Abstract
Traumatic and non-traumatic brain injury results from severe disruptions in the cellular microenvironment leading to massive loss of neuronal populations and increased neuroinflammation. The progressive cascade of secondary events, including ischemia, inflammation, excitotoxicity and free radical release contribute to neural tissue damage. NLRX1 is a member of the NLR family of pattern recognition receptors and is a potent negative regulator of several pathways that significantly modulate many of these events. Thus, we hypothesized that NLRX1 limits immune system signaling in the brain following trauma. To evaluate this hypothesis, we utilized Nlrx1−/− mice in a controlled cortical impact (CCI) injury murine model of traumatic brain injury (TBI). Here, we show that the Nlrx1−/− mice exhibited significantly larger brain lesions and increased motor deficits following CCI injury. Mechanistically, our data indicate that the NF-κB signaling cascade is significantly up-regulated in the Nlrx1−/− animals. This up-regulation is associated with increased microglia and macrophage populations in the cortical lesion. Utilizing a mouse neuroblastoma cell line (N2A), we also found that NLRX1 significantly reduced apoptosis under hypoxic conditions. In human patients, we identify 15 NLRs that are significantly dysregulated, including significant downregulation of NLRX1 in brain injury following aneurysm. We further demonstrate a concurrent increase in NF-κB signaling that is correlated with aneurysm severity in these human subjects. Together, our data extend the function of NLRX1 beyond its currently characterized role in host-pathogen defense and identify this highly novel NLR as a significant modulator of brain injury progression.
Keywords: inflammation, brain injury, Nod-like receptor, NLR, NF-κB, microglia, neuron, inflammasome
INTRODUCTION
Traumatic brain injury (TBI) is a complex neurological condition that has emerged as an important cause of morbidity and mortality in the adolescent and young adult populations. It is defined differently throughout the literature, but is generally accepted as any external force that causes injury to the brain. These events may or may not involve injury to the skull or overlying tissues. Conversely, non-traumatic brain injuries have a wide range of causes, but are not directly associated with physical trauma. Examples of non-traumatic brain injury can include brain tumors, meningitis, hypoxic/anoxic brain injury, stroke, or aneurysm. In both traumatic and non-traumatic brain injury, the resulting morbidity and mortality seen clinically is not typically due to the actual primary injury itself, but rather, the secondary changes that occur in the brain as a result of the injury. These secondary changes are associated with the activation of both the innate and adaptive immune system and include inflammation, infiltration of immune cells, release of excitatory neurotransmitters, cerebral edema, vasospasm, ischemia, hypoxia, free radical damage, and others (1–3). A great deal of research has looked into the role of the adaptive immune system in brain injury and how it can modulate these various secondary processes. However, only relatively recently have researchers begun to focus on the role of the innate immune system (1). To date, the majority of studies have been focused on the role of microglial cells in the progression of the injury, as these are the predominant innate immune cell type in the brain (2). Somewhat similar to macrophages, microglial cells express a diverse variety of pattern recognition receptors (PRRs) that modulate their response to injury and drive many of the critical secondary changes seen in the brain following injury (2).
PRRs are proteins associated with plasma and endosomal membranes, as well as, within the cytosol itself. These receptors and sensors are responsible for recognition of various foreign and host molecular motifs (known as DAMPs or PAMPs). Once these proteins bind or sense their respective ligands, they are responsible for initiating a variety of cellular responses including the activation of key inflammatory signaling pathways, such as the NF-κB signaling cascade. NF-κB signaling has previously been shown to be important in the pathogenesis of brain injury. For example, Lian et al. showed that mice lacking IĸBɑ, an NF-κB inhibitory protein, showed significantly increased neuroinflammation when assessed in a model of TBI (4). Moreover, in another TBI model, suppressing the NF-κB signaling pathway through exogenous VEGI treatment attenuated brain injury (5). Thus, these studies illustrate that unrestricted NF-κB signaling is an important component in the pathophysiology of brain injury.
NOD-like receptors (NLRs) are intracellular PRRs that are generally classified as either inflammasome-forming NLRs or regulatory NLRs. The inflammasome-forming NLRs are well-studied and are characterized by their capacity to initiate the formation of the multi-protein inflammasome complex. Inflammasome formation ultimately facilitates the maturation and activation of the pro-inflammatory cytokines IL-18 and IL-1β. Several inflammasome forming NLRs have been evaluated in the context of brain injury. For example, multiple studies have suggested that NLRP1 and NLRP3 may play critical regulatory roles in TBI in both humans and rodents (6–8). Moreover, NLRC4 and AIM2 have also been implicated in contributing to non-traumatic brain injury in stroke models (9). However, while the inflammasome forming NLRs have been well studied in multiple types of brain injury, there is currently a paucity of data pertaining to the contribution of the regulatory NLRs. This sub-group includes both positive and negative regulatory NLRs that function through the direct regulation of inflammatory signaling pathways, such as NF-κB, AKT, and interferon (IFN) signaling (10–12). Three NLRs have been shown to function as negative regulators, including NLRP12, NLRC3, and NLRX1. NLRX1 is of particular interest in the context of brain injury as it has been shown to play a role in a variety of different cellular processes important to injury pathogenesis in a diverse range of cell types and tissues. For example, NLRX1 negatively regulates NF-κB signaling, type I interferon (IFN) signaling and reactive oxygen species (ROS) production, as well as, acts as a positive regulator of autophagy in macrophages and fibroblasts (11, 13–15). NLRX1 has been best characterized in the context of pathogen recognition (13, 15, 16). However, recent studies have extended the function of NLRX1 beyond this initial role in modulating host-pathogen interactions and identified contributions to cancer, chronic obstructive pulmonary disease, inflammatory bowel disease, and the modulation of cell death (11, 17–19).
The purpose of this study was to investigate the role of NLRX1 in the pathogenesis of brain injury. We hypothesized that loss of NLRXI would exacerbate NF-κB signaling, and tissue damage following TBI. To test this, we used an Nlrx1−/− mouse in a model of controlled cortical impact (CCI) and evaluated both quantitative and qualitative measures of brain injury. Consistent with our hypothesis, mice lacking NLRX1 demonstrated increased pathophysiological features consistent with increased brain injury compared to wild type control mice. Increased lesion volume in Nlrx1−/− mice was associated with increased NF-κB signaling and influx of CD11b+ microglia and/or macrophage populations in the lesion site. These findings correlate with NLRX1 dependent effects identified in a relevant neuroblast cell line in which we performed cell death assays. Gene expression analysis from the cortex of CCI-injured Nlrx1−/− mice show increased changes in mRNA expression levels of genes involved in NF-κB signaling. Similar correlations were found in gene expression data from human patients following brain injury associated with ruptured aneurysms. Collectively, these studies provide evidence of NLRX1’s role beyond host-pathogen interactions and further our knowledge regarding the underlying mechanisms involved in brain injury.
MATERIALS AND METHODS
Animals
The generation and characterization of Nlrx1−/− mice have been previously described (15). All mice were maintained on the C57BL/6J background. All animals used in experiments were male and between 2–4 months of age. C57BL/6J and Nlrx1−/− mice were maintained as separate colonies. All studies involving mice were repeated at least 3 independent times, with 3–7 mice per genotype and treatment group. All experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were conducted under the approval of the Virginia Tech Institutional Animal Care and Use Committee (IACUC) and the Virginia Maryland College of Veterinary Medicine.
Cell culture and Cell Lines
All mouse neuroblastoma (N2A) cell lines were generated as previously described (32). Briefly, we utilized Origene TrueORF cDNA Clones with TurboFectin to generate Nlrx1 stable knock-in cells and Origene shRNA plasmid against Nlrx1 to generate the knockdown cells. Cell death was evaluated by flow cytometry using Annexin V/ Propidium Iodine (PI) staining. H2O2 was utilized to stimulate neuron cell death. All experiments were performed 4 independent times and each experiment included all of the experimental groups. We evaluated 10,000 events, excluding cellular debris and cellular clumps based on side and forward scatter. The analysis was performed using automatic assignment of the set gates to all samples within the FloJo workspace.
CCI Injury and Behavioral Assessment
Mice were anesthetized using intraperitoneal (i.p.) injection of ketamine and xylazine. Following the loss of consciousness, mice were positioned in a stereotaxic frame (20, 21). Body temperature was maintained at 37°C and monitored via rectal probe throughout the surgery. During surgery, a skin incision was made followed by a 5 mm craniotomy using a portable drill over the right parietal-temporal cortex (−2.5 mm A/P and 2.0 mm lateral from bregma). Subsequently, CCI was induced by application of the eCCI- 6.3 device (Custom Design & Fabrication; 4mm impounder) at a velocity of 3.5 m/s, depth of 0.5 mm, and 150 ms impact duration (20, 21). Animals designated as sham controls were placed under anesthesia as described above and, following loss of consciousness, received a skin incision and closure only. Skin incisions were closed using Vetbond tissue adhesive (3M). Following surgery, all animals were placed into a heated cage to maintain body temperature for 1 hour. At 1, 3 or 14 days post-CCI injury, mice were euthanized and brain tissue was removed following decapitation. Fresh frozen tissue was embedded in OCT and coronally sectioned at 30 µm thick. Serial sections were taken at 300µm apart and stained for Nissl substance (21). Rotarod behavior assessment was performed as previously described (21, 22).
Evaluation of Contusion Volume and CD11b-positive cells
Contusion volume was assessed by a blinded investigator using Cavalieri Estimator from StereoInvestigator (MicroBrightField) and an Olympus BX51TRF motorized microscope (Olympus America). Contusion volume (mm3) was determined as previously described (21). Briefly, volume analysis was performed by estimating the area of tissue loss in the ipsilateral cortical hemisphere for five coronal serial sections at or around the epicenter (−1.1 to −2.6 mm posterior from bregma) of injury. Nissl stained serial sections were viewed under brightfield illumination at a magnification of 4×. A random sampling scheme was used that estimates every 10th section from rostral to caudal, yielding five total sections to be analyzed. A randomly placed grid with 100 µm spaced points was placed over the ipsilateral hemisphere and the area of contusion was marked within each grid. Contusion boundaries were identified by loss of Nissl staining, pyknotic neurons and tissue hemorrhage. The contoured area, using grid spacing, was then used to estimate total tissue volume based on section thickness, section interval and total number of sections within the Cavalieri program. Data is represented as volume of tissue loss or contusion volume (mm3) for wild type and Nlrx1−/− mice. StereoInvestigator optical fractionator was performed on serial coronal brain sections to estimate the total number of CD11b-positive cells within 1500µm (−0.2 A/P to −2.5 A/P) of injured cortical tissue at previously described (21, 23).
Expression Profiling
Total RNA was collected from brain specimens following mechanical homogenization, lysis, and extraction using Trizol and the manufacturers protocols. The purified RNA was quantified and 1µg was pooled from 3 randomly chosen brains prior to the cDNA reaction. Expression profiles were assessed using the RT2 Profiler PCR Array Platform PAMM-025Z (Qiagen) following the manufacturer’s protocols. Ingenuity Pathways Analysis (IPA) software was utilized to assess the array data. In addition to the profiling studies, RNA samples (5 µg) were archived using a cDNA Archive Kit (ABI) and evaluated via rtPCR using specifically targeted, commercially available primer/probe sets (ABI).
Human Metadata Analysis
Human NLRX1 expression was evaluated using a publicly accessible microarray meta-analysis search engine (http://www.nextbio.com/b/search/ba.nb), as previously described (24). Gene expression and pathway analysis in human subjects and rodents were conducted using the following array data series (available through the National Center for Biotechnology Information: https://www.ncbi.nlm.nih.gov/): GSE11686; GSE3307; GSE58294; GSE36233; GSE1767; GSE21079; GSE66573; GSE43591; and GSE20141.
Statistical analysis
Data was analyzed using GraphPad Prism, version 6 (GraphPad Software, Inc., San Diego, CA). Student’s two-tailed t test was used for comparison of two experimental groups. Multiple comparisons were done using one-way and two-way ANOVA where appropriate followed by Tukey post-test for multiple pairwise examinations. Correlation was also computed using GraphPad Prism. Changes were identified as statistically significant if P was less than 0.05. Mean values were reported together with the standard error of mean (SEM).
RESULTS
NLRX1 Attenuates Damage and Motor Deficits Following Controlled Traumatic Brain Injury
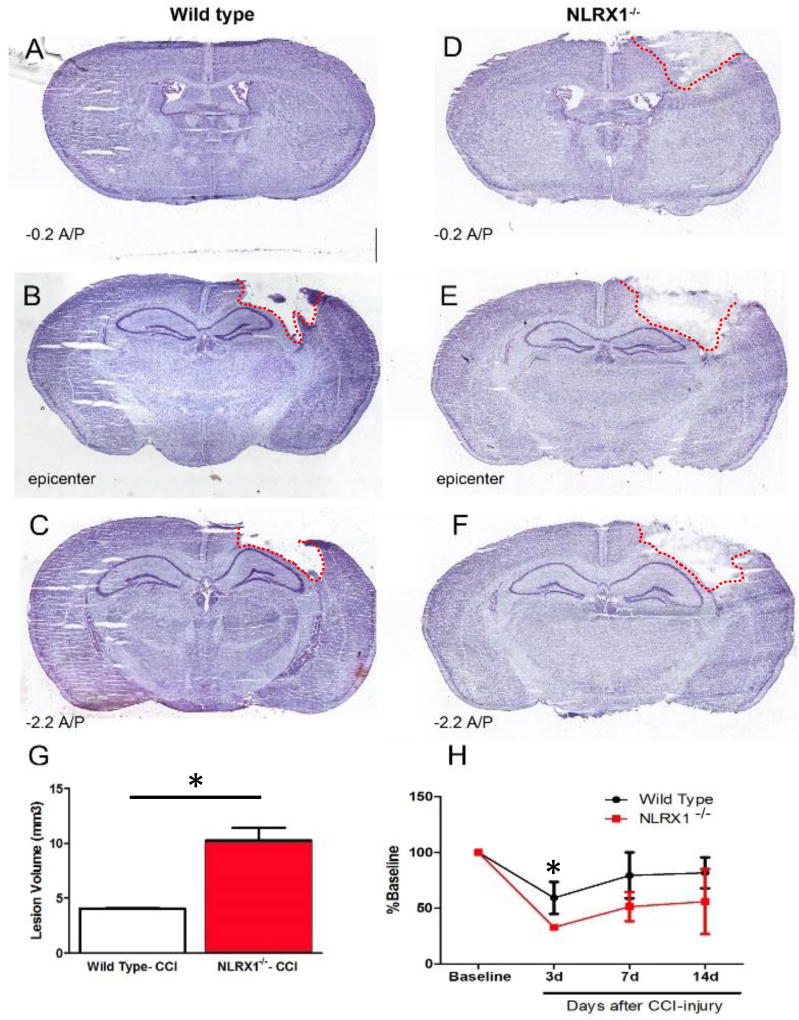
NLRX1 functions as a negative regulator of inflammation and modulates a variety of pathways associated with brain injury (10, 12, 25). Thus, we hypothesized that Nlrx1−/− mice would demonstrate significantly increased tissue damage following TBI. To evaluate this hypothesis, we utilized previously characterized Nlrx1−/− mice in a controlled cortical impact (CCI) model (20, 21). Wild type and Nlrx1−/− animals were subjected to a moderate CCI using a 4mm impounder at a velocity of 3.5m/s, depth of 0.5mm, and 150ms impact duration, as previously described (6, 20, 21). TBI progression was evaluated 3 days post-injury, which was empirically determined to be the peak time point for acute neural tissue damage and pathophysiology in prior studies utilizing wild type C57BL/6J animals in our CCI model (6, 20, 21). Upon necropsy, Nlrx1−/− mice were observed to have significantly larger gross lesions and hemorrhage compared to the wild type animals (data not shown). Serial sections were generated from the whole brain and subjected to Nissl staining. Contusion boundaries were identified based on the loss of Nissl stain, pyknotic neurons and tissue hemorrhage. Consistent with the gross lesion observations, Nlrx1−/− mice were found to have more expansive contusion boundaries, which radiated out significantly further from the impact epicenter compared to the injuries observed in the wild type animals (Figure 1A–F). Using the Cavelieri estimator, we found significant differences in contusion volume between the Nlrx1−/− mice (10.28 ± 1.15mm3) and the wild type animals (4.55 ± 0.47mm3) post-CCI injury (Figure 1G). These data support a role for NLRX1 in limiting neural tissue loss and TBI pathogenesis in the cortex following CCI-injury.
Figure 1. Increased lesion volume and motor deficits in Nlrx1−/− mice following CCI-injury.
A–C) Nissl staining of wild type brains at 3 days post-CCI compared to Nlrx1−/−(D–F) shows increased lesion volume (G) in the absence of NLRX1. (H) Motor deficits are also more prominent in Nlrx1−/− mice compared to wild type at 3–14 days post-CCI. (n=5–7 per group; **p<0.05).
We next sought to determine if the increased damage following TBI in the Nlrx1−/− mice resulted in any broad behavioral effects. Here, we utilized Rotarod behavioral analysis to determine if motor deficit and recovery were affected following CCI-injury. Mice were trained on the Rotarod 4 days prior to CCI-injury and the animals were then subjected to motor assessments at 3, 7, and 14 days post-CCI injury or post-sham operation. The time to fall was determined and then normalized to the average baseline time for each animal. No significant differences were observed between the Nlrx1−/− and wild type sham operated mice (Nlrx1−/−: 110.70% ± 11.42; wild type: 115.37% ± 5.24) (data not shown). However, following CCI-injury, Nlrx1−/− mice performed significantly worse on the Rotarod (Figure 1H). Both wild type and Nlrx1−/− mice demonstrated motor deficits following injury. However, at 3 days post-CCI injury, the Nlrx1−/− mice had a significant increase in motor deficits on the Rotarod compared to the wild type animals (Nlrx1−/−: 32.76% ± 1.0; wild type: 59.17% ± 5.45) (Figure 1H). This trend continued through days 7 (Nlrx1−/−: 51.36% ± 7.6; wild type: 79.35% ± 7.8) and 14 (Nlrx1−/−: 55.8% ± 16.8; wild type: 81.73% ± 5.21) (Figure 1H). Together, these data indicate that Nlrx1−/− mice experience increased motor impairments following CCI-injury and recovery is attenuated compared to wild type animals. The Rotarod data correlates with the contusion volume estimates and confirms that loss of NLRX1 significantly impacts neural tissue damage and subsequent motor function recovery following CCI-injury.
