Abstract
Very low birth weight (VLBW: ≤ 1500 g) individuals have an increased risk of white matter alterations and neurodevelopmental problems, including fine and gross motor problems. In this hospital-based follow-up study, the main aim was to examine white matter microstructure and its relationship to fine and gross motor function in 31 VLBW young adults without cerebral palsy compared with 31 term-born controls, at mean age 22.6 ± 0.7 years. The participants were examined with tests of fine and gross motor function (Trail Making Test-5: TMT-5, Grooved Pegboard, Triangle from Movement Assessment Battery for Children-2: MABC-2 and High-level Mobility Assessment Tool: HiMAT) and diffusion tensor imaging (DTI). Probabilistic tractography of motor pathways of the corticospinal tract (CST) and corpus callosum (CC) was performed. Fractional anisotropy (FA) was calculated in non-crossing (capsula interna in CST, body of CC) and crossing (centrum semiovale) fibre regions along the tracts and examined for group differences. Associations between motor test scores and FA in the CST and CC were investigated with linear regression. Tract-based spatial statistics (TBSS) was used to examine group differences in DTI metrics in all major white matter tracts. The VLBW group had lower scores on all motor tests compared with controls, however, only statistically significant for TMT-5. Based on tractography, FA in the VLBW group was lower in non-crossing fibre regions and higher in crossing fibre regions of the CST compared with controls. Within the VLBW group, poorer fine motor function was associated with higher FA in crossing fibre regions of the CST, and poorer bimanual coordination was additionally associated with lower FA in crossing fibre regions of the CC. Poorer gross motor function was associated with lower FA in crossing fibre regions of the CST and CC. There were no associations between motor function and FA in non-crossing fibre regions of the CST and CC within the VLBW group. In the TBSS analysis, the VLBW group had lower FA and higher mean diffusivity compared with controls in all major white matter tracts. The findings in this study may indicate that the associations between motor function and FA are caused by other tracts crossing the CST and CC, and/or by alterations in the periventricular white matter in the centrum semiovale. Some of the associations were in the opposite direction than hypothesized, thus higher FA does not always indicate better function. Furthermore, widespread white matter alterations in VLBW individuals persist into young adulthood.
Abbreviations: AD, axial diffusivity; CC, corpus callosum; CST, corticospinal tract; DTI, diffusion tensor imaging; FA, fractional anisotropy; HiMAT, high-level mobility assessment tool; MABC-2, movement assessment battery for children-2; MD, mean diffusivity; MNI, Montreal neurological institute; MRI, magnetic resonance imaging; NICU, neonatal intensive care unit; RD, radial diffusivity; ROI, region-of-interest; TBSS, tract-based spatial statistics; SES, socioeconomic status; TMT-5, Trail Making Test-5; VLBW, very low birth weight; VOI, volume-of-interest
Keywords: Preterm, Brain, Diffusion tensor imaging, Tractography, Motor function, Young adulthood
Highlights
-
•
Motor function was associated with FA in crossing fibre regions of CST and CC in VLBW young adults
-
•
In crossing fibre regions of CST, FA was higher in VLBW than in control young adults
-
•
TBSS showed lower FA and higher MD in white matter tracts in VLBW than in control young adults
1. Introduction
Being born preterm with very low birth weight (VLBW: ≤ 1500 g) is associated with increased risk of disturbances in perinatal brain development (Volpe, 2009) and later neurodevelopmental problems (Aarnoudse-Moens et al., 2009, de Kieviet et al., 2009). Fine and gross motor problems are common and have been found to persist into young adulthood, also when excluding participants with cerebral palsy (Husby et al., 2013).
The preterm brain is particularly susceptible to diffuse white matter injury, related to a disrupted maturation of pre-oligodendrocytes into oligodendrocytes, important for axonal myelination (Volpe, 2009). Myelination deficits may affect structural and functional connectivity, leading to slower signalling in the brain. In normal brain development, diffusion tensor imaging (DTI) (Basser and Pierpaoli, 1996) shows that fractional anisotropy (FA) increases and mean diffusivity (MD) decreases as white matter pathways mature and myelinate, starting during gestation and continuing into adulthood (Cascio et al., 2007, Lebel et al., 2008). Preterm birth disrupts the normal maturation of white matter, and the preterm brain typically exhibits lower FA and higher MD compared with term-born controls at birth, adolescence and young adulthood (Li et al., 2014, Pandit et al., 2013). Several white matter tracts are affected, including motor pathways such as the corticospinal tract (CST) and the corpus callosum (CC) (Eikenes et al., 2011, Groeschel et al., 2014). Although most studies have reported lower FA in VLBW individuals compared with controls, segmented analyses of the CST have demonstrated that FA varies significantly along the tract. In crossing fibre regions, such as the corona radiata and centrum semiovale, FA has been shown to be higher in VLBW young adults than controls, while FA was lower in the VLBW group in non-crossing fibre regions, such as the capsula interna (Groeschel et al., 2014, Jurcoane et al., 2016). This indicates that crossing fibres affect FA differently in the two groups.
To find a structural explanation for the commonly seen fine and gross motor problems in VLBW individuals, some studies have searched for microstructural correlates to motor problems in the whole brain and in specific motor tracts. Better fine motor performance at two years has been found to correlate with higher FA in all major white matter tracts by TBSS in very preterm infants at term equivalent age (van Kooij et al., 2012). Another study did not find any correlation between motor function and FA at two years of age in very preterm children without major motor problems and focal lesions (Counsell et al., 2008). In older VLBW children and adolescents, reduced FA in several white matter tracts has been shown to be associated with motor problems (de Kieviet et al., 2014, Li et al., 2014, Skranes et al., 2007), while FA in the CST of VLBW adults was not associated with motor problems at eight years (Jurcoane et al., 2016). Hence, the few existing studies investigating structural white matter correlates to motor problems yield mixed findings, possibly due to variation in DTI analysis and motor assessments. Furthermore, there are no studies examining associations between fine and gross motor function and DTI metrics in segmented regions of motor tracts in preterm populations.
The aim of this study was to investigate associations between fine and gross motor function and FA in non-crossing and crossing fibre regions of the CST and CC with probabilistic tractography in VLBW young adults without cerebral palsy compared with a term-born control group. Moreover, we wanted to examine group differences in DTI metrics in the whole brain by tract-based spatial statistics (TBSS). We hypothesized that, within the VLBW group, poorer motor function would be associated with lower FA in motor pathways of the CST and CC, especially in non-crossing fibre regions. More specifically, we hypothesized that poorer basic fine motor function was associated with lower FA in the CST from the primary motor cortex and that poorer complex fine motor function was associated with lower FA in the CST from the primary and premotor cortices. Furthermore, we hypothesized that poorer bimanual coordination was associated with lower FA in the CC connecting the primary motor and premotor cortices in addition to FA in the CST from the primary motor and premotor cortices. For gross motor function, we hypothesized that lower scores on automatic tasks were associated with lower FA in the CST from the primary motor cortex, and that lower scores on tasks involving planning were associated with lower FA in the CST from the premotor cortex. As for group differences in DTI metrics, we hypothesized that VLBW young adults had lower FA and higher MD in all major white matter tracts by TBSS compared with controls.
2. Materials and methods
2.1. Study design
The present study is part of a prospective hospital-based study of a preterm born VLBW group (birth weight ≤ 1500 g) and a term-born control group (gestational age ≥ 37 weeks). Clinical tests and cerebral magnetic resonance imaging (MRI) have been carried out at 1, 5, 14 and 20 years of age (Eikenes et al., 2011, Skranes et al., 2007, Skranes et al., 1997, Skranes et al., 1993). The VLBW children were born in 1986–1988 and admitted to the Neonatal Intensive Care Unit (NICU) at St. Olavs Hospital, Trondheim University Hospital, Norway. The control children were included at birth in the same period. They were born to a 10% random sample of women living in the Trondheim region, originally selected for follow-up during pregnancy in a multicentre study (Bakketeig et al., 1993). At 23 years, we aimed to include the VLBW and control participants from the 14-year follow-up in order to compare motor function longitudinally (Husby et al., 2013).
2.2. Participants
2.2.1. VLBW group
At age 23, we contacted 54 VLBW young adults, whereof 18 (33%) did not consent, leaving 36 (67%) VLBW young adults for motor and MRI examinations. Four (11%) of these had cerebral palsy and were therefore excluded from the study. Additionally, one VLBW participant was excluded due to image artefacts, leaving data from 31 VLBW participants suitable for analyses.
2.2.2. Control group
At age 23, we contacted 48 controls matched to the VLBW participants by age and sex. Two of the contacted controls were not testable due to pregnancy, five had moved too far away and four did not consent. Thus, 37 (77%) controls underwent motor and MRI examinations, whereof six were excluded due to image artefacts, leaving data from 31 control participants suitable for analyses.
2.2.3. Non-participants
There were no significant differences between those who participated and those who did not consent to or were not contacted for participation at age 23 in either group with regard to perinatal data, parental socioeconomic status (SES) or motor skills at 14 years (data not shown).
2.3. Clinical characteristics
Perinatal data included birth weight, gestational age, birth head circumference, Apgar scores, days in NICU, days on mechanical ventilator, proportion of infants with intraventricular haemorrhage and maternal age. At 14 years, parental SES was calculated according to Hollingshead's Two factor index of social position (Hollingshead, 1957), rated from 1 (lowest) to 5 (highest) based on a combination of parents' education and occupation.
2.4. Motor assessments
The motor assessments were carried out by experienced examiners, blinded to neonatal history, clinical characteristics and results from previous follow-up. The dominant hand was defined as the writing hand and the dominant foot was self-reported or defined as the preferred foot for one leg stand.
2.4.1. Trail Making Test-5 (TMT-5)
TMT-5 is part of the standardized Delis-Kaplan Executive Function System and measures motor speed (Delis et al., 2001). The task is to draw a line as fast as possible (time in seconds) between 32 circles in the order directed by a dotted line. The test is performed with the dominant hand only and we considered it as a test of basic fine motor function.
2.4.2. Grooved Pegboard
Grooved Pegboard requires complex visual-motor coordination and measures how quickly (time in seconds) the participants can insert pegs into 25 keyhole-shaped holes with various orientations in a 5 × 5 matrix with each hand separately (Grooved Pegboard Test. User instructions, 2002). We considered this test as a test of complex fine motor function.
2.4.3. Triangle from Movement Assessment Battery for Children-2 (MABC-2)
MABC-2 is a comprehensive motor test battery that identifies and evaluates children's motor development (Henderson et al., 2007). We used the item Triangle as a test for bimanual coordination. The task is to form a triangle out of three strips, nuts and bolts with both hands as fast as possible. Raw scores (time in seconds) were used in this study.
2.4.4. High-level Mobility Assessment Tool (HiMAT)
HiMAT examines gross motor function (Williams et al., 2005a, Williams et al., 2005b) and raw scores of the seven timed items (in seconds) were used in this study. We grouped these items in two categories based on the involvement of automatic or planned movements; HiMAT automatic (Walk, Walk on toes, Run) and HiMAT planned (Walk backwards, Walk over obstacle, Skip, Hop forward).
2.5. Image acquisition
At 23 years, DTI was acquired on a 3 T Siemens Trio with Quantum gradients (30 mT/m) and a 12-channel head matrix coil (Siemens AG, Erlangen, Germany) at St. Olavs Hospital, Trondheim University Hospital, Norway. To reduce movement, foam pads were placed around the participants' heads. DTI was acquired with a single-shot balanced-echo EPI sequence with b = 1000 s/mm2 in 30 non-collinear directions using the following parameters: TR = 6800 ms, TE = 84 ms, FOV = 240 × 240 mm, slice thickness 2.5 mm, acquisition matrix 96 × 96, giving isotropic voxels of 2.5 mm. Full brain coverage was obtained with 55 transversal slices with no gap. For each slice, six images without diffusion weighting (b = 0) were acquired. The DTI sequence was repeated two times for increased signal-to-noise ratio. To correct for image distortion caused by magnetic susceptibility artefacts (Holland et al., 2010), two additional b0 images were acquired with opposite phase-encode polarity.
2.6. Image analyses
The DTI analyses were performed with the tools of the FMRIB Software Library (FSL; Oxford Centre for Functional MRI of the Brain, UK; www.fmrib.ox.ac.uk/fsl). Image artefacts due to motion and eddy current distortions were minimized by registration of all DTI acquisitions to the mean b = 0 image using affine registration. Image distortion caused by magnetic susceptibility artefacts was minimized with a nonlinear B0-unwarping method using paired images with opposite phase-encode polarities (Holland et al., 2010). The brain was extracted using Brain Extraction Tool (BET, part of FSL). FMRIB's Diffusion Toolbox (FDT) was used to fit a diffusion tensor model to the raw diffusion data in each voxel. Voxel-wise maps of FA, MD, axial (AD; λ1) and radial diffusivity (RD; (λ2 + λ3) / 2) were calculated for the VLBW and control group.
2.6.1. Probabilistic tractography
Fibre tractography of the CST and CC was performed using BedpostX, a probabilistic tractography routine implemented in FSL based on a multifibre model (Behrens et al., 2007). The following parameters were used: 5000 streamline samples, 0.5 mm step lengths, curvature thresholds = 0.2.
2.6.1.1. Corticospinal tract (CST)
A region-of-interest (ROI) approach was used to track the CST fibres connecting from a seed ROI in the cerebral peduncles to three different target ROIs in the primary motor and premotor cortices. The seed ROI was placed manually in three contiguous slices in the cerebral peduncle on each participant's colour-coded FA map in individual space based on anatomical landmarks in the FA map (Fig. 1).
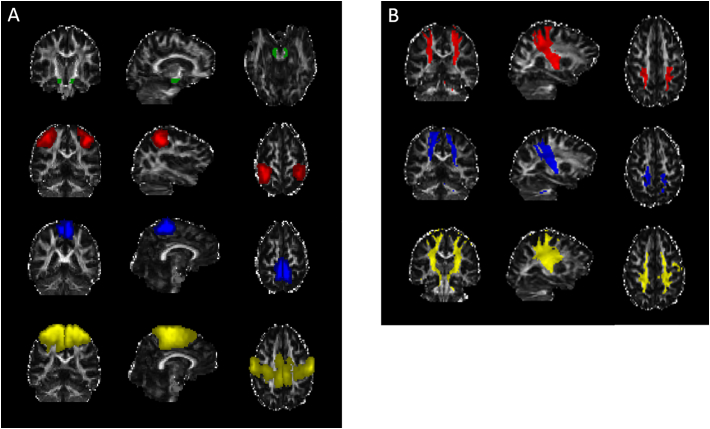
Fig. 1.
A) ROIs used for the probabilistic tractography of the left and right corticospinal tract (CST): Seed ROI in the cerebral peduncles (green) and target ROIs in the hand area in the primary motor cortex (red), foot area in the primary motor cortex (blue) and premotor cortex (yellow). B) Probabilistic tractography results of the CSThand (red), CSTfoot (blue) and CSTpremotor (yellow). Underlying coronal, sagittal and axial gray scaled image is the FA map for one of the participants in the study in individual space.
Abbreviations: ROI, region-of-interest; CST, corticospinal tract; FA, fractional anisotropy. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For the primary motor cortex, we wanted to select the hand and foot areas specifically as target ROIs for tracking of CSThand and CSTfoot. Due to the difficulty of depicting the foot area of the primary motor cortex based on anatomy or template based atlases, both foot and hand ROIs were based on functional ROIs obtained in a group of young healthy adults in MNI space (Berntsen et al., 2008).
For the CSTpremotor tracking, the target ROI was placed in the premotor cortex as defined by the Juelich Histological atlas in FSL (Eickhoff et al., 2005) in MNI space. The target ROIs in the primary motor and premotor cortices were transformed from MNI space to each participant's individual FA space using linear and non-linear registration (FLIRT and FNIRT, FMRIB's Linear and Non-Linear Image Registration Tool) between each participant's FA map and the MNI FA-template (FMRIB58_FA_1mm).
Only streamlines passing through the seed ROI and the target ROI were included in the analyses. The CST tracking was performed separately for the left and right hemispheres. An exclusion mask (not shown) was included in the midline of the brain dividing the two hemispheres, and streamlines were discarded if they entered the exclusion mask.
2.6.1.2. Corpus callosum (CC)
For tracking of the CC, a three ROI approach was used to select the CC fibres connecting the primary motor (CCmotor) and the premotor (CCpremotor) cortices (Fig. 2). The seed ROIs were placed manually on each participant's colour-coded FA map in individual space in three contiguous sagittal slices of CC region number II and III according to Hofer and Frahm (2006), comprising premotor and motor fibres, respectively (Hofer and Frahm, 2006). The target ROIs were placed in the primary motor and the premotor cortices in the left and right cerebral hemispheres, as defined by the Juelich Histological atlas in FSL (Eickhoff et al., 2005). The target ROIs were given in MNI space, and were transformed from MNI to each participant's individual FA space using FLIRT and FNIRT. Only streamlines passing through the seed ROI and the target ROIs were included in the analyses. An exclusion mask (not shown) was included in the axial slice inferior to the sagittal seed ROIs, and streamlines were discarded if entering the exclusion mask. We chose to use the whole primary motor cortex instead of the hand and foot areas in the CC tracking, because pilot testing showed that few streamlines passed through the CC to the hand area lateral in the primary motor cortices. The difficulty of resolving lateral projections of the CC has been described earlier (Hofer and Frahm, 2006).
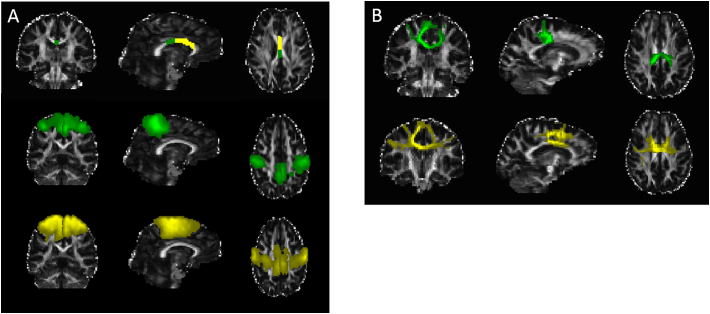
Fig. 2.
A) ROIs used for probabilistic tractography of the corpus callosum (CC): Seed ROIs in the CC corresponding to areas connecting the primary motor (green) and premotor cortices (yellow), target ROIs in the primary motor cortex (green) and premotor cortex (yellow). B) Probabilistic tractography results of the CCmotor (green) and CCpremotor (yellow). Underlying coronal, sagittal and axial gray scaled image is the FA map for one of the participants in the study in individual space.
Abbreviations: ROI, region-of-interest; CC, corpus callosum; FA, fractional anisotropy. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.6.1.3. Quality control
The registration of the ROIs from MNI to individual space and resulting fibre tracts were visually inspected for each participant, and the tractography results were thresholded (50 for the CST and 300 for the CC) in order to include only anatomical plausible pathways. All manually placed ROIs were drawn by the same researcher, blinded to neonatal history, clinical characteristics and results from previous follow-up.
2.6.1.4. Segmentation of the CST and CC
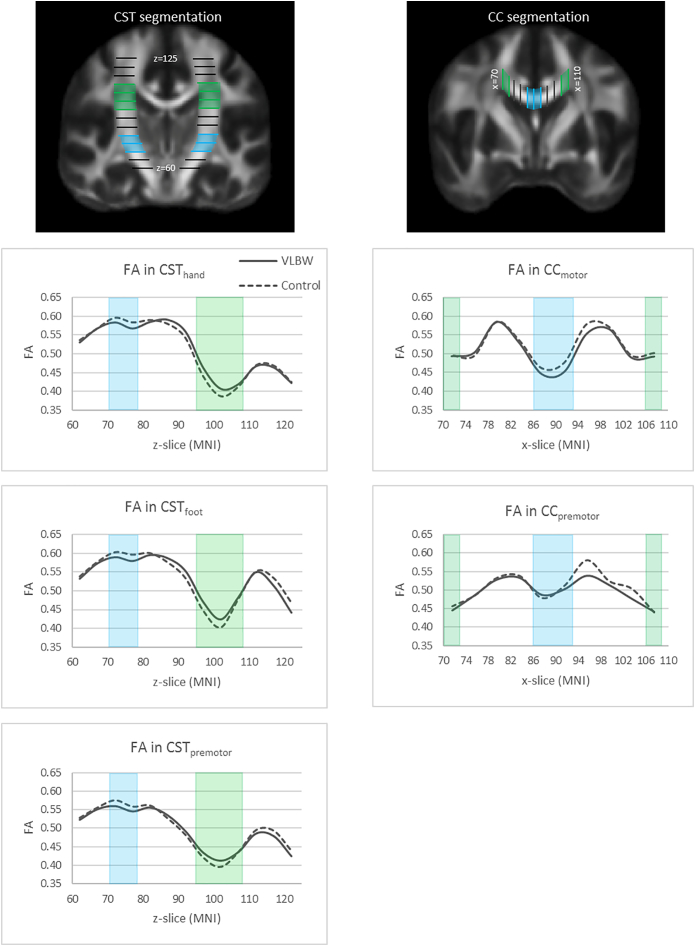
Segmentation of the tracts was performed by generating slice-wise tract profiles along the z-axis (inferior-superior) for the CSThand, CSTfoot and CSTpremotor and along the x-axis (left-right) for the CCmotor and CCpremotor in order to calculate FA at equivalent levels across individuals. These tract profiles are shown in Fig. 3. The slices were defined in MNI space and warped into each participant's individual space using the corresponding warp field computed in the TBSS procedure with FLIRT/FNIRT.
Fig. 3.
Segmentation of the corticospinal tract (CST) and the corpus callosum (CC) are shown in MNI template space in the first row. The graphs show fractional anisotropy (FA) (y-axis) in the contralateral CST to the dominant hand and CC slice-wise along the tracts (x-axis) in MNI template space for the VLBW group (solid lines) and the control group (dashed lines). Blue and green areas are volumes of interest indicating regions of the tracts with predominantly non-crossing and crossing fibres, respectively.
For the CST tracts, the slice-wise measurements were acquired for five contiguous slices from the cerebral peduncles (z = 60) to above the lateral ventricles (z = 124), thus generating 13 volumes-of-interest (VOIs). For the CC tracts, the slice-wise measurements were acquired for four contiguous slices between the lateral parts of the CC from left to right (x = 70–109), thus generating 10 VOIs. Furthermore, the slice-wise measurements were divided into regions of predominantly non-crossing and crossing fibres. For the CST, non-crossing regions were defined as an area with few large crossing fibres, corresponding to the capsula interna (z = 70–79), and crossing fibre regions were defined as the area where the superior longitudinal fascicle crosses the CST, corresponding to the centrum semiovale and corona radiata (z = 95–109) (Fig. 3). For the CC, the non-crossing region was defined as the medial part, corresponding to the body of the CC where there are less crossing fibres (x = 86–93) (Groeschel et al., 2014), while the crossing fibre regions were defined as the lateral parts of the CC, corresponding to the centrum semiovale (x = 70–73 and x = 106–109) (Fig. 3). FA was measured at the intersection of the VOIs and the streamlines. Mean FA was calculated for non-crossing and crossing fibre regions for the CSThand, CSTfoot, CSTpremotor, CCmotor and CCpremotor tractography results in all VLBW and control individuals.
2.6.2. Tract-based spatial statistics (TBSS)
Voxel-wise statistical analysis was performed using TBSS (part of FSL) (Smith et al., 2006). A detailed description is given elsewhere (Eikenes et al., 2011). Voxel-wise statistics of the skeletonized FA, MD, AD and RD were carried out on the white matter skeleton using Randomise (part of FSL) to test for group differences between the VLBW and control group. Randomise performs non-parametric permutation-based testing and inference using Threshold-Free Cluster Enhancement (Nichols and Holmes, 2002) with a correction for multiple comparisons (p < 0.05, corrected for sex and age at MRI).
2.7. Statistical analyses
Student's t-test was used for approximately normally distributed data; else the Mann–Whitney U test was applied. Normality was assessed by visual inspection of Q-Q plots of the residuals. Linear regression was applied to explore associations between motor test scores and FA in non-crossing and crossing fibre regions of the CST and CC. Motor test scores were entered separately as dependent variables, whereas FA, group, sex, age and the interaction FA x group were entered as independent variables. The interaction term was added to test if the effect of FA was different in VLBW and control participants. We re-categorized the left and right CST into contralateral CST to the dominant and non-dominant hand. For motor tests performed with one hand (TMT-5 and Grooved Pegboard), only the contralateral CST in the brain was included in the association analyses to reduce the number of analyses. For motor tests performed with two hands (MABC-2 Triangle) or the whole body (HiMAT), the right and left CST were averaged in the association analyses as this gave similar results as separate analyses of each side. Two-sided p-values < 0.05 were considered statistically significant. Given the relatively small sample size, no correction for multiple comparisons was applied for linear regression. SPSS 22.0 was used for statistical analyses.
2.8. Ethics
The project complies with the principles of the Declaration of Helsinki and the study protocol was approved by the Regional Committee for Medical and Health Research Ethics in Central Norway (REK number 4.2005.2605). Written informed consent was obtained from the participants.
3. Results
3.1. Clinical characteristics
Table 1 shows the clinical characteristics of the VLBW and the control group. Birth weight and gestational age differed by definition between the groups and, as expected, head circumference at birth and Apgar scores were lower in the VLBW group. There were no statistically significant group differences in maternal age, parental SES, age at follow-up or sex distribution.
Table 1.
Clinical characteristics of the VLBW group compared with the control group at 23 years of age.
|
VLBW (n = 31) |
Control (n = 31) |
p-value | |||
|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | ||
| Birth weight (g) | 1238 | (219) | 3603 | (364) | < 0.001 |
| Gestational age (weeks) | 29.4 | (2.7) | 39.5 | (1.1) | < 0.001 |
| Birth head circumference (cm)a | 27.4 | (2.3) | 35.3 | (1.2) | < 0.001 |
| Apgar score after 1 minb | 6.7 | (1.9) | 8.9 | (0.4) | < 0.001 |
| Apgar score after 5 minc | 8.4 | (1.7) | 9.7 | (1.7) | 0.005 |
| Maternal age | 28.8 | (5.2) | 30.2 | (4.2) | 0.262 |
| Parental SES at 14 yearsc | 3.4 | (1.2) | 3.7 | (1.0) | 0.462 |
| Age at follow-up | 22.5 | (0.7) | 22.7 | (0.7) | 0.253 |
| n | (%) | n | (%) | ||
| Boys | 11 | (35.5) | 14 | (42.2) | 0.437 |
| Intraventricular haemorrhaged | 2 | (6.7) | NA | NA | |
| Median | (Range) | Median | (Range) | ||
| Stay in NICU (days)e | 60 | (25–386) | NA | NA | |
| Mechanical ventilation (days)e | 1 | (0–44) | NA | NA | |
Analyses performed with Student's t-test and Mann-Whitney U test.
Abbreviations: VLBW, very low birth weight; SES, socioeconomic status; NICU, neonatal intensive care unit; NA, not applicable.
Data missing for seven VLBW and two control participants.
Data missing for three control participants.
Data missing for two control participants.
Grade I and II.
Data missing for two VLBW participants.
3.2. Motor function
Table 2 shows fine and gross motor test scores in the VLBW and the control group. The VLBW group used longer time than controls on all fine motor tests, however only significant for TMT-5 (Table 2). On gross motor tests, there were no significant group differences for HiMAT automatic and HiMAT planned. However, VLBW young adults were significantly slower than controls on the items Run and Hop forward (Table 2).
Table 2.
Motor test scores in the VLBW group compared with the control group at 23 years of age.
| VLBW (n = 31) |
Control (n = 31) |
||||
|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | p-value | |
| Fine motor tests (sec) | |||||
| Trail Making Test-5 | 28.5 | (11.1) | 19.7 | (5.7) | < 0.001 |
| Grooved Pegboard dom. hand | 68.6 | (17.2) | 63.5 | (9.9) | 0.161 |
| Grooved Pegboard non-dom. hand | 73.6 | (13.5) | 68.1 | (11.0) | 0.080 |
| MABC-2 triangle | 36.5 | (12.0) | 32.8 | (7.4) | 0.152 |
| Gross motor tests (sec) | |||||
| HiMAT automatic | 10.6 | (1.8) | 10.1 | (1.6) | 0.247 |
| Walk | 3.9 | (0.7) | 3.9 | (0.6) | 0.614 |
| Walk on toes | 4.8 | (1.0) | 4.5 | (1.0) | 0.234 |
| Run | 1.9 | (0.3) | 1.7 | (0.2) | 0.045 |
| HiMAT planned | 16.6 | (3.5) | 15.3 | (2.4) | 0.088 |
| Walk backwards | 5.4 | (1.8) | 4.8 | (0.8) | 0.071 |
| Walk obstacle | 4.1 | (0.7) | 4.0 | (0.7) | 0.377 |
| Skip | 3.0 | (0.6) | 2.9 | (0.6) | 0.227 |
| Hop forward | 4.7 | (2.7) | 3.6 | (0.8) | 0.039 |
Analyses performed with Student's t-test.
Abbreviations: VLBW, very low birth weight; dom, dominant; MABC-2, Movement Assessment Battery for Children-2; HiMAT, High-level Mobility Assessment Tool.
3.3. Probabilistic tractography
Table 3 shows that FA was lower in non-crossing fibre regions and higher in crossing fibre regions of the CST in the VLBW group compared with the control group. There were no group differences in FA of the CC (Table 3). Fig. 3 shows graphs of FA in the segmented parts of the CST and CC.
Table 3.
Mean fractional anisotropy in predominantly crossing and non-crossing fibre regions of the corticospinal tract (CST)a and the corpus callosum (CC)b in the VLBW group and the control group.
| VLBW (n = 31) |
Control (n = 31) |
|||||
|---|---|---|---|---|---|---|
| Motor tract | Tract region | Mean | (SD) | Mean | (SD) | p-value |
| CSThand dom | Non-crossing | 0.575 | (0.038) | 0.589 | (0.030) | 0.100 |
| Crossing | 0.430 | (0.040) | 0.413 | (0.030) | 0.063 | |
| CSThand non-dom | Non-crossing | 0.572 | (0.037) | 0.596 | (0.037) | 0.012 |
| Crossing | 0.440 | (0.039) | 0.423 | (0.025) | 0.046 | |
| CSTfoot dom | Non-crossing | 0.584 | (0.039) | 0.600 | (0.033) | 0.097 |
| Crossing | 0.459 | (0.045) | 0.443 | (0.030) | 0.108 | |
| CSTfoot non-dom | Non-crossing | 0.587 | (0.040) | 0.609 | (0.031) | 0.017 |
| Crossing | 0.468 | (0.043) | 0.442 | (0.026) | 0.006 | |
| CSTpremotor dom | Non-crossing | 0.552 | (0.040) | 0.568 | (0.031) | 0.090 |
| Crossing | 0.428 | (0.027) | 0.418 | (0.024) | 0.127 | |
| CSTpremotor non-dom | Non-crossing | 0.544 | (0.033) | 0.544 | (0.042) | 0.995 |
| Crossing | 0.434 | (0.025) | 0.424 | (0.019) | 0.086 | |
| CCmotor | Non-crossing | 0.448 | (0.049) | 0.469 | (0.039) | 0.066 |
| Crossing | 0.492 | (0.042) | 0.499 | (0.284) | 0.456 | |
| CCpremotor | Non-crossing | 0.495 | (0.030) | 0.495 | (0.037) | 0.949 |
| Crossing | 0.444 | (0.034) | 0.448 | (0.032) | 0.671 | |
Analyses performed with Student's t-test. Dom and non-dom indicate the contralateral CST to the dominant and non-dominant hand, respectively.
Abbreviations: CST, corticospinal tract; CC, corpus callosum; VLBW, very low birth weight; dom, dominant.
CSThand, CST from hand area in primary motor cortex; CSTfoot, CST from foot area in primary motor cortex; CSTpremotor, CST from premotor cortex.
CCmotor, CC connecting the primary motor cortices; CCpremotor, CC connecting the premotor cortices.
3.4. Associations between motor function and fractional anisotropy of CST and CC by probabilistic tractography
There were significant between-group differences for some of the associations between fine motor test scores and FA in non-crossing and crossing fibre regions of the CST and CC (Table 4). Within the VLBW group, there were no significant associations between motor tests and FA in non-crossing fibre regions. However, there were several significant associations in crossing fibre regions of the CST and CC within the VLBW group, both positive and negative. For fine motor function, poorer performance on TMT-5 was associated with higher FA in crossing fibre regions of CSThand and poorer performances on Grooved Pegboard dominant hand and MABC-2 Triangle were associated with higher FA in crossing fibre regions of CSThand and CSTpremotor. In contrast, poorer performance on MABC-2 Triangle was associated with lower FA in crossing fibre regions of CCmotor. For gross motor function, poorer performance on HiMAT planned was associated with lower FA in crossing fibre regions of CSTfoot and CCpremotor. Within the control group, poorer performance on Grooved Pegboard non-dominant hand was associated with lower FA in non-crossing fibre regions of CSThand and CSTpremotor, and poorer performance on MABC-2 Triangle was associated with higher FA in crossing fibre regions of CCmotor.
Table 4.
Associations between motor test scores and mean FA in non-crossing and crossing fibre regions of the corticospinal tract (CST)a and corpus callosum (CC)b in the VLBW group and the control group at 23 years of age.
| VLBW (n = 31) |
Control (n = 31) |
FA × group |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean FA of motor tracts | B | (95% CI) | p-value | B | (95% CI) | p-value | p-value | ||
| Fine motor tests (sec) | Motor tract | Tract region | |||||||
| Trail Making Test-5 dominant hand |
CSThand | Non-crossing | − 11.7 | (− 96.6 to 73.2) | 0.783 | 24.5 | (− 82.2 to 125.2) | 0.679 | 0.623 |
| Crossing | 100.8 | (24.0 to 177.6) | 0.011 | 45.4 | (− 50.4 to 141.2) | 0.346 | 0.370 | ||
| CSTpremotor | Non-crossing | − 22.1 | (− 101.0 to 56.8) | 0.577 | 31.2 | (− 68.8 to 131.2) | 0.535 | 0.407 | |
| Crossing | 100.1 | (− 16.7 to 217.0) | 0.092 | 17.6 | (− 108.4 to 143.7) | 0.780 | 0.336 | ||
| Grooved Pegboard dominant hand |
CSThand | Non-crossing | − 75.3 | (− 220.0 to 69.4) | 0.302 | − 7.7 | (− 184.4 to 169.0) | 0.931 | 0.558 |
| Crossing | 177.7 | (45.5 to 310.0) | 0.009 | − 42.1 | (− 206.9 t 122.7) | 0.611 | 0.042 | ||
| CSTpremotor | Non-crossing | − 71.5 | (− 206.4 to 63.4) | 0.293 | − 7.3 | (− 178.3 to 163.8) | 0.933 | 0.558 | |
| Crossing | 207.8 | (10.1 to 405.4) | 0.040 | − 61.6 | (− 274.7 to 151.5) | 0.565 | 0.066 | ||
| Grooved Pegboard non-dominant hand |
CSThand | Non-crossing | − 70.1 | (− 193.2 to 53.1) | 0.259 | − 125.1 | (− 248.1 to − 2.1) | 0.046 | 0.526 |
| Crossing | 88.2 | (− 27.1 to 203.5) | 0.131 | − 180.5 | (− 363.5 to 2.5) | 0.053 | 0.015 | ||
| CSTpremotor | Non-crossing | 2.9 | (− 137.2 to 143.1) | 0.967 | − 121.5 | (− 233.1 to − 9.8) | 0.034 | 0.173 | |
| Crossing | 122.9 | (− 62.3 to 308.1) | 0.189 | − 225.3 | (− 466.7 to 16.0) | 0.067 | 0.024 | ||
| MABC-2 Triangle | CSThand | Non-crossing | 8.2 | (− 116.7 to 133.1) | 0.895 | 29.9 | (− 96.5 to 156.4) | 0.637 | 0.807 |
| Crossing | 139.8 | (46.5 to 233.1) | 0.004 | 62.7 | (− 66.9 to 192.3) | 0.337 | 0.336 | ||
| CSTpremotor | Non-crossing | 45.5 | (− 81.8 to 172.8) | 0.477 | − 1.7 | (− 122.5 to 119.1) | 0.978 | 0.594 | |
| Crossing | 174.9 | (23.9 to 326.0) | 0.024 | 97.5 | (− 74.2 to 269.3) | 0.260 | 0.494 | ||
| CCmotor | Non-crossing | − 42.3 | (− 121.8 to 37.1) | 0.290 | 11.5 | (− 82.7 to 105.7) | 0.807 | 0.371 | |
| Crossing | − 102.0 | (− 179.6 to − 24.4) | 0.011 | 155.6 | (40.6 to 270.7) | 0.009 | 0.000 | ||
| CCpremotor | Non-crossing | 46.3 | (− 76.2 to 168.8) | 0.452 | 51.8 | (− 46.2 to 149.8) | 0.294 | 0.944 | |
| Crossing | 8.9 | (− 97.4 to 115.2) | 0.868 | 57.6 | (− 55.8 to 171.1) | 0.313 | 0.533 | ||
| Gross motor tests (sec) | |||||||||
| HiMAT automatic | CSTfoot | Non-crossing | − 12.6 | (− 31.1 to 5.9) | 0.178 | 6.4 | (− 15.6 to 28.4) | 0.561 | 0.189 |
| Crossing | − 2.8 | (− 19.3 to 13.7) | 0.737 | − 16.7 | (− 41.0 to 7.5) | 0.173 | 0.335 | ||
| CSTpremotor | Non-crossing | − 7.8 | (− 29.7 to 14.1) | 0.477 | 6.0 | (− 14.8 to 26.8) | 0.565 | 0.365 | |
| Crossing | − 2.3 | (− 29.2 to 24.5) | 0.862 | − 24.9 | (− 55.5 to 5.6) | 0.108 | 0.264 | ||
| CCmotor | Non-crossing | 6.8 | (− 6.8 to 20.5) | 0.320 | − 6.6 | (− 22.8 to 9.6) | 0.417 | 0.196 | |
| Crossing | − 4.2 | (− 18.9 to 10.6) | 0.576 | 12.3 | (− 9.6 to 34.2) | 0.266 | 0.220 | ||
| CCpremotor | Non-crossing | 7.4 | (− 13.9 to 28.6) | 0.491 | − 5.3 | (− 22.3 to 11.7) | 0.538 | 0.355 | |
| Crossing | − 8.9 | (− 27.1 to 9.3) | 0.332 | − 9.3 | (− 28.7 to 10.1) | 0.342 | 0.976 | ||
| HiMAT plannedc | CSTfoot | Non-crossing | − 13.3 | (− 44.9 to 18.3) | 0.404 | 4.6 | (− 32.9 to 42.2) | 0.805 | 0.465 |
| Crossing | − 36.0 | (− 65.5 to − 6.6) | 0.017 | − 30.0 | (− 70.2 to 10.2) | 0.140 | 0.805 | ||
| CSTpremotor | Non-crossing | − 0.6 | (− 37.5 to 36.4) | 0.975 | 5.6 | (− 29.5 to 40.7) | 0.751 | 0.810 | |
| Crossing | − 40.3 | (− 86.1 to 5.4) | 0.083 | − 40.1 | (− 91.0 to 10.8) | 0.120 | 0.994 | ||
| CCmotor | Non-crossing | 17.1 | (− 5.2 to 39.4) | 0.131 | − 19.2 | (− 45.9 to 7.4) | 0.154 | 0.036 | |
| Crossing | − 8.7 | (− 33.5 to 16.1) | 0.485 | 18.0 | (− 19.2 to 55.2) | 0.337 | 0.239 | ||
| CCpremotor | Non-crossing | 2.8 | (− 33.1 to 38.7) | 0.876 | − 14.4 | (− 43.3 to 14.5) | 0.321 | 0.455 | |
| Crossing | − 35.6 | (− 65.3 to − 5.9) | 0.020 | − 22.2 | (− 53.2 to 8.8) | 0.157 | 0.534 | ||
Regression coefficient B for FA in a linear regression with motor tests as dependent variables and FA, group and FA x group (indicating between-group differences) as independent variables. Adjusted for sex and age. For motor tests performed with one hand, FA in the contralateral CST was used in the analyses. For Triangle and HiMAT, mean FA in CST right and left was used in the analyses.
Abbreviations: FA, fractional anisotropy; CST, corticospinal tract; CC, corpus callosum; VLBW, very low birth weight; MABC-2, Movement Assessment Battery for Children-2; HiMAT, High-level Mobility Assessment Tool.
CSThand, CST from hand area in primary motor cortex; CSTfoot, CST from foot area in primary motor cortex; CSTpremotor, CST from premotor cortex.
CCmotor, CC connecting the primary motor cortices; CCpremotor, CC connecting the premotor cortices.
Data missing for one participant in each group.
3.5. Tract-based spatial statistics (TBSS)
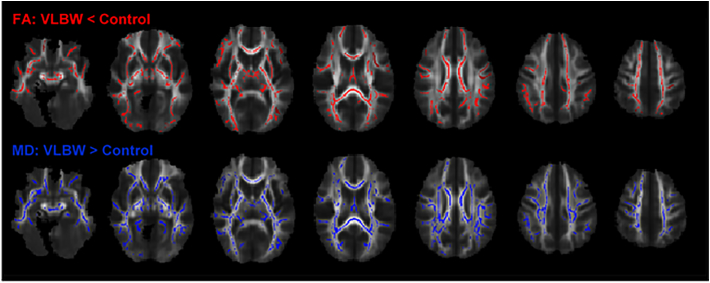
TBSS analysis demonstrated lower FA and higher MD in the VLBW group compared with controls in all major white matter tracts (Fig. 4). FA was lower in 34% and MD higher in 45% of the total voxels on the white matter skeleton at the group level. Lower FA was mainly caused by higher RD (83% of the voxels with lower FA) (data not shown). There were no voxels with higher FA and/or lower MD in the VLBW group compared with the control group.
Fig. 4.
TBSS analysis demonstrated significantly lower fractional anisotropy (red) and higher mean diffusivity (blue) in the VLBW group compared with the control group (p < 0.05, nonparametric permutation test, corrected for multiple comparisons, sex and age at MRI). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In this study, fine and gross motor function were associated with FA only in crossing fibre regions of the CST and CC in VLBW young adults without cerebral palsy. The associations between fine motor function and FA in the CST were in the opposite direction than expected, with poorer motor function being associated with higher FA. Moreover, the VLBW group had lower FA and higher MD in all major white matter tracts compared with controls at 23 years of age.
As hypothesized, the basic fine motor test (TMT-5) was associated with FA in the CST from the primary hand motor cortex, and the complex fine motor test (Grooved Pegboard) was additionally associated with FA in the CST from the premotor cortex. The test for bimanual coordination (MABC-2 Triangle) was as hypothesized associated with FA in the CST from the primary hand and premotor cortices, and with FA in the CC connecting the primary motor cortices. We expected this test to also be associated with FA in the CC connecting the premotor cortices, as the supplementary motor cortex is important for bimanual coordination (Johansen-Berg et al., 2007). For gross motor function, only HiMAT planned was significantly associated with FA in the CST and CC within the VLBW group, possibly indicating that the items of HiMAT automatic were too easy to detect differences between the groups. The finding of timed motor performances being associated with FA may support our former speculations that reduced motor speed is one of the key issues in preterm populations and is related to white matter microstructure in the preterm brain (Husby et al., 2013).
As we found an association between motor function and FA only in crossing fibre regions (centrum semiovale) of the CST, this may indicate that crossing tracts are causing the association and/or that the periventricular white matter is an especially vulnerable region in VLBW individuals (Volpe, 2003), affecting motor function. Furthermore, the direction of the associations was both positive and negative, which is difficult to explain. We speculate that the association between poorer motor function and higher FA may be related to the effect of crossing fibres. The superior longitudinal fasciculus is the largest tract crossing the CST in centrum semiovale, and was found to have lower FA in VLBW young adults than controls in the present study as well as other studies (Allin et al., 2011, Eikenes et al., 2011). These crossing fibres with lower FA will to a lesser extent than in controls reduce the apparent AD and thereby reduce the FA value in the voxels of this crossing fibre region. These voxels are therefore likely to show higher FA in VLBW individuals compared with controls, which was found in our study and has also been shown in other studies (Groeschel et al., 2014, Jurcoane et al., 2016). In crossing fibre regions, higher FA in VLBW individuals compared with controls is therefore less likely to be a sign of improved white matter integrity, but rather a general sign of axon loss, poorer branching and dysmyelination, which may lead to poorer motor function. Thus, higher FA does not always indicate better function, as also shown in other studies (Hoeft et al., 2007, Steele et al., 2012). However, we also found associations between poorer motor function and lower FA in crossing fibre regions of CSTfoot and CC. These fibres are located closer to the lateral ventricles than the CSThand, and may therefore be more affected by periventricular white matter damage. However, we do not know if and how this may affect the direction of the associations. Interesting future work could be to address these crossing fibre regions more closely by either performing tractography of selected crossing fibres in the areas of CST and CC where we found correlations between motor function and FA, performing other whole-brain tractography analysis methods, or by employing more advanced diffusion acquisitions and analysis which may give the opportunity to study multiple fibre orientations in each voxel.
Other studies have found an association between better motor function and higher FA in VLBW populations at different ages (de Kieviet et al., 2014, Skranes et al., 2007, Sripada et al., 2015, van Kooij et al., 2012), while some studies have not found any association between motor function and FA (Counsell et al., 2008, Jurcoane et al., 2016). However, it is difficult to compare the results from the various studies due to large differences in methodologies and age at examination. The relationship between motor function and FA might change during the course of development, and two of the studies did not assess motor function and DTI at the same time (Jurcoane et al., 2016, van Kooij et al., 2012). Furthermore, a large variety of motor assessments were used, possibly affecting the associations with FA. The former studies have also used various tractography and whole-brain voxel-based methods, thereby incorporating a different selection and extent of the white matter studied. Probabilistic tractography is likely to be more sensitive to the effects of crossing fibres on FA than TBSS, as also regions with low FA are included in the analyses.
In our study, TBSS was used to test for voxel-wise group differences in diffusion measures of white matter tracts in the whole brain. The finding of reduced FA and increased MD in all major white matter tracts in VLBW young adults compared with controls is in line with earlier findings in this cohort at age 15 and 20 years (Eikenes et al., 2011, Vangberg et al., 2006), as well as other studies of preterm born individuals at different ages (Li et al., 2014, Meng et al., 2016). The reduction of FA was caused by a significant increase in RD, possibly indicating reduced and aberrant myelination or poor axonal packing (Beaulieu, 2002, Song et al., 2005). Hence, these alterations may be related to the “encephalopathy of prematurity”, involving diffuse periventricular leukomalacia with a disturbed maturation of myelin-producing oligodendrocytes and injury of axons and subplate neurons (Volpe, 2009). However, other explanations for reduced anisotropy, like decreased fibre organization and increased membrane permeability, may not be excluded (Beaulieu, 2002).
Strengths of this study are the long-term follow-up of the two study groups, which were recruited at birth and equivalent in terms of maternal age and parental socioeconomic status. The groups have been followed prospectively with comprehensive multidisciplinary clinical, cognitive and MRI assessments at different ages. At 23 years, motor assessments were performed by experienced examiners and manual ROIs were drawn by the same researcher, all of whom were blinded to neonatal history and clinical outcome, thus reducing the risk of information bias. Only participants from the 14-year follow-up were invited, potentially increasing the risk of selection bias due to loss to follow-up from the original cohort. However, we found no differences in clinical characteristics at birth or motor skills at 14 years between those who participated and those who did not participate or were not invited to follow-up at 23 years in either of the groups. Due to the limited sample size, we chose not to correct for multiple comparisons for the association and tractography analyses to avoid potential type II errors. By using linear regression to examine associations between motor function and FA, both the VLBW and control group are included in the analysis, thereby increasing statistical power. The main findings were consistent, indicating that they were not due to chance.
4.1. Conclusion
Our findings showed that individual variability in motor function has structural correlates in VLBW young adults. Motor function was associated with FA only in crossing fibre regions of the CST and CC in the VLBW group, indicating that other tracts crossing the CST and CC are causing this association and/or that the vulnerable periventricular white matter in the centrum semiovale affect motor function. As poorer fine motor function was associated with higher FA, our results show that higher FA does not always indicate better function. Furthermore, VLBW young adults still showed widespread white matter alterations by TBSS, demonstrating that white matter abnormalities persist into young adulthood.
Acknowledgments
Acknowledgements
We would like to thank all the participants for their engagement in the project. We are grateful to Sigrun Flækken and Inger Helene Hamborg for assistance with motor assessments and Erik Magnus Berntsen for access to the functional ROIs.
Funding
The study was funded by the Research Council of Norway (Researcher program: NEVRONOR. Project Number: 182663) and was independent of commercial interest.
References
- Aarnoudse-Moens C.S., Weisglas-Kuperus N., van Goudoever J.B., Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- Allin M.P., Kontis D., Walshe M., Wyatt J., Barker G.J., Kanaan R.A.…Nosarti C. White matter and cognition in adults who were born preterm. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0024525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakketeig L.S., Jacobsen G., Hoffman H.J., Lindmark G., Bergsjø P., Molne K., Rødsten J. Pre-pregnancy risk factors of small-for-gestational age births among parous women in Scandinavia. Acta Obstet. Gynecol. Scand. 1993;72(4):273–279. doi: 10.3109/00016349309068037. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Behrens T.E., Berg H.J., Jbabdi S., Rushworth M.F., Woolrich M.W. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? NeuroImage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsen E.M., Samuelsen P., Lagopoulos J., Rasmussen I.A., Jr., Haberg A.K., Haraldseth O. Mapping the primary motor cortex in healthy subjects and patients with peri-rolandic brain lesions before neurosurgery. Neurol. Res. 2008;30(9):968–973. doi: 10.1179/016164108X323753. [DOI] [PubMed] [Google Scholar]
- Cascio C.J., Gerig G., Piven J. Diffusion tensor imaging: application to the study of the developing brain. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46(2):213–223. doi: 10.1097/01.chi.0000246064.93200.e8. [DOI] [PubMed] [Google Scholar]
- Counsell S.J., Edwards A.D., Chew A.T.M., Anjari M., Dyet L.E., Srinivasan L.…Cowan F.M. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131(12):3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- Delis D., Kaplan E., Kramer J. 2001. Examiner's Manual. [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K., Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eikenes L., Løhaugen G.C., Brubakk A.M., Skranes J., Håberg A.K. Young adults born preterm with very low birth weight demonstrate widespread white matter alterations on brain DTI. NeuroImage. 2011;54(3):1774–1785. doi: 10.1016/j.neuroimage.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Groeschel S., Tournier J.D., Northam G.B., Baldeweg T., Wyatt J., Vollmer B., Connelly A. Identification and interpretation of microstructural abnormalities in motor pathways in adolescents born preterm. NeuroImage. 2014;87:209–219. doi: 10.1016/j.neuroimage.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Grooved Pegboard Test. User instructions . Lafayette Instrument Company, Inc.; IN: 2002. Lafayette. [Google Scholar]
- Henderson S.E., Sugden D.A., Barnett A.L. 2nd ed. Pearson Assessment; London: 2007. Movement Assessment Battery for Children-2. Examiner's Manual. [Google Scholar]
- Hoeft F., Barnea-Goraly N., Haas B.W., Golarai G., Ng D., Mills D., Reiss A.L. More is not always better: increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. J. Neurosci. 2007;27(44):11960–11965. doi: 10.1523/JNEUROSCI.3591-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S., Frahm J. Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32(3):989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Holland D., Kuperman J.M., Dale A.M. Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. NeuroImage. 2010;50(1):175–183. doi: 10.1016/j.neuroimage.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A.B. Yale University; New Haven, CT: 1957. Two Factor Index of Social Position. [Google Scholar]
- Husby I.M., Skranes J., Olsen A., Brubakk A.-M., Evensen K.A.I. Motor skills at 23years of age in young adults born preterm with very low birth weight. Early Hum. Dev. 2013;89(9):747–754. doi: 10.1016/j.earlhumdev.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H., Della-Maggiore V., Behrens T.E., Smith S.M., Paus T. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. NeuroImage. 2007;36(Suppl. 2):T16–21. doi: 10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurcoane A., Daamen M., Scheef L., Bauml J.G., Meng C., Wohlschlager A.M.…Boecker H. White matter alterations of the corticospinal tract in adults born very preterm and/or with very low birth weight. Hum. Brain Mapp. 2016;37(1):289–299. doi: 10.1002/hbm.23031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kieviet J.F., Piek J.P., Aarnoudse-Moens C.S., Oosterlaan J. Motor development in very preterm and very low-birth-weight children from birth to adolescence a meta-analysis. JAMA. 2009;302(20):2235–2242. doi: 10.1001/jama.2009.1708. [DOI] [PubMed] [Google Scholar]
- de Kieviet J.F., Pouwels P.J., Lafeber H.N., Vermeulen R.J., van Elburg R.M., Oosterlaan J. A crucial role of altered fractional anisotropy in motor problems of very preterm children. Eur. J. Paediatr. Neurol. 2014;18(2):126–133. doi: 10.1016/j.ejpn.2013.09.004. [DOI] [PubMed] [Google Scholar]
- van Kooij B.J., de Vries L.S., Ball G., van Haastert I.C., Benders M.J., Groenendaal F., Counsell S.J. Neonatal tract-based spatial statistics findings and outcome in preterm infants. AJNR Am. J. Neuroradiol. 2012;33(1):188–194. doi: 10.3174/ajnr.A2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Li K., Sun Z., Han Y., Gao L., Yuan L., Zeng D. Fractional anisotropy alterations in individuals born preterm: a diffusion tensor imaging meta-analysis. Dev. Med. Child Neurol. 2014 doi: 10.1111/dmcn.12618. [DOI] [PubMed] [Google Scholar]
- Meng C., Bauml J.G., Daamen M., Jaekel J., Neitzel J., Scheef L.…Sorg C. Extensive and interrelated subcortical white and gray matter alterations in preterm-born adults. Brain Struct. Funct. 2016;221(4):2109–2121. doi: 10.1007/s00429-015-1032-9. [DOI] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit A.S., Ball G., Edwards A.D., Counsell S.J. Diffusion magnetic resonance imaging in preterm brain injury. Neuroradiology. 2013;55(Suppl. 2):65–95. doi: 10.1007/s00234-013-1242-x. [DOI] [PubMed] [Google Scholar]
- Skranes J.S., Vik T., Nilsen G., Smevik O., Andersson H.W., Rinck P., Brubakk A.M. Cerebral magnetic resonance imaging (MRI) and mental and motor function of very low birth weight infants at one year of corrected age. Neuropediatrics. 1993;24(05):256–262. doi: 10.1055/s-2008-1071553. [DOI] [PubMed] [Google Scholar]
- Skranes J.S., Vik T., Nilsen G., Smevik O., Andersson H.W., Brubakk A.M. Cerebral magnetic resonance imaging and mental and motor function of very low birth weight children at six years of age. Neuropediatrics. 1997;28(3):149–154. doi: 10.1055/s-2007-973692. [DOI] [PubMed] [Google Scholar]
- Skranes J.S., Vangberg T.R., Kulseng S., Indredavik M.S., Evensen K.A.I., Martinussen M.…Brubakk A.-M. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130(3):654–666. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E.…Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song S.K., Yoshino J., Le T.Q., Lin S.J., Sun S.W., Cross A.H., Armstrong R.C. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26(1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sripada K., Løhaugen G.C., Eikenes L., Bjørlykke K.M., Håberg A.K., Skranes J., Rimol L.M. Visual-motor deficits relate to altered gray and white matter in young adults born preterm with very low birth weight. NeuroImage. 2015;109:493–504. doi: 10.1016/j.neuroimage.2015.01.019. [DOI] [PubMed] [Google Scholar]
- Steele C.J., Scholz J., Douaud G., Johansen-Berg H., Penhune V.B. Structural correlates of skilled performance on a motor sequence task. Front. Hum. Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangberg T.R., Skranes J., Dale A.M., Martinussen M., Brubakk A.M., Haraldseth O. Changes in white matter diffusion anisotropy in adolescents born prematurely. NeuroImage. 2006;32(4):1538–1548. doi: 10.1016/j.neuroimage.2006.04.230. [DOI] [PubMed] [Google Scholar]
- Volpe J.J. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics. 2003;112(1 Pt 1):176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- Volpe J.J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet. Neurol. 2009;8(1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G., Robertson V., Greenwood K., Goldie P., Morris M.E. The high-level mobility assessment tool (HiMAT) for traumatic brain injury. Part 1: item generation. Brain Inj. 2005;19(11):925–932. doi: 10.1080/02699050500058687. [DOI] [PubMed] [Google Scholar]
- Williams G., Robertson V., Greenwood K.M., Goldie P.A., Morris M.E. The high-level mobility assessment tool (HiMAT) for traumatic brain injury. Part 2: content validity and discriminability. Brain Inj. 2005;19(10):833–843. doi: 10.1080/02699050500058711. [DOI] [PubMed] [Google Scholar]