Abstract
Objective
To evaluate B-type natriuretic peptide (BNP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) and cardiac structure and function in normal women through pregnancy and the postpartum.
Methods
In this prospective observational study, we obtained serial transthoracic echocardiograms, BNP, and NT-proBNP at seven intervals from 6 weeks’ gestation through 12 months postpartum. Women with hypertension or cardiac disease were excluded. Using 6–12 months postpartum as reference for non-pregnant levels, echocardiogram measurements and BNP/NT-proBNP were compared over time using linear mixed models with Tukey-Kramer adjustment for multiple comparisons.
Results
Of 116 patients, data was available for 78–114 healthy pregnant or postpartum women within each time interval, and 102 patients provided data for ≥ 4 intervals. Compared to 6–12 months postpartum, BNP and NT-proBNP remained stable through pregnancy and delivery, increased within 48 h postpartum (P < 0.0001), then returned to baseline. Left ventricular volume increased within 48 h postpartum (P = 0.021) while left atrial volume increased at 18–24 weeks (P = 0.0002), 30–36 weeks (P < 0.0001) and within 48 h postpartum (P = 0.002). The transmittal early/late diastolic velocity (E/A) ratio, transmittal early/peak mitral annulus diastolic velocity (E/E′) ratio, isovolumic relaxation times, and mitral valve deceleration times were similar within 48 h and 6–12 months postpartum.
Conclusion
In normal women, BNP/NT-proBNP, left atrial, and left ventricular volumes increase within 48 h postpartum without indications of altered diastolic function.
Keywords: Brain, echocardiography, natriuretic peptide, pregnancy, postpartum period
Introduction
In the US, cardiovascular disease in pregnancy is increasing, making this a leading cause of maternal mortality [1]. This rise in cardiovascular disease suggests that diagnostic tools in cardiology, such as B-type natriuretic peptide (BNP) and echocardiography, will become increasingly important in obstetrics. BNP and its amino-terminal co-metabolite N-terminal pro-B-type natriuretic peptide (NT-proBNP) are biomarkers that have established roles in the diagnosis, prognosis, and risk stratification of heart failure outside of pregnancy [2, 3]. Secreted predominantly by left ventricular cardiac myocytes in response to increased left ventricular wall tension, BNP increases diuresis and natriuresis and decreases vascular tone [4]. The byproduct of BNP production is the biologically inactive NT-proBNP [5]. The utility of measuring NT-proBNP is its longer half-life of 60–90 min compared to 20 min for BNP [5], with possibly increased sensitivity for left ventricular dysfunction [6].
For obstetricians, BNP and NT-proBNP may have diagnostic value for worsening existing cardiomyopathy or new onset peripartum cardiomyopathy in the pregnant or postpartum patient presenting with acute dyspnea [1]. In general, studies have found BNP to be relatively stable during normal pregnancy [7, 8] but there is less data on BNP and NT-proBNP during labor and the early postpartum period, a time of substantial intravascular fluid shifts with altered preload. In addition, clear thresholds for BNP and NT-proBNP levels in pregnancy that are diagnostic for heart failure are not yet available [9].
Echocardiography is a first line cardiac evaluation in pregnancy, especially as it avoids radiation exposure [9]. However, as with BNP, there is little data on echo-cardiographic findings in the early postpartum period. The objective of this study was to examine BNP and NT-proBNP levels and to correlate them with cardiac structure and function through pregnancy and the postpartum period in normal women.
Methods
We conducted a prospective cohort study of pregnant women recruited from the community between August 2007 and January 2011. Women were included if they were 18–45 years old with a singleton pregnancy of 6–12 weeks’ gestational age at the time of recruitment. Gestational age was confirmed by ultrasound. Women with preexisting heart disease, hypertension, pulmonary edema, and delivery prior to 32 weeks’ gestational age were excluded. This study was approved by the University of Hawaii Institutional Review Board, Queens Medical Center Research and Institutional Review Committee, and Western Institutional Review Board, and informed consent was obtained from all participants. Transthoracic echocardiography and serum BNP/NT-proBNP levels were measured at seven time points during the study interval: (1) 6–12 weeks’ gestational age, (2) 18–24 weeks’, (3) 30–36 weeks’, (4) intrapartum (during labor or immediately prior to delivery), (5) the first 48 h postpartum, (6) 6–12 weeks postpartum, and (7) 6–12 months postpartum. The 6–12 month postpartum interval was also used to represent the non-pregnant state. Plasma BNP was measured via chemiluminescent microparticle immunoassay by the Abbott Architect ci8200 (Abbott Laboratories, Abbott Park IL) (range 10–5000 pg/mL, intraassay and interassay CV of 0.9%–5.6% and 1.7%–6.7%, respectively). NT-proBNP was measured by Roche Cobas e411 (Roche Diagnostics, Indianapolis IN) (range 5–35,000 pg/mL, intraassay and interassay CV of 1.3%–4.2% and 1.8%–4.6°/o, respectively). Samples were collected into EDTA tubes, immediately stored at − 80°C, and assayed within 6 months of collection.
A registered cardiac sonographer performed all echocardiograms using a General Electric Vivid e machine (GE Healthcare, Wauwatosa, Wisconsin) with the patient in the left lateral supine position. Structural measurements obtained at each echocardiogram included left atrial volume, left ventricular mass, ascending aortic diameter, and left ventricular volumes. Physiologic echocardiogram measurements collected included stroke volume, cardiac output, transmitral flow velocities in early (E) and late (A) diastole, diastolic peak velocity at the medial mitral annulus (E′), isovolumic relaxation time, and mitral valve deceleration time. From these measurements, the transmitral E/A and E/E′ ratios were calculated indicators of diastolic function. Isovolumic relaxation time and mitral valve deceleration time were also reported as indicators of left ventricular diastolic dysfunction, with increasing isovolumic relaxation time and decreasing mitral valve deceleration time correlating with dysfunction [10,11].
Demographic and clinical information were summarized by mean and standard deviation for continuous variables and frequency and percentage for categorical variables. BNP and NT-proBNP measures were also summarized by mean and standard deviation as well as median and interquartile range at every time point. Echocardiogram measurements, NT-proBNP, and BNP were compared over time using linear mixed models. A Tukey-Kramer adjustment for multiple comparisons was performed, and a P-value of < 0.05 was considered statistically significant. Pearson correlations between BNP or NT-proBNP and echocardiogram measurements were also calculated at every time point. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
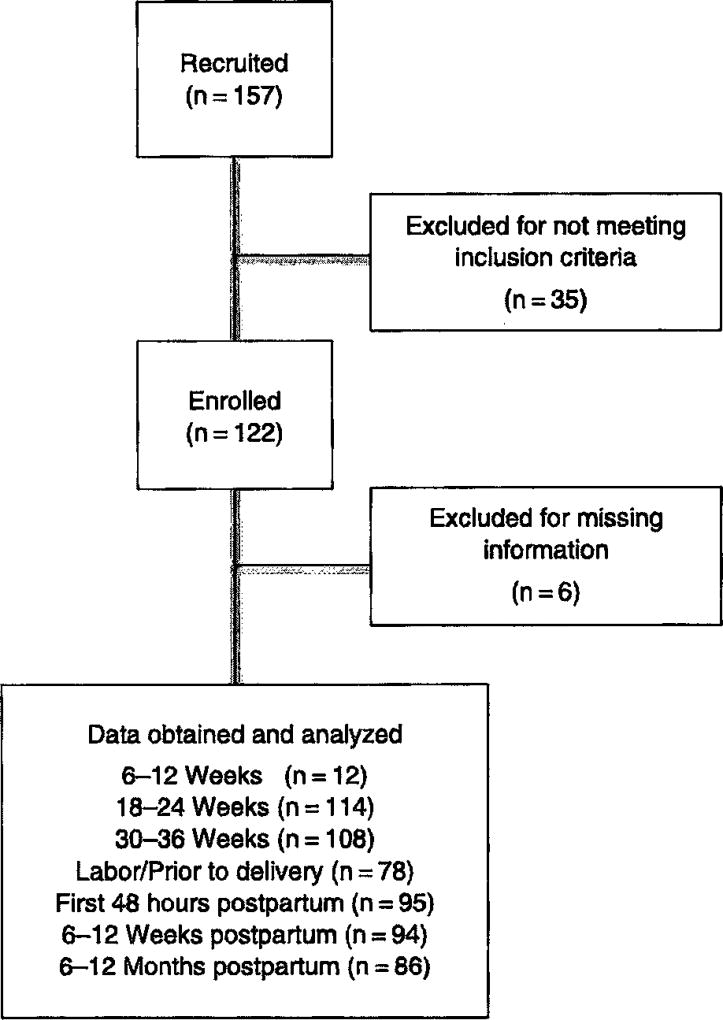
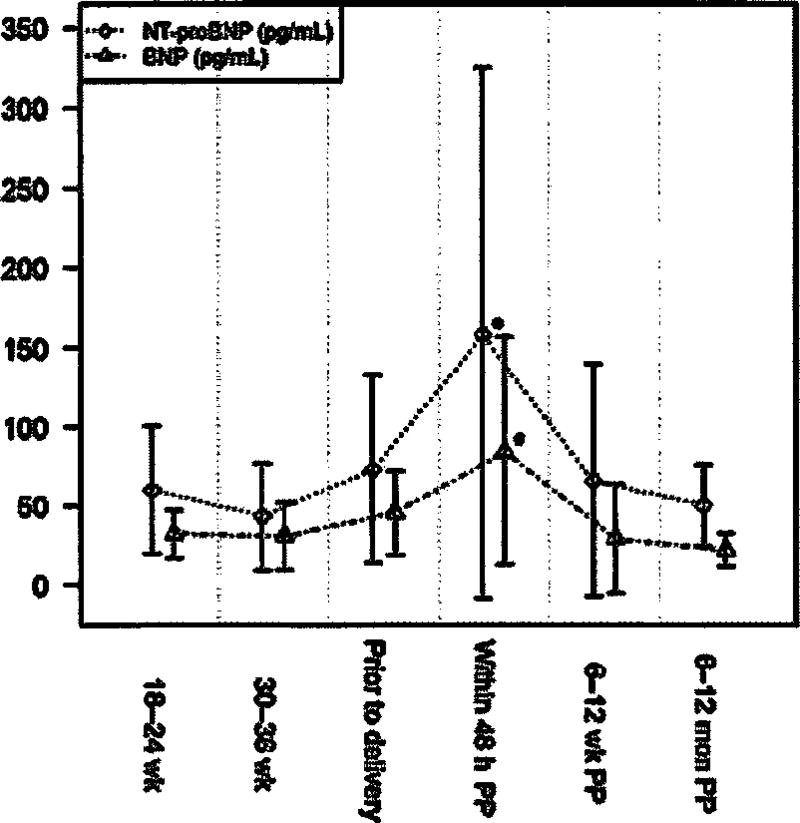
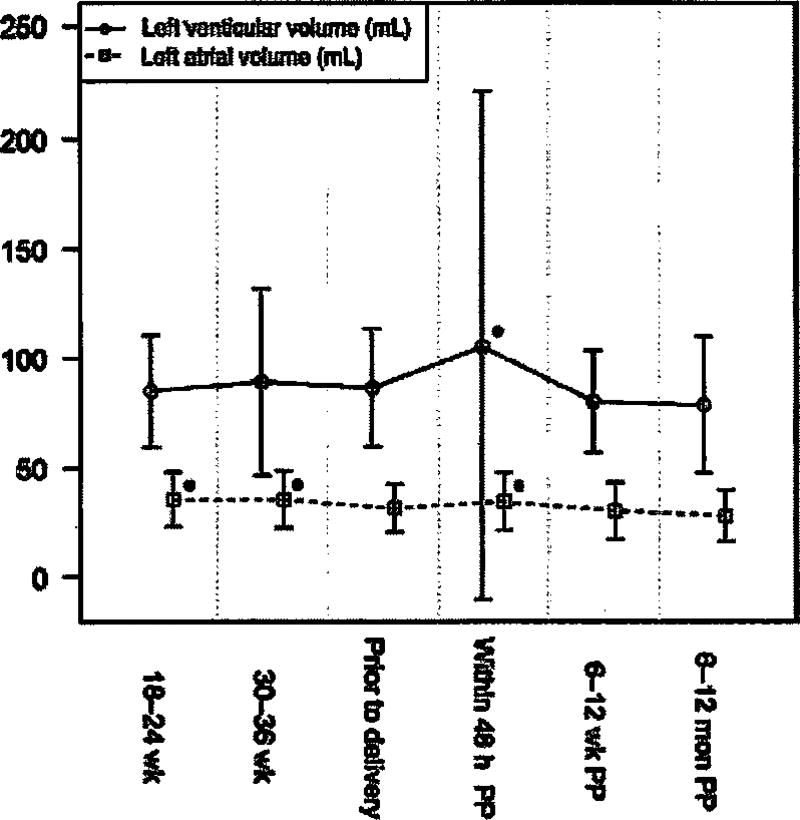
One hundred and fifty-seven women were recruited, of whom 41 were excluded due to preexisting cardiac disease, hypertension, or missing information, leaving 116 patients for analysis (Figure 1). For 102 patients, data was available for at least four of the seven time intervals. However, data was obtained for only 12 subjects at 6–12 weeks’ gestation; therefore, this time interval was excluded from the analysis. Demographic data is described in Table 1 while BNP and NT-proBNP and levels are shown in Table 2 and Figure 2. Compared to non-pregnant levels (6–12 months postpartum), BNP and NT-pro BNP remained stable through pregnancy and delivery, then increased in the first 48 h after delivery (P < 0.0001). Within 48 h of delivery the mean BNP was 84.6 pg/mL and 2 standard deviations above the mean was 144.3 pg/mL. Left ventricular volume also demonstrated an isolated increase in the first 48 h postpartum during the study interval (P = 0.021). Left atrial volume was increased at 18–24 weeks (P = 0.0002) and 30–36 weeks (P < 0.0001) and then again in the first 48 h postpartum (P = 0.002) (Figure 3). There were no correlations between BNP or NT-proBNP and echocardiogram parameters within a single study visit. However, NT-proBNP prior to delivery was positively correlated with both left atrial (P = 0.008) and left ventricular (P = 0.015) volume in the first 48 h postpartum. There were no such relationships with BNP.
