Abstract
Influenza A virus (IAV) causes an acute infection in humans that is normally eliminated by CD8+ cytotoxic T lymphocytes. Individuals expressing the MHC class I molecule HLA-A2 produce cytotoxic T lymphocytes bearing T-cell receptors (TCRs) that recognize the immunodominant IAV epitope GILGFVFTL (GIL). Most GIL-specific TCRs utilize α/β chain pairs encoded by the TRAV27/TRBV19 gene combination to recognize this relatively featureless peptide epitope (canonical TCRs). However, ∼40% of GIL-specific TCRs express a wide variety of other TRAV/TRBV combinations (non-canonical TCRs). To investigate the structural underpinnings of this remarkable diversity, we determined the crystal structure of a non-canonical GIL-specific TCR (F50) expressing the TRAV13-1/TRBV27 gene combination bound to GIL–HLA-A2 to 1.7 Å resolution. Comparison of the F50–GIL–HLA-A2 complex with the previously published complex formed by a canonical TCR (JM22) revealed that F50 and JM22 engage GIL–HLA-A2 in markedly different orientations. These orientations are distinguished by crossing angles of TCR to peptide–MHC of 29° for F50 versus 69° for JM22 and by a focus by F50 on the C terminus rather than the center of the MHC α1 helix for JM22. In addition, F50, unlike JM22, uses a tryptophan instead of an arginine to fill a critical notch between GIL and the HLA-A2 α2 helix. The F50–GIL–HLA-A2 complex shows that there are multiple structurally distinct solutions to recognizing an identical peptide–MHC ligand with sufficient affinity to elicit a broad anti-IAV response that protects against viral escape and T-cell clonal loss.
Keywords: crystal structure, influenza virus, major histocompatibility complex (MHC), protein complex, surface plasmon resonance (SPR), T-cell receptor, epitope
Introduction
CD8+ T cells play a critical role in the immune response to viruses by recognizing and eliminating infected cells (1). Recognition is mediated by αβ T-cell receptors (TCRs),2 which bind viral peptides presented by major histocompatibility complex (MHC) class I molecules on infected cells. TCRs engage peptide–MHC (pMHC) through their six complementarity-determining region (CDR) loops, three from the variable α (Vα) domain, and three from Vβ. The first and second CDRs (CDR1 and CDR2) are encoded within the Vα and Vβ gene segments. CDR3 is formed by DNA recombination involving juxtaposition of Vα and Jα segments for the α chain genes and of Vβ, D, and Jβ segments for the β chain genes. Estimates of TCR diversity in humans have placed the number of unique structures in the range of 105–108 (2–5).
The human CD8+ T-cell response to influenza A virus (IAV) has been studied extensively (6–11). The dominant epitope in individuals expressing the MHC class I molecule HLA-A*0201 (HLA-A2) corresponds to residues 58–66 of matrix protein M1 (GILGFVFTL; referred to as GIL) (12). Initial studies of GIL-specific CD8+ T-cell responses in HLA-A2+ subjects indicated that the Vβ repertoire is highly biased toward usage of the TRBV19 gene (up to 98%) with a highly conserved CDR3β motif, 97XRSX100 (6–9). The Vα gene segment, although not as strongly selected as Vβ, showed a strong preference for TRAV27 (up to 80%) (6, 7, 13). This canonical TRAV27/TRBV19 gene combination is observed in multiple HLA-A2-matched but otherwise genetically unrelated individuals. More recently, however, high-throughput sequencing and single-cell TCR analysis have revealed substantially greater repertoire diversity than previously realized, with only ∼60% of GIL-specific TCRs expressing the TRBV19 gene and ∼20% expressing TRAV27 (14). These non-canonical TCRs utilize a wide variety of TRAV and TRBV genes, including TRAV13-1, TRAV17, TRAV29, TRAV38-2, TRBV14, TRBV24-1, TRBV27, and TRBV29-1, among others. Indeed, 2,406 unique TCRα and 2,437 TCRβ sequences have been identified for TCRs recognizing the GIL peptide presented by HLA-A2 (14). These sequences include 461 Vα-Jα and 359 Vβ-Jβ combinations, as well as dozens of distinct CDR3α and CDR3β consensus motifs. Broad TCR repertoire diversity has also been documented for other defined viral antigens, including ones from cytomegalovirus, Epstein–Barr virus, and HIV (14–17). Such diversity ensures robust T-cell responses to single viral epitopes that would not be possible if a single epitope could only elicit a few TCR clonotypes (18). Moreover, TCR diversity provides protection against viral escape (19–21).
In most pMHC structures, one or more residues in the central portion of the antigenic peptide (P4–P6) feature solvent-exposed side chains that facilitate TCR binding (22). However, the GIL peptide is unusual in that only the side chain of P8 Thr, at the C-terminal end of the peptide, is substantially exposed to solvent, making GIL a challenging target for TCR recognition (23). The crystal structure of a canonical GIL-specific TRAV27/TRBV19 TCR (JM22) bound to GIL–HLA-A2 revealed what appears to be the most efficient solution to recognizing the featureless GIL peptide with sufficient affinity to permit selection of engaging T cells. In the JM22–GIL–HLA-A2 complex, the side chain of the conserved βArg98 residue of the 97XRSX100 CDR3β consensus motif fills a notch between the peptide and the HLA-A2 α2 helix (23). This structural solution is also adopted by other GIL-specific TCRs expressing TRBV19 and the 97XRSX100 CDR3β motif, even when TRBV19 is paired with α chains different from TRAV27 (14, 24). However, ∼40% of GIL-specific TCRs do not use the canonical TRBV19 gene, and not all TRBV19-expressing TCRs contain the 97XRSX100 CDR3β motif (14, 24). Hence, multiple solutions exist for binding the featureless GIL peptide. Here we investigated the structural basis for the surprising diversity of the TCR response to this dominant IAV epitope by determining the structure of a GIL-specific TCR (F50) expressing a completely different, non-canonical TRAV/TRBV gene combination (TRAV13-1/TRBV27) in complex with GIL–HLA-A2.
Results
Interaction of TCR F50 with GIL–HLA-A2
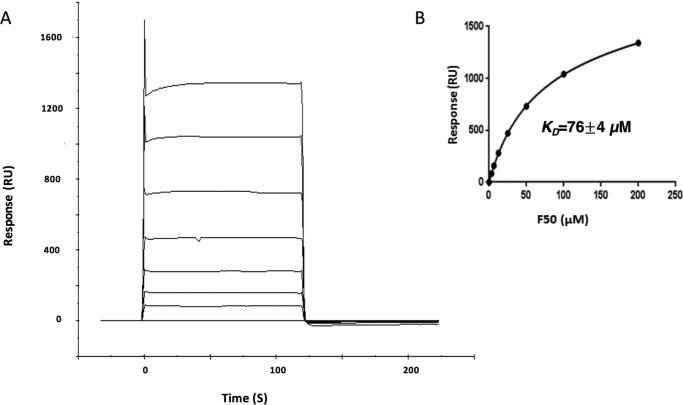
The IAV GIL-specific TCR F50 was isolated from CD8+ T cells from the peripheral blood of an HLA-A2+ healthy male donor following in vitro stimulation with the GIL peptide as described under “Experimental procedures.” F50 utilizes gene segments TRAV13-1 and TRAJ54 for the α chain, and TRBV27 and TRBJ1-1 for the β chain, whereas JM22 utilizes TRAV27 and TRAJ37 for the α chain and TRBV19 and TRBJ7–2 for the β chain. We used surface plasmon resonance (SPR) to measure the affinity of TCR F50 for HLA-A2 loaded with the GIL peptide (Fig. 1A). To characterize the interaction of F50 with GIL–HLA-A2, we expressed these proteins by in vitro folding from inclusion bodies produced in Escherichia coli. Biotinylated GIL–HLA-A2 was directionally coupled to a streptavidin-coated biosensor surface, and different concentrations of F50 were sequentially flowed over the immobilized pMHC ligand. A dissociation constant (KD) of 76 ± 4 μm was obtained by fitting equilibrium data to a 1:1 binding model (Fig. 1B). This affinity is ∼25-fold weaker than that of JM22 for GIL–HLA-A2 (KD = 3.2 μm) (25), with the caveat that we did not independently measure the affinity of JM22 in our experimental system. It is, however, well within the range of 0.5–500 μm for natural TCR-pMHC interactions (26). Moreover, it is consistent with the much lower representation among GIL-specific TCRs of the TRBV27 β chain expressed by F50 than the dominant TRBV19 β chain expressed by JM22 (14, 24), in agreement with the concept of affinity-driven clonal expansion of T cell repertoires.
Figure 1.
Surface plasmon resonance analysis of the binding of TCR F50 to GIL–HLA-A2. A, TCR F50 at concentrations of 0, 2.5, 5, 9.5, 19, 37.5, 75, and 150 μm was injected over immobilized GIL–HLA-A2 (1000 RU). B, increased RU relative to the control channel are plotted against the TCR F50 concentration. The KD (± S.D.) from fitting to a single-site equilibrium binding equation is 76 ± 4 μm for two independent experiments.
Overview of the F50–GIL–HLA-A2 complex
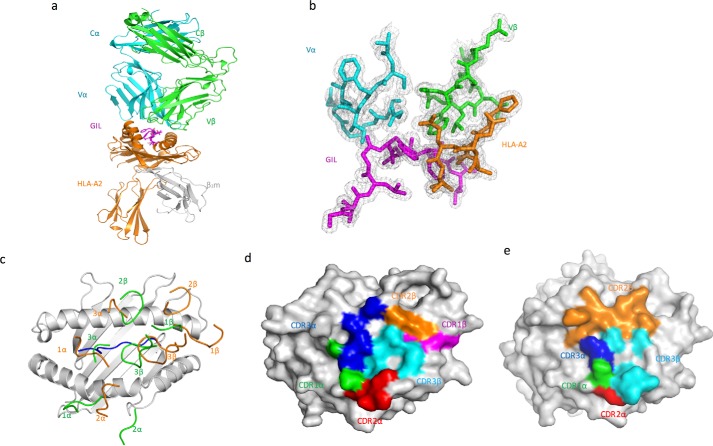
To understand how TCR F50 recognizes GIL–HLA-A2 and to compare its recognition mode with that of JM22, we determined the structure of the F50–GIL–HLA-A2 complex to 1.7 Å resolution (Table 1 and Fig. 2a). The interface between F50 and GIL–HLA-A2 was in unambiguous electron density for the single complex molecule in the asymmetric unit of the crystal (Fig. 2b). Of note, the resolution of the F50–GIL–HLA-A2 complex is one of the highest reported for any TCR-pMHC class I or II complex (>60 unique structures), whose resolutions seldom exceed 2.5 Å (22). Indeed, the 1.7 Å resolution of the F50–GIL–HLA-A2 complex is second only to that of the JM22–GIL–HLA-A2 complex itself at 1.4 Å (23). The comparably high resolutions of these two structures justify detailed comparisons between them.
Table 1.
Data collection and structure refinement statistics
| F50–GIL–HLA-A2 | |
|---|---|
| Data collection | |
| Space group | P1211 |
| Cell dimensions | |
| a (Å) | 66.3 |
| b (Å) | 71.1 |
| c (Å) | 100.7 |
| α, β, γ (°) | 90, 96.2, 90 |
| Resolution range (Å)a | 37.9–1.70 (1.76–1.70) |
| Unique reflectionsa | 100,320 (9,832) |
| Rmergea,b | 0.055 (0.647) |
| Mean I/σ(I) a | 7.52 (1.2) |
| Completeness (%)a | 98.1 (96.6) |
| Refinement | |
| Resolution range (Å)a | 37.9–1.70 |
| Rwork (%)/Rfree (%)a,c | 19.1 (28.9)/21.3 (28.0) |
| No. of protein atoms | 6,662 |
| No. of water molecules | 808 |
| Root mean square deviation from ideality | |
| Bond lengths (Å) | 0.013 |
| Bond angles (°) | 1.18 |
| Ramachandran statistics (%) | |
| Most favored | 98.0 |
| Allowed | 2.0 |
| Disallowed | 0.0 |
a The values in parentheses are statistics for the highest resolution shell.
b Rmerge = Σ|Ij − <I> |/ΣIj, where Ij is the intensity of an individual reflection, and <I> is the average intensity of that reflection.
c Rwork = Σ||Fo| − |Fc||/Σ|Fo|, where Fc is the calculated structure factor. Rfree is as for Rwork but calculated for a randomly selected 5.0% of reflections not included in the refinement.
Figure 2.
Structure of the TCR F50–GIL–HLA-A2 complex. a, side view of the F50–GIL–HLA-A2 complex (ribbon diagram). Cyan, TCR α chain; green, TCR β chain; orange, HLA-A2 heavy chain; gray, β2 microglobulin (βs2m); magenta, GIL peptide. b, electron density in the interface of the F50–GIL–HLA-A2 complex. Density from the final 2Fo − Fc map at 1.7 Å resolution is contoured at 1σ. c, positions of CDR loops of TCRs F50 and JM22 (Protein Data Bank accession code 1OGA) (23) on GIL–HLA-A2. CDRs of F50 are orange. CDRs of JM22 are green. GIL peptide is blue. d, footprint of TCR F50 on GIL–HLA-A2. The top of the MHC molecule is depicted as a gray surface. The areas contacted by individual CDR loops are color-coded: green, CDR1α; red, CDR2α; blue, CDR3α; magenta, CDR1β; orange, CDR2β; cyan, CDR3β. e, footprint of TCR JM22 on GIL–HLA-A2.
F50 docks symmetrically over GIL–HLA-A2 in a diagonal orientation, with a crossing angle of TCR to pMHC (27) of 29° compared with 69° for JM22 (Fig. 2c). Upon binding GIL–HLA-A2, F50 buries 73% (215 Å2) of the peptide solvent-accessible surface. This percentage is significantly less than the 85% (258 Å2) of peptide surface buried by JM22 (23), which may contribute to the lower affinity of F50 compared with JM22. As shown by the footprint of F50 on the pMHC surface (Fig. 2d), F50 establishes contacts with the N-terminal half of the GIL peptide mainly via the CDR1α and CDR3α loops, whereas the CDR1β and CDR3β loops mostly contact the C-terminal half. F50 utilizes all six CDR loops to interact with the MHC molecule, with CDR1α and CDR2α positioned over the HLA-A2 α2 helix, and CDR1β and CDR2β positioned over the HLA-A2 α1 helix (Fig. 2c).
Interaction of TCR F50 with HLA-A2
The F50–GIL–HLA-A2 complex buries a total solvent-accessible surface of 1760 Å2, comparable with that in other TCR-pMHC complexes (22). The Vα and Vβ domains bury 46% (333 Å2) and 54% (391 Å2) of HLA-A2 surface area, respectively. This roughly equal contribution of Vα and Vβ is typical of TCR-pMHC complexes (22). In sharp contrast to the F50–GIL–HLA-A2 complex, MHC recognition in the JM22–GIL–HLA-A2 complex is dominated by Vβ, which accounts for 67% of the buried surface on HLA-A2 (23). Thus, F50 and JM22 use different strategies to engage an identical pMHC ligand.
