Abstract
Objective
To compare risk of re-intervention, long-term clinical outcomes, and health care utilization among women who have bulk symptoms from leiomyoma and who underwent following procedures: hysterectomy, myomectomy, uterine artery embolization (UAE), and magnetic resonance–guided, focused ultrasound surgery.
Methods
This was a retrospective analysis of administrative claims from a large U.S. commercial insurance database. Women aged 18–54 years undergoing any of the above leiomyoma procedures between 2000 and 2013 were included. We assessed following outcome measures: risk of re-intervention between uterine-sparing procedures, risk of other surgical procedures or complications of the index procedure, 5-year health care utilization; pregnancy rates, and reproductive outcomes. Propensity score matching along with Cox proportional hazard models were used to adjust for differences in baseline characteristics between study cohorts.
Results
Among the 135,522 study-eligible women with mean follow-up of 3.4 years, hysterectomy was the most common first-line procedural therapy (111,324; 82.2%) followed by myomectomy (19,965; 14.7%), UAE (4,186; 3.1%) and magnetic resonance–guided focused ultrasound surgery (47; 0.0003%). Small but statistically significant differences were noted for UAE and myomectomy in re-intervention rate (17.1% versus 15.0%, p=0.02), subsequent hysterectomy rates (13.2% versus 11.1%, p<0.01) and subsequent complications from index procedures (18.1% versus 24.6%, p<0.001). During follow-up, women undergoing myomectomy had lower leiomyoma-related health care utilization, but had higher all-cause outpatient services. Pregnancy rates were 7.5% and 2.2% among myomectomy and UAE cohorts, respectively (p<0.001), with both cohorts having similar rate of adverse reproductive outcome (69.4%).
Conclusions
While the overwhelming majority of women having leiomyoma with bulk symptoms underwent hysterectomy as their first treatment procedure, among those undergoing uterine-sparing index procedures, approximately one seventh had a re-intervention, and one tenth ended up undergoing hysterectomy during follow-up. Compared with women undergoing myomectomy, women undergoing UAE had a higher risk of re-intervention, lower risk of subsequent complications, but similar rate of adverse reproductive outcomes.
INTRODUCTION
Uterine myomas (leiomyomas) are very common in reproductive-age women with cumulative incidence reaching up to 70% in white women and 80% in black women by the age 50.1 Leiomyomas impose significant economic burden with annual medical cost for women with leiomyomas reaching $8,463 higher than those without (in 2005 dollars).2 The overall economic burden of leiomyomas in the U.S. ranges from $5.9 to $34.4 billion (in 2010 dollars),3,4 with such a wide range indicating difficulty in measuring leiomyoma-related costs, particularly indirect costs (e.g., lost wages).
Leiomyomas can cause debilitating symptoms including heavy menstrual bleeding, bulk symptoms such as pelvic pain, urinary problems, and constipation.5–7 Hysterectomy, which eliminates most leiomyoma symptoms and the possibility of formation of new leiomyomas, is the mainstay of treatment for leiomyomas. Up to 50% of all hysterectomies performed in the U.S. have a discharge diagnosis of leiomyomas.8–10 However, hysterectomy is not acceptable to many women, especially to those desiring fertility. Alternatives to hysterectomy for treating leiomyoma-related bulk symptoms include the following uterine-sparing options:myomectomy, uterine artery embolization (UAE), and magnetic resonance–guided, focused ultrasound surgery. However, the real-world evidence on the comparative effectiveness between alternative procedural treatments for leiomyoma-related bulk symptoms is sparse.11 Specifically, extant evidence demonstrates substantial variation in the re-intervention rates and reproductive outcomes between different leiomyoma treatment procedures.12–19
Therefore, the primary objective of this study was to compare the risk of re-intervention between different uterine-sparing leiomyoma procedures. The secondary objectives were to compare long-term health care utilization and reproductive outcomes between leiomyoma procedures.
MATERIALS AND METHODS
We conducted a retrospective analysis of administrative claims data from a large U.S. commercial insurance database, Optum Labs Data Warehouse (OLDW), which includes both privately insured and Medicare Advantage enrollees throughout the U.S. OLDW contains longitudinal health information on over 100 million enrollees over the last 20 years, from geographically diverse regions across the U.S., with greatest representation from the South and Midwest20. Healthcare claims data are generated as part of usual clinical practice in which care providers submit claims to payer organizations for reimbursement in standardized format (e.g., UB-04 form or CMS 1500 form). Payer organizations conduct internal checks to validate consistency and accuracy of the claims submitted. Given that the OLDW captures data from one of the largest commercial insurance companies in the U.S., the population of women with leiomyoma drawn from this database will resemble the commercially insured leiomyoma patient population in the U.S. The health plans included in the database provide claims for professional (e.g., physician), facility (e.g., hospital), and outpatient prescription medication services. Medical (professional, facility) claims include International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes, ICD-9 procedure codes, Current Procedural Terminology, Version 4 (CPT-4) procedure codes and Healthcare Common Procedure Coding System (HCPCS) procedure codes. Study data were accessed using techniques compliant with the Health Insurance Portability and Accountability Act of 1996 (HIPAA). Because this study involved analysis of pre-existing, de-identified data, it was exempt from Institutional Review Board approval.
