Abstract
Schizophrenia is characterized by deficits in gesturing that is important for nonverbal communication. Research in healthy participants and brain-damaged patients revealed a left-lateralized fronto-parieto-temporal network underlying gesture performance. First evidence from structural imaging studies in schizophrenia corroborates these results. However, as of yet, it is unclear if cortical thickness abnormalities contribute to impairments in gesture performance. We hypothesized that patients with deficits in gesture production show cortical thinning in 12 regions of interest (ROIs) of a gesture network relevant for gesture performance and recognition. Forty patients with schizophrenia and 41 healthy controls performed hand and finger gestures as either imitation or pantomime. Group differences in cortical thickness between patients with deficits, patients without deficits, and controls were explored using a multivariate analysis of covariance. In addition, the relationship between gesture recognition and cortical thickness was investigated. Patients with deficits in gesture production had reduced cortical thickness in eight ROIs, including the pars opercularis of the inferior frontal gyrus, the superior and inferior parietal lobes, and the superior and middle temporal gyri. Gesture recognition correlated with cortical thickness in fewer, but mainly the same, ROIs within the patient sample. In conclusion, our results show that impaired gesture production and recognition in schizophrenia is associated with cortical thinning in distinct areas of the gesture network.
Keywords: Schizophrenia, Cortical thickness, Gesture, Praxis, Nonverbal communication
Highlights
-
•
Impairments in gesture production and recognition in schizophrenia are related to altered brain structure.
-
•
Brain alterations in schizophrenia are located in areas that are generally damaged in apraxia.
-
•
Schizophrenia patients with gesture deficits show cortical thinning of several regions in the gesture network.
-
•
Deficits of gesture production and recognition are both related to a fronto-parieto-temporal gesture network.
1. Introduction
Gestures are an important and integral part of communication (Goldin-Meadow and Alibali, 2013, Hostetter, 2011). They are not only important for language production and comprehension in verbal communication, but also play a crucial role in nonverbal communication, as they may transmit information on their own (Goldin-Meadow and Alibali, 2013).
Research on gestures may either focus on recognition and interpretation of gestures, or on gesture production (Walther and Mittal, 2016). The production of gestures can be investigated in two domains: imitation (following demonstration) and pantomime (after verbal instruction). The semantic categories of gestures can be meaningless, communicative (intransitive), or object-related (transitive) (Vanbellingen et al., 2010).
Gestures are compound actions that involve the coordinated interplay of several brain regions. The neural correlates of gesture processing have been extensively studied in fMRI experiments in healthy participants (Andric and Small, 2012, Yang et al., 2015). Planning, pantomime of tool use, and communicative gestures activate a left-hemispheric fronto-parieto-temporal network (Bohlhalter et al., 2009, Hermsdorfer et al., 2007, Johnson-Frey et al., 2005, Kroliczak and Frey, 2009, Niessen et al., 2014). Here, we refer to it as “gesture network”, that includes superior and inferior frontal as well as parietal areas and superior and middle temporal cortices (see Fig. 1) and also contributes to gesture recognition and interpretation. However, various gesture types seem to depend on different regions within this network. For example, pantomime of tool use activates some additional regions compared to imitation of meaningless gestures, such as the triangular part of the inferior frontal gyrus (IFG), the middle temporal gyrus (MTG), the supramarginal gyrus (SMG), and the intraparietal sulcus (Vry et al., 2015).
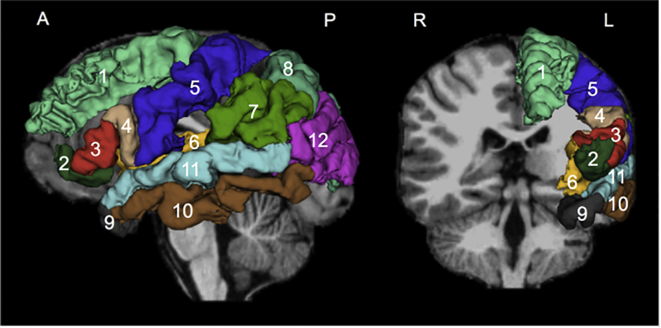
Fig. 1.
Cortical regions of interest. The model is shown on a sagittal and coronal view and was created from a randomly selected case using the model maker module of Slicer 4.5. It is superimposed on the individual T1-weighted image. The regions of interest are based on FreeSurfer colors: superior frontal gyrus: 1, pars orbitalis of IFG: 2, pars triangularis of IFG: 3, pars opercularis of IFG: 4, precentral gyrus: 5, insula: 6, supramarginal gyrus: 7, superior parietal lobe: 8, temporal pole: 9, middle temporal gyrus: 10, superior temporal gyrus: 11, inferior parietal lobe: 12.
Studies investigating gesture recognition and comprehension have focused on varying contextual information, demonstrating distinct involvement of brain areas within the gesture network. For example, a meta-analysis suggests three different networks: a perceptual-motor network (including the premotor cortex as well as parietal and temporal regions), a semantic network (consisting of temporal and frontal regions) and a social emotive network (comprising of the IFG, the insula, and the putamen) (Yang et al., 2015).
Taken together, fMRI studies in healthy participants reveal associations to the left-lateralized gesture network for gesture production (planning or execution), recognition and comprehension.
Gesturing is severely impaired in patients with apraxia following left hemispheric brain damage. Lesion mapping studies in apraxia have investigated pantomime of tool use and imitation of meaningless and meaningful gestures. Pantomime of tool use has been associated with the IFG, the occipito-temporal cortex, the parietal cortex, premotor and (pre-) central regions, and the insula (Goldenberg et al., 2007, Hoeren et al., 2014, Manuel et al., 2013, Weiss et al., 2016). Imitation of meaningful gestures can be attributed to the left IFG, the middle frontal gyrus, the premotor cortex, the SMG, and pre-central regions, whereas imitation of meaningless gestures relies predominantly on areas of the parietal lobe, for example on the angular gyrus, postcentral areas, and only small parts of the IFG (Goldenberg, 2009, Hoeren et al., 2014, Mengotti et al., 2013, Weiss et al., 2016).
To summarize, as in healthy participants, all apraxic deficits in brain-damaged patients can be attributed to damage in the left-lateralized gesture network (Buxbaum et al., 2014, Weiss et al., 2016), and different gesture types show associations with distinct regions within this network.
Patients with schizophrenia show both fewer and disturbed nonverbal behaviors (Lavelle et al., 2013, Lavelle et al., 2014). They have specific impairments in gesture recognition and production (imitation and pantomime) (Matthews et al., 2013, Park et al., 2008, Walther et al., 2015, Walther et al., 2013a, White et al., 2016). A high correlation between both tasks argues further for a generalized gesture deficit (Walther et al., 2015). Gesture impairments are present at disease onset (Stegmayer et al., 2016b), are related to reduced social competence (Park et al., 2008), and gesture production and recognition predict functional outcome and negative symptoms after 6 months (Walther et al., 2016).