Figure 1.
Consort diagram.
Table 1.
Study participant demographics.
| Maternal age, years (mean [SD]) | 31.2 [5.8] |
| Body mass Index, kg/m2 (mean [SD]) | 28.5 [6.5] |
| Gestational age at delivery, weeks (mean [SD]) | 38.8 [1.9] |
| Nulliparity (n [%]) | 44 [37.9] |
| Race (n [%]) | |
| White | 16 [13.8] |
| Mixed | 47 [40.5] |
| Aslan | 37 [31.9] |
| Native Hawaiian and other Pacific Islander | 5 [4.3] |
| Unknown | 11 [9.5] |
| Regional anesthesia (epidural or spinal) (n [%]) | 97 [83.6] |
| Cesarean delivery (n [%]) | 39 [33.6] |
Table 2.
B-type natriuretic (BNP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels through pregnancy and the postpartum.
| 18–24 Weeks | 30–36 Weeks | In labor or immediately prior to delivery |
Within 48 h deliverya | 6–12 Weeks postpartum |
6–12 Months postpartum |
|
|---|---|---|---|---|---|---|
| BNP (pg/mL) | ||||||
| Mean [SD] | 31.93 [14.83] | 30.68 [21.39] | 45.59 [26.94] | 84.58a [72.14] | 29.05 [34.93] | 21.8 [10.26] |
| Median [25%–75%] | 26.3 [21.8–42.3] | 24.7 [17.8–34.7] | 36.40 [28.35–60.30] | 77.00a [31.20–108.55] | 16.90 [14.30–33.20] | 18.50 [15.80–25.70] |
| NT-proBNP (pg/mL) | ||||||
| Mean [SD] | 60.07 [40.19] | 43.10 [33.99] | 73.50 [59.81] | 157.98a [166.68] | 66.07 [73.58] | 50.08 [25.88] |
| Median [25%–75%] | 45.80 [32.35–83.35] | 35.60 [22.40–56.00] | 57.65 [34.90–93.30] | 107.50a [60.90–201.80] | 49.10 [35.20–60.90] | 40.75 [34.90–54.85] |
Levels increased compared to 6–12 months postpartum (P < 0.0001).
Figure 2.
B-type natriuretic peptide and N terminal-pro B-type natriuretic peptide through pregnancy and the postpartum period. P-value < 0.05 for a single time point compared to 6–12 months postpartum (non-pregnant). BNP=B-type natriuretic peptide; NT-proBNP=N terminal-pro B-type natriuretic peptide.
Figure 3.
Left atrial and left ventricular volumes through pregnancy and the postpartum period. P-value < 0.05 for a single time point compared to 6–12 months postpartum (non-pregnant).
Table 3 describes other cardiac structural and functional measurements obtained over the study interval. Heart rate increased significantly from 18 weeks through the first 48 h postpartum before returning to the nonpregnant state. There were no significant changes in mean arterial pressure, stroke volume, cardiac output, left ventricular mass, or aortic ascendance.
Table 3.
Cardiac structure and function from 18 weeks gestation to 12 months postpartum.
| Mean ± SD (n) | P-valuea | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 18–24 Weeks | 30–36 Weeks | In labor or Immediately prior to delivery |
Within 48 h of delivery |
6–12 Weeks postpartum |
6–12 Weeks postpartum |
||
| Mean arterial pressure (mm Hg) | 81.9 ± 8.6 (114) | 84.0 ± 8.9 (108) | 86.4 ± 11.3 (78)b | 85.5 ± 10.3 (95) | 85.0 ± 10.0 (94) | 83.3 ± 9.6 (86) | < 0.0001 |
| P = 0.51 | P = 0.99 | P = 0.0493 | P = 0.40 | P = 0.84 | reference | ||
| Heart rate (bpm) | 77.7 ± 12.3 (101)b | 82.6 ± 10.6 (105)b | 80.0 ± 14.6 (59)b | 78.2 ± 11.1 (89)b | 67.9 ± 9.3 (94) | 71.5 ± 10.5 (86) | < 0.0001 |
| P = 0.0002 | P < 0.0001 | P < 0.0001 | P = 0.0002 | P = 0.16 | reference | ||
| Stroke volume (mL) | 80.9 ± 70.4 (100) | 76.4 ± 52.6 (105) | 74.0 ± 17.5 (59) | 72.4 ± 14.9 (89) | 72.7 ± 17.4 (94) | 73.1 ± 18.1 (85) | 0.72 |
| P = 0.78 | P = 0.99 | P = 0.99 | P = 0.99 | P = 0.99 | reference | ||
| Cardiac output (L/min) | 6.3 ± 5.3 (100) | 6.3 ± 4.4 (105) | 5.8 ± 1.4 (59) | 5.6 ± 1.3 (89) | 4.9 ± 1.2 (94) | 5.2 ± 1.5 (85) | 0.017 |
| P = 0.23 | P = 0.19 | P = 0.86 | P = 0.96 | P = 0.99 | reference | ||
| Left ventricular volume (mL) | 85.16 ± 25.41 (101) | 89.56 ± 42.76 (105) | 86.89 ± 26.83 (58) | 105.47 ± 115.92 (88)b | 80.38 ± 23.01 (94)a | 79.05 ± 30.99 (86) | 0.021 |
| P = 0.98 | P = 0.76 | P = 0.96 | P = 0.021 | P = 0.016 | reference | ||
| Left ventricular mass (g) | 117.2 ± 42.2 (101) | 115.1 ± 57.8 (105) | 117.2 ± 34.8 (58) | 113.4 ± 142.6 (88) | 119.6 ± 32.9 (94) | 105.6 ± 38.0 (86) | 0.9 |
| P = 0.90 | P = 0.95 | P = 0.93 | P = 0.98 | P = 0.76 | reference | ||
| Left atrial volume (mL) | 35.66 ± 12.65 (100)b | 35.78 ± 13.06 (105)b | 31.72 ± 11.1 (57) | 34.75 ± 13.28 (88)b | 30.34 ± 13.05 (93) | 28.04 ± 11.94 (86) | < 0.0001 |
| P = 0.0002 | P < 0.0001 | P = 0.27 | P = 0.002 | P = 0.85 | reference | ||
| Aortic ascendance (cm) | 2.6 ± 0.3 (101) | 2.6 ± 0.3 (105) | 2.7 ± 0.3 (58) | 3.0 ± 2.7 (89) | 2.6 ± 0.3 (94) | 2.6 ± 0.3 (85) | 0.14 |
| P = 0.99 | P = 0.99 | P = 0.99 | P = 0.20 | P = 0.99 | reference | ||
P-value < 0.05 testing whether there is any difference among all visits.
P-value < 0.05 single time point compared to 6–12 months postpartum (non-pregnant).
Among echocardiographic diastolic indices, the transmittal E/A ratio decreased significantly at 30–36 weeks (P = 0.008) and intrapartum (P = 0.0004) and then returned to non-pregnant levels (Table 4). Isovolumic relaxation times were decreased at 18–24 (P = 0.006) weeks and intrapartum (P = 0.008). Transmittal E/E′ ratios and mitral valve deceleration times were unchanged over time.
Table 4.
Diastolic measurements from 18 weeks gestation to 12 months postpartum.
| Mean ± SD (n) | P-valuea | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 18–24 Weeks | 30–36 Weeks | In labor or Immediately prior to delivery |
Within 48 h of delivery |
6–12 Weeks postpartum |
6–12 Months postpartum |
||
| Transmitral E/A | 1.6 ± 0.7 (101) | 1.4 ± 0.4 (105)b | 1.3 ± 0.4 (58)b | 1.5 ± 0.4 (89) | 1.6 ± 0.4 (94) | 1.6 ± 0.5 (86) | < 0.0001 |
| P = 0.99 | P = 0.008 | P = 0.0004 | P = 0.80 | P = 0.99 | reference | ||
| Transmitral E/E′ | 8.6 ± 11.2 (95) | 7.6 ± 2.5 (99) | 7.8 ± 2.5 (57) | 8.6 ± 3.7 (84) | 6.8 ± 1.9 (86) | 7.0 ± 1.8 (80) | 0.057 |
| P = 0.31 | P = 0.97 | P = 0.89 | P = 0.29 | P = 0.99 | reference | ||
| Isovolumic relaxation time (ms) | 67.7 ± 15.5 (101)b | 73.9 ± 14.0 (105) | 66.7 ± 15.34 (59)b | 70.5 ± 12.7 (89) | 78.3 ± 17.5 (94) | 75.7 ± 17.0 (85) | <0.0001 |
| P = 0.006 | P = 0.97 | P = 0.008 | P = 0.23 | P = 0.85 | reference | ||
| Mitral valve deceleration time (ms) | 204.6 ± 60.6 (101) | 210.9 ± 65.4 (105) | 207.7 ± 77.1 (58) | 216.6 ± 173.0 (89) | 221.8 ± 63.4 (94) | 196.9 ± 70.1 (86) | 0.53 |
| P = 0.99 | P = 0.91 | P = 0.99 | P = 0.78 | P = 0.51 | reference | ||
P-value < 0.05 testing whether there is any difference among all visits.
P-value < 0.05 (shaded) single time point compared to 6–12 months postpartum (non-pregnant).
Discussion
This paper is among the first to report BNP and NT-proBNP levels in association with echocardiogram measurements in a longitudinal fashion through normal pregnancy and the postpartum period. We found an isolated increase in BNP and NT-proBNP in the first 48 h postpartum with a return to non-pregnant levels by 6–12 weeks postpartum. Left atrial and ventricular volumes were increased in the first 48 h postpartum as well. Echocardiographic indices of diastolic function were not significantly different within 48 h postpartum compared to the non-pregnant state.
Given the normal clinical course of our subjects, these findings suggest a normal physiologic increase in BNP in the first 48 h postpartum. In addition, the concurrent increases in left atrial and ventricular volumes support the idea of augmented myocardial stretch during this time period. Increased cardiac preload immediately after delivery occurs as a result of uteroplacental autotransfusion, release of vena caval obstruction, and mobilization of extravascular fluid [12] and could account for these findings. However, cardiac output in the first 48 h postpartum was not increased compared to the non-pregnant state, which is consistent with prior findings that cardiac output returns to baseline within 1 h after delivery [13]. BNP in the early postpartum interval could also reflect fluid status, with elevated levels serving as a marker for volume overload.
We also found NT-proBNP prior to delivery to be a predictor of left atrial and ventricular volumes in the first 48 h postpartum. The relationship with NT-proBNP but not BNP could reflect the longer half-life of NT-proBNP. BNP has been shown to be a predictor of left ventricular volume following myocardial infarction [14] and further study could assess the potential role of NT-proBNP as a predictor of postpartum left cardiac dysfunction.
While this study supports previous findings of stable BNP levels during pregnancy and similar levels to that of the non-pregnant state [8,15,16], there has been little data on BNP and NT-proBNP in the early postpartum period. Our findings are consistent with those of Yoshimura et al. [17] who found that BNP increased at 5–72 h postpartum with mean levels of 116 pg/mL. BNP and NT-proBNP levels during the early postpartum period may be relevant in the evaluation of peripartum cardiomyopathy. Peripartum cardiomyopathy is a dilated cardiomyopathy with onset from the last month of pregnancy to 5 months postpartum in the absence of other etiologies and is associated with an elevated BNP [18]. Outside of pregnancy a BNP greater than 100 pg/mL is an independent predictor of diastolic dysfunction [19] and may be considered as a diagnostic threshold in the acute evaluation of heart failure [2]. In this study, 11 of 32 patients had BNP levels greater than 100 pg/mL within 48 h postpartum, the highest level reaching 380.6 pg/mL. Therefore, while our results do not dispute the utility of BNP during pregnancy and the postpartum period, we suggest that modestly elevated levels, while perhaps pathologic in other clinical situations, should be interpreted with caution in the immediate postpartum period. Within our cohort, a BNP of 144.3 pg/mL represented the upper 95th percentile within 48 h postpartum, and levels above this value could warrant increased concern for abnormal cardiac function. However, while all subjects had normal clinical outcomes during the study, the elevated postpartum BNPs could also represent sub-clinical disease, a potential area of future research.
Though longitudinal data on echocardiogram findings during pregnancy are limited, our prenatal echocardiogram indices of diastolic function are largely consistent with previous studies [20–23]. However, as with BNP, this study is among the first to report echocardiogram findings in healthy women in the immediate postpartum period. As previously noted, while our findings suggest that increases in left cardiac volumes may be a normal physiologic phenomenon, echocardiographic markers of diastolic dysfunction would not be expected in the absence of cardiac pathology or significant resistance in the aorta.
While previous longitudinal studies generally report data up to 6–12 weeks postpartum [8, 24, 25], this study describes long-term biomarker and echocardiographic changes up to 12 months after delivery. Given the length of follow-up, our findings at 6–12 months postpartum may represent the best available proxy for non-pregnant baseline levels in a longitudinal study. However, it is unclear if cardiac changes during pregnancy ever completely resolve postpartum [25]. We did find BNP and NT-proBNP levels and echocardiogram measurements at 6–12 months postpartum to be statistically similar to first trimester values. While the similarity indicates that maternal cardiac structure and function may return to prepregnancy levels, this study was not powered for this comparison.
A limitation of this study includes missing data at multiple time intervals, though our statistical analysis was selected to account for this. High dropout rates are common in these types of longitudinal studies [26]. In addition, conditions other than myocardial stretch may increase BNP, including cardiac inflammation or infection, sepsis, renal failure, liver cirrhosis, and intracranial disease [5]. Intrauterine growth restriction has also been associated with elevated BNP levels, even in normotensive patients [27], demonstrating the multifactorial nature of BNP secretion. Therefore, factors other than preload may have contributed to our reported BNP and NT-proBNP findings. Finally, our findings are limited to patients without chronic cardiovascular disease. Women with heart disease, even if asymptomatic, have demonstrated significantly higher peak BNP levels during pregnancy than healthy women [16]. Such data indicates that BNP and echocardiographic trends in this population warrant further research.
This study contributes to the knowledge of cardiac structure and function in pregnancy in association with BNP and NT-proBNP. The information highlights the importance of understanding the unique physiology of pregnancy in the obstetric evaluation of cardiac pathology.
Acknowledgments
The research described was supported in part by the NIH grants U54MD007584, G12MD007601, R01HL103931, and P20GM103466. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or The Queen’s Medical Center.
Footnotes
Conflict of interest: Authors state no conflict of interest.
Material and methods: Informed consent: Informed consent has been obtained from all individuals included in this study.
Ethical approval: The research related to human subject use has complied with all the relevant national regulations, and institutional policies, and is in accordance with the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
Contributor Information
Janet M. Burlingame, University of Hawaii John A. Burns School of Medicine, Department of Obstetrics, Gynecology and Women’s Health, Honolulu, HI, USA
Hyeong Jun Ahn, Office of Biostatistics and Quantitative Health Sciences, University of Hawaii, John A Burns School of Medicine, Honolulu, HI, USA.
Todd Seto, The Queen's Medical Center, Center for Outcomes Research and Evaluation, Honolulu, HI, USA.
W. H. Wilson Tang, Department of Cardiovascular Medicine, Cleveland Clinic Foundation, Cleveland, OH, USA.
References
- 1.Kumari M, Tang WH, Maroo AP. Natriuretic peptide testing in high-risk pregnancy: A preventative opportunity? Curr Heart Fail Rep. 2014;11:471–6. doi: 10.1007/s11897-014-0228-2. [DOI] [PubMed] [Google Scholar]
- 2.Tang WHW, Francis GS, Morrow DA, Newby K, Cannon CP, Jesse RL, et al. National academy of clinical biochemistry laboratory medicne practice guidelines: Clinical utilization of cardiac biomarker testing In heart failure. Circulation. 2007;116:99–109. doi: 10.1161/CIRCULATIONAHA.107.185267. [DOI] [PubMed] [Google Scholar]
- 3.Clerico A, Passino C, Franzini M, Emdin M. Cardiac biomarker testing in the clinical laboratory: Where do we stand? General overview of the methodology with special emphasis on natriuretic peptides. Clin Chim Acta. 2015;443:17–24. doi: 10.1016/j.cca.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Suttner S, Boldt J. Natriuretic peptide system: physiology and clinical utility. Curr Opin Crit Care. 2004;10:336–41. doi: 10.1097/01.ccx.0000135513.26376.4f. [DOI] [PubMed] [Google Scholar]
- 5.Tsai SH, Lin YY, Chu SJ, Hsu CW, Cheng SM. interpretation and use of natriuretic peptides in non-congestive heart failure settings. Yonsei Med J. 2010;51:151–63. doi: 10.3349/ymj.2010.51.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfister R, Scholz M, Wielckens K, Erdmann E, Schneider C. Use of NT-proBNP in routine testing and comparison to BNP. Eur J Heart Fail. 2004;6:289–93. doi: 10.1016/j.ejheart.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Resnik J, Hong C, Resnik R, Kazanegra R, Beede J, Bhalla V, et al. Evaluation of B-type natriuretic peptdide (BNP) levels in normal and preeclamptic women. Am J Obstet Gynecol. 2005;193:450–4. doi: 10.1016/j.ajog.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Hameed A, Chan K, Ghamsary M, Elkayam U. Longitudinal changes In the B-natriuretic peptide levels in normal pregnancy and postpartum. Clin Cardiol. 2009;32:E60–2. doi: 10.1002/clc.20391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howlett J, McKelvie R, Costigan J, Ducharme A, Estrella-Holder E, Ezekowitz J, et al. The 2010 Canadian Cardiovascular Society guidelines for the diagnosis and management of heart failure update: Heart failure in ethnic minority populations, heart failure and pregnancy, disease management, and quality improvement/assurance programs. Can J Cardiol. 2010;26:185–202. doi: 10.1016/s0828-282x(10)70367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaid R, Barker C, Little S, Nagueh S. Pre- and post-operative diastolic dysfunction in patients with valvular heart disease: diagnosis and therapeutic implications. J Am Coll Cardiol. 2013;62:1922–30. doi: 10.1016/j.jacc.2013.08.1619. [DOI] [PubMed] [Google Scholar]
