Abstract
Arginine has been considered as the most potent nutraceutics discovered ever, due to its powerful healing property, and it's been known to scientists as the Miracle Molecule. Arginine detection in fermented food products is necessary because, high level of arginine in foods forms ethyl carbamate (EC) during the fermentation process. Therefore, L-arginine detection in fermented food products is very important as a control measure for quality of fermented foods, food supplements and beverages including wine. In clinical analysis arginine detection is important due to their enormous inherent versatility in various metabolic pathways, topmost in the synthesis of Nitric oxide (NO) and tumor growth. A number of methods are being used for arginine detection, but biosensors technique holds prime position due to rapid response, high sensitivity and high specificity. However, there are many problems still to be addressed, including selectivity, real time analysis and interference of urea presence in the sample. In the present review we aim to emphasize the significant role of arginine in human physiology and foods. A small attempt has been made to discuss the various techniques used for development of arginine biosensor and how these techniques affect their performance. The choice of transducers for arginine biosensor ranges from optical, pH sensing, ammonia gas sensing, ammonium ion-selective, conductometric and amperometric electrodes because ammonia is formed as a final product.
Keywords: Arginine, Biosensor, Immobilization, Conducting Materials, Nanocomposite, Quantum dots etc
Graphical abstract
Highlights
-
•
First ever review on arginine biosensors.
-
•
Description of significance role of arginine in food and human physiology.
-
•
Comparison of different immobilizations, transducers and biological components used for the development of arginine biosensors.
-
•
Critically reviewed all the biosensors developed for arginine detection, discussed the possible challenges and recommendations.
1. Introduction
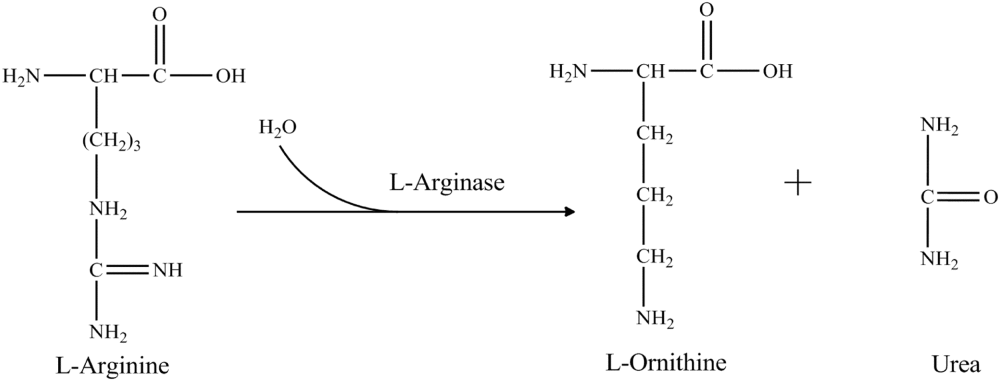
L-arginine is a molecule offering immense clinical and quality control significance. It is predominantly found in active sites of many proteins. Its structure is favorable to aid binding of phosphate anion and therefore catalyzes phosphorylation reactions [1]. Also, arginine plays a significant role in maintaining charge of many proteins [2]. During nitrogen metabolism, arginine hydrolyzed into urea and ornithine by the action of arginase. Arginine also aids ammonia detoxification, hormone secretion, and has a role in maintenance of the immune system [3]. It is reported that conversion of arginine into nitric oxide by nitric oxide synthase may help in the treatment of various physiological conditions such as cardiovascular diseases, peripheral vascular disease, erectile dysfunction, atherosclerosis, vascular headaches and chest pain, by enhancing vasodilation [4]. Arginine induces production of proteins, checking tissue wasting in people with critical illness and also helps to enhance sperm production.
Arginine is a biomarker for certain auxotrophic tumors since these tumors require arginine as a sole component for growth. Melanoma and hepatocellular carcinoma (HCC) do not express the enzyme arginosuccinate synthase (ASS) and therefore, are unable to synthesize arginine [5]. Tumor cells require more arginine to generate nitric oxide, thereby promoting tumor angiogenesis [6], [7], [8]. In leukemic patients the levels of arginine are lower as compared to normal subjects. The reported concentration of plasma arginine in different types of cancer are, breast cancer and colon cancer 80±3 µM, pancreatic cancer 76 ± 5 µM and esophageal cancer 41.9 ± 13.4 µM, in compared to a normal level of 90–150 µM [9], [10]. A significantly reduced level of arginine, due to excessive arginase activity, has also been reported in various other clinical conditions like reperfusion injury, asthma, arthritis and psoriasis [11], [12], [13], [14], [15], [16], [17], [18], [19]. Therefore, it is evident that low arginine levels are indicative of physiological abnormalities, endorsing its significance as a biomarker.
Detection of arginine in foods is imperative as a control for quality, particularly in juices & wine. In winery bioprocess, L-arginine degrades to urea and ornithine by the enzyme L-arginase. Some urea is assimilated by the yeast, and some is released into the fermentation medium. If the grapes used for wine production have excessive arginine, the accumulated urea in the medium can spontaneously react with ethanol to form ethyl carbamate, which is a potent carcinogen [20], [21], [22], [23]. To avoid this potential health hazard detection of arginine levels in grape juice is an essential pre-requisite. Fig. 1, Fig. 2 elucidate the potential role of arginine in tumor cell growth and fermentation process during wine production.
Fig. 1.
Diagrammatic representation of arginine utilization by tumor cells.
Fig. 2.
Bioprocess of ethyl carbamate formation from arginine during fermentation in wine production.
1.1. Traditional method of arginine analysis
A simple and accurate method for determining arginine levels in grape juice is the prerequisite for winemakers. The enzymatic reaction to determine Arginine is simple, specific and sensitive, and has been used for determining trace amount of arginine in biological materials shown in Fig. 3 [104]. The Sakaguchi reaction comprises of the reaction of alkaline solution of arginine with α-naphthol and hypochlorite giving a red color. Since its inception [24] many variations of the Sakaguchi reaction have been reported where the reaction was suitably modified by the addition of hypobromite and oxine (8-hydroxyquinoline) by [25], [26] respectively. Earlier, conventional methods for arginine determination are accounted as a number of amino acids including arginine were determined through micro titration method. In this, amino acids were allowed to react with ninhydrin and the resulting ammonia was finally aerated over into boric acid and titrated with standard acid [27]. One of the earliest methods of arginine determination via ornithine formation is Chinard's reaction, whereby ornithine when treated with a mixture of glacial acetic acid and ninhydrin reagent forms a red color product which can be measured spectrophotometrically at a wavelength of 515 nm. For determination of amino acids and related compounds, Moore and Stein [28] used a modified ninhydrin reagent composed of 2% ninhydrin and 0.3% hydrindantin in 3: 1 methyl Cellosolve- N sodium acetate buffer (pH 5.5). Rosenberg et al. [29] describes another method of the reaction of arginine with n-propanol and diacetyl in presence of increased concentration of α-naphthol and gives red color product with maximum absorbance at 535 nm. But still there is very less specificity for a particular amino acid so further work is required. These methods were not specific for arginine so quantification of arginine still compromised.
Fig. 3.
Enzymatic reaction to determine L-arginine.
Further, improvement was made by introducing a little modification in Sakaguchi reaction for quantitative determination of L-arginine using sulfosalicylic acid and oxine solution with hypobromite, it shows better specificity to arginine with other amino acid but arginine derived materials like methyl arginine also shows good response with this methods [30]. Notenboon et al. [31] proposed fluorescent modification of the Sakaguchi reaction using 2,4 dichloro α-naphthol for reaction with arginine and it exhibited good response with higher sensitivity. Many others analytical methods using chromatographic technology were applied to determine the arginine content. Arginine content in plasma and urine was determined by Chinard's ninhydrine reaction and found Plasma arginine level in adults in the range of 56–98 µmol/1, and urine arginine in the range of 0.58–1.77 mg/day in a healthy person [32]. Konings [33] described an interesting procedure for arginine determination in serum sample. This method involved transformation of arginine and ATP into phospho-arginine and ADP by the action of enzymes arginine kinase. The ADP formed is measured by two coupling reactions involving pyruvate kinase and lactate dehydrogenase with measurement of NADH consumption at 340 nm. A flow-injection technique for L-arginine determination was reported by Alonso et al. [34] in this technique the enzyme L-arginase was immobilized on an epoxy matrix and during the reaction produced urea was measured spectrophotometrically through indophenols formation at an absorbance of 629 nm. Other reported methods for arginine analysis involves Spectrophotometry [35], [36], [37], turbidimetry [38], Ionization mass spectrometry [39], Pulsed amperometry [40], [41], amino-acid analyzer and capillary electrophoresis [42], high performance liquid chromatography (HPLC) [43], [44], [45], [46], [47], [48], [49], [50], Hydrophilic interaction liquid chromatography electrospray tandem mass spectrometry (HILC-ETMS) [51], reverse phase HPLC [52]. Orduna [53] reported an enzymatic end point arginine analysis method by using enzymes arginase, urease, and glutamate dehydrogenase.
