modified by other genotypes and environmental exposures. The later situation may be occurring in the case of polymorphisms in the gene for manganese superoxide dismutase (MnSOD/SOD2), an anti-oxidant enzyme located in the mitochondria that detoxifies superoxide generated as a byproduct of oxidative phosphorylation to the less reactive hydrogen peroxide (H2O2). MnSOD is an essential, nuclear-encoded, mitochondrial enzyme. The expression of MnSOD is activated by a myriad of environmental and biological factors such as alcohol, smoking, ROS and cytokines [1–3]. A polymorphism in the mitochondrial targeting sequence that results in an alanine (Ala) rather than a valine (Val) at codon 16 results in increased MnSOD mRNA stability and targets the enzyme more efficiently into the mitochondria, altering the secondary structure of the protein from a beta sheet (Val) to alpha helix (Ala) which affects its import into the mitochondrial membrane [4]. Thus, the Val MnSOD genotype is expected to result in less absolute enzyme activity and this was experimentally demonstrated in human breast cancer cell lines [5]. Moreover, mouse embryonic fibroblasts engineered to express similar levels of each of the MnSOD alleles did not demonstrate a difference in reactive oxygen levels [5].
There has been considerable effort examining whether MnSOD plays a role in breast cancer (reviewed in [6]). The impact of the Val16Ala polymorphism on breast cancer risk has been examined with two studies showing a moderate increase in risk [7–9] while others report no association or reduced risk among women homozygous for the Ala/Ala genotype [10–12]. An interaction between the MnSOD Val16Ala polymorphism and radiation exposure and smoking in increasing breast cancer risk was reported [13] and a meta-analysis revealed that breast cancer risk was elevated for premenopausal women who are homozygous for the Ala/Ala genotype and had low intake of dietary antioxidants [14]. A similar observation was reported for prostate cancer where the Val16Ala polymorphism was associated with a 10-fold increase in prostate cancer risk in men with the lowest level of dietary anti-oxidant intake [15], and individuals who consumed less dietary anti-oxidants, including selenium, had the greatest risk of prostate cancer. In addition, a 3-fold increase in aggressive prostate cancer was observed in men homozygous for Ala/Ala genotype and low dietary carotenoid status [16].
A consequence of MnSOD enzyme activity is the production of H2O2, which can diffuse through the mitochondrial membrane and affect cellular proliferation, metabolism and apoptosis [17]. GPX1 is a ubiquitously expressed enzyme that is localized in both the cytoplasm and the mitochondria [18] and detoxifies H2O2 using reducing equivalents from glutathione [19]. A polymorphism in the GPX1 gene resulting in a leucine (Leu) instead of a proline (Pro) at position 198 is associated with elevated risk of cancer and other diseases [19], including breast cancer [20, 21]. While not all studies have indicated this association, a meta-analysis consisting of 31 publications including 14,372 cases and 18,081 controls estimated an overall elevated cancer risk associated with the GPX1 Pro198Leu polymorphism in individuals who carried the Leu allele (Pro/Leu and Leu/Leu) with an odds ratio (OR) 1.12 and 95% confidence interval (CI) of 1.02–1.23 [22]. The at-risk Leu allele was found to encode a protein that is less responsive to selenium, this being determined by examining the GPX enzyme activity of MCF-7 breast cancer cell lines transfected with GPX1 allelic-specific expression constructs and cultured in media supplemented with varying amounts of selenium [23]. GPX1 proteins encoded by these different alleles also partitions differentially between the cytoplasm and mitochondria [24]. Lower levels of GPX1 enzyme activity have been associated with the Leu allele in human clinical samples as well [20, 25]. Initially, Cox et al. reported that there was no association between the Leu allele of GPX1 and risk of breast cancer in participants of the Nurse's Health Study [26]. The researchers later reported that there was indeed a significant increased risk for breast cancer when MnSOD genotypes were considered [27]. Individuals homozygous for both the MnSODAla and the GPX1Leu genotypes were at greater risk of breast cancer [OR 1.87, CI 1.09–3.19][27] indicating an interaction between MnSOD and GPX1 in modulating cancer risk.
To examine the molecular mechanism behind the epidemiological observation that GPX1 genotype modifies the risk of cancer associated with MnSOD genotypes, studies manipulating the genotypes and levels of MnSOD and GPX1 in a cell culture model were conducted, taking advantage of the observation that MCF-7 breast cancer cells are null for GPX1 [23, 28] and have negligible levels of endogenous MnSOD. These cells were used to exclusively express different GPX1 and MnSOD alleles by transfection of allele specific GPX1 and MnSOD expression constructs. The molecular consequences of polymorphisms in the antioxidant enzymes were investigated to determine outcomes related to oxidative stress and cellular signaling.
Materials and Methods
Cells and culturing conditions
MCF-7 human breast cancer cells were obtained from ATCC (Manassas, VA) and were verified by analyzing 15 autosomal short tandem repeat loci and sex specific amelogenin locus to identify gender (Genetica DNA Lavoratories, Burlington, NC). MCF-7 engineered to express MnSOD and GPX1 allele specific constructs were maintained in minimum essential media (MEM, Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS, Gemini Biosciences, West Sacramento, CA), 100 units/ml penicillin and 100 ug/ml streptomycin (Gibco, Grand Island, NY) and incubated at 37°C with 5% CO2. The selenium concentration of the serum used was 152 nM as determined by graphite furnace atomic absorption spectrometry (Texas A&M Veterinary Diagnostic Laboratory at College Station, Texas), resulting in a final selenium concentration of 15.2 nmol/L in media supplemented with 10% serum.
Generation of allele-specific GPX1 and MnSOD gene constructs
MCF-7 cells transfected with GPX1 expression constructs encoding 5 or 7 alanine repeats at the -NH2 terminus and either a proline or leucine at codon 198 were previously generated [23]. Both MnSOD Ala16 and Val16 expression constructs were transfected into MCF-7 cells using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA) and selected with 500 ug/mL G418 (Sigma, St. Louis, MO). GPX1 and MnSOD double transfectants were generated by sequential transfection of MnSOD allele specific constructs into previously generated MCF-7GPX1 engineered cell lines using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA) and selected with 400 µg/mL G418 (Sigma, St. Louis, MO). The MCF-7GPX1 transfectants containing either 5 or 7 alanine repeat codons (A5 or A7) or either a proline (P) or leucine (L) at codon 198 were generated and referred to as GPX1A5P and GPX1A7L and the MCF-7 transfectants containing either MnSOD Val or Ala at codon 16 are referred to as MnSODVal or MnSODAla. Control MCF-7 cells transfected with the empty pLNCX vector are referred to as C for "controls".
GPX activity assay
GPX enzyme activity was determined on whole cell lysates using a coupled spectrophotometric assay that determines the GPX dependent consumption of NADPH [29]. Enzyme activity was expressed as nmole oxidized NADPH per minute per milligram of total protein. For all experiments, the transfectants were plated in triplicates and maintained in G418, which was removed from culture media 3 days before analysis to reduce the impact of antibiotic supplementation on selenoprotein synthesis [30, 31]. Statistical analysis (paired t test, two-tailed) was performed with Graphpad Instat. A value of P<0.05 was considered statistically significant.
Western blot analyses
Protein samples were prepared in NuPAGE LDS sample buffer (Invitrogen) containing NuPAGE sample reducing agent, boiled at 100°C for 10 minutes. The samples were electrophoresed on 4% to 12% Bis-Tris denaturing polyacrylamide gels (Invitrogen) and transferred to polyvinylidene difluoride membranes (Millipore). Antibodies against the following proteins were used: GPX1 and SBP1 (MBL International), MnSOD (Millipore), E Cadherin (Santa Cruz), Phospho-Akt Serine 473 (Cell Signaling Technology), Bcl-2 (Abcam) and Sirt3 (Aviva). Protein levels were quantified by densitometry or fluorescence detection using the LI-COR Odyssy Imaging System. Statistical analysis (paired t test, two-tailed) was performed with Graphpad Instat. A value of P<0.05 was considered statistically significant.
Amplex Red Assay
H2O2 production was measured using the Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Invitrogen/Life Sciences). Cells were grown to 80% confluence and incubated with the provided Amplex Red reaction buffer for 30 min at 37°C. The supernatant was then transferred to a black-walled 96-well plate and fluorescence was quantified at 560EX/590EM on a SpectraMax M5 spectrophotometer (Molecular Devices, Sunnyvale, CA) and normalized to protein content as determined using the bicinchoninic acid (BCA) protein assay reagent (Thermo Fisher Scientific, Rockford, IL). Statistical analysis (paired t test, two-tailed) was performed with Graphpad Instat. A value of P<0.05 was considered statistically significant.
Results
Derivative MCF-7 cell lines express specific MnSOD and GPX1 alleles
The human MCF-7 breast carcinoma cell line does not express detectable levels of GPX1 mRNA or protein [23, 32], and expresses very low levels of MnSOD, making it an ideal host for the ectopic expression of specific alleles of these proteins. Allele-specific MnSOD and GPX1 expression constructs were transfected into MCF-7 cells individually or in combination, single clones were selected, expanded and examined to determine the expression of the transfected genes. The MnSOD expression constructs encoded either a valine or alanine at codon 16. The GPX1 expression constructs encoded either a leucine or proline at codon 198, but in addition, this polymorphism was paired with a trinucleotide repeat variation that results in a variable number of alanine codons in the amino terminus of the protein (proline/5 alanine codons, GPX1A5P; leucine/7 alanine codons; GPX1A7L) as these have been extensively studied in the past and shown to elicit different biological responses [24, 28].
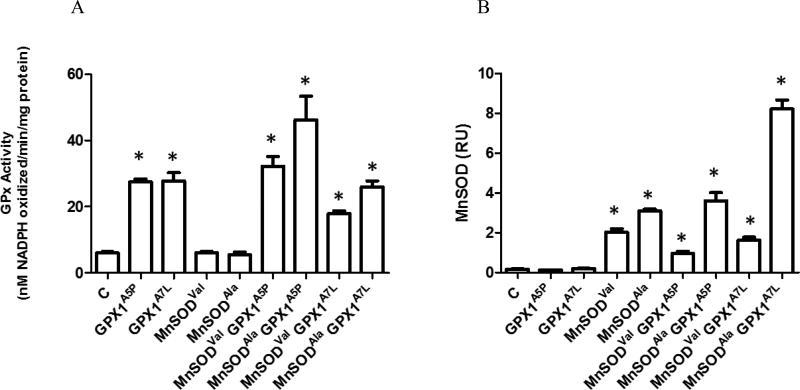
GPX activities in transfectants were determined by the enzyme assay and MnSOD by western blotting (Figures 1, S1, S2). GPX activity of the selected transfectants is shown in Figure 1A and the determined enzyme activity generally corresponded to the levels of GPX protein determined by western blotting (Figure S2). The low levels of total GPX activity seen in clones that were not transfected with a GPX1 expression construct reflects the levels of expression of other GPX family members, presumably GPX2, as no GPX1 was detectable in protein extracts prepared from these cells, even after extended exposure of the western blots. MnSOD levels were very low in transfectants that were not transfected with the MnSOD expression constructs. Clones expressing lower levels of MnSODval than the MnSODala were selected to reflect the physiological circumstance as described in the Introduction.
Figure 1. GPX enzyme activity and MnSOD levels are expressed in MCF-7 transfectants.
A. Lysates from MCF-7 transfected with the empty vector (C), MCF-7 GPX1, MCF-7 MnSODVal and MCF-7 MnSODAla cell lines were used to determine GPX enzyme activity using a spectrophotometric assay that determines the GPX dependent consumption of NADPH. B. Lysates from MCF-7, GPX1, MnSODVal, and MnSODAla cells were analyzed for MnSOD protein levels by western blot using anti-MnSOD antibodies. β-Actin was used as an endogenous protein loading control. Protein levels were quantified using fluorescence detection and normalized to β-Actin. Error bars indicate the standard deviation (n=3) (* = p<0.05).
GPX1 modulates the MnSOD-dependent effect of signaling proteins
Signaling molecules involved in the redox signaling pathway, cell growth, metabolism, and cell-cell adhesion, may be differentially affected by the GPX1 and MnSOD proteins encoded by specific alleles that alter their levels or enzyme activity, presumably by the balance between the production of H2O2 by MnSOD and its elimination by GPX1 [33]. It was specifically shown that women expressing both the MnSOD allele encoding an alanine at position 16 and the GPX1 allele encoding a leucine at position 198 were at greater risk of breast cancer [27]. Here, the effect of the expression of GPX1 and MnSOD gene variants on the levels of proteins implicated in cancer such as E-cadherin, phosphorylated-Akt (p-Akt) Serine 473, Bcl-2, and Sirt3 were studied by immunoblotting using protein specific antibodies.
E-Cadherin and Akt
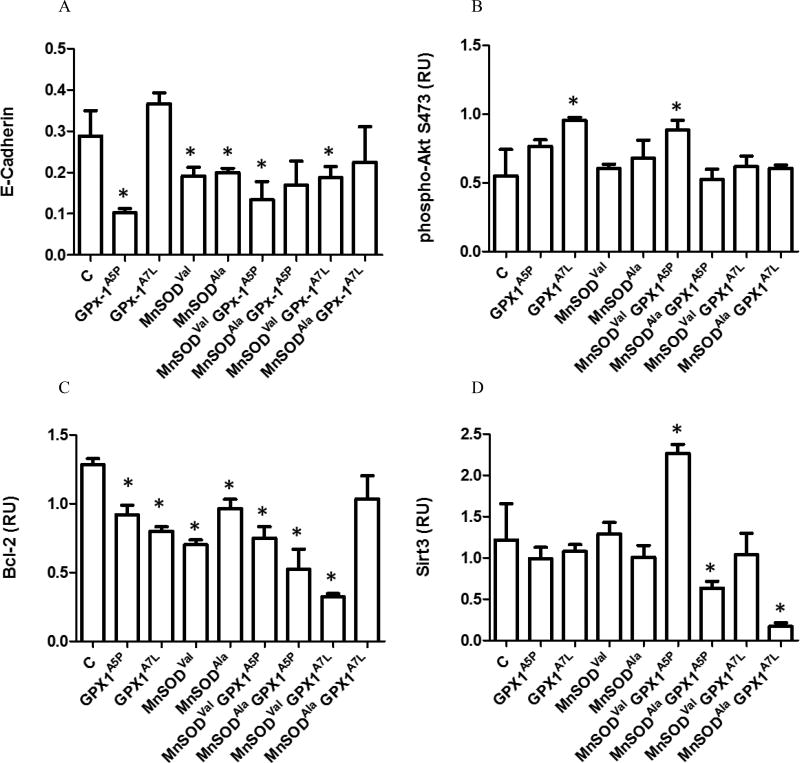
An inverse relationship was previously reported between MnSOD levels and expression of E-cadherin, a calcium-dependent cell-cell adhesion molecule with roles in tissue formation and tumor metastasis [34–37]. In addition, H2O2, the product of the reaction catalyzed by MnSOD and the substrate of GPX1, mediates the epidermal growth factor induced down regulation of E-cadherin [38]. In order to assess whether allelic variations in GPX1 and MnSOD modulate the expression of E-cadherin, whole cell lysates were obtained from the generated MCF-7 cell lines and analyzed by western blotting with anti-E-cadherin monoclonal antibodies. There were lower levels of E-cadherin due to the ectopic expression of the common GPX1 allele, GPX1A5P, either MnSOD allele or the combination of either MnSODVal or MnSODAla with GPX1A5P is shown in Figure 2A, compared to vector-only transfected cells, although the difference didn’t always reach statistical significance. In contrast, the expression of GPX1A7L did not change E-cadherin levels when expressed alone but decreased the levels of the protein when co-expressed with MnSODVal (Figures 2A, S3).
Figure 2. The effects of the expression of GPX1 and MnSOD on the levels of E-Cadherin, p-Akt, Bcl-2 and Sirt3.
Lysates from MCF-7 cells transfected with the empty vector (C), GPX1, MnSOD allele-specific constructs were analyzed for selected protein levels by western blot using protein-specific antibodies. β-Actin was used as an endogenous protein loading control. Protein levels were quantified using fluorescence detection and normalized to β-Actin. Error bars indicate the standard deviation (n=3) (* = p<0.05). Proteins analyzed were A) E-Cadherin, B) p-Akt, C) Bcl-2 and D) Sit3.
Akt is a protein kinase that enhances proliferation and cell survival, and its activation has been shown to be predictive of poor clinical outcome in prostate cancer [39–42]. The effects of the enhanced expression of anti-oxidant enzymes resulted in contrasting effects on Akt phosphorylation. For example, over-expression of GPX1 in Chang liver cells decreased p-Akt levels but Akt activation was also reduced in neurons of GPX1 knock-out mice [19, 30, 43]. The ectopic expression of GPX1A5P or MnSOD alleles had little effect on the phosphorylation of Akt at position 473 while the GPX1A7L and the co-expression of GPX1A5P with MnSODVal resulted in a significant increase in p-Akt levels (Figures 2B, S4).
Bcl-2
Bcl-2 negatively regulates apoptosis by binding to and inactivating pro-apoptotic binding partners and preserving mitochondrial membrane permeability [44–46]. Both GPX1 and MnSOD have been shown to up-regulate the expression of Bcl-2 to promote a more anti-apoptotic environment [19, 47]. In order to investigate whether GPX1 and MnSOD expression affects Bcl-2 levels, whole cell lysates were obtained from the generated MCF-7 cell lines and analyzed by western blotting using anti-Bcl-2 antibodies. Cells expressing either GPX1 or MnSOD alleles have decreased Bcl-2 levels compared to the control (C) (Figures 2C, S5). A contrasting pattern was apparent, however, where the co-expression of GPX1A5P did not have a significant effect on the reduction of Bcl-2 levels further than what was seen when expressed with either MnSODAla allele, while co-expression of GPX1A7L with MnSODVal resulted in a significant further decline in Bcl-2 levels.
Sirt3
Sirt3 is a member of the mammalian sirtuin family of protein that functions as a mitochondrial deacetylase implicated as a possible tumor suppressor [48–50]. Among its many substrates, Sirt3 deacetyles MnSOD at lysine 122 resulting in increased MnSOD enzyme activity [51] and Sirt3 ablation leads to hyper-acetylation of mitochondrial and cytoplasm localized GPX1 in response to stress [52]. Expression of the MnSOD or GPX1 alleles singularly did not alter the levels of Sirt3 in the transfected cells (Figure 2(D)). There was an apparent interaction between MnSOD and GPX1 alleles such that the ectopic expression of either GPX1 alleles significantly reduced the levels of Sirt3 when co-expressed with the MnSODAla but not MnSODVal. In fact, co-expression of MnSODVal and GPX1A5P resulted in an increase in Sirt3 levels (Figures 2(D), S6).
H2O2
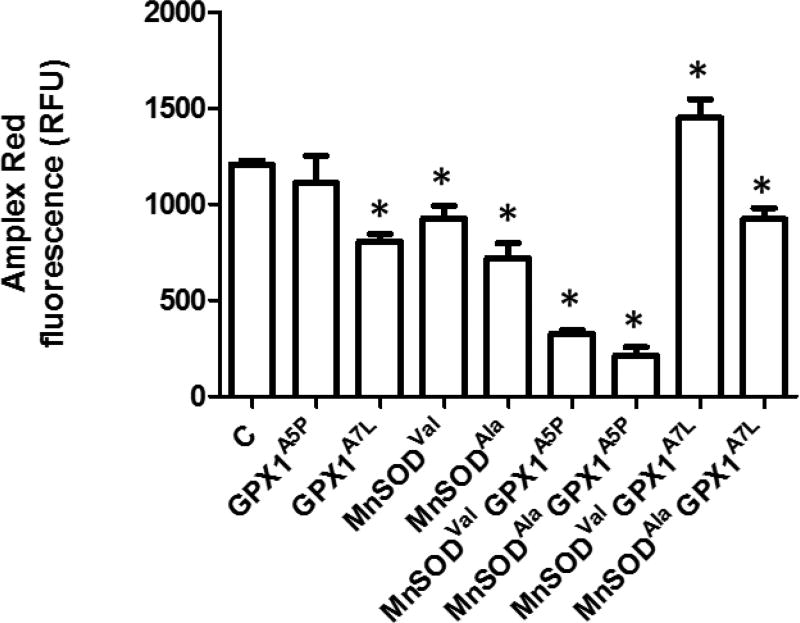
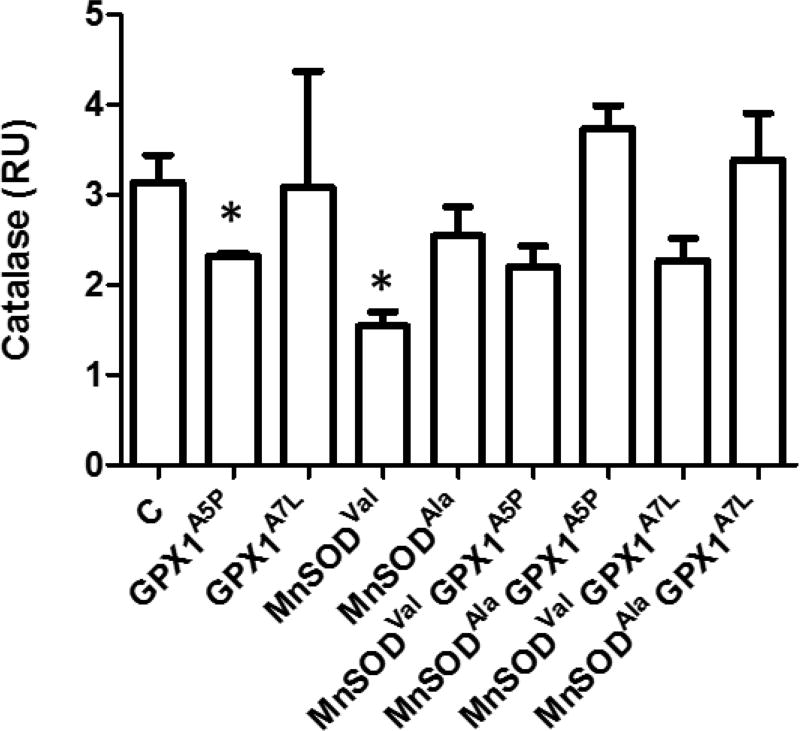
The studies described above were predicated on the potential dynamics between the production of H2O2 by MnSOD and the removal of H2O2 by GPX1, this being influenced by both the levels and cellular localization of these proteins. H2O2 levels were assessed in the transfectants using the Amplex Red indicator (Figure 3). There was a difference in the efficacy by which GPX1 alleles impacted steady state H2O2 levels, with the expression of GPX1A7L and each of the MnSOD proteins resulting in somewhat less H2O2 than that seen in control and GPX1A5P cells. A more dramatic reduction in H2O2 levels was surprisingly observed when GPX1A5P cells were co-expressed with MnSODVal or MnSODAla. These results are unlikely to be explained by effects on catalase as levels of catalase measured by western blotting varied among the transfectants (Figures 4, S7) but in a way that could not directly be associated with the levels of H2O2 shown in Figure 3.
Figure 3. H2O2 levels in cells expressing GPX1A7L and MnSOD variants.
H2O2 levels were assessed in the transfectants using the Amplex Red indicator. Fluorescence was quantified and normalized to protein content as determined using the BCA protein assay. Error bars indicate the standard error (n=4) (* = p<0.05).
Figure 4. Catalase levels in MCF-7 GPX1 and MnSOD transfectants.
Lysates from MCF-7 transfected with the empty vector (C), GPX1, MnSOD transfected MCF-7 cells were analyzed for catalase levels by western blot using anti-catalase antibodies. β-Actin was used as an endogenous protein loading control. Protein levels were quantified using fluorescence detection and normalized to β-Actin. Error bars indicate the standard deviation (n=3) (* = p<0.05).
Discussion
This study was initiated to investigate the molecular consequences of polymorphisms in the genes for GPX1 and MnSOD on the expression of cancer related proteins in order to gain a better understanding of the epidemiological data indicating an interaction between the proteins encoded by these alleles and breast cancer risk. Our approach took advantage of the lack of detectable levels of GPX1 and the low activity of MnSOD in MCF-7 cells, thus permitting the exclusive expression (GPX1) or predominant expression (MnSOD) of specific alleles individually or in combination. Transfectants were selected for further study that expressed these two proteins at levels similar to what we have seen in human breast tissue (data not shown).
In some instances, the expression of a specific GPX1 allele resulted in no or relatively small changes in the levels of the examined proteins. This is apparent for the levels of the activated, phosphorylated form of Akt and consistent with our previous data examining the effect of GPX1 expression on phosphorylation at this same site in Akt (473) [53]. Previously published data indicated that an enhanced expression of GPX1 in Chang liver cells did not change the baseline pAkt levels although GPX1 significantly attenuated the induction of pAkt when the cells were challenged with H2O2 [30]. Both GPX1 alleles reduced the levels of Bcl-2 (Figure 2C), which differs from what was reported when GPX1 was expressed in human epithelial cells where no effect was observed [54]. In contrast to these results, the expression of GPX1A5P resulted in a dramatic reduction in E-cadherin levels while there was no affect when GPX1A7L was expressed (Figure 2A). Previous work in tongue squamous cell carcinoma cells indicated that MnSOD generated H2O2 was able to repress E-cadherin levels [35]. The data presented in Figure 2A and our previous data indicating that GPX1A7L was preferentially located in the cytoplasm [24] might be interpreted as indicating that GPX1A7L was less efficient in scavenging H2O2 at its cellular site of action.
The enhanced expression of MnSOD encoded by different alleles had very similar effects on the levels of the investigated protein, having no effect on p-Akt or Sirt3, and causing a reduction in the levels of E-cadherin and Bcl-2. Again, the E-cadherin result is [55] consistent to that previously reported by Liu et al. [35] while the reduction in Bcl-2 is contrary to what has been reported when MnSOD was expressed in lung carcinoma cells [56]. In all cases, differences due to the expression of distinct MnSOD alleles were minimal or not evident.
The most striking interactions between MnSOD and GPX1 alleles occurred when the levels of Bcl-2 and Sirt3 were examined in transfectants. For example, co-expression of MnSODVal and GPX1A7L resulted in a significant decline in Bcl-2 levels while co-expression of MnSODAla and GPX1A7L had no effect (Figure 2C). In the case of Sirt3 where expression of the individual MnSOD or GPX1 alleles had no effect on Sirt3 levels, the co-expression of the MnSODAla allele, but not the MnSODVal with either GPX1 resulted in a much lower level of Sirt3. These results cannot be explained by the final levels of H2O2 determined by using Amplex Red (Figure 3) as that analysis indicated that the lowest levels of H2O2 detected occurred when either MnSOD alleles were co-expressed with GPX1A5P, the version of GPX1 that is more mitochondrially located [24].
The physiological impact of the expression of different alleles of MnSOD and GPX1 described herein remain unknown. It is therefore interesting to note that GPX1 levels are lower and MnSOD levels are higher in the MDA-MB-231 cell line derived from a triple negative metastatic adenocarcinoma as compared to the levels of those proteins in MCF7 cells that exhibit a less aggressive phenotype [55, 57]. Recent data indicated that higher levels of MnSOD are associated with increased malignancy in human breast cancer samples and the effects of MnSOD are likely mediated through the production of the GPX1 substrate, H2O2 [33]. The data presented here indicates interactions between MnSOD and GPX1 genotypes exists, however there is a need for greater understanding of the interactive consequences of these proteins in breast cancer etiology.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health [Grant # RO1CA127943, R21CA182103] and a UIC Cancer Center Pilot Grant to AMD and MB and a Research Supplement to Promote Diversity in Health-Related Research to AMD.
References
- 1.Xu Y, et al. An intronic NF-kappaB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-alpha and interleukin-1beta. DNA Cell Biol. 1999;18(9):709–22. doi: 10.1089/104454999314999. [DOI] [PubMed] [Google Scholar]
- 2.Koch OR, et al. Ethanol treatment up-regulates the expression of mitochondrial manganese superoxide dismutase in rat liver. Biochem Biophys Res Commun. 1994;201(3):1356–65. doi: 10.1006/bbrc.1994.1853. [DOI] [PubMed] [Google Scholar]
- 3.Perera CS, St Clair DK, McClain CJ. Differential regulation of manganese superoxide dismutase activity by alcohol and TNF in human hepatoma cells. Arch Biochem Biophys. 1995;323(2):471–6. doi: 10.1006/abbi.1995.0069. [DOI] [PubMed] [Google Scholar]
- 4.Sutton A, et al. The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenetics. 2003;13(3):145–57. doi: 10.1097/01.fpc.0000054067.64000.8f. [DOI] [PubMed] [Google Scholar]
- 5.McAtee BL, Yager JD. Manganese superoxide dismutase: effect of the ala16val polymorphism on protein, activity, and mRNA levels in human breast cancer cell lines and stably transfected mouse embryonic fibroblasts. Mol Cell Biochem. 2010;335(1–2):107–18. doi: 10.1007/s11010-009-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becuwe P, et al. Manganese superoxide dismutase in breast cancer: from molecular mechanisms of gene regulation to biological and clinical significance. Free Radic Biol Med. 2014;77:139–51. doi: 10.1016/j.freeradbiomed.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Ambrosone CB, et al. Manganese superoxide dismutase (MnSOD) genetic polymorphisms, dietary antioxidants, and risk of breast cancer. Cancer Res. 1999;59(3):602–6. [PubMed] [Google Scholar]
- 8.Mitrunen K, et al. Association between manganese superoxide dismutase (MnSOD) gene polymorphism and breast cancer risk. Carcinogenesis. 2001;22(5):827–9. doi: 10.1093/carcin/22.5.827. [DOI] [PubMed] [Google Scholar]
- 9.Bergman M, et al. Polymorphism in the manganese superoxide dismutase (MnSOD) gene and risk of breast cancer in young women. J Cancer Res Clin Oncol. 2005;131(7):439–44. doi: 10.1007/s00432-004-0663-7. [DOI] [PubMed] [Google Scholar]
- 10.Tamimi RM, et al. Manganese superoxide dismutase polymorphism, plasma antioxidants, cigarette smoking, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13(6):989–96. [PubMed] [Google Scholar]
- 11.Cai Q, et al. Genetic polymorphism in the manganese superoxide dismutase gene, antioxidant intake, and breast cancer risk: results from the Shanghai Breast Cancer Study. Breast Cancer Res. 2004;6(6):R647–55. doi: 10.1186/bcr929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egan KM, et al. MnSOD polymorphism and breast cancer in a population-based case-control study. Cancer Lett. 2003;199(1):27–33. doi: 10.1016/s0304-3835(03)00349-5. [DOI] [PubMed] [Google Scholar]
- 13.Millikan RC, et al. Manganese superoxide dismutase Ala-9Val polymorphism and risk of breast cancer in a population-based case-control study of African Americans and whites. Breast Cancer Res. 2004;6(4):R264–74. doi: 10.1186/bcr786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai SH, et al. Sirt3 attenuates hydrogen peroxide-induced oxidative stress through the preservation of mitochondrial function in HT22 cells. Int J Mol Med. 2014;34(4):1159–68. doi: 10.3892/ijmm.2014.1876. [DOI] [PubMed] [Google Scholar]
- 15.Li H, et al. Manganese superoxide dismutase polymorphism, prediagnostic antioxidant status, and risk of clinical significant prostate cancer. Cancer Res. 2005;65(6):2498–504. doi: 10.1158/0008-5472.CAN-04-3535. [DOI] [PubMed] [Google Scholar]
- 16.Mikhak B, et al. Manganese superoxide dismutase (MnSOD) gene polymorphism, interactions with carotenoid levels and prostate cancer risk. Carcinogenesis. 2008;29(12):2335–40. doi: 10.1093/carcin/bgn212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahlos K, et al. Proliferation, apoptosis, and manganese superoxide dismutase in malignant mesothelioma. Int J Cancer. 2000;88(1):37–43. doi: 10.1002/1097-0215(20001001)88:1<37::aid-ijc6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Arthur JR. The glutathione peroxidases. Cell Mol Life Sci. 2000;57(13–14):1825–35. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15(7):1957–97. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravn-Haren G, et al. Associations between GPX1 Pro198Leu polymorphism, erythrocyte GPX activity, alcohol consumption and breast cancer risk in a prospective cohort study. Carcinogenesis. 2006;27(4):820–5. doi: 10.1093/carcin/bgi267. [DOI] [PubMed] [Google Scholar]
- 21.Meplan C, et al. Association between polymorphisms in glutathione peroxidase and selenoprotein P genes, glutathione peroxidase activity, HRT use and breast cancer risk. PLoS One. 2013;8(9):e73316. doi: 10.1371/journal.pone.0073316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, et al. GPx-1 polymorphism (rs1050450) contributes to tumor susceptibility: evidence from meta-analysis. J Cancer Res Clin Oncol. 2011;137(10):1553–61. doi: 10.1007/s00432-011-1033-x. [DOI] [PubMed] [Google Scholar]
- 23.Hu YJ, Diamond AM. Role of glutathione peroxidase 1 in breast cancer: loss of heterozygosity and allelic differences in the response to selenium. Cancer Res. 2003;63(12):3347–51. [PubMed] [Google Scholar]
- 24.Bera S, et al. Natural allelic variations in glutathione peroxidase-1 affect its subcellular localization and function. Cancer Res. 2014;74(18):5118–26. doi: 10.1158/0008-5472.CAN-14-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shuvalova YA, et al. Association between polymorphisms of eNOS and GPx-1 genes, activity of free-radical processes and in-stent restenosis. Mol Cell Biochem. doi: 10.1007/s11010-012-1419-3. [DOI] [PubMed] [Google Scholar]
- 26.Cox DG, et al. No association between GPX1 Pro198Leu and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2004;13(11 Pt 1):1821–2. [PubMed] [Google Scholar]
- 27.Cox DG, Tamimi RM, Hunter DJ. Gene × Gene interaction between MnSOD and GPX-1 and breast cancer risk: a nested case-control study. BMC Cancer. 2006;6:217. doi: 10.1186/1471-2407-6-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhuo P, et al. Molecular consequences of genetic variations in the glutathione peroxidase 1 selenoenzyme. Cancer Res. 2009;69(20):8183–90. doi: 10.1158/0008-5472.CAN-09-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samuels BA, et al. Increased glutathione peroxidase activity in human sarcoma cell line with inherent doxorubicin resistance. Cancer Research. 1991;51:521–527. [PubMed] [Google Scholar]
- 30.Handy DE, et al. Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses. J Biol Chem. 2009;284:11913–21. doi: 10.1074/jbc.M900392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tobe R, et al. High error rates in selenocysteine insertion in mammalian cells treated with the antibiotic doxycycline, chloramphenicol, or geneticin. J Biol Chem. 2013;288(21):14709–15. doi: 10.1074/jbc.M112.446666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu FF, Doroshow JH, Esworthy RS. Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase, GSHPx-GI. J Biol Chem. 1993;268(4):2571–6. [PubMed] [Google Scholar]
- 33.Hart PC, et al. MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat Commun. 2015;6:6053. doi: 10.1038/ncomms7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65(23):3756–88. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, et al. Manganese superoxide dismutase induces migration and invasion of tongue squamous cell carcinoma via H2O2-dependent Snail signaling. Free Radic Biol Med. 2012;53(1):44–50. doi: 10.1016/j.freeradbiomed.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onder TT, et al. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68(10):3645–54. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 37.Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5(6):R217–22. doi: 10.1186/bcr651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng JC, Klausen C, Leung PC. Hydrogen peroxide mediates EGF-induced down-regulation of E-cadherin expression via p38 MAPK and snail in human ovarian cancer cells. Mol Endocrinol. 2010;24(8):1569–80. doi: 10.1210/me.2010-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 40.Ayala G, et al. High levels of phosphorylated form of Akt-1 in prostate cancer and non-neoplastic prostate tissues are strong predictors of biochemical recurrence. Clinical Cancer Research. 2004;10:6572–6578. doi: 10.1158/1078-0432.CCR-04-0477. [DOI] [PubMed] [Google Scholar]
- 41.Kreisberg JI, et al. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Research. 2004;64:5232–5236. doi: 10.1158/0008-5472.CAN-04-0272. [DOI] [PubMed] [Google Scholar]
- 42.Choi JH, et al. Potential inhibition of PDK1/Akt signaling by phenothiazines suppresses cancer cell proliferation and survival. Ann N Y Acad Sci. 2008;1138:393–403. doi: 10.1196/annals.1414.041. [DOI] [PubMed] [Google Scholar]
- 43.Taylor JM, et al. Diminished Akt phosphorylation in neurons lacking glutathione peroxidase-1 (Gpx1) leads to increased susceptibility to oxidative stress-induced cell death. J Neurochem. 2005;92(2):283–93. doi: 10.1111/j.1471-4159.2004.02863.x. [DOI] [PubMed] [Google Scholar]
- 44.Lindsay J, Esposti MD, Gilmore AP. Bcl-2 proteins and mitochondria--specificity in membrane targeting for death. Biochim Biophys Acta. 2011;1813(4):532–9. doi: 10.1016/j.bbamcr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 45.Hockenbery DM. bcl-2 in cancer, development and apoptosis. J Cell Sci Suppl. 1994;18:51–5. doi: 10.1242/jcs.1994.supplement_18.7. [DOI] [PubMed] [Google Scholar]
- 46.Hockenbery DM, et al. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75(2):241–51. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 47.Hart PC, et al. MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat Commun. 2015;6 doi: 10.1038/ncomms7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giralt A, Villarroya F. SIRT3, a pivotal actor in mitochondrial functions: metabolism, cell death and aging. Biochem J. 2012;444(1):1–10. doi: 10.1042/BJ20120030. [DOI] [PubMed] [Google Scholar]
- 49.Kim HS, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17(1):41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bell EL, et al. SirT3 suppresses hypoxia inducible factor 1alpha and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011;30(26):2986–96. doi: 10.1038/onc.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tao R, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40(6):893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fritz KS, et al. Mitochondrial acetylome analysis in a mouse model of alcohol-induced liver injury utilizing SIRT3 knockout mice. J Proteome Res. 2012;11(3):1633–43. doi: 10.1021/pr2008384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nasr MA, et al. GPx-1 modulates Akt and P70(S6K) phosphorylation and Gadd45 levels in MCF-7 cells. Free Radic Biol Med. 2004;37(2):187–95. doi: 10.1016/j.freeradbiomed.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 54.Faucher K, et al. Overexpression of human GPX1 modifies Bax to Bcl-2 apoptotic ratio in human endothelial cells. Mol Cell Biochem. 2005;277(1–2):81–7. doi: 10.1007/s11010-005-5075-8. [DOI] [PubMed] [Google Scholar]
- 55.Kwiatkowska E, et al. Effect of 3-bromopyruvate acid on the redox equilibrium in non-invasive MCF-7 and invasive MDA-MB-231 breast cancer cells. J Bioenerg Biomembr. 2016;48(1):23–32. doi: 10.1007/s10863-015-9637-5. [DOI] [PubMed] [Google Scholar]
- 56.Chen PM, et al. MnSOD overexpression confers cisplatin resistance in lung adenocarcinoma via the NF-kappaB/Snail/Bcl-2 pathway. Free Radic Biol Med. 2015;79:127–37. doi: 10.1016/j.freeradbiomed.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Piotrowska H, Kucinska M, Murias M. Expression of CYP1A1, CYP1B1 and MnSOD in a panel of human cancer cell lines. Mol Cell Biochem. 2013;383(1–2):95–102. doi: 10.1007/s11010-013-1758-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.