- 11.Oh J, Hatle L, Tajik A, Little W. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol. 2006;47:500–6. doi: 10.1016/j.jacc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Monga M, Mastrobattlsta MJ. Maternal cardiovascular, respiratory, and renal adaptation to pregnancy. In: Creasy RK, Resnik JK, Iams JD, editors. Creasy and Resnik’s maternal-fetal medicine: principles and practice. 7. Philadelphia, PA: Elsevier; 2014. pp. 93–9. [Google Scholar]
- 13.Ouzounian JG, Elkayam U. Physiologic changes of normal pregnancy and delivery. Cardiol Clin. 2012;30:317–29. doi: 10.1016/j.ccl.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Weir RA, Balmain S, Steedman T, Ng LL, Squire IB, Rumley A, et al. Tissue plasminogen activator antigen predicts medium-term left ventricular end-systolic volume after acute myocardial infarction. J Thromb Thrombolysis. 2010;29:421–8. doi: 10.1007/s11239-009-0383-6. [DOI] [PubMed] [Google Scholar]
- 15.Yuteri-Kaplan L, Saber S, Zamudio S, Srinivasan D, Nyirenda T, Alvarez M, et al. Brain natriuretic peptide in term pregnancy. Reprod Sci. 2012;19:520–5. doi: 10.1177/1933719111426598. [DOI] [PubMed] [Google Scholar]
- 16.Tanous D, Siu SC, Mason J, Greutmann M, Wald RM, Parker JD, et al. B-type natriuretic peptide in pregnant women with heart disease. J Am Coll Cardiol. 2010;56:1247–53. doi: 10.1016/j.jacc.2010.02.076. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimura T, Yoshimura M, Yasue H, Ito M, Okamura H, Mukoyama M, et al. Plasma concentration of atrial natriuretic peptide and brain natriuretic peptide during normal human pregnancy and the postpartum period. J Endocrinol. 1994;140:393–7. doi: 10.1677/joe.0.1400393. [DOI] [PubMed] [Google Scholar]
- 18.Capriola M. Peripartum cardiomyopathy: a review. Int J Women’s Health. 2013;5:1–8. doi: 10.2147/IJWH.S37137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grewal J, McKelvie R, Lonn E, Tait P, Carlsson J, Gianni M, et al. BNP and NT-proBNP predict echocardiographic severity of diastolic dysfunction. Eur J Heart Fail. 2008;10:252–9. doi: 10.1016/j.ejheart.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Fok W, Chan L, Wong J, Yu C, Lau T. Left ventricular diastolic function during normal pregnancy: assessment by spectral tissue Doppler imaging. Ultrasound Obstet Gynecol. 2006;28:789–93. doi: 10.1002/uog.3849. [DOI] [PubMed] [Google Scholar]
- 21.Mesa A, Jessurun C, Hernandez A, Adam K, Brown D, Vaughn W, et al. Left ventricular diastolic function in normal human pregnancy. Circulation. 1999;99:511–7. doi: 10.1161/01.cir.99.4.511. [DOI] [PubMed] [Google Scholar]
- 22.Bamfo J, Kametas N, Nicolaides K, Chambers J. Maternal left ventricular diastolic and systolic long-axis function during normal pregnancy. Eu J Echocardiogr. 2007;8:360–8. doi: 10.1016/j.euje.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Araoye M, Rubler S, Holford F. Isovoiumic relaxation time in normal subjects and patients with cardiac disease: comparison of determination made with echocardiographic techniques and apex cardiography. Angiology. 1978;29:7–15. doi: 10.1177/000331977802900102. [DOI] [PubMed] [Google Scholar]
- 24.Easterling T, Benedetti T, Schmucker B, Carlson K, Millard S. Maternal hemodynamics and aortic diameter in normal and hypertensive pregnancies. Obstet Gynecol. 1991;78:1073–7. [PubMed] [Google Scholar]
- 25.Capeless E, Clapp J. When do cardiovascular parameters return to their preconception values? Am J Obstet Gynecol. 1991;165:883–6. doi: 10.1016/0002-9378(91)90432-q. [DOI] [PubMed] [Google Scholar]
- 26.Naqvi T, Elkayam U. Serial echocardiographic assessment of the human heart in normal pregnancy. Circ Cardiovasc Imaging. 2012;5:283–5. doi: 10.1161/CIRCIMAGING.112.974808. [DOI] [PubMed] [Google Scholar]
- 27.Garofoli F, Mannarino S, Montanari L, Cerbo R, Tzialla C, Mazzucchelli I, et al. Variation of B-type natriuretic peptide concentrations and intrauterine growth restriction: mother, fetus, and newborn. J Biol Regul Homeost Agents. 2012;26:733–9. [PubMed] [Google Scholar]