Studies of neural correlates of gesture production and recognition in schizophrenia have been sparse, but corroborate the results found in studies in healthy or brain-damaged participants. Patients with schizophrenia show aberrant brain function during gesture recognition, particularly with functional dysconnectivity between the superior temporal sulcus (STS) and the IFG when processing metaphoric gestures (Straube et al., 2014). Likewise, an fMRI study of gesture production found impaired gesture planning and execution to be linked to reduced activation in the dorsolateral prefrontal cortex (DLPFC) and increased activation of the inferior parietal lobe (IPL) (Stegmayer et al., 2017). Furthermore, patients with schizophrenia with gesture deficits had reduced grey matter volume in the left IFG, the right insula, the temporal pole (TP), and the anterior cingulate cortex, compared to healthy controls (Stegmayer et al., 2016a).
Taken together, the evidence suggests that structural and functional alterations in the gesture network contribute to the pathophysiology of impaired gesture performance in schizophrenia. So far, structural brain imaging analyses were limited to grey matter volume. However, schizophrenia is also associated with reduced cortical thickness (Goldman et al., 2009). Despite the fact that grey matter volume is a composite of cortical thickness and surface area, volume is more closely linked to surface area than to thickness (Winkler et al., 2010). Measurements of cortical thickness and surface area, in turn, are genetically and phenotypically independent (Panizzon et al., 2009, Winkler et al., 2010) and each shows a different pattern of development during adolescence, especially in regions important for social cognition (Vijayakumar et al., 2016). Furthermore, surface area seems to be more influenced by genetic factors than thickness, as most volumetric differences between first-degree relatives of schizophrenia and healthy controls are related to surface area rather than thickness (Goghari et al., 2007).
Even though cortical thinning in schizophrenia may be influenced by genetic effects, it is more associated to disease factors, as thinning occurs around the time of illness onset and is absent before onset (Sprooten et al., 2013). In addition, cortical thinning might not be progressive over the course of the illness, again arguing that pathological changes occur in a limited vulnerable phase around illness onset (Kubota et al., 2011). Therefore, cortical thickness can reveal additional information and important insights into the underlying pathophysiology.
Our goal in this study was thus to examine cortical thickness correlates in the gesture network for gesture production and recognition in patients with schizophrenia. Gesture production is impaired in approximately 50% of patients with schizophrenia (Walther et al., 2015, Walther et al., 2013a, Walther et al., 2013b). Patients with gesture deficits and without demonstrate clinical and demographic differences, for example patients with deficits were older, had poorer frontal cortex function and more severe psychopathology (Walther et al., 2013b). Therefore, we divided our clinical sample in patients with and without gesture deficits.
Given the findings of gesture production or recognition in healthy controls, in patients with brain damage, and from the few studies focusing on patients with schizophrenia, we hypothesized that we would find associations between reduced cortical thickness and disturbed gesture production and recognition in a comprehensive gesture network, consisting of the superior parietal lobe (SPL), the IPL, the superior temporal gyrus (STG), the MTG, the superior frontal gyrus (SFG), the IFG (pars opercularis, triangularis, and orbitalis), the precentral gyrus, the SMG, the TP, and the insula. As data decidedly indicates a predominantly left hemisphere network (Bohlhalter et al., 2009, Goldenberg, 2009, Hermsdorfer et al., 2013, Johnson-Frey et al., 2005, Kroliczak and Frey, 2009, Manuel et al., 2013), we concentrated exclusively on the left hemisphere. Results for the right hemisphere can be found in the supplementary table S1.
2. Materials and methods
2.1. Participants
Forty patients with schizophrenia spectrum disorder (25 men, 15 women) were recruited at the inpatient and outpatient departments at the University Hospital of Psychiatry, Bern, Switzerland. Forty-one healthy controls (23 men, 18 women) were recruited among staff and were matched for age, sex, and education. Patients were split into two groups according to gesture performance (with deficits and without deficits, according to the previously published cut-off scores (Walther et al., 2013a). Demographic and clinical characteristics of the participants are presented in Table 1. All patients and controls were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971).
Table 1.
Demographic and clinical characteristics.
| Variablesa |
Controls (n = 41), Mean (SD) |
Patients with deficits (n = 19), Mean (SD) |
Patients without deficits (n = 21), Mean (SD) |
Statisticsb |
||||
|---|---|---|---|---|---|---|---|---|
| Chi-square test |
||||||||
| ×2 (df = 2) | p | |||||||
| Sex (men/women) | 23/18 | 8/11 | 17/4 | 6.579 | 0.037⁎ | |||
| One-way ANOVA | ||||||||
| F (df = 2/78) | p | Post hoc (p) | ||||||
| Controls vs. patients with deficits | Controls vs. patients without deficits | Patients with vs. without deficits | ||||||
| Age (years) | 38.93 (13.69) | 44.84 (11.13) | 32.38 (7.34) | 5.623 | 0.005⁎⁎ | 0.222 | 0.124 | 0.004⁎⁎ |
| Education (years) | 14.20 (2.73) | 12.5 (3.13) | 14.62 (2.96) | 3.100 | 0.051 | 0.113 | 1.000 | 0.069 |
| TULIA total score | 225.86 (7.86) | 192.86 (16.32) | 223.24 (7.54) | 70.050 | 0.001⁎⁎ | 0.001⁎⁎ | 1.000 | 0.001⁎⁎ |
| PKT total score | 27.37 (1.46) | 21.84 (6.50) | 25.90 (2.21) | 16.422 | 0.001⁎⁎ | 0.001⁎⁎ | 0.365 | 0.001⁎⁎ |
| t-test | ||||||||
| T (df = 38) | p | |||||||
| CPZ (mg) 5 years | 349.86 (343.02) | 127.57 (189.40) | 2.570 | 0.003⁎⁎ | ||||
| DOI (months) | 212.84 (170.57) | 80.38 (83.80) | 3.164 | 0.002⁎⁎ | ||||
| PANSS positive | 19.47 (7.65) | 17.05 (5.00) | 1.199 | 0.048⁎ | ||||
| PANSS negative | 17.05 (3.81) | 18.05 (5.05) | − 0.698 | 0.241 | ||||
| PANSS total | 72.05 (19.24) | 70.00 (16.34) | 0.365 | 0.806 | ||||
CPZ, average chlorpromazine equivalents for the last five years; DOI, duration of illness; PANSS, positive and negative syndrome scale.
SD, standard deviation; df, degrees of freedom; post hoc tests of the ANOVA were Bonferroni-corrected.
p value is significant at the 0.05 level (2-tailed).
p value is significant at the 0.01 level (2-tailed).
Patients were diagnosed with schizophrenia, schizoaffective disorder, or schizophreniform disorder according to the structured clinical interview (SCID) and DSM-5 criteria. The Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) was additionally used to assess schizophrenia symptoms. Thirty-six of 40 patients received antipsychotic medication. The dosages of these antipsychotics were calculated as the average chlorpromazine equivalents (CPZ) (Woods, 2003) per day for the last five years.
Exclusion criteria were substance-related addictions (except nicotine), a past or current medical or neurological condition, head trauma with loss of consciousness, electroconvulsive treatment, and any contraindications to MRI (magnetic resonance imaging). Specific exclusion criteria for controls were a history of psychiatric disorders and first-degree relatives with psychotic disorders. Written informed consent was acquired from all participants and the study was approved by the local ethics committee, “Kantonale Ethikkommission Bern” (KEK-BE 025/13).
First, we conducted the clinical interview and assessments of psychpathology. Afterwards, on the same day, participants underwent structural MRI scanning. The assessment of the gesture tests was performed either on the same day or the day after the MRI scan.
2.2. Gesture tests
We used the test of upper limb apraxia (TULIA) (Vanbellingen et al., 2010) to assess performance of hand and finger gestures. The test was videotaped and later analyzed by a rater who was blind to the diagnoses. Possible errors included temporal-spatial errors or content errors. The maximum total score is 240, based on 48 items in either the imitation domain (performance after demonstration by the examiner) or the pantomime domain (performance after verbal instruction). A higher score indicates superior performance. Further, three semantic types of gestures were included: meaningless, intransitive, and transitive gestures.
The Postural Knowledge Test (PKT) (Bohlhalter et al., 2011, Mozaz et al., 2002) was used to assess gesture recognition. Participants were presented with cartoons of a person with a missing hand, performing transitive or intransitive gestures. For each cartoon, the participants were asked to indicate the missing hand gesture, choosing between three options. Total scores range from 0 to 30. Again, a higher score indicates better performance.
2.3. MRI acquisition
Imaging was carried out on a 3T MRI scanner (Siemens Magnetom Trio; Siemens Medical Solutions, Erlangen, Germany) with a 12-channel head coil. We acquired a T1-weighted MDEFT (modified driven equilibrium Fourier transform pulse) sequence (Deichmann et al., 2004) for anatomical brain imaging (176 sagittal slices, 1 × 1 × 1 mm3, matrix size 256 × 256), field of view (FOV) 256 × 256, 7,92 ms repetition time (TR), 2,48 ms echo time (TE), 910 ms inversion time (TI), and a flip angle (FA) of 16°.
2.4. Data analyses
2.4.1. Cortical thickness analyses
We used an internal pipeline (see https://github.com/pnlbwh/pnlutil) to process the data. The T1-weighted images were transformed from DICOM to NRRD file format by creating a nhdr header file for all participants. Then the images were aligned to the AC-PC axis and centered. All scans were visually examined for image quality and orientation. A brain masking technique based on multi-atlas brain segmentation (MABS) was implemented to exclude non-brain areas (Del Re et al., 2016). This technique has shown to be more accurate than the brain masks generated using FreeSurfer (Del Re et al., 2016).
The masked MR images were processed using FreeSurfer version 5.3. (Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, Massachusetts, USA). The fully automated FreeSurfer pipeline performs cortical reconstruction and subcortical volumetric segmentation using an automated algorithm. A detailed description of this process has been described in previous publications (Dale et al., 1999, Fischl, 2012, Fischl et al., 1999) and is available on the FreeSurfer website (http://ftp.nmr.mgh.harvard.edu/fswiki/recon-all). The FreeSurfer parcellation was then quality-controlled with a special focus on our ROIs. The following 12 ROIs (see Fig. 1) were selected using the Desikan-Killiany atlas (Desikan et al., 2006): SPL, IPL, STG, MTG, SFG, IFG (pars opercularis, triangularis, and orbitalis), precentral gyrus, SMG, TP, and insula. As gesture performance is a function of a left-lateralized brain network, we focused on ROIs in the left hemisphere (Goldenberg, 2009).
The model is shown on a sagittal and coronal view and was created from a randomly selected case using the model maker module of Slicer 4.5. It is superimposed on the individual T1-weighted image. The regions of interest are based on FreeSurfer colors: superior frontal gyrus: 1, pars orbitalis of IFG: 2, pars triangularis of IFG: 3, pars opercularis of IFG: 4, precentral gyrus: 5, insula: 6, supramarginal gyrus: 7, superior parietal lobe: 8, temporal pole: 9, middle temporal gyrus: 10, superior temporal gyrus: 11, inferior parietal lobe: 12.
2.4.2. Statistical analyses
All statistical analyses were computed using IBM SPSS for Macintosh, version 24. Demographic and clinical data were compared using a chi-square test, t-tests, or one-way analyses of variance (ANOVA) where appropriate.
For cortical thickness analyses, a multivariate analysis of covariance (MANCOVA) with age, sex, and intracranial volumes (ICV) as covariates was performed on the overall TULIA score to determine the presence of group differences in the cortical thickness of the ROIs. Post hoc tests were performed between the three groups for which p values were Sidak-corrected. In a next step, partial correlations, corrected for age, sex, ICV, and antipsychotic dosage were conducted between the ROIs and the TULIA and PKT scores within both the patient and the control group. See the supplementary material for additional cortical thickness analyses of the right hemisphere.
3. Results
3.1. Behavioral and clinical data
Demographic and clinical characteristics are given in Table 1. We used TULIA cut-off scores (Walther et al., 2013a) to separate patients with deficits in gesture production from patients without deficits. Splitting the patients into a deficits and a non-deficits group revealed significant differences in sex distribution between groups (X2 (2) = 6.579, p = 0.037): fewer women were found in the group without deficits. Furthermore, patients with deficits were older than patients without deficits, received higher antipsychotic medication dosages, and had longer duration of illness as well as a higher PANSS positive score. However, the two groups had comparable negative and total PANSS scores.
According to the cut-off, 19 patients showed relevant impairments in gesture production, whereas 21 patients showed no deficits. Patients with deficits performed worse compared to patients without deficits or controls in the gesture production test. However, patients without deficits and controls did not differ significantly (p > 0.05).
3.2. Group differences in cortical thickness
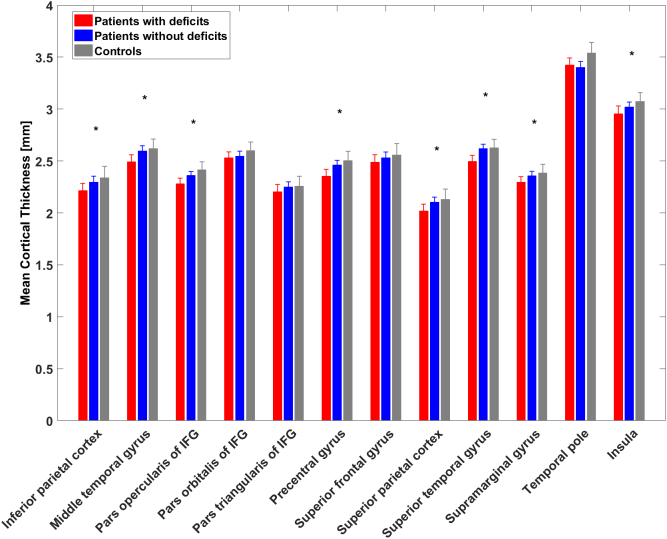
Group differences in the cortical thickness of the ROIs were evaluated with a MANCOVA, treating the group variable as fixed factor, the ROIs as dependent variables, and sex, age, and ICV as covariates (Wilks Lambda = 0.574, p = 0.031) (see Table 2 and Fig. 2). Post hoc tests (Sidak-corrected) showed significant differences for group in the following ROIs: IPL, MTG, pars opercularis of IFG, precentral gyrus, SMG, SPL, STG, and insula. Patients with deficits showed reduced cortical thickness in all ROIs compared to controls. However, no significant group differences were found in the pars orbitalis and triangularis of IFG, SFG, and TP, and no other group comparisons showed significant differences.
Table 2.
MANCOVA with post hoc tests.
| ROI |
Statisticsa |
||
|---|---|---|---|
|
F (df = 2/75) |
p |
Sign. post hoc tests |
|
| Controls vs. patients with deficits | |||
| Inferior parietal lobe | 6.921 | 0.002⁎⁎ | 0.002⁎⁎ |
| Middle temporal gyrus | 4.183 | 0.019⁎ | 0.015⁎ |
| Pars opercularis of IFGb | 5.218 | 0.008⁎⁎ | 0.015⁎ |
| Pars orbitalis of IFG | 1.227 | 0.299 | |
| Pars triangularis of IFG | 0.891 | 0.414 | |
| Precentral gyrus | 6.655 | 0.002⁎⁎ | 0.003⁎⁎ |
| Superior frontal gyrus | 1.526 | 0.224 | |
| Supramarginal gyrus | 3.533 | 0.034⁎ | 0.041⁎ |
| Superior parietal lobe | 5.847 | 0.004⁎⁎ | 0.008⁎⁎ |
| Superior temporal gyrus | 3.771 | 0.028⁎ | 0.023⁎ |
| Temporal pole | 2.056 | 0.135 | |
| Insula | 5.023 | 0.009⁎⁎ | 0.036⁎ |
Note: Post hoc comparisons between patients with and without deficits and between patients without deficits and controls were not significant.
F, F-value; df, degrees of freedom; post hoc tests of the MANCOVA were Sidak-corrected.
IFG, inferior frontal gyrus.
p value is significant at the 0.05 level (2-tailed).
p value is significant at the 0.01 level (2-tailed).
Fig. 2.
Illustration of the mean cortical thickness of each ROI in each group.
Significant group differences in post hoc tests between patients with deficits and controls are marked with asterisks (*); error bars indicate standard deviations.
3.3. Associations of impaired gesture production and recognition with cortical thickness
Within patients and controls, we computed partial correlations between the TULIA or PKT scores and the thickness of the ROIs, corrected for age, sex, ICV, and, within the patient sample, for antipsychotic dosage (see Table 3). In patients, we found significant correlations between TULIA scores and the precentral gyrus, the SPL, and the TP. For PKT scores, we detected significant associations within patients for the IPL, the pars opercularis of the IFG, the precentral gyrus, the SFG, the SPL, and the STG. We further correlated the two TULIA domains pantomime and imitation with cortical thickness separately within the patients and controls using the same covariates. In patients, we detected associations between pantomime and cortical thickness in the precentral gyrus as well as for the SPL, and between imitation and the temporal pole. In controls, pantomime was also correlated with the precentral gyrus (see Table 4).
Table 3.
Partial correlations between the ROIs and task scores for patients and controls, corrected for age, sex, ICV, and in patients additionally for CPZ.
| ROI |
Patients |
Controls |
||||||
|---|---|---|---|---|---|---|---|---|
| TULIAa |
PKTb |
TULIA |
PKT |
|||||
| r | p | r | p | r | p | r | p | |
| Inferior parietal lobe | 0.210 | 0.220 | 0.375⁎ | 0.024 | 0.133 | 0.428 | 0.370⁎ | 0.022 |
| Middle temporal gyrus | − 0.014 | 0.935 | 0.227 | 0.183 | 0.085 | 0.612 | 0.171 | 0.305 |
| Pars opercularis of IFGc | 0.096 | 0.577 | 0.407⁎ | 0.014 | 0.217 | 0.191 | 0.109 | 0.513 |
| Pars orbitalis of IFG | 0.092 | 0.595 | − 0.078 | 0.651 | 0.047 | 0.779 | 0.020 | 0.906 |
| Pars triangularis of IFG | − 0.102 | 0.553 | 0.064 | 0.711 | 0.184 | 0.268 | 0.285 | 0.083 |
| Precentral gyrus | 0.404⁎ | 0.014 | 0.332⁎ | 0.048 | 0.232 | 0.161 | 0.318 | 0.051 |
| Superior frontal gyrus | 0.117 | 0.497 | 0.438⁎⁎ | 0.008 | 0.166 | 0.319 | 0.170 | 0.309 |
| Supramarginal gyrus | 0.205 | 0.231 | 0.325 | 0.053 | − 0.097 | 0.564 | 0.065 | 0.697 |
| Superior parietal lobe | 0.417⁎ | 0.011 | 0.329⁎ | 0.050 | 0.088 | 0.598 | 0.142 | 0.395 |
| Superior temporal gyrus | 0.197 | 0.250 | 0.389⁎ | 0.019 | 0.077 | 0.647 | 0.129 | 0.440 |
| Temporal pole | − 0.444⁎⁎ | 0.007 | − 0.165 | 0.336 | − 0.074 | 0.657 | 0.092 | 0.581 |
| Insula | − 0.048 | 0.780 | 0.004 | 0.982 | 0.095 | 0.570 | 0.107 | 0.524 |
TULIA, test of upper limb apraxia.
PKT, postural knowledge test.
IFG, inferior frontal gyrus.
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
Table 4.
Partial correlations between the ROIs of the left hemisphere and TULIA domain for patients and controls, corrected for age, sex, ICV and in patients additionally for CPZ.
| ROI |
Patients |
Controls |
||||||
|---|---|---|---|---|---|---|---|---|
| TULIAa Pantomime |
TULIA Imitation |
TULIA Pantomime |
TULIA Imitation |
|||||
| r | p | r | p | r | p | r | p | |
| Inferior parietal lobe | 0.275 | 0.104 | 0.008 | 0.965 | 0.256 | 0.120 | − 0.061 | 0.715 |
| Middle temporal gyrus | 0.030 | 0.862 | − 0.089 | 0.606 | 0.249 | 0.132 | − 0.140 | 0.403 |
| Pars opercularis of IFGb | 0.201 | 0.240 | − 0.132 | 0.443 | 0.313 | 0.055 | 0.026 | 0.878 |
| Pars orbitalis of IFG | 0.209 | 0.220 | − 0.159 | 0.355 | 0.058 | 0.730 | 0.018 | 0.916 |
| Pars triangularis of IFG | − 0.024 | 0.891 | − 0.205 | 0.231 | 0.283 | 0.085 | 0.003 | 0.987 |
| Precentral gyrus | 0.404⁎ | 0.014 | 0.058 | 0.735 | 0.367⁎ | 0.023 | − 0.010 | 0.952 |
| Superior frontal gyrus | 0.226 | 0.185 | − 0.128 | 0.457 | 0.316 | 0.053 | − 0.070 | 0.676 |
| Supramarginal gyrus | 0.282 | 0.096 | − 0.016 | 0.928 | − 0.003 | 0.988 | − 0.175 | 0.294 |
| Superior parietal lobe | 0.396⁎ | 0.017 | 0.289 | 0.087 | 0.250 | 0.130 | − 0.135 | 0.419 |
| Superior temporal gyrus | 0.301 | 0.074 | − 0.071 | 0.679 | 0.230 | 0.165 | − 0.133 | 0.428 |
| Temporal pole | − 0.279 | 0.099 | − 0.570⁎⁎ | 0.001 | − 0.042 | 0.802 | − 0.087 | 0.604 |
| Insula | − 0.040 | 0.817 | − 0.044 | 0.799 | 0.139 | 0.405 | 0.009 | 0.955 |
TULIA, test of upper limb apraxia.
IFG, inferior frontal gyrus.
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
All correlations (except the one between TULIA scores and the TP) were positive, indicating inferior performance in gesture production and recognition with reduced cortical thickness.
In controls, only PKT scores and cortical thickness of the IPL were correlated, demonstrating that increased thickness of this ROI is related to superior gesture recognition.
Partial correlations for PKT subscores transitive and intransitive in patients and controls are presented in the Supplementary Table S2.
4. Discussion
This study aimed to investigate cortical thickness correlates in the gesture network of gesture production and recognition in patients with schizophrenia. We found cortical thinning of this network, including the pars opercularis of the IFG, the precentral gyrus, the insula, the SPL, the IPL, the SMG, the STG, and the MTG in patients with gesture deficits, compared to healthy controls. In contrast, cortical thickness in patients without deficits did not significantly differ from controls. Furthermore, impaired gesture recognition in patients was also related to cortical thinning within the gesture network.
The significantly thinned regions in the frontal regions included the pars opercularis of the IFG, the precentral gyrus, and the insula in patients with gesture production deficits. In addition, reduced cortical thickness in the precentral gyrus correlated with inferior gesture production in all patients. The two frontal regions (IFG, precentral gyrus) and the insula have been associated with gesture tasks in healthy and brain-damaged participants. Previous work in healthy participants demonstrated activation of the precentral gyrus, the IFG, and the insula during planning or execution of pantomime and imitation gestures (Bohlhalter et al., 2009, Fridman et al., 2006, Kroliczak and Frey, 2009, Vry et al., 2015). Lesion studies corroborate those results and show that impaired production of pantomime tool use or imitation of meaningful and meaningless gestures were associated with the pars opercularis and other parts of the IFG, the insula, and the precentral gyrus (Goldenberg et al., 2007, Goldenberg and Karnath, 2006, Manuel et al., 2013, Weiss et al., 2016). In schizophrenia, reduced grey matter volume has been found in frontal areas including the IFG, the insula, and the precentral gyrus in pantomime performance (Stegmayer et al., 2016a). More specifically, there is considerable evidence that the IFG plays a key role in gesture production. The pars opercularis of the IFG is a part of Broca's area that is not just important for language, but also for higher order motor functions, because it integrates sensory stimuli with motor hand actions (Binkofski and Buccino, 2004). Therefore, the pars opercularis of the IFG serves as an important area for interplay between language and praxis, and lesions of this area have been shown to lead to concurrent aphasia and apraxia (Weiss et al., 2016).
In our study, patients with deficits in gesture production showed reduced cortical thickness compared to healthy controls in the temporal lobe, i.e. the MTG and the STG. Compatible with our results, studies of healthy participants revealed activation of the left MTG during planning of tool use pantomime and intransitive gestures (Kroliczak and Frey, 2009, Vry et al., 2015), and an association between the activation and volume of the left STG during planning of transitive or pantomime of intransitive gestures and production of metaphoric gestures (Bernard et al., 2015, Bohlhalter et al., 2009, Vry et al., 2015). In addition, lesion studies revealed associations of the left MTG with imitation of meaningless hand gestures (Goldenberg and Karnath, 2006) and of the left MTG and STG with imitation and pantomime of tool use gestures (Buxbaum et al., 2014). Patients with schizophrenia showed reduced activity in the STS during imitation of finger movements (Thakkar et al., 2014), and reduced grey matter volume in the left MTG has been found to be associated with pantomime performance in patients with deficits (Stegmayer et al., 2016a). The temporal lobe has been shown to be important for gesture knowledge and production. For example, the posterior part of the temporal lobe is relevant for the processing of semantic object information (Chao et al., 1999, Johnson-Frey et al., 2005, Martin et al., 1996) and for arm and hand positioning in the production of tool-related gestures (Buxbaum et al., 2014).
In the parietal lobe, thinning of the IPL, the SPL, and the SMG was present in patients with deficits in gesture production, compared to healthy controls. In addition, reduced cortical thickness of the SPL was correlated with inferior gesture production in all patients. Our results are consistent with studies of healthy participants that reveal an involvement of the left SPL, IPL, and SMG in planning or executing intransitive and transitive pantomime or imitation gestures (Bohlhalter et al., 2009, Fridman et al., 2006, Kroliczak and Frey, 2009, Vry et al., 2015). In accordance with studies of healthy participants, research on brain-damaged patients often indicates the importance of the parietal lobe for imitation of meaningless and meaningful gestures (Goldenberg and Karnath, 2006, Mengotti et al., 2013), whereas the role of the parietal lobe for pantomime of tool use has been questioned (Goldenberg, 2009). However, recent lesion studies in large sample sizes showed that the integrity of the SPL and the IPL is essential for pantomime of tool use gestures (Hoeren et al., 2014, Weiss et al., 2016). Grey matter volume or activity of the IPL or the SPL has also been linked to pantomime, non-imitative actions, or action observation in patients with schizophrenia (Stegmayer et al., 2016a, Thakkar et al., 2014). Taken together, findings suggest that the parietal lobe, particularly the IPL and the SPL combine sensory, motor, and cognitive inputs and play an important role in goal-directed actions such as grasping and execution of tool-related actions (Binkofski and Buxbaum, 2013, Gottlieb, 2007).
In separate correlation analyses, we tested whether TULIA domains had a specific pattern of association with brain structure. Indeed, in patients we detected higher correlations for pantomime than for imitation with cortical thickness (see Table 4). This effect might be due to limited variance in imitation compared to pantomime scores in patients, as only a few patients show imitation deficits.
In additional correlation analyses, we then tested if the PKT subdomains are differently associated with brain structure (see Table S2). In controls, the recognition of transitive gestures was associated with the pars triangularis of the IFG and the STG. In contrast, in patients we detected very similar patterns of correlations for transitive and intransitive gestures of several associations within the gesture network.
Cortical thinning in schizophrenia has been found in various brain regions, predominantly in temporal and frontal areas (Goldman et al., 2009, Oertel-Knochel et al., 2013, Rimol et al., 2010). Our results partly support these findings. However, there were no differences in cortical thickness of patients without deficits and controls. In addition, patients with and without deficits did not differ regarding cortical thickness in any ROI. This suggests that cortical thinning within patients with gesture deficits, compared to controls, is related neither to changes of cortical thickness in schizophrenia alone, nor exclusively to a gesture impairment. On the one hand, if cortical thinning in the gesture network in patients with deficits would only represent general cortical thinning in schizophrenia, we would also expect differences in ROIs between patients without deficits and controls. On the other hand, if the reduced cortical thickness in patients with deficits would solely represent the gesture deficits, we should also see a difference between patients with and without deficits. As neither scenario is reflected in our findings, we conclude that the cortical thinning in patients with deficits compared to controls not only represents general cortical thinning in schizophrenia, but is also associated with the gesture impairment.
Our findings may also be in part associated with illness chronicity. While cortical thickness in frontal and temporal lobes is reduced in recent-onset participants (Ziermans et al., 2012), longitudinal investigations suggest progressive decline in cortical thickness in these areas in schizophrenia (van Haren et al., 2011). We have investigated a patient group with a considerable age span. Thus, our findings may be influenced by, but not limited to, illness chronicity.
In eight out of 12 ROIs, we found differences in cortical thinning in patients with deficits compared to healthy controls. However, in four ROIs, namely the pars orbitalis and triangularis of the IFG, the SFG, and the TP, we found no group differences in cortical thickness. In contrast, recent evidence from gesture studies in schizophrenia suggest an involvement of those four ROIs in schizophrenia (Stegmayer et al., 2016a, Straube et al., 2013). There are several possible explanations for the observed discrepancy between studies. Depending on gesture category and domain, different brain regions can be affected. Furthermore, specific gesture errors can also be associated with different brain regions (Manuel et al., 2013). We applied validated tests of gesture behavior, including blinded ratings (TULIA) or unequivocal multiple choice tests (PKT). Our composite score of gesture production included both imitation and pantomime of meaningful (transitive or intransitive) and meaningless gestures. In addition, gesture recognition was solely measured as the ability to recognize correct hand gestures, whereas comprehension was not tested. Therefore, findings can be inconsistent depending on the investigated gesture task or underlying construct.
In addition to the variety of gesture tests, the selection of a suitable atlas and therefore the definition of the ROIs may have some intrinsic limitations. For example, the SFG can be divided into three separable subregions that are part of distinct functional networks (Li et al., 2013). Arbitrarily combining those functionally separate parts into one entity may lead to distorted or negative findings. Finally, studies of grey matter volume and functional activations do not always lead to similar results, and volume and cortical thickness provide not entirely the same information about grey matter structure (Winkler et al., 2010). So far, this is the first study in patients with schizophrenia investigating cortical thickness abnormalities in gesture production and recognition.
Surprisingly, in schizophrenia patients, superior gesture production was associated with reduced cortical thickness in the temporal pole. This negative correlation was only detected for the left side of this ROI and it is most likely driven by the TULIA imitation subscore (see Table 4 and S1). The result is quite counter-intuitive and if replicated requires clarification in future studies.
On the behavioral level, gesture production and recognition are strongly correlated in patients with schizophrenia (Walther et al., 2015). Based on this finding, we expected a pattern of associations between cortical thinning and gesture recognition similar to that found in gesture production. However, our analyses differed from these expectations. Gesture production was tested categorically based on a cut-off, whereas gesture recognition was tested as a continuous variable within a correlation with cortical thickness. We found associations of reduced cortical thickness in several regions, including the IPL, the opercular part of the IFG, the STG, and the SFG with inferior performance in the gesture recognition task in patients. The slightly different pattern of correlations for gesture production and recognition deficits in our study (see Table 3) indicates that our tests for gesture production and recognition do not measure the exact same underlying mechanisms. Our results are in line with a study that found a functional dysconnectivity of the left STS to the bilateral IFG during the processing of metaphoric gestures in patients with schizophrenia (Straube et al., 2014). In addition, a meta-analysis revealed the involvement of an interplay of several, mainly left-lateralized brain regions, including the IFG, the STG, the MTG, the SPL, and the IPL, in gesture recognition (Yang et al., 2015). Areas of the parietal lobe, comprising the SMG and the IPS, are involved in perceiving hand gestures (Andric and Small, 2012), whereas temporal and frontal areas, including the MTG, the STG, and the IFG are involved in the processing of gesture meanings and action recognition (Andric and Small, 2012, Buccino et al., 2004).
Some limitations of this study need to be addressed. First, we used a predefined atlas to map the ROIs on the brains (Desikan-Killiany atlas). Each ROI, for example the SFG (Li et al., 2013), can include several regions that are functionally independent, and a measurement across the entire ROI may cause distortions. Second, although some of our patients have a long medical history, our sample presents a large variance from first-episode to chronic patients. We controlled for the effect of medication and therefore indirectly also for duration of illness, but the heterogeneity of our sample nevertheless reduces the generalizability of our results. Third, age not only affects cortical thickness of frontal and temporal areas in schizophrenia patients (van Haren et al., 2011), but is also strongly associated with the gesture performance. Indeed, schizophrenia patients with gesture performance deficits are often the older patients. Even though we controlled for age in all of our analyses, one should bear in mind this closely linked relation between defective gesture performance and age.
5. Conclusion
In summary, this study explored, for the first time, cortical thickness abnormalities in gesture production and recognition in patients with schizophrenia. Our findings indicate that impaired gesture performance in schizophrenia may partly result from structural alterations, such as cortical thinning. These findings have significant implications for the understanding of the nature of gesture deficits in schizophrenia. Clearly, further studies are needed to specify the exact functions of the different regions and connecting fibers in specific gesture tasks. In addition, longitudinal studies are needed to disentangle the influence of illness chronicity from the gesture deficits in schizophrenia.
Funding Source
This study received funding from the Bangerter-Rhyner Foundation (to Sebastian Walther), the Swiss National Science Foundation (SNF grant 152,619/1 to Sebastian Walther, Andrea Federspiel, and Stephan Bohlhalter), and from the National Institute of Mental Health (NIMH T32MH016259-35 to Amanda E. Lyall).
Conflict of interest
The authors declare that there are no conflicts of interest regarding the subject of this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2017.10.017.
Appendix A. Supplementary data
Supplementary tables
References
- Andric M., Small S.L. 2012. Gesture's neural language. (1664–1078 Electronic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard J.A., Millman Z.B., Mittal V.A. Beat and metaphoric gestures are differentially associated with regional cerebellar and cortical volumes. Hum. Brain Mapp. 2015;36:4016–4030. doi: 10.1002/hbm.22894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F., Buccino G. Motor functions of the Broca's region. Brain Lang. 2004;89:362–369. doi: 10.1016/S0093-934X(03)00358-4. [DOI] [PubMed] [Google Scholar]
- Binkofski F., Buxbaum L.J. Two action systems in the human brain. Brain Lang. 2013;127:222–229. doi: 10.1016/j.bandl.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlhalter S., Hattori N., Wheaton L., Fridman E., Shamim E.A. Gesture subtype-dependent left lateralization of praxis planning: an event-related fMRI study. Cereb. Cortex. 2009;19:1256–1262. doi: 10.1093/cercor/bhn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlhalter S., Vanbellingen T., Bertschi M., Wurtz P., Cazzoli D. Interference with gesture production by theta burst stimulation over left inferior frontal cortex. Clin. Neurophysiol. 2011;122:1197–1202. doi: 10.1016/j.clinph.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Buccino G., Binkofski F., Riggio L. The mirror neuron system and action recognition. Brain Lang. 2004;89:370–376. doi: 10.1016/S0093-934X(03)00356-0. [DOI] [PubMed] [Google Scholar]
- Buxbaum L.J., Shapiro A.D., Coslett H.B. Critical brain regions for tool-related and imitative actions: a componential analysis. Brain. 2014;137:1971–1985. doi: 10.1093/brain/awu111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L.L., Haxby J.V., Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat. Neurosci. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Deichmann R., Schwarzbauer C., Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. NeuroImage. 2004;21:757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Del Re E.C., Gao Y., Eckbo R., Petryshen T.L., Blokland G.A. A new MRI masking technique based on multi-atlas brain segmentation in controls and schizophrenia: a rapid and viable alternative to manual masking. J. Neuroimaging. 2016;26:28–36. doi: 10.1111/jon.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fridman E.A., Immisch I., Hanakawa T., Bohlhalter S., Waldvogel D. The role of the dorsal stream for gesture production. NeuroImage. 2006;29:417–428. doi: 10.1016/j.neuroimage.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Goghari V.M., Rehm K., Carter C.S., AW MacDonald., 3rd Regionally specific cortical thinning and gray matter abnormalities in the healthy relatives of schizophrenia patients. Cereb. Cortex. 2007;17:415–424. doi: 10.1093/cercor/bhj158. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Apraxia and the parietal lobes. Neuropsychologia. 2009;47:1449–1459. doi: 10.1016/j.neuropsychologia.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Goldenberg G., Karnath H.O. The neural basis of imitation is body part specific. J. Neurosci. 2006;26:6282–6287. doi: 10.1523/JNEUROSCI.0638-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G., Hermsdorfer J., Glindemann R., Rorden C., Karnath H.O. Pantomime of tool use depends on integrity of left inferior frontal cortex. Cereb. Cortex. 2007;17:2769–2776. doi: 10.1093/cercor/bhm004. [DOI] [PubMed] [Google Scholar]
- Goldin-Meadow S., Alibali M.W. Gesture's role in speaking, learning, and creating language. Annu. Rev. Psychol. 2013;64:257–283. doi: 10.1146/annurev-psych-113011-143802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman A.L., Pezawas L., Mattay V.S., Fischl B., Verchinski B.A. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch. Gen. Psychiatry. 2009;66:467–477. doi: 10.1001/archgenpsychiatry.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb J. From thought to action: the parietal cortex as a bridge between perception, action, and cognition. Neuron. 2007;53:9–16. doi: 10.1016/j.neuron.2006.12.009. [DOI] [PubMed] [Google Scholar]
- van Haren N.E., Schnack H.G., Cahn W., van den Heuvel M.P., Lepage C. Changes in cortical thickness during the course of illness in schizophrenia. Arch. Gen. Psychiatry. 2011;68:871–880. doi: 10.1001/archgenpsychiatry.2011.88. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J., Terlinden G., Muhlau M., Goldenberg G., Wohlschlager A.M. Neural representations of pantomimed and actual tool use: evidence from an event-related fMRI study. NeuroImage. 2007;36(Suppl. 2):T109–18. doi: 10.1016/j.neuroimage.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J., Li Y., Randerath J., Roby-Brami A., Goldenberg G. Tool use kinematics across different modes of execution. Implications for action representation and apraxia. Cortex. 2013;49:184–199. doi: 10.1016/j.cortex.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Hoeren M., Kummerer D., Bormann T., Beume L., Ludwig V.M. Neural bases of imitation and pantomime in acute stroke patients: distinct streams for praxis. Brain. 2014;137:2796–2810. doi: 10.1093/brain/awu203. [DOI] [PubMed] [Google Scholar]
- Hostetter A.B. When do gestures communicate? A meta-analysis. Psychol. Bull. 2011;137:297–315. doi: 10.1037/a0022128. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey S.H., Newman-Norlund R., Grafton S.T. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb. Cortex. 2005;15:681–695. doi: 10.1093/cercor/bhh169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kroliczak G., Frey S.H. A common network in the left cerebral hemisphere represents planning of tool use pantomimes and familiar intransitive gestures at the hand-independent level. Cereb. Cortex. 2009;19:2396–2410. doi: 10.1093/cercor/bhn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota M., Miyata J., Yoshida H., Hirao K., Fujiwara H. Age-related cortical thinning in schizophrenia. Schizophr. Res. 2011;125:21–29. doi: 10.1016/j.schres.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Lavelle M., Healey P.G., McCabe R. Is nonverbal communication disrupted in interactions involving patients with schizophrenia? Schizophr. Bull. 2013;39:1150–1158. doi: 10.1093/schbul/sbs091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle M., Healey P.G., McCabe R. Nonverbal behavior during face-to-face social interaction in schizophrenia: a review. J. Nerv. Ment. Dis. 2014;202:47–54. doi: 10.1097/NMD.0000000000000031. [DOI] [PubMed] [Google Scholar]
- Li W., Qin W., Liu H., Fan L., Wang J. Subregions of the human superior frontal gyrus and their connections. NeuroImage. 2013;78:46–58. doi: 10.1016/j.neuroimage.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Manuel A.L., Radman N., Mesot D., Chouiter L., Clarke S. Inter- and intrahemispheric dissociations in ideomotor apraxia: a large-scale lesion-symptom mapping study in subacute brain-damaged patients. Cereb. Cortex. 2013;23:2781–2789. doi: 10.1093/cercor/bhs280. [DOI] [PubMed] [Google Scholar]
- Martin A., Wiggs C.L., Ungerleider L.G., Haxby J.V. Neural correlates of category-specific knowledge. Nature. 1996;379:649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Matthews N., Gold B.J., Sekuler R., Park S. Gesture imitation in schizophrenia. Schizophr. Bull. 2013;39:94–101. doi: 10.1093/schbul/sbr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengotti P., Corradi-Dell'Acqua C., Negri G.A., Ukmar M., Pesavento V., Rumiati R.I. Selective imitation impairments differentially interact with language processing. Brain. 2013;136:2602–2618. doi: 10.1093/brain/awt194. [DOI] [PubMed] [Google Scholar]
- Mozaz M., Rothi L.J., Anderson J.M., Crucian G.P., Heilman K.M. Postural knowledge of transitive pantomimes and intransitive gestures. J. Int. Neuropsychol. Soc. 2002;8:958–962. doi: 10.1017/s1355617702870114. [DOI] [PubMed] [Google Scholar]
- Niessen E., Fink G.R., Weiss P.H. Apraxia, pantomime and the parietal cortex. Neuroimage Clin. 2014;5:42–52. doi: 10.1016/j.nicl.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel-Knochel V., Knochel C., Rotarska-Jagiela A., Reinke B., Prvulovic D. Association between psychotic symptoms and cortical thickness reduction across the schizophrenia spectrum. Cereb. Cortex. 2013;23:61–70. doi: 10.1093/cercor/bhr380. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Panizzon M.S., Fennema-Notestine C., Eyler L.T., Jernigan T.L., Prom-Wormley E. Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Matthews N., Gibson C. Imitation, simulation, and schizophrenia. Schizophr. Bull. 2008;34:698–707. doi: 10.1093/schbul/sbn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol L.M., Hartberg C.B., Nesvag R., Fennema-Notestine C., Hagler D.J., Jr. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol. Psychiatry. 2010;68:41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Sprooten E., Papmeyer M., Smyth A.M., Vincenz D., Honold S. Cortical thickness in first-episode schizophrenia patients and individuals at high familial risk: a cross-sectional comparison. Schizophr. Res. 2013;151:259–264. doi: 10.1016/j.schres.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Stegmayer K., Bohlhalter S., Vanbellingen T., Federspiel A., Moor J. Structural brain correlates of defective gesture performance in schizophrenia. Cortex. 2016;78:125–137. doi: 10.1016/j.cortex.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Stegmayer K., Moor J., Vanbellingen T., Bohlhalter S., Muri R.M. Gesture performance in first- and multiple-episode patients with schizophrenia spectrum disorders. Neuropsychobiology. 2016;73:201–208. doi: 10.1159/000446116. [DOI] [PubMed] [Google Scholar]
- Stegmayer K., Bohlhalter S., Vanbellingen T., Federspiel A., Wiest R. Limbic interference during social action planning in Schizophrenia. Schizophr. Bull. 2017 doi: 10.1093/schbul/sbx059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube B., Green A., Sass K., Kirner-Veselinovic A., Kircher T. Neural integration of speech and gesture in schizophrenia: evidence for differential processing of metaphoric gestures. Hum. Brain Mapp. 2013;34:1696–1712. doi: 10.1002/hbm.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube B., Green A., Sass K., Kircher T. Superior temporal sulcus disconnectivity during processing of metaphoric gestures in schizophrenia. Schizophr. Bull. 2014;40:936–944. doi: 10.1093/schbul/sbt110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar K.N., Peterman J.S., Park S. Altered brain activation during action imitation and observation in schizophrenia: a translational approach to investigating social dysfunction in schizophrenia. Am. J. Psychiatry. 2014;171:539–548. doi: 10.1176/appi.ajp.2013.13040498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanbellingen T., Kersten B., Van Hemelrijk B., Van de Winckel A., Bertschi M. Comprehensive assessment of gesture production: a new test of upper limb apraxia (TULIA) Eur. J. Neurol. 2010;17:59–66. doi: 10.1111/j.1468-1331.2009.02741.x. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N., Allen N.B., Youssef G., Dennison M., Yucel M. Brain development during adolescence: a mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum. Brain Mapp. 2016;37:2027–2038. doi: 10.1002/hbm.23154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vry M.S., Tritschler L.C., Hamzei F., Rijntjes M., Kaller C.P. The ventral fiber pathway for pantomime of object use. NeuroImage. 2015;106:252–263. doi: 10.1016/j.neuroimage.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Walther S., Mittal V.A. Why we should take a closer look at gestures. Schizophr. Bull. 2016;42:259–261. doi: 10.1093/schbul/sbv229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S., Vanbellingen T., Muri R., Strik W., Bohlhalter S. Impaired gesture performance in schizophrenia: particular vulnerability of meaningless pantomimes. Neuropsychologia. 2013;51:2674–2678. doi: 10.1016/j.neuropsychologia.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Walther S., Vanbellingen T., Muri R., Strik W., Bohlhalter S. Impaired pantomime in schizophrenia: association with frontal lobe function. Cortex. 2013;49:520–527. doi: 10.1016/j.cortex.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Walther S., Stegmayer K., Sulzbacher J., Vanbellingen T., Muri R. Nonverbal social communication and gesture control in schizophrenia. Schizophr. Bull. 2015;41:338–345. doi: 10.1093/schbul/sbu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S., Eisenhardt S., Bohlhalter S., Vanbellingen T., Muri R. Gesture performance in schizophrenia predicts functional outcome after 6 months. Schizophr. Bull. 2016;42:1326–1333. doi: 10.1093/schbul/sbw124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P.H., Ubben S.D., Kaesberg S., Kalbe E., Kessler J. Where language meets meaningful action: a combined behavior and lesion analysis of aphasia and apraxia. Brain Struct. Funct. 2016;221:563–576. doi: 10.1007/s00429-014-0925-3. [DOI] [PubMed] [Google Scholar]
- White T.P., Borgan F., Ralley O., Shergill S.S. You looking at me?: interpreting social cues in schizophrenia. Psychol. Med. 2016;46:149–160. doi: 10.1017/S0033291715001622. [DOI] [PubMed] [Google Scholar]
- Winkler A.M., Kochunov P., Blangero J., Almasy L., Zilles K. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S.W. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Yang J., Andric M., Mathew M.M. The neural basis of hand gesture comprehension: a meta-analysis of functional magnetic resonance imaging studies. Neurosci. Biobehav. Rev. 2015;57:88–104. doi: 10.1016/j.neubiorev.2015.08.006. [DOI] [PubMed] [Google Scholar]
- Ziermans T.B., Schothorst P.F., Schnack H.G., Koolschijn P.C., Kahn R.S. Progressive structural brain changes during development of psychosis. Schizophr. Bull. 2012;38:519–530. doi: 10.1093/schbul/sbq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables