Abstract
It has been proposed that pain competes with other attention-demanding stimuli for cognitive resources, and many chronic pain patients display significant attention and mental flexibility deficits. These alterations may result from disruptions in the functioning of the default mode network (DMN) which plays a critical role in attention, memory, prospection and self-processing, and recent investigations have found alterations in DMN function in multiple chronic pain conditions. Whilst it has been proposed that these DMN alterations are a characteristic of pain that is chronic in nature, we recently reported altered oscillatory activity in the DMN during an acute, 5 minute noxious stimulus in healthy control subjects. We therefore hypothesize that altered DMN activity patterns will not be restricted to those in chronic pain but instead will also occur in healthy individuals during tonic noxious stimuli. We used functional magnetic resonance imaging to measure resting state infra-slow oscillatory activity and functional connectivity in patients with chronic orofacial pain at rest and in healthy controls during a 20-minute tonic pain stimulus. We found decreases in oscillatory activity in key regions of the DMN in patients with chronic pain, as well as in healthy controls during tonic pain in addition to changes in functional connectivity between the posterior cingulate cortex and areas of the DMN in both groups. The results show that similar alterations in DMN function occur in healthy individuals during acute noxious stimuli as well as in individuals with chronic pain. These DMN changes may reflect the presence of pain per se and may underlie alterations in attentional processes that occur in the presence of pain.
Abbreviations: ALFF, amplitude of low-frequency fluctuations; fALFF, fractional amplitude of low-frequency fluctuations; DMN, default mode network; IPC, inferior parietal cortex; ISO, infra-slow oscillations; mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex
Keywords: Precuneus, Posterior cingulate cortex, Attention, Prefrontal cortex, Chronic orofacial pain, Functional magnetic resonance imaging
Highlights
-
•
Default mode network dynamics were measured in chronic and acute pain.
-
•
Altered infra-slow activity and connectivity occurred in chronic and acute pain.
-
•
Default mode network changes characterize pain per se.
1. Introduction
Chronic pain is a disease state with an enormous socioeconomic burden. Whilst most investigations focus on understanding the underlying pathophysiology of chronic pain largely center on sensory aspects, many chronic pain patients also report significant alterations in their sleep-wake cycle, family and social relations and cognitive function (Iverson and McCracken, 1997, Smith-Seemiller et al., 2003). It has been proposed that chronic pain is attention demanding, competing with other attention-demanding stimuli for cognitive resources (Eccleston and Crombez, 1999). Indeed, attention deficits are displayed in individuals with chronic pain conditions (Eccleston, 1994, Grisart and Van der Linden, 2001, Van Damme et al., 2010), and significant associations between chronic pain and sustained attention and mental flexibility have been reported (Karp et al., 2006, Sjogren et al., 2000, Verdejo-Garcia et al., 2009).
One cortical network that is thought to be involved in higher order functions is the default mode network (DMN). This network consists of a set of regions that are functionally highly connected and include the medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), inferior parietal cortex (IPC) and precuneus (Raichle et al., 2001, Shulman et al., 1997). This network was firstly shown to be more active during passive task conditions than during numerous goal-directed tasks. More recently it has been hypothesized that in addition to a role in memory, prospection and self-processing (Cavanna and Trimble, 2006, Spreng and Grady, 2010), all of which require a continuous level of background activity, the DMN also may act as a sentinel by monitoring the external environment (Buckner et al., 2008).
Some recent investigations using resting state functional magnetic resonance imaging (fMRI) have reported significant changes in DMN function in many chronic pain conditions (Baliki et al., 2008, Baliki et al., 2014, Letzen et al., 2013, Napadow et al., 2012, Tagliazucchi et al., 2010). These studies clearly show that chronic pain is associated with altered DMN dynamics, and it has been strongly suggested that chronic pain reorganizes “the dynamics of the DMN and as such reflect the maladaptive physiology of different types of chronic pain” (Baliki et al., 2008). Whilst this may be true, acute pain in a healthy, non-chronic pain individual is unquestionably attention demanding, competing with other attention-demanding stimuli for cognitive resources. Though Seminowicz and Davis (2007a) found little effect of acute pain on cognitive performance, this lack of effect might be due to the short stimulus duration (14 s). Indeed, there is considerable brain imaging evidence showing that pain and cognitive-related brain activities interact, possibly due to similar neural circuitries (Bantick et al., 2002, Petrovic et al., 2000, Remy et al., 2003, Valet et al., 2004).
In a separate investigation, Seminowicz and Davis also found that whilst performing an attention-demanding cognitive task during acute noxious stimuli, activity in the DMN was anti-correlated (one signal increased whilst the other decreased) to activity in a task-positive network and that these networks were operating at a frequency between 0.03 and 0.08 Hz (Seminowicz and Davis, 2007b). Curiously, although not the focus of the investigation, we recently reported significant decreases in oscillatory power in areas of the DMN during a 6 minute muscle pain stimulus in healthy control subjects (Alshelh et al., 2016). Although we did not explore these oscillation decreases in detail, given the evidence presented above, it is possible that altered DMN dynamics also occur during acute pain stimuli in healthy controls; these acute pain stimuli simply need to be of long enough duration to allow for reliable detection. The aim of this study is to use resting state fMRI to investigate infra-slow oscillations and connectivity within the DMN in individuals with chronic orofacial neuropathic pain and in healthy individuals during tonic orofacial pain. We hypothesize that altered DMN activity patterns will not be restricted to those in chronic pain but will also occur in healthy individuals during tonic noxious stimuli.
2. Materials and methods
2.1. Subjects
Forty-three subjects with chronic orofacial neuropathic pain (32 females; mean age 47.3 ± 2.3 years [± SEM]) and 57 pain-free controls (35 females; mean age 43.4 ± 1.7 years) were recruited for the study. There was no significant difference in age (t-test; p = 0.08) or gender composition (chi-squared test, p = 0.25) between groups. All chronic pain subjects were diagnosed using the Liverpool criteria as having post-traumatic trigeminal neuropathy (Nurmikko and Eldridge, 2001).
During the seven days prior to the MRI session, each chronic pain subject kept a pain diary recording the intensity of their on-going pain, three times a day using a 10 cm horizontal visual analogue scale (VAS) with 0 indicating “no pain” and 10 indicating “the most intense imaginable pain”. These pain intensity scores were then averaged over the 7-day period to create a mean diary pain intensity score. On the day of the MRI scanning, each chronic pain subject outlined the location of their on-going pain on a standard drawing of the head, completed a McGill Pain Questionnaire and listed their current medication use. Informed written consent was obtained for all procedures according to the Declaration of Helsinki and the study was approved by our local Institutional Human Research Ethics Committees. Data from 27 of the 43 chronic pain subjects were used in previous investigations (Alshelh et al., 2016, Gustin et al., 2011, Henderson et al., 2013, Wilcox et al., 2015).
2.2. MRI acquisition
All subjects lay supine on the bed of a 3 Tesla MRI scanner (Philips, Achieva) with their head immobilized in a tight-fitting head coil. With each subject relaxed and at rest, a series of 180 gradient echo echo-planar functional MRI image volumes using BOLD contrast were collected. Each image volume contained 35 axial slices covering the entire brain (repetition time = 2000 ms; echo time = 30 ms, flip angle = 90°, raw voxel size = 3 × 3 × 4 mm). In each subject, a high-resolution 3D T1-weighted anatomical image set, covering the entire brain, was collected (turbo field echo; repetition time = 5600 ms; echo time = 2.5 ms, flip angle = 8°, raw voxel size 0.87 mm3).
In 16 of the 57 control subjects (5 females, age 24.9 ± 1.2 years) a tonic pain stimulus was delivered. A catheter connected to a syringe filled with hypertonic saline (5%) was placed into the right masseter muscle midway between its upper and lower borders. The catheter was attached to an infusion pump with a 10 ml syringe placed outside the scanner room. With the subject relaxed, 500 gradient echo echo-planar fMRI image volumes using BOLD contrast were collected over a 25-minute period. Each image volume contained 42 axial slices covering the entire brain (repetition time = 3000 ms, echo time = 30 ms, flip angle = 90°, raw voxel size = 1.5 × 1.5 × 4 mm3). Following a 5-minute baseline period (100 fMRI volumes), a 0.2 ml bolus of hypertonic saline was injected into the masseter muscle followed by a continuous infusion for the remaining 20 min. During the infusion we aimed to keep a moderate pain intensity of approximately 5 out of 10. This was achieved by instructing each subject to push a buzzer once if the pain fell below 5 and twice if it increased above 5, and the infusion rate was adjusted accordingly to maintain the pain intensity at 5 out of 10. Following the scan, each subject was asked to rate the average pain during the scan on a 10 cm horizontal VAS, outline the location of the pain on a standard drawing of the head and complete a McGill Pain Questionnaire describing the qualities of the pain.
2.3. MRI analysis
2.3.1. Overall changes in infra-slow oscillatory power
Using SPM12 (Friston et al., 1995), all fMRI images were motion corrected, global signal drifts removed using the detrending method described by Macey et al. (2004), spatially normalized to the Montreal Neurological Institute (MNI) template, and spatially smoothed using a 6 mm full-width-half-maximum Gaussian filter. In no subject was there significant movement (> 0.5 mm in any direction). Furthermore, comparison of the mean movement in 6 directions (X, Y, Z, roll, yaw, tilt) revealed no significant differences between chronic pain and control groups (p > 0.05, two-sample t-test, Bonferroni corrected for multiple comparisons), or between the baseline and pain periods in the tonic pain group (p > 0.05, paired t-test, Bonferroni corrected for multiple comparisons).
2.3.2. Identifying the default mode network using independent components analysis
To identify the DMN we used ICA on the control (n = 57), chronic pain (n = 43) and tonic pain (n = 16) groups using the Group ICA toolbox (Calhoun et al., 2001). This technique can extract independent sources of activity within a recorded mixture of sources and can therefore detect various networks (Brown et al., 2001, Frigyesi et al., 2006). Using the Infomax ICA algorithm, we estimated 20 independent component maps in which signal intensity within multiple regions covaried. All components were displayed (p < 0.05, false discovery rate corrected for multiple comparisons with a minimum of 10 contiguous voxels) and for each group, the component map that included the major regions that define the DMN such as the medial prefrontal cortex, inferior parietal cortex, precuneus and posterior cingulate cortex was selected. The DMN component map for each of the three groups was then added together to create a single DMN mask, which was used in further analyses.
2.3.3. Voxel-by-voxel infra-slow frequency amplitude analysis
To explore regional differences in infra-slow oscillation (ISO) power we used the SPM toolbox REST (Song et al., 2011) to calculate the sum of amplitudes of low frequency fluctuations (ALFF) in the frequency band 0.03–0.06 Hz. We also divided these ALFF values by total power over the entire frequency range to obtain fractional ALFF (fALFF) values for each voxel. Significant differences within the DMN between control and chronic pain subjects were then determined using a two-sample random-effects procedure with age and gender added as nuisance variables (p < 0.05, false discovery rate corrected for multiple comparisons, minimum 10 contiguous voxels). For each of the comparisons, ALFF and fALFF values from significant clusters were extracted from the control and chronic pain groups and plotted. The effect of pain intensity and pain duration was determined by comparing its relationship with 0.03–0.06 Hz power in significant clusters in chronic pain subjects (p < 0.05, Bonferroni corrected for the number of correlations examined). The effects of medication on infra-slow oscillations were determined by comparing 0.03–0.06 Hz power in each significant cluster in chronic pain subjects taking analgesic medications (n = 16) with those not taking medication (n = 27) (p < 0.05, 2-tailed, 2-sample t-test).
For the tonic pain group, ALFF power was calculated for the baseline and tonic pain periods and significant differences within the DMN were determined using a paired random effects procedure (p < 0.05, false discovery rate corrected for multiple comparisons, minimum 10 contiguous voxels). These comparisons were made between the baseline period (100 volumes) and each of the four 5-minute periods (100 volumes each) during the tonic pain period. Using the significant clusters derived from the baseline versus the first 5-minute pain period, ALFF values were extracted from the baseline and each of the 5-minute pain periods and plotted. Significant differences between baseline and pain periods were then determined (paired t-test, p < 0.05, Bonferroni corrected for multiple comparisons). In addition, for each significant cluster, the percentage change in signal intensity relative to the baseline period was calculated for each of the four 5-minute pain periods in each subject and plotted. Significant differences between signal intensity during the baseline and each of the pain periods were determined (paired t-test, p < 0.05, Bonferroni corrected).
2.3.4. Voxel-by-voxel functional connectivity analysis
Results from the infra-slow frequency analysis revealed an area of the posterior cingulate cortex (PCC) in which both chronic and tonic pain groups displayed significant decreases in infra-slow frequency amplitude relative to controls and baseline, respectively. This PCC region was used as a “seeding area” to assess the effects of pain on PCC functional connectivity within the DMN. For the control and chronic pain groups, PCC signal intensity was extracted for each subject and the strength of covariation between this signal and each voxel within the DMN was determined. Cerebrospinal fluid signal intensity, calculated as the average signal intensity of a 4 mm diameter sphere placed in the 4th ventricle in each individual subject, in addition to the 6-direction movement parameters derived from the realignment step, were included as nuisance variables. Comparisons of PCC resting connectivity strength between controls and chronic pain subjects were determined using a two-group random effects analysis. For the tonic pain group, differences in PCC connectivity strength between the baseline and each of the tonic pain periods were determined using paired random effects analysis. For each of these analyses, following an initial threshold of p < 0.001 with a minimum cluster extent of 10 contiguous voxels, we used cluster correction to correct for multiple comparisons (p < 0.05, Bonferroni corrected for the number of voxels in each cluster). For the chronic pain analysis, functional connectivity values were extracted from each significant cluster and plotted. The effect of pain intensity and pain duration was determined by comparing its relationship with PCC connectivity strengths in significant clusters in chronic pain subjects (p < 0.05, Bonferroni corrected for the number of correlations examined). For the tonic pain analysis, PCC connectivity strength values were extracted from each significant cluster for the baseline and each of the 5-minute pain periods and plotted. Significant differences between baseline and each pain period were then determined (paired t-test, p < 0.05, Bonferroni corrected).
3. Results
3.1. Subject characteristics
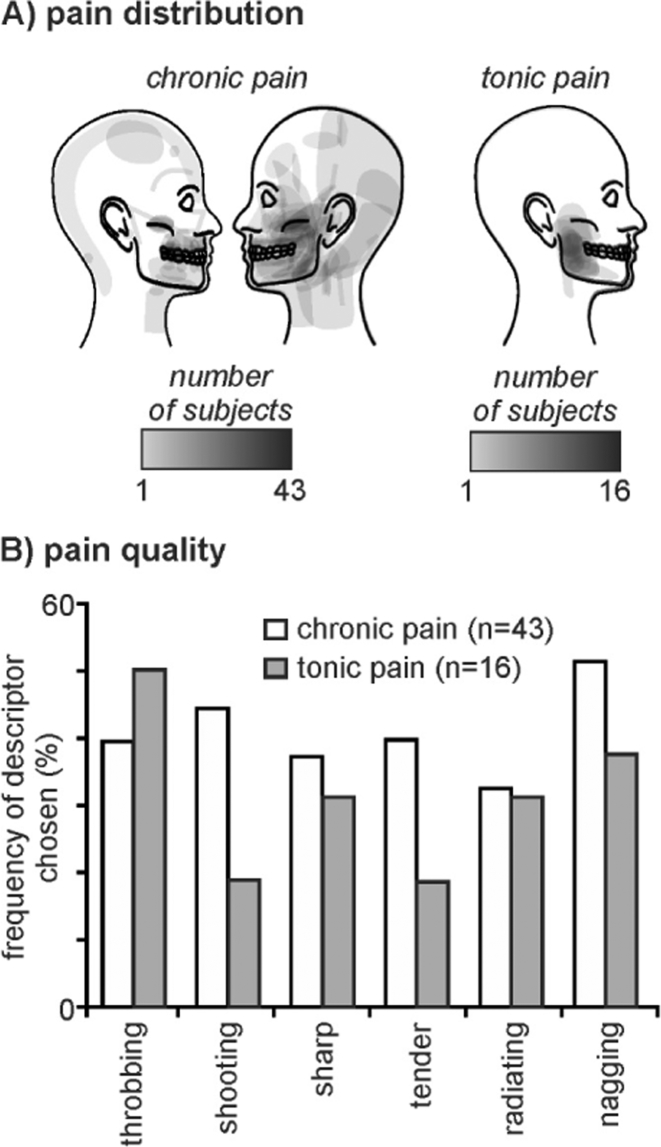
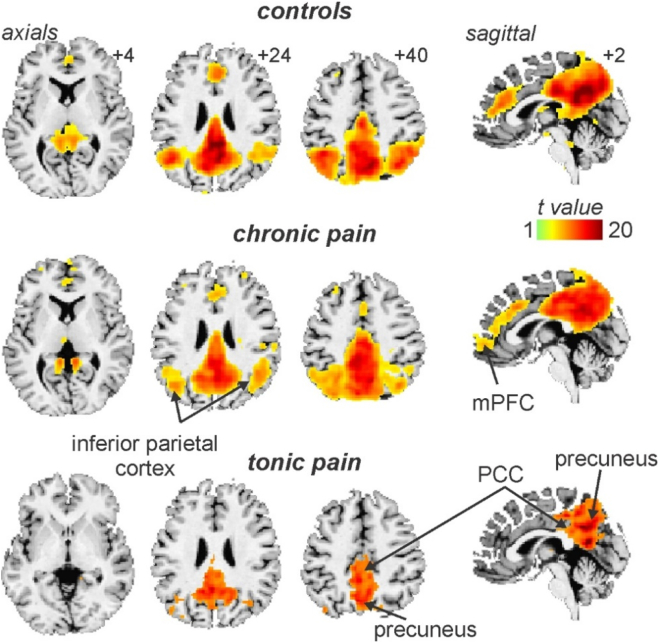
The mean (± SEM) diary pain of the chronic pain group was 3.8 ± 0.4 and the mean VAS scores for the tonic pain group was 4.8 ± 0.3. Both chronic and tonic pain groups reported pain encompassing the maxillary and mandibular distributions of the trigeminal nerve, although the chronic pain subjects generally reported a wider spread of on-going pain that often included the ophthalmic trigeminal division (Fig. 1A). Furthermore, 29 of the chronic pain subjects reported left sided only pain, and 14 right side only. Chronic pain subjects most often described their on-going pain as “shooting”, “throbbing” and “nagging”, and similarly the tonic pain was described as “throbbing”, “annoying” and “nagging” (Fig. 1B). The mean pain duration of the chronic pain group was 6.0 ± 0.9 years and 16 of the chronic pain subjects were taking some form of daily analgesic medication. ICA revealed a component comprising of the DMN in the control, chronic pain and tonic pain groups. This component encompassed vast regions of the precuneus, PCC, anterior cingulate cortex, medial prefrontal cortex (mPFC) and the inferior parietal cortex (IPC) (Fig. 2).
Fig. 1.
Pain distribution and quality of pain in chronic pain subjects and in individuals during tonic pain. A) Individual pain distribution patterns in 43 chronic pain subjects and 16 healthy controls during experimentally induced tonic pain. B) Frequency (percentage of subjects) of descriptors chosen from the McGill Pain Questionnaire to describe the on-going pain in chronic pain subjects (white bars) and in healthy controls during tonic pain (grey bars).
Fig. 2.
The default mode network in controls, chronic pain and tonic pain groups as defined by an independent component analysis. Areas displaying significant signal intensity covariation are indicated by the hot colour scale and overlaid onto an individual's T1-weighted anatomical image set. The default mode network consists of the precuneus, posterior cingulate cortex (PCC), inferior parietal cortex and the medial prefrontal cortex (mPFC). Slice locations in Montreal Neurological Institute space are indicated at the top right of each slice in the top panel.
3.2. Voxel-by-voxel infra-slow frequency amplitude analysis
3.2.1. Chronic pain
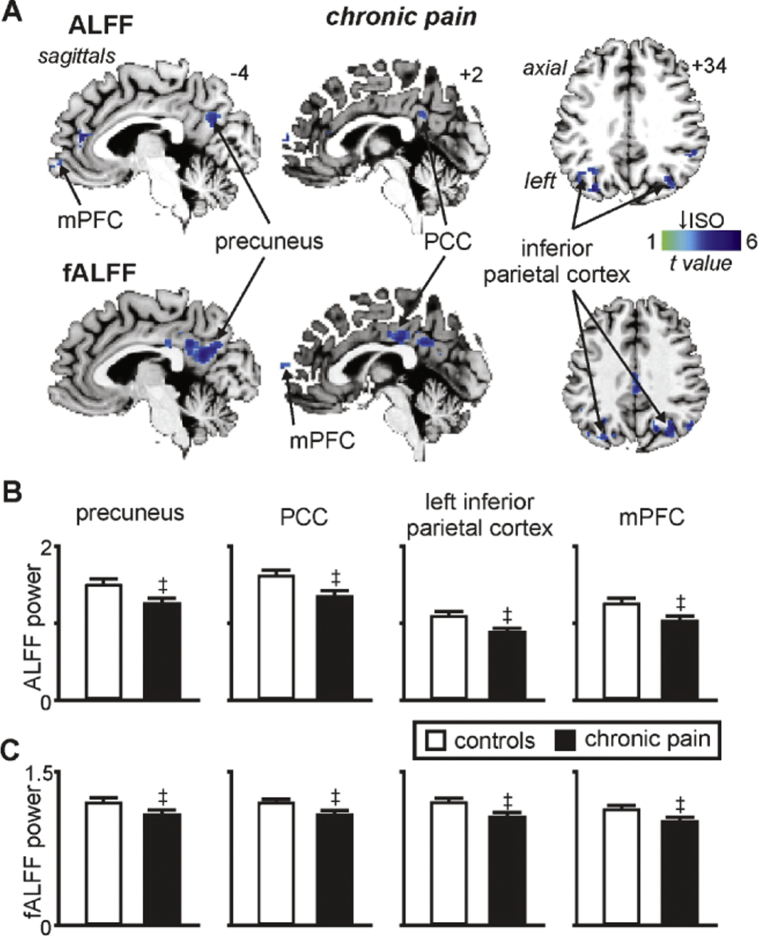
A comparison of infra-slow oscillation power between 0.03 and 0.06 Hz revealed significantly reduced power in chronic pain subjects in multiple regions of the DMN (Fig. 3A, Table 1A). That is, decreased power occurred in the region encompassing the precuneus (mean ± SEM 0.03–0.06 Hz ALFF: controls: 1.52 ± 0.04, chronic pain: 1.26 ± 0.05), PCC (controls: 1.64 ± 0.05, chronic pain: 1.37 ± 0.06), left and right IPC (left: controls: 1.12 ± 0.02, chronic pain: 0.91 ± 0.03; right: controls: 1.28 ± 0.04, chronic pain: 1.01 ± 0.05) and the mPFC (controls: 1.28 ± 0.05, chronic pain: 1.05 ± 0.05) (Fig. 3B). Similarly, comparison of infra-slow oscillation power relative to an individual's total power (fALFF) also revealed significantly reduced power in chronic pain subjects compared with controls in the precuneus (controls: 1.22 ± 0.02 chronic pain: 1.10 ± 0.03), PCC (controls: 1.23 ± 0.02, chronic pain: 1.10 ± 0.03), left and right IPC (left: controls: 1.21 ± 0.02, chronic pain: 1.07 ± 0.02; right: controls: 1.30 ± 0.02, chronic pain: 1.16 ± 0.02) and the mPFC (controls: 1.14 ± 0.02, chronic pain: 1.03 ± 0.03) (Fig. 3C). In no region of the DMN was power greater in chronic pain subjects compared with controls.
Fig. 3.
Significant changes in infra-slow oscillatory power (ISO: 0.03–0.06 Hz) in individuals with chronic pain. A) Regions where chronic pain subjects have significantly reduced amplitude of low-frequency fluctuations (ALFF) and fractional ALFF (fALFF) compared with controls within the default mode network. Power reductions are indicated by the cool colour scale and overlaid onto an individual's T1-weighted anatomical image set. Note the decreases within the precuneus, posterior cingulate cortex (PCC), inferior parietal cortex and medial prefrontal cortex (mPFC). Slice locations in Montreal Neurological Institute space are indicated at the top right of each slice in the top panel. B) Plots of mean ± SEM ALFF and C) mean ± SEM fALFF in controls and chronic pain groups for significant clusters. ‡ significant differences derived from the voxel-by-voxel random effects analysis (p < 0.05, false discovery rate corrected).
Table 1.
Location of significant infra-slow oscillation power decreases within the default mode network in individuals with chronic pain (A) and during tonic pain (B). Locations are in Montreal Neurological Institute (MNI) space.
| Cluster size | t value | MNI co-ordinate |
|||
|---|---|---|---|---|---|
| X | Y | Z | |||
| (A) Chronic pain | |||||
| ALFF | |||||
| Precuneus | 16 | 3.49 | − 4 | − 66 | 26 |
| 10 | 3.44 | 8 | − 68 | 38 | |
| Posterior cingulate cortex | 32 | 3.26 | 4 | − 48 | 28 |
| Inferior parietal cortex | |||||
| Left | 51 | 4.26 | − 30 | − 76 | 32 |
| Right | 53 | 3.73 | − 42 | − 58 | 22 |
| 36 | 3.88 | 34 | − 66 | 36 | |
| 40 | 3.80 | 46 | − 60 | 42 | |
| Medial prefrontal cortex | 38 | 4.94 | − 6 | 38 | 12 |
| 25 | 4.62 | − 12 | 38 | 42 | |
| fALFF | |||||
| Precuneus | 663 | 4.75 | − 8 | − 54 | 20 |
| Posterior cingulate cortex | 144 | 3.86 | 4 | − 28 | 32 |
| Inferior parietal cortex | |||||
| Left | 75 | 4.68 | − 42 | − 54 | 18 |
| 94 | 4.10 | − 32 | − 76 | 32 | |
| Right | 139 | 4.26 | 44 | − 60 | 42 |
| 77 | 4.15 | 36 | − 70 | 28 | |
| Medial prefrontal cortex | 12 | 3.39 | 2 | 66 | 8 |
| (B) Tonic pain | |||||
| ALFF | |||||
| Precuneus | 585 | 4.32 | − 6 | − 70 | 38 |
| Posterior cingulate cortex | 24 | 4.78 | − 10 | − 54 | 10 |
| 12 | 4.58 | − 4 | − 22 | 36 | |
| 68 | 4.55 | − 6 | − 44 | 44 | |
| Inferior parietal cortex | |||||
| Left | 411 | 6.63 | − 38 | − 74 | 36 |
| Right | 18 | 3.91 | − 48 | − 60 | 46 |
| 29 | 4.89 | 46 | − 72 | 26 | |
| Medial prefrontal cortex | 12 | 4.11 | 8 | 60 | 6 |
In no region was either ALFF or fALFF power significantly correlated to diary pain (ALFF: precuneus r = − 0.13, PCC r = − 0.17, left IPC r = − 0.14, right IPC r = 0.01, mPFC r = 0.11; fALFF: precuneus r = − 0.17, PCC r = − 0.18, left IPC r = − 0.11, right IPC r = − 0.07, mPFC r = 0.06; all p > 0.05) or pain duration (ALFF: precuneus r = 0.17, PCC r = 0.13, left IPC r = 0.01, right IPC r = − 0.04, mPFC r = 0.03; fALFF: precuneus r = 0.22, PCC r = 0.23, left IPC r = 0.01, right IPC r = 0.03, mPFC r = 0.32; all p > 0.05). In addition, in no region was there a significant difference in power between those taking medication compared with those that were not (mean ± SEM power medication versus no medication: ALFF: precuneus 1.22 ± 0.06 vs 1.29 ± 0.04, PCC 1.31 ± 0.05 vs 1.40 ± 0.06, left IPC 0.90 ± 0.03 vs 0.92 ± 0.03, right IPC 0.97 ± 0.04 vs 1.03 ± 0.04, mPFC 1.14 ± 0.06 vs 0.99 ± 0.03; fALFF: precuneus 1.16 ± 0.03 vs 1.07 ± 0.02, PCC 1.16 ± 0.03 vs 1.07 ± 0.02, left IPC 1.10 ± 0.02 vs 1.06 ± 0.02, right IPC 1.13 ± 0.02 vs 1.17 ± 0.02, mPFC 1.01 ± 0.03 vs 1.04 ± 0.02; all p > 0.05).
3.2.2. Tonic pain
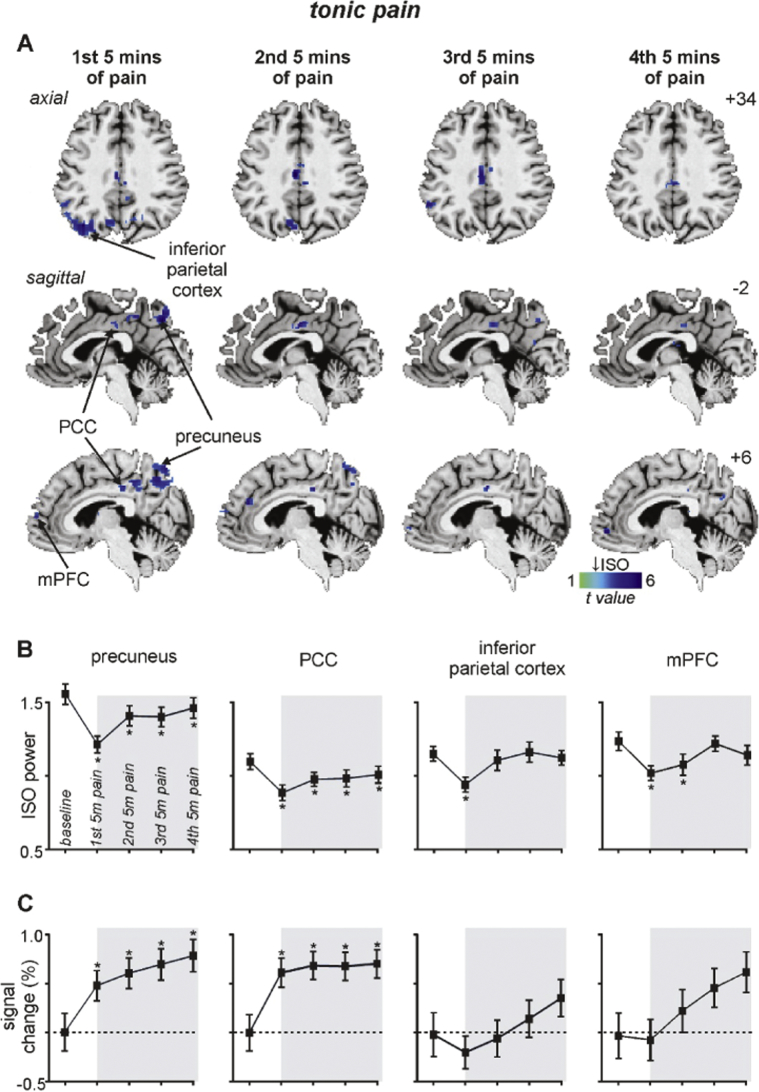
A comparison of infra-slow oscillation power during tonic pain with baseline revealed significant reductions in the same DMN regions as those that occurred in individuals with chronic pain (Fig. 4A, Table 1B). That is, decreased power occurred in the region of the precuneus (mean ± SEM 0.03–0.06 Hz ALFF: baseline: 1.59 ± 0.07, 1st 5 min tonic pain: 1.24 ± 0.06, 2nd 5 min: 1.44 ± 0.07, 3rd 5 min: 1.43 ± 0.07, 4th 5 min: 1.49 ± 0.07), PCC (baseline: 1.10 ± 0.05, 1st 5 min tonic pain: 0.89 ± 0.05, 2nd 5 min: 0.98 ± 0.05, 3rd 5 min: 0.98 ± 0.06, 4th 5 min: 1.00 ± 0.06), left IPC (baseline: 1.15 ± 0.05, 1st 5 min tonic pain: 0.94 ± 0.05, 2nd 5 min: 1.11 ± 0.07, 3rd 5 min: 1.17 ± 0.07,4th 5 min: 1.13 ± 0.05) and the mPFC (baseline: 1.23 ± 0.06, 1st 5 min tonic pain: 1.02 ± 0.05, 2nd 5 min: 1.08 ± 0.07, 3rd 5 min: 1.22 ± 0.05, 4th 5 min: 1.14 ± 0.07). In the precuneus and PCC, the significant reduction in power was sustained for the tonic pain period, whereas in the IPC and mPFC the power reductions were transient and returned to baseline by the 3rd 5-minute pain period.
Fig. 4.
Significant changes in infra-slow oscillatory power (ISO: 0.03–0.06 Hz) during tonic pain. A) Regions of the default mode network where the amplitude of low-frequency fluctuations (ALFF) are significantly reduced during the 1st, 2nd, 3rd and 4th 5 minute periods of tonic pain compared with a baseline period. Power reductions are indicated by the cool colour scale and overlaid onto an individual's T1-weighted anatomical image set. Note the decreases within the precuneus, posterior cingulate cortex (PCC), inferior parietal cortex and medial prefrontal cortex (mPFC). Slice locations in Montreal Neurological Institute space are indicated at the top right of each slice in panel A. B) Plots of mean ± SEM ALFF during baseline and the four 5 minute pain periods for significant clusters. C) Plots of mean ± SEM % signal intensity changes relative to baseline for the four 5 minute pain periods for clusters with significantly reduced ALFF power. Note that only the precuneus and PCC display both reduced ALFF and increased signal intensity changes during all of the tonic pain periods. The grey shading indicates the tonic pain period. * p < 0.05, 2-tailed paired t-test.
The reduction in infra-slow oscillation power within the precuneus and PCC was accompanied by significant signal intensity increases that were sustained for the tonic pain period (mean ± SEM %signal intensity increase: precuneus: 1st 5 min tonic pain: 0.47 ± 0.15, 2nd 5 min: 0.60 ± 0.16, 3rd 5 min: 0.69 ± 0.16, 4th 5 min: 0.78 ± 0.16; PCC: 1st 5 min tonic pain: 0.62 ± 0.15, 2nd 5 min: 0.69 ± 0.14, 3rd 5 min: 0.68 ± 0.14, 4th 5 min: 0.71 ± 0.14: all p < 0.05). In contrast, within the IPC and mPFC, signal intensity did not change significantly during the tonic pain period (IPC: 1st 5 min tonic pain: − 0.19 ± 0.17, 2nd 5 min: − 0.06 ± 0.19, 3rd 5 min: 0.15 ± 0.19, 4th 5 min: 0.35 ± 019; mPFC: 1st 5 min tonic pain: − 0.05 ± 0.23, 2nd 5 min: 0.28 ± 0.24, 3rd 5 min: 0.54 ± 0.22, 4th 5 min: 0.71 ± 0.23: all p > 0.05).
3.3. Functional connectivity of PCC
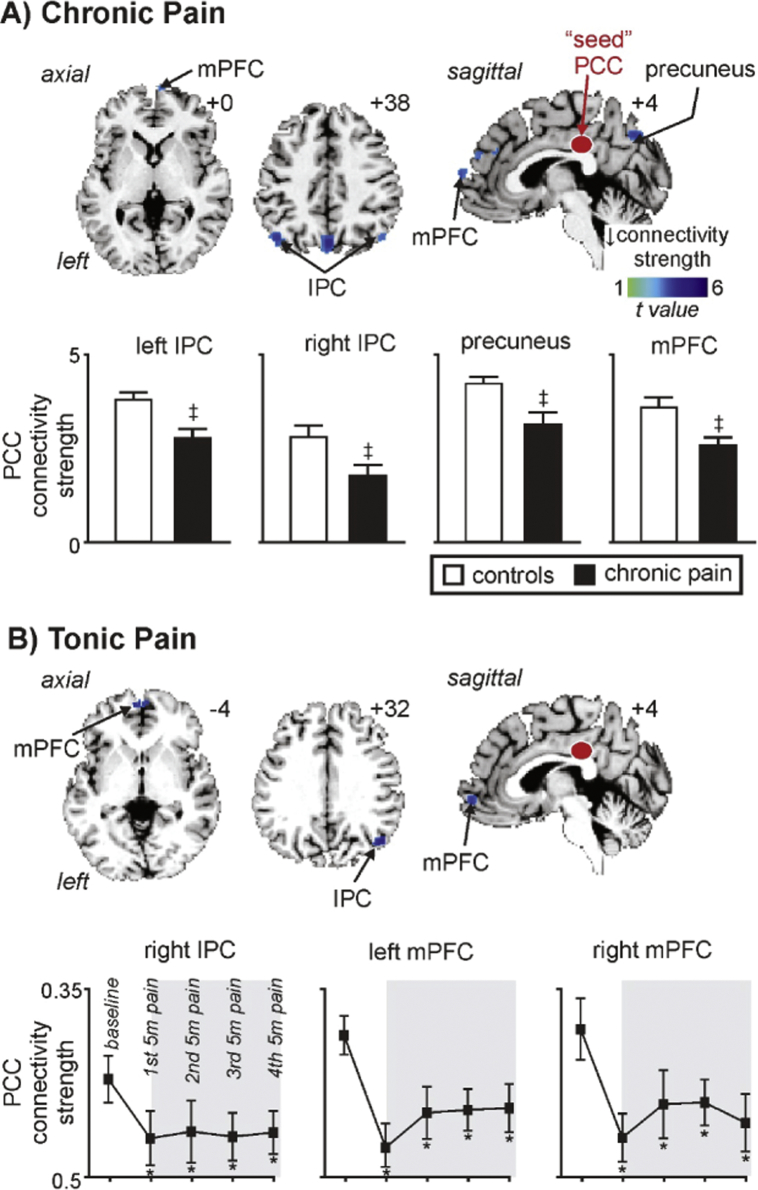
The PCC region that displayed decreased infra-slow oscillation power in both the chronic and tonic pain groups and also a sustained increase in signal intensity during tonic pain was used to explore connectivity changes within the DMN. Similar to the infra-slow oscillation power changes, chronic pain subjects displayed significantly reduced PCC connectivity strengths compared with controls in the precuneus (mean ± SEM PCC connectivity strength: controls: 0.42 ± 0.02, chronic pain: 0.32 ± 0.02), left and right IPC (left: controls: 0.39 ± 0.02, chronic pain: 0.29 ± 0.03; right: controls: 0.29 ± 0.02, chronic pain: 0.18 ± 0.03) and the mPFC (controls: 0.37 ± 0.02, chronic pain: 0.26 ± 0.02) (Fig. 5A, Table 2A). In no DMN region was PCC connectivity strength greater in the chronic pain subjects compared with controls.
Fig. 5.
Significant changes in posterior cingulate cortex (PCC) functional connectivity in chronic and tonic pain groups. A) Regions where PCC connectivity strength within the default mode network is significantly reduced in chronic pain subjects compared with controls. Connectivity strength reductions are indicated by the cool colour scale and overlaid onto an individual's T1-weighted anatomical image set. Note decreases in the precuneus, inferior parietal cortex (IPC) and medial prefrontal cortex (mPFC). Slice locations in Montreal Neurological Institute space are indicated at the top right of each slice. Plots of mean ± SEM PCC connectivity strength are also shown. ‡ significant differences derived from the voxel-by-voxel random effects analysis (p < 0.05, false discovery rate corrected). B) Regions where PCC connectivity strength is significantly reduced during tonic pain compared with the baseline period. Connectivity strength reductions are indicated by the cool colour scale and overlaid onto an individual's T1-weighted anatomical image set. Note the decreases within the IPC and mPFC. Plots of mean ± SEM PCC connectivity strength are also shown for the baseline and each of the 5 min tonic pain periods. The grey shading indicates the tonic pain period. * p < 0.05, 2-tailed paired t-test.
Table 2.
Location of significant posterior cingulate cortex connectivity decreases within the default mode network in individuals with chronic pain (A) and during tonic pain (B). Locations are in Montreal Neurological Institute (MNI) space.
| Cluster size | t value | MNI co-ordinate |
|||
|---|---|---|---|---|---|
| X | Y | Z | |||
| (A) Chronic pain | |||||
| Precuneus | 48 | 3.87 | 0 | − 72 | 40 |
| Inferior parietal cortex | |||||
| Left | 24 | 3.41 | − 46 | − 68 | 36 |
| Right | 10 | 2.90 | 50 | − 68 | 40 |
| Medial prefrontal cortex | 29 | 3.76 | 12 | 40 | 50 |
| 10 | 3.48 | 6 | 66 | 8 | |
| (B) Tonic pain | |||||
| Right inferior parietal cortex | 21 | 4.05 | 48 | − 68 | 30 |
| Medial prefrontal cortex | |||||
| Left | 16 | 3.91 | − 2 | 58 | − 4 |
| Right | 18 | 3.00 | 2 | 56 | − 6 |
In no region was PCC connectivity strength significantly correlated to diary pain (precuneus r = − 0.01, left IPC r = 0.08, right IPC r = 0.01, mPFC r = − 0.09; all p > 0.05) or pain duration (precuneus r = − 0.12, left IPC r = 0.18, right IPC r = − 0.11, mPFC r = 0.01; all p > 0.05). In addition, in no region was there a significant difference in PCC connectivity strength between those taking medication compared with those that were not (mean ± SEM PCC connectivity strength medication versus no medication: precuneus 0.30 ± 0.03 vs 0.33 ± 0.03, left IPC 0.28 ± 0.04 vs 0.29 ± 0.04, right IPC 0.13 ± 0.06 vs 0.22 ± 0.03, mPFC 0.25 ± 0.04 vs 0.27 ± 0.03; all p > 0.05).
The tonic pain group displayed reduced PCC connectivity strength during pain compared to baseline in the right IPC (baseline: 0.18 ± 0.05; 1st 5 min tonic pain: 0.07 ± 0.05, 2nd 5 min: 0.08 ± 0.06, 3rd 5 min: 0.07 ± 0.04, 4th 5 min: 0.08 ± 0.04), left mPFC (baseline: 0.26 ± 0.04; 1st 5 min tonic pain: 0.04 ± 0.05, 2nd 5 min: 0.12 ± 0.05, 3rd 5 min: 0.12 ± 0.04, 4th 5 min: 0.12 ± 0.05) and right mPFC (baseline: 0.27 ± 0.06; 1st 5 min tonic pain: 0.07 ± 0.05, 2nd 5 min: 0.14 ± 0.06, 3rd 5 min: 0.14 ± 0.04, 4th 5 min: 0.10 ± 0.0.05) (Fig. 5B, Table 1B). These significant reductions in PCC connectivity were sustained for the entire tonic pain period. In no DMN region was PCC connectivity strength greater during tonic pain compared with baseline.
4. Discussion
Consistent with our overall hypothesis, we found that DMN functional properties were significantly altered in both subjects with chronic pain and in healthy individuals during tonic painful stimuli. Indeed, we found decreased ISO power in both chronic pain and tonic pain groups over most of the DMN, including in the precuneus, PCC, IPC and mPFC. Additionally, in healthy controls, tonic pain evoked sustained ISO decreases in the precuneus and PCC which were associated with sustained overall signal intensity increases. Finally, both chronic and tonic pain groups displayed significant reductions in PCC connectivity within the DMN, and these changes were sustained for the entire pain period in the tonic pain group.
Consistent with previous investigations we found altered DMN dynamics in individuals with chronic pain (Baliki et al., 2008, Baliki et al., 2014, Letzen et al., 2013, Loggia et al., 2013, Napadow et al., 2012, Tagliazucchi et al., 2010). Although in one of these previous studies, ISOs were investigated and no significant differences at low frequencies (0.01–0.05 Hz) were found within the DMN (Baliki et al., 2014), ISO power was averaged over the entire DMN network, which would eliminate the opportunity of finding changes within discrete regions of the DMN. More importantly, we found that similar DMN changes to those in chronic pain subjects also occurred in healthy individuals during a tonic noxious stimulus. Both chronic and tonic pain were associated with reduced ISOs within multiple regions of the DMN, including the precuneus, PCC, mPFC and IPC. This clearly indicates that DMN functional changes are not a unique characteristic of chronic pain but instead likely represent the presence of pain itself. It has been proposed that ISO activity is a fundamental property of brain function that is maintained by adenosine receptor-mediated signalling (Hughes et al., 2011, Lorincz et al., 2009). Adenosine is likely released by astrocytes since they can display spontaneous intracellular infra-slow calcium oscillations (Parri and Crunelli, 2001), are responsive to glutamate and acetylcholine, and a link between adenosine and infra-slow oscillatory activity has been demonstrated in the cortex (Cunningham et al., 2006). Whilst we have previously speculated that increased ISO activity in the ascending pain pathways of chronic orofacial pain patients results from astrocyte activation (Alshelh et al., 2016, Henderson and Di Pietro, 2016), what ISO power reductions represent is unclear.
The rapid nature of the ISO decreases during tonic pain suggests that they do not result from astrogliosis. During tonic pain, decreased ISOs were sustained for the entire 20 min pain period in the precuneus and PCC, two regions which also displayed sustained increases in overall signal intensity. In contrast, ISOs within the inferior parietal cortex and mPFC were transient, and overall signal intensity in these two regions did not change. Similarly, we have previously shown that chronic orofacial pain is associated with increased on-going activity in the DMN as indicated by increased cerebral blood flow in the DMN, particularly within the precuneus (Youssef et al., 2014). These data suggest that within the DMN, decreased ISOs are coupled with overall activity increases which together may result from increased afferent drive rather than longer-term changes mediated by mechanisms such as astrogliosis. As mentioned above, Seminowicz and Davis found that during an attention-demanding cognitive task in the presence of acute pain, the DMN and task-positive networks displayed anti-correlated ISO fluctuations within a similar frequency range as those explored in this study (Seminowicz and Davis, 2007b). This further supports the notion that changes in ISO power may represent a fundamental property of brain function.
In addition to altered ISO and overall activity levels, we found that both chronic and tonic pain groups displayed decreased PCC connectivity strengths with other DMN regions. Decreased PCC connectivity with the mPFC and IPC occurred in both chronic and tonic pain groups, and also with the precuneus in chronic pain subjects. These decreases are consistent with previous studies reporting decreased DMN connectivity in individuals with chronic back pain, complex regional pain syndrome and osteoarthritis (Baliki et al., 2014), although in individuals with temporomandibular disorder, DMN connectivity strengths reportedly increase (Kucyi et al., 2014). The PCC is a key neural hub in the DMN (Fransson and Marrelec, 2008) and is activated during the regulation of attention (Gusnard et al., 2001, Hahn et al., 2007, Small et al., 2003) and displays hypometabolism in individuals with cognitive impairment (Liang et al., 2008, Minoshima et al., 1997, Valla et al., 2001). Since there is evidence of decreased connectivity between the PCC and other DMN areas, such as the precuneus, in attention deficit hyperactivity disorder, a condition characterised by a reduced ability in paying attention (Castellanos et al., 2008, Rubia et al., 2005, Uddin et al., 2008), it is possible that reduced PCC connectivity during pain also underlies an altered ability to attend to particular tasks.
If as hypothesized, the DMN is acting as a sentinel by monitoring the external environment (Buckner et al., 2008), ISO and connectivity changes within the DMN may underpin such monitoring during pain and reduce the ability of other stimuli to attract an individual's attention away from the pain. Indeed, connectivity strength within the DMN in chronic pain subjects is correlated to pain rumination, a measure of an individual's continual focus on his/her pain and its potential negative outcomes (Kucyi et al., 2014). Consistent with this previous study, we did not find any significant relationships between either ISO power or PCC connectivity strengths with either on-going pain intensity or pain duration in chronic pain subjects. However, others have reported relationships between DMN connectivity and ongoing pain intensity and duration and there is evidence that connectivity strengths between the DMN and areas such as insular cortex are related to the on-going pain intensity in individuals with chronic lower back pain (Loggia et al., 2013) and also in those with fibromyalgia (Napadow et al., 2012). These data raise the prospect that differences in the relationships between DMN connectivity and clinical pain variables may depend on the specific type of pain investigated, the modality and/or analysis of the acquired data or the specific circuitry examined. Our study does however, show that DMN changes occur during experimentally applied painful stimuli in healthy controls, suggesting that it is the presence of pain per se that is more likely responsible for such DMN changes. Furthermore, it is curious that this decreased DMN connectivity strength is coupled to decreased ISO power, raising the prospect that DMN functional coupling at rest is significantly influenced by signal coupling within the 0.03–0.06 Hz range.
Whilst we have clearly shown that altered DMN dynamics occur in healthy individuals during experimentally applied painful stimuli, the interpretation of what these changes represent remain unknown.
One limitation of this study is that during the tonic pain experiment, we asked each subject to indicate when deviations from the target pain intensity percept occurred. Although this could have introduced a potential motor confound, given that most subjects indicated a deviation no more than 5 times over the course of the entire 20 min pain period, we suggest that this potential for such a confound is negligible. Furthermore, we did not measure behavioural changes such as changes in cognitive or attention-demanding tasks, particularly in the tonic pain group. Future investigations could explore the effect of changes in DMN function evoked by both tonic and chronic pain.
5. Conclusions
We have found that altered DMN dynamics is not unique to chronic pain but also occurs during tonic pain in healthy controls. Both chronic and tonic pain are attention demanding stimuli competing for cognitive resources, and functional changes within the DMN may underlie some of the attentional and cognitive alterations that occur in the presence of pain.
Acknowledgments
Acknowledgements
This work was supported by funding from the Australian National Health and Medical Research Council (G160279 and G182968) and the NGW Macintosh Memorial Fund.
References
- Alshelh Z., Di Pietro F., Youssef A.M., Reeves J.M., Macey P.M., Vickers E.R., Peck C.C., Murray G.M., Henderson L.A. Chronic neuropathic pain: It's about the rhythm. J. Neurosci. 2016;36:1008–1018. doi: 10.1523/JNEUROSCI.2768-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Geha P.Y., Apkarian A.V., Chialvo D.R. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J. Neurosci. 2008;28:1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Mansour A.R., Baria A.T., Apkarian A.V. Functional reorganization of the default mode network across chronic pain conditions. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantick S.J., Wise R.G., Ploghaus A., Clare S., Smith S.M., Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- Brown G.D., Yamada S., Sejnowski T.J. Independent component analysis at the neural cocktail party. Trends Neurosci. 2001;24:54–63. doi: 10.1016/s0166-2236(00)01683-0. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Pearlson G.D., Pekar J.J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F.X., Margulies D.S., Kelly C., Uddin L.Q., Ghaffari M., Kirsch A., Shaw D., Shehzad Z., Di Martino A., Biswal B., Sonuga-Barke E.J.S., Rotrosen J., Adler L.A., Milham M.P. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cunningham M.O., Pervouchine D.D., Racca C., Kopell N.J., Davies C.H., Jones R.S., Traub R.D., Whittington M.A. Neuronal metabolism governs cortical network response state. Proc. Natl. Acad. Sci. U. S. A. 2006;103:5597–5601. doi: 10.1073/pnas.0600604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston C. Chronic pain and attention: a cognitive approach. Br J Clin Psychol. 1994;33(Pt 4):535–547. doi: 10.1111/j.2044-8260.1994.tb01150.x. [DOI] [PubMed] [Google Scholar]
- Eccleston C., Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol. Bull. 1999;125:356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- Fransson P., Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. NeuroImage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Frigyesi A., Veerla S., Lindgren D., Hoglund M. Independent component analysis reveals new and biologically significant structures in micro array data. BMC Bioinformatics. 2006;7:290. doi: 10.1186/1471-2105-7-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Worsley K.P., Proline J.B., Frith C.D., Frackowiak R.S.J. Statistical parametric maps in functional imaging: a general imaging approach. Journal of Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Grisart J.M., Van der Linden M. Conscious and automatic uses of memory in chronic pain patients. Pain. 2001;94:305–313. doi: 10.1016/S0304-3959(01)00366-9. [DOI] [PubMed] [Google Scholar]
- Gusnard D.A., Raichle M.E., Raichle M.E. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Gustin S.M., Peck C.C., Wilcox S.L., Nash P.G., Murray G.M., Henderson L.A. Different pain, different brain: thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes. J. Neurosci. 2011;31:5956–5964. doi: 10.1523/JNEUROSCI.5980-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B., Ross T.J., Stein E.A. Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cereb. Cortex. 2007;17:1664–1671. doi: 10.1093/cercor/bhl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L.A., Di Pietro F. How do neuroanatomical changes in individuals with chronic pain result in the constant perception of pain? Pain Manag. 2016;6:147–159. doi: 10.2217/pmt.15.67. [DOI] [PubMed] [Google Scholar]
- Henderson L.A., Peck C.C., Petersen E.T., Rae C.D., Youssef A.M., Reeves J.M., Wilcox S.L., Akhter R., Murray G.M., Gustin S.M. Chronic pain: lost inhibition? J. Neurosci. 2013;33:7574–7582. doi: 10.1523/JNEUROSCI.0174-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S.W., Lorincz M.L., Parri H.R., Crunelli V. Infraslow (< 0.1 Hz) oscillations in thalamic relay nuclei basic mechanisms and significance to health and disease states. Prog. Brain Res. 2011;193:145–162. doi: 10.1016/B978-0-444-53839-0.00010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson G.L., McCracken L.M. 'Postconcussive' symptoms in persons with chronic pain. Brain Inj. 1997;11:783–790. doi: 10.1080/026990597122990. [DOI] [PubMed] [Google Scholar]
- Karp J.F., Reynolds C.F., 3rd, Butters M.A., Dew M.A., Mazumdar S., Begley A.E., Lenze E., Weiner D.K. The relationship between pain and mental flexibility in older adult pain clinic patients. Pain Med. 2006;7:444–452. doi: 10.1111/j.1526-4637.2006.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A., Moayedi M., Weissman-Fogel I., Goldberg M.B., Freeman B.V., Tenenbaum H.C., Davis K.D. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J. Neurosci. 2014;34:3969–3975. doi: 10.1523/JNEUROSCI.5055-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzen J.E., Craggs J.G., Perlstein W.M., Price D.D., Robinson M.E. Functional connectivity of the default mode network and its association with pain networks in irritable bowel patients assessed via lidocaine treatment. J. Pain. 2013;14:1077–1087. doi: 10.1016/j.jpain.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W.S., Reiman E.M., Valla J., Dunckley T., Beach T.G., Grover A., Niedzielko T.L., Schneider L.E., Mastroeni D., Caselli R., Kukull W., Morris J.C., Hulette C.M., Schmechel D., Rogers J., Stephan D.A. Alzheimer's disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4441–4446. doi: 10.1073/pnas.0709259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia M.L., Kim J., Gollub R.L., Vangel M.G., Kirsch I., Kong J., Wasan A.D., Napadow V. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154:24–33. doi: 10.1016/j.pain.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz M.L., Geall F., Bao Y., Crunelli V., Hughes S.W. ATP-dependent infra-slow (< 0.1 Hz) oscillations in thalamic networks. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey P.M., Macey K.E., Kumar R., Harper R.M. A method for removal of global effects from fMRI time series. NeuroImage. 2004;22:360–366. doi: 10.1016/j.neuroimage.2003.12.042. [DOI] [PubMed] [Google Scholar]
- Minoshima S., Giordani B., Berent S., Frey K.A., Foster N.L., Kuhl D.E. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann. Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Napadow V., Kim J., Clauw D.J., Harris R.E. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. 2012;64:2398–2403. doi: 10.1002/art.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmikko T.J., Eldridge P.R. Trigeminal neuralgia–pathophysiology, diagnosis and current treatment. Br. J. Anaesth. 2001;87:117–132. doi: 10.1093/bja/87.1.117. [DOI] [PubMed] [Google Scholar]
- Parri H.R., Crunelli V. Pacemaker calcium oscillations in thalamic astrocytes in situ. Neuroreport. 2001;12:3897–3900. doi: 10.1097/00001756-200112210-00008. [DOI] [PubMed] [Google Scholar]
- Petrovic P., Petersson K.M., Ghatan P.H., Stone-Elander S., Ingvar M. Pain-related cerebral activation is altered by a distracting cognitive task. Pain. 2000;85:19–30. doi: 10.1016/s0304-3959(99)00232-8. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy F., Frankenstein U.N., Mincic A., Tomanek B., Stroman P.W. Pain modulates cerebral activity during cognitive performance. NeuroImage. 2003;19:655–664. doi: 10.1016/s1053-8119(03)00146-0. [DOI] [PubMed] [Google Scholar]
- Rubia Katya, Smith Anna B., Brammer Michael J., Toone Brian, Taylor Eric. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am. J. Psychiatr. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Seminowicz D.A., Davis K.D. Interactions of pain intensity and cognitive load: the brain stays on task. Cereb. Cortex. 2007;17:1412–1422. doi: 10.1093/cercor/bhl052. [DOI] [PubMed] [Google Scholar]
- Seminowicz D.A., Davis K.D. Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. J. Neurophysiol. 2007;97:3651–3659. doi: 10.1152/jn.01210.2006. [DOI] [PubMed] [Google Scholar]
- Shulman G.L., Corbetta M., Buckner R.L., Fiez J.A., Miezin F.M., Raichle M.E., Petersen S.E. Common blood flow changes across visual tasks: I. Increases in subcortical structures and cerebellum but not in nonvisual cortex. J. Cogn. Neurosci. 1997;9:624–647. doi: 10.1162/jocn.1997.9.5.624. [DOI] [PubMed] [Google Scholar]
- Sjogren P., Thomsen A.B., Olsen A.K. Impaired neuropsychological performance in chronic nonmalignant pain patients receiving long-term oral opioid therapy. J. Pain Symptom Manag. 2000;19:100–108. doi: 10.1016/s0885-3924(99)00143-8. [DOI] [PubMed] [Google Scholar]
- Small D.M., Gitelman D.R., Gregory M.D., Nobre A.C., Parrish T.B., Mesulam M.M. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. NeuroImage. 2003;18:633–641. doi: 10.1016/s1053-8119(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Smith-Seemiller L., Fow N.R., Kant R., Franzen M.D. Presence of post-concussion syndrome symptoms in patients with chronic pain vs mild traumatic brain injury. Brain Inj. 2003;17:199–206. doi: 10.1080/0269905021000030823. [DOI] [PubMed] [Google Scholar]
- Song X.W., Dong Z.Y., Long X.Y., Li S.F., Zuo X.N., Zhu C.Z., He Y., Yan C.G., Zang Y.F. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Grady C.L. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J. Cogn. Neurosci. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Tagliazucchi E., Balenzuela P., Fraiman D., Chialvo D.R. Brain resting state is disrupted in chronic back pain patients. Neurosci. Lett. 2010;485:26–31. doi: 10.1016/j.neulet.2010.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Kelly A.M.C., Biswal B.B., Margulies D.S., Shehzad Z., Shaw D., Ghaffari M., Rotrosen J., Adler L.A., Castellanos F.X., Milham M.P. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J. Neurosci. Methods. 2008;169:249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Valet M., Sprenger T., Boecker H., Willoch F., Rummeny E., Conrad B., Erhard P., Tolle T.R. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain–an fMRI analysis. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Valla J., Berndt J.D., Gonzalez-Lima F. Energy Hypometabolism in posterior cingulate cortex of Alzheimer's patients: superficial laminar cytochrome oxidase associated with disease duration. J. Neurosci. 2001;21:4923–4930. doi: 10.1523/JNEUROSCI.21-13-04923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme S., Legrain V., Vogt J., Crombez G. Keeping pain in mind: a motivational account of attention to pain. Neurosci. Biobehav. Rev. 2010;34:204–213. doi: 10.1016/j.neubiorev.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A., Lopez-Torrecillas F., Calandre E.P., Delgado-Rodriguez A., Bechara A. Executive function and decision-making in women with fibromyalgia. Arch. Clin. Neuropsychol. 2009;24:113–122. doi: 10.1093/arclin/acp014. [DOI] [PubMed] [Google Scholar]
- Wilcox S.L., Gustin S.M., Macey P.M., Peck C.C., Murray G.M., Henderson L.A. Anatomical changes at the level of the primary synapse in neuropathic pain: evidence from the spinal trigeminal nucleus. J. Neurosci. 2015;35:2508–2515. doi: 10.1523/JNEUROSCI.3756-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef A.M., Gustin S.M., Nash P.G., Reeves J.M., Petersen E.T., Peck C.C., Murray G.M., Henderson L.A. Differential brain activity in subjects with painful trigeminal neuropathy and painful temporomandibular disorder. Pain. 2014;155:467–475. doi: 10.1016/j.pain.2013.11.008. [DOI] [PubMed] [Google Scholar]