Abstract
We investigate the presence of complex organic molecules (COMs) in strongly UV-irradiated interstellar molecular gas. We have carried out a complete millimetre (mm) line survey using the IRAM 30 m telescope towards the edge of the Orion Bar photodissociation region (PDR), close to the H2 dissociation front, a position irradiated by a very intense far-UV (FUV) radiation field. These observations have been complemented with 8.5″ resolution maps of the H2CO JKa,Kc = 51,5 → 41,4 and C18O J = 3 → 2 emission at 0.9 mm. Despite being a harsh environment, we detect more than 250 lines from COMs and related precursors: H2CO, CH3OH, HCO, H2CCO, CH3CHO, H2CS, HCOOH, CH3CN, CH2NH, HNCO, and HC3N (in decreasing order of abundance). For each species, the large number of detected lines allowed us to accurately constrain their rotational temperatures (Trot) and column densities (N). Owing to subthermal excitation and intricate spectroscopy of some COMs (symmetric- and asymmetric-top molecules such as CH3CN and H2CO, respectively), a correct determination of N and Trot requires building rotational population diagrams of their rotational ladders separately. The inferred column densities are in the 1011 – 1013cm−2 range. We also provide accurate upper limit abundances for chemically related molecules that might have been expected, but are not conclusively detected at the edge of the PDR (HDCO, CH3O, CH3NC, CH3CCH, CH3OCH3, HCOOCH3, CH3CH2OH, CH3CH2CN, and CH2CHCN). A non-thermodynamic equilibrium excitation analysis for molecules with known collisional rate coefficients suggests that some COMs arise from different PDR layers but we cannot resolve them spatially. In particular, H2CO and CH3CN survive in the extended gas directly exposed to the strong FUV flux (Tk = 150 – 250 K and Td ≳ 60 K), whereas CH3OH only arises from denser and cooler gas clumps in the more shielded PDR interior (Tk = 40 – 50 K). The non-detection of HDCO towards the PDR edge is consistent with the minor role of pure gas-phase deuteration at very high temperatures. We find a HCO/H2CO/CH3OH ≃ 1/5/3 abundance ratio. These ratios are different from those inferred in hot cores and shocks. Taking into account the elevated gas and dust temperatures at the edge of the Bar (mostly mantle-free grains), we suggest the following scenarios for the formation of COMs: (i) hot gas-phase reactions not included in current models; (ii) warm grain-surface chemistry; or (iii) the PDR dynamics is such that COMs or precursors formed in cold icy grains deeper inside the molecular cloud desorb and advect into the PDR.
Keywords: astrochemistry, surveys, ISM: photon-dominated region (PDR), ISM: molecules, ISM: abundances
1. Introduction
Almost 200 molecules have been detected in the interstellar medium (ISM) and circumstellar shells. A very large fraction of them are complex organic molecules (COMs). COMs are traditionally defined as carbon-based molecular species with more than six atoms in their structure (Herbst & van Dishoeck 2009; Caselli & Ceccarelli 2012). To date, the largest organic molecules detected in the ISM (excluding PAHs and fullerenes) are propyl cyanide (C3H7CN; Belloche et al. 2009, 2014) and benzene (c-C6H6; Cernicharo et al. 2001).
COMs have been detected in the ISM since the 1970s (e.g. CH3CHO, Gottlieb 1973; or HCOOCH3, Brown et al. 1975). Most of the detections have been reported towards hot cores, that is, dense gas surrounding high-mass protostars in massive star-forming regions such as Sgr B2 and Orion KL (e.g. Bisschop et al. 2007; Ziurys & McGonagle 1993; Tercero et al. 2013, 2015; Ikeda et al. 2001), and towards hot corinos, that is, the low-mass analogs of hot cores such as IRAS 16293-2422 (Cazaux et al. 2003) and NGC 1333 IRAS 4A (Bottinelli et al. 2004). The high degree of chemical complexity (e.g. C2H5CN, CH3CCH, HCOOCH3, CH3OCH3…) found in these protostellar sources is thought to result from thermal desorption of the ice mantles coating dust grains. Herbst & van Dishoeck (2009) have reviewed the subject. They classify the observed COMs towards protostars in three different generations depending on the time they are produced. The zeroth-generation species, such as H2CO and CH3OH, are formed through grain-surface reactions in a previous cold interstellar phase in which icy mantles built up around granular cores of carbon and silicate grains. The first-generation species, such as HCOOCH3, are formed during the passive warm-up of the inner envelope of the protostar. During this phase, zeroth-generation species are photodissociated producing radicals such as HCO and CH3O, which can associate through surface reactions to form larger molecules. Finally, the second-generation species are formed once the core has become a hot core or hot corino. The dust temperature at this stage is high enough (Td ≳ 100 K) to sublimate the mantles completely, and new molecules are formed through warm gas-phase reactions (ion molecule and endothermic neutral-neutral reactions).
When protostellar outflows impact the ambient envelope material, grains can be eroded, and because of the high temperatures reached in shocks, icy mantles can sublimate. In sufficiently high-velocity shocks, ices can also be sputtered and directly injected into the gas phase (e.g. υs > 20 − 25 km s−1 for water ice, Draine et al. 1983). Several observations of the L1157 outflow have revealed the existence of H2CO, H2CS, CH3OH, HC3N, HNCO, NH2CHO, and CH3CHO in shocked gas (Bachiller & Pérez Gutiérrez 1997; Mendoza et al. 2014; Codella et al. 2015). In addition, Requena-Torres et al. (2006) have shown that the galactic centre contains dense clouds that are rich in CH3OH, HCOOCH3, and CH3OCH3. Widespread shocks have been invoked to explain their abundance and extended spatial distribution. The relative importance of the gas-phase reactions immediately after ice mantle sublimation compared to a formation of COMs on the grain surfaces is however far from being understood.
Recent observations towards UV-shielded cold cores (e.g. TMC 1, L1689B, or Barnard 1-b) have revealed molecules once considered to be present only in hot molecular cores (e.g. Remijan et al. 2006; Bacmann et al. 2012; Cernicharo et al. 2012). In these cold environments, COMs are thought to form on the surface of grains and to be released through non-thermal desorption processes, chemical desorption, direct desorption by cosmic ray impacts, or secondary photon induced processes (Cernicharo et al. 2012). Finally, a number of organic species have been identified in circumstellar envelopes around evolved stars (e.g. IRC+10216, Cernicharo et al. 2000), towards extragalactic sources (e.g. Meier & Turner 2005, 2012; Aladro et al. 2011), meteorites (e.g. Cronin & Chang 1993), and comets (e.g. Bockelée-Morvan et al. 2004).
Studies of environments permeated by stellar far-UV photons (FUV, 6.0 eV < hν < 13.6 eV) are more scarce. Guzmán et al. (2014) and Gratier et al. (2013) presented the unexpected detection of HCOOH, CH2CO, CH3CN, CH3OH, CH3CHO, and CH3CCH in the Horsehead photodissociation region (PDR; a relatively low-FUV-flux dense PDR, χ ≈ 60 times the mean interstellar field in Draine units), finding enhanced abundances compared to a nearby cold and dense core shielded from external FUV radiation. Guzmán et al. (2014) proposed that owing to the cold grain temperatures, ice-mantle photodesorption processes dominate the formation of COMs in the Horsehead. In lower-density translucent clouds (χ ≈ 1), only H2CO has been unambiguously detected (Liszt et al. 2006). These observations might suggest that, in FUV-irradiated environments, the presence of COMs diminishes as the χ/nH ratio increases. Thus, COMs might not be present in strongly FUV-irradiated gas.
In this work we test the above hypothesis and investigate the presence and abundances of COMs at the high FUV-illuminated edge of the Orion Bar, with an impinging radiation field of a few 104 times the mean interstellar field (Marconi et al. 1998). Because of its proximity (414 ± 7 pc, Menten et al. 2007) and nearly edge-on orientation, the Orion Bar provides an excellent template to determine the chemical content and also to investigate the structure and dynamics of strongly FUV-irradiated molecular gas (e.g. Tielens et al. 1993; Hogerheijde et al. 1995; Cuadrado et al. 2015, 2016; Goicoechea et al. 2016).
Multi-wavelength observations towards different positions of the Orion Bar have been historically used in the development of PDR models (e.g. Tielens & Hollenbach 1985a,b) and today they are still used as a template to understand the unresolved emission from sources as different as the nuclei of distant star-burst galaxies (e.g. Fuente et al. 2008) or the illuminated surfaces of protoplanetary disks (e.g. Agúndez et al. 2008). The transition from ionised to neutral gas in the Orion Bar has been extensively mapped, generally at low angular resolution, in various atomic and molecular tracers (see e.g. Tielens et al. 1993; Hogerheijde et al. 1995; van der Werf et al. 1996; Walmsley et al. 2000; Leurini et al. 2010; Ossenkopf et al. 2013). The detailed analysis of these observations suggested an inhomogeneous density distribution. The most commonly accepted scenario is that an extended gas component, with mean gas densities of 104 − 105 cm−3, causes the chemical stratification seen perpendicular to the dissociation front as the FUV field is attenuated (Hogerheijde et al. 1995; Jansen et al. 1995; Simon et al. 1997; van der Wiel et al. 2009; Habart et al. 2010; van der Tak et al. 2013). In addition, another component of clumpy material with higher densities (≳ 106 cm−3) and more shielded from FUV radiation is embedded in the interclump gas (Burton et al. 1990; Parmar et al. 1991; Stoerzer et al. 1995; Young Owl et al. 2000; Lis & Schilke 2003; Batrla & Wilson 2003; Andree-Labsch et al. 2017). Previous observations of H2CO and CH3OH in the Orion Bar have shown that the two molecules trace these two different environments: CH3OH, the denser and cooler clumps seen deeper inside the Bar, and H2CO, the warmer interclump medium directly exposed to the strong FUV-field (Leurini et al. 2006, 2010).
We have performed a complete millimetre line survey using the IRAM 30 m telescope towards the edge of Orion Bar, a high FUV-illuminated position close to what Stoerzer et al. (1995) call the “CO+ peak”, near the H2 dissociation front (see Fig. 1). This position shows a distinctive chemistry that can only be understood due to the presence of a strong flux of FUV photons (e.g. compared to that in the more shielded clumps deeper inside the Bar): enhanced abundances of simple reactive ions (e.g. CH+, CO+, and HOC+) and small hydrocarbon ions (e.g. l-C3H+) that are only abundant in the presence of C+; vibrationally excited H2; and high gas temperatures (e.g. Nagy et al. 2013; Guzmán et al. 2015; Cuadrado et al. 2015). Indeed, high-angular resolution ALMA-ACA images do show that reactive ions such as SH+ or HOC+ do not emit from the more shielded clumps (Goicoechea et al. 2017). Therefore, their chemistry is different to that of the PDR edge observed in this work. Our survey covers ~220 GHz of bandwidth, between 80 GHz and 360 GHz. These observations have been complemented with several 8.5″ resolution maps of different molecules at 0.9 mm to put our line survey position in the context of the Bar large-scale emission. In this paper we focus on the detection of rotational lines from COMs and related precursors.
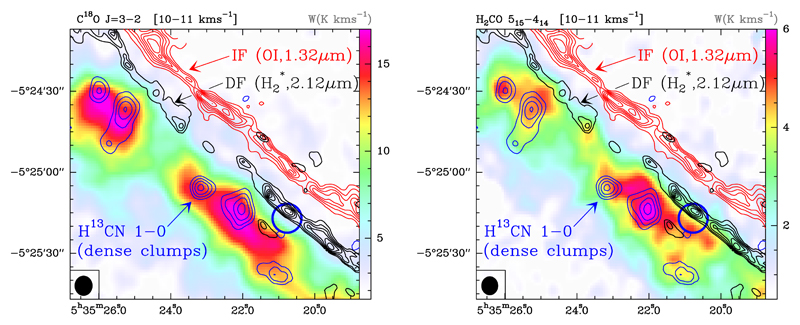
Fig. 1.
C18O J = 3 → 2 (left panel) and H2CO JKa,Kc = 51,5 → 41,4 (right panel) line integrated intensity maps (W in K km s−1) in the 10 − 11 km s−1 velocity interval observed with the IRAM 30 m telescope at ~329 GHz and ~351 GHz, respectively (colour scale). Black contours are the υ = 1 → 0 S (1) emission delineating the H2 dissociation front (DF, Walmsley et al. 2000). The red contours represent the O i fluorescent line at 1.32 µm (Walmsley et al. 2000) marking the position of the ionisation front (IF) that separates the H ii region and the neutral cloud. The blue contours represent the H13CN J = 1 → 0 emission tracing dense molecular clumps inside the Bar (Lis & Schilke 2003). The target position of the Orion Bar survey, close to the DF, is indicated with a blue circle. The IRAM 30 m beam at 1 mm is plotted in the bottom left corner (black circle).
The paper is organised as follows. In Sect. 2 we describe the line survey and the mapping observations. In Sect. 3 we report the observational features of the detected organic molecules, while in Sect. 4 we present the C18O and H2CO integrated line intensity maps at 0.9 mm. The data analysis is explained in Sect. 5. In Sect. 6 we discuss the results, and finally in Sect. 7 we summarise the main conclusions.
2. Observations and data reduction
2.1. Line survey
We performed a complete millimetre line survey towards the edge of Orion Bar using the IRAM 30 m telescope. The target position is at α2000 = 05h 35m 20.8s, δ2000 = − 05°25′17.0″, close to the dissociation front. This position is at (Δα, Δδ) = (3″, −3″) from the “CO+ peak” of Stoerzer et al. (1995).
Our observations cover a total of 217 GHz along 3, 2, 1, and 0.9 mm bands of the EMIR receivers using the WILMA (2 MHz spectral resolution) and FTS (200 kHz spectral resolution) backends. The observing procedure was position switching (PSW) with the reference position located at an offset (−600″, 0″) to avoid the extended molecular emission from the Orion Molecular Cloud (OMC-1). The antenna temperature, was converted to the main beam temperature, TMB , through the relation, where ηMB is the antenna efficiency. All intensities in tables and figures are in main beam temperature. A local standard of rest (LSR) of 10.7 km s−1 has been used in the line survey target position in the Orion Bar dissociation front. Table 1 shows an overview of the frequency ranges observed with each backend, the spectral resolution in velocity units (δυ) in the observed frequency ranges, as well as the variation in the telescope efficiencies, ηMB , and the half power beam width (HPBW) across the covered frequency range.
Table 1.
Observed frequency ranges and telescope parameters.
| Rec.a | Backend | Freq.b [GHz] |
δυc [km s-1] |
ηMB d | HPBWe [arcsec] |
|---|---|---|---|---|---|
| E0 | FFTS | 80–117 | 0.75–0.51 | 0.87–0.82 | 31–21 |
| E1 | WILMA | 128–176 | 4.7–3.4 | 0.80–0.74 | 19–14 |
| E2 | FFTS | 202–275 | 0.30–0.22 | 0.70–0.56 | 12–9 |
| E3 | FFTS | 275–305 | 0.22–0.20 | 0.56–0.50 | 9–8 |
| FFTS | 328–359 | 0.18–0.17 | 0.46–0.40 | 8–7 | |
Notes.
Emir receiver.
Observed frequency range.
Spectral resolution in velocity units (δυ) in the observed frequency ranges.
Antenna efficiencies.
The half power beam width can be well fitted by HPBW[arcsec]≈2460/Frequency[GHz].
In Appendix A we study the possible contamination of bright molecular line emission from the Orion BN/KL region (located at a distance of ~20′) through the beam side lobes (several arcmin, see Greve et al. 1998). Although the contribution to the detected power ranges from ~12% (3 mm) to ~30% (0.8 mm), most of the emission from the hot core region arises at different velocities (~8 km s−1) than those of the Orion Bar (~10 − 11 km s−1) and thus can be easily separated.
Data reduction and spectral analysis were done using the CLASS software of the GILDAS package1 developed by IRAM. Weighted spectra were averaged and calibrated, and a polynomial baseline of low order (typically second or third order) was subtracted from each ~200 MHz wide spectrum. Finally, Gaussian profiles were fitted to all the detected lines (Appendix B). The rms noise of our observations obtained after integration during ~4 h ranges between 4 mK and 20 mK per resolution channel.
2.2. Maps
The 0.9 mm line emission from different molecules was mapped with the IRAM 30 m telescope in January 2014 under excellent winter conditions (<1 mm of precipitable water vapour). The E3 receiver and the FTS backend at 200 kHz spectral resolution were used. On-the-fly (OTF) scans were obtained along and perpendicular to the Bar over a 170″ × 170″ region, with an OFF position at (−600″,0″) relative to the map centre at α2000 = 05h 35m 20.1s, δ2000 = − 05°25′07.0″, which is slightly different from that of the line survey (see above). The HPBW at this frequency is ~7″. Data processing consisted in a linear baseline subtraction in each observed spectra. The resulting spectra were gridded to a data cube through convolution with a Gaussian kernel providing a final resolution of 8.5″. The total integration time was approximately 6 h, and the achieved rms noise is ~1 K per ~0.2 km s−1 channel. Figure 1 shows the C18O J = 3 → 2 and H2CO JKa,Kc = 51,5 → 41,4 integrated line intensity maps in the range υLSR = 10 − 11 km s−1 in which the Orion Bar shows prominent emission. We note that van der Wiel et al. (2009) also presented a smaller H2CO JKa,Kc = 51,5 → 41,4 map at lower angular resolution (a factor of ~2).
3. Results: complex organic molecule detections
We have identified more than 250 lines from COMs and related organic precursors. The detected lines are attributed to ten different molecules with up to seven atoms: HCO, HNCO, H2CO, H2CS, CH2NH, H2CCO, HC3N, CH3OH, CH3CN, and CH3CHO. We also detect several lines of the isotopologue We note that among the organic molecules with up to seven atoms, we also identified several lines of formic acid, trans- and cis-HCOOH, in the Orion Bar PDR. Cuadrado et al. (2016) have recently reported the first detection of the cis conformer of HCOOH in the interstellar medium as well as a detailed analysis of the detected lines of both HCOOH conformers in the 3 mm spectral band. Line profiles peak at υLSR = 10 − 11 km s−1, the velocity of the Bar, thus confirming their PDR origin. The detected organic molecules and their dipole moments are listed in Table 2.
Table 2.
Dipole moments (μ), number of detected lines of complex organic molecules and related organic precursors, and their corresponding Figure and Table numbers.
| Molecule | No. of lines | μ [Debyes] | Fig. | Table | ||
|---|---|---|---|---|---|---|
| HCO | 16 | μa=1.363 | μb=0.700a | 2 | B.1 | |
| H2CO | 15 | μa=2.332b | 3 | B.2 | ||
| 8 | μa=2.332b | 4 | B.3 | |||
| H2CS | 26 | μa=1.649b | 5 | B.4 | ||
| HNCO | 6 | μa=1.602 | μb=1.350c | 6 | B.5 | |
| CH2NH | 6 | μa=1.340 | μb=1.446d | 7 | B.6 | |
| H2CCO | 30 | μa=1.422b | 8 | B.7 | ||
| HC3N | 11 | μa=3.732e | 9 | B.8 | ||
| CH3CN | 44 | μa=3.922f | 11 | B.9 | ||
| CH3OH | 55 | μa=0.896 | μb=1.412g | 12 | B.10 | |
| CH3CHO | 36 | μa=2.423 | μb=1.260h | 13 | B.11 | |
References.
Previous studies of the Orion Bar have already reported the presence of HCO, H2CO, and CH3OH in several positions close to the “CO+ peak”: (i) Schilke et al. (2001) detected one hyperfine component of HCO N = 1 → 0 using the IRAM 30 m telescope; (ii) Hogerheijde et al. (1995) detected nine lines of H2CO and three lines of CH3OH in several positions along the Bar using the JCMT, IRAM 30 m, and CSO telescopes; (iii) Nagy et al. (2017) detected ten submm H2CO lines with Herschel/HIFI. In addition, H2CO, HDCO, H2CS, and CH3OH have been detected towards the colder, denser, and more FUV-shielded clumps: (iv) Leurini et al. (2006) detected seven lines of H2CO, four lines of H2CS, and ten lines of CH3OH using APEX between 279 GHz and 361.5 GHz towards clump #1 of Lis & Schilke (2003); (v) Parise et al. (2009) also detected two isotopologues, one line of and one line of HDCO, towards clump #3 of Lis & Schilke (2003) using the IRAM 30 m telescope. To our knowledge, our work presents the first detection of CH2NH, H2CCO, HC3N, CH3CN, and CH3CHO in the Orion Bar PDR.
Line assignment was carried out using J. Cernicharo’s spectral catalogue (MADEX, Cernicharo 2012), and the JPL (Pickett et al. 1998)2 and CDMS (Müller et al. 2001, 2005)3 public databases. Although some lines have peak intensities lower than 3σ noise level, we included them in our results as tentative detections because other transitions from the same molecule are well detected in this survey, and these 3σ limits are in agreement with our excitation models. Given their intricate spectroscopy, below we summarise the main spectroscopic information of the detected species.
3.1. Formyl radical: HCO
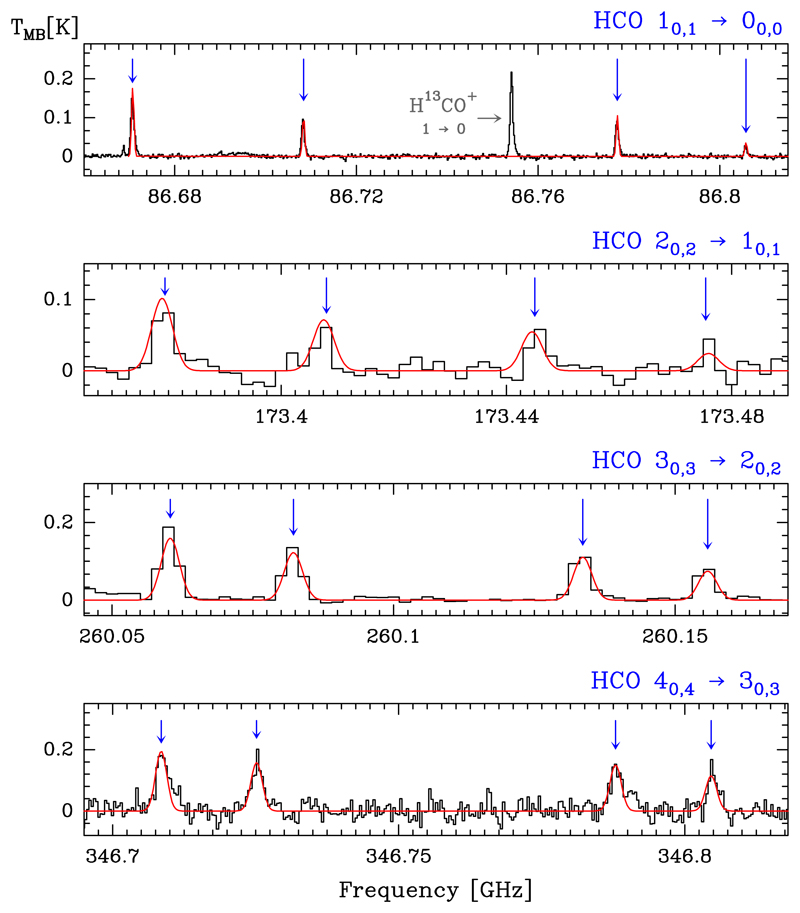
The formyl radical, HCO, is a light asymmetric rotor with one unpaired electron and one non-zero nuclear spin. The quantum numbers designating the energy levels are N, J, and F. Spin doubling (J = N + S ) is produced by the coupling between the rotational angular momentum, N, and the unpaired electron spin, S , while the hyperfine structure (F = J + I) is due to the coupling of the angular momentum, J, and the spin of the hydrogen nucleus, I. Each rotational level NKa,Kc is split into a doublet by the spin-rotation interaction, the levels of which are further split into doublets by magnetic hyperfine interactions, with the final energy levels labelled by the quantum number F = J ± 1/2 (e.g. Bowater et al. 1971; Saito 1972; Austin et al. 1974; Blake et al. 1984). a-type (ΔKa = 0, ±2...) and b-type (ΔKa = ±1, ±3...) transitions are allowed, with a stronger dipole moment for the a-type transitions (see Table 2).
We identified a total of 16 lines of HCO. They consist of four sets of rotational transitions corresponding to the hyperfine splitting of the N = 1 → 0 to 4 → 3 transitions. All transitions observed here are a-type. The quantum numbers of the detected HCO transitions, their spectroscopic parameters, and the results from fitting the line profiles with Gaussians are listed in Table B.1. In Fig. 2 we present the spectra of the N = 1 → 0 to 4 → 3 rotational lines.
Fig. 2.
Detected HCO hyperfine structure (HFS) lines of the N = 1 → 0, 2 → 1, 3 → 2, and 4 → 3 rotational transitions (black histogram spectra). A single excitation temperature model is shown overlaid in red (see Sect. 5.4). HFS lines are indicated by the blue arrows. The other spectral features appearing in the selected windows are labelled with their corresponding identification.
3.2. Formaldehyde: H2CO
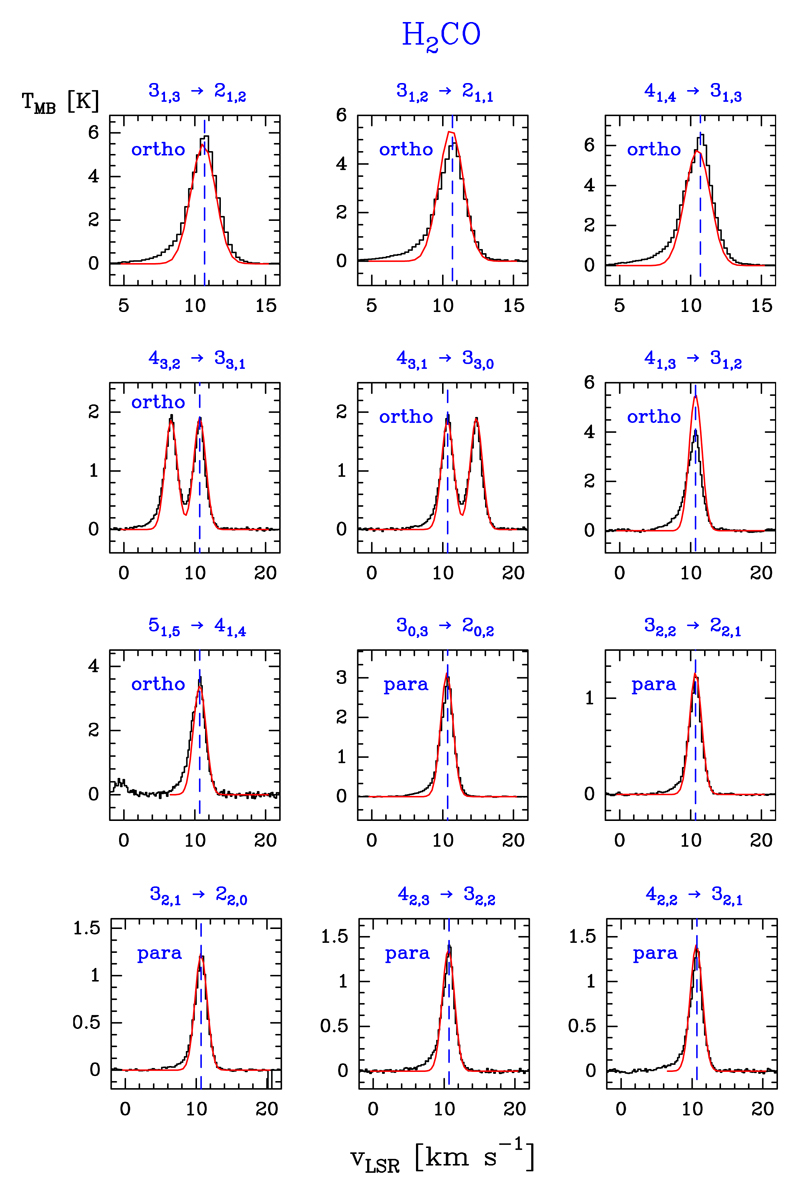
Formaldehyde, H2CO, was the first polyatomic organic molecule found in the ISM (Snyder et al. 1969). It is a slightly asymmetric prolate rotor molecule with K-type doublets and an ortho-para symmetry because of the two indiscernible off-axis hydrogen atoms. The ortho states are those with Ka odd, and the para states with Ka even. The nuclear spin weights are 3 and 1 for o-H2CO and p-H2CO, respectively. The K-type doublets are the result of the slight asymmetry in the H2CO produced by the light H atoms. There are no line doublets in the Ka = 0 para state (e.g. Bocquet et al. 1996; Brünken et al. 2003; Eliet et al. 2012).
We detected nine lines of the ortho species (in the Ka = 1 and 3 ladders) with Eu/k ≤ 125.8 K, and six lines for the para species (in the Ka = 0 and 2 ladders) with Eu/k ≤ 82.1 K. Line profiles are shown in Fig. 3. Table B.2 gives the observed line parameters.
Fig. 3.
Example of ortho- and para-H2CO lines (black histogram spectra). A non-LTE LVG model (Tk ≃ 200 K, n(H2) ≃ 1 × 106 cm−3, N(o-H2CO) = 4.0 × 1013 cm−2, and N(p-H2CO) = 1.8 × 1013 cm−2) is shown overlaid in red (see Sect. 5.2). The dashed lines indicate the LSR velocity (10.7 km s−1) of the Orion Bar PDR.
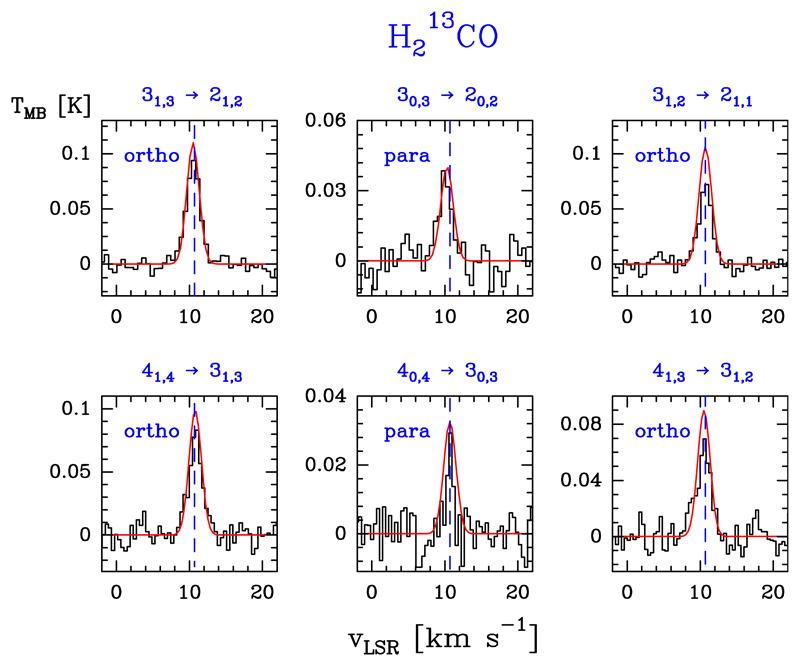
We also detected eight lines of consisting of six lines of ortho species and two lines of the para species, in the Ka = 0 and 1 ladders, respectively (Eu/k ≤ 34.0 K). Line profiles are shown in Fig. 4. Table B.3 gives the observed line parameters. No lines of deuterated formaldehyde were detected.
Fig. 4.
Example of ortho- and para- lines (black histogram spectra). A non-LTE LVG model (Tk ≃ 200 K, n(H2) ≃ 1 × 106 cm−3, and is shown overlaid in red (see Sect. 5.2).
3.3. Thioformaldehyde: H2CS
Thioformaldehyde, H2CS, has a formaldehyde-like structure. It is a slightly asymmetric prolate rotor, with only a-type transitions and ortho-para symmetry (e.g. Johnson et al. 1971; Beers et al. 1972; Maeda et al. 2008).
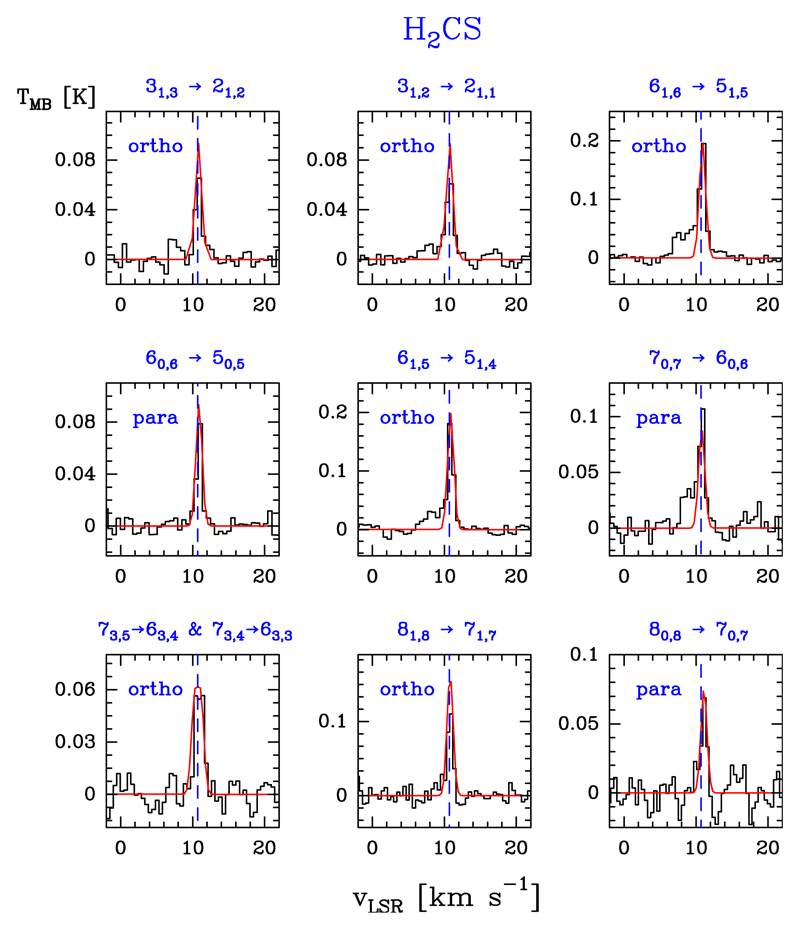
We detected 15 lines of ortho species (in the Ka = 1 and 3 ladders) with Eu/k ≤ 149.8 K, and 11 lines of para species (in the Ka = 0 and 2 ladders) with Eu/k ≤ 112.0 K. Figure 5 shows a selection of ortho and para detected lines. Table B.4 gives the observed line parameters.
Fig. 5.
Example of ortho- and para-H2CS lines (black histogram spectra). Single excitation temperature models are shown overlaid in red (see Sect. 5.4).
3.4. Isocyanic acid: HNCO
Isocyanic acid, H-N=C=O, is the simplest molecule containing carbon, hydrogen, oxygen, and nitrogen. It is a slightly asymmetric prolate rotor with a- and b-type transitions. Its rotational levels are designated as JKa,Kc (e.g. Kukolich et al. 1971; Hocking et al. 1975; Lapinov et al. 2007).
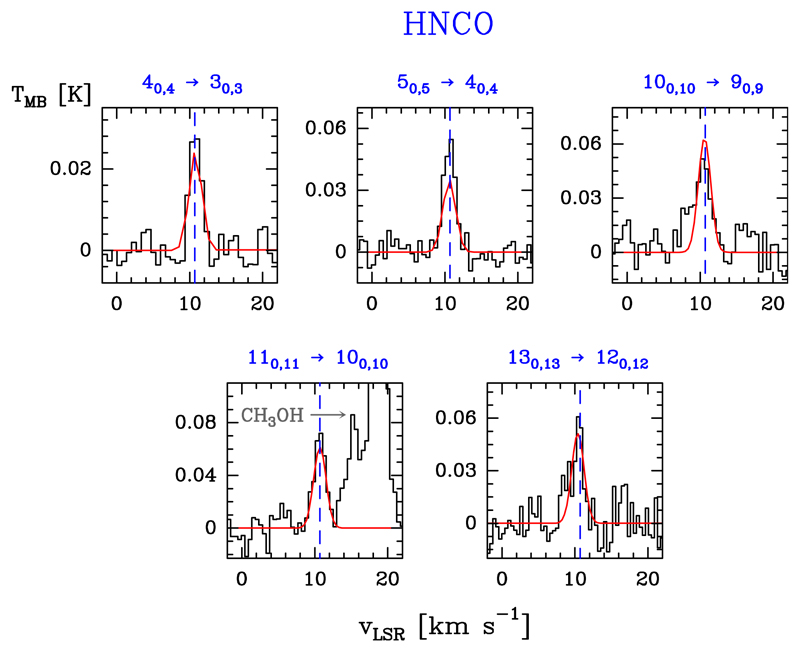
We detect six a-type transitions inside the Ka = 0 rotational ladder (Eu/k ≤ 96.0 K). The observed lines do not show evidence for hyperfine splittings. Line profiles are shown in Fig. 6. Table B.5 gives the observed line parameters.
Fig. 6.
Example of HNCO detected lines (black histogram spectra). A single excitation temperature model is shown overlaid in red (see Sect. 5.4).
3.5. Methanimine: CH2NH
Methanimine is the simplest molecule containing a carbon-nitrogen double bond (H2C=NH). It is a planar molecule and a nearly prolate asymmetric rotor with components of the electric dipole moment along both the a and b molecular axes (e.g. Kirchhoff et al. 1973; Dore et al. 2010, 2012).
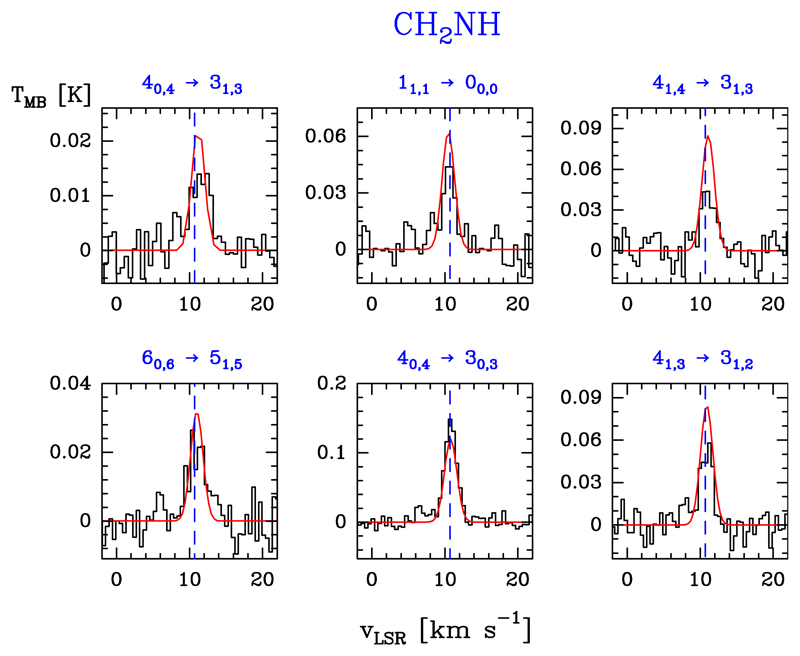
We detected six lines of CH2NH consisting of (i) three a-type transitions (in the Ka = 0 and 1 ladders) and (ii) three b-type transitions, with Eu/k ≤ 64.1 K. The observed lines do not show evidence for hyperfine splittings. The line profiles are shown in Fig. 7. Table B.6 gives the observed line parameters.
Fig. 7.
CH2NH detected lines (black histogram spectra). A single excitation temperature model is shown overlaid in red (see Sect. 5.4).
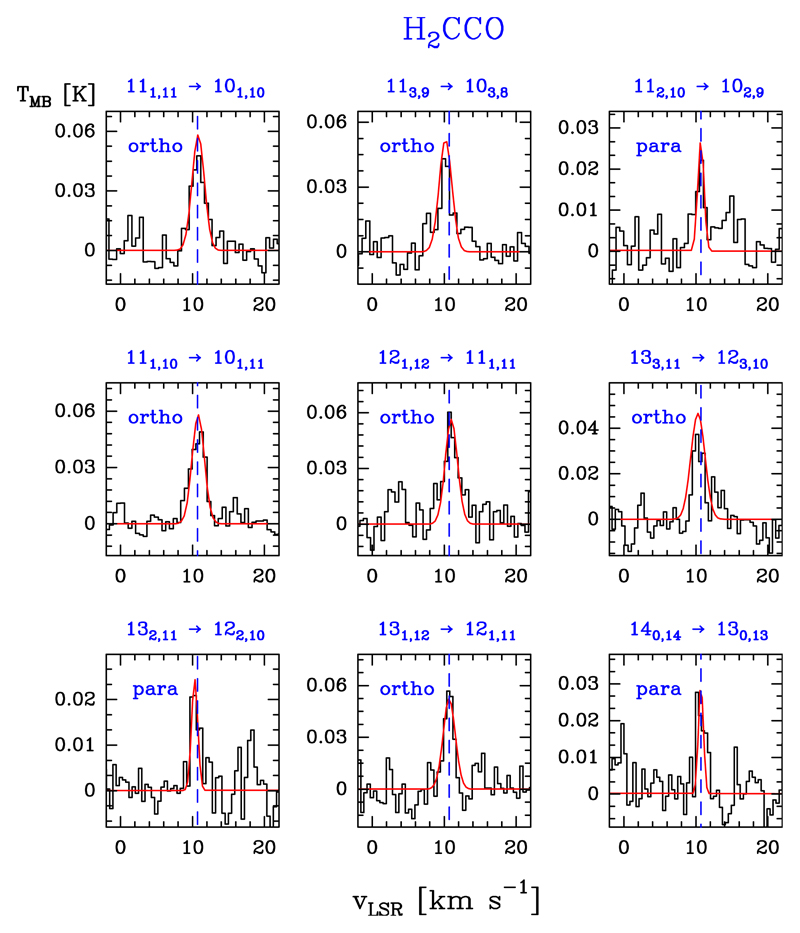
3.6. Ketene: H2CCO
Ketene, H2C=C=O, is a slightly asymmetric prolate rotor with only a-type transitions and ortho-para symmetry (e.g. Johnson & Strandberg 1952; Fabricant et al. 1977; Brown et al. 1990; Johns et al. 1992). We detected 20 lines of ortho species (in the Ka = 1 and 3 ladders) with Eu/k ≤ 205.2 K, and ten lines for the para species (in the Ka = 0 and 2 ladders) with Eu/k ≤ 154 K. Examples of the line profiles of ortho and para lines are shown in Fig. 8. Table B.7 gives the observed line parameters.
Fig. 8.
Example of ortho- and para-H2CCO lines (black histogram spectra). Single excitation temperature models are shown overlaid in red (see Sect. 5.4).
3.7. Cyanoacetylene: HC3N
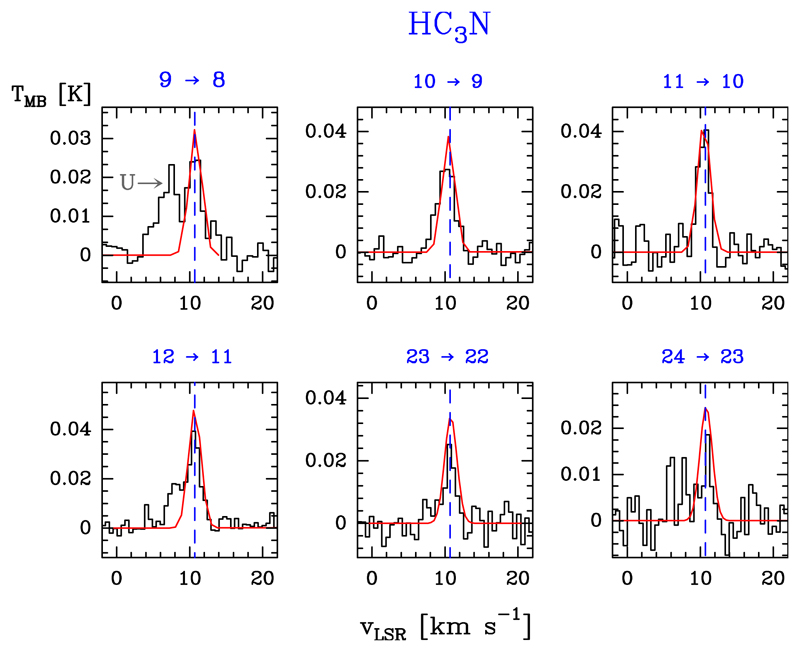
Cyanoacetylene, H–C≡C–C≡N, is a linear molecule with a large dipole moment of μa = 3.732 D (DeLeon & Muenter 1985). It is the simplest example of cyanopolyynes, HCnN (with n = 3, 5, 7…; see e.g. Turner 1971; Avery et al. 1976; Cernicharo et al. 1986). The rotational transitions show hyperfine splittings owing to the interaction of the electric-quadrupole moment of the nitrogen nuclear spin with the electronic-charge distribution (e.g. de Zafra 1971; Mbosei et al. 2000; Chen et al. 1991; Yamada et al. 1995; Thorwirth et al. 2000). This hyperfine splitting is only fully resolved in the lowest rotational transitions (e.g. Turner 1971; Dickinson 1972), therefore, HC3N is described here by a simple rotational spectrum, with J+1 → J transitions.
We detected 11 lines of HC3N, from J = 9 → 8 to 24 → 23, with Eu/k ≤ 131.0 K. Figure 9 shows a selection of detected lines and Table B.8 gives the observed line parameters.
Fig. 9.
Example of HC3N detected lines (black histogram spectra). A non-LTE LVG model (Tk ≃ 80 K, n(H2) ≃ 1 × 106 cm−3, N(HC3N) = 4.0 × 1011 cm−2) is shown overlaid in red (see Sect. 5.2).
3.8. Methyl cyanide: CH3CN
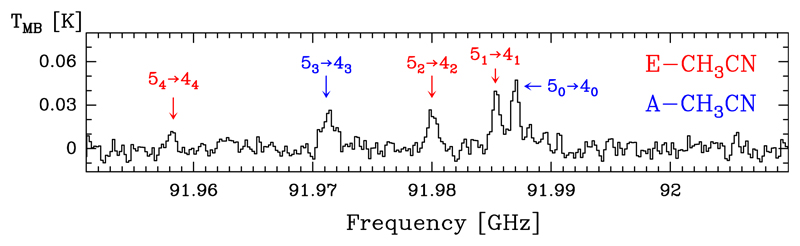
Methyl cyanide, CH3CN, is a strongly prolate symmetric rotor, therefore, the permitted radiative transitions are all ΔK = 0. The internal rotation of the methyl group gives rise to two non-interacting torsional substates, denoted A and E. The A state levels are described by K = 3n, and those of E state by K = 3n ± 1, with n ≥ 0 (e.g. Kukolich et al. 1973; Kukolich 1982; Boucher et al. 1977; Anttila et al. 1993; Šimečková et al. 2004; Cazzoli & Puzzarini 2006; Müller et al. 2009). Transitions with different K but the same J occur in narrow frequency regions but they have quite different energies (see Fig. 10).
Fig. 10.
Observed emission lines from the J = 5 → 4 transitions of CH3CN. Transitions with different K but the same J occur in narrow frequency regions despite they have different level energies.
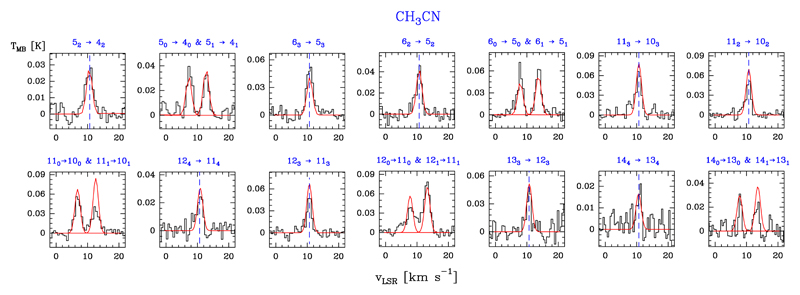
We detected 18 lines of A-CH3CN (in the K = 0 and 3 ladders) with Eu/k ≤ 184.4 K, 23 lines for the E-CH3CN (in the K = 1, 2, and 4 ladders) with Eu/k ≤ 199.0 K, and three lines corresponding to several fully overlapped A-E transitions. Examples of line profiles of A- and E-CH3CN lines are shown in Fig. 11. Table B.9 gives the observed line parameters. We did not detect the CH3NC isomer (see Sect. 5.6).
Fig. 11.
Example of A- and E-CH3CN lines (black histogram spectra). A non-LTE LVG model (Tk ≃ 150 K, n(H2) ≃ 1 × 106 cm−3, N(A-CH3CN) = 5 × 1011 cm−2, and N(E-CH3CN) = 6 × 1011 cm−2) is shown overlaid in red (see Sect. 5.2).
3.9. Methanol: CH3OH
Methanol, CH3OH, is the simplest alcohol molecule. It is a slightly asymmetric rotor showing hindered internal rotation. Its energy levels are classified as a symmetric rotor with quantum number JK and a threefold barrier that causes two states in the molecule, A and E; the latter being doubly degenerate (e.g. Lees & Baker 1968; Lees et al. 1973; Xu et al. 2008).
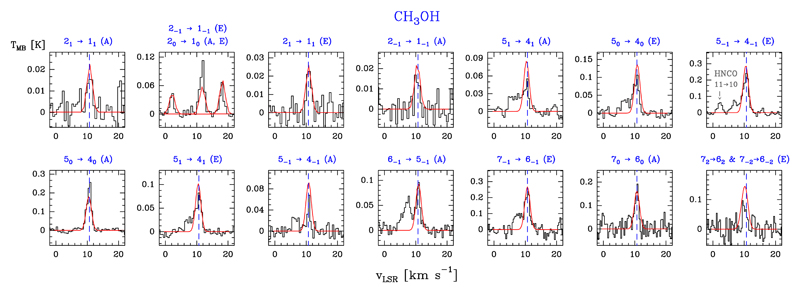
We detected 20 lines of A-CH3OH (in the |K| = 0 and 1 ladders) with Eu/k ≤ 104.4 K, 33 lines for the E-CH3OH (in the |K| = 1, 2, 3, and 4 ladders) with Eu/k ≤ 114.8 K, and two lines corresponding to several fully overlapped A-E transitions. Examples of line profiles of A- and E-CH3OH lines are shown in Fig. 12. Table B.10 gives the observed line parameters.
Fig. 12.
Example of A- and E-CH3OH lines (black histogram spectra). A non-LTE LVG model (Tk ≃ 40 K, n(H2) ≃ 3 × 107 cm−3, N(A-CH3OH) = 1.2 × 1013 cm−2, and N(E-CH3OH) = 1.9 × 1013 cm−2) is shown overlaid in red (see Sect. 5.2).
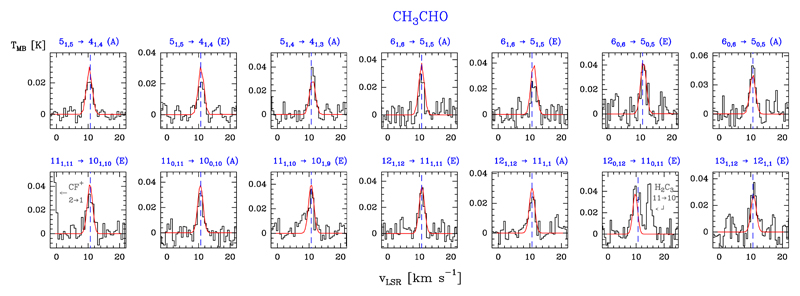
3.10. Acetaldehyde: CH3CHO
Acetaldehyde, CH3CHO, is an asymmetric-top molecule with A and E symmetry states and a- and b-type transitions (e.g. Kleiner et al. 1996, and references therein).
We detected 18 lines of A-CH3CHO, 15 lines of E-CH3CHO, and three lines corresponding to a fully overlapped A-E transition (a-type transitions with Eu/k ≤ 109.7 K). Figure 13 shows a selection of detected lines and Table B.11 gives the observed line parameters.
Fig. 13.
Examples of A- and E-CH3CHO lines (black histogram spectra). Single excitation temperature models are shown overlaid in red (see Sect. 5.4).
4. Spatial distribution of C18O and H2CO
Figure 1 shows the spatial distribution of the rotationally excited C18O J = 3 → 2 (Eu/k ≃32 K, Aul ≃ 2 × 10−6 s−1) and H2CO JKa,Kc = 51,5 → 41,4 (Eu/k ≃ 47 K, Aul ≃ 1 × 10−3s−1) lines along the Bar. The molecular dissociation front (DF) is traced by the vibrationally excited H2 ν = 1 → 0 S (1) line emission ( black contours in Fig. 1; Walmsley et al. 2000). The red contours represent the O i fluorescent line at 1.32 µm (Walmsley et al. 2000) marking the position of the ionisation front (IF) that separates the H ii region and the neutral cloud. The blue contours represent the H13CN J = 1 → 0 emission tracing dense molecular clumps inside the Bar (Lis & Schilke 2003).
The emission from the Orion Bar can be distinguished from the extended OMC-1 cloud component by the emission LSR velocity. While OMC-1 is brighter in the 8 − 10 km s−1 velocity range, the Orion Bar emits predominantly in the 10 − 11 km s−1 range. The C18O and H2CO line emissions shown in Fig. 1 are integrated in this latter interval. In these maps, H2CO peaks at the position of the dense (n(H2) ≃ 6 × 106 cm−3) and lukewarm (Tk ≃ 50 K) clumps inside the Bar (Lis & Schilke 2003). In addition, our maps reveal extended H2CO emission along the PDR and close to the DF. This conclusion was initially inferred by Leurini et al. (2010) from ~11″ resolution observations of lower-excitation H2CO lines.
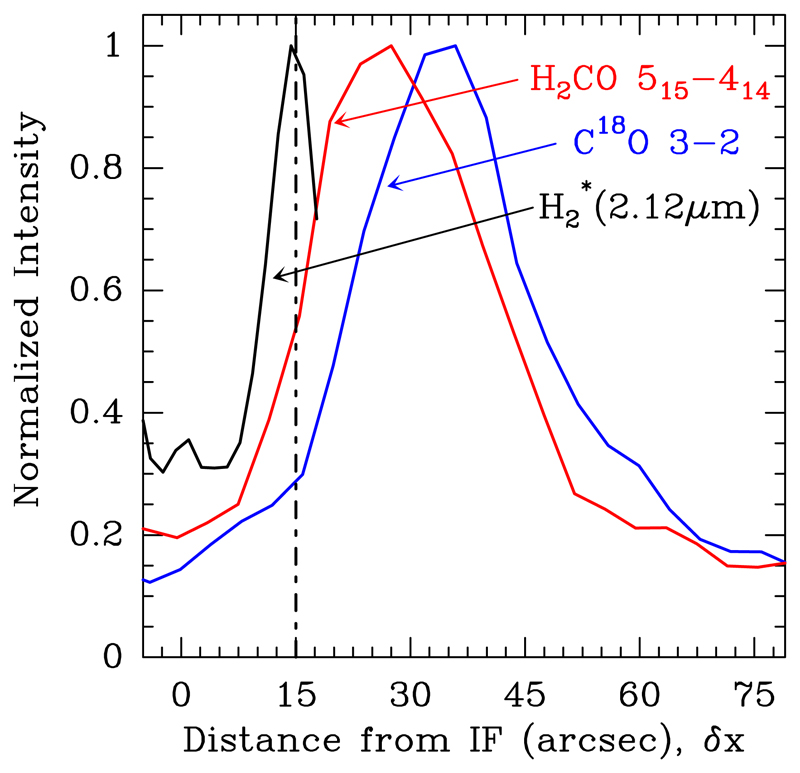
To investigate the chemical stratification as a function of FUV flux attenuation, we built averaged line intensity crosscuts perpendicular to the Bar. The maps were rotated by 50° to bring the incident FUV radiation from left, and then, line intensities in the mapped area were averaged parallel to the IF. In Fig. 14 we plot the resulting normalised intensity cuts as a function of distance to the IF position. Distances are in arcseconds and increase with depth into the molecular cloud. Although these cuts do not represent true abundance variations (different lines have different excitation conditions), to first order they can be used to study the chemical stratification in the PDR. The emission peak is at ~15″ (vertical dot-dashed line) from the IF, and shows a narrow emission profile. The H2CO JKa,Kc = 51,5 → 41,4 emission peak appears at ~27″, followed by C18O J = 3 → 2 that peaks at ~35″.
Fig. 14.
Normalised intensity crosscuts for C18O J = 3 → 2 (blue curve), H2CO JKa,Kc = 51,5 → 41,4 (red curve), and (black curve) as a function of distance from the IF (in arcsec). The vertical dot-dashed line indicates the H2 dissociation front. FUV radiation comes from left.
We note that the different position of the emission peaks of the C18O J = 3 → 2 and H2CO JKa,Kc = 51,5 → 41,4 lines shown in Fig. 14 could be related to a sharp thermal gradient in the region, with the gas temperature increasing from ~50 K inside the dense clumps seen in the PDR interior (Lis & Schilke 2003), to ~300 K near the DF. The H2CO JKa,Kc = 51,5 → 41,4 line requires higher excitation conditions (higher temperature/density) and thus it would naturally peak closer to the DF than the C18O J = 3 → 2 line, even for constant H2CO and C18O abundances throughout the PDR. Still, the main point is that observations show that H2CO is present in the warm interclump gas and close to the PDR edge where the FUV flux is strong.
5. Analysis
5.1. Line parameter fitting procedure
Detected lines show simple Gaussian line profiles centred at the Orion Bar LSR velocity and thus Gaussian profiles were fitted to all detected lines. For the resolved and unblended COM lines detected with significant S/N, the mean line width is Δυ = 1.8 km s−1, with a standard deviation of σ = 0.6 km s−1.
The spectroscopic and observational line parameters of the detected lines are given in Tables B.1-B.11. We provide line frequencies (in MHz), energy of the upper level of each transition (Eu/k in K), Einstein coefficient for spontaneous emission (Aul in s−1), intrinsic line strength (S ul), and the level degeneracy (gu) from the MADEX spectral catalogue, and the JPL and CDMS molecular databases. The velocity-integrated intensity (∫ TMB dυ in mK km s−1), LSR velocity (vLSR in km s−1), FWHM (full width at half maximum) line width (Δυ in km s−1), and the line peak temperature (TMB in mK) were obtained from Gaussian fits. Parentheses indicate the uncertainty. When two or more transitions were found to be overlapping, the total profile was fitted. Fully overlapping transitions are marked with connecting symbols in the tables.
5.2. Non-LTE LVG excitation analysis: physical conditions
We estimate the beam-averaged physical conditions towards the line survey position from non-LTE (local thermodynamic equilibrium) excitation models for molecules with known inelastic collisional rate coefficients (H2CO, HC3N, CH3CN, and CH3OH)4. In particular, we run a large grid of non-LTE excitation and radiative transfer models in which the statistical equilibrium equations are explicitly solved in the LVG (large velocity gradient) approximation using MADEX (Cernicharo 2012, see also Appendix A in Cuadrado et al. 2015).
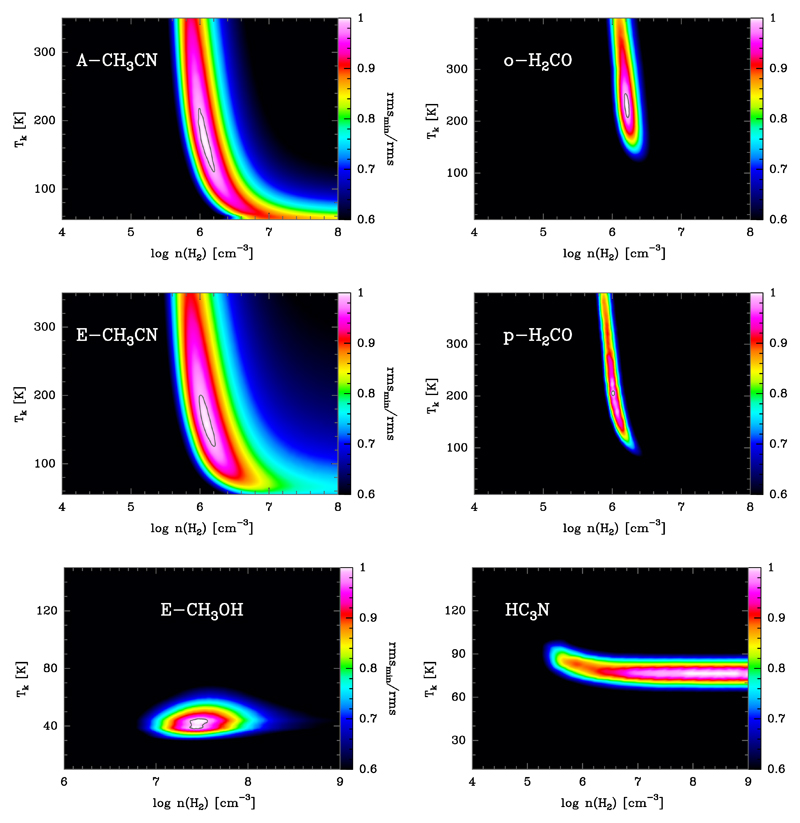
We tested a broad range of column density (N), H2 density (n(H2) = 103 − 109 cm−3), and gas temperature (Tk = 10 − 1000 K) values. We adopted Δυ = 2 km s−1 line widths. In order to constrain the physical conditions that reproduce the observed line intensities and line profiles towards the observed position, we compared the observed H2CO, CH3OH, CH3CN, and HC3N lines to synthetic lines obtained from the grid of LVG models. The best fit model (assuming uniform beam filling emission) was obtained by finding the minimum root mean square (rms); we refer to Cuadrado et al. (2015) for a detailed explanation. The range of column densities that we used as an input in the models is around the value derived from the rotational diagrams analysis (see Sect. 5.4). In fact, the best fit models have column densities within a factor of 2 (see Table 3) of the inferred value from the rotational diagram analysis (we note that in these models the observed lines are optically thin). Figure 15 represents the rmsmin/rms ratio as a function of Tk and n(H2) for a grid of excitation models trying to fit the observed lines towards the PDR position.
Table 3.
Best fit LVG model parameters and comparison with the column density obtained with the rotational diagram (RD) analysis.
| LVG calculations |
RD analysis* |
|||
|---|---|---|---|---|
| Tk [K] | n(H2) [cm−3] | N(X)LVG [cm−2] | N(X)RD [cm−2] | |
| o-H2CO | ~200 | ~106 | 4.0 × 1013 | (4.4 ± 0.6) × 1013 |
| p-H2CO | ~200 | ~106 | 1.8 × 1013 | (1.6 ± 0.1) × 1013 |
| HC3N | ~80 | ~106 | 4.0 × 1011 | (4.2 ± 0.3) × 1011 |
| A-CH3CN | ~150 | ~106 | 5.0 × 1011 | (5.8 ± 0.8) × 1011 |
| E-CH3CN | ~150 | ~106 | 6.0 × 1011 | (5.7 ± 0.7) × 1011 |
| E-CH3OH | ~40 | ~3×107 | 1.9 × 1013 | (1.9 ± 0.2) × 1013 |
Notes.
Rotational diagram analysis assuming uniform beam filling (see Sect. 5.4).
Fig. 15.
Grid of LVG excitation and radiative transfer models for CH3CN, H2CO, CH3OH, and HC3N lines towards the PDR position assuming uniform beam filling and using column densities shown in Table 3. The best fits are those with the higher rmsmin / rms value.
From the excitation analysis it seems that not all COMs arise from the same PDR layers (i.e. same cloud depth). The set of beam-averaged physical conditions that best fit the CH3CN and H2CO lines lie within Tk = 150 − 250 K and n(H2) = (1 − 3) × 106 cm−3, similar (although slightly denser gas) to that obtained for C2H in Cuadrado et al. (2015). Figures 3 and 11 show the best fit models overlaid over the observed lines. The HC3N detected lines (see Fig. 9) fit within Tk = 70 − 90 K and n(H2) > 1 × 106 cm−3. The physical conditions that fit the CH3OH detected lines (see Fig. 12) are colder and denser (Tk = 40 − 50 K and n(H2) ≃ 5 × 107 cm−3). The best-fit parameters (n(H2), Tk, and N) of the LVG models fitting the lines in Fig. 3, 9, 11, and 12 are shown in Table 3