NLRX1 Regulates CD11b+ Cell Proliferation and Influx Following TBI
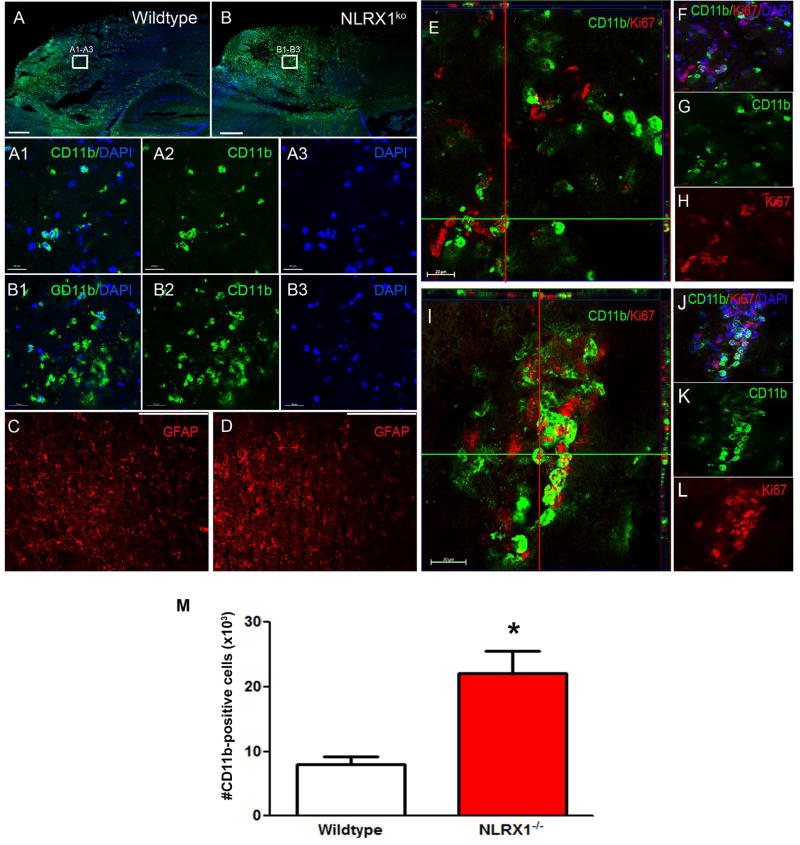
NLRX1 is broadly expressed in the brain and in a variety of cells known to influence TBI pathogenesis, including neurons, microglia, and astrocytes (Supplemental Figure 1A–B). To better characterize the increased CCI-injury observed in the Nlrx1−/− mice, we next sought to determine the effects of NLRX1 loss on microglia and astrocyte populations in the lesion following TBI. Frozen sections were prepared for immunohistochemistry (IHC) and specimens were evaluated for CD11b and glial fibrillary acid protein (GFAP) expression at 3 days post-CCI injury (Figure 2A–D). In the intact brain, the microglia should be the only CD11b+ cells. However, following injury, disruption to the blood brain barrier can allow an increased influx of macrophages and neutrophils into the lesion that will also contribute to the CD11b+ cell population. Increased CD11b+ cells were observed in all of the mice following CCI-injury compared to the sham-operated animals. However, an increase in CD11b+ cells were observed in the Nlrx1−/− mice compared to the wild type animals following CCI-injury (Figure 2A:A1–A3 – 2B:B1–B3). In addition, astrocytes can also significantly impact TBI pathogenesis [reviewed in (26)]. Here, we utilized the prototypical astrocyte marker GFAP to evaluate astrogliosis in the peri-lesion cortex after CCI injury. No significant differences were observed in density or distribution of GFAP+ cells between the Nlrx1−/− mice and wild type animals following CCI injury (Figure 2C – 2D). Together, these data suggest that NLRX1 functions either directly or indirectly to modulate microglia proliferation and/or macrophage influx with no effects on astrocyte populations following CCI-injury.
Figure 2. Analysis of microglia and astrocyte activation 3 days post-CCI injury in wild type and Nlrx1−/− mice.
(A:A1–A3) Confocal image analysis shows CD11b+ microglia (green) are seen in the lesioned cortex of wild type mice. (B:B1–3) Increased numbers of CD11b+ cells are seen in Nlrx1−/− mice. (C–D) Activated GFAP+ astrocytes are found in the peri-lesion site following CCI-injury. No differences in astrocytes are observed between wild type (C) and Nlrx1−/− mice (D). Scale bar= 1mm in A–D and 20µm in A1–A3 and B1–B3. (E–L) Ortho view from confocal imaging shows dividing CD11b+ cells co-labeled with anti-Ki67 staining (red) and nuclear DAPI staining (blue). There is greatly enhanced staining in Nlrx1−/− mice (I–L) compared to wild type mice (E–H). (M) StereoInvestigator, optical fractionator analysis showed a significant increase in CD11b+ cells in the cortex of Nlrx1−/− mice compared wild type. *P<0.05. Data is graphed as mean ± SEM. Scale bar = 20µm in E–H and I–L.
Prior studies evaluating NLRX1 in cancer have found a role for NLRX1 in the regulation of macrophage proliferation (11). Specifically, macrophages harvested from Nlrx1−/− mice demonstrated a significant increase in proliferation under specific physiological conditions (11). Following TBI, microglia in the lesion area undergo proliferation in response to injury and play vital roles in both the modulation of secondary injury and recovery (26, 27). While microglia are essential for proper sub-acute resolution of damage in the CNS, acutely these cells can also negatively impact pathogenesis by propagating inflammation to tissues adjacent to the site of injury and instigating significant collateral damage if dysregulated (28). Thus, we next sought to determine if the increase in CD11b+ cells was associated with increased microglia proliferation or macrophage influx following CCI-injury. Frozen sections were prepared for IHC and specimens were evaluated for CD11b and Ki67 reactivity 3 days post-CCI injury. Interestingly, we observed a significant increase in CD11b+ and Ki67+ cells in both Nlrx1−/− and wild type mice in the injured cortex (Figure 2E–L). However, we observed a greater density of these cells in the Nlrx1−/− mice compared to the wild type animals (Figure 2E–L). Indeed, in the wild type mice very few double-positive cells were observed (Figure 2E–H). Quantitative assessment of CD11b+ cells using optical fractionator showed an increase in the number of CD11b+ cells present in the injured cortex of Nlrx1−/− mice (22,133 ± 3458 cells) compared to wild type mice (7966 ± 1270 cells) at 3 days post-CCI injury (Figure 2M). Consistent with the increased CD11b immunoreactivity, these Ki67 findings suggest that NLRX1 regulates microglia proliferation following TBI. It is also possible that infiltrating macrophages may also contribute to this cell population. However, in either case, these findings are consistent with increased TBI pathogenesis and could contribute either directly or indirectly to enhanced injury progression and motor deficits.
NLRX1 Negatively Regulates NF-κB Signaling Following TBI
NLRX1 has been previously shown to negatively regulate NF-κB signaling and this attenuation is correlated with increased proliferation and tumorigenesis in cancer models (11, 29). Thus, we next profiled gene expression to evaluate NF-κB signaling following TBI in the Nlrx1−/− and wild type brain following CCI injury. Fresh 4 × 4 mm cortical tissue was dissected from the ipsilateral and contralateral hemispheres of each animal at 3 days post-injury. Total RNA was extracted from 3 randomly chosen wild type or Nlrx1−/− brains and the RNA from each genotype was pooled in equal concentrations for cDNA synthesis (Supplemental Figure 2). Changes in gene expression were evaluated using a panel of Superarrays (Qiagen) chosen to evaluate pathways associated with inflammatory signaling, cell death, and proliferation as previously described (11, 15). Gene expression was determined using the ΔΔCt method to calculate fold change, as described by the manufacturer. A panel of 8 housekeeping genes were used to normalize the expression of each gene on the array and the fold change in gene expression between the respective CCI-injured versus uninjured Nlrx1−/− and wild type brains were determined (Supplemental Figure 3). Gene expression data was further analyzed using Ingenuity Pathway Analysis (IPA) to better define and visualize the signaling pathways influenced by the loss of NLRX1 (Figure 3).
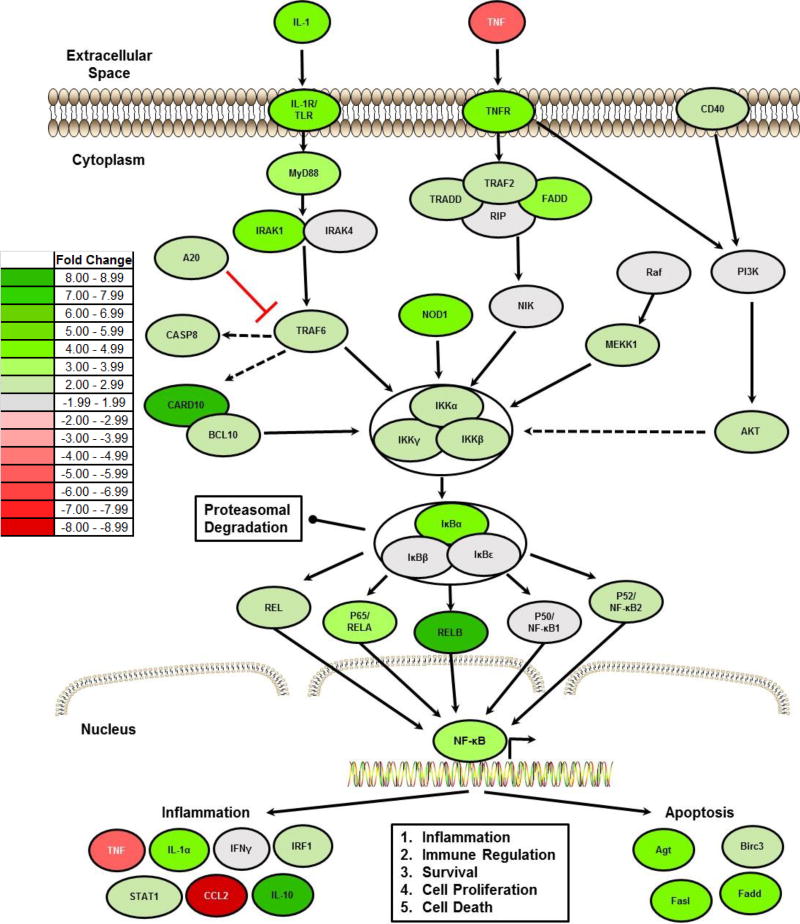
Figure 3. NLRX1 Negatively Regulates NF-κB Signaling Following TBI.
A) Heatmap schematic illustrating fold change in expression of all genes associated with NF-κB signaling that were identified as being significantly up- or down-regulated in the brain following TBI in Nlrx1−/− mice compared to the TBI wild type animals. Analysis was based on the ΔΔCt method, where all data was standardized to the average gene expression for a panel of 8 housekeeping genes and normalized to the respective non-lesion, contralateral region of each respective animal. Greater than a 2-fold change in gene expression was considered significant. Three randomly selected brains from each genotype and treatment group were selected and pooled for profiling studies. Pathway assessments were based on Ingenuity Pathway Analysis. (n=3 per group).
The gene expression studies and pathway analysis revealed a significant increase in NF-κB signaling in the cortical brain tissue from both Nlrx1−/− and wild type mice following injury. However, canonical NF-κB signaling was significantly up-regulated in Nlrx1−/− lesions compared to those in wild type animals (Figure 3; Supplemental Figure 3). This evaluation revealed that 47 genes associated with inflammation and NF-κB signaling were significantly up-regulated, defined as ≥ two-fold increase in gene expression over wild type (Supplemental Figure 3). The genes with the greatest increase in expression in the Nlrx1−/− brains included: Card10; Relb; and Il10, all three of which were up-regulated > 7 fold compared to wild type. Conversely, the following 3 genes were found significantly down-regulated in the Nlrx1−/− brains: Slc20a1; Tnf; and Ccl2 (Supplemental Figure 3). IPA analysis of the global changes in gene expression revealed a significant increase in NF-κB signaling in the Nlrx1−/− lesions (Figure 3). This increase was observed in genes categorized across each functional group in the NF-κB signaling cascade, including ligands and receptors, downstream signaling mediators, kinases, cytoplasmic sequestering and release factors, and 4 of the 5 NF-κB transcription factors (Figure 3). Significant differences were also observed in NF-κB responsive genes associated with the immune response. While generally these NF-κB responsive genes were significantly up-regulated, notable exceptions included Tnf and Ccl2 that were significantly downregulated following CCI injury in the Nlrx1−/− mice (Figure 3; Supplemental Figure 3). Finally, several genes in the NF-κB signaling cascade and associated with apoptosis were also found to be significantly up-regulated in lesions from the Nlrx1−/− brains compared to wild type lesions (Figure 3; Supplemental Figure 3).
NLRX1 Ablation Results in Increased NF-κB Signaling in CD11b+ Cell Populations Following TBI
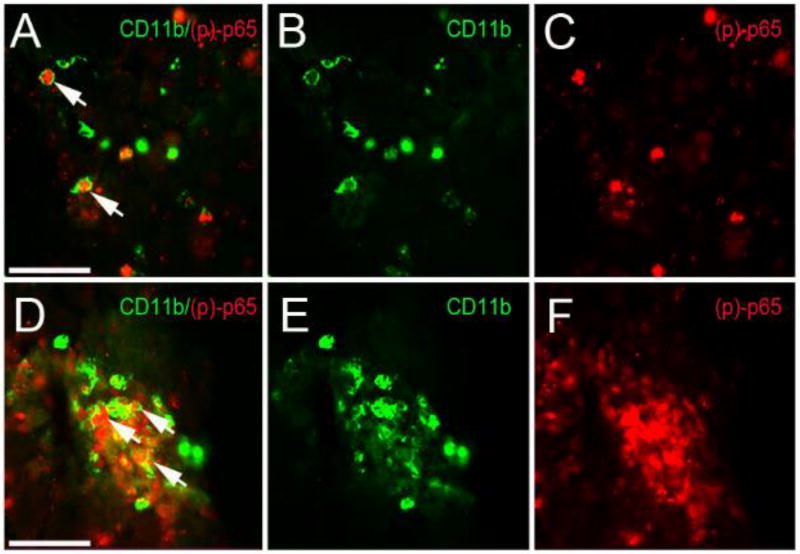
NLRX1 is broadly expressed in the brain and NF-κB is a master regulator of gene transcription in cells throughout the CNS (Supplementary Figure 1)(30, 31). Thus, we next sought to identify the cell types associated with the increased NF-κB signaling in the CCI-injured cortical tissue of Nlrx1−/− mice. To identify these cells, we utilized IHC targeting (p)p-65, which is a direct assessment of the activation of the canonical NF-κB signaling cascade. Likewise, p65 was found to be significantly up-regulated in the gene expression and pathway analysis studies in the Nlrx1−/− mice (Figure 3). IHC analysis revealed increased immunoreactivity for (p)p-65 in the cortical lesions following CCI injury in both Nlrx1−/− and wild type animals (Figure 4). In both genotypes, the (p)p-65 staining was localized to CD11b+ cells (Figure 4A and 4D). Indeed, despite their lower numbers compared to the Nlrx1−/− mice, the overwhelming majority of (p)p-65-positive cells in the wild type animals were also double positive for CD11b (Figure 4A–C). Consistent with the gene expression findings, we observed significantly increased numbers of CD11b+, (p)p-65+ double positive cells in the Nlrx1−/− mice compared to the wild type animals (Figure 4D–F). These data suggest that the CD11b+ cell population is a major source of NF-κB signaling, which may be suppressed by NLRX1 in the cortex following CCI injury. Together, these findings are consistent with NLRX1’s role as a negative regulator of canonical NF-κB signaling observed in other diseases and model systems and may underlie the increased proliferation, recruitment and function of these cells in the brain following trauma (11, 15).
Figure 4. Microglia expression of (p)-p65 at 3 days post-CCI injury in the cortex of wild type and Nlrx1−/− mice.
(A–C) High magnification fluorescence image of CD11b (green) and phospho(p)-p65 (red) in wild type injured cortex. (D–F) Increased CD11b/ phospho(p)-p65-positive cells are seen Nlrx1−/− mice. Scale bar = 100µm in images A and D.
NLRX1 Protects Cultured Neurons from Oxidative Damage
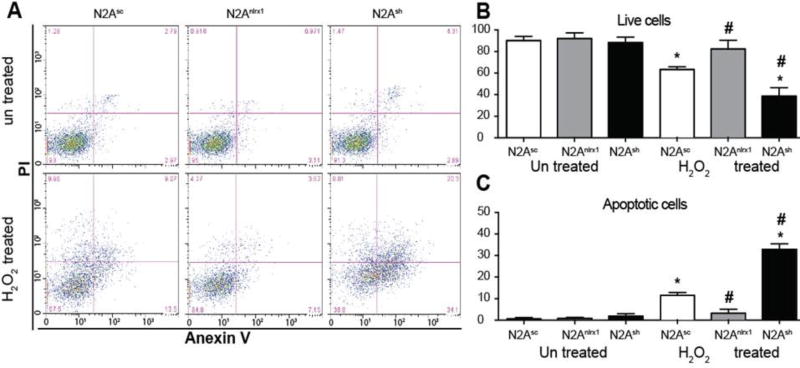
In addition to regulating NF-κB signaling and CD11b+ cell proliferation and recruitment, NLRX1 has also been shown to modulate cell death in neurons (32). Neuronal death is a critical component of the lesion outcome observed in the CCI injury model, which was exacerbated in the Nlrx1−/− mice. Likewise, we observed significantly increased expression of several genes associated with cell death and apoptosis in the Nlrx1−/− lesions, namely Fadd, Fasl, and Agt, compared to those in the wild type animals (Figure 3; Supplemental Figure 3). To evaluate the contribution of NLRX1 to neuronal cell death, we next employed an in vitro model based on mouse N2A cells (32). N2A cells are a murine neuroblastoma cell line shown in previous studies to derive multiple neuronal cell types (33, 34). NLRX1 was either knocked down or over expressed and cell death was evaluated following H2O2 treatment. ROS has been suggested to be an important mediator of acute brain injury following TBI. Here, we demonstrate that treatment of N2A cells with 100mM H2O2 for 18 hrs resulted in a significant decrease in cell viability in all cell lines except N2ANlrx1 cells that overexpress Nlrx1 (Figure 5A–C). Increased NLRX1 in N2A cells led to a significant reduction in H2O2 induced cell death compared to control cells or cells with shRNA knockdown of Nlrx1 (N2Ash), which conversely display enhanced cytotoxicity (Figure 5B–C). Annexin V and propidium iodide (PI) staining allows for the quantification of cells in early stages of apoptosis, when phosphatidylserine is flipped and faces outside of the cell increasing its availability for Annexin V binding. To exclude necrotic cells and cells at the late stages of apoptosis, we used PI stain that penetrates all dead cells. Using this technique, we observed a significant increase in apoptosis after treatment with H2O2 in all cell lines except N2ANlrx1 (Figure 5A). Our data indicates that apoptosis is significantly reduced in N2ANlrx1 cells and significantly increased in N2Ash cells (Figure 5A). Thus, we can conclude that NLRX1 plays a key anti-apoptotic role in neuroblasts under oxidative stress like that occurring acutely following TBI and suggest that NLRX1 may function to attenuate neuron loss in vivo. However, it should be noted that future in vivo and ex vivo studies utilizing primary neurons are needed to provide additional insight necessary to confirm this hypothesis.
Figure 5. NLRX1 protects neurons from oxidative damage.
A) Representative flow cytometry plots of N2Asc, N2Anlrx1 and N2Ash cells stained with Annexin V/PI stain before and after H2O2 treatment. B) Quantification of flow cytometry (bottom left quadrant) data showing % of live cells. C) Quantification of flow cytometry (bottom right quadrant) data showing % of live cells. Transfected with scrambled control (N2sc), with nlrx1 (N2Nlrx1), and shRNA for nlrx1 (N2Ash) were treated with 100mM H2O2 and stained with Annexin V PI stain. These cells were characterized previously (32). Cell were analyzed by flow cytometry using Cytoflex 20 from Beckman Coulter. Data was analyzed using FloJo software. Means of different groups were compared using two way ANOVA followed by Tukey’s test. Significance were established at p<0.05. *, different from untreated control, #, different from treated control, n=4 experiments were repeated 4 times, for analysis 10000 events were collected excluding cellular debris and cell clumps based on size.
NF-κB Signaling is Significantly Dysregulated Following Non-Traumatic Brain Injury in Human Subjects
Our findings that NF-κB signaling is significantly up-regulated in Nlrx1−/− mice following TBI is consistent with prior studies linking NLRX1 as a negative regulator of inflammation and its protective role in attenuating neuronal cell death (32). In human patients, increased NF-κB signaling has been shown to promote neurodegenerative mechanisms during Parkinson’s Disease and Alzheimer’s Disease (35–39). However, there have been few human studies directly evaluating NF-κB signaling following trauma and/or TBI. Likewise, there is a paucity of data associated with non-inflammasome forming NLRs, such as NLRX1, in human patients following brain injury. To address these shortcomings and evaluate the relevance of our findings in human patients, we conducted a retrospective analysis of gene expression data archived as NIH GEO datasets from human patients following ruptured brain aneurysms (GSE26969; GSE54083)(24). Ruptured brain aneurysms are considered non-traumatic brain injuries that have several clinical features in common with traumatic contusion injury and our CCI injury model, including massive hemorrhage, infiltration of peripheral-derived immune cells, neuronal and glial apoptosis, reduced cerebral blood flow and blood-brain barrier permeability (40–42). Also, there are several well-characterized and controlled human brain aneurysm datasets publicly available to evaluate our hypotheses. Here, we mined data from several independent studies evaluating gene expression changes in specimens collected from the aneurysmal dome following either ruptured, superficial, or un-ruptured intracranial aneurysms in up to 10 human subjects per condition (43, 44). Each study evaluated gene expression on >41,000 transcripts from 3–10 patients in each group. Expression data was normalized to GAPDH and fold change in expression was compared against the superficial aneurysm data.
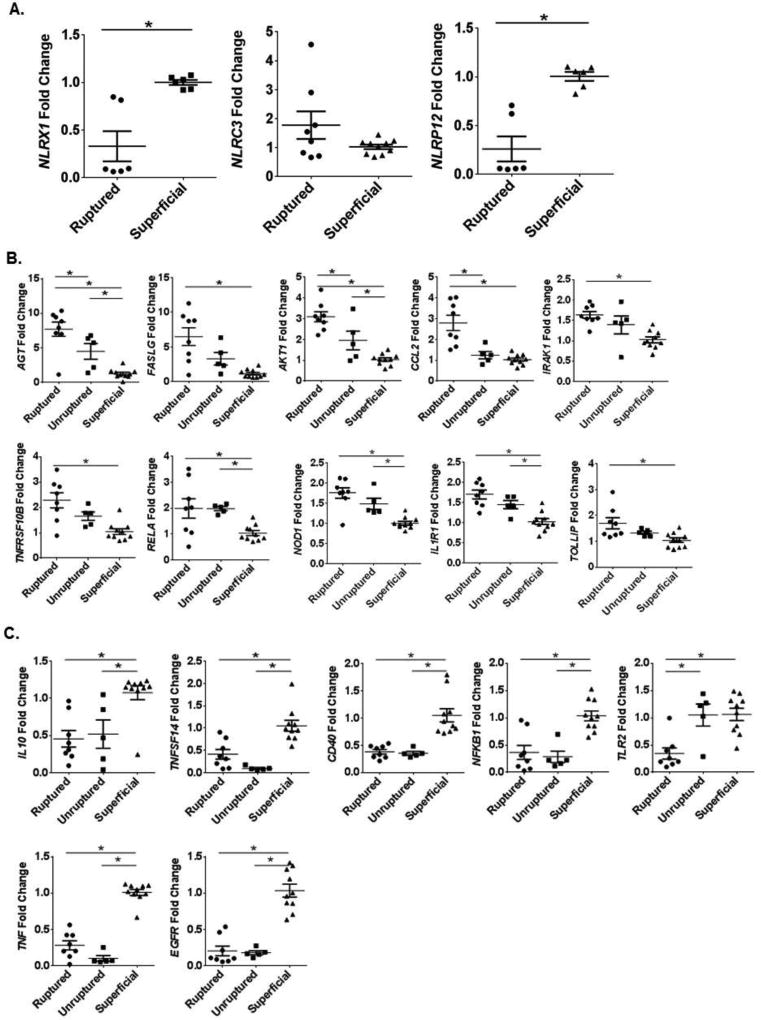
As mentioned above, several studies have evaluated the contribution of inflammasome forming NLRs in TBI in both human and rodent models (6–9, 45). However, data pertaining to non-inflammasome forming regulatory NLR family members has yet to be evaluated. This group of NLRs includes NLRX1, which has functional characteristics similar to 2 additional family members, NLRP12 and NLRC3. All three of these NLRs have been suggested to exert negative regulatory pressure on signaling pathways associated with the activation of other families of pattern recognition receptors, such as Toll-like Receptors (TLRs) and Rig-I Like Helicase Receptors (RLRs; RLHs)(12, 15, 46, 47). Here, we evaluated the expression of these 3 NLRs in the human aneurysm patient subsets and found that both NLRX1 (0.33 ± 0.16 aneurysm vs 1.00 ± 0.03 superficial) and NLRP12 (0.26 ± 0.13 aneurysm vs 1.01 ± 0.05 superficial) were significantly down-regulated in these human patient populations compared to NLRC3 (1.46 ± 0.90 aneurysm vs 0.72 ± 0.53 superficial) (Figure 6A). In addition to these 3 negative regulatory NLRs, we also evaluated expression patterns for the remaining 19 NLR family members (Supplemental Figure 4). Here, we found significant dysregulation among the majority of NLRs evaluated. Among the pyrin family NLRs, we found that NLRP1, NLRP2, NLRP3, NLRP4, and NLRP13 were significantly down-regulated following aneurysm (Supplemental Figure 4A). Whereas, NLRP6, NLRP7, NLRP10, and NLRP11 were significantly up-regulated in these human patients (Supplemental Figure 4A). Likewise, among the card family NLRs, NOD1 and NLRC5 were significantly up-regulated, while NLRC4 was significantly down-regulated (Supplemental Figure 4B). Finally, assessments of CIITA and NAIP revealed that both of these NLRs were significantly down-regulated following aneurysm (Supplemental Figure 4C). Together, these data show broad dysregulation among the NLR family members in the context of brain injury in human subjects. However, consistent with the findings from the Nlrx1−/− mice in the CCI injury model, it appears that loss or attenuation of NLRX1 is associated with increased brain injury and pathogenesis in both human and rodent models.
Figure 6. Genes associated with NF-κB signaling were significantly dysregulated following non-traumatic brain injury in human subjects.
Retrospective metadata analysis of gene expression changes in specimens collected from the aneurysmal dome following either ruptured, unruptured, or superficial intracranial aneurysms in human subjects were analyzed (GSE26969; GSE54083). Genes associated with NF-κB signaling were evaluated. A) The 10 genes with the largest changes in gene expression compared to the superficial specimens. B) Genes that were downregulated compared to specimens from superficial injuries. (n=5–10 specimens per group; *p<0.05).
In addition to the NLR genes, we also analyzed the expression of the genes associated with inflammation and NF-κB signaling in the mouse CCI injury model (shown in Supplemental Figure 3) in the human aneurysm datasets. Similar to the mouse findings, the NF-κB signaling cascade was significantly up-regulated in the human subjects. Indeed, there was a positive correlation between increased NF-κB gene expression and aneurysm severity (superficial vs unruptured: r = 0.405, p = 0.002; superficial vs ruptured: r = 0.439, p = 0.0008). The 10 genes with the largest differences in expression between the two aneurysm groups are shown in Figure 6B. Similar to the mouse TBI model, the majority of genes associated with NF-κB signaling were either significantly up-regulated or un-changed in the human subjects (Figure 6B; data not shown). However, we did observe a few notable differences in the human subject data. Specifically, in human subjects, CCL2 expression is significantly up-regulated following ruptured aneurysm (2.80 fold increase), even though NLRX1 is significantly down-regulated (Figure 6A–B). Conversely, in the mouse, when NLRX1 is ablated, Ccl2 was significantly down-regulated following CCI (−8.61 fold decrease) (Figure 3). In total, 7 genes were found to be significantly down-regulated in the human subjects. This included TNF expression, which was significantly downregulated in Nlrx1−/− mice following CCI and in the human aneurysm subjects (Figures 3 and 6C). Conversely, IL-10, EGFR, TNFSF14, CD40, and TLR2 expression were all significantly down-regulated in the human subjects, but significantly up-regulated in the mouse CCI model in the absence of NLRX1 (Figures 3 and 6C). However, despite these relatively few discrepancies, the sum of these data reveal that NF-κB signaling is significantly up-regulated in both mouse and human and identifies a diverse range of mediators associated with this signaling cascade that impact the pathogenesis of brain injury.
DISCUSSION
Here we provide evidence that NLRX1 negatively regulates NF-κB signaling, apoptosis and infiltration of CD11b+ cells following TBI. Genetic ablation of Nlrx1 in mice results in significantly increased brain pathophysiology following CCI. Mechanistically, our findings suggest that NLRX1 attenuates brain injury through the regulation of NF-κB signaling, the recruitment and expansion of CD11b+ cell populations in the lesion area, and potentially by limiting apoptosis in neuronal cell populations. In human patients, the down-regulation of NLRX1 expression and concomitant up-regulation of NF-κB signaling is correlated with injury severity following aneurysm. Thus, the findings from the mouse and human models appear consistent and further support the importance of NLRX1 and the pathways regulated by this unique protein in the brain following injury.
There is a growing appreciation that NLR family members have diverse functions in diseases beyond those directly associated with pathogens. Indeed, studies utilizing both human clinical samples and/or mouse models have shown a role for these unique proteins in the modulation of neuroinflammation (48, 49). To date, the vast majority of studies have focused on inflammasome forming NLRs in mice and their contribution to the pathophysiology of neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis (49–51). Beyond neurodegenerative diseases, inflammasome forming NLRs have also been found to significantly modulate various pathologic features of both traumatic and non-traumatic brain injury. Of the inflammasome forming NLRs in brain injury, the NLRP1 and NLRP3 inflammasomes are the best characterized in mice and the majority of studies have focused on mechanisms associated with regulating IL-1β/IL-18 and pyroptosis (6–8, 45, 50). However, aside from the inflammasome, there is a paucity of data pertaining to the role of non-inflammasome forming regulatory NLRs in brain injury. The regulatory NLR sub-groups can be divided into positive regulators that promote inflammation (i.e. NOD1/NLRC1 and NOD2/NLRC2) or negative regulators (i.e. NLRP12, NLRC3, and NLRX1)(52). To date, NOD2 is the only regulatory NLR that has been evaluated in the context of brain injury (53–55). In models of ischemia-reperfusion injury, NOD2 was found to be significantly up-regulated in microglia and astrocyte populations following focal cerebral ischemia- reperfusion injury and pretreatment with NOD2 agonists significantly increased infarct volume and neurological dysfunction in wild type mice and rats (53–55). Furthermore, genetic ablation of Nod2 significantly improved stroke outcomes and attenuated neuroinflammation (53). Nod2−/− mice demonstrated significantly reduced levels of IL-1β, IL-6, and TNF associated with attenuated NF-κB, MAPK, and JNK signaling (53). All of these findings are consistent with the role of NOD2 as a positive regulator of inflammation signaling.