Chemiluminescence is an attractive analytical method due to its sensitivity, ease of operation and simple instrumentation, especially to trace biological substances analysis, but this method has not been widely used in practice [54]. Some studies involved enzymes which made the experiment more expensive and required more care for maintaining the activity of the enzymes [55], [56], [57]. Other methods of arginine determination include the use of High performance liquid chromatography techniques (HPLC) such as those reported where amino acids are derivatized with 2-chlorobenzoxazole to yield highly fluorescent N-(2-benzoxazolyl)-amino acids (BOX-AAs) whose separation on a C18 reversed phase column is done for quantitative estimation [58], [59] and discussed the involving pre-column derivatization with o-phthaldialdehyde. Fluorescence in the latter report is monitored at excitation and emission wavelengths of 340 and 455 nm, respectively.
These all above said methods needed pretreatment of sample, mostly slow and not commonly available to small-scale wineries. Hua et al. [60] used a rapid and accurate method for the determination of arginine in grape juice. It based on Sakaguchi reaction following the separation of arginine with Amberlite IR120 strong cation-exchange resin. This method successfully applied for the quick and accurate quantification of arginine in grape juice for the control formation of the ethyl carbamate level in wine, because the ethyle carbamate formation depend on the concentration of arginine in grape juice; the maximum arginine adsorption capacity of Amberlite IR120 was 0.683 ± 0.020 mg/g. But many limitations associated with this method like as sample preparation, interference of cations and high maintenance.
2. Arginine biosensors
2.1. Immobilization methods
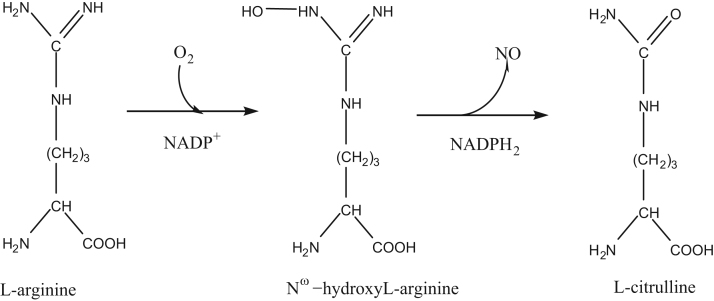
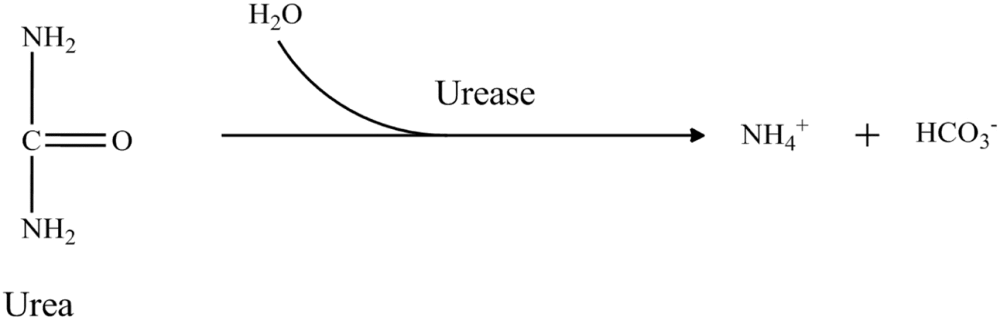
Most of the arginine biosensors were developed by immobilizing two enzymes arginase (EC 3.5.3.1) and urease (EC 3.5.1.5). These enzymes catalyzed the hydrolysis of arginine to ammonium and bicarbonate ions by two consecutive steps. The reaction sequence is as follow:
 |
(1) |
 |
(2) |
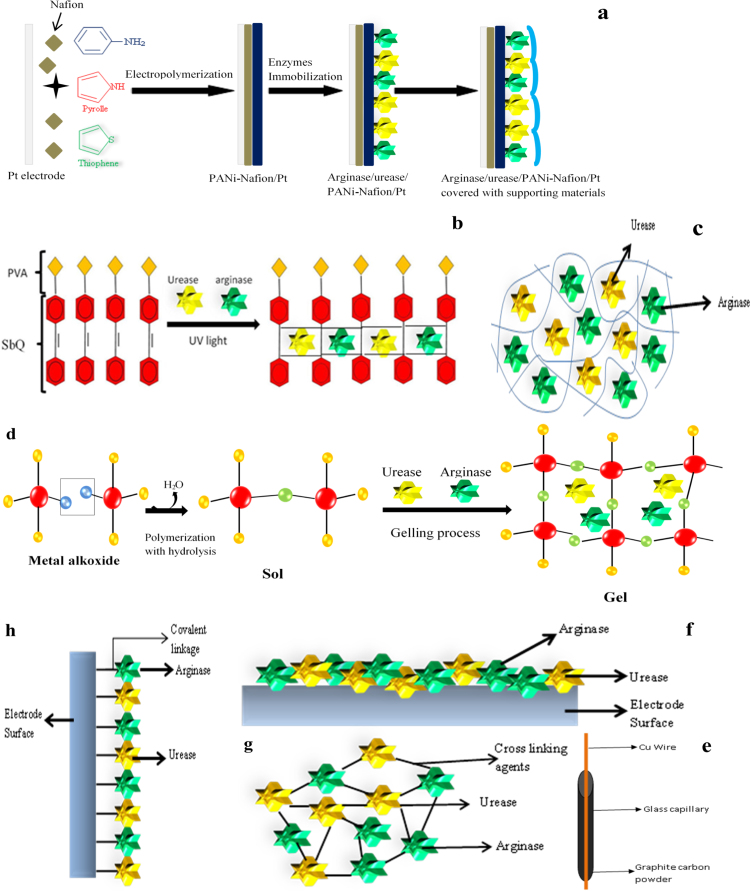
Enzyme based arginine biosensors have been developed using several types of transducers like an ammonia gas electrode [61], [62], [63], [64], [65], a pH electrode [66], ammonium ion selective electrode [67], [68] and so for. But the main aspect regarding the practical application of arginine biosensor is moreover depending on the operational life of biomolecules. A variety of immobilization strategies have been applied for the construction of L-arginine biosensors are shown in Fig. 4.
Fig. 4.
Different immobilization methods to develop arginine biosensor, entrapment: (a) electro polymerization, (b) photopolymerization, (c) polysaccharides based gel, (d) sole gel techniques, (e) carbon paste electrode, Physical adsorption (f), cross linking (g) and covalent binding (h).
The major problem associated with bio-component immobilization is about the stability. Stability is reduced due to leakage of biomolecules from the matrices or lose of activity due to change in conformation in micro environment [69], [70]. Other reason like significant diffusion limitations of substrates or products formed during specific biochemical reaction. So, further efforts have been made by researcher to minimized these problems by using some restricted conditions like leakage of biomolecule can be abridged by lowering the pore size of the matrices or the membrane cut off molecular weight. Some approaches are to control micro environment conditions utilizing different kind of matrices, changing processing conditions and reducing particle size [71], [72]. The comparative account of different strategies for immobilization of biological molecules for the fabrication of arginine biosensor is shown in Table 1. Table 1 clearly reflects entrapment methods are the most popular used methods as compared to other methods, because of long term stability and high throughput activity.
Table 1.
Comparison of immobilization techniques used for the construction of arginine biosensor.
| S.No. | Method of Immobilizations | Supporting materials | Bio components | Merits | Demerits | References |
|---|---|---|---|---|---|---|
| 1 | Adsorption | Circular cellophane dialysis membrane | Streptococcus faecium | Simple, easy and limited loss of enzyme activity | Desorption and non-specific adsorption | [63] |
| 2 | Covalent binding | Gas permeable membrane | Arg/urs | No diffusion barrier and stable | Matrix not re-generable and toxic chemical used for coupling | [67] |
| Gold nanoparticle /calcium alginate beads | Arg/urs | Stable and not affected in adverse condition like pH change | High enzyme activity loss | [73] | ||
| 3 | Cross linking | GA/Biologically active membrane | Arginase | Simple and stable | High enzyme activity loss and involvement of toxic chemicals | [74] |
| GA/Bio-membrane | Arg/urs | Used in combination with entrapment to lower the loss of enzyme activity | Toxic chemical treatment | [75] | ||
| GA/BSA/Glycerol | Ars/urs | Simple and enzyme activity retain as such during immobilization process | Less stable and loading capacity also very low | [76] | ||
| 4 | Entrapment | Electrodeposition with GA | Arg/urs | Gentle treatment, specificity with enzyme and substrate retained | Involvement of toxic chemicals and insufficient immobilization | [77] |
| Polypyrrole and polyion | Arg/urs | Easy to construct by simple dropping and drying methods | Chance of leakage high for the enzyme | [68] | ||
| Graphite-Teflon electrode matrix | Arg/urs | Simple physical inclusion and good for co-immobilization of many enzymes | Need optimized condition highly affected with external condition like pH temp. etc. | [78] | ||
| PVC and aromatic polyurethane | Arg/urs | Higher loading of active enzyme | Complex procedure and loss of enzyme activity | [102] | ||
| Glutaraldehyde and gelatin | Arg/urs | Easy to use and fix on to the transducers | Involvement of toxic chemicals | [66] | ||
| PANi/Nafion membrane | Arg/urs | Easy to use and no direct chemical modification | No longer storage stability, biosensor activity become half after 72 h | [79] | ||
| Gelatin/Agar/Polyacrylamide/Calcium alginate beads | Arg/urs | Easy and simple and best for optical transducers | No longer storage stability of enzymes and damage problem | [80], [81] | ||
| Sol-gel nylon membrane | Arg/urs | Simple preparation, chemical inertness, low tem encapsulation and mechanical stability | Leaching of entrapped biomolecules and also less storage stability | [82] | ||
| PVA –SbQ membrane | Arg/urs | Easy to use and less time required for immobilization process highly stable over three months | Treatment of toxic chemicals | [76] |
GA: Gluteraldehyde; Arg/urs: Arginase/urease; PVC: poly-vinyl chloride; PANi: poly aniline; PVA-SbQ: poly(vinyl alcohol)-styrylpyridinium.
The working principle of biosensor is based on a specific bioassay principle. Two main components one is biological or bio-components and second is physical component also known as transducer or transducing elements. In general, the biological component has to be immobilized on best way on to the transducer. The selection of immobilization methods is based on the nature of bio-component that has to be immobilized, the type of transducer used and the physical state of the analyte.
Table 2 provides a comparison of the various biosensor developed for arginine detection. From the Table 2 it is found that most of the arginine biosensors are based on the electrochemical transducers and most of the biosensors were developed by using two enzymes system (arginase and urease). The linear range and limit of detection for arginine was quite comparable in all the type of biosensors. The lowest response time was found in optical biosensor, while highest in potentiometric and optical biosensors. In terms of storage stability maximum storage stability was observed in conductometric arginine biosensor immobilized in cross linked matrix by using glutaraldehyde.
Table 2.
Comparison of the biosensors developed for the detection of L- arginine.
| Bio-components | Transducers | Working electrode | Immobilization methods | Detection range (M) | Limit of Detection (M) | Optimum pH | Response time (Minutes) | Storage stability (days) | References |
|---|---|---|---|---|---|---|---|---|---|
| Streptococcus faecium | Potentiometric | NH3 gas electrode | Physical adsorption | 10−3 – 10−5 | 1.6 × 10−5 | 7.4 | – | 2–20 | [63] |
| Streptococcus lactis | Potentiometric | NH3 gas electrode | Cell suspension | 10−3 – 10−6 | 8 × 10−6 | 7.8 | 6–8 | – | [64] |
| Arg/urs | Potentiometric | NH3 gas electrode | Physical adsorption | 10−3− 10−5 | – | 5 | [65] | ||
| Arg/urs | Potentiometric | GCE | Electropolymerization | 10−3 – 10−5 | – | 6.8 | – | – | [77] |
| Decarboxylase/ autotrophic bacteria | Amperometric | Clark oxygen electrode | Entrapment | – | – | 5.2 | 0.5 to 30 | – | [83] |
| Arg/urs | Potentiometric | NH4+ ISE | Covalent | 10−2 – 10−4 | 10−5 | 8.5 | 1.5 − 4 | 7 | [67] |
| Arg/urs | Potentiometric | NH4+ ISE | Physical adsorption | 10−3 – 10−5 | – | 7.5 | – | – | [68] |
| L/D- arginine oxidase | Amperometric | SPE | Cross-linking | – | – | 7.8 | 56 | [84] | |
| L/D- arginine oxidase | Amperometric | Graphite Teflon electrode | Physical adsorption | 10−3 − 10−4 | 1.6 × 10−4 and 3.3 × 10−5a | 9.0 | – | – | [78] |
| Arg/urs | Potentiometric | NH3 ISE | Cross-linking | 10−3 – 10−6 | – | 8.0 | – | – | [102] |
| Arg/urs | pH electrode | Entrapment | 3.5× 10−4 − 2.5× 10−5 | – | 8.5 | 10 | 1 | [66] | |
| Arg/urs | Potentiometric | NH3 ISE | Covalent | 4× 10−2 – 1.2× 10−4 | < 10−4 | 9.5 | 3–5 | 14 | [73] |
| Arg/urs | Optical | – | Entrapment | 10−1 – 10−10 | 10−10 | 7.0 | 0.1 | 60 | [80] |
| Arg/urs | Conductometric | Au coated ceramic plate | Cross-linking | 5 × 10−3 − 2.5× 10−5 | – | 6.0 | – | 14 | [75] |
| Arg/urs | Conductometric | Au coated ceramic plate | Entrapment | 1.4 ×10−3 – 10−4 | 2.5 × 10−5 | 6.0 | 0.5 | 90 | [76] |
| Cross linked | 4.0× 10−3− 1.0 × 10−5 | 5.0 × 10−7 | 6.0 | 2 | 45 | ||||
| Arg/urs | Conductometric | Au coated ceramic plate | Cross-linking | 6 × 10−3 – 10−5 | 1.0 × 10−5 | 6.0 | 0.46–0.55 | >120 | [85] |
| Arg/urs | Amperometric | PANi-composite Pl electrode | Physical adsorption | 10−4 – 10−6 | 3.8 × 10−5 | 7.5 | 0.16 | 3 | [79] |
| Arg/urs | Potentiometric | NH3 ISE | Physical adsorption | 10−1 − 10−9 | 10−9 | 6.5 | 5 | 60 | [82] |
| Arg/urs | Optical | – | Entrapment | 10−1 − 10−9 | 10−9 | 6.5 | 10 | 60 | [82] |
| Urease | Potentiometric | ISFET | Cross-linking | 1.0 × 10−4− 2.0 × 10−3 | 5× 10−5 | 7.4 | – | – | [86] |
| – | Amperometric | Copper nanoparticle modified | – | 2.6× 10−4− 2.0 × 10−5/ (7.0 × 10−4 – 10 × 10−5)a | 4.3 × 10−6 /2.0 × 10−5 | – | – | – | [87] |
| Arg/urs | Amperometric | PANi composite Pt electrode | Physical adsorption | 1.0–0.6 × 10−3 | 0.085 × 10−3 | 7.5 | 1 | 3 | [88] |
| ADI | Potentiometric | PAG | Entrapment | 10−1 – 10−9 | 10−9 | 7.2 | 0.5 | 30 | [81] |
| ADI | Amperometric | PANi composite Pt-SPE | Cross linking | 3 × 10−6 – 2.0× 10−4 | 10−6 | 0.25 | 30 | [102]` | |
| Crude ADI | Optical | – | Cross linking | 1.0–10−4 | 10−5 | 8.0 | – | – | [103] |
In flow injection analysis mode, Arg/Urs: Arginase/ urease, GCE: Glossy Carbon Electrode, ISE: Ion selective Electrode, SPE: Screen Printed Electrode, PANi: Polyanyline, ISFET: Ion Selective Field Effect Transistor, ADI: arginine Deiminase, PAG: Polyacrylamide gel.
2.1.1. Entrapment methods
2.1.1.1. Electropolymerization
Electropolymerization is a simple technique, it is performed by applying suitable potential or current to the working electrode immersed in a medium containing monomer which has to be polymerized. Ivnitskii and Rishpon [77] used electropolymerization technique for the immobilization of arginase and urease in combination of gluteraldehyde for the development of arginine biosensor. But, as the direct enzymes membrane is formed on the electrode surface, hence creates snag to the conductivity of the electrode. So, later on conducting polymers such as polyaniline, polypyrrole, polythiophene and polyurethane electropolymerized films were used for immobilization of enzymes (Fig. 4a).
Polypyrrole (PPy), in particular, presents a relatively stable electrical conductivity and can be electropolymerized under biocompatible conditions such as low oxidation potential and neutral pH. The electropolymerized polypyrrole (PPy) on a polyion complex (PIC) containing urease and arginase was fabricated for arginine detection [68]. In this method simply the change in pH of the medium is occurred due to the formation of ammonium ion in two successive reaction catalyzed by arginase and urease. Electropolymerized matrices of PVC and aromatic polyurethane (ArPU) doped with various membrane active components were also used for arginine biosensor [102]. Recently, Polyaniline has attracted much attention due to various remarkable characteristics as stable conductivity, charge transfer capability and environmental stability. An arginine biosensor has been developed featuring electropolymerized polyaniline film on the Pt electrode by immobilizing urease and arginase-I. A layer calcium alginate gel (CAG) was applied for physical support to the enzymes immobilized in to electropolymerzed film on to the electrode [79]. Advantages of these methods are easy and generate high conductive surface for electronic processing. The main problem associated with this method is led to change in topography of enzymes and resulted the loss of activity and stability.
2.1.1.2. Photopolymerization
Photopolymerization is a process where a phot sensitive monomer gets polymerized when it is exposed to light, often ultraviolet or visible light (Fig. 4b). A photopolymerized poly vinyle alcohol-styrylpyridinium (PVA-SbQ) matrix for the entrapment of arginase and urease was applied for the construction of arginine biosensor [75]. Advantage of this method over other immobilization methods is providing high storage stability (90 days) of entrapped enzymes. It is found that response time of biosensor was also influenced with immobilization techniques. Saiapina et al. [76] found that enzymes entrapped in photopolymerized matrix showed less response time 30 s as in cross-linked found 120 s, but the major constrain in this technique is use of toxic materials.
2.1.1.3. Entrapment in a Polysaccharide-based gel
Enzymes can also be entrapped in a polysaccharide-based gel (e.g. gelatin, chitosan or agarose) that works as a semipermeable membrane (Fig. 4c). Contrary to synthetic polymers such as polyacrylamide, these matrices are biocompatible, nontoxic, provide a natural microenvironment to the enzyme and also give sufficient accessibility to electrons to shuttle between the enzyme and the electrode. The enzyme arginase and urease both were co-immobilized in the gelatin membrane and cross-linked with gluteraldehyde, on the surface of pH electrode for the development of arginine biosensor [66]. Main disadvantage of their work that is stability of the biosensor. Only one day is reported to get stable and reproducible reading, after a decreased was observed.
Kumar et al. [80] has also used different immobilization matrices for arginase and urease enzyme such as polyacrylamide, calcium alginate, gelatin and agar to develop arginine biosensor. The calcium alginate method was found to be best in terms of storage stability around 60 days and also showed lower response time 14 s as compared with other immobilization matrices less than 30 days. The main hurdle with these methods is chance of damage during operation as being soft matrices.
2.1.1.4. Sol–Gel method
The Sol–gel method is based on the ability to form solid metal or semimetal oxides via the aqueous process of hydrolytically labile precursors such as: ester of silicic, polysilicic acid, alkoxide, halide of aluminum in Fig. 4d. [89], [90], [91], [92]. Sol-gel co-immobilization of enzymes (arginase, urease) and dye on to the nylon membrane was used for the development of arginine biosensor [82]. This approach has appeared to be quite promising as it allows the association of a soft biocompatible, thermo stable and non-swelling component. The storage stability was found to be around 60 days.
2.1.1.5. Entrapment in Carbon Paste Matrix (CPM)
CPM is a mixture of carbon (graphite) powders and a binder (pasting liquid) is a popular electrode material used for the preparation of various electrodes in Fig. 4e [93], [94]. An amperometric biosensor designed by applying two enzymes, amino acid oxidase and horseradish peroxidase both were co-immobilized with a mediator into graphite and 70% Teflon electrode matrix. This biosensor is used for the selective determination of L- or D-amino acids [78].
2.1.2. Physical adsorption
In the adsorption, biomolecules are physically adsorbed on to the polymer matrix with the help of Vander Waals and some hydrogen bonds Fig. 4f. The core advantage of this immobilization method is to retain the native form of biomolecules. This method has widely been used to develop enzyme and whole cell based biosensors. Arginine biosensor has been developed by immobilization of living cells (Streptococcus faecium) on circular cellophane dialysis membrane [63]. Although this method is good due to negligible loss of enzyme activity, but the basic problem appeared is desorption of enzyme due to change in temperature, pH, and ionic strength of working solution and results in drop off the sensor stability [71].
2.1.3. Immobilization by cross-Linking
There are bi-functional compounds having ability to bind one functional end with biomolecule and other end to the solid supports (Fig. 4g). The commonly used cross linking agents is glutaraldehyde (both ends have aldehyde group), corbodiimide (having both side alkane group), cyanogen bromide (nitrogen and bromine help in functional reaction) and ethyl chloroformate (chloride and alkane takes part in chemical reaction). The immobilization of enzyme arginase with the help of amino group of NH2-column activated with glutaraldehyde, a most promising amine-reactive cross linker [95]. The bi-enzyme solution containing arginase, urease and a working solution of gluteraldehyde for cross-linking was used to construct the arginine biosensor [75], [76]. The storage stability of biosensor was found to be >120 days.
2.1.4. Covalent immobilization
A very widespread chemical immobilization method is being used for enzyme immobilization with polymeric supports to the development enzymatic biosensors. Covalent immobilization can be performed directly onto the transducer surface or onto a thin membrane fixed onto the transducer Fig. 4h. Carbo-di-imides allow the binding between the carboxyl groups of a support and the amino function of an enzyme. This procedure is widely used to develop enzymatic biosensors.
A biosensor for arginine and creatine were developed by using two enzymes: one is urease and second is creatinase or arginase depending on the analyte: creatine or arginine. Immobilization was carried out by using polyvinyl chloride (PVC-COOH) as the membrane matrix material. It provides carboxylic groups on the surface of the ion-selective membrane which makes covalent binding of enzymes possible. The covalent binding of enzymes to the matrix of the ion-selective membrane made-up of the carboxylated poly (viny1 chloride) was achieved using carbodiimide and glutaraldehyde [67]. Stability of the immobilized enzymes was obtained few days to one week. Another approach of covalent immobilization was arginase immobilized on an ethylenediamine (EDA) monolithic convective interaction media (CIM) disk derivatized with gluteraldehyde [95]. Recombinant arginase-I was immobilized using the carbodiimide pentafluoro phenol method on the surface of NPs functionalized with ω-mercapto hexadecanoic acid [96]. The storage stability was found up to 14 days.
2.2. Transducer for arginine biosensor
2.2.1. Electrochemical transducers
Electrochemical biosensors are providing an attractive means to detect arginine in any biological sample. Over the past decades several sensing concepts and related devices have been developed for the detection of arginine. In the above reaction sequence (1), (2) there is formation of ionic molecules that triggers the potential of the electrode or electrical properties of the conducting polymers, which can be easily analyzed by the sensor response. It can be analyzed by using various types of transducers like amperometric, conductometric and potentiometric. Which is being explained bellow in detail format.
2.2.1.1. Amperometric
Amperometric biosensor is a type of electrochemical biosensor, most of the amperometric arginine biosensor was developed using two enzymes arginase and urease. Enzyme amino acid di-decarboxylase and autotrophic bacteria were co-immobilized on a micro-fabricated Clark oxygen electrode based biosensor for the detection of variety of amino acids including L-arginine has been developed [83]. The response time of the developed biosensor was found to be 0.5–30 min for different amino acid. Sarkar et al. [84] developed screen printed electrodes based amperometric biosensor for monitoring L & D- amino acids using enzymes D-amino acid oxidase (porcine kidney) & L-amino acid oxidase (Crotalus adamateus). Both L- arginine and D-arginine were determined by these electrodes. Domınguez et al. [78] has developed an amperometric biosensor based on graphite filled Teflon electrode which contain L/D- AAOD (amino acid oxidase), HRP (horseradish peroxidase) and ferrocene for the detection of various amino acids such as: L-arginine, L-phenylalanine, L-leucine, L-methionine, L-tryptophan, D-leucine, D-methionine, D-serine, and D-valine. The developed biosensor has shown a good linear range for concentrations of 1.0 × 10−4 to 20 × 10−3 M for arginine. Another, amperometric biosensor has been fabricated using polyaniline/nafion composite film on platinum electrode as a working electrode. The developed method has shown a good specificity towards analyte L-arginine. The sensitivity of the biosensor was found to be 110 ± 1.3 nA/mM.mm2 with the apparent Michaelis–Menten constant (Kmapp) derived from L-arginine calibration curve of 1.27 ± 0.29 mM. A linear concentration range was observed from 7.0 × 10−5 to 6.0 × 10−4 M concentration of L-arginine, and LOD was found to be 3.8 × 10−5 M with response time of 10 s [79]. The proposed biosensor working principle is shown in Fig. 5.
Fig. 5.
The amperometric detection of arginine using arginase/urease composite PANI-Nafion film.
These amperometric arginine biosensors have shown excellent performance regarding many aspects like long storage stability (upto 60 days), high sensitivity, quick response time, very less requirement of bio-component for immobilization. In addition, some of constrains that is associated with the amperometric arginine biosensors is using two-enzyme system that after immobilization onto the electrode surface reduces the sensitivity by increasing the resistance.
2.2.1.2. Conductometric
In conductometric transducer based biosensor, the conducting polymer are used for the interactions between biochemical reaction and microelectrode, and the generated conductance or impedance is recorded. Liu et al. [97] has developed a biosensor system based on the surface acoustic wave (SAW)/conductance for the detection of L-arginine. The bioassay principle was based on two coupling reactions involving arginase and urease with measurement of frequency shift due to the change in conducting ions produced. Other conductometric biosensor for L-arginine determination was compared with two types of enzyme immobilization (cross-linking with glutaraldehyde (GA) and entrapment in the polymeric membrane). The optimum features determined in terms of sensitivity, linearity and the detection limit are 4.2 µS/mM, 1.0 × 10−5 to 4.0 × 10−3 M, and 5.0× 10−7 M, respectively, for L-arginine biosensor based on enzymes immobilized having cross-linked with GA. They observed that entrapment in polymeric membrane showed fast response time but detection limit was not lower than achieved in cross linking with GA which showed longer response time. The storage stability was also influenced by the immobilization methods. GA showed better stability as polymeric membrane entrapment, its might be occurred due to leaching of enzymes from the polymeric membrane with time [76].
2.2.1.3. Potentiometric
Potentiometric based transducers measure the charge potential or ion activity in the electrochemical reaction. Potentiometric techniques involve a non-faradaic electrode process and measurement of the potential difference between a working electrode and reference electrode.
2.2.1.3.1. Ammonia/Ammonium ion sensing probe
The first potentiometric arginine biosensor has been developed by coupling intact microorganisms (Streptococcus faecium) with an ammonia gas-sensing membrane electrode. The resulting electrode provides a linear response to arginine over the concentration range 5.0 × 10−5 to 1.0 × 10−3 M in phosphate buffer pH 7.4, with good selectivity over other amino acids. The slope of the calibration graph is −45 to −40 mV/decade during the period 2–20 days [63]. The bacterial cells Streptococcus lactis have been employed for the conversion of L-arginine into ammonia. The produced ammonia was detected by using ammonia gas-electrode as a transducer. The combination of bacteria1 action and the gas electrode responds linearly to L-arginine over the concentration range 8.0 × 10−6 to 1.0 × 10−3M, with a slope of 59.0 mV/decade and specific with respect to other L-amino acids [64]. An Ammonium ion selective electrode has been prepared by electropolymerization technique of the enzymes urease and arginase onto the inert electrode [77]. Similarly, another ammonium ion selective electrode enzymatically sensitized for detection of creatine and L-arginine has been reported. Enzyme layers were formed for two enzymes urease and arginase/creatinase dependent on the analyte (substrate): creatine or arginine. The linear ranges of both biosensors were 1.0 × 10−4 to 3.0 × 10−2 M and the detection limit was below 10−5 M [67]. The bi-enzymatic approach for the detection of arginine was carried out and both enzymes arginase and urease were co-immobilized onto the ion selective electrode based transducer, and L-arginine was measured in the range of 1.0 ×10−5 to 1.0 ×10−3 M with a sensitivity of 50 mV/ decade [68]. Some other immobilization approaches have been developed for arginine biosensor here both enzymes such as arginase and urease are co-immobilized into the gelatin membrane and cross-linked with glutaraldehyde. The developed biosensor showed good linear response on L-arginine concentration ranging from 2.5 × 10−5 to 3.1× 10−4 M with response time 10 min [66]. Lvova et al. [102] demonstrated all-solid-state potentiometric electronic tongue microsystem for L-arginine detection in korean green tea. Recently, a novel potentiometric arginine biosensor has been developed based on bacteria producing arginine deiminase, the cell free extract was immobilized on to the ammonium ion selective electrode [81]. The first ever single enzyme based approach for specific detection of arginine has been proposed. The biosensor showed good linear range from 1to 10−9 M with 30 s response time. These systems have the advantage of simplicity because they require only one electrode where directly bio component is immobilized. Easy to avoiding interfering compounds using selective membrane either for substrate or for product. One more advantage of this biosensor is there is no need to be correct urea interference.
2.2.1.3.2. CO2 sensing electrode
The development of a plug-flow based bioreactor for determining arginine by immobilizing arginine decarboxylase on controlled pore glass beads and monitoring of the evolved CO2 by CO2 sensing electrode and the process was used for monitoring arginine in peanuts to assess their maturity [98]. The linear response for arginine was found to be 3 × 10−4 to 3 × 10−3 M. the storage stability of arginine estimation bioreactor was up to 30 days was obtained.
2.2.1.4. Ion-selective field effect transistors (ISFET)
A novel approach based on ion-selective field effect transistors (ISFET) was used for the construction of arginine biosensor [86]. Detection limit for arginine was found to be 0.05 mM and selectivity towards other amino acids was also studied.
2.2.2. Optical transducers
2.2.2.1. Fluorescence based
Fluorescence based biosensor development for arginine is the breakdown of arginine into ammonia by two step reaction as shown above in Eqs. (1), (2). The formation of ammonium ions causes the protonation of pH sensitive indicator (Rhodamine 6 G) which changes its fluorescence spectrum upon deprotonation [82]. The linear range was achieved up to nanomolar (~10−9 M) range of arginine with response time of 10 min. The standard reference chart was used for quantifying arginine in various food samples. Application into the real samples of Pineapple Juice, Orange Juice and Green Tea were taken in separate glass cells (5 ml each of the samples) for the estimation of arginine contents. A very recent paper on arginine deiminase co-immobilized with ZnS quantum dots based biosensor for the detection of arginine has been reported [103]. In this method a linear response 1.0–10−4 M was obtained for arginine with response time 2 min the developed system was successfully applied for arginine estimation in fruit juices samples.
2.2.2.2. Absorption based
L-Arginase converts arginine into urea and ornithine and subsequent deamination of urea by the second enzyme urease into ammonia and carbon dioxide. The formation of ammonia leads to increase pH of the medium, which is detected by phenol red dye present in medium and change color yellow to red. The concentration of the arginine presence in the medium is directly proportional to the color produced by the dye and detected as a change in absorbance by the spectrophotometric transducers [80], [81], [82]. The detection range of these biosensors were found to be 1–10−9M for arginine with response time 5 min.
2.3. Biological components for construction of arginine biosensor
2.3.1. Enzyme based
First enzyme based arginine biosensor was developed by using arginine hydrolytic enzymes (arginase & urease) having specific activity 150U/mg and 1200U/mg respectively. The effect of Mn2+ ion on the enzyme arginase showed significant response on the enzyme activity and found that the Mn2+ ions raised the activity of arginase enzyme [77]. Later, some other enzymatic approach was used for the development of arginine biosensors. Enzyme amino acid decarboxylases was also utilized as biological material and co-immobilized with some bacterial cells for the development of arginine biosensor [83]. Some other multi-enzymatic method has been taken in consideration for the construction of arginine biosensor, one of them is L/D-amino acid oxidase (AAO) and horseradish peroxidase (HRP) were co-immobilized on the working electrode of the biosensor [78]. Most popular multi-enzymatic approach is using two enzymes arginase and urease both co-immobilized onto the surface of different transducer for the development of arginine biosensor [65–68,73–76,79,85,102]. The sequence of reaction is as given earlier in Eqs. (1), (2).
A novel arginine biosensor was developed by using urease inhibition by arginine molecules. In this approach, the concentration of urea and arginine were optimized and then inhibition of urease enzyme was studied under the presence of arginine, and also in the presence of some other amino acid to evaluate biosensor specificity [84]. Briefly the arginine detection was achieved by calculating % level of inhibition of urease enzyme.
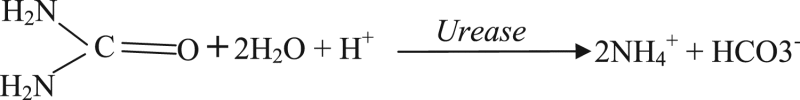
Where Y is the % inhibition and Ia and I is the steady state signal with and without arginine.
2.3.2. Whole cell based
Whole cell based arginine biosensor having some possible advantages such as the exclusion of enzyme purification steps, minimize the chances of trailing enzyme activity [99] and also enhance the life of the electrode. First whole cell based arginine biosensor was developed by using Streptococcus faecium ATCC 9790. The biosensor was fabricated by immobilization of intact cells of S. faecium ATCC 9790 onto electrode modified with ammonium gas sensing membrane [63]. This membrane electrode with bacterial cells may also serve as a model for the development of other new sensing approach. Later, another whole cell based arginine biosensor has been approached using Streptococcus lactis ATCC 19435 immobilized ammonia gas-electrode [64]. The reaction is as follows:
But the problem linked with whole cell based arginine biosensor is regarding selectivity and specificity of the biosensor. Because, in whole cell biosensors, the cells are able to interact or utilize some other amino acid or related substances present in the sample medium. A biosensor for monitoring arginine in broad range samples like in food samples such as a variety of fruit juices and beverages, other in clinical samples such as body fluids like serum, urine and blood. So the chances of interference in detection is quite high in whole cell based biosensor. To overcome these type of problems, a biosensor for specific arginine detection was developed by immobilizing Bacillus subtilis (Arginase) with urease enzyme and a pH sensitive indicator in calcium alginate or poly-acrylamide gel. The biosensor showed rapid detection of arginine in real sample (response time of 40 s to 4 min) because the signal transduction machinery (indicator) has been co-immobilized along with Bacillus subtilis and urease. The linear range of detection was found to be up to nanomolar (1 × 10−1− 1 × 10−9 M) level of arginine [100].
2.3.3. Tissue based
The first tissue based biosensor was developed by co-immobilization of the enzyme urease and a thin slice of bovine liver tissue on the surface of ammonia gas sensing electrode. The bovine liver tissue provides a large quantity of the enzyme arginase and the combined biocatalytic activities of arginase and urease led to generate ammonia from arginine [103]. This arginine biosensor serves to demonstrate the concept of employing whole sections of mammalian tissue as an effective biocatalytic layer and provide certain advantages over purified enzymes. Oungpipat and Lenghor [101] developed an arginine biosensor based on immobilization of bovine liver arginase and Cajanus cajan tissue was used as source of urease. A stainless steel electrode was used for monitoring the pH change during the reaction. The hybrid arginine biosensor possesses a linear dynamic range from 3.0 × 10−3 to 0.3 M with a detection limit of 3.0 × 10−3 M for arginine. The biosensor responds to arginine, with steady state responses achieved within 12 min. The sensitivity of the biosensor decreased to 50% of the original value after 5 days of continuous use. The selective detection of L-arginine from different biological fluids, food samples and pharmaceuticals are listed in Table 3.
Table 3.
Application of developed arginine biosensors in different samples.
3. Nanomaterial based arginine sensors
Nanomaterial based sensors have certain advantages over traditional biosensors such as high sensitivity and specificity, which provides a potential application in analysis of clinical samples. Nanomaterials particularly nanoparticles, offer an excellent way to increases the bio-recognition area, because high surface area-to-volume ratio of nanoparticles make a large number of sites available for molecular interaction [105]. Although most of the traditional biosensors shown good selectivity and were responsive to the target analyte, but poor precision/ insufficient stability and low sensitivity make it less acceptable against nanomaterial based biosensors and sensors. Martínez-Periñán et al. [106] developed the electrochemical sensor for the detection of L-Arginine, L-ornithine and L-citrulline in urine and serum samples, based on the use of Ni(OH)2 nanoparticle-modified carbon nanotubes. However, the main drawback of this method was useful for detection of a group of amino acids and was not found to be selective for L-Arginine. Another, copper nanoparticle based arginine sensor has been developed for the determination of arginine in urine sample [87]. Table 4 shown a comparative analysis of arginine sensors based on nanoparticles.
Table 4.
Comparison of the analytical property of nanoparticle based arginine sensors.
| S.No. | Support of immobilization | Working electrode | Working pH | LOD (µM) | Linear range (µM) | Response time (s) | Sensitivity (µA.M−1.cm−1) | Potential applied | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ni(OH)2NPs/SWCNT | SPE | 11 | 0.515 | up to 30 | 50 | 201 ± 2 | +0.55 V | 106 |
| 2 | CuNPs/CPE | CPE | 8 | 4.3 | 20–263 | 30 | 4.8 ×102 | +0.72 V | 87 |
SPE: screen printed electrode, CPE: carbon paste electrode.
4. Conclusion and future recommendation
In this review, we have provided a comprehensive knowledge of L-arginine in medical and foods systems along with the insight view of development biosensor devices for arginine detection. The L-arginine detection is critically important to diagnosis and for the treatment of many diseases including cancer. In food the quantification of arginine is used as a quality control parameter. A variety of biosensors have been developed based on transducers ranging from amperometric, potentiometric, conductometric and optical. We found that the electrochemical techniques specially amperometric has gained much attention due to high throughput, high sensitivity, long storage stability (120 days), easy to use, good linear range and low level of detection over other transducers based biosensor. Although, potentiometric is too simple over amperometric technique because of direct detection of ammonium ion by using ion selective electrodes and easily combined with the continuous system, but stability and reliability is not truly realized. The introduction of nanomaterials and derived materials with amperometric technique make it more refine and reliable, it will eventually make its way from the laboratory to enter commercial markets like clinics and industries. In spite of this rapid advancement into the development of L-arginine biosensor, some improvement is still needed to achieve specificity of the system as urea present in sample may interfere. Hence single enzyme approach would be a better option in the terms of specificity, selectivity and cost. Miniaturized arginine biosensor could be developed by using hand held fiber optic and mini-potentiostat electrochemical. Lab-on chip concept would be a better choice for the fast detection of arginine, and easy to use as the diagnosis kit for many clinical conditions as well as food and food processing systems.
Acknowledgments
The authors are grateful to the Department of Biotechnology, Punjabi University Patiala for providing facilities to carry out this work.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.10.006.
Appendix A. Transparency document
Supplementary Material. Conflict of interest.
References
- 1.Fuhrmann J., Schmidt A., Spiess S., Lehner A., Turgay K., Mechtler K., Charpentier E., Clausen T. McsB is a protein arginine kinase that phosphorylates and inhibits the heat-shock regulator CtsR. Science. 2009;324:1323–1327. doi: 10.1126/science.1170088. [DOI] [PubMed] [Google Scholar]
- 2.Riordan J.F., McElvany K.D., Borders C.L., Jr Arginyl residues: anion principles of protein conformation. Science. 1977;195:884–886. doi: 10.1126/science.190679. [DOI] [PubMed] [Google Scholar]
- 3.Coman D., Yaplito-Lee J., Boneh A. New indications and controversies in arginine therapy. Clin. Nutr. 2008;27(4):489–496. doi: 10.1016/j.clnu.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Boger R.H. The pharmacodynamics of L-Arginine. J. Nutr. 2007;137(6):1650S–1655S. doi: 10.1093/jn/137.6.1650S. [DOI] [PubMed] [Google Scholar]
- 5.Ensor C.M., Holtsberg F.W., Bomalaski J.S., Clark M.A. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002;62:5443–5450. [PubMed] [Google Scholar]
- 6.Wheatley D.N., Scott L., Lamb J., Smith S. Single amino acid (Arginine) restriction: growth and death of cultured HeLa and human diploid fibroblasts. Physiol Biochem. 2000;10:37–55. doi: 10.1159/000016333. [DOI] [PubMed] [Google Scholar]
- 7.Wheatley D.N. Controlling cancer by restricting arginine availability – arginine catabolizing enzymes as anticancer agents. Anticancer Drugs. 2004;15:825–833. doi: 10.1097/00001813-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Morbidelli L., Donnini S., Ziche M. Role of nitric oxide in tumor angiogenesis. Cancer Treat. Res. 2004;117:155–167. doi: 10.1007/978-1-4419-8871-3_11. [DOI] [PubMed] [Google Scholar]
- 9.Vissers Y.L.J., Dejong C.H.C., Luiking Y.C., Fearon K.C.H., von Meyenfeldt M.F., Deutz N.E.P. Plasma arginine concentrations are reduced in cancer patients: evidence for arginine deficiency? Am. J. Clin. Nutr. 2005;81:1142–1146. doi: 10.1093/ajcn/81.5.1142. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan I., Aydin Y., Bilen Y., Genc F., Keles M.S., Eroglu A. Th e evaluation of plasma arginine, arginase, and nitric oxide levels in patients with esophageal cancer. Turk J. Med. Sci. 2005;42(3):403–409. [Google Scholar]
- 11.Wheatley D.N., Campbell E. Arginine catabolism, liver extracts and cancer. Pathol. Oncol. Res. 2001;8:18–25. doi: 10.1007/BF03033696. [DOI] [PubMed] [Google Scholar]
- 12.Wheatley D.N., Campbell E. Arginine deprivation and tumor cell death: 1. arginase and its inhibition. Mol. Cell Biochem. 2002;244:177–185. [PubMed] [Google Scholar]
- 13.Wheatley D.N., Campbell E. Arginine deprivation, growth inhibition and tumor cell death: 3. Deficient utilization of citrulline by malignant cells. British J. Cancer. 2003;89:573–576. doi: 10.1038/sj.bjc.6601134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corraliza I., Moncada S. Increased expression of arginase II in patients with different forms of arthritis. Implications of the regulation of nitric oxide. J. Rheumatol. 2002;29:2261–2265. [PubMed] [Google Scholar]
- 15.Bruch-Gerharz D., Schnorr O., Suschek C., Beck K.-F., Pfeilschifter J., Ruzicka T., Kolb-Bachofen V. Arginase I overexpression in psoriasis. Am. J. Pathol. 2003;162:203–211. doi: 10.1016/S0002-9440(10)63811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hein T.W., Zhang C., Wang W., Chang C.I., Thengchaisri N., Kuo L. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: counteracting role of arginase. FASEB J. 2003;17:2328–2330. doi: 10.1096/fj.03-0115fje. [DOI] [PubMed] [Google Scholar]
- 17.Meurs H., Maarsingh H., Zaagsma J. Arginase and asthma: novel insights into nitric oxide homeostasis and airway hyperresponsiveness. Trends Pharmacol. Sci. 2003;24:450–455. doi: 10.1016/S0165-6147(03)00227-X. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann N., King N.E., Laporte J., Yang M., Mishra A., Pope S.M., Muntel E.E., Witte D.P., Pegg A.A., Foster P.S. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J. Clin. Invest. 2003;111:1863–1874. doi: 10.1172/JCI17912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porembska Z., Luboinski G., Chrzanowska A., Mielczarek M., Magnuska J., Baranczyk-Kuzma A. Arginase in patients with breast cancer. Clin. Chim. Acta. 2003;328:105–111. doi: 10.1016/s0009-8981(02)00391-1. [DOI] [PubMed] [Google Scholar]
- 20.Ough C.S., Crowell E.A., Gutlove B.R. Carbamyl compound reactions with ethanol. Am. J. Enol. Vitic. 1988;39:239–242. [Google Scholar]
- 21.Ough C.S., Stevens D., Sendovski T. Factors contributing to urea formation in commercially fermented wines. Am. J. Enol. Vitic. 1990;41:68–73. [Google Scholar]
- 22.Kodama S., Suzuki T., Fujinawa S. Urea contribution to ethyl carbamate formation in commercial wines during storage. Am. J. Enol. Vitic. 1994;45:17–24. [Google Scholar]
- 23.Uthurry C.A., Lepe J.A. Suárez. Ethyl carbamate production by selected yeasts and lactic acid bacteria in red wine. Food Chem. 2006;94:262–270. [Google Scholar]
- 24.Sakaguchi S. A new color reaction of protein and arginine. J. Biochem. 1925;5:25–31. [Google Scholar]
- 25.Weber C.J. A modification in Sakaguchi's reaction for the quantitative determination of arginine. J. Biol. Chem. 1930;86:217–222. [Google Scholar]
- 26.Sakaguchi S. A new method for the colorimetric determination of arginine. J. Biochem. 1950;37(2):231–236. [Google Scholar]
- 27.Sobel A.E., Hirschman A., Besman L. A convient microtitration method for the estimation of amino acids. Federation Proc. 1945;4:99–103. [PubMed] [Google Scholar]
- 28.Moore S., Stein W.H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J. Biol. Chem. 1954;211:907–913. [PubMed] [Google Scholar]
- 29.Rosenberg H., Ennor A.H., Morrison J.F. The estimation of arginine. Biochem. J. 1956;63:153–159. doi: 10.1042/bj0630153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akamatsu S., Watanable T. The quantitative determination of arginine. J. Biochem. 1961;49(6):566–569. doi: 10.1093/oxfordjournals.jbchem.a127345. [DOI] [PubMed] [Google Scholar]
- 31.Notenboon C.D., van de Veerdonk F.C.G., van de Karner J.C. A fluorescent modification of the sakaguchi reaction on arginine. Histochem. Cell Biol. 1967;9(2):117–121. doi: 10.1007/BF00305854. [DOI] [PubMed] [Google Scholar]
- 32.Gopalkrishna R., Nagarajan B. A simplified procedure for the estimation of arginine in plasma and urine using arginase. Clin. Chim. Acta. 1980;106:333–337. doi: 10.1016/0009-8981(80)90319-8. [DOI] [PubMed] [Google Scholar]
- 33.Konings C.H. A kinetic procedure for the estimation of arginine in serum using arginine kinase. Clinica. Chimica. Acta. 1988;176:185–193. doi: 10.1016/0009-8981(88)90206-9. [DOI] [PubMed] [Google Scholar]
- 34.Alonso A., Almendral M.J., Baez M.D., Porras M.J., Alonso C. Enzyme immobilization on an epoxy matrix for determination of L-arginine by flow injection techniques. Anal. Chim. Acta. 1995;308:164–169. [Google Scholar]
- 35.Tahboub Y.R., Pardue H.L. A predictive-kinetic method for the quantitation of amino acids with ninhydrin. Anal. Chim. Acta. 1985;173:23–32. [Google Scholar]
- 36.Medien H.A. Spectrophotometric method for determination and kinetics of amino acids through their reaction with syringaldehyde. Spectrochim. Acta Part A. 1998;54:359–365. [Google Scholar]
- 37.Snezana S., Mitic G.Z., Miletic A., Pavlovic N., Snezana B.T., Velimirovic D.S. Development and evaluation of a Kinetic-spectrophotometric method for determination of arginine. J. Chinese. Chem. Soc. 2007;54:47–54. [Google Scholar]
- 38.Ballesteros E., Gallego M., Valcarcel M., Grases F. Continuous kinetic method for the quantitative resolution of structural isomers of arginine and ornithine. Anal. Chim. Acta. 1995;315:145–151. [Google Scholar]
- 39.Krause E., Wenschuh H., Jungbult P.R. The dominance of arginine-containing peptides in MALDI-derived tryptic mass fingerprints of proteins. Anal. Chem. 1999;71:4160–4165. doi: 10.1021/ac990298f. [DOI] [PubMed] [Google Scholar]
- 40.Bhavana A.D., Hirosai S., Tsutomu N. Pulsed amperometric detection of underivatized amino acids using polypyrrole modified copper electrode in acidic solution. Talanta. 2002;58:1203–1211. doi: 10.1016/s0039-9140(02)00204-7. [DOI] [PubMed] [Google Scholar]
- 41.Awawdeh M.A., Legako A.J., Harmon J.H. Solid-state optical detection of amino acids. Sensor Actuat. 2003;91:227–230. [Google Scholar]
- 42.Zhang L., Liu Y., Chen G. Simultaneous determination of allantoin, choline and L-arginine in Rhizoma Dioscoreae by capillary electrophoresis. J. Chromatogr. A. 2004;1043:317–321. doi: 10.1016/j.chroma.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Huang Z., Ough C.S. Effect of vineyard locations, varieties, and rootstocks on the juice amino acid composition of several cultivars. Am. J. Enol. Vitic. 1989;40:135–139. [Google Scholar]
- 44.Spayd S.E., Wample R.L., Evans R.G. Nitrogen fertilization of white riesling grapes in Washington. Must and wine composition. Am. J. Enol. Vitic. 1994;45:34–42. [Google Scholar]
- 45.Meyer J., Richter N., Hecker M. High-performance liquid chromatographic determination of nitric oxide synthase-related arginine derivatives in vitro and in vivo. Anal. Biochem. 1997;247:11–16. doi: 10.1006/abio.1997.2008. [DOI] [PubMed] [Google Scholar]
- 46.Pettersson A., Uggla L., Backman V. Determination of dimethylated arginines in human plasma by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1997;692:257–262. doi: 10.1016/s0378-4347(96)00525-7. [DOI] [PubMed] [Google Scholar]
- 47.Teerlink T., Nijveldt R.J., Jong S. Determination of arginine, asymmetric dimethylarginine, and symmetric dimethylarginine in human plasma and other biological samples by high-performance liquid chromatography. Anal. Biochem. 2002;303:131–137. doi: 10.1006/abio.2001.5575. [DOI] [PubMed] [Google Scholar]
- 48.Marra M., Bonfigli A.R., Testa R. Highperformance liquid chromatographic assay of asymmetric dimethylarginine, symmetric dimethylarginine, and arginine in human plasma by derivatization with naphthalene-2, 3-dicarboxaldehyde. Anal. Biochem. 2003;318:13–17. doi: 10.1016/s0003-2697(03)00157-x. [DOI] [PubMed] [Google Scholar]
- 49.Zhang W.Z., Kaye D.M. Simultaneous determination of arginine and seven metabolites in plasma by reversed-phase liquid chromatography with a time-controlled ortho-phthaldialdehyde precolumn derivatization. Anal. Biochem. 2004;326:87–92. doi: 10.1016/j.ab.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Bhandare P., Madhavan P., Rao B.M., Rao S.N. Determination of arginine, lysine and histidine in drug substance and drug product without derivatisation by using HILIC column LC technique. J. Chem. Pharm. Res. 2010;2:580–586. [Google Scholar]
- 51.Jens M.L., Stefanie M.B.B. Fast and efficient determination of arginine, symmetric dimethylarginine, and asymmetric dimethylarginine in biological fluids by hydrophilic-interaction liquid chromatography electrospray tandem mass spectrometry. Clin. Chem. 2004;52:488–493. doi: 10.1373/clinchem.2005.060152. [DOI] [PubMed] [Google Scholar]
- 52.Baxter J.H., Johns P.W. Determination of free arginine, glutamine, and β-alanine in nutritional products and dietary supplements. Food Anal. Meth. 2012;5:821–827. [Google Scholar]
- 53.Orduna R.M. Quantitative determination of L-arginine by enzymatic end point analysis. J. Agric. food Chem. 2001;49:549–552. doi: 10.1021/jf000522y. [DOI] [PubMed] [Google Scholar]
- 54.Zhou G., Chen H. Flow injection chemiluminescence determination of amino acids by oxidation with N-bromosuccinimide. Anal. Sci. 2002;18:693–696. doi: 10.2116/analsci.18.693. [DOI] [PubMed] [Google Scholar]
- 55.Kiba N., Kato A., Furusawa M. Determination of branched-chain L-amino acids by flow-injection analysis with co-immobilized leucine dehydrogenase/NADH oxidase and chemiluminescence detection. Anal. Chim. Acta. 1995;311:71–76. [Google Scholar]
- 56.Kiba N., Tachibana M., Tani K., Miwa T. Chemiluminometric branched chain amino acids determination with immobilized enzymes by flow-injection analysis. Anal. Chim. Acta. 1998;375:65–70. [Google Scholar]
- 57.Wada M., Kuroda N., Akiyama S., Nakashima K. A sensititve and rapid FIA with an immobilized enzyme column reactor and peroxyoxalate chemiluminescence detection for the determinationof total D-amino acids in human plasma. Anal. Sci. 1997;13:945–950. [Google Scholar]
- 58.K.R. Anumula, Method for quantitative determination of amino acids, United State Patent 6800486, 2000.
- 59.Wu G., Meininger C.J. Analysis of citrulline, arginine and methylarginine using high- performance liquid chromatography. Methods Enzymol. 2008;440:177–189. doi: 10.1016/S0076-6879(07)00810-5. [DOI] [PubMed] [Google Scholar]
- 60.Hua L., Liang X., Lidan F., Yanlin L., Wang H. A simple and fast method for arginine determination in grape juice. J. Food Drug Anal. 2008;16:53–58. [Google Scholar]
- 61.Rechnitz G.A., Kobos R.K., Riechel S.J., Gebauer C.R. A bio-selective membrane electrode prepared with living bacterial cells. Anal. Chim. Acta. 1977;94:357–365. doi: 10.1016/S0003-2670(01)84537-2. [DOI] [PubMed] [Google Scholar]
- 62.Grobler S.R., Basson N., Van Wyk C.W. Bacterial electrode for L-arginine. Talanta. 1982;29:49–51. doi: 10.1016/0039-9140(82)80135-5. [DOI] [PubMed] [Google Scholar]
- 63.Nikolelis D.P., Hadjiioannou T.P. Construction of an arginine enzyme electrode and determination of arginine in biological materials. Anal. Chim. Acta. 1983;147:33–39. [Google Scholar]
- 64.Karacaoglu S., Timur S., Telefoncu A. Arginine selective biosensor based on arginase-urease immobilized in gelatin. Art. Cell Blood Sub. Immob. Biotechnol. 2003;31:357–363. doi: 10.1081/bio-120023164. [DOI] [PubMed] [Google Scholar]
- 65.Koncki R., Walcerz I., Ruckruh F., Glab S. Bienzymatic potentiometric electrodes for creatine and L-arginine determination. Anal. Chim. Acta. 1996;333:215–222. [Google Scholar]
- 66.Komaba S., Fujino Y., Matsuda T., Osaka T., Satoh I. Biological determination of Ag(I) ion and arginine by using the composite film of electroinactive polypyrrole and polyion comp. Sensor Actuat. B. 1998;52:78–83. [Google Scholar]
- 67.Singh M., Verma N., Garg A.K., Redhu N. Urea biosensor: review. Sensor Actuat. B-Chem. 2008;134:345–351. [Google Scholar]
- 68.Singh A.K., Verma N. Quartz crystal microbalance based approach for food quality. Curr. Biotechnol. 2014;2(4):127–132. [Google Scholar]
- 69.Sassolas A., Blum L.J., Leca-Bouvier B.D. Immobilization strategies to develop enzymatic biosensors. Biotech. Adv. 2012;30:489–511. doi: 10.1016/j.biotechadv.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Ariga K., Ji Q., Mori T., Naito M., Yamauchi Y., Abe H., Hill J.P. Enzyme nanoarchitectonics: organization and device application. Chem. Soc. Rev. 2013;42:6322. doi: 10.1039/c2cs35475f. [DOI] [PubMed] [Google Scholar]
- 71.Stasyuk N., Smutok O., Gayda G., Gonchar M., Kovalchuk Y., New Bi-enzyme A. Potentiometric sensor for arginine analysis based on recombinant human arginase I and commercial urease. J. Mat. Sci. Eng. 2011;1:819–827. [Google Scholar]
- 72.Andre C., Agiovlasileti D., Guillaume Y.C. Peculiarities of a novel bioenzymatic reactor using carbon nanotubes as enzyme activity enhancers: application to arginase. Talanta. 2011;85:2703–2706. doi: 10.1016/j.talanta.2011.07.077. [DOI] [PubMed] [Google Scholar]
- 73.Saiapina O.Y., Dzyadevych S.V., Jaffrezic-Renault N. Potentiality of application of the conductometric L-arginine biosensors for the real sample analysis. Biopol. Cell. 2012;28:441–448. [Google Scholar]
- 74.Saiapina O.Y., Dzyadevych S.V., Jaffrezic-Renault N., Soldatkin O.P. Development and optimization of a novel conductometric bi-enzyme biosensor for L-arginine determination. Talanta. 2012;92:58–64. doi: 10.1016/j.talanta.2012.01.041. [DOI] [PubMed] [Google Scholar]
- 75.Ivnitskii D.M., Rishpon J. Biosensor based on direct detection of membrane potential induced by immobilized hydrolytic enzymes. Anal. Chim. Acta. 1993;282:517–525. [Google Scholar]
- 76.Dominguez R., Serra B., Reviejo A.J., Pingarron J.M. Chiral analysis of amino acids using electrochemical composite bienzyme biosensors. Anal. Biochem. 2001;298:275–282. doi: 10.1006/abio.2001.5371. [DOI] [PubMed] [Google Scholar]
- 77.Stasyuk N., Smutok O., Gayda G., Bohdan V., Yevgen K., Gonchar M. Bi-enzyme L-arginine-selective Amperometric biosensor based on ammonium-sensing polyaniline-modified electrode. Biosens. Bioelectron. 2012;37:46–52. doi: 10.1016/j.bios.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 78.Kumar K., Phathak T., Shefali W. L-arginase based biosensor for detection of L-arginine in juice samples. J. Nat. Prod. Plant. Resour. 2012;2:494–499. [Google Scholar]
- 79.Verma N., Singh A.K., Kaur P. Biosensor based on ion selective electrode for detection of L-Arginine in Fruit Juices. J. Anal. Chem. 2015;70(9):1111–1115. [Google Scholar]
- 80.G. Kaur 〈http://hdl.handle.net/10603/2897S〉.
- 81.Suzuki H., Tamia E., Karube I. Integrated amino acid sensors for detection of L-glutamate, L-lysine, L-arginine, and L-histidine. Electroanal. 1994;6:299–304. [Google Scholar]
- 82.Sarkar P., Tothill I.E., Setford S.J., Turner A.P.F. Screen-printed amperometric biosensors for the rapid measurement of L- and D-amino acids. Analyst. 1999;124:865–870. doi: 10.1039/a901404g. [DOI] [PubMed] [Google Scholar]
- 83.Saiapina O.Y., Matsishin N.J., Pyeshkov V.M., Soldatkin O.P., Melik V.G., Walcarius A., Jaffrezic-Renault N., Dzyadevych S.V. Application of ammonium selective zeolite for enhancement of conductometric Bi-enzyme biosensor for L-arginine detection. Sens. Electron. Мicrosys. Technol. 2012;3(9):49–66. [Google Scholar]
- 84.Sheliakina M., Arkhypova V., Soldatkin O., Saiapina O., Akata B., Dzyadevych S. Urease-based ISFET biosensor for arginine determination. Talanta. 2014;121:18–23. doi: 10.1016/j.talanta.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 85.Gill I., Ballesteros A. Bioencapsulation within synthetic polymers (part 1): sol–gel encapsulated biological. Tibtech. 2000;18:282–296. doi: 10.1016/s0167-7799(00)01457-8. [DOI] [PubMed] [Google Scholar]
- 86.Stasyuk O.A., Szatylowicz H., Krygowski T.M. Tautomerisation of thymine acts against the Hückel 4N + 2 rule. The effect of metal ions and H-Bond complexations on the electronic structure of thymine. Org. Biomol. Chem. 2014;12:6476–6483. doi: 10.1039/c4ob00964a. [DOI] [PubMed] [Google Scholar]
- 87.Heli H., Sattarahmady N., Hajjizadeh M. Electrocatalytic oxidation and electrochemical detection of guanine, Larginine and Llysine at a copper nanoparticles modified electrode. Anal. Methods. 2014;6:6981–6989. [Google Scholar]
- 88.Verma N., Kumar S., Kaur H. Fiber optic biosensor for the detection of Cd in milk. J. Biosens. Bioelectron. 2010;1:102. [Google Scholar]
- 89.Verma N., Kaur H., Kumar S., Multipurpose A. Biopolymer membrane for heavy metal preconcentration and enzyme immobilization. J. Biol. Sci. 2011;11(5):388–393. [Google Scholar]
- 90.Verma N., Kaur G. Zinc finger peptide based optic sensor for detection of zinc ions. Biosens. Bioelectron,. 2016;86:466–477. doi: 10.1016/j.bios.2016.06.088. [DOI] [PubMed] [Google Scholar]
- 91.Svancara I., Vytras K., Kalcher K., Walcarius A., Wang J. Carbon paste electrodes in facts, numbers, and notes: a review on the occasion of the 50-years jubilee of carbon paste in electrochemistry and electroanalysis. Electroanal. 2009;21:7–28. [Google Scholar]
- 92.Verma N., Kaur H., Kumar S. Whole cell based electrochemical biosensor for monitoring lead in milk. Biotechnol. 2011;10(3):259–266. [Google Scholar]
- 93.Andre C., Herlem G., Gharbi T., Guillaume Y.C. A new arginase enzymatic reactor: development and application for the research of plant-derived inhibitors. J. Pharm. Biomed. Anal. 2011;55:48–53. doi: 10.1016/j.jpba.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 94.Stasyuk N., Serkiz R., Mudry S., Gayda G., Zakalskiy A., Koval’chuk Y., Gonchar M., Nisnevitch M. Recombinant human arginase I immobilized on gold and silver nanoparticles: preparation and properties. Nanotechnol. Develop. 2011;1:11–15. [Google Scholar]
- 95.Vega-Valle P., Young C., Swaisgoo H.E. Arginase-urease electrode for determination of arginine and peanut maturity. J. Food Sci. 1980;45:1026–1030. [Google Scholar]
- 96.Liu D., Yin A., Ge K., Chen K., Nie L., Yao S. Enzymatic analysis of arginine with the saw/conductance sensor system. Enz. Microbial Technol. 1995;17:856–863. [Google Scholar]
- 97.Kumar S., Verma N., Singh A.K. Development of cadmium specific recombinant biosensor and its application in milk samples. Sens. Actuat. B. 2017;240:248–254. [Google Scholar]
- 98.N. Verma, G. Kaur, D.P. Singh, Microbial arginine biosensor. India Patent: 275416, 2016.
- 99.Oungpipat W., Narong L.N. Hybrid arginine biosensor based on bovine liver, cajanus cajan & stainless steel electrode. 25th Congress on Science & Technology of Thailand. 1999 [Google Scholar]
- 100.Lvova L., Legin A., Vlasov Y., Cha G.S., Nam H. Multicomponent analysis of Korean green tea by means of disposable all-solid-state potentiometric electronic tongue microsystem. Sensor. Actuat B. 2003;95:391–395. [Google Scholar]
- 101.Arnold M.A. Potentiometric sensor using whole tissue sections. Ion Select Elect. Rev. 1986;8:85–113. [Google Scholar]
- 102.Zhybak M.T., Fayura L.Y., Boretsky Y.R., Gonchar M.V., Sibirny A.A., Dempsey E., Turner A.P.F., Korpan Y.I. Amperometric L-arginine biosensor based on a novel recombinant arginine deiminase. Microchim Acta. 2017;184:2679–2686. [Google Scholar]
- 103.Verma N., Singh A.K., Saini N. Synthesis and characterization of ZnS quantum dots and application for development of arginine biosensor. Sensing and Biosensing Research. 2017;15:41–45. [Google Scholar]
- 104.Crane B.R., Arvai A.S., Ghosh D.K., Wu C., Getzoff E.D., Stuehr D.J., Tainer J.A. Structure of nitric oxide synthase oxygenase dimer with pterin and substrate. Science. 1998;279:2121–2126. doi: 10.1126/science.279.5359.2121. [DOI] [PubMed] [Google Scholar]
- 105.Pundir C.S., Yadav S., Kumar A. Creatinine sensors. Trends in Analytical chemistry. 2013;50:42–52. [Google Scholar]
- 106.Martínez-Periñán E., Revenga-Parra M., Zamora F., Pariente F., Lorenzo E. Nanostructured electrochemical detector for the quantification of amino acids related to metabolic diseases. Sensors Actuators B Chem. 2016;236(2016):773–780. [Google Scholar]