We identified women aged 18–54 years who had diagnoses of leiomyoma and bulk symptoms, and who underwent either a uterine-sparing procedure that was expected to reduce bulk or size-related symptoms (e.g., myomectomy, UAE and magnetic resonance–guided focused ultrasound surgery) or hysterectomy between 1/1/2000–12/31/2013 (Appendix 1, available online at http://links.lww.com/xxx, for relevant diagnosis codes, and Appendix 2, also available online at http://links.lww.com/xxx, for relevant procedure codes). Bulk symptoms include pelvic pain or pressure, urinary problems, nocturia, constipation, and dyspareunia. Women’s first procedural therapy after the leiomyoma and bulk diagnoses during the study period was defined as the “index procedure”, which also defined their study cohorts. For example, “myomectomy cohort” refers to women who received myomectomy as their index procedure. The date of the index procedure was defined as the “index date”. The 2-week period prior to the index date was considered as “pre-operative period”. The 12-month period before the pre-operative period was defined as the “baseline”. All women included in the study were required to have continuous health insurance during the pre-operative and baseline periods and also for a minimum of 6 months after the index date. Beyond the minimum required enrollment of 6 months in the follow-up, women in the study were followed until they disenrolled from the health plans or the end of the study period (June 30th, 2014). We excluded women who received any procedural therapies for leiomyoma prior to the index date as well as those with uterine cancer (see Appendix 1, http://links.lww.com/xxx, for codes).
The two primary outcomes of the study were (i) whether women undergoing any of the uterine-sparing leiomyoma procedures (myomectomy, UAE and magnetic resonance–guided, focused ultrasound surgery) as the index procedure, underwent any subsequent leiomyoma procedure during follow-up (re-intervention); and (ii) whether they underwent other surgical procedures that could have been done to treat potential complications of the index procedure. In defining the first primary outcome, if a woman had a subsequent claim for the same procedure as the index procedure within 30 days, we considered the latter as part of the initial treatment rather than a separate procedure or re-intervention. For example, if a women underwent myomectomy as the index procedure, and a second myomectomy was observed within a month, we considered the latter procedure as being related to the index procedure, and did not count it as re-intervention. But if the woman underwent a UAE or hysterectomy within 30 days following the index myomectomy, we considered it as a re-intervention. We also examined whether women eventually received a hysterectomy at any point during follow-up. The second outcome of interest was subsequent surgical procedures or complications related to the primary procedure following the index leiomyoma procedure. (See Appendix 2, http://links.lww.com/xxx)
We assessed three secondary outcomes. First, we compared long-term health care utilization, including leiomyoma-related outpatient, inpatient and emergency department (ED) visits, all-cause outpatient, inpatient and ED visits between the leiomyoma procedures. Unique visits were defined by health care claims with dates of service on different days for outpatient and ED, and number of different episodes of inpatient stay. Long-term refers to a minimum of 5 years of follow-up from the index procedure with continuous insurance. Leiomyoma-related health care utilization was defined as a claim with a leiomyoma diagnosis. While this definition of leiomyoma-related utilization may include visits for unrelated concerns (e.g., pelvic inflammatory disease) but with a fibroid diagnosis attached, this possibility is rather minimal given that these outcome measures are assessed following the index leiomyoma procedure. Multiple claims on the same day were counted as one event. Second, we assessed health care utilization between women who received hysterectomy as the index procedure with those that received any of the three uterine-sparing procedures as the index procedure but underwent hysterectomy during follow-up. Addressing this sub-aim will help quantify the difference in health care utilization between those who undergo hysterectomy as the first procedure vs. those who first try uterine-sparing procedures but eventually undergo hysterectomy. Third, we compared reproductive outcomes between women undergoing myomectomy and UAE as index procedure. Specifically, we assessed the rates of pregnancies in these two cohorts following the index procedure, and among those who became pregnant, the rates of other reproductive outcomes including term delivery, preterm delivery and spontaneous abortion.
For each study woman, the database provided information on demographic and socioeconomic characteristics as of the index date (age, race, household income, and residence region), her co-morbidities, Charlson-Deyo comorbidity index,21 and health care utilization measures at baseline. Categorical variables were reported in terms of counts and percents, and compared between the cohorts using chi-square tests. For continuous variables, means and standard deviations (SD) were reported and were compared between the study cohorts using t-test. For non-normal variables such as follow-up time and duration, median and inter-quartile range (IQR) were presented, and Kruskal-Wallis test was used to compare these outcomes between the cohorts.
To compare outcomes between the study cohorts, we use propensity score (PS) matching method. The PS is the probability that a subject receives a specific treatment under the study, which in our case will be the probability of undergoing a specific leiomyoma treatment procedure. The PS is estimated using a logistic regression, accounting for the baseline covariates that influence the receipt of a procedure. Two subjects from two different cohorts (procedures) but with similar PS have the similar probability of receiving any of the two procedures, and therefore they can be statistically interchanged as control for each other. In essence, the PS method enables creation of study cohorts that are balanced in terms of observed patient characteristics in a very simple and straightforward manner.22 The other advantages of the PS method compared to traditional multivariate methods have been described elsewhere.22 One-to-one propensity score matching was used to balance the differences in baseline characteristics between the study cohorts.23,24 Patient characteristics reported in Table 1 (barring patient counts, and follow-up duration) were adjusted in the PS matching.
Table 1.
Patient baseline characteristics, stratified by index procedure (N=135,475)
| Myomectomy | UAE | Hysterectomy | Overall | p-value | |
|---|---|---|---|---|---|
|
| |||||
| Patients, N (%) | 19,965 (14.7%) | 4,186 (3.1%) | 111,324 (82.2%) | 135,522 | |
| Age, years, mean (SD) | 38.4 (6.6) | 44.0 (5.4) | 44.1 (5.5) | 43.2 (6.0) | <0.01 |
| Race, N (%) | <0.01 | ||||
| Asian | 714 (3.6%) | 106 (2.5%) | 1762 (1.6%) | 2582 (1.9%) | |
| Black | 2744 (13.7%) | 823 (19.7%) | 9528 (8.6%) | 13099 (9.7%) | |
| Hispanic | 1564 (7.8%) | 224 (5.4%) | 8203 (7.4%) | 9994 (7.4%) | |
| White | 8443 (42.3%) | 1702 (40.7%) | 56747 (51.0%) | 66913 (49.4%) | |
| Unknown | 6500 (32.6%) | 1331 (31.8%) | 35084 (31.5%) | 42934 (31.7%) | |
| Income level, N (%) | <0.01 | ||||
| < $40,000 | 2234 (11.2%) | 490 (11.7%) | 12743 (11.4%) | 15472 (11.4%) | |
| $40,000 – $49,999 | 1695 (8.5%) | 349 (8.3%) | 11226 (10.1%) | 13270 (9.8%) | |
| $50,000 – $59,999 | 1621 (8.1%) | 374 (8.9%) | 10575 (9.5%) | 12574 (9.3%) | |
| $60,000 – $74,999 | 2119 (10.6%) | 430 (10.3%) | 12676 (11.4%) | 15232 (11.2%) | |
| $75,000 – $99,999 | 2724 (13.6%) | 562 (13.4%) | 15048 (13.5%) | 18341 (13.5%) | |
| ≥ $100,000 | 3198 (16.0%) | 687 (16.4%) | 14749 (13.2%) | 18640 (13.8%) | |
| Unknown | 6374 (31.9%) | 1294 (30.9%) | 34307 (30.8%) | 41993 (31.0%) | |
| Region, N (%) | <0.01 | ||||
| Midwest | 3516 (17.6%) | 891 (21.3%) | 27599 (24.8%) | 32023 (23.6%) | |
| Northeast | 4538 (22.7%) | 897 (21.4%) | 9759 (8.8%) | 15198 (11.2%) | |
| South | 9301 (46.6%) | 1924 (46.0%) | 57046 (51.2%) | 68290 (50.4%) | |
| West | 2561 (12.8%) | 467 (11.2%) | 16565 (14.9%) | 19600 (14.5%) | |
| Unknown | 49 (0.2%) | 7 (0.2%) | 355 (0.3%) | 411 (0.3%) | |
| Charlson Index at baseline, N (%) | <0.01 | ||||
| 0 | 15031 (75.3%) | 2676 (63.9%) | 68990 (62.0%) | 86734 (64.0%) | |
| 1 | 3539 (17.7%) | 1026 (24.5%) | 28992 (26.0%) | 33565 (24.8%) | |
| ≥2 | 1395 (7.0%) | 484 (11.6%) | 13342 (12.0%) | 15223 (11.2%) | |
| Baseline Comorbidities, N (%) | |||||
| Pelvic pain | 13759 (68.9%) | 2636 (63.0%) | 71317 (64.1%) | 87737 (64.7%) | <0.01 |
| Anemias | 4416 (22.1%) | 1470 (35.1%) | 24601 (22.1%) | 30499 (22.5%) | <0.01 |
| Inflammatory diseases | 1058 (5.3%) | 89 (2.1%) | 5165 (4.6%) | 6313 (4.7%) | <0.01 |
| Noninflammatory diseases | 6370 (31.9%) | 1164 (27.8%) | 35362 (31.8%) | 42905 (31.7%) | <0.01 |
| Endometriosis | 1639 (8.2%) | 161 (3.8%) | 10634 (9.6%) | 12436 (9.2%) | <0.01 |
| Benign neoplasm of the uterus/ovary | 674 (3.4%) | 132 (3.2%) | 5258 (4.7%) | 6065 (4.5%) | <0.01 |
| Disorders of uterus | 4201 (21.0%) | 1097 (26.2%) | 25051 (22.5%) | 30361 (22.4%) | <0.01 |
| Urinary problems | 3597 (18.0%) | 1045 (25.0%) | 23624 (21.2%) | 28280 (20.9%) | <0.01 |
| Constipation or gas | 1891 (9.5%) | 448 (10.7%) | 9654 (8.7%) | 11997 (8.9%) | <0.01 |
| Dyspareunia | 1094 (5.5%) | 158 (3.8%) | 7178 (6.4%) | 8433 (6.2%) | <0.01 |
| Pregnancy | 2567 (12.9%) | 195 (4.7%) | 2999 (2.7%) | 5761 (4.3%) | <0.01 |
| Baseline Health Care Utilization, Mean (SD) | |||||
| UF-Related Outpatient | 2.5 (2.7) | 3.5 (2.4) | 1.5 (1.8) | 1.7 (2.0) | <0.01 |
| UF-Related ED & Inpatient | 0.1 (0.4) | 0.1 (0.4) | 0.1 (0.3) | 0.1 (0.3) | <0.01 |
| Any Outpatient | 14.9 (12.8) | 15.0 (13.0) | 14.0 (12.1) | 14.2 (12.2) | <0.01 |
| Any ED & Inpatient | 0.5 (1.1) | 0.6 (1.2) | 0.6 (1.4) | 0.6 (1.3) | <0.01 |
|
| |||||
| Follow-up time(# years), Median(IQR) | 2.6 (1.3–4.8) | 2.6 (1.4–4.6) | 2.6 (1.3–4.8) | 2.6 (1.3–4.8) | 0.34 |
Note: IQR – Inter Quartile Range.
We created four different propensity-score matched cohorts to assess different outcomes. First, in order to compare rates of re-intervention, subsequent other surgical procedures (which could be a consequence or complication of the index procedure), and pregnancy occurrences among women undergoing myomectomy and UAE, we matched women undergoing myomectomy and UAE. Second, to compare 5-year health care utilization, we again matched women undergoing myomectomy and UAE but required at least 5 years of follow-up with continuous health insurance. Third, in order to compare health care utilization between women undergoing hysterectomy as the initial procedure and those undergoing hysterectomy as a subsequent procedure following an index uterine-sparing leiomyoma procedure, we matched these two cohorts of women through propensity score matching that had at least 5 years of follow-up. Fourth, to compare reproductive outcomes following pregnancy after myomectomy or UAE, we matched women who underwent myomectomy with those who underwent UAE and became pregnant during follow-up. As will be explained in the Results section, due to small sample size, only descriptive statistics were provided for the outcomes associated with magnetic resonance–guided, focused ultrasound surgery cohort.
In order to account for variable follow-up, we further analyzed the risks of primary outcomes – subsequent leiomyoma and other surgical procedures – through Cox proportional hazard model on the corresponding matched cohorts. All analyses were conducted using SAS 9.3 (SAS Institute Inc, Cary, North Carolina) and Stata 13.1 (Stata Corp, College Station, TX).
RESULTS
We identified 135,522 women with leiomyoma and bulk symptoms who received one of the four procedural therapies between 1/1/2000 and 12/31/2013. The flow diagram in Figure 1 indicates how the final study sample was obtained from the OLDW database after apply the inclusion and exclusion criteria. The overwhelming majority of women received hysterectomy as the index procedure (111,324 or 82.2%), while 19,965 (14.7%) and 4,186 (3.1%) received myomectomy and UAE, respectively (Table 1). While not shown in the table due space constraint, the percentage of women with leiomyoma receiving hysterectomy as the index procedure decreased from 87% in 2000 to 82% in 2002, which stabilized around the latter percentage for the remainder of the study period. Since only a very small number of women (n=47; 0.0003%) underwent magnetic resonance–guided, focused ultrasound surgery, we restricted their analysis to a limited number of descriptive outcomes, and relevant data pertaining to the magnetic resonance–guided, focused ultrasound surgery cohort are presented in Appendixes 3–5, available online at http://links.lww.com/xxx. The mean age for the overall study sample was 43.2 years (SD 6.0). Myomectomy cohort was younger (mean age 38.4 years) and a higher proportion of them had a pregnancy during the baseline period (12.9% for myomectomy cohort compared to 4.3% of the whole cohort). On average, women in the study were followed for about 3.4 years. Other notable findings are that Black women opted for UAE at a substantially higher rate than other treatment options, and that women seeking magnetic resonance–guided, focused ultrasound surgery and UAE appear to have the highest leiomyoma-related outpatient utilization. (see Table 1)
Figure 1.
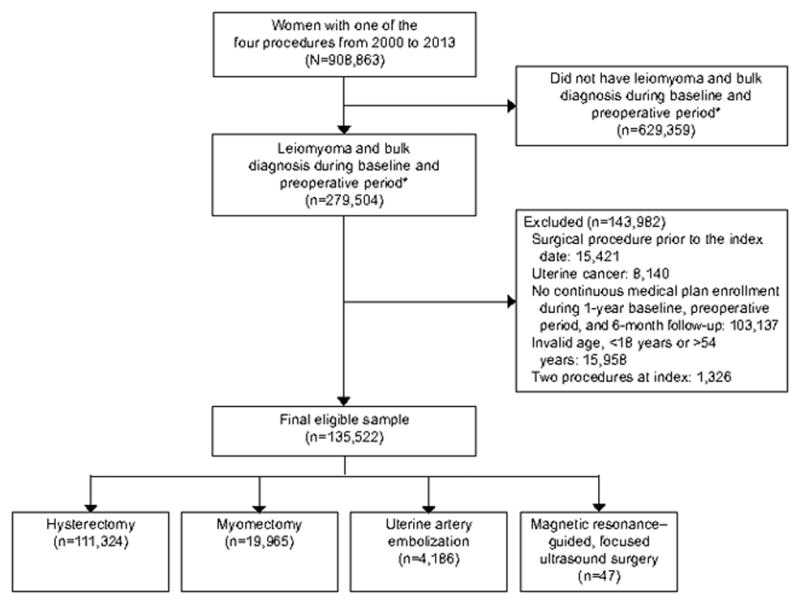
Patient selection. *379 days (365+14) prior to the index procedure.
As presented in Table 2, among the 24,151 women receiving a uterine-sparing index procedure other than magnetic resonance–guided, focused ultrasound surgery, 3,358 (13.9%) received a subsequent leiomyoma procedure (Table 2). Hysterectomy was the most common subsequent leiomyoma procedure, with 2,150 (64.0%) undergoing hysterectomy, followed by myomectomy (29.2%) and UAE (6.7%). The average time between the index procedure and the subsequent leiomyoma procedure was 2.2 (SD 2.1) years. Overall, 2,316 (9.6%) women ended up having a hysterectomy during follow-up, which includes not only the 2,150 women described above who had hysterectomy as their second procedure subsequent to the index procedure but also those who had a leiomyoma procedure other than hysterectomy as the second procedure but ended up having hysterectomy beyond the second procedure. The average time between the index procedure and the subsequent hysterectomy was 2.4 (SD 2.3) years.
Table 2.
Subsequent leiomyoma procedures, stratified by index procedure
| Myomectomy (N=19965) | UAE (N=4186) | Overall (N=24,151) | p-value | |
|---|---|---|---|---|
|
| ||||
| Subsequent leiomyoma procedure, N (%) | 2648 (13.3%) | 689 (16.5%) | 3358 (13.9%) | 0.01 |
|
| ||||
| Leiomyoma procedure type1 among those with a second procedure | n=2648 | n=689 | n=3358 | <0.01 |
|
| ||||
| MRgFUS, n (%) | 0 (0.0%) | 0 (0.0%) | 4 (0.1%) | |
| Myomectomy, n (%) | 866 (32.7%) | 109 (15.8%) | 980 (29.2%) | |
| UAE, n (%) | 132 (5.0%) | 91 (13.2%) | 224 (6.7%) | |
| Hysterectomy, n (%) | 1650 (62.3%) | 489 (71.0%) | 2150 (64.0%) | |
|
| ||||
| Duration2 between index leiomyoma procedure and any subsequent leiomyoma procedure (# years), Mean (SD) | 1.6 (0.6–3.2) | 1.3 (0.7–2.7) | 1.5 (0.6–3.1) | 0.04 |
|
| ||||
| Subsequent hysterectomy3, N (%) | 1773 (8.9%) | 530 (12.7%) | 2316 (9.6%) | <0.01 |
|
| ||||
| Duration between index leiomyoma procedure and subsequent hysterectomy (# years), Mean(SD) | 1.8 (0.7–3.6) | 1.6 (0.8–2.9) | 1.8 (0.7–3.4) | 0.27 |
Leiomyoma procedure types were based on the type of the second procedure among those who had a subsequent leiomyoma procedure(s).
Duration refers to the duration between the index leiomyoma procedure and the second leiomyoma procedure.
Hysterectomy here includes both second procedures and any subsequent procedures that were hysterectomies.
Table 3 presents the re-intervention rates between propensity-score-matched women in the myomectomy and UAE cohorts. In the propensity-score-matched cohorts of women undergoing myomectomy and UAE, small but statistically significant difference in overall re-intervention rate myomectomy and UAE cohort was found (15.0% vs. 17.1%; p=0.02). While the rates of subsequent leiomyoma procedure were similar in year 1 and 2 following the index procedure at approximately 7% and 12–13% for both myomectomy and UAE cohorts, the differences became statistically significant from the third year onward (15.0% vs. 19.4%, p=0.03 for third year). Similar patterns were observed when examining subsequent hysterectomies. Overall, 11.1% of the women undergoing myomectomy had hysterectomy compared to 13.2% of women who underwent UAE (p<0.01). The rates of hysterectomies were similar between myomectomy and UAE cohorts in the first two years at approximately 5% and 9% respectively. However, by the third year, significant difference emerged, with the rates of hysterectomy in women undergoing myomectomy being 10.5% compared to 14.6% for women undergoing UAE (p=0.02). When analyzed by a Cox proportional hazard model, UAE cohort was 22% more likely to receive a subsequent leiomyoma procedure (HR=1.22, 95% CI: 1.09–1.37; p<0.001), and 32% more likely to receive a subsequent hysterectomy (HR=1.32, 95% CI: 1.16–1.50; p<0.001) than myomectomy cohort (data not shown in the tables).
Table 3.
Re-intervention rates at different follow-up time points after propensity score matching (N=7,752)
| Myomectomy (N=3876) | UAE (N=3876) | p-value | |
|---|---|---|---|
|
| |||
| Any subsequent leiomyoma procedure | |||
| Overall (N=7,752)i | 583 (15.0%) | 661 (17.1%) | 0.02 |
| 1 year (N=5,626)ii | 187 (6.6%) | 192 (6.8%) | 0.79 |
| 2 year (N=2,932)iii | 173 (11.8%) | 201 (13.7%) | 0.12 |
| 3 year (N=1,546)iv | 116 (15.0%) | 150 (19.4%) | 0.03 |
| 4 year (N=812)v | 74 (18.2%) | 94 (23.2%) | 0.08 |
| 5 year (N=444)vi | 50 (22.5%) | 59 (26.6%) | 0.33 |
|
| |||
| Any subsequent Hysterectomy | |||
| Overall (N=7,752)i | 11.0% | 13.2% | <0.01 |
| 1 year (N=5,626)ii | 131 (4.7%) | 130 (4.6%) | 0.95 |
| 2 year (N=2,932)iii | 124 (8.5%) | 144 (9.8%) | 0.20 |
| 3 year (N=1,546)iv | 81 (10.5%) | 113 (14.6%) | 0.02 |
| 4 year (N=812)v | 53 (13.1%) | 70 (17.2%) | 0.10 |
| 5 year (N=444)vi | 37 (16.7%) | 42 (18.9%) | 0.54 |
Overall numbers does not account for the variable length of follow-up beyond the minimum six months.
Requires that patients had a minimum of 1 year of continuous insurance.
Requires that patients had a minimum of 2 years of continuous insurance.
Requires that patients had a minimum of 3 years of continuous insurance.
Requires that patients had a minimum of 4 years of continuous insurance.
Requires that patients had a minimum of 5 years of continuous insurance.
Significantly more women in myomectomy cohort underwent at least one subsequent surgical procedure than in UAE cohort: 24.6% versus 18.1% (p<0.001), with corresponding hazard of 1.38 (95% CI: 1.25 to 1.52). In addition, 18.6% women who underwent myomectomy and 13.2% who underwent UAE (p<0.001) had subsequent surgical procedures other than leiomyoma procedures, with corresponding hazard of 1.39 (95% CI: 1.24 to 1.56) (Table 4).
Table 4.
Subsequent surgical procedures or complications after propensity score matching (N=7752)
| Myomectomy (N=3876) | UAE (N=3876) | p value | HR (95% CI) | |
|---|---|---|---|---|
|
| ||||
| Esophagus/stomach | 15 (0.4%) | 21 (0.5%) | 0.16 | 0.62 (0.32, 1.20) |
| Intestine/appendix/rectum/anus | 311 (8.0%) | 193 (5.0%) | <0.01 | 1.55 (1.30, 1.86) |
| Liver/gallbladder/pancreas | 47 (1.2%) | 61 (1.6%) | 0.09 | 0.71 (0.49, 1.05) |
| Hernia | 68 (1.8%) | 48 (1.2%) | 0.15 | 1.31 (0.91, 1.91) |
| Urinary bladder | 75 (1.9%) | 65 (1.7%) | 0.70 | 1.07 (0.77, 1.49) |
| Urinary | 72 (1.9%) | 72 (1.9%) | 0.67 | 0.93 (0.67, 1.29) |
| Ovary/fallopian tubes | 352 (9.1%) | 227 (5.9%) | <0.01 | 1.50 (1.27, 1.77) |
| Cervix/uterus | 560 (14.4%) | 458 (11.8%) | 0.01 | 1.18 (1.05, 1.34) |
| Vagina | 67 (1.7%) | 32 (0.8%) | <0.01 | 1.99 (1.30, 3.04 |
|
| ||||
| Any of the above procedures | 954 (24.6%) | 702 (18.1%) | <0.01 | 1.38 (1.25, 1.52) |
| Any of the above procedures except uterine or cervix | 720 (18.6%) | 513 (13.2%) | <0.01 | 1.39 (1.24, 1.56) |
Within our cohort, 31,294 (23%) women had at least 5 years of follow-up. We compared their mean (±SD) health care utilization in terms of number of visits, including both leiomyoma-related, and all-cause health care utilization during the 5 years following the index procedure (Table 5). As expected, the hysterectomy cohort had little leiomyoma-related health care utilization during follow-up, and they also had lower utilization of any leiomyoma-related outpatient services than UAE and myomectomy cohorts. UAE cohort had more leiomyoma-related outpatient services and higher leiomyoma-related ED and inpatient visits, but lower utilization of all-cause outpatient services than myomectomy cohort.
Table 5.
Five-year healthcare utilization (N=31,277), Median (IQR)
| Myomectomy (N=4,684) | UAE (N=910) | Hysterectomy (N=25,683) | p-value | |
|---|---|---|---|---|
| Leiomyoma-Related Outpatient Visits | 1 (0–4) | 4 (2–6) | 0 (0–0) | <0.01 |
| Leiomyoma-Related Emergency Department and Inpatient Visits | 0 (0–0) | 0 (0–0) | 0 (0–0) | <0.01 |
| All-Cause Outpatient Visits | 53 (32–86) | 47 (28–76) | 46 (27–79) | <0.01 |
| All-Cause Emergency Department and Inpatient Visits | 1 (0–3) | 1 (0–2) | 1 (0–2) | <0.01 |
In the propensity-score-matched cohorts of myomectomy and UAE who had at least 5 years of follow-up, UAE cohort had higher leiomyoma-related outpatient services (4.6 vs. 2.7, p<0.001), but lower utilization of all-cause outpatient services (62.5 vs. 68.6, p=0.009) (Appendix 6, available online at http://links.lww.com/xxx).
In the propensity-score-matched cohorts of women who underwent hysterectomy as their first (index) leiomyoma procedure versus those who underwent a uterine-sparing procedure as the first procedure but subsequently received hysterectomy, the former had significantly lower leiomyoma-related or all-cause health care utilizations during the 5-year follow-up period. For example, women who underwent hysterectomy as their first procedure had 0.2 leiomyoma-related outpatient visits compared to 4.2 visits (p<0.001) for those who first had a uterine-sparing procedure followed by hysterectomy; the former group of women had 59.2 all-cause outpatient visits vs. 72.0 all-cause outpatient visits for the latter group (p<0.001) (see Appendix 7, available online at http://links.lww.com/xxx).
The percentage of women in myomectomy cohort who became pregnant was 17.8% compared to 2.0% in the corresponding UAE cohort (7.5% versus 2.2% in the matched cohorts, p<0.001; data not shown in Tables). Among women who became pregnant following the index myomectomy or UAE, we did a propensity score matching between these two cohorts (see Table 6). However, we did not find any significant differences between the two groups for any reproductive outcomes. In fact, the percentages of women who experienced any adverse reproductive outcome were identical in the two cohorts (69.4%, Table 6).
Table 6.
Reproductive Outcomes after propensity score matching (N=170)
| Reproductive Outcomes | Myomectomy (N=85) | UAE (N=85) | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| Spontaneous abortion | 10 (11.8%) | 14 (16.5%) | 0.38 | ||
| Ectopic pregnancy | 4 (4.7%) | 4 (4.7%) | <0.99 | ||
| Cesarean section | 38 (44.7%) | 39 (45.9%) | 0.88 | ||
| Postpartum hemorrhage | 2 (2.4%) | 7 (8.2%) | 0.09 | ||
| Abnormal placentation | 8 (9.4%) | 11 (12.9%) | 0.47 | ||
| Preterm delivery | 10 (11.8%) | 4 (4.7%) | 0.09 | ||
| Malpresentation | 11 (12.9%) | 6 (7.1%) | 0.20 | ||
| Small for gestational age | 11 (12.9%) | 5 (5.9%) | 0.12 | ||
| Any of the above adverse outcomes | 59 (69.4%) | 59 (69.4%) | <0.99 | ||
|
| |||||
| Therapeutic abortion | 3 (3.5%) | 5 (5.9%) | 0.47 | ||
| Normal delivery | 12 (14.1%) | 17 (20.0%) | 0.31 | ||
|
| |||||
| Number of Pregnancy, Median (IQR) | 1 (1–1) | 1 (1–1) | 0.54 | ||
|
| |||||
| # Follow-up years, Median (IQR) | 3.1 (1.9–5.1) | 4.0 (2.6–5.7) | 0.07 | ||
DISCUSSION
This study provides new information regarding myomectomy and UAE, two widely used alternatives to hysterectomy for leiomyomas. Consistent with previous studies,16–18 we found that a higher percentage of women undergoing UAE needed re-intervention. In contrast to the overall re-intervention rates for UAE (17.1%) and myomectomy (15.0%) found in our study, previously reported re-intervention rates for UAE (14.0% to 36.7%) and myomectomy (2.7% to 6.1%) varied considerably; our study found substantially higher re-intervention rate for UAE than previously reported.16–18 The current evidence on the complication rates between UAE and myomectomy is mixed.14,18,19 Our finding of higher complication rates for the myomectomy cohort aligns with those studies that found higher complication rate in myomectomy than UAE.18,19 However, note that such mixed evidence may stem from differences in complication definitions and the time periods during which complications were captured.
Reproductive outcomes including pregnancy are key criteria for many women and providers while deciding on leiomyoma treatment options. In this study, a much smaller percentage of women undergoing UAE became pregnant compared to those undergoing myomectomy. There have been concerns about ovarian failure after UAE,25 although the loss of ovarian function after UAE was found primarily in women aged 45 or older.26 It is noteworthy that women who underwent index UAE were more likely to undergo hysterectomy subsequently than those undergoing index myomectomy (13.1% vs. 11.0%), potentially suggesting that myomectomy patients likely had a greater interest in subsequent pregnancy. A limitation of this study is that health care claims data do not capture women’s pregnancy intent or other relevant outcomes such as ovarian reserve reduction, and therefore the study could not make any definitive inference on pregnancy rates between UAE and myomectomy. The lower pregnancy rate for UAE was likely due in part to both patient and provider preference toward myomectomy for pregnancy optimization, which is also supported by the American College of Obstetricians and Gynecologists.27 This is also reflected in our data that women in myomectomy cohort were more likely to have a pregnancy in the baseline period. A previous study found women who underwent UAE were less likely to attempt to get pregnant, but those who did had a higher success rate than women undergoing myomectomy.19 Our study could not confirm this finding, but it was clear that between propensity-matched women from UAE and myomectomy cohorts who became pregnant, the likelihoods of experiencing an array of adverse reproductive outcomes and delivery rates were similar albeit the small sample size following propensity-matching.
While alternatives to hysterectomy have become increasingly available in recent years, this study confirms that an overwhelming majority of the commercially-insured women with leiomyoma-related bulk symptoms underwent hysterectomy. Given that hysterectomy is the definitive treatment for leiomyoma, the rate of hysterectomy with underlying leiomyoma diagnosis continues to be high at around 80%, with a small decline in the early 2000s.8,28 This finding has been confirmed in our study as well with the rate stabilizing at around 82% since 2002. While hysterectomy leads to lower health care utilization over 5 years compared to women who undergo uterine-sparing procedures, there may be long-term adverse consequences of hysterectomy including fracture risk, pelvic organ prolapse, cardiovascular disease risk and risk of dementia, which may often take 20 to 30 years to manifest.29–32
Our study has the usual limitations of claims-based analyses including the general limitation that that claims are generated for reimbursement purpose but not for research. Nevertheless, we adopted appropriate safeguards including a minimum of one year of wash-out period to ensure that the study subjects are new comers to leiomyoma procedures. The very large database used in the study provides confidence in the generalizability of the results, particularly to commercially insured women in the U.S. Given that Black women were only about 10% in our study, and the fact that cumulative incidence of leiomyoma can be up to 80% in Black women,1 additional studies including cohorts with higher proportion of Black women (e.g., Medicaid data) are warranted. Unobserved confounding factors not captured in the data may violate propensity-matching assumption and potentially bias the results.
In conclusion, over 80% of the women with leiomyoma-related bulk symptoms underwent hysterectomy as the first-line procedural therapy. Among women who underwent uterine-sparing procedures, about one in seven women had a second uterine-sparing leiomyoma procedure, and one in ten underwent hysterectomy over an average of 3.4 years. Compared to myomectomy cohort, women who underwent UAE had a higher risk of re-intervention, lower risk of other surgical procedure but similar rate of adverse reproductive outcomes.
Supplementary Material
Acknowledgments
Supported by Kern Center for Science of Health Care Delivery, Mayo Clinic, Rochester, MN, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD060503), NIH/NCRR CTSA Grant Number UL1 RR024150 and a grant from the Focused Ultrasound Foundation.
Financial Disclosure: Shannon K. Laughlin-Tommaso has received NIH funding (5K12HD065987-O2) and research funding, paid to Mayo Clinic, from Truven Health Analytics and Insightec (Israel) for a focused ultrasound ablation clinical trial. She is on the data safety monitoring board for the ULTRA trial (Halt Medical, CA). Elizabeth A. Stewart has received NIH funding (R01HD060503, P50 HS023418, R01HD074711) and served as a consultant to AbbVie, Astellas Pharma, Bayer Health Care, Gynesonics and Viteava for consulting related to uterine leiomyoma, and to GlaxoSmithKline for consulting related to adenomyosis and to Welltwigs for consulting related to infertility. She has also received royalties from UpToDate and Massachusetts Medical Society. Bijan J. Borah, Xiaoxi Yao and Herbert C. Heien did not report any potential conflicts of interest.
Footnotes
Each author has indicated that he or she has met the journal’s requirements for authorship.
Presented as poster in AcademyHealth Annual Research Meeting in Minneapolis, MN, June 15, 2015.
References
- 1.Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. American journal of obstetrics and gynecology. 2003;188(1):100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 2.Lee DW, Ozminkowski RJ, Carls GS, Wang S, Gibson TB, Stewart EA. The direct and indirect cost burden of clinically significant and symptomatic uterine fibroids. Journal of occupational and environmental medicine. 2007;49(5):493–506. doi: 10.1097/JOM.0b013e31805f6cf2. [DOI] [PubMed] [Google Scholar]
- 3.Flynn M, Jamison M, Datta S, Myers E. Health care resource use for uterine fibroid tumors in the United States. American journal of obstetrics and gynecology. 2006;195(4):955–964. doi: 10.1016/j.ajog.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. American journal of obstetrics and gynecology. 2012;206(3):211. e211–211. e219. doi: 10.1016/j.ajog.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laughlin SK, Stewart EA. Uterine leiomyomas: individualizing the approach to a heterogeneous condition. Obstetrics and gynecology. 2011;117(2 Pt 1):396. doi: 10.1097/AOG.0b013e31820780e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart EA. Uterine fibroids. The Lancet. 2001;357(9252):293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 7.Stewart EA. Uterine fibroids: the complete guide. Johns Hopkins University Press; 2007. [Google Scholar]
- 8.Merrill RM. Hysterectomy surveillance in the United States, 1997 through 2005. Medical science monitor: international medical journal of experimental and clinical research. 2008;14(1):CR24–31. [PubMed] [Google Scholar]
- 9.Moore BJ, Steiner CA, Davis PH, Stocks C, Barrett ML. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): 2006. Trends in Hysterectomies and Oophorectomies in Hospital Inpatient and Ambulatory Settings, 2005–2013: Statistical Brief #214. [PubMed] [Google Scholar]
- 10.Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 Pt 1):233–241. doi: 10.1097/AOG.0b013e318299a6cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gliklich RE, Leavy MB, Velentgas P, Campion DM, Mohr P. Identification of Future Research Needs in the Comparative Management of Uterine Fibroid Disease A Report on the Priority-Setting Process, Preliminary Data Analysis, and Research Plan. 2011 [Google Scholar]
- 12.McLucas B, Goodwin S, Adler L, Rappaport A, Reed R, Perrella R. Pregnancy following uterine fibroid embolization. International Journal of Gynecology & Obstetrics. 2001;74(1):1–7. doi: 10.1016/s0020-7292(01)00405-2. [DOI] [PubMed] [Google Scholar]
- 13.Pron G, Mocarski E, Bennett J, Vilos G, Common A, Vanderburgh L. Pregnancy After Uterine Artery Embolization for Leiomyomata: The Ontario Multicenter Trial. Obstetrics & Gynecology. 2005;105(1):67–76. doi: 10.1097/01.AOG.0000149156.07061.1f. [DOI] [PubMed] [Google Scholar]
- 14.Mara M, Maskova J, Fucikova Z, Kuzel D, Belsan T, Sosna O. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovascular and interventional radiology. 2008;31(1):73–85. doi: 10.1007/s00270-007-9195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg J, Pereira L. Pregnancy outcomes following treatment for fibroids: uterine fibroid embolization versus laparoscopic myomectomy. Current Opinion in Obstetrics and Gynecology. 2006;18(4):402–406. doi: 10.1097/01.gco.0000233934.13684.cb. [DOI] [PubMed] [Google Scholar]
- 16.Broder MS, Goodwin S, Chen G, et al. Comparison of long-term outcomes of myomectomy and uterine artery embolization. Obstetrics & Gynecology. 2002;100(5):864–868. doi: 10.1016/s0029-7844(02)02182-8. [DOI] [PubMed] [Google Scholar]
- 17.Mara M, Fucikova Z, Maskova J, Kuzel D, Haakova L. Uterine fibroid embolization versus myomectomy in women wishing to preserve fertility: preliminary results of a randomized controlled trial. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2006;126(2):226–233. doi: 10.1016/j.ejogrb.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Manyonda IT, Bratby M, Horst JS, Banu N, Gorti M, Belli A-M. Uterine artery embolization versus myomectomy: impact on quality of life—results of the FUME (Fibroids of the Uterus: Myomectomy versus Embolization) Trial. Cardiovascular and interventional radiology. 2012;35(3):530–536. doi: 10.1007/s00270-011-0228-5. [DOI] [PubMed] [Google Scholar]
- 19.Narayan A, Lee AS, Kuo GP, Powe N, Kim HS. Uterine artery embolization versus abdominal myomectomy: a long-term clinical outcome comparison. Journal of Vascular and Interventional Radiology. 2010;21(7):1011–1017. doi: 10.1016/j.jvir.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum Labs: building a novel node in the learning health care system. Health Aff (Millwood) 2014;33(7):1187–1194. doi: 10.1377/hlthaff.2014.0038. [DOI] [PubMed] [Google Scholar]
- 21.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 22.Borah BJ, Moriarty JP, Crown WH, Doshi JA. Applications of propensity score methods in observational comparative effectiveness and safety research: where have we come and where should we go? J Comp Eff Res. 2014;3(1):63–78. doi: 10.2217/cer.13.89. [DOI] [PubMed] [Google Scholar]
- 23.d’Agostino RB. Tutorial in biostatistics: propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate behavioral research. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hehenkamp WJ, Volkers NA, Broekmans FJ, et al. Loss of ovarian reserve after uterine artery embolization: a randomized comparison with hysterectomy. Human reproduction. 2007;22(7):1996–2005. doi: 10.1093/humrep/dem105. [DOI] [PubMed] [Google Scholar]
- 26.Kaump GR, Spies JB. The impact of uterine artery embolization on ovarian function. Journal of Vascular and Interventional Radiology. 2013;24(4):459–467. doi: 10.1016/j.jvir.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Alternatives to hysterectomy in the management of leiomyomas. ACOG Practice Bulletin No. 96. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2008;112:387–400. doi: 10.1097/AOG.0b013e318183fbab. [DOI] [PubMed] [Google Scholar]
- 28.Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000–2004. American journal of obstetrics and gynecology. 2008;198(1):34. e31–34. e37. doi: 10.1016/j.ajog.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 29.Melton LJ, Achenbach SJ, Gebhart JB, Babalola EO, Atkinson EJ, Bharucha AE. Influence of hysterectomy on long-term fracture risk. Fertility and sterility. 2007;88(1):156–162. doi: 10.1016/j.fertnstert.2006.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blandon RE, Bharucha AE, Melton LJ, et al. Incidence of pelvic floor repair after hysterectomy: a population-based cohort study. American journal of obstetrics and gynecology. 2007;197(6):664. e661–664. e667. doi: 10.1016/j.ajog.2007.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howard BV, Kuller L, Langer R, et al. Risk of Cardiovascular Disease by Hysterectomy Status, With and Without Oophorectomy The Women’s Health Initiative Observational Study. Circulation. 2005;111(12):1462–1470. doi: 10.1161/01.CIR.0000159344.21672.FD. [DOI] [PubMed] [Google Scholar]
- 32.Phung T, Waltoft BL, Laursen TM, et al. Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dementia and geriatric cognitive disorders. 2009;30(1):43–50. doi: 10.1159/000314681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