Due to these early studies associated with inflammasome forming NLRs and NOD2 modulation of ischemia-reperfusion injury and stroke pathophysiology, we sought to expand upon the clinical relevance of these findings and evaluate the expression patterns of all 22 NLR family members in human patients (Figure 6 and Supplemental Figure 4). Here, we took advantage of the robust, publicly available gene expression datasets for ruptured, unruptured, or superficial intracranial aneurysms in human subjects and utilized bioinformatics based approaches to mine these data (43, 44). We chose aneurysm due to our joint interests in both traumatic (TBI) and non-traumatic (ruptured aneurysm or therapeutic anticoagulation) progressive hemorrhagic brain injury. Aneurysms have similar pathophysiological features that are relevant to TBI and the respective animal models; including the CCI injury model used in the current study and previously utilized to evaluate NLR function in mice (6, 9). As in severe cases of traumatic intracranial hemorrhage following TBI, intercerebral hemorrhage in patients with ruptured aneurysms results in increased cranial pressure, hypoxia/ischemia, necrosis and infiltration of the immune-derived compartment, blood - brain barrier disruption and oxidative stress. These common pathological features are also well-established in animal models of brain contusion injury (Oxidative Stress, Brain Edema, Blood-Brain Barrier Permeability, and Autonomic Dysfunction from Traumatic Brain Injury). Our data demonstrates that a large number of NLRs, 15 of the 22 identified in humans, are significantly dysregulated in human aneurysm specimens, with the majority (9 of the 15) up-regulated with increasing aneurysm severity (Figure 6 and Supplemental Figure 4). Interestingly, we did not observe any significant differences in NOD2 expression in the aneurysm specimens (Supplemental Figure 4B). This is counter to our anticipated results based on the previous pre-clinical mouse model data for Nod2−/− animals (53–55). However, we did observe a significant increase in NOD1 gene expression in the aneurysm specimens (Supplemental Figure 4B). NOD1 and NOD2 are functionally related and modulate similar biochemical pathways. Thus, it is possible that NOD1 may have a more prevalent role in human brain injury and future studies utilizing Nod1−/− mice may shed insight into this intriguing observation.
While NOD1 and NOD2 are regulatory NLRs that function to promote inflammation, NLRP12, NLRC3, and NLRX1 are regulatory NLRs that negatively regulate inflammatory signaling cascades activated by other classes of pattern recognition receptors. Interestingly, the gene expression data analysis from the human aneurysm studies revealed that both NLRX1 and NLRP12 are significantly downregulated in ruptured aneurysms compared to the superficial, whereas we did not observe a significant difference in NLRC3 expression (Figure 6A). NLRX1 and NLRC3 negatively regulate canonical NF-κB signaling, whereas NLRP12 attenuates both canonical and non-canonical NF-κB signaling pathways (15, 46, 47, 56). Thus, consistent with the findings that NLRX1 and NLRP12 were down-regulated, our expanded analysis of genes associated with inflammation revealed that a large number of genes associated with NF-κB signaling were significantly up-regulated in the human aneurysm specimens and this up-regulation was correlated with injury severity (Figure 6B). The role of NF-κB signaling in both ruptured aneurysm and traumatic brain injury is quite complex. In the CNS, NF-κB signaling regulates inflammation and apoptotic cell death following nerve injury and damage. This pathway has also been found to contribute to infarction and cell death in various stroke models and patients (57, 58). However, NF-κB signaling is also a critical component for neuronal survival, the attenuation of neurodegeneration, and activation of this cascade also facilitates recovery post-injury (59). Ultimately, the impact of increased NF-κB signaling in brain injury is likely a matter of which NF-κB factors are activated, where the injury occurs, and what cell-types are involved. Likewise, it is reasonable to speculate that the temporal dynamics of pathway activation are also significant factors. The current data demonstrates that NF-κB signaling is a common feature that is dysregulated following both ruptured aneurysm in humans and brain contusion injury in the rodent suggesting a potential common target based on known similarities in the pathological progression of these disorders.
To date, NLRX1 has been predominately studied in the context of inflammation associated with either virus or bacteria exposure (52, 60, 61). However, recent studies have emerged that reveal a more dynamic role for NLRX1 in modulating pathological conditions that extend beyond specific host-pathogen interactions (11, 18, 32). To date, there have been very few studies evaluating NLRX1 function in the CNS. To our knowledge, the only prior in vivo study of NLRX1 in the CNS characterized its role in modulating experimental autoimmune encephalomyelitis (EAE)(62). EAE is a common autoimmune mouse model of multiple sclerosis and is associated with the loss of immunological tolerance to CNS-derived antigens. In the EAE model, NLRX1 was found to play a robust protective role (62). Nlrx1−/− mice were shown to have significantly increased clinical parameters associated with disease progression and corresponding increases in tissue damage during EAE (62). The Nlrx1−/− animals were also more susceptible to myelin-reactive T cells following adoptive transfer (62). The mechanism associated with NLRX1 protection in the EAE model was further associated with the attenuation of macrophage and microglia activation (62). Indeed, microglia and macrophages cultured from Nlrx1−/− mice have been previously shown to generate excessive amounts of various pro-inflammatory cytokines, including IL-6 and CCL2, following PAMP stimulation (10, 11, 15, 62). This excessive activation of the microglia and macrophages in the Nlrx1−/− mice resulted in chronic inflammatory signaling and ultimately enhanced neurodegeneration.
In general, the findings from the prior EAE studies are consistent with our findings here for TBI. Similar to EAE, the Nlrx1−/− mice have significantly enhanced injury progression, tissue damage, and behavioral abnormalities consistent with increased brain lesion volume (Figure 1). Likewise, also consistent with the prior EAE findings, we also observed increased CD11b+ cells in the lesion that is consistent with either increased microglia proliferation and/or macrophage influx following damage (Figure 2). These findings are consistent with the increased cell recruitment observed in the EAE studies, as well as, prior observations that CD11b+ cells from Nlrx1−/− mice are hyper-proliferative under controlled ex vivo conditions (11). The CD11b+ proliferative cells appear to be a major contributing source of the increased NF-κB signaling at the 3 day time-point following TBI (Figure 4). The significant increase in gene expression associated with NF-κB signaling in the TBI mouse model corresponds with the findings from the human ruptured aneurysm specimens and the prior data associated with EAE, with a few notable exceptions. For example, in the mouse EAE model, IL-6 and CCL2 expression was significantly up-regulated and correlated with disease severity in the Nlrx1−/− mice (62). However, 3 days following TBI, CCL2 is significantly down-regulated in the Nlrx1−/− animals and expression of Il-6 was not significantly changed in the NLRX1 deficient animals compared to wild type (Figure 3; data not shown). Similarly, in the EAE model, no differences were observed in TNF generation; whereas in the TBI model TNF was significantly down-regulated (Figure 3). These few discrepancies are likely associated with the fundamental differences in the two models. Specifically, the EAE model is considered a model of “sterile inflammation” and is inherently driven by immune system hyper-activation (62). Whereas, in the TBI model, the resultant inflammation is driven purely by acute mechanical damage recognition in the CNS and the resultant injury response and resolution. Despite the few discrepancies in specific cytokine levels, the overall consistency between the phenotypes and proposed mechanisms support the conclusion that NLRX1 limits inflammation in the CNS and its down-regulation following CCI injury may contribute to cellular apoptosis and tissue loss. Furthermore, the two studies suggest that the attenuation in pathology is associated through the modulation of inflammatory signaling cascades in CD11b+ cell populations.
Beyond inflammatory signaling cascades and CD11b+ cells, it is also critical to consider the impact of NLRX1 on neuronal cell death following TBI. In addition to dysregulated inflammation, neuronal cell death is a hallmark feature associated with the severity of injury. As our data indicates, we observed significantly increased cortical tissue loss in the Nlrx1−/− mice compared to the wild type animals following TBI (Figure 1). The increase in apoptotic gene expression in Nlrx1−/− mice partially correlates with increased NF-κB signaling in these animals (Figure 3). A variety of genes associated with cell death and apoptosis were significantly up-regulated in our brain profiling studies (Figure 3; Supplemental Figure 3). These observations are consistent with previous work from members of our team evaluating NLRX1 in N2A cell death using an in vitro model system (32). In these prior studies, NLRX1 was either knocked down or over-expressed in N2A cell lines and mechanisms associated with cell death were evaluated. Using this system, NLRX1 was found to modulate the balance between necrosis and apoptosis in N2A cells following rotenone treatment (32). Attenuation of NLRX1 was found to promote apoptosis, rather than necrosis, through the enhancement of DRP1 phosphorylation and regulation of mitochondrial fission (32). While these data support our in vivo findings, we sought to expand upon these prior studies using an in vitro model with greater relevance to TBI. Thus, we utilized the previously described N2A cells and exposed them to H2O2. Similar to the previous rotenone findings, overexpression of NLRX1 attenuated cell death, while knockdown of NLRX1 enhanced cell death following H2O2 exposure (Figure 5). These findings support the previous observations with rotenone and provide additional mechanistic insight into the in vivo loss of neurons observed in the Nlrx1−/− mice following TBI. Several prior studies have evaluated the role of NLRX1 in cell death and proliferation in monocytes, fibroblasts, and epithelial cells (11, 19, 29, 63). Likewise, NLRX1 has also been shown to modulate ROS signaling and autophagy in these diverse cells following activation via pathogen associated molecular pattern stimulation and/or pathogen exposure (14, 16, 29, 64, 65). Together, our findings and the results of these prior studies reveal a relationship between NLRX1, mitochondria, and cell death. However, the exact mechanism of NLRX1 in these processes remains elusive. Our findings associated with NLRX1 in N2A cells, and likely neurons, is consistent with many of these prior studies, and provides additional mechanistic insight associated with the enhanced injury progression observed in the Nlrx1−/− mice. As this is an area of intense research focus, future studies will no doubt reveal additional mechanistic insight associated with NLRX1 in neuronal cell death.
NLRX1 is a unique member of the NLR family and has diverse regulatory functions that are associated with a myriad of human diseases beyond pathogen infections. The contribution of NLRX1 and other non-inflammasome forming regulatory NLRs in the modulation of brain pathophysiology is an emerging area of research interest with tremendous potential. Here, we have shown that NLRX1 protects against brain injury in mouse models and human subjects, in part through the negative regulation of NF-κB signaling. Our findings are consistent with the limited other studies conducted thus far with NLRX1 in the CNS. We anticipate that future studies will better define the role of NLRX1 in other human neurological disorders. Likewise, our gene expression and profiling studies have uncovered a broad range of genes and pathways that have not previously been associated with brain injury in either humans or mice. Thus, future studies of these genes should reveal novel targets and pathways for future therapeutic strategies to treat brain injury.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Jenny P.Y. Ting (UNC Chapel Hill) for kindly providing the Nlrx1−/− mice used in this study. We would also like to acknowledge Dr. Andrea Bertke and Angela Ives (Virginia Maryland College of Veterinary Medicine) for technical assistance and support.
References
- 1.Rivest S. Regulation of innate immune responses in the brain. Nature reviews. Immunology. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- 2.Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. The Mount Sinai journal of medicine, New York. 2009;76:97–104. doi: 10.1002/msj.20104. [DOI] [PubMed] [Google Scholar]
- 4.Lian H, Shim DJ, Gaddam SS, Rodriguez-Rivera J, Bitner BR, Pautler RG, Robertson CS, Zheng H. IkappaBalpha deficiency in brain leads to elevated basal neuroinflammation and attenuated response following traumatic brain injury: implications for functional recovery. Molecular neurodegeneration. 2012;7:47. doi: 10.1186/1750-1326-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao W, Zhao Z, Yu G, Zhou Z, Zhou Y, Hu T, Jiang R, Zhang J. VEGI attenuates the inflammatory injury and disruption of blood-brain barrier partly by suppressing the TLR4/NF-kappaB signaling pathway in experimental traumatic brain injury. Brain research. 2015;1622:230–239. doi: 10.1016/j.brainres.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Brickler T, Gresham K, Meza A, Coutermarsh-Ott S, Williams TM, Rothschild DE, Allen IC, Theus MH. Nonessential Role for the NLRP1 Inflammasome Complex in a Murine Model of Traumatic Brain Injury. Mediators of inflammation. 2016;2016:6373506. doi: 10.1155/2016/6373506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu HD, Li W, Chen ZR, Hu YC, Zhang DD, Shen W, Zhou ML, Zhu L, Hang CH. Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochemical research. 2013;38:2072–2083. doi: 10.1007/s11064-013-1115-z. [DOI] [PubMed] [Google Scholar]
- 8.Ma J, Xiao W, Wang J, Wu J, Ren J, Hou J, Gu J, Fan K, Yu B. Propofol Inhibits NLRP3 Inflammasome and Attenuates Blast-Induced Traumatic Brain Injury in Rats. Inflammation. 2016;39:2094–2103. doi: 10.1007/s10753-016-0446-8. [DOI] [PubMed] [Google Scholar]
- 9.Denes A, Coutts G, Lenart N, Cruickshank SM, Pelegrin P, Skinner J, Rothwell N, Allan SM, Brough D. AIM2 and NLRC4 inflammasomes contribute with ASC to acute brain injury independently of NLRP3. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:4050–4055. doi: 10.1073/pnas.1419090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia X, Cui J, Wang HY, Zhu L, Matsueda S, Wang Q, Yang X, Hong J, Songyang Z, Chen ZJ, Wang RF. NLRX1 negatively regulates TLR-induced NF-kappaB signaling by targeting TRAF6 and IKK. Immunity. 2011;34:843–853. doi: 10.1016/j.immuni.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coutermarsh-Ott S, Simmons A, Capria V, LeRoith T, Wilson JE, Heid B, Philipson CW, Qin Q, Hontecillas-Magarzo R, Bassaganya-Riera J, Ting JP, Dervisis N, Allen IC. NLRX1 suppresses tumorigenesis and attenuates histiocytic sarcoma through the negative regulation of NF-kappaB signaling. Oncotarget. 2016;7:33096–33110. doi: 10.18632/oncotarget.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei Y, Wen H, Ting JP. The NLR protein, NLRX1, and its partner, TUFM, reduce type I interferon, and enhance autophagy. Autophagy. 2013;9:432–433. doi: 10.4161/auto.23026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, Accavitti-Loper MA, Madden VJ, Sun L, Ye Z, Lich JD, Heise MT, Chen Z, Ting JP. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–577. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- 14.Tattoli I, Carneiro LA, Jehanno M, Magalhaes JG, Shu Y, Philpott DJ, Arnoult D, Girardin SE. NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-kappaB and JNK pathways by inducing reactive oxygen species production. EMBO reports. 2008;9:293–300. doi: 10.1038/sj.embor.7401161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, Scull MA, Gris D, Roney KE, Zimmermann AG, Bowzard JB, Ranjan P, Monroe KM, Pickles RJ, Sambhara S, Ting JP. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappaB signaling pathways. Immunity. 2011;34:854–865. doi: 10.1016/j.immuni.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdul-Sater AA, Said-Sadier N, Lam VM, Singh B, Pettengill MA, Soares F, Tattoli I, Lipinski S, Girardin SE, Rosenstiel P, Ojcius DM. Enhancement of reactive oxygen species production and chlamydial infection by the mitochondrial Nod-like family member NLRX1. The Journal of biological chemistry. 2010;285:41637–41645. doi: 10.1074/jbc.M110.137885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang MJ, Yoon CM, Kim BH, Lee CM, Zhou Y, Sauler M, Homer R, Dhamija A, Boffa D, West AP, Shadel GS, Ting JP, Tedrow JR, Kaminski N, Kim WJ, Lee CG, Oh YM, Elias JA. Suppression of NLRX1 in chronic obstructive pulmonary disease. The Journal of clinical investigation. 2015;125:2458–2462. doi: 10.1172/JCI71747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koblansky AA, Truax AD, Liu R, Montgomery SA, Ding S, Wilson JE, Brickey WJ, Muhlbauer M, McFadden RM, Hu P, Li Z, Jobin C, Lund PK, Ting JP. The Innate Immune Receptor NLRX1 Functions as a Tumor Suppressor by Reducing Colon Tumorigenesis and Key Tumor-Promoting Signals. Cell reports. 2016;14:2562–2575. doi: 10.1016/j.celrep.2016.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh K, Poteryakhina A, Zheltukhin A, Bhatelia K, Prajapati P, Sripada L, Tomar D, Singh R, Singh AK, Chumakov PM, Singh R. NLRX1 acts as tumor suppressor by regulating TNF-alpha induced apoptosis and metabolism in cancer cells. Biochimica et biophysica acta. 2015;1853:1073–1086. doi: 10.1016/j.bbamcr.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Baumann G, Travieso L, Liebl DJ, Theus MH. Pronounced hypoxia in the subventricular zone following traumatic brain injury and the neural stem/progenitor cell response. Experimental biology and medicine (Maywood, N.J.) 2013;238:830–841. doi: 10.1177/1535370213494558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theus MH, Ricard J, Bethea JR, Liebl DJ. EphB3 limits the expansion of neural progenitor cells in the subventricular zone by regulating p53 during homeostasis and following traumatic brain injury. Stem cells (Dayton, Ohio) 2010;28:1231–1242. doi: 10.1002/stem.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamm RJ, Pike BR, O'Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. Journal of neurotrauma. 1994;11:187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- 23.Theus MH, Ricard J, Glass SJ, Travieso LG, Liebl DJ. EphrinB3 blocks EphB3 dependence receptor functions to prevent cell death following traumatic brain injury. Cell death & disease. 2014;5:e1207. doi: 10.1038/cddis.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kupershmidt I, Su QJ, Grewal A, Sundaresh S, Halperin I, Flynn J, Shekar M, Wang H, Park J, Cui W, Wall GD, Wisotzkey R, Alag S, Akhtari S, Ronaghi M. Ontology-based meta-analysis of global collections of high-throughput public data. PloS one. 2010;5 doi: 10.1371/journal.pone.0013066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnoult D, Soares F, Tattoli I, Castanier C, Philpott DJ, Girardin SE. An N-terminal addressing sequence targets NLRX1 to the mitochondrial matrix. Journal of cell science. 2009;122:3161–3168. doi: 10.1242/jcs.051193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karve IP, Taylor JM, Crack PJ. The contribution of astrocytes and microglia to traumatic brain injury. British journal of pharmacology. 2016;173:692–702. doi: 10.1111/bph.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muccigrosso MM, Ford J, Benner B, Moussa D, Burnsides C, Fenn AM, Popovich PG, Lifshitz J, Walker FR, Eiferman DS, Godbout JP. Cognitive deficits develop 1month after diffuse brain injury and are exaggerated by microglia-associated reactivity to peripheral immune challenge. Brain, behavior, and immunity. 2016;54:95–109. doi: 10.1016/j.bbi.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loane DJ, Kumar A, Stoica BA, Cabatbat R, Faden AI. Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. Journal of neuropathology and experimental neurology. 2014;73:14–29. doi: 10.1097/NEN.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soares F, Tattoli I, Rahman MA, Robertson SJ, Belcheva A, Liu D, Streutker C, Winer S, Winer DA, Martin A, Philpott DJ, Arnoult D, Girardin SE. The mitochondrial protein NLRX1 controls the balance between extrinsic and intrinsic apoptosis. The Journal of biological chemistry. 2014;289:19317–19330. doi: 10.1074/jbc.M114.550111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao F, Ding B, Zhou L, Gao X, Guo H, Xu H. Magnesium sulfate provides neuroprotection in lipopolysaccharide-activated primary microglia by inhibiting NF-kappaB pathway. The Journal of surgical research. 2013;184:944–950. doi: 10.1016/j.jss.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 31.He Y, Jackman NA, Thorn TL, Vought VE, Hewett SJ. Interleukin-1beta protects astrocytes against oxidant-induced injury via an NF-kappaB-dependent upregulation of glutathione synthesis. Glia. 2015;63:1568–1580. doi: 10.1002/glia.22828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imbeault E, Mahvelati TM, Braun R, Gris P, Gris D. Nlrx1 regulates neuronal cell death. Molecular brain. 2014;7:90. doi: 10.1186/s13041-014-0090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saragoni L, Hernandez P, Maccioni RB. Differential association of tau with subsets of microtubules containing posttranslationally-modified tubulin variants in neuroblastoma cells. Neurochemical research. 2000;25:59–70. doi: 10.1023/a:1007587315630. [DOI] [PubMed] [Google Scholar]
- 34.Tremblay RG, Sikorska M, Sandhu JK, Lanthier P, Ribecco-Lutkiewicz M, Bani-Yaghoub M. Differentiation of mouse Neuro 2A cells into dopamine neurons. Journal of neuroscience methods. 2010;186:60–67. doi: 10.1016/j.jneumeth.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M, Faucheux BA, Agid Y, Hirsch EC. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7531–7536. doi: 10.1073/pnas.94.14.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boissiere F, Hunot S, Faucheux B, Duyckaerts C, Hauw JJ, Agid Y, Hirsch EC. Nuclear translocation of NF-kappaB in cholinergic neurons of patients with Alzheimer's disease. Neuroreport. 1997;8:2849–2852. doi: 10.1097/00001756-199709080-00009. [DOI] [PubMed] [Google Scholar]
- 38.Kaltschmidt B, Uherek M, Volk B, Baeuerle PA, Kaltschmidt C. Transcription factor NF-kappaB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2642–2647. doi: 10.1073/pnas.94.6.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lukiw WJ, Bazan NG. Strong nuclear factor-kappaB-DNA binding parallels cyclooxygenase-2 gene transcription in aging and in sporadic Alzheimer's disease superior temporal lobe neocortex. Journal of neuroscience research. 1998;53:583–592. doi: 10.1002/(SICI)1097-4547(19980901)53:5<583::AID-JNR8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 40.Uchiyama Y, Abe T, Hirohata M, Tanaka N, Kojima K, Nishimura H, Norbash AM, Hayabuchi N. Blood brain-barrier disruption of nonionic iodinated contrast medium following coil embolization of a ruptured intracerebral aneurysm. AJNR. American journal of neuroradiology. 2004;25:1783–1786. [PMC free article] [PubMed] [Google Scholar]
- 41.Hayman EG, Wessell A, Gerzanich V, Sheth KN, Simard JM. Mechanisms of Global Cerebral Edema Formation in Aneurysmal Subarachnoid Hemorrhage. Neurocritical care. 2016 doi: 10.1007/s12028-016-0354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sehba FA, Hou J, Pluta RM, Zhang JH. The importance of early brain injury after subarachnoid hemorrhage. Progress in neurobiology. 2012;97:14–37. doi: 10.1016/j.pneurobio.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, Yang X, Jiang F, Dusting GJ, Wu Z. Transcriptome-wide characterization of gene expression associated with unruptured intracranial aneurysms. European neurology. 2009;62:330–337. doi: 10.1159/000236911. [DOI] [PubMed] [Google Scholar]
- 44.Nakaoka H, Tajima A, Yoneyama T, Hosomichi K, Kasuya H, Mizutani T, Inoue I. Gene expression profiling reveals distinct molecular signatures associated with the rupture of intracranial aneurysm. Stroke. 2014;45:2239–2245. doi: 10.1161/STROKEAHA.114.005851. [DOI] [PubMed] [Google Scholar]
- 45.Fann DY, Lee SY, Manzanero S, Tang SC, Gelderblom M, Chunduri P, Bernreuther C, Glatzel M, Cheng YL, Thundyil J, Widiapradja A, Lok KZ, Foo SL, Wang YC, Li YI, Drummond GR, Basta M, Magnus T, Jo DG, Mattson MP, Sobey CG, Arumugam TV. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell death & disease. 2013;4:e790. doi: 10.1038/cddis.2013.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider M, Zimmermann AG, Roberts RA, Zhang L, Swanson KV, Wen H, Davis BK, Allen IC, Holl EK, Ye Z, Rahman AH, Conti BJ, Eitas TK, Koller BH, Ting JP. The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-kappaB. Nature immunology. 2012;13:823–831. doi: 10.1038/ni.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaki MH, Vogel P, Malireddi RK, Body-Malapel M, Anand PK, Bertin J, Green DR, Lamkanfi M, Kanneganti TD. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer cell. 2011;20:649–660. doi: 10.1016/j.ccr.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lukens JR, Gurung P, Shaw PJ, Barr MJ, Zaki MH, Brown SA, Vogel P, Chi H, Kanneganti TD. The NLRP12 Sensor Negatively Regulates Autoinflammatory Disease by Modulating Interleukin-4 Production in T Cells. Immunity. 2015;42:654–664. doi: 10.1016/j.immuni.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lukens JR, Dixit VD, Kanneganti TD. Inflammasome activation in obesity-related inflammatory diseases and autoimmunity. Discovery medicine. 2011;12:65–74. [PMC free article] [PubMed] [Google Scholar]
- 50.Saresella M, La Rosa F, Piancone F, Zoppis M, Marventano I, Calabrese E, Rainone V, Nemni R, Mancuso R, Clerici M. The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer's disease. Molecular neurodegeneration. 2016;11:23. doi: 10.1186/s13024-016-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, Lu M, Du RH, Qiao C, Jiang CY, Zhang KZ, Ding JH, Hu G. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson's disease. Molecular neurodegeneration. 2016;11:28. doi: 10.1186/s13024-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allen IC. Non-Inflammasome Forming NLRs in Inflammation and Tumorigenesis. Frontiers in immunology. 2014;5:169. doi: 10.3389/fimmu.2014.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu H, Wei X, Kong L, Liu X, Cheng L, Yan S, Zhang X, Chen L. NOD2 is involved in the inflammatory response after cerebral ischemia-reperfusion injury and triggers NADPH oxidase 2-derived reactive oxygen species. International journal of biological sciences. 2015;11:525–535. doi: 10.7150/ijbs.10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bai X, Zhang X, Chen L, Zhang J, Zhang L, Zhao X, Zhao T, Zhao Y. Protective effect of naringenin in experimental ischemic stroke: down-regulated NOD2, RIP2, NF-kappaB, MMP-9 and up-regulated claudin-5 expression. Neurochemical research. 2014;39:1405–1415. doi: 10.1007/s11064-014-1326-y. [DOI] [PubMed] [Google Scholar]
- 55.Li H, Hu J, Ma L, Yuan Z, Wang Y, Wang X, Xing D, Lei F, Du L. Comprehensive study of baicalin down-regulating NOD2 receptor expression of neurons with oxygen-glucose deprivation in vitro and cerebral ischemia-reperfusion in vivo. European journal of pharmacology. 2010;649:92–99. doi: 10.1016/j.ejphar.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 56.Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, Arthur JC, Woodford RM, Davis BK, Uronis JM, Herfarth HH, Jobin C, Rogers AB, Ting JP. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity. 2012;36:742–754. doi: 10.1016/j.immuni.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simmons LJ, Surles-Zeigler MC, Li Y, Ford GD, Newman GD, Ford BD. Regulation of inflammatory responses by neuregulin-1 in brain ischemia and microglial cells in vitro involves the NF-kappa B pathway. Journal of neuroinflammation. 2016;13:237. doi: 10.1186/s12974-016-0703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hwang CJ, Yun HM, Jung YY, Lee DH, Yoon NY, Seo HO, Han JY, Oh KW, Choi DY, Han SB, Yoon DY, Hong JT. Reducing effect of IL-32alpha in the development of stroke through blocking of NF-kappaB, but enhancement of STAT3 pathways. Molecular neurobiology. 2015;51:648–660. doi: 10.1007/s12035-014-8739-0. [DOI] [PubMed] [Google Scholar]
- 59.Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell death and differentiation. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- 60.Coutermarsh-Ott S, Eden K, Allen IC. Beyond the inflammasome: regulatory NOD-like receptor modulation of the host immune response following virus exposure. The Journal of general virology. 2016;97:825–838. doi: 10.1099/jgv.0.000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lupfer C, Kanneganti TD. The expanding role of NLRs in antiviral immunity. Immunological reviews. 2013;255:13–24. doi: 10.1111/imr.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eitas TK, Chou WC, Wen H, Gris D, Robbins GR, Brickey J, Oyama Y, Ting JP. The nucleotide-binding leucine-rich repeat (NLR) family member NLRX1 mediates protection against experimental autoimmune encephalomyelitis and represses macrophage/microglia-induced inflammation. The Journal of biological chemistry. 2014;289:4173–4179. doi: 10.1074/jbc.M113.533034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jaworska J, Coulombe F, Downey J, Tzelepis F, Shalaby K, Tattoli I, Berube J, Rousseau S, Martin JG, Girardin SE, McCullers JA, Divangahi M. NLRX1 prevents mitochondrial induced apoptosis and enhances macrophage antiviral immunity by interacting with influenza virus PB1-F2 protein. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E2110–2119. doi: 10.1073/pnas.1322118111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lei Y, Kansy BA, Li J, Cong L, Liu Y, Trivedi S, Wen H, Ting JP, Ouyang H, Ferris RL. EGFR-targeted mAb therapy modulates autophagy in head and neck squamous cell carcinoma through NLRX1-TUFM protein complex. Oncogene. 2016;35:4698–4707. doi: 10.1038/onc.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lei Y, Wen H, Yu Y, Taxman DJ, Zhang L, Widman DG, Swanson KV, Wen KW, Damania B, Moore CB, Giguere PM, Siderovski DP, Hiscott J, Razani B, Semenkovich CF, Chen X, Ting JP. The mitochondrial proteins NLRX1 and TUFM form a complex that regulates type I interferon and autophagy. Immunity. 2012;36:933–946. doi: 10.1016/j.immuni.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.