Abstract
Acute kidney injury (AKI) and chronic kidney disease (CKD) are inter-connected. While AKI-to-CKD transition has been intensively studied, the information of AKI on CKD is very limited. Nonetheless, AKI, when occurring in CKD patients, is known to be more severe and difficult to recover. CKD is associated with significant changes in cell signaling in kidney tissues, including the activation of TGF-β, p53, HIF, and major developmental pathways. At the cellular level, CKD is characterized by mitochondrial dysfunction, oxidative stress, and aberrant autophagy. At the tissue level, CKD is characterized by chronic inflammation and vascular dysfunction. These pathological changes may contribute to the heightened sensitivity of, and non-recovery from, AKI in CKD patients.
Keywords: acute kidney injury, chronic kidney disease, fibrosis, inflammation, mitochondria, cell signaling
I. Introduction
Chronic kidney disease (CKD) is characterized by the gradual loss of renal function over a period of months to years. In addition to renal deficiency, CKD is a major risk multiplier in patients with diabetes, hypertension, heart diseases, and stroke1. The prevalence of CKD has reached a staggering high level around the world. In the United Sates, across-sectional analysis of the National Health and Nutrition Examination Surveys shows that the prevalence of CKD stages 1 to 4 increased to 13.1% in 1999–20042. In China, over 10% adults suffer from CKD according to the analysis of a nationwide representative sample of 47,024 adults3. Acute kidney injury (AKI), in contrast, is defined as a kidney disease with rapid loss of renal function. While previously thought to be two separate syndromes, AKI and CKD have been recognized to be closely associated or inter-connected by both clinical and experimental studies in the last decade4–6. On one hand, AKI may contribute to the development and progression of CKD. On the other hand, CKD is known to predispose or sensitize patients to AKI. While AKI-to-CKD transition has been intensively studied in recent years, very limited work has been directed to investigate how CKD affects AKI. In this review, we aim to briefly summarize the inter-relationship between AKI and CKD, and then focus on the potential mechanisms that underlie the poor prognosis of AKI in CKD patients.
II. The AKI-CKD connection
Despite some disputes, recent epidemiology and experimental studies have demonstrated that AKI contributes to the development and progression of CKD. AKI in both adults and children is closely associated with the increased risk of developing CKD and the risk of CKD incidence shows a dependence on the severity of AKI7–10. In a meta-analysis of 13 cohort studies, Cocaet al. showed that AKI patients had significantly higher risks of developing CKD and end stage renal disease (ESRD) and higher mortality as compared to the patients without AKI11. More recently, Kim et al. reported that persistent AKI (defined as incomplete reduction of serum creatinine at 7 days) after gastric surgery was associated with CKD progression 1 year later12. Similarly, the duration of AKI after cardiac surgery was also regarded as a strong independent risk factor for CKD development13. In addition, a recent retrospective study indicated that 29% of patients with elective cardiac surgery and cardiopulmonary bypass experienced AKI and notably, half of these AKI patient developed CKD14. In diabetic patients, Thakar et al. reported that AKI was a factor that doubled the risk of reaching stage 4 CKD and significantly reduced the survival of patients15. Comparing to the CKD patients who did not experience superimposed AKI, those who did also had 30% higher long-term risk of death or ESRD. Moreover, based on a large community-based cohort of patients with CKD, Hsu et al. showed that 49% of CKD patients who sustained severe AKI reached ESRD status within 30 days after discharge, while the percentage for CKD patients without severe AKI was only 1.5%16. Experimentally, severe and repeated episodes of AKI led to renal interstitial fibrosis that was associated with gradual loss of renal function over time, a characteristic of CKD17–19. These studies have provided compelling evidence for a role of AKI in the development and progression of CKD11. Mechanistically, AKI-CKD transition is related to a complex interplay between injured or stressed renal tubular cells with vasculature, immune system, and interstitial fibroblasts4–6, 20, 21.
Conversely, CKD is also an important risk factor for the development of AKI. James et al. conducted a meta-analysis in 2015 showing that CKD was a risk factor of developing AKI in patients with diabetes or hypertension22. Consistently, CKD was shown to be an independent risk factor of AKI in patients of major cardiac surgery23. Similar results were shown in a multicenter study of a large cohort of postoperative patients by The National Taiwan University Surgical ICU Associated Renal Failure group24. In this study, among the patients who survived to hospital discharge after major surgery, 46.8% patients without prior CKD experienced AKI, whereas significantly higher percentage (67.0%) of patients with prior CKD did. In addition, Wilson et al. conducted a systematic review of risk prediction models for AKI following major non-cardiac surgery and found that renal insufficiency was an important risk factor of AKI occurrence25. In animal models, there is evidence that ischemic AKI is significantly more severe in diabetic mice than in non-diabetic mice26, 27. Together, these studies indicate that CKD predisposes and sensitizes patients to AKI.
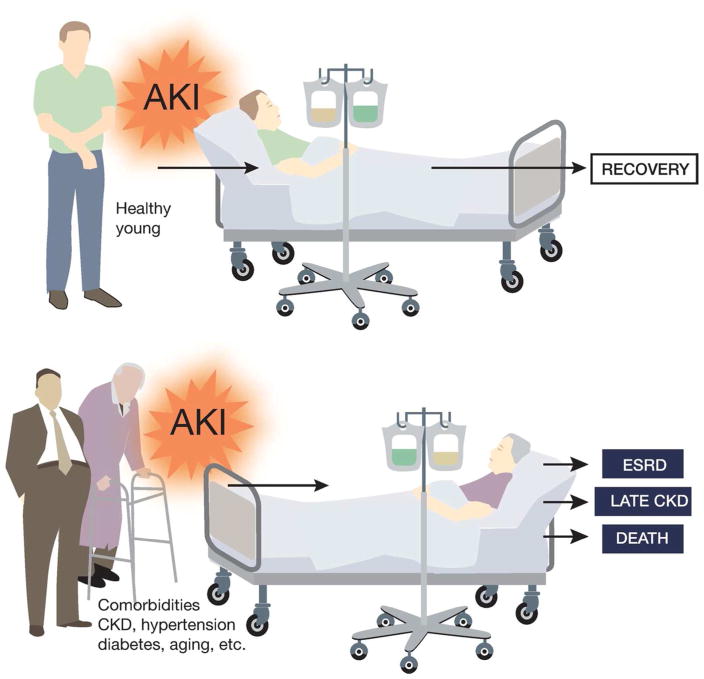
Furthermore, CKD may adversely affect kidney repair in, and the recovery from, AKI. In 2004, Go et al. showed that reduced baseline kidney function due to proteinuria, diabetes, hypertension or other diseases was a strong and independent risk factor for hospitalization, cardiovascular events, and death28. More recently, Zhou et al. showed that the pre-existence of CKD worsened renal function in AKI patients and delayed the renal function recovery after AKI; in fact, acute on chronic kidney injury (ACKI) was frequently associated with higher risk of mortality29. Findlayet al. further examined the information of patient with renal replacement therapy requiring AKI in Southwest Scotland between 1994 and 2005. The data from this study indicate that the underlying CKD rather than illness severity can predict the medium- to long-term mortality30. Macedoet al. followed 84 adult survivors of AKI for a median time of 4.1 years and showed that age and serum creatinine levels at hospital discharge were independent factors associated with non-recovery of renal function31. Consistently, a large cohort study of 43,008 hospitalized patients by Pannu et al. revealed that a lower baseline estimated glomerular filtration rate (eGFR) pre-AKI was associated with higher risk of ESRD and death32. These studies are consistent with the general observation in clinical practice that in young and otherwise healthy patients, AKI is mostly reversible or recoverable, whereas AKI in patients with CKD and related comorbid conditions (eg. diabetes, hypertension, aging) is severe and hard to recover (Figure 1). Experimentally, Polichnowski et al. demonstrated that kidney repair following ischemic AKI was impaired and renal fibrosis increased in rats with severe renal mass reduction or nephrectomy, a model of CKD33. Despite these clinical and experimental studies, the mechanism of non-recovery from AKI in CKD patients remains largely elusive.
Figure 1.
Poor prognosis of AKI in patients with CKD and related co-morbidities.
III. Potential mechanisms underlying the susceptibility and non-recovery of AKI in CKD patients
a. CKD-associated changes in signaling pathways
Pathologically, AKI is characterized by cell injury and death in renal tubules34. Interestingly, CKD is also associated with tubular damage and dysfunction. It has been known for years that tubulointerstitial pathology is a defining feature of virtually all types of CKD35. Although abnormal glomerular filtration may impact on CKD progression, the tubulointerstitial pathology involving mainly tubular epithelial cells and interstitial fibroblasts, plays a major role in the initiation of CKD36. Drastic alterations of cell signaling in renal tubular cells during the initiation and progression of CKD may contribute to the susceptibility of CKD kidneys to AKI.
TGF-β
In CKD, TGF-β signaling pathway is activated and consequently induces renal cells to produce fibrotic extracellular matrix proteins, leading to glomerulosclerosis as well as tubulointerstitial fibrosis37, 38. However, the role of TGF-β signaling in AKI is paradoxical probably due to its multiple patho-physiological functions in different cells and at different stages of AKI. Some early studies reported the transient activation of TGF-β signaling in AKI that may promote kidney tubular cell proliferation and apoptosis39, 40. In sharp contrast, most recent studies suggested that TGF-β is an injurious factor and inhibition of TGF-β pathway may reduce renal tubular injury41–44. Using a mouse model of inducible TGF-β expression in kidney tubular cells, Koesters R et al demonstrated that tubular TGF-β activation may initially stimulate interstitial cell proliferation, but sustained TGF-β induction leads to tubular degeneration through autophagy-mediated tubular decomposition45. Therefore, the persistently elevated TGF-β signaling in CKD may have synergistic or additive injury effect at the acute phase of AKI. Furthermore, activated TGF-β signaling in CKD may prevent the recovery of AKI. In this regard, TGF-β is known to trigger fibrogenic foci and initiate progressive fibrogenesis in kidney injury46. In addition, the activation of TGF-β signaling in renal tubular cells may prevent their re-differentiation and ensuing regeneration of functional renal tubules after AKI47.
P53
P53 is a major mediator of tubular cell injury and death in AKI48–50. More recent studies have further verified the role of p53 in AKI by testing renal tubule-specific p53 ablation mouse models51, 52. Interestingly, CKD may also be associated with the increase or activation of p53 as a consequence of TGF-β up-regulation or PTEN down-regulation53, 54. Such “pre-activation” of p53, though at low to moderate levels, may greatly enhance tubular damage upon AKI. In support, Peng et al. detected marginal p53 activation in hyperglycemic renal tubular cells and in diabetic kidneys, which was dramatically increased upon ischemic AKI; importantly, these cells and tissues showed significantly more injury that could be ameliorated by p53 inhibitors and genetic knockout26. However, p53 may also play a dual role in AKI as it may suppress renal inflammation and related inflammatory injury by increasing the infiltration of anti-inflammatory M2 macrophages55. Recent studies have further indicated the involvement of p53 in renal fibrosis. Pifithrin-α, a pharmacological inhibitor of p53, was shown to stimulate post-ischemia renal fibrosis56, whereas targeted ablation of p53 from renal proximal tubules led to the suppression of renal fibrosis52, suggesting that p53 in different renal cell types may contribute differently to kidney repair.
Developmental signaling pathways
Multiple signaling pathways in kidney development are activated in both CKD and AKI, and contribute to their pathogeneses. Especially, these pathways play an important role in kidney repair following injury, including fibrogenesis, as reviewed recently57–59. Briefly, Notch signaling pathway is known for its indispensable role in kidney development for long time60. Recently, Notch activation was detected in ischemic AKI and was shown to prevent renal tubular epithelial cells from proliferation and regeneration, resulting in a delay of the recovery from AKI61, 62. In addition, Notch activation was also reported to increase inflammation and apoptosis in ischemic AKI63. In CKD, Notch is also activated in renal tubular cells where it may promote renal fibrosis and inflammation64–66. The elevated Notch signaling in CKD may therefore exaggerate tubular damage upon AKI and delay kidney repair or recovery, contributing to the susceptibility of, and non-recovery from, AKI in CKD patients.
The Hedgehog signaling is also a critical pathway in kidney development that promotes renal fibrosis in AKI and CKD-related conditions. Specifically, Liu and colleagues demonstrated that Sonic Hedgehog from injured renal tubular cells may activate fibroblasts for proliferation, suggesting a model of epithelial-mesenchymal communication67, 68. The recent studies by Kramann, Humphreys and colleagues have further pinpointed the critical role of the Hedgehog transcriptional activator Gli1 in specifying perivascular mesenchymal stem cells in kidneys as the main origin of myofibroblasts in renal fibrosis69. Moreover, Gli2 is a key to myofibroblast proliferation by driving cell cycle progression, further supporting a pivotal role of Hedgehog signaling in renal fibrosis70. In contrast, less is known about Hedgehog signaling in the acute phase of AKI. Nonetheless, inhibition of Hedgehog signaling in obstructively injured kidneys promoted renal tubular cell apoptosis and inhibited tubular cell proliferation71, suggesting a cytoprotective function of this pathway. Thus, Hedgehog signaling in CKD and associated fibrosis may antagonize AKI acutely, but it may prevent kidney repair and enhance fibrosis in the long term.
Other kidney developmental signaling pathways that are activated in various CKD conditions include the canonical WNT/β-catenin pathway, which also promotes renal fibrosis72–77. Interestingly, this pathway is also activated during and following AKI. While WNT/β-catenin signaling protects renal tubular cells and promotes their proliferation78, 79, sustained WNT/β-catenin activation contributes to renal fibrosis during the recovery or repair phase of AKI80, 81. Thus, CKD-associated WNT/β-catenin signaling may have paradoxical effects on AKI: protecting against acute injury initially but enhancing renal fibrosis thereafter.
HIF
Kidney tissues, especially renal tubules, are highly oxygen demanding and susceptible to hypoxic injury. In both AKI and CKD conditions, hypoxia is an important pathogenic factor that is accompanied with the activation of HIF (including HIF-1, -2, -3) to modulate gene expression82. In AKI, HIF activation in renal proximal tubules does not have a major protective role83, 84; however, global stabilization of HIF or selective regulation of HIF in other kidney cell types may reduce AKI85–87, suggesting that HIF activation in other cells may have renoprotective effects. In the repair phase of AKI, HIF may also play a perplexing role, likely depending on the expressing cell types. While HIF-1 activation did not significantly improve wound healing in renal tubular cells in some studies81, 88, there is evidence that HIF-1 may enhance renal tubular cell migration after wounding89, 90. HIF signaling was also shown to promote renal fibrosis by trans-activating genes for ECM turnover, co-operating with TGF-β1, and by promoting the change of tubular cells to a pro-fibrotic phenotype91. As HIF is activated in CKD, it is important to understand whether HIF protects CKD patients from AKI and/or further promotes fibrosis following AKI for accelerated progression of CKD.
b. Mitochondrial dysfunction and oxidative stress
Mitochondria are the main energy source in kidney cells, especially renal tubule cells. However, after damage, mitochondria may turn into active “killer” of these cells. In this regard, permeabilization of mitochondrial outer membrane by pro-apoptotic Bcl-2 family proteins can directly activate the intrinsic pathway of apoptosis. Ablation of these pro-apoptotic genes, such as Bax and Bak, results in the amelioration of ischemic as well as cisplatin nephrotoxic AKI50, 92, 93. Interestingly, prior to membrane permeabilization, mitochondria experience a dramatic change in morphology and become fragmented. Now it is clear that mitochondria are dynamic organelles that constantly undergo fission and fusion, and during cell stress mitochondrial fusion is arrested while fission activated, culminating in mitochondrial fragmentation94. Fragmented mitochondria are highly susceptible to Bax insertion and consequent outer membrane permeabilization95. Thus, mitochondrial fragmentation and outer membrane permeabilization are considered to be two critical events in triggering the intrinsic pathway of apoptosis96. In ischemic and nephrotoxic models of AKI, inhibition of mitochondrial fragmentation pharmacologically or genetically has significant renoprotective effects93, 97, 98, further verifying the pathogenic role of mitochondrial fragmentation. Cell death can also be triggered by damages at the mitochondrial inner membrane. In this case, a well-documented phenomenon is mitochondrial permeability transition (MPT) that leads to necrosis in a variety of cell types. MPT has been implicated in tubular necrosis in AKI for a long time, which has been verified recently by using gene knockout mouse models99–101. Mitochondrial dysfunction occurs in kidney tissues and cells during the development and progression of CKD102, 103. Both high glucose and albumin overload can stimulate mitochondria-mediated intrinsic pathway of apoptosis, which may result from the activation of p53, PKC-δ, and oxidative stress under CKD condition104. Mitochondrial fragmentation, as a result of pathological alterations in mitochondrial dynamics, has also been detected in experimental models of diabetic kidney disease105, 106. Fragmented mitochondria are more vulnerable to damage, including Bax attack95. Indeed, the severity of ischemic AKI in diabetic mice is associated with heightened activation of the mitochondrial pathway of apoptosis. Thus, the loss of mitochondrial dynamics in diabetic kidneys and other CKD conditions is expected to contribute to the AKI sensitivity in CKD.
Mitochondria in CKD kidneys are also low in function. For example, Sharma and colleagues107 analyzed the metabolites in urine from diabetic patients with or without CKD. Interestingly, the diabetic patients with CKD had less water-soluble organic anions in urine that are related to kidney mitochondrial function. Moreover, these patients had less mitochondrial protein and DNA, and lower levels of PGC1α (key transcription factor for mitochondrial biogenesis) in kidneys. Together, these data indicate an overall down-regulation of mitochondrial function and biogenesis in kidneys during diabetic nephropathy. Interestingly, kidney repair or recovery from AKI is associated with and may depend on mitochondrial biogenesis. In this regard, Schnellmann and colleagues demonstrated that pharmacological stimulation of mitochondrial biogenesis may promote kidney recovery from AKI108–111. In addition, Parikh and colleagues demonstrated worsened septic AKI in PGC1α-knockout mice112, providing further support for the beneficial effect of PGC1α and associated mitochondrial biogenesis in AKI and its recovery. In view of these studies, it is concluded that CKD-associated suppression of mitochondrial function and biogenesis would sensitize kidney cells and tissues to AKI and prevent kidney repair or recovery from AKI.
Oxidative stress is a common feature of CKD. By comparing CKD patients with healthy people, Oberg et al. revealed significantly elevated oxidative stress in stage 3–5 CKD patient blood samples by measuring oxidative stress biomarkers, including increases in plasma levels of protein-associated carbonyl content and decreases in plasma protein–reduced thiol content113. Similar increases of oxidative stress were shown in CKD patient blood samples by Ramos et al, who further reported an interesting correlation of oxidative stress with body fat deposition114. The oxidative stress in CKD may have multiple origins including, but not limited to, dysfunctional mitochondria102, 103, 115. For example, it may also involve the down-regulation of Nrf2 in CKD, a key transcriptional factor of anti-oxidant genes116.
The oxidative status in CKD may adversely affect AKI because oxidative stress is a well-known pathogenic factor in AKI. In critically ill patients with AKI, Himmelfarb J et al. showed that plasma protein oxidation is significantly increased and it cannot be substantially improved by hemodialysis117. In an orthopedic trauma-induced model, obese rats with higher oxidative stress developed more severe AKI118. In addition, the pre-existing CKD, such as diabetic nephropathy, is the most prominent risk factor of contrast medium-induced AKI119, 120. A major factor in the susceptibility of the CKD patients to contrast medium-induced AKI is considered to be oxidative stress in kidney tissues, which may result in enzymatic and vascular/endothelial dysfunction to markedly increase the incidence of AKI121. Altogether, these studies implicate that existing oxidative stress, such as that in obesity and CKD, may exacerbate AKI.
c. Changes of autophagy
Autophagy is a cellular process of degradation of cytoplasmic contents, including protein aggregates and dysfunctional organelles. Functionally, autophagy is an important mechanism for the maintenance of cellular homeostasis by removing potentially toxic components and recycling degraded substances. In AKI, autophagy is rapidly activated in renal tubular cells. Blockade of autophagy pharmacologically or genetically results in more severe AKI while induction of autophagy protects kidneys, supporting a protective role of autophagy in AKI122–127. After AKI, autophagy decreases in some renal tubular cells but it is still maintained at high levels in other cells128; however, the role of autophagy in kidney repair following AKI remains unclear. Baisantryet al. reported that specific ablation of Atg5 from renal proximal tubules increased ischemic AKI at earlier time-points, but suppressed renal fibrosis during kidney recovery or repair129. Consistently, Livingston et al. showed that renal fibrosis in obstructed kidneys was ameliorated in renal proximal tubule Atg7-knockout mice and interesting, this was associated with decreased production of specific pro-fibrotic factors130. However, Li et al. reported higher levels of renal fibrosis in obstructed kidneys of proximal tubule Atg5-knockout mice131. Thus, while the protective role of autophagy in AKI is well-established, it is inconclusive whether autophagy is beneficial or detrimental to kidney repair.
Currently, the information of autophagy regulation in CKD is limited and sometimes controversial, likely due to the complexity of the etiologies of CKD. In this regard, autophagy in diabetic nephropathy has received much attention as it is considered a metabolic disorder. The accumulating evidence indicates that the nutrient-sensing pathways in kidneys are altered under diabetic conditions, including particularly the activation of mTOR and the inhibition of SIRT1 and AMPK132–134. Changes of these nutrient-sensing pathways may further impair the autophagic response in kidneys135. For example, accumulation of the autophagy substrate p62 in kidneys has been shown in both STZ-induced type 1 diabetic mice136 and Wistar fatty type 2 diabetic rats137. Moreover,p62 accumulation has also been observed in renal tubules of kidney biopsy samples from patients with type 2 diabetes, suggesting the inhibition of autophagy138. In addition, autophagy reduction occurs in the kidney biopsies from patients with focal segmental glomerulosclerosis (FSGS), although the main affected cells are glomerular podocytes139. Autophagy regulation in other CKD models or conditions is less clear. In the 5/6th nephrectomy model of CKD, Li H et al. showed evidence of autophagy suppression in kidneys, including decreases in LC3II and accumulation of P62140. However, in the same model Fedorova et al. detected the up-regulation of autophagy genes (eg. beclin 1 and BNIP3)115. In Leishmania infantum-associated glomerulonephritis, Eschet al. showed autophagy induction in both glomeruli and renal tubules with induction141.
Would the autophagy changes in CKD affect AKI and kidney repair? In other words, does autophagy contribute to the AKI sensitivity and non-recovery in CKD? Based on the protective role of autophagy in AKI, it is predicted that autophagy inhibition in some CKD conditions, such as diabetic nephropathy and FSGS, would sensitize kidney cells and tissues to AKI. However, in other CKD conditions where autophagy is induced, autophagy may have an opposite role. It is more difficult to predict the effect on kidney repair, because, as presented above, the role of autophagy in kidney repair and fibrosis remains elusive. Again, it is important to emphasize that this depends on the etiology of CKD, which not only determines if autophagy is up- or down-regulated but also the repair response after AKI: normal vs. maladaptive repair. Normal repair fully restores tubular structure and function, whereas maladaptive repair includes a series of pathological changes including tubular atrophy, persistent inflammation, capillary rarefaction, expansion of myofibroblasts, and development of fibrosis that prevent the recovery of AKI142–144. Thus, the changes of autophagy in CKD likely contribute to the AKI sensitivity and non-recovery/repair in CKD patients. However, the effects may largely depend on the etiology or types of CKD.
d. Chronic inflammation
Chronic inflammation is a common feature in CKD patients due to multiple factors such as the induction of pro-inflammatory cytokines, oxidative stress, uremia, and high chances of infection. In a study of 687 patients with myocardial infarction-induced CKD, Tonelli M et al. showed the significant induction of two inflammatory biomarkers (C-reactive protein and soluble tumor necrosis factor receptor II) and its association with the progression of CKD145. More recently, AmdurRL et al. examined the association of inflammatory biomarkers and the progression of CKD in the Chronic Renal Insufficiency Cohort (CRIC) Study, which enrolled 3430 adults with a wide range of kidney function, and found that elevated plasma levels of TNF-α was closely related to the rapid loss of kidney function in patients with CKD146. CKD patients are also more susceptible to infection and related inflammation. In fact, pre-operative CKD was shown to a strong predictor of post-operative infection, AKI, and in-hospital death147.
Importantly, the pro-inflammatory cytokines that are up-regulated in CKD also play critical roles in the induction and progression of AKI. Firstly, the cardiovascular events in CKD patients are closely related to proinflammatory cytokine induction148–151. As such, the CKD patients with chronic inflammation have high rates of cardiovascular problems, which lead to decreased blood supply to kidneys resulting in ischemic AKI. Among the various cytokines up-regulated in CKD, IL-10 is closely associated with and has been suggested to be responsible for the risk of cardiovascular events in CKD151. CKD is also accompanied with the induction of other major cytokines, such as TNF-α, TNF-like weak inducer of apoptosis (TWEAK) and IL-6152–156. TNF-α and TWEAK can reduce Kotho expression through the activation of NF-kB pathway, which may contribute to kidney functional loss in folic acid-induced AKI157. TNF-α is also important to the increased severity of ischemic AKI in diabetic mice158. In addition, the activation of fibroblast growth factor-inducible 14 (Fn14) by TWEAK in pericytes may further promote the release of inflammatory cytokines and capillary vasoconstriction, contributing to AKI159. In AKI, IL-6 can activate STAT3 pathway, which not only induces more tubular cell death but also increases renal fibrosis and glomerulosclerosis in post-AKI kidneys160, 161. Notably, CKD is associated with high levels of IL-6, which is a critical risk factor of AKI in sepsis patients154, 162.
There is also an increase of High-mobility group box1 (HMGB1) in CKD. For example, in a cross-sectional study Bruchfeld et al. analyzed 177 CKD patients to reveal the significant increase of serum HMGB1. In this study, HMGB1 showed a correlation with the decline of renal function or glomerular filtration rate and increases in markers of inflammation and malnutrition163. Interestingly, HMGB1 may also be a major player in the inflammatory response and associated tissue damage in AKI164. In AKI patients, there is a marked increase in HMGB1 that correlates with inflammation165. In animal models, neutralizing antibodies of HMGB1 protect against ischemic AKI and reduced renal inflammation, whereas recombinant HMGB1 have opposite effects166, 167. Quite relevant to the focus of our discussion, Star and colleagues demonstrated the release of HMGB1 from apoptotic cells in spleen in the 5/6th nephrectomy mouse model of progressive CKD, and notably, cecal ligation and puncture-induced AKI in this model was attenuated by anti-HMGB1 antibodies, suggesting a critical role of HMGB1 in septic AKI under CKD conditions168.
Of note, increases of other inflammatory cytokines (eg. IL-8) and interferons have also been reported in CKD patients, although less is clear about their involvement in AKI169–171. Also, in addition to induced expression, renal clearance of cytokines is decreased in CKD, which may contributes to the accumulation of pro-inflammatory cytokines in kidney tissues and the susceptibility of and non-recovery from AKI168.
The increases of chemokines and cytokines in CKD are often associated with the infiltration of inflammatory cells into kidneys. The infiltration of these cells is generally believed to predispose the kidneys to AKI. In this regard, macrophages have received much attention. Due to the differences in surface antigens and polarization status, macrophages may present as M1, M2, and various subtypes inbetween that may react differently to stimuli 172, 173. M1 macrophages are pro-inflammatory and mediate kidney damage during the early phase of AKI. In contrast, M2 macrophages generally contribute to kidney tissue remodeling following AKI by enhancing tubular cell proliferation and repair as well as inducing maladaptive repair of fibrosis174. Macrophage infiltration occurs in CKD in both human patients and animal models175–177, and notably, the infiltration is followed by M1 macrophage polarization whereas M2 polarization is impaired177, predisposing the kidneys to inflammation and tissue damage in AKI.
e. Vascular dysfunction
Depending on its progression, CKD is associated with various degrees of vascular dysfunction that occurs in both renal vasculature and other peripheral vasculature. In kidneys, CKD induces alterations in the tissue microenvironment that may directly induce vascular pathology. The dysfunction of endothelial cells as well as vascular calcification in kidneys during CKD have been noticed for long time178–180. In fact, the extent and histoanatomic type of vascular calcification are good predictors of subsequent cardiovascular mortality in CKD patients181. The endothelial dysfunction in CKD leads to changes in microcirculation, adhesion of inflammatory cells for inflammation, vascular permeability, and abnormal glomerular leakage. Especially at late stages of CKD when patients are experiencing proteinuria, renal vasculature damage and changes of glomerular architecture can adversely affect the blood supply to renal tubular cells and result in further reduction of GFR. Such loss of renal function leads to the accumulation of multiple pathogenic factors, such as uremic toxins, oxidized proteins, and advanced glycation end products (AGE), to name just a few182–186. Those injurious factors are delivered through blood to all over the body to cause further peripheral vasculature damage and major cardiovascular defects, which may directly decrease blood support to kidneys to induce ischemic AKI28, 187, 188.
In addition, vasculature damage may significantly enhance the risk of AKI. A good example in this aspect is contrast medium-induced AKI189. Pre-existence of CKD is known to significantly increase the incidence of AKI following the administration of contrast medium in patients190. Though the detailed mechanism of contrast medium-induced AKI is not fully understood, it is closely related to the vascular dysfunction in addition to the direct toxic effect in renal tubular cells. Contrast medium has been reported to have vasoconstriction effect, resulting in the reduction of vasa recta blood supply and decreases in the blood flow rate in afferent arteriole, which further lead to renal tubular damage and decline of glomerular filtration191–193. The pre-existence of vascular dysfunction in CKD can certainly augment these pathological changes, inducing more severe AKI.
In 2001, Basile and colleagues demonstrated the loss of peritubular capillary or vascular rarefaction within weeks after ischemic AKI in rats194. Subsequent studies further established a role of vascular rarefaction in the development of renal fibrosis and chronic kidney problems after AKI18. Remarkably, Polichnowski et al. recently demonstrated that kidney repair and recovery after ischemic AKI was disproportionately impaired in rats with severe (75%) renal mass reduction (RMR) compared to rats without RMR33. In these animals, renal function remained lower and greater numbers of renal tubules failed to differentiate after 4 weeks of recovery, which was associated with more severe tubulointerstitial fibrosis and capillary rarefaction, with development of hypertension and proteinuria. The most hypertensive rats showed the most proteinuria and in addition, had glomerulosclerosis. The findings indicate that pre-existing CKD may compromise tubule repair, induce greater capillary dropout, and promote fibrosis with hypertension development after superimposed AKI. In a later study195, Polichnowski and coworkers quantified tubulointerstitial fibrosis and glomerulosclerosis at 4 weeks and at a later time-point of 16 weeks after IRI in rats with 50% RMR. Whereas averaged tubulointerstitial fibrosis early after AKI at 4 weeks decreased with time, averaged glomerulosclerosis scores were increased. Plotted as a function of systolic blood pressure measured by radio telemetry in conscious rats, severe tubulointerstitial fibrosis was observed only in animals exhibiting marked glomerulosclerosis, proteinuria and kidney hypertrophy as well as systolic blood pressures in excess of 127 mm3Hg. The strong association of tubulointerstitial fibrosis and glomerulosclerosis with modest blood pressure increase over time when AKI is superimposed upon CKD (in the RMR model), together with impaired autoregulation of pre-glomerular arterioles known to occur after RMR suggests that increased transmission of even small increases of arterial pressure through less responsive pre-glomerular arterioles is sufficient to cause progression of renal disease after AKI in settings of preexisting CKD6,195. Therefore, fibrosis associated with tubule atrophy may actually decrease at the late phase of AKI recovery unless vascular pathologies and associated tubule dysfunction cause sufficient increase of arterial pressure that is transmitted directly to glomeruli because of impaired glomerular arteriolar autoregulation. Of note, and relevant to human disease, in a large retrospective cohort study, elevated blood pressure was observed in patients with preexisting CKD recovering from AKI and even after AKI alone196.
Mechanistically, post-AKI vascular defects may involve the impairment of proliferation and phenotypic change in endothelial cells18. As discussed above, CKD is associated with vascular dysfunction, which may further prevent vascular repair and regeneration in post-AKI kidneys, resulting in worsened vascular defects, the suppression of normal kidney repair, and the induction of maladaptive repair or renal fibrosis.
f. Epigenetic regulation
CKD is frequently associated with significant changes in epigenetics in kidneys, such as DNA methylation, histone acetylation, and expression of non-coding RNAs197, 198. DNA methylation changes in CKD are characterized by hypermethylation and hypomethylation of different genes. Smyth LJ et al identified different loci of genes either hypermethylated or hypomethylated in CKD by genome-wide profiling199. The development of diabetic nephropathy is also associated with DNA methylation changes in specific genes200. Interestingly, significant changes in DNA methylation occurs cisplatin-induced AKI and blockade of DNA methylation sensitizes kidney injury, suggesting a protective role of DNA methylation in AKI201. In terms of specific genes, the hypermethylation of klotho gene promoter in CKD conditions has been reported by several laboratories202–207. The suppression of klotho by hypermethylation is a potential risk factor for AKI development since klotho plays a protective role in AKI208–210. Chromatin modifications, especially histone acetylation, may also contribute to AKI sensitivity of CKD patients because inhibition of histone deacetylases showed beneficial effects in both CKD and AKI conditions211–214. As to the regulation of non-coding RNAs, genomic wide profiling has identified the dysregulation of multiple microRNAs in CKD, although the information about long non-coding RNAs is still limited215–217. The roles of specific microRNAs, such as mir-21, −192, and −29, have been delineated in CKD218–223, providing a foundation for further investigation of their involvement in AKI susceptibility of CKD patients.
IV. Conclusions and Perspectives
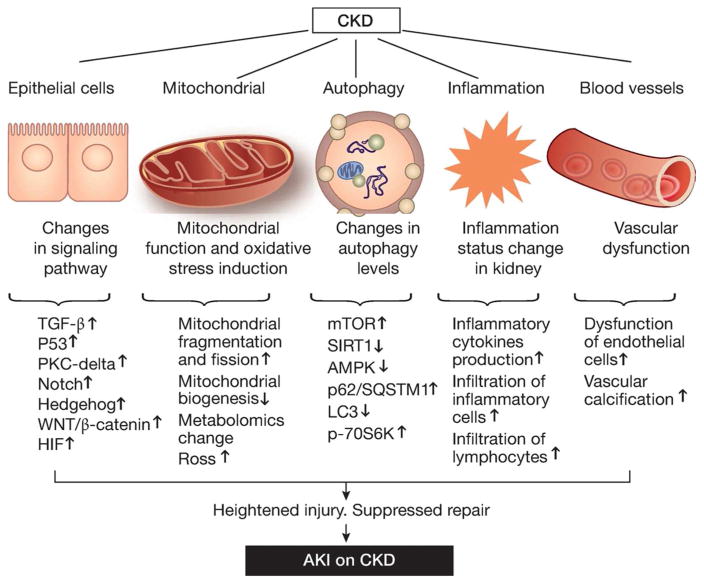
AKI and CKD are interconnected syndromes. On one hand, AKI contributes to the initiation and progression of CKD; on the other hand, CKD predisposes patients to AKI, and AKI on CKD has a poor prognosis. While much has learned about AKI-CKD transition, the underlying mechanism of AKI on CKD remains largely elusive. In this review, we have analyzed the major changes in CKD that may increase the sensitivity or susceptibility of AKI and suppress kidney repair or recovery from AKI in CKD patients (Figure 2). Although the molecular, cellular and tissue alterations in CKD are discussed separately, they are related and most likely cooperate to result in a pro-AKI condition. In addition, other pathological changes in CKD may be involved as well. For example, the kidney is a production site of several growth factors, such as epidermal growth factor, hepatocyte growth factor, and insulin-like growth factor I224. These growth factors not only affect the severity of AKI but also play important roles in the regulation of kidney repair and fibrosis225. In CKD, the production and function of these growth factors may be altered. Thus, AKI sensitivity, severity and non-recovery in CKD may involve multiple mechanisms at epigenetic, gene expression, signaling, organellar, cellular and tissue levels. Elucidation of these mechanisms may identify effective therapeutic targets to reduce AKI and promote kidney repair and recovery in CKD patients.
Figure 2.
Potential mechanisms underlying AKI sensitivity and non-recovery in CKD patients.
Acknowledgments
This work was supported in part by grants from the National Natural Science Foundation of China (81430017, 81370791), and the National Institutes of Health and Department of Veterans Administration of USA.
Footnotes
DISCLOSURE
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Couser WG, Remuzzi G, Mendis S, et al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 4.Venkatachalam MA, Griffin KA, Lan R, et al. Acute kidney injury: a springboard for progression in chronic kidney disease. American journal of physiology Renal physiology. 2010;298:F1078–1094. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawla LS, Eggers PW, Star RA, et al. Acute kidney injury and chronic kidney disease as interconnected syndromes. The New England journal of medicine. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatachalam MA, Weinberg JM, Kriz W, et al. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. Journal of the American Society of Nephrology: JASN. 2015;26:1765–1776. doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171:226–233. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 8.Garg AX, Suri RS, Barrowman N, et al. Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: a systematic review, meta-analysis, and meta-regression. JAMA. 2003;290:1360–1370. doi: 10.1001/jama.290.10.1360. [DOI] [PubMed] [Google Scholar]
- 9.Askenazi DJ, Feig DI, Graham NM, et al. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. 2006;69:184–189. doi: 10.1038/sj.ki.5000032. [DOI] [PubMed] [Google Scholar]
- 10.Mammen C, Al Abbas A, Skippen P, et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59:523–530. doi: 10.1053/j.ajkd.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 11.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim CS, Bae EH, Ma SK, et al. Impact of Transient and Persistent Acute Kidney Injury on Chronic Kidney Disease Progression and Mortality after Gastric Surgery for Gastric Cancer. Plos One. 2016:11. doi: 10.1371/journal.pone.0168119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palomba H, Castro I, Yu L, et al. The duration of acute kidney injury after cardiac surgery increases the risk of long-term chronic kidney disease. J Nephrol. 2016 doi: 10.1007/s40620-016-0351-0. [DOI] [PubMed] [Google Scholar]
- 14.Legouis D, Galichon P, Bataille A, et al. Rapid Occurrence of Chronic Kidney Disease in Patients Experiencing Reversible Acute Kidney Injury after Cardiac Surgery. Anesthesiology. 2016 doi: 10.1097/ALN.0000000000001400. [DOI] [PubMed] [Google Scholar]
- 15.Thakar CV, Christianson A, Himmelfarb J, et al. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011;6:2567–2572. doi: 10.2215/CJN.01120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu CY, Chertow GM, McCulloch CE, et al. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4:891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grgic I, Campanholle G, Bijol V, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney international. 2012;82:172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basile DP, Friedrich JL, Spahic J, et al. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. American journal of physiology Renal physiology. 2011;300:F721–733. doi: 10.1152/ajprenal.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nath KA, Vercellotti GM, Grande JP, et al. Heme protein-induced chronic renal inflammation: suppressive effect of induced heme oxygenase-1. Kidney international. 2001;59:106–117. doi: 10.1046/j.1523-1755.2001.00471.x. [DOI] [PubMed] [Google Scholar]
- 20.Lan R, Geng H, Singha PK, et al. Mitochondrial Pathology and Glycolytic Shift during Proximal Tubule Atrophy after Ischemic AKI. Journal of the American Society of Nephrology: JASN. 2016;27:3356–3367. doi: 10.1681/ASN.2015020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nature reviews Nephrology. 2015;11:264–276. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James MT, Grams ME, Woodward M, et al. A Meta-analysis of the Association of Estimated GFR, Albuminuria, Diabetes Mellitus, and Hypertension With Acute Kidney Injury. Am J Kidney Dis. 2015;66:602–612. doi: 10.1053/j.ajkd.2015.02.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung CU, Nelson JA, Fischer JP, et al. Acute kidney injury after open ventral hernia repair: an analysis of the 2005–2012 ACS-NSQIP datasets. Hernia. 2015 doi: 10.1007/s10029-015-1395-0. [DOI] [PubMed] [Google Scholar]
- 24.Wu VC, Huang TM, Lai CF, et al. Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int. 2011;80:1222–1230. doi: 10.1038/ki.2011.259. [DOI] [PubMed] [Google Scholar]
- 25.Wilson T, Quan S, Cheema K, et al. Risk prediction models for acute kidney injury following major noncardiac surgery: systematic review. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2016;31:231–240. doi: 10.1093/ndt/gfv415. [DOI] [PubMed] [Google Scholar]
- 26.Peng J, Li X, Zhang D, et al. Hyperglycemia, p53, and mitochondrial pathway of apoptosis are involved in the susceptibility of diabetic models to ischemic acute kidney injury. Kidney Int. 2015;87:137–150. doi: 10.1038/ki.2014.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao G, Zhang B, Ramesh G, et al. TNF-alpha mediates increased susceptibility to ischemic AKI in diabetes. American journal of physiology Renal physiology. 2013;304:F515–521. doi: 10.1152/ajprenal.00533.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Q, Zhao C, Xie D, et al. Acute and acute-on-chronic kidney injury of patients with decompensated heart failure: impact on outcomes. BMC Nephrol. 2012;13:51. doi: 10.1186/1471-2369-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Findlay M, Donaldson K, Robertson S, et al. Chronic kidney disease rather than illness severity predicts medium- to long-term mortality and renal outcome after acute kidney injury. Nephrol Dial Transplant. 2015;30:594–598. doi: 10.1093/ndt/gfu185. [DOI] [PubMed] [Google Scholar]
- 31.Macedo E, Zanetta DMT, Abdulkader RCRM. Long-term follow-up of patients after acute kidney injury: patterns of renal functional recovery. PloS one. 2012;7:e36388. doi: 10.1371/journal.pone.0036388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pannu N, James M, Hemmelgarn BR, et al. Modification of outcomes after acute kidney injury by the presence of CKD. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2011;58:206–213. doi: 10.1053/j.ajkd.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Polichnowski AJ, Lan R, Geng H, et al. Severe renal mass reduction impairs recovery and promotes fibrosis after AKI. Journal of the American Society of Nephrology: JASN. 2014;25:1496–1507. doi: 10.1681/ASN.2013040359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linkermann A, Chen G, Dong G, et al. Regulated cell death in AKI. Journal of the American Society of Nephrology: JASN. 2014;25:2689–2701. doi: 10.1681/ASN.2014030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20:1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 36.Hodgkins KS, Schnaper HW. Tubulointerstitial injury and the progression of chronic kidney disease. Pediatr Nephrol. 2012;27:901–909. doi: 10.1007/s00467-011-1992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loeffler I, Wolf G. Transforming growth factor-beta and the progression of renal disease. Nephrol Dial Transplant. 2014;29(Suppl 1):i37–i45. doi: 10.1093/ndt/gft267. [DOI] [PubMed] [Google Scholar]
- 38.Gewin L, Zent R. How does TGF-beta mediate tubulointerstitial fibrosis? Semin Nephrol. 2012;32:228–235. doi: 10.1016/j.semnephrol.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mark A, Hock T, Kapturczak MH, et al. Induction of heme oxygenase-1 modulates the profibrotic effects of transforming growth factor-beta in human renal tubular epithelial cells. Cell Mol Biol (Noisy-le-grand) 2005;51:357–362. [PubMed] [Google Scholar]
- 40.Guan Q, Nguan CY, Du C. Expression of transforming growth factor-beta1 limits renal ischemia-reperfusion injury. Transplantation. 2010;89:1320–1327. doi: 10.1097/TP.0b013e3181d8e9dc. [DOI] [PubMed] [Google Scholar]
- 41.Gewin L, Vadivelu S, Neelisetty S, et al. Deleting the TGF-beta receptor attenuates acute proximal tubule injury. J Am Soc Nephrol. 2012;23:2001–2011. doi: 10.1681/ASN.2012020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soofi A, Zhang P, Dressler GR. Kielin/chordin-like protein attenuates both acute and chronic renal injury. J Am Soc Nephrol. 2013;24:897–905. doi: 10.1681/ASN.2012070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nath KA, Croatt AJ, Warner GM, et al. Genetic deficiency of Smad3 protects against murine ischemic acute kidney injury. Am J Physiol Renal Physiol. 2011;301:F436–442. doi: 10.1152/ajprenal.00162.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gentle ME, Shi S, Daehn I, et al. Epithelial cell TGFbeta signaling induces acute tubular injury and interstitial inflammation. J Am Soc Nephrol. 2013;24:787–799. doi: 10.1681/ASN.2012101024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koesters R, Kaissling B, Lehir M, et al. Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol. 2010;177:632–643. doi: 10.2353/ajpath.2010.091012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bottinger EP. TGF-beta in renal injury and disease. Semin Nephrol. 2007;27:309–320. doi: 10.1016/j.semnephrol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Lan RP, Geng H, Polichnowski AJ, et al. PTEN loss defines a TGF-beta-induced tubule phenotype of failed differentiation and JNK signaling during renal fibrosis. Am J Physiol-Renal. 2012;302:F1210–F1223. doi: 10.1152/ajprenal.00660.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelly KJ, Plotkin Z, Vulgamott SL, et al. P53 mediates the apoptotic response to GTP depletion after renal ischemia-reperfusion: protective role of a p53 inhibitor. Journal of the American Society of Nephrology: JASN. 2003;14:128–138. doi: 10.1097/01.asn.0000040596.23073.01. [DOI] [PubMed] [Google Scholar]
- 49.Jiang M, Yi X, Hsu S, et al. Role of p53 in cisplatin-induced tubular cell apoptosis: dependence on p53 transcriptional activity. American journal of physiology Renal physiology. 2004;287:F1140–1147. doi: 10.1152/ajprenal.00262.2004. [DOI] [PubMed] [Google Scholar]
- 50.Wei Q, Dong G, Franklin J, et al. The pathological role of Bax in cisplatin nephrotoxicity. Kidney international. 2007;72:53–62. doi: 10.1038/sj.ki.5002256. [DOI] [PubMed] [Google Scholar]
- 51.Zhang D, Liu Y, Wei Q, et al. Tubular p53 regulates multiple genes to mediate AKI. J Am Soc Nephrol. 2014;25:2278–2289. doi: 10.1681/ASN.2013080902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ying Y, Kim J, Westphal SN, et al. Targeted deletion of p53 in the proximal tubule prevents ischemic renal injury. Journal of the American Society of Nephrology: JASN. 2014;25:2707–2716. doi: 10.1681/ASN.2013121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samarakoon R, Helo S, Dobberfuhl AD, et al. Loss of tumour suppressor PTEN expression in renal injury initiates SMAD3- and p53-dependent fibrotic responses. J Pathol. 2015;236:421–432. doi: 10.1002/path.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Overstreet JM, Samarakoon R, Meldrum KK, et al. Redox control of p53 in the transcriptional regulation of TGF-beta1 target genes through SMAD cooperativity. Cell Signal. 2014;26:1427–1436. doi: 10.1016/j.cellsig.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutton TA, Hato T, Mai E, et al. p53 Is Renoprotective after Ischemic Kidney Injury by Reducing Inflammation. Journal of the American Society of Nephrology. 2013;24:113–124. doi: 10.1681/ASN.2012050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dagher PC, Mai EM, Hato T, et al. The p53 inhibitor pifithrin-alpha can stimulate fibrosis in a rat model of ischemic acute kidney injury. Am J Physiol-Renal. 2012;302:F284–F291. doi: 10.1152/ajprenal.00317.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edeling M, Ragi G, Huang S, et al. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nature reviews Nephrology. 2016;12:426–439. doi: 10.1038/nrneph.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kramann R. Hedgehog Gli signalling in kidney fibrosis. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2016;31:1989–1995. doi: 10.1093/ndt/gfw102. [DOI] [PubMed] [Google Scholar]
- 59.Tan RJ, Zhou D, Liu Y. Signaling Crosstalk between Tubular Epithelial Cells and Interstitial Fibroblasts after Kidney Injury. Kidney Dis (Basel) 2016;2:136–144. doi: 10.1159/000446336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCright B, Gao X, Shen L, et al. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development. 2001;128:491–502. doi: 10.1242/dev.128.4.491. [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi T, Terada Y, Kuwana H, et al. Expression and function of the Delta-1/Notch-2/Hes-1 pathway during experimental acute kidney injury. Kidney Int. 2008;73:1240–1250. doi: 10.1038/ki.2008.74. [DOI] [PubMed] [Google Scholar]
- 62.Gupta S, Li S, Abedin MJ, et al. Effect of Notch activation on the regenerative response to acute renal failure. Am J Physiol Renal Physiol. 2010;298:F209–215. doi: 10.1152/ajprenal.00451.2009. [DOI] [PubMed] [Google Scholar]
- 63.Huang R, Zhou Q, Veeraragoo P, et al. Notch2/Hes-1 pathway plays an important role in renal ischemia and reperfusion injury-associated inflammation and apoptosis and the gamma-secretase inhibitor DAPT has a nephroprotective effect. Ren Fail. 2011;33:207–216. doi: 10.3109/0886022X.2011.553979. [DOI] [PubMed] [Google Scholar]
- 64.Bielesz B, Sirin Y, Si H, et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest. 2010;120:4040–4054. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Djudjaj S, Chatziantoniou C, Raffetseder U, et al. Notch-3 receptor activation drives inflammation and fibrosis following tubulointerstitial kidney injury. J Pathol. 2012;228:286–299. doi: 10.1002/path.4076. [DOI] [PubMed] [Google Scholar]
- 66.Walsh DW, Roxburgh SA, McGettigan P, et al. Co-regulation of Gremlin and Notch signalling in diabetic nephropathy. Biochim Biophys Acta. 2008;1782:10–21. doi: 10.1016/j.bbadis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 67.Ding H, Zhou D, Hao S, et al. Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol. 2012;23:801–813. doi: 10.1681/ASN.2011060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou D, Li Y, Zhou L, et al. Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J Am Soc Nephrol. 2014;25:2187–2200. doi: 10.1681/ASN.2013080893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kramann R, Schneider RK, DiRocco DP, et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kramann R, Fleig SV, Schneider RK, et al. Pharmacological GLI2 inhibition prevents myofibroblast cell-cycle progression and reduces kidney fibrosis. The Journal of clinical investigation. 2015;125:2935–2951. doi: 10.1172/JCI74929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rauhauser AA, Ren C, Lu D, et al. Hedgehog signaling indirectly affects tubular cell survival after obstructive kidney injury. Am J Physiol Renal Physiol. 2015;309:F770–778. doi: 10.1152/ajprenal.00232.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tan RJ, Zhou D, Zhou LL, et al. Wnt/beta-catenin signaling and kidney fibrosis. Kidney International Supplements. 2014;4:84–90. doi: 10.1038/kisup.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Banon-Maneus E, Rovira J, Ramirez-Bajo MJ, et al. Wnt Pathway Activation in Long Term Remnant Rat Model. Biomed Research International. 2014 doi: 10.1155/2014/324713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Al-Chaqmaqchi HAM, Moshfegh A, Dadfar E, et al. Activation of Wnt/beta-Catenin Pathway in Monocytes Derived from Chronic Kidney Disease Patients. Plos One. 2013:8. doi: 10.1371/journal.pone.0068937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shkreli M, Sarin KY, Pech MF, et al. Reversible cell-cycle entry in adult kidney podocytes through regulated control of telomerase and Wnt signaling. Nat Med. 2012;18:111–119. doi: 10.1038/nm.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He WC, Tan RJ, Li YJ, et al. Matrix Metalloproteinase-7 as a Surrogate Marker Predicts Renal Wnt/beta-Catenin Activity in CKD. Journal of the American Society of Nephrology. 2012;23:294–304. doi: 10.1681/ASN.2011050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. Journal of the American Society of Nephrology. 2005;16:2373–2384. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- 78.Terada Y, Tanaka H, Okado T, et al. Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. Journal of the American Society of Nephrology. 2003;14:1223–1233. doi: 10.1097/01.asn.0000060577.94532.06. [DOI] [PubMed] [Google Scholar]
- 79.Zhou D, Li YJ, Lin L, et al. Tubule-specific ablation of endogenous beta-catenin aggravates acute kidney injury in mice. Kidney International. 2012;82:537–547. doi: 10.1038/ki.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiao LX, Zhou D, Tan RJ, et al. Sustained Activation of Wnt/beta-Catenin Signaling Drives AKI to CKD Progression. Journal of the American Society of Nephrology. 2016;27:1727–1740. doi: 10.1681/ASN.2015040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peng JP, Ramesh G, Sun L, et al. Impaired Wound Healing in Hypoxic Renal Tubular Cells: Roles of Hypoxia-Inducible Factor-1 and Glycogen Synthase Kinase 3 beta/beta-Catenin Signaling. Journal of Pharmacology and Experimental Therapeutics. 2012;340:176–184. doi: 10.1124/jpet.111.187427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eckardt KU, Bernhardt WM, Weidemann A, et al. Role of hypoxia in the pathogenesis of renal disease. Kidney Int Suppl. 2005:S46–51. doi: 10.1111/j.1523-1755.2005.09909.x. [DOI] [PubMed] [Google Scholar]
- 83.Schley G, Klanke B, Schodel J, et al. Selective Stabilization of HIF-1 alpha in Renal Tubular Cells by 2-Oxoglutarate Analogues. American Journal of Pathology. 2012;181:1595–1606. doi: 10.1016/j.ajpath.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 84.Wei QQ, Liu Y, Liu PY, et al. MicroRNA-489 Induction by Hypoxia-Inducible Factor-1 Protects against Ischemic Kidney Injury. Journal of the American Society of Nephrology. 2016;27:2784–2796. doi: 10.1681/ASN.2015080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kapitsinou PP, Jaffe J, Michael M, et al. Preischemic targeting of HIF prolyl hydroxylation inhibits fibrosis associated with acute kidney injury. Am J Physiol-Renal. 2012;302:F1172–F1179. doi: 10.1152/ajprenal.00667.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kapitsinou PP, Sano H, Michael M, et al. Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. Journal of Clinical Investigation. 2014;124:2396–2409. doi: 10.1172/JCI69073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schley G, Klanke B, Schodel J, et al. Hypoxia-Inducible Transcription Factors Stabilization in the Thick Ascending Limb Protects against Ischemic Acute Kidney Injury. Journal of the American Society of Nephrology. 2011;22:2004–2015. doi: 10.1681/ASN.2010121249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Botusan IR, Sunkari VG, Savu O, et al. Stabilization of HIF-1 alpha is critical to improve wound healing in diabetic mice. P Natl Acad Sci USA. 2008;105:19426–19431. doi: 10.1073/pnas.0805230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Higgins DF, Kimura K, Bernhardt WM, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haase VH. Hypoxia-inducible factor signaling in the development of kidney fibrosis. Fibrogenesis Tissue Repair. 2012;5:S16. doi: 10.1186/1755-1536-5-S1-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Higgins DF, Kimura K, Iwano M, et al. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle. 2008;7:1128–1132. doi: 10.4161/cc.7.9.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang M, Pabla N, Murphy RF, et al. Nutlin-3 protects kidney cells during cisplatin therapy by suppressing Bax/Bak activation. J Biol Chem. 2007;282:2636–2645. doi: 10.1074/jbc.M606928200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wei Q, Dong G, Chen J-K, et al. Bax and Bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney international. 2013;84:138–148. doi: 10.1038/ki.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhan M, Brooks C, Liu F, et al. Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney international. 2013;83:568–581. doi: 10.1038/ki.2012.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brooks C, Cho S-G, Wang C-Y, et al. Fragmented mitochondria are sensitized to Bax insertion and activation during apoptosis. Am J Physiol Cell Physiol. 2011;300:C447–455. doi: 10.1152/ajpcell.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brooks C, Dong Z. Regulation of mitochondrial morphological dynamics during apoptosis by Bcl-2 family proteins: a key in Bak? Cell cycle (Georgetown, Tex ) 2007;6:3043–3047. doi: 10.4161/cc.6.24.5115. [DOI] [PubMed] [Google Scholar]
- 97.Brooks C, Wei Q, Cho S-G, et al. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. The Journal of clinical investigation. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xiao X, Hu Y, Quiros PM, et al. OMA1 mediates OPA1 proteolysis and mitochondrial fragmentation in experimental models of ischemic kidney injury. American journal of physiology Renal physiology. 2014;306:F1318–1326. doi: 10.1152/ajprenal.00036.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Linkermann A, Brasen JH, Darding M, et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2013;110:12024–12029. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Devalaraja-Narashimha K, Diener AM, Padanilam BJ. Cyclophilin D gene ablation protects mice from ischemic renal injury. American journal of physiology Renal physiology. 2009;297:F749–759. doi: 10.1152/ajprenal.00239.2009. [DOI] [PubMed] [Google Scholar]
- 101.Park JS, Pasupulati R, Feldkamp T, et al. Cyclophilin D and the mitochondrial permeability transition in kidney proximal tubules after hypoxic and ischemic injury. American journal of physiology Renal physiology. 2011;301:F134–150. doi: 10.1152/ajprenal.00033.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Granata S, Zaza G, Simone S, et al. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genomics. 2009;10:388. doi: 10.1186/1471-2164-10-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Che RC, Yuan YG, Huang SM, et al. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am J Physiol-Renal. 2014;306:F367–F378. doi: 10.1152/ajprenal.00571.2013. [DOI] [PubMed] [Google Scholar]
- 104.Li X, Pabla N, Wei Q, et al. PKC-delta promotes renal tubular cell apoptosis associated with proteinuria. J Am Soc Nephrol. 2010;21:1115–1124. doi: 10.1681/ASN.2009070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang W, Wang Y, Long J, et al. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 2012;15:186–200. doi: 10.1016/j.cmet.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhan M, Usman IM, Sun L, et al. Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. Journal of the American Society of Nephrology: JASN. 2015;26:1304–1321. doi: 10.1681/ASN.2014050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sharma K, Karl B, Mathew AV, et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol. 2013;24:1901–1912. doi: 10.1681/ASN.2013020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith JA, Stallons LJ, Collier JB, et al. Suppression of mitochondrial biogenesis through toll-like receptor 4-dependent mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling in endotoxin-induced acute kidney injury. J Pharmacol Exp Ther. 2015;352:346–357. doi: 10.1124/jpet.114.221085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garrett SM, Whitaker RM, Beeson CC, et al. Agonism of the 5-hydroxytryptamine 1F receptor promotes mitochondrial biogenesis and recovery from acute kidney injury. J Pharmacol Exp Ther. 2014;350:257–264. doi: 10.1124/jpet.114.214700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Whitaker RM, Wills LP, Stallons LJ, et al. cGMP-selective phosphodiesterase inhibitors stimulate mitochondrial biogenesis and promote recovery from acute kidney injury. J Pharmacol Exp Ther. 2013;347:626–634. doi: 10.1124/jpet.113.208017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stallons LJ, Whitaker RM, Schnellmann RG. Suppressed mitochondrial biogenesis in folic acid-induced acute kidney injury and early fibrosis. Toxicol Lett. 2014;224:326–332. doi: 10.1016/j.toxlet.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tran M, Tam D, Bardia A, et al. PGC-1 alpha promotes recovery after acute kidney injury during systemic inflammation in mice. Journal of Clinical Investigation. 2011;121:4003–4014. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 114.Ramos LF, Shintani A, Ikizler TA, et al. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol. 2008;19:593–599. doi: 10.1681/ASN.2007030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fedorova LV, Tamirisa A, Kennedy DJ, et al. Mitochondrial impairment in the five-sixth nephrectomy model of chronic renal failure: proteomic approach. BMC Nephrol. 2013;14:209. doi: 10.1186/1471-2369-14-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ruiz S, Pergola PE, Zager RA, et al. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83:1029–1041. doi: 10.1038/ki.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Himmelfarb J, McMonagle E, Freedman S, et al. Oxidative stress is increased in critically ill patients with acute renal failure. J Am Soc Nephrol. 2004;15:2449–2456. doi: 10.1097/01.ASN.0000138232.68452.3B. [DOI] [PubMed] [Google Scholar]
- 118.Mittwede PN, Xiang L, Lu S, et al. Oxidative stress contributes to orthopedic trauma-induced acute kidney injury in obese rats. Am J Physiol Renal Physiol. 2015;308:F157–163. doi: 10.1152/ajprenal.00537.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pflueger A, Abramowitz D, Calvin AD. Role of oxidative stress in contrast-induced acute kidney injury in diabetes mellitus. Med Sci Monit. 2009;15:RA125–136. [PubMed] [Google Scholar]
- 120.Heyman SN, Rosenberger C, Rosen S, et al. Why is diabetes mellitus a risk factor for contrast-induced nephropathy? Biomed Res Int. 2013;2013:123589. doi: 10.1155/2013/123589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tasanarong A, Vohakiat A, Hutayanon P, et al. New strategy of alpha- and gamma-tocopherol to prevent contrast-induced acute kidney injury in chronic kidney disease patients undergoing elective coronary procedures. Nephrol Dial Transplant. 2013;28:337–344. doi: 10.1093/ndt/gfs525. [DOI] [PubMed] [Google Scholar]
- 122.Periyasamy-Thandavan S, Jiang M, Wei Q, et al. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int. 2008;74:631–640. doi: 10.1038/ki.2008.214. [DOI] [PubMed] [Google Scholar]
- 123.Jiang M, Wei Q, Dong G, et al. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82:1271–1283. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jiang M, Liu K, Luo J, et al. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol. 2010;176:1181–1192. doi: 10.2353/ajpath.2010.090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kimura T, Takabatake Y, Takahashi A, et al. Autophagy Protects the Proximal Tubule from Degeneration and Acute Ischemic Injury. Journal of the American Society of Nephrology. 2011;22:902–913. doi: 10.1681/ASN.2010070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu SY, Hartleben B, Kretz O, et al. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy. 2012;8:826–837. doi: 10.4161/auto.19419. [DOI] [PubMed] [Google Scholar]
- 127.Kaushal GP, Shah SV. Autophagy in acute kidney injury. Kidney International. 2016;89:779–791. doi: 10.1016/j.kint.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin F, Wang ZV, Hill JA. Seeing is believing: dynamic changes in renal epithelial autophagy during injury and repair. Autophagy. 2014;10:691–693. doi: 10.4161/auto.27749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baisantry A, Bhayana S, Rong S, et al. Autophagy Induces Prosenescent Changes in Proximal Tubular S3 Segments. Journal of the American Society of Nephrology. 2016;27:1609–1616. doi: 10.1681/ASN.2014111059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Livingston MJ, Ding HF, Huang S, et al. Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy. 2016;12:976–998. doi: 10.1080/15548627.2016.1166317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li H, Peng X, Wang Y, et al. Atg5-mediated autophagy deficiency in proximal tubules promotes cell cycle G2/M arrest and renal fibrosis. Autophagy. 2016;12:1472–1486. doi: 10.1080/15548627.2016.1190071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kume S, Thomas MC, Koya D. Nutrient sensing, autophagy, and diabetic nephropathy. Diabetes. 2012;61:23–29. doi: 10.2337/db11-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Godel M, Hartleben B, Herbach N, et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest. 2011;121:2197–2209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mori H, Inoki K, Masutani K, et al. The mTOR pathway is highly activated in diabetic nephropathy and rapamycin has a strong therapeutic potential. Biochem Biophys Res Commun. 2009;384:471–475. doi: 10.1016/j.bbrc.2009.04.136. [DOI] [PubMed] [Google Scholar]
- 135.Ding Y, Choi ME. Autophagy in diabetic nephropathy. J Endocrinol. 2015;224:R15–30. doi: 10.1530/JOE-14-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vallon V, Rose M, Gerasimova M, et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol. 2013;304:F156–167. doi: 10.1152/ajprenal.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kitada M, Takeda A, Nagai T, et al. Dietary restriction ameliorates diabetic nephropathy through anti-inflammatory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty (fa/fa) rats: a model of type 2 diabetes. Exp Diabetes Res. 2011;2011:908185. doi: 10.1155/2011/908185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yamahara K, Kume S, Koya D, et al. Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. Journal of the American Society of Nephrology: JASN. 2013;24:1769–1781. doi: 10.1681/ASN.2012111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zeng C, Fan Y, Wu J, et al. Podocyte autophagic activity plays a protective role in renal injury and delays the progression of podocytopathies. The Journal of pathology. 2014;234:203–213. doi: 10.1002/path.4382. [DOI] [PubMed] [Google Scholar]
- 140.Li H, Satriano J, Thomas JL, et al. Interactions between HIF-1alpha and AMPK in the regulation of cellular hypoxia adaptation in chronic kidney disease. Am J Physiol Renal Physiol. 2015;309:F414–428. doi: 10.1152/ajprenal.00463.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Esch KJ, Schaut RG, Lamb IM, et al. Activation of autophagy and nucleotide-binding domain leucine-rich repeat-containing-like receptor family, pyrin domain-containing 3 inflammasome during Leishmania infantum-associated glomerulonephritis. Am J Pathol. 2015;185:2105–2117. doi: 10.1016/j.ajpath.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Grgic I, Campanholle G, Bijol V, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Basile DP, Bonventre JV, Mehta R, et al. Progression after AKI: Understanding Maladaptive Repair Processes to Predict and Identify Therapeutic Treatments. J Am Soc Nephrol. 2016;27:687–697. doi: 10.1681/ASN.2015030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11:264–276. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tonelli M, Sacks F, Pfeffer M, et al. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005;68:237–245. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 146.Amdur RL, Feldman HI, Gupta J, et al. Inflammation and Progression of CKD: The CRIC Study. Clin J Am Soc Nephro. 2016;11:1546–1556. doi: 10.2215/CJN.13121215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Minakata K, Bando K, Tanaka S, et al. Preoperative chronic kidney disease as a strong predictor of postoperative infection and mortality after coronary artery bypass grafting. Circ J. 2014;78:2225–2231. doi: 10.1253/circj.cj-14-0328. [DOI] [PubMed] [Google Scholar]
- 148.Carrero JJ, Stenvinkel P. Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: a hypothesis proposal. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S49–55. doi: 10.2215/CJN.02720409. [DOI] [PubMed] [Google Scholar]
- 149.Miyamoto T, Carrero JJ, Stenvinkel P. Inflammation as a risk factor and target for therapy in chronic kidney disease. Curr Opin Nephrol Hypertens. 2011;20:662–668. doi: 10.1097/MNH.0b013e32834ad504. [DOI] [PubMed] [Google Scholar]
- 150.Rao M, Wong C, Kanetsky P, et al. Cytokine gene polymorphism and progression of renal and cardiovascular diseases. Kidney Int. 2007;72:549–556. doi: 10.1038/sj.ki.5002391. [DOI] [PubMed] [Google Scholar]
- 151.Yilmaz MI, Solak Y, Saglam M, et al. The relationship between IL-10 levels and cardiovascular events in patients with CKD. Clin J Am Soc Nephrol. 2014;9:1207–1216. doi: 10.2215/CJN.08660813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Navarro-Gonzalez JF, Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 153.Anders HJ, Muruve DA. The inflammasomes in kidney disease. J Am Soc Nephrol. 2011;22:1007–1018. doi: 10.1681/ASN.2010080798. [DOI] [PubMed] [Google Scholar]
- 154.Oh DJ, Kim HR, Lee MK, et al. Profile of human beta-defensins 1,2 and proinflammatory cytokines (TNF-alpha, IL-6) in patients with chronic kidney disease. Kidney Blood Press Res. 2013;37:602–610. doi: 10.1159/000355740. [DOI] [PubMed] [Google Scholar]
- 155.Alexandraki K, Piperi C, Kalofoutis C, et al. Inflammatory process in type 2 diabetes: The role of cytokines. Ann N Y Acad Sci. 2006;1084:89–117. doi: 10.1196/annals.1372.039. [DOI] [PubMed] [Google Scholar]
- 156.Sanz AB, Sanchez-Nino MD, Ortiz A. TWEAK, a multifunctional cytokine in kidney injury. Kidney Int. 2011;80:708–718. doi: 10.1038/ki.2011.180. [DOI] [PubMed] [Google Scholar]
- 157.Moreno JA, Izquierdo MC, Sanchez-Nino MD, et al. The inflammatory cytokines TWEAK and TNFalpha reduce renal klotho expression through NFkappaB. J Am Soc Nephrol. 2011;22:1315–1325. doi: 10.1681/ASN.2010101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gao G, Zhang B, Ramesh G, et al. TNF-alpha mediates increased susceptibility to ischemic AKI in diabetes. Am J Physiol Renal Physiol. 2013;304:F515–521. doi: 10.1152/ajprenal.00533.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gomez IG, Roach AM, Nakagawa N, et al. TWEAK-Fn14 Signaling Activates Myofibroblasts to Drive Progression of Fibrotic Kidney Disease. Journal of the American Society of Nephrology. 2016;27:3639–3652. doi: 10.1681/ASN.2015111227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nechemia-Arbely Y, Barkan D, Pizov G, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol. 2008;19:1106–1115. doi: 10.1681/ASN.2007070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ranganathan P, Jayakumar C, Ramesh G. Proximal tubule-specific overexpression of netrin-1 suppresses acute kidney injury-induced interstitial fibrosis and glomerulosclerosis through suppression of IL-6/STAT3 signaling. Am J Physiol Renal Physiol. 2013;304:F1054–1065. doi: 10.1152/ajprenal.00650.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chawla LS, Seneff MG, Nelson DR, et al. Elevated plasma concentrations of IL-6 and elevated APACHE II score predict acute kidney injury in patients with severe sepsis. Clin J Am Soc Nephrol. 2007;2:22–30. doi: 10.2215/CJN.02510706. [DOI] [PubMed] [Google Scholar]
- 163.Bruchfeld A, Qureshi AR, Lindholm B, et al. High Mobility Group Box Protein-1 correlates with renal function in chronic kidney disease (CKD) Molecular medicine (Cambridge, Mass ) 2008;14:109–115. doi: 10.2119/2007-00107.Bruchfeld. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Goligorsky MS. TLR4 and HMGB1: partners in crime? Kidney international. 2011;80:450–452. doi: 10.1038/ki.2011.170. [DOI] [PubMed] [Google Scholar]
- 165.Zakiyanov O, Kriha V, Vachek J, et al. Placental growth factor, pregnancy-associated plasma protein-A, soluble receptor for advanced glycation end products, extracellular newly identified receptor for receptor for advanced glycation end products binding protein and high mobility group box 1 levels in patients with acute kidney injury: a cross sectional study. BMC nephrology. 2013;14:245. doi: 10.1186/1471-2369-14-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wu H, Ma J, Wang P, et al. HMGB1 contributes to kidney ischemia reperfusion injury. Journal of the American Society of Nephrology: JASN. 2010;21:1878–1890. doi: 10.1681/ASN.2009101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li J, Gong Q, Zhong S, et al. Neutralization of the extracellular HMGB1 released by ischaemic damaged renal cells protects against renal ischaemia-reperfusion injury. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26:469–478. doi: 10.1093/ndt/gfq466. [DOI] [PubMed] [Google Scholar]
- 168.Leelahavanichkul A, Huang Y, Hu X, et al. Chronic kidney disease worsens sepsis and sepsis-induced acute kidney injury by releasing High Mobility Group Box Protein-1. Kidney international. 2011;80:1198–1211. doi: 10.1038/ki.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Perlman AS, Chevalier JM, Wilkinson P, et al. Serum Inflammatory and Immune Mediators Are Elevated in Early Stage Diabetic Nephropathy. Ann Clin Lab Sci. 2015;45:256–263. [PubMed] [Google Scholar]
- 170.Fassett RG, Robertson IK, Ball MJ, et al. Effects of atorvastatin on biomarkers of inflammation in chronic kidney disease. Clin Nephrol. 2014;81:75–85. doi: 10.5414/CN108090. [DOI] [PubMed] [Google Scholar]
- 171.Almquist T, Jacobson SH, Mobarrez F, et al. Lipid-lowering treatment and inflammatory mediators in diabetes and chronic kidney disease. Eur J Clin Invest. 2014;44:276–284. doi: 10.1111/eci.12230. [DOI] [PubMed] [Google Scholar]
- 172.Wermuth PJ, Jimenez SA. The significance of macrophage polarization subtypes for animal models of tissue fibrosis and human fibrotic diseases. Clin Transl Med. 2015;4:2. doi: 10.1186/s40169-015-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80:915–925. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- 175.Eardley KS, Kubal C, Zehnder D, et al. The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int. 2008;74:495–504. doi: 10.1038/ki.2008.183. [DOI] [PubMed] [Google Scholar]
- 176.Ma LJ, Corsa BA, Zhou J, et al. Angiotensin type 1 receptor modulates macrophage polarization and renal injury in obesity. Am J Physiol Renal Physiol. 2011;300:F1203–1213. doi: 10.1152/ajprenal.00468.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li C, Ding XY, Xiang DM, et al. Enhanced M1 and Impaired M2 Macrophage Polarization and Reduced Mitochondrial Biogenesis via Inhibition of AMP Kinase in Chronic Kidney Disease. Cell Physiol Biochem. 2015;36:358–372. doi: 10.1159/000430106. [DOI] [PubMed] [Google Scholar]
- 178.Futrakul N, Panichakul T, Sirisinha S, et al. Glomerular endothelial dysfunction in chronic kidney disease. Renal failure. 2004;26:259–264. doi: 10.1081/jdi-120039524. [DOI] [PubMed] [Google Scholar]
- 179.Qunibi WY, Nolan CA, Ayus JC. Cardiovascular calcification in patients with end-stage renal disease: a century-old phenomenon. Kidney international Supplement. 2002:S73–80. doi: 10.1046/j.1523-1755.62.s82.15.x. [DOI] [PubMed] [Google Scholar]
- 180.Ameer OZ, Boyd R, Butlin M, et al. Abnormalities associated with progressive aortic vascular dysfunction in chronic kidney disease. Front Physiol. 2015;6:150. doi: 10.3389/fphys.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. J Am Soc Nephrol. 2009;20:1453–1464. doi: 10.1681/ASN.2008070692. [DOI] [PubMed] [Google Scholar]
- 182.Linden E, Cai W, He JC, et al. Endothelial dysfunction in patients with chronic kidney disease results from advanced glycation end products (AGE)-mediated inhibition of endothelial nitric oxide synthase through RAGE activation. Clinical journal of the American Society of Nephrology: CJASN. 2008;3:691–698. doi: 10.2215/CJN.04291007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bolton CH, Downs LG, Victory JG, et al. Endothelial dysfunction in chronic renal failure: roles of lipoprotein oxidation and pro-inflammatory cytokines. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2001;16:1189–1197. doi: 10.1093/ndt/16.6.1189. [DOI] [PubMed] [Google Scholar]
- 184.Martens CR, Edwards DG. Peripheral vascular dysfunction in chronic kidney disease. Cardiol Res Pract. 2011;2011:267257. doi: 10.4061/2011/267257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Malyszko J. Mechanism of endothelial dysfunction in chronic kidney disease. Clin Chim Acta. 2010;411:1412–1420. doi: 10.1016/j.cca.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 186.Masereeuw R, Mutsaers HAM, Toyohara T, et al. The kidney and uremic toxin removal: glomerulus or tubule? Seminars in nephrology. 2014;34:191–208. doi: 10.1016/j.semnephrol.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 187.Kuznik A, Mardekian J, Tarasenko L. Evaluation of cardiovascular disease burden and therapeutic goal attainment in US adults with chronic kidney disease: an analysis of national health and nutritional examination survey data, 2001–2010. BMC nephrology. 2013;14:132. doi: 10.1186/1471-2369-14-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Goldfarb M, Rosenberger C, Abassi Z, et al. Acute-on-chronic renal failure in the rat: functional compensation and hypoxia tolerance. Am J Nephrol. 2006;26:22–33. doi: 10.1159/000091783. [DOI] [PubMed] [Google Scholar]
- 189.Sadat U. Radiographic contrast-media-induced acute kidney injury: pathophysiology and prophylactic strategies. ISRN Radiol. 2013;2013:496438. doi: 10.5402/2013/496438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Morabito S, Pistolesi V, Benedetti G, et al. Incidence of contrast-induced acute kidney injury associated with diagnostic or interventional coronary angiography. J Nephrol. 2012;25:1098–1107. doi: 10.5301/jn.5000101. [DOI] [PubMed] [Google Scholar]
- 191.Sendeski M, Patzak A, Persson PB. Constriction of the vasa recta, the vessels supplying the area at risk for acute kidney injury, by four different iodinated contrast media, evaluating ionic, nonionic, monomeric and dimeric agents. Invest Radiol. 2010;45:453–457. doi: 10.1097/RLI.0b013e3181d77eed. [DOI] [PubMed] [Google Scholar]
- 192.Sendeski MM, Persson AB, Liu ZZ, et al. Iodinated contrast media cause endothelial damage leading to vasoconstriction of human and rat vasa recta. American journal of physiology Renal physiology. 2012;303:F1592–1598. doi: 10.1152/ajprenal.00471.2012. [DOI] [PubMed] [Google Scholar]
- 193.Liu ZZ, Viegas VU, Perlewitz A, et al. Iodinated contrast media differentially affect afferent and efferent arteriolar tone and reactivity in mice: a possible explanation for reduced glomerular filtration rate. Radiology. 2012;265:762–771. doi: 10.1148/radiol.12120044. [DOI] [PubMed] [Google Scholar]
- 194.Basile DP, Donohoe D, Roethe K, et al. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. American journal of physiology Renal physiology. 2001;281:F887–899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 195.Picken M, Long J, Williamson GA, et al. Progression of Chronic Kidney Disease After Acute Kidney Injury: Role of Self-Perpetuating Versus Hemodynamic-Induced Fibrosis. Hypertension. 2016;68:921–928. doi: 10.1161/HYPERTENSIONAHA.116.07749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hsu C-y, Hsu RK, Yang J, et al. Elevated BP after AKI. Journal of the American Society of Nephrology: JASN. 2016;27:914–923. doi: 10.1681/ASN.2014111114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Reddy MA, Natarajan R. Recent developments in epigenetics of acute and chronic kidney diseases. Kidney international. 2015;88:250–261. doi: 10.1038/ki.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Susztak K. Understanding the epigenetic syntax for the genetic alphabet in the kidney. Journal of the American Society of Nephrology: JASN. 2014;25:10–17. doi: 10.1681/ASN.2013050461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Smyth LJ, McKay GJ, Maxwell AP, et al. DNA hypermethylation and DNA hypomethylation is present at different loci in chronic kidney disease. Epigenetics. 2014;9:366–376. doi: 10.4161/epi.27161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sapienza C, Lee J, Powell J, et al. DNA methylation profiling identifies epigenetic differences between diabetes patients with ESRD and diabetes patients without nephropathy. Epigenetics. 2011;6:20–28. doi: 10.4161/epi.6.1.13362. [DOI] [PubMed] [Google Scholar]
- 201.Guo C, Xiao PL, Wei X, Chen Q, Ding J-K, Huang H, Fan S, Shi G, Dong HZ. DNA methylation protects against cisplatin-induced kidney injury by regulating specific genes including Irf8. Kidney Int. 2017 doi: 10.1016/j.kint.2017.03.038. in press. [DOI] [PMC free article] [PubMed]
- 202.Zhang Q, Liu L, Lin W, et al. Rhein reverses Klotho repression via promoter demethylation and protects against kidney and bone injuries in mice with chronic kidney disease. Kidney Int. 2017;91:144–156. doi: 10.1016/j.kint.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 203.Zhang C, Liang Y, Lei L, et al. Hypermethylations of RASAL1 and KLOTHO is associated with renal dysfunction in a Chinese population environmentally exposed to cadmium. Toxicol Appl Pharmacol. 2013;271:78–85. doi: 10.1016/j.taap.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 204.Young GH, Wu VC. KLOTHO methylation is linked to uremic toxins and chronic kidney disease. Kidney Int. 2012;81:611–612. doi: 10.1038/ki.2011.461. [DOI] [PubMed] [Google Scholar]
- 205.Hu Y, Mou L, Yang F, et al. Curcumin attenuates cyclosporine Ainduced renal fibrosis by inhibiting hypermethylation of the klotho promoter. Mol Med Rep. 2016;14:3229–3236. doi: 10.3892/mmr.2016.5601. [DOI] [PubMed] [Google Scholar]
- 206.Chen J, Zhang X, Zhang H, et al. Indoxyl Sulfate Enhance the Hypermethylation of Klotho and Promote the Process of Vascular Calcification in Chronic Kidney Disease. Int J Biol Sci. 2016;12:1236–1246. doi: 10.7150/ijbs.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chen J, Zhang X, Zhang H, et al. Elevated Klotho promoter methylation is associated with severity of chronic kidney disease. PLoS One. 2013;8:e79856. doi: 10.1371/journal.pone.0079856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hu MC, Shi M, Zhang J, et al. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78:1240–1251. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sugiura H, Yoshida T, Tsuchiya K, et al. Klotho reduces apoptosis in experimental ischaemic acute renal failure. Nephrol Dial Transplant. 2005;20:2636–2645. doi: 10.1093/ndt/gfi165. [DOI] [PubMed] [Google Scholar]
- 210.Panesso MC, Shi M, Cho HJ, et al. Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int. 2014;85:855–870. doi: 10.1038/ki.2013.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cianciolo Cosentino C, Skrypnyk NI, Brilli LL, et al. Histone deacetylase inhibitor enhances recovery after AKI. J Am Soc Nephrol. 2013;24:943–953. doi: 10.1681/ASN.2012111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shi Y, Xu L, Tang J, et al. Inhibition of HDAC6 protects against rhabdomyolysis-induced acute kidney injury. Am J Physiol Renal Physiol. 2017;312:F502–F515. doi: 10.1152/ajprenal.00546.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pang M, Kothapally J, Mao H, et al. Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2009;297:F996–F1005. doi: 10.1152/ajprenal.00282.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Imai N, Hishikawa K, Marumo T, et al. Inhibition of histone deacetylase activates side population cells in kidney and partially reverses chronic renal injury. Stem Cells. 2007;25:2469–2475. doi: 10.1634/stemcells.2007-0049. [DOI] [PubMed] [Google Scholar]
- 215.Szeto CC, Ching-Ha KB, Ka-Bik L, et al. Micro-RNA expression in the urinary sediment of patients with chronic kidney diseases. Dis Markers. 2012;33:137–144. doi: 10.3233/DMA-2012-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rudnicki M, Perco P, BDH, et al. Renal microRNA- and RNA-profiles in progressive chronic kidney disease. Eur J Clin Invest. 2016;46:213–226. doi: 10.1111/eci.12585. [DOI] [PubMed] [Google Scholar]
- 217.Liu Y, Guo R, Hao G, et al. The expression profiling and ontology analysis of noncoding RNAs in peritoneal fibrosis induced by peritoneal dialysis fluid. Gene. 2015;564:210–219. doi: 10.1016/j.gene.2015.03.050. [DOI] [PubMed] [Google Scholar]
- 218.Kato M, Zhang J, Wang M, et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A. 2007;104:3432–3437. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chung AC, Huang XR, Meng X, et al. miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J Am Soc Nephrol. 2010;21:1317–1325. doi: 10.1681/ASN.2010020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Krupa A, Jenkins R, Luo DD, et al. Loss of MicroRNA-192 promotes fibrogenesis in diabetic nephropathy. J Am Soc Nephrol. 2010;21:438–447. doi: 10.1681/ASN.2009050530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Long J, Wang Y, Wang W, et al. MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J Biol Chem. 2011;286:11837–11848. doi: 10.1074/jbc.M110.194969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gomez IG, MacKenna DA, Johnson BG, et al. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J Clin Invest. 2015;125:141–156. doi: 10.1172/JCI75852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Denby L, Ramdas V, Lu R, et al. MicroRNA-214 antagonism protects against renal fibrosis. J Am Soc Nephrol. 2014;25:65–80. doi: 10.1681/ASN.2013010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hammerman MR, Miller SB. Therapeutic use of growth factors in renal failure. J Am Soc Nephrol. 1994;5:1–11. doi: 10.1681/ASN.V511. [DOI] [PubMed] [Google Scholar]
- 225.Nigame S, Lieberthal W. Acute renal failure. III. The role of growth factors in the process of renal regeneration and repair. Am J Physiol Renal Physiol. 2000;279:F3–F11. doi: 10.1152/ajprenal.2000.279.1.F3. [DOI] [PubMed] [Google Scholar]