Overall, Vα of F50 makes 27 van der Waals contacts and three hydrogen bonds with HLA-A2, compared with 25 van der Waals contacts and two hydrogen bonds by Vβ. These interactions are mediated by six Vα and six Vβ residues and involve twelve MHC residues, eight of which are also contacted by JM22 (Table 2). Of the total buried surface on HLA-A2, excluding the GIL peptide, CDR1α, CDR2α, and CDR3α contribute 6, 22, and 19%, respectively, compared with 9, 20, and 28% for CDR1β, CDR2β, and CDR3β, respectively. Thus, MHC recognition by F50 involves both germline-encoded and somatically generated interactions. The contributions of CDR2α and CDR2β to the MHC buried surface in the F50–GIL–HLA-A2 complex (22 and 20%, respectively) are substantially greater than the average for 34 other TCR-pMHC class I complexes (11 and 12%, respectively) (22). In particular, CDR2α of the canonical JM22 TCR accounts for only 7% of the buried surface on HLA-A2. Unlike JM22, F50 does not recruit water molecules to the interface with HLA-A2 (see below).
Table 2.
Interactions between TCR and MHC in the F50–GIL–HLA-A2 and JM22–GIL–HLA-A2 complexes
| Hydrogen bonds | van der Waals contacts | Hydrogen bonds | van der Waals contacts | |
|---|---|---|---|---|
| HLA-A2 | F50 | F50 | JM22 | JM22 |
| R65H | Q58β | |||
| K66H | N98α | |||
| K68H | D56β | |||
| A69H | I96β | D56β | ||
| G99β | ||||
| Q72H | M50β | I53β | ||
| G99β | N55β | |||
| T73H | I53β | |||
| R75H | N55β | |||
| V76H | M50β | I53β | ||
| T80H | A80H (Oγ1) N51β (Nδ2) | N51β | ||
| Y84H | Y84H(OH) E30β(Oϵ2) | E30β | ||
| K146H | L95β | |||
| A149H | Y101β | |||
| A150H | R52α | A150H(O) R98β(Nϵ) | R98β | |
| L96β | A150H(O) R98β(Nη2) | Y101β | ||
| W99β | ||||
| H151H | R52α | V51α | ||
| R98β | ||||
| Y101β | ||||
| V152H | W99β | R98β | ||
| E154H | E154H (Oϵ2) N54α (Nδ2) | N54α | S31α | |
| V51α | ||||
| Q155H | Q155H (Nϵ2) N32α (Nδ2) | N32α | Q155H (Oϵ1) R98β (Nη1) | S31α |
| Q155H (Oϵ1) S53α (Oγ) | R52α | Q155H (Oϵ1) R98β (Nη2) | G94α | |
| S53α | R98β | |||
| N54α | S100β | |||
| W99β |
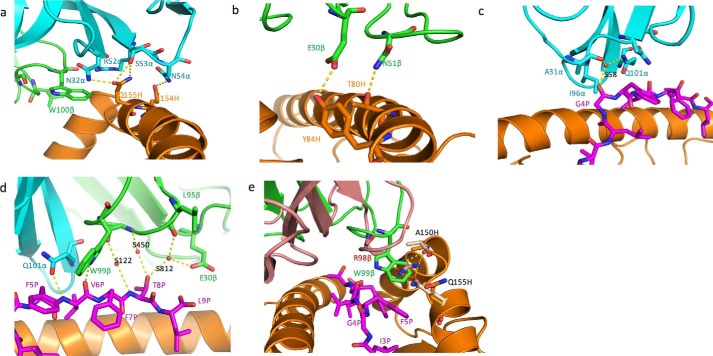
F50 engages the HLA-A2 α2 helix mainly through CDR1α and CDR2α. Thus, residues αAsn32, αSer53, and αAsn54 form a network of three side-chain–side-chain hydrogen bonds linking F50 to residues Glu154H and Gln155H of the HLA-A2 α2 helix (Table 2 and Fig. 3a). These hydrogen bonds are reinforced by 16 germline-encoded van der Waals contacts with CDR1α and CDR2α that further anchor Vα to helix α2. Because of the acute crossing angle of F50 to GIL–HLA-A2 (29° compared with 69° for JM22), CDR3α of F50 contacts the HLA-A2 α1 helix instead of the α2 helix contacted by CDR3α of JM22 (Table 2) (23). Indeed, interactions between CDR3α and the MHC α1 helix are characteristic of TCRs docking on pMHC ligands with acute crossing angles (22).
Figure 3.
Interactions of TCR F50 with HLA-A2 and the GIL peptide. a, interactions between CDR2α (cyan) of F50 and the HLA-A2 α2 helix (orange). The side chains of contacting residues are drawn in stick representation with carbon atoms in cyan (CDR2α) or orange (HLA-A2), nitrogen atoms in blue, and oxygen atoms in red. Hydrogen bonds are indicated by yellow dashed lines. b, interactions of CDR1β and CDR2β (green) with the HLA-A2 α1 helix (orange). c, interactions of CDR1α and CDR3α with the GIL peptide. A bridging water molecule is depicted as a red sphere. d, interactions of CDR1β and CDR3β with the GIL peptide, including bridging water molecules (red spheres). e, interactions between CDR3β (green) of F50 and GIL–HLA-A2. The side chain of βTrp99 occupies a notch between the GIL peptide (magenta) and the HLA-A2 α2 helix (orange). Superposed onto CDR3β of F50 is CDR3β of JM22 (pink). In the JM22–GIL–HLA-A2 complex (23), the pocket between GIL and the HLA-A2 α2 helix is filled by the side chain of βArg98, which makes hydrogen bonds with HLA-A2 Ala150H and Gln155H (beige).
In addition to CDR3α, F50 engages the HLA-A2 α1 helix through CDR1β, CDR2β, and CDR3β (Table 2). Notably, these interactions focus on the C terminus of the α1 helix, as illustrated by the positions of CDR1β and CDR2β on the pMHC surface (Fig. 2, c and d). This C-terminal site is rarely targeted by other TCRs, including JM22 (Fig. 2, c and e), which typically dock more toward the center of the MHC α1 helix (22). In particular, F50 residues βGlu30 and βAsn51 make two side-chain–side-chain hydrogen bonds with C-terminal HLA-A2 α1 residues Tyr84H and Thr80H, respectively (Fig. 3b). Neither of these HLA-A2 residues contacts JM22, which instead targets the central portion of helix α1 via its CDR2β (Fig. 2c and Table 2). Interestingly, the HLA-A24-restricted HIV-specific TCR T36-5 (28), which utilizes the same Vβ (TRBV27) as F50, nevertheless engages the MHC α1 helix via a different set of germline-encoded interactions. For example, whereas βAsn51 hydrogen bonds with Thr80H in the F50–GIL–HLA-A2 complex, this same residue hydrogen bonds with Ala69H and Thr73H in the T36-5–HIV–HLA-A24 complex, in agreement with the idea of flexibility in evolutionarily selected contacts between TCR and MHC (29, 30).
Peptide recognition by TCR F50
Except for a few contacts made by CDR1α and CDR1β (5 of 36), all interactions between F50 and the GIL peptide are mediated by the somatically generated CDR3 loops, with CDR3α and CDR3β accounting for 18 and 13 contacts, respectively. Peptide specificity is conferred mainly by shape complementarity, because the F50-GIL interface features only two hydrogen bonds: F50 αGln101 Oϵ1-N P6 Val and F50 βTrp99 Nϵ1-O P6 Val (Table 3 and Fig. 3, c and d). Of note, JM22 also makes two hydrogen bonds with the main chain of P6 Val, but using different CDR residues: JM22 βGln52 Oϵ1-N P6 Val and JM22 βSer99 Oγ–O P6 Val. F50 engages the central and C-terminal portions of the GIL peptide (P4 Gly, P5 Phe, P6 Val, P8 Thr, and P9 Leu) with the principal focus on P5 Phe and P6 Val, whereas N-terminal residues P1–P3 do not contact TCR.
Table 3.
Interactions between TCR and GIL peptide in the F50–GIL–HLA-A2 and JM22–GIL–HLA-A2 complexes
| Hydrogen bonds | van der Waals contacts | Hydrogen bonds | van der Waals contacts | |
|---|---|---|---|---|
| GIL | F50 | F50 | JM22 | JM22 |
| G4P | A30α | G4P(O) Q52β (Nϵ2) | S95α | |
| I96α | Q96α | |||
| Q52β | ||||
| F5P | N32α | S95α | ||
| I96α | G97α | |||
| Q101α | Q52β | |||
| W99β | R98β | |||
| S100β | ||||
| V6P | V6P(N) Q101α(Oϵ1) | Q101α | V6P(N) Q52β(Oϵ1) | Q52β |
| V6P(O) W99β(Nϵ1) | W99β | V6P(O) S99β(Oγ) | S99β | |
| T8P | L96β | T8P (Oγ1) D32β (Oδ2) | D32β | |
| I53β | ||||
| L9P | E30β |
In most unliganded pMHC structures, one or more residues in the central portion of the antigenic peptide (P4–P6) feature protruding side chains that facilitate TCR binding (22). However, the GIL peptide presented by HLA-A2 is atypical in that only the side chain of P8 Thr, at the C-terminal end of the peptide, is substantially exposed to solvent. In the JM22–GIL–HLA-A2 complex (23), the guanidinium moiety of CDR3β Arg98, which is conserved in most GIL-specific TCRs expressing the canonical TRBV19 gene segment (6–9, 14, 24), inserts into a notch between the peptide and the HLA-A2 α2 helix. In the F50–GIL–HLA-A2 complex, by contrast, this same notch is occupied by the aromatic side chain of CDR3β Trp99, which makes 10 hydrophobic contacts and one hydrogen bond (F50 βTrp99 Nϵ1-O P6 Val) with the GIL peptide (Table 3 and Fig. 3e). These interactions are reinforced by eight contacts with HLA-A2 α2 residues Ala150H, Val152H, and Gln155H, further anchoring the βTrp99 side chain in the mainly hydrophobic notch. These same MHC residues are also contacted by βArg98 of JM22, indicating a certain degree of structural mimicry. However, F50 βTrp99, unlike JM22 βArg98β, cannot form hydrogen bonds with HLA-A2 Ala150H or Gln155H (Table 3). Indeed, mutating βTrp99 to arginine completely abolished binding of F50 to GIL–HLA-A2, as determined by SPR (not shown), demonstrating that these residues are not functionally interchangeable.
The side chain of HLA-A2 Gln155H adopts different rotamer conformations in the F50–GIL–HLA-A2 and JM22–GIL–HLA-A2 structures (Fig. 3e). In the JM22–GIL–HLA-A2 complex, the Gln155H side chain shifts by 3.6 Å compared with its position in unbound GIL–HLA-A2 to open the notch between the peptide and the α2 helix, into which βArg98 docks (23). In the F50–GIL–HLA-A2 complex, the gatekeeper Gln155H side chain shifts an additional 1.8 Å from its unbound position to further open this notch to avoid steric clashes with the bulky βTrp99 side chain and optimize shape complementarity and hydrogen bonding with pMHC (Fig. 3e).
Comparison with GIL-specific TCRs LS01 and LS10
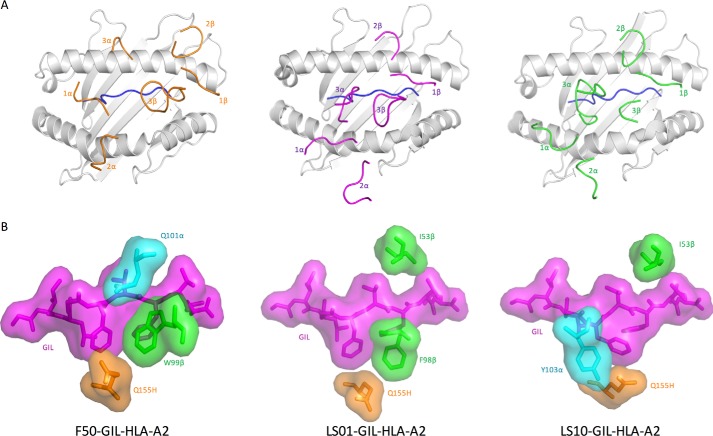
A recent crystallographic study of two GIL-specific TCRs (LS01 and LS10) expressing the canonical TRBV19 gene, but different TRAV genes showed that both TCRs maintain the same overall docking orientation on pMHC as JM22, because of the preservation of specific contacts between the conserved CDR1β and CDR2β loops and HLA-A2 (Fig. 4A) (24). This orientation is distinct from that found in the non-canonical F50–GIL–HLA-A2 complex, which features a much more acute crossing angle, as described above.
Figure 4.
Different structural solutions to binding the featureless GIL peptide. A, positions of CDR loops of TCRs F50, LS01 (Protein Data Bank accession code 5ISZ), and LS10 (Protein Data Bank accession code 5JHD) (24) on GIL–HLA-A2. CDRs of F50 are orange. CDRs of LS01 are magenta. CDRs of LS10 are green. GIL peptide is blue. LS01 and LS10 dock similarly on pMHC and differently from F50. B, top views of the F50–GIL–HLA-A2, LS01–GIL–HLA-A2, and LS10–GIL–HLA-A2 complexes in the region of the pocket between GIL (magenta) and HLA-A2 Gln155H (orange). Structures are displayed as stick/surface representations.
The CDR3β motifs of LS01 (97XFX99) and LS10 (97XGXY100) differ from the dominant 97XRSX100 motif of other GIL-specific TCRs expressing TRBV19 (24). Consequently, LS01, LS10, JM22, and F50 employ different strategies to recognize the critical notch between GIL and the MHC α2 helix near P5 Phe (Fig. 4B). Whereas JM22 and F50 use Arg98 and Trp99 of CDR3β, respectively, to fill this common pocket, LS01 uses Phe98. By contrast, binding of LS10 induces a conformational change in GIL, whereby the P5 Phe side chain moves into the notch. This shift creates a new notch that is occupied by αAla98 and αGly99 and covered by αTyr103.
Water interactions at TCR-pMHC interfaces
Bound water molecules have been localized in the interfaces of many antigen–antibody (and other protein–protein) complexes, where they act as molecular adaptors to bridge the protein partners and improve the fit between them (31). However, the contribution of bound waters to mediating TCR-pMHC interactions has not been examined in detail, in large measure because relatively few TCR-pMHC crystal structures have been determined at sufficiently high resolution (≤2.5 Å) to permit the identification of ordered waters with a reasonable degree of accuracy.
The high resolution of the F50–GIL–HLA-A2 complex allowed the inclusion of many bound water molecules, including ones in the interface between TCR and pMHC. In particular, four waters are mostly or completely buried in the interface, where they mediate hydrogen bonding interactions between F50 and the GIL peptide (Table 4 and Fig. 3d). No buried waters were found between F50 and HLA-A2. By contrast, the JM22–GIL–HLA-A2 structure includes bridging waters both between JM22 and GIL and between JM22 and HLA-A2 (23). The shape correlation statistic (Sc) (32) for the F50–GIL–HLA-A2 complex with interfacial waters is 0.65 (Sc = 1.0 for interfaces with perfect geometrical fits), which is the same as the Sc without such waters (Table 5). Thus, bound water molecules do not contribute appreciably to the overall interfacial shape complementarity of this particular TCR-pMHC complex, although they do fill a gap between F50 and GIL. In marked contrast, the corresponding Sc values for the JM22–GIL–HLA-A2 complex (23) are 0.77 with interfacial waters versus 0.64 without waters (ΔSc = 0.13), indicating a substantial contribution to improving the overall fit. Similarly, for the F6–GIL–HLA-A2 complex (14), in which F6 uses the same β chain as JM22 but a different α chain, the Sc values are 0.61 and 0.54 with and without interfacial waters, respectively.
Table 4.
Water bridges between TCR and GIL peptide in the F50–GIL–HLA-A2 and JM22–GIL–HLA-A2 complexes
| GIL | Water | F50 | B factor | Water | JM22 | B factor |
|---|---|---|---|---|---|---|
| G4(O) | S58 | A31α(O) | 22.3 | S1 | Q52β(O) | 17.2 |
| Q101α (Nϵ2) | S99β(N) | |||||
| T8(N) | S122 | G97β(O) | 23.9 | S6 | R98β (Nη1) | 18.4 |
| S99β (Oγ) | ||||||
| T8(Oγ1) | S450 | L95β(O) | 30.1 | S10 | Q58β (Nϵ2) | 20.5 |
| T8(Oγ1) | S812 | G97β(N) | 35.9 | S14 | S95α(O) | 19.0 |
| S19 | D32β (Oδ1) | 18.7 |
Table 5.
Shape complementarity (Sc) of TCR–pMHC class I complexes in the presence and absence of interfacial water molecules
| TCR–pMHC class I complexa | PDB | Sc, with waters | Sc, without waters | ΔSc | Number of interfacial watersb | Reference |
|---|---|---|---|---|---|---|
| F50–GIL–HLA-A2 | 5TEZ | 0.65 | 0.65 | 0.00 | 4 (0 MHC; 4 peptide) | This paper |
| JM22–GIL–HLA-A2 | 1OGA | 0.77 | 0.64 | 0.13 | 10 (5 MHC; 5 peptide) | Ref. 23 |
| F6–GIL–HLA-A2 | 5EUO | 0.61 | 0.54 | 0.07 | 8 (4 MHC; 4 peptide) | Ref. 14 |
| LS01–GIL–HLA-A2 | 5ISZ | 0.74 | 0.71 | 0.03 | 11 (6 MHC; 5 peptide) | Ref. 24 |
| LS10–GIL–HLA-A2 | 5JHD | 0.68 | 0.66 | 0.02 | 2 (2 MHC; 2 peptide) | Ref. 24 |
| B7–Tax–HLA-A2 | 1BD2 | 0.64 | 0.64 | 0.00 | 3 (3 MHC; 0 peptide) | Ref. 35 |
| AHIII–p1049–HLA-A2 | 1LP9 | 0.74 | 0.71 | 0.03 | 8 (6 MHC; 3 peptide) | Ref. 49 |
| LC13–EBV–HLA-A8 | 1MI5 | 0.67 | 0.61 | 0.06 | 6 (4 MHC; 3 peptide) | Ref. 50 |
| 1G4–ESO 9V–HLA-A2 | 2BNQ | 0.78 | 0.75 | 0.03 | 9 (5 MHC; 4 peptide) | Ref. 51 |
| 1G4–ESO 9C–HLA-A2 | 2BNR | 0.75 | 0.75 | 0.00 | 7 (5 MHC; 2 peptide) | Ref. 51 |
| 2C–QL9–H-2Ld | 2E7L | 0.67 | 0.64 | 0.03 | 2 (1 MHC; 1 peptide) | Ref. 52 |
| AGA1–KF11–HLA-B57 | 2YPL | 0.77 | 0.81 | −0.04 | 3 (3 MHC; 0 peptide) | Ref. 48 |
| H27–14–Nef–HLA-A24 | 3VXR | 0.76 | 0.75 | 0.01 | 7 (5 MHC; 3 peptide) | Ref. 28 |
| C25–NLV–HLA-A2 | 5D2N | 0.70 | 0.70 | 0.00 | 8 (6 MHC; 2 peptide) | Ref. 34 |
a Only TCR–pMHC class I structures of at 2.5 Å resolution or better were considered in this analysis.
b The first value is the total number of water molecules in the corresponding TCR–pMHC interface. In parentheses are the numbers of waters bridging TCR and MHC or TCR and peptide in each complex. These numbers may in some cases add up to more than the total number of interfacial waters because a single water molecule can sometimes bridge TCR to both MHC and peptide.
An analysis of 11 other TCR-pMHC structures of ≤2.5 Å resolution revealed little or no effect of interfacial waters on overall shape complementarity (ΔSc < 0.05), except in one case (LC13–EBV–HLA-A8) (ΔSc = 0.06) (Table 5). Nevertheless, these waters help correct imperfections in the interface by filling small cavities or channels between TCR and pMHC. More importantly, they contribute to complex stability by forming bridging hydrogen bonds to enhance polar interactions and neutralize unpaired hydrogen-bonding groups, as observed in other protein-protein complexes (33). Indeed, most TCR-pMHC complexes contain comparable numbers of water-mediated and direct hydrogen bonds (e.g. 5 versus 7 for F50–GIL–HLA-A2, 14 versus 8 for JM22–GIL–HLA-A2 (23), and 11 versus 11 for F6–GIL–HLA-A2 (14)). For the 14 TCR-pMHC structures analyzed here (Table 5), the number of interfacial waters ranged from as few as 2 to as many as 11, with an average of 6/complex, in line with antigen-antibody complexes (31).
Discussion
The application of high-throughput sequencing and single-cell TCR analysis to interrogate CD8+ T cell repertoires to single viral epitopes has revealed far greater TCR sequence diversity than previously appreciated for immune responses to IAV, cytomegalovirus, HIV, and other viruses (14, 15, 17, 24). Given this diversity, understanding the structural basis for recognition of an identical pMHC complex by thousands of different TCRs represents a considerable challenge.
To date, ∼25 structures of TCRs with different Vα and/or Vβ gene usage bound to an identical (or nearly identical) pMHC ligand have been reported, involving both MHC class I and class II molecules (14, 22, 24, 34). These complexes may be divided into three categories: 1) those in which the TCRs use the same Vα gene segment but different Vβs (30), 2) those in which TCRs use the same Vβ but different Vαs (14, 24, 35–38), and 3) those in which the TCRs use different Vαs and Vβs (34, 39). The third category, which includes TCR F50, has considerably fewer examples than the first two categories, where the TCRs use the same Vα or Vβ region to engage the same pMHC. Nevertheless, some general conclusions may be drawn regarding Vα/Vβ gene usage and TCR docking orientation. Thus, TCRs that use the same Vβ but different Vαs typically bind pMHC in the same overall orientation because of the preservation of most (but not necessarily all) germline-encoded interactions between the conserved CDR1β and CDR2β loops and the MHC α-helices. That is, use of a different Vα has not been observed to reposition Vβ on pMHC in an appreciably different way, although small adjustments do occur. Conversely, TCRs that use the same Vα but different Vβs engage pMHC in very similar orientations because most germline-encoded interactions between CDR1α and CDR2α and the MHC ligand are maintained, irrespective of the Vβ partner.
By contrast, TCRs using different Vα and Vβ regions can employ very different strategies to bind an identical pMHC, as seen here and in previous studies (34, 39). For example, a comparison of two TCRs expressing unrelated Vα/Vβ gene combinations in complex with a bulged peptide from Epstein–Barr virus presented by HLA-B8 revealed two distinct binding modes: one in which the TCR straddles the bulged peptide but makes few contacts with MHC and one in which the TCR is positioned toward the N-terminal end of the peptide binding groove of HLA-B8, thereby largely bypassing the bulged peptide (39). In another case, human cytomegalovirus-specific TCRs C7 (TRAV24/TRBV7-2) and C25 (TRAV26-2/TRBV7-6) were found to dock over pMHC with crossing angles of 29° and 61°, respectively, with C7-HLA-A2 interactions dominated by Vα and C25-HLA-A2 interactions dominated by Vβ (34). Here we have shown that F50 (TRAV13-1/TRBV27) engages GIL–HLA-A2 in a decidedly different orientation than do canonical TCRs JM22 (TRAV27/TRBV19), F6 (TRAV27/TRBV19), LS01 (TRAV24/TRBV19), and LS10 (TRAV38–2/TRBV19) (14, 23, 24). This binding mode is characterized by a more acute crossing angle and focus on the C terminus rather than the center of the MHC α1 helix. In addition, the critical notch between the peptide and MHC α2 helix is occupied by a tryptophan rather than arginine residue as in most canonical GIL-specific TCRs. These TCR-pMHC structures, together with the remarkable diversity of TCRs expressing non-canonical TRAV/TRBV combinations in GIL-specific repertoires (14), demonstrate that there are many ways for TCRs to bind even a featureless peptide such as GIL with sufficient affinity to elicit broad anti-viral responses that provide protection against T-cell clonal loss and viral escape.
Experimental procedures
Isolation of GIL-specific TCR F50
To obtain TCR F50, GIL-specific CD8+ T cells were isolated from the peripheral blood of a HLA-A2+ healthy male donor (71 years old) after 14 days of in vitro stimulation with GIL–HLA-A2 using an artificial antigen presenting system as previously described (14). Briefly, peripheral blood mononuclear cells were separated from leukopheresis cells by Ficoll gradient centrifugation. CD8+ T cells were isolated from peripheral blood mononuclear cells by immunomagnetic enrichment (34, 40). GIL-specific CD8+ T cells were expanded in an artificial antigen-presenting system using GIL peptide (GILGFVFTL) (BioMer Technology) (41). The expanded cells were stained with APC- and FITC-conjugated GIL dextramer (Immudex). FITC and APC double-positive cells were sorted by flow cytometry. Single-cell analysis was used to identify the paired α and β chains of GIL-specific TCRs, including F50 (14, 34).
Expression and purification of TCR F50
Soluble TCR F50 for affinity measurements and structure determination was prepared by in vitro folding from inclusion bodies produced in E. coli. The Vα and Vβ regions of F50 (residues 1–209 and 1–244, respectively) were cloned into the expression vector pET26b (Novagen) containing Cα and Cβ regions. An interchain disulfide (CαCys163–CβCys171) was engineered to increase yield of the TCR αβ heterodimer (42). The F50 α and β chains were expressed separately as inclusion bodies in BL21(DE3) E. coli cells (Agilent Technologies). Bacteria were grown at 37 °C in LB medium to A600 = 0.6–0.8 and induced with 1 mm isopropyl-β-d-thiogalactoside. After incubation for 3 h, the bacteria were harvested by centrifugation and resuspended in 50 mm Tris-HCl (pH 8.0) containing 0.1 m NaCl and 2 mm EDTA; cells were disrupted by sonication. Inclusion bodies were washed extensively with 50 mm Tris-HCl (pH 8.0) and 5% (v/v) Triton X-100 and then dissolved overnight in 8 m urea, 50 mm Tris-HCl (pH 8.0), 10 mm EDTA, and 10 mm DTT. For in vitro folding, the TCR α and β chains were mixed in a 1.2:1 molar ratio for 30 min prior to dilution into ice-cold folding buffer containing 5 m urea, 0.4 m l-arginine HCl, 100 mm Tris-HCl (pH 8.0), 5 mm EDTA, 3.7 mm cystamine, and 6.6 mm cysteamine to a final protein concentration of 80 mg/liter. The folding mixture was dialyzed against 10 mm Tris-HCl (pH 8.0) for 72 h at 4 °C. The mixture was concentrated 20-fold and dialyzed against 25 mm Tris-HCl (pH 8.0). Disulfide-linked TCR F50 heterodimers were purified using sequential Superdex 200 GL and Mono Q columns (GE Healthcare).
Production of GIL–HLA-A2
Soluble HLA-A2 loaded with GIL peptide (GILGFVFTL) (GenScript) was prepared by in vitro folding. The HLA-A*0201 heavy chain (residues 1–275) and β2-microglobulin (residues 1–99) were produced separately as inclusion bodies in BL21(DE3) E. coli cells transformed by pET26b containing the corresponding genes. Inclusion bodies were dissolved in 8 m urea, 50 mm Tris-HCl (pH 8.0), 10 mm EDTA, and 10 mm DTT. For in vitro folding, the HLA-A*0201 heavy chain (30 mg), β2m (30 mg), and GIL peptide (20 mg) were mixed and added dropwise to 1 liter of a folding solution containing 5 m urea, 0.4 m l-arginine HCl, 100 mm Tris-HCl (pH 8.0), 5 mm EDTA, 3.7 mm cystamine, and 6.6 mm cysteamine. The folding solution was dialyzed against distilled water for 24 h and then against 10 mm Tris-HCl (pH 8.0) for 48 h at 4 °C. After 20-fold concentration and further dialysis against 20 mm Tris-HCl (pH 8.0) and 20 mm NaCl, correctly folded GIL–HLA-A2 was purified using a Mono Q column.
Crystallization and data collection
TCR F50 was mixed with GIL–HLA-A2 in a 1:1 molar ratio and concentrated to 10 mg/ml. Crystals of the F50–GIL–HLA-A2 complex grew in 10–15% (w/v) polyethylene glycol 3350, 0.1 m imidazole (pH 8.0), and 0.2 m sodium malonate. For data collection, crystals were transferred to a cryoprotectant solution of mother liquor containing 25% (v/v) glycerol prior to flash cooling in a nitrogen stream. X-ray diffraction data for the F50–GIL–HLA-A2 complex were collected at Beamline 24ID-E of the Advanced Photon Source of the Argonne National Laboratory with an ADSC Q315 CCD detector. Diffraction data were indexed, integrated, and scaled with the program HKL2000 (43). The data collection statistics are summarized in Table 1.
Structure determination and refinement
The structure of the F50–GIL–HLA-A2 complex was solved by molecular replacement with the program Phaser (44). An HIV-specific TCR (Protein Data Bank accession code 3VXU) (28) and GIL–HLA-A2 (Protein Data Bank accession code 1OGA) (23) were used as search models with the CDRs and peptide removed, respectively. One complex molecule was located in the asymmetric unit. Structure refinement was performed using rigid body and simulated annealing via Phenix (45). The model was further refined by manual model building with Coot (46) based on 2Fo − Fc and Fo − Fc maps with the GIL peptide omitted in the initial refinement. The final Rwork and Rfree values for the F50–GIL–HLA-A2 complex are 19.1 and 21.3%, respectively. Refinement statistics are presented in Table 1. Stereochemical parameters were evaluated by PROCHECK (47).
Surface plasmon resonance analysis
The interaction of TCR F50 with GIL–HLA-A2 was assessed by SPR using a BIAcore T100 biosensor at 25 °C. Biotin-tagged GIL–HLA-A2 (NIH Tetramer Core Facility) was immobilized on a streptavidin-coated BIAcore SA chip (GE Healthcare) at 1000 resonance units (RU), followed by blocking the remaining streptavidin sites with 20 μm biotin solution. An additional flow cell was injected only with free biotin to serve as a blank control. For analysis of TCR binding, solutions containing different concentrations of F50 were flowed sequentially over the chips immobilized with GIL–HLA-A2 and the blank. Injections of TCR were stopped at 30 s after SPR signals reached a plateau. Equilibrium data were fitted with a 1:1 binding model using BIAevaluation 3.1 software to obtain the KD.
Protein Data Bank accession code
Coordinates and structure factors for the F50–GIL–HLA-A2 complex have been deposited in the Protein Data Bank under accession code 5TEZ.
Author contributions
X. Y. determined the crystal structure. G. C. isolated TCR genes. X. Y., G. C., N.-P. W., and R. A. M. analyzed the data and wrote the manuscript.
Acknowledgments
We acknowledge the National Institutes of Health Tetramer Core Facility for providing biotinylated GIL–HLA-A2. The X-ray SER-CAT beamlines at the Advanced Photon Source are supported by the US Department of Energy, Basic Energy Sciences, Office of Science, under Contract W-31-109-Eng-38. We thank Edwin Pozharski and Swarna Lakshmi Pigudu for generous assistance with X-ray data collection.
This work was supported by National Institutes of Health Grant AI036900 (to R. A. M.) and the Intramural Research Program of the National Institute on Aging (to N.-P. W.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The atomic coordinates and structure factors (code 5TEZ) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- TCR
- T-cell receptor
- IAV
- influenza A virus
- MHC
- GIL, GILGFVFTL epitope of IAV
- pMHC
- peptide–MHC
- CDR
- complementarity-determining region
- V
- variable
- SPR
- surface plasmon resonance
- RU
- resonance units.
References
- 1. Zhang N., and Bevan M. J. (2011) CD8+ T cells: foot soldiers of the immune system. Immunity 35, 161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arstila T. P., Casrouge A., Baron V., Even J., Kanellopoulos J., and Kourilsky P. (1999) A direct estimate of the human αβ T cell receptor diversity. Science 286, 958–961 [DOI] [PubMed] [Google Scholar]
- 3. Robins H. S., Srivastava S. K., Campregher P. V., Turtle C. J., Andriesen J., Riddell S. R., Carlson C. S., and Warren E. H. (2010) Overlap and effective size of the human CD8+ T cell receptor repertoire. Sci. Transl. Med. 2, 47ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qi Q., Liu Y., Cheng Y., Glanville J., Zhang D., Lee J. Y., Olshen R. A., Weyand C. M., Boyd S. D., and Goronzy J. J. (2014) Diversity and clonal selection in the human T-cell repertoire. Proc. Natl. Acad. Sci. U.S.A. 111, 13139–13144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li H. M., Hiroi Z., Y., Shi A., Chen G., De S., Metter E. J., Wood W. H. 3rd, Sharov A., Milner J. D., Becker K. G., Zhan M., and Weng N. P. (2016) TCRβ repertoire of CD4+ and CD8+ T cells is distinct in richness, distribution, and CDR3 amino acid composition. J. Leukoc. Biol. 99, 505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moss P. A., Moots R. J., Rosenberg W. M., Rowland-Jones S. J., Bodmer H. C., McMichael A. J., and Bell J. I. (1991) Extensive conservation of α and β chains of the human T-cell antigen receptor recognizing HLA-A2 and influenza A matrix peptide. Proc. Natl. Acad. Sci. U.S.A. 88, 8987–8990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lehner P. J., Wang E. C., Moss P. A., Williams S., Platt K., Friedman S. M., Bell J. I., and Borysiewicz L. K. (1995) Human HLA-A0201-restricted cytotoxic T lymphocyte recognition of influenza A is dominated by T cells bearing the Vβ17 gene segment. J. Exp. Med. 181, 79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naumov Y. N., Naumova E. N., Clute S. C., Watkin L. B., Kota K., Gorski J., and Selin L. K. (2006) Complex T cell memory repertoires participate in recall responses at extremes of antigenic load. J. Immunol. 177, 2006–2014 [DOI] [PubMed] [Google Scholar]
- 9. Naumov Y. N., Naumova E. N., Yassai M. B., and Gorski J. (2011) Selective T cell expansion during aging of CD8 memory repertoires to influenza revealed by modeling. J. Immunol. 186, 6617–6624 [DOI] [PubMed] [Google Scholar]
- 10. La Gruta N. L., and Turner S. J. (2014) T cell mediated immunity to influenza: mechanisms of viral control. Trends Immunol. 35, 396–402 [DOI] [PubMed] [Google Scholar]
- 11. Gil A., Yassai M. B., Naumov Y. N., and Selin L. K. (2015) Narrowing of human influenza A virus-specific T cell receptor α and β repertoires with increasing age. J. Virol. 89, 4102–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gotch F., Rothbard J., Howland K., Townsend A., and McMichael A. (1987) Cytotoxic T lymphocytes recognize a fragment of influenza virus matrix protein in association with HLA-A2. Nature 326, 881–882 [DOI] [PubMed] [Google Scholar]
- 13. Naumov Y. N., Naumova E. N., Yassai M. B., Kota K., Welsh R. M., and Selin L. K. (2008) Multiple glycines in TCR α-chains determine clonally diverse nature of human T cell memory to influenza A virus. J. Immunol. 181, 7407–7419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen G., Yang X., Ko A., Sun X., Gao M., Zhang Y., Shi A., Mariuzza R. A., and Weng N. P. (2017) Sequence and structural analyses reveal distinct and highly diverse human CD8+ TCR repertoires to immunodominant viral antigens. Cell Rep. 19, 569–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klarenbeek P. L., Remmerswaal E. B., ten Berge I. J., Doorenspleet M. E., van Schaik B. D., Esveldt R. E., Koch S. D., ten Brinke A., van Kampen A. H., Bemelman F. J., Tak P. P., Baas F., de Vries N., and van Lier R. A. (2012) Deep sequencing of antiviral T-cell responses to HCMV and EBV in humans reveals a stable repertoire that is maintained for many years. PLoS Pathog. 8, e1002889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang G. C., Dash P., McCullers J. A., Doherty P. C., and Thomas P. G. (2012) T cell receptor αβ diversity inversely correlates with pathogen-specific antibody levels in human cytomegalovirus infection. Sci. Transl. Med. 4, 128ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miconnet I. (2012) Probing the T-cell receptor repertoire with deep sequencing. Curr. Opin. HIV AIDS 7, 64–70 [DOI] [PubMed] [Google Scholar]
- 18. La Gruta N. L., and Thomas P. G. (2013) Interrogating the relationship between naïve and immune antiviral T cell repertoires. Curr. Opin. Virol. 3, 447–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Messaoudi I., Guevara Patiño J. A., Dyall R., LeMaoult J., and Nikolich-Zugich J. (2002) Direct link between MHC polymorphism, T cell avidity, and diversity in immune defense. Science 298, 1797–1800 [DOI] [PubMed] [Google Scholar]
- 20. Meyer-Olson D., Shoukry N. H., Brady K. W., Kim H., Olson D. P., Hartman K., Shintani A. K., Walker C. M., and Kalams S. A. (2004) Limited T cell receptor diversity of HCV-specific T cell responses is associated with CTL escape. J. Exp. Med. 200, 307–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yager E. J., Ahmed M., Lanzer K., Randall T. D., Woodland D. L., and Blackman M. A. (2008) Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J. Exp. Med. 205, 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rossjohn J., Gras S., Miles J. J., Turner S. J., Godfrey D. I., and McCluskey J. (2015) T cell antigen receptor recognition of antigen-presenting molecules. Annu. Rev. Immunol. 33, 169–200 [DOI] [PubMed] [Google Scholar]
- 23. Stewart-Jones G. B., McMichael A. J., Bell J. I., Stuart D. I., and Jones E. Y. (2003) A structural basis for immunodominant human T cell receptor recognition. Nat. Immunol. 4, 657–663 [DOI] [PubMed] [Google Scholar]
- 24. Song I., Gil A., Mishra R., Ghersi D., Selin L. K., and Stern L. J. (2017) Broad TCR repertoire and diverse structural solutions for recognition of an immunodominant CD8+ T cell epitope. Nat. Struct. Mol. Biol. 24, 395–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ishizuka J., Stewart-Jones G. B., van der Merwe A., Bell J. I., McMichael A. J., and Jones E. Y. (2008) The structural dynamics and energetics of an immunodominant T cell receptor are programmed by its Vβ domain. Immunity 28, 171–182 [DOI] [PubMed] [Google Scholar]
- 26. Sewell A. K. (2012) Why must T cells be cross-reactive? Nat. Rev. Immunol. 12, 669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rudolph M. G., Stanfield R. L., and Wilson I. A. (2006) How TCRs bind MHCs, peptides, and coreceptors. Annu. Rev. Immunol. 24, 419–466 [DOI] [PubMed] [Google Scholar]
- 28. Shimizu A., Kawana-Tachikawa A., Yamagata A., Han C., Zhu D., Sato Y., Nakamura H., Koibuchi T., Carlson J., Martin E., Brumme C. J., Shi Y., Gao G. F., Brumme Z. L., Fukai S., et al. (2013) Structure of TCR and antigen complexes at an immunodominant CTL epitope in HIV-1 infection. Sci. Rep. 3, 3097–3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garcia K. C. (2012) Reconciling views on T cell receptor germline bias for MHC. Trends Immunol. 33, 429–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deng L., Langley R. J., Wang Q., Topalian S. L., and Mariuzza R. A. (2012) Structural insights into the editing of germ-line-encoded interactions between T-cell receptor and MHC class II by Vα CDR3. Proc. Natl. Acad. Sci. U.S.A. 109, 14960–14965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sundberg E. J., and Mariuzza R. A. (2002) Molecular recognition in antibody–antigen complexes. Adv. Protein Chem. 61, 119–160 [DOI] [PubMed] [Google Scholar]
- 32. Lawrence M. C., and Colman P. M. (1993) Shape complementarity at protein/protein interfaces. J. Mol. Biol. 234, 946–950 [DOI] [PubMed] [Google Scholar]
- 33. Wodak S. J., and Janin J. (2002) Structural basis of macromolecular recognition. Adv. Protein Chem. 61, 9–73 [DOI] [PubMed] [Google Scholar]
- 34. Yang X., Gao M., Chen G., Pierce B. G., Lu J., Weng N. P., and Mariuzza R. A. (2015) Structural basis for clonal diversity of the public T cell response to a dominant human cytomegalovirus epitope. J. Biol. Chem. 290, 29106–29119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ding Y. H., Smith K. J., Garboczi D. N., Utz U., Biddison W. E., and Wiley D. C. (1998) Two human T cell receptors bind in a similar diagonal mode to the HLA-A2/Tax peptide complex using different TCR amino acids. Immunity 8, 403–411 [DOI] [PubMed] [Google Scholar]
- 36. Feng D., Bond C. J., Ely L. K., Maynard J., and Garcia K. C. (2007) Structural evidence for a germline-encoded T cell receptor-major histocompatibility complex interaction “codon.” Nat. Immunol. 8, 975–983 [DOI] [PubMed] [Google Scholar]
- 37. Dai S., Huseby E. S., Rubtsova K., Scott-Browne J., Crawford F., Macdonald W. A., Marrack P., and Kappler J. W. (2008) Crossreactive T cells spotlight the germline rules for αβ T cell-receptor interactions with MHC molecules. Immunity 28, 324–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stadinski B. D., Trenh P., Smith R. L., Bautista B., Huseby P. G., Li G., Stern L. J., and Huseby E. S. (2011) A role for differential variable gene pairing in creating T cell receptors specific for unique major histocompatibility ligands. Immunity 35, 694–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu Y. C., Miles J. J., Neller M. A., Gostick E., Price D. A., Purcell A. W., McCluskey J., Burrows S. R., Rossjohn J., and Gras S. (2013) Highly divergent T-cell receptor binding modes underlie specific recognition of a bulged viral peptide bound to a human leukocyte antigen class I molecule. J. Biol. Chem. 288, 15442–15454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Araki Y., Wang Z., Zang C., Wood W. H. 3rd, Schones D., Cui K., Roh T. Y., Lhotsky B., Wersto R. P., Peng W., Becker K. G., Zhao K., and Weng N. P. (2009) Genome-wide analysis of histone methylation reveals chromatin state-based regulation of gene transcription and function of memory CD8+ T cells. Immunity 30, 912–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oelke M., Maus M. V., Didiano D., June C. H., Mackensen A., and Schneck J. P. (2003) Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat. Med. 9, 619–624 [DOI] [PubMed] [Google Scholar]
- 42. Boulter J. M., Glick M., Todorov P. T., Baston E., Sami M., Rizkallah P., and Jakobsen B. K. (2003) Stable, soluble T-cell receptor molecules for crystallization and therapeutics. Protein Eng. 16, 707–711 [DOI] [PubMed] [Google Scholar]
- 43. Otwinowski Z., and Minor W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 44. Storoni L. C., McCoy A. J., and Read R. J. (2004) Likelihood-enhanced fast rotation functions. Acta Crystallogr. D Biol. Crystallogr. 60, 432–438 [DOI] [PubMed] [Google Scholar]
- 45. Afonine P. V., Grosse-Kunstleve R. W., Echols N., Headd J. J., Moriarty N. W., Mustyakimov M., Terwilliger T. C., Urzhumtsev A., Zwart P. H., and Adams P. D. (2012) Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Emsley P., Lohkamp B., Scott W. G., and Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Laskowski R. A., MacArthur M. W., Moss D. S., and Thornton J. M. (1993) PROCHECK: a program to check the stereo chemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 [Google Scholar]
- 48. Stewart-Jones G. B., Simpson P., van der Merwe P. A., Easterbrook P., McMichael A. J., Rowland-Jones S. L., Jones E. Y., and Gillespie G. M. (2012) Structural features underlying T-cell receptor sensitivity to concealed MHC class I micropolymorphisms. Proc. Natl. Acad. Sci. U.S.A. 109, E3483–E3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buslepp J., Wang H., Biddison W. E., Appella E., and Collins E. J. (2003) A correlation between TCR Vα docking on MHC and CD8 dependence: implications for T cell selection. Immunity 19, 595–606 [DOI] [PubMed] [Google Scholar]
- 50. Kjer-Nielsen L., Clements C. S., Purcell A. W., Brooks A. G., Whisstock J. C., Burrows S. R., McCluskey J., and Rossjohn J. (2003) A structural basis for the selection of dominant αβ T cell receptors in antiviral immunity. Immunity 18, 53–64 [DOI] [PubMed] [Google Scholar]
- 51. Chen J. L., Stewart-Jones G., Bossi G., Lissin N. M., Wooldridge L., Choi E. M., Held G., Dunbar P. R., Esnouf R. M., Sami M., Boulter J. M., Rizkallah P., Renner C., Sewell A., van der Merwe P. A., et al. (2005) Structural and kinetic basis for heightened immunogenicity of T cell vaccines. J. Exp. Med. 201, 1243–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Colf L. A., Bankovich A. J., Hanick N. A., Bowerman N. A., Jones L. L., Kranz D. M., and Garcia K. C. (2007) How a single T cell receptor recognizes both self and foreign MHC. Cell 129, 135–146 [DOI] [PubMed] [Google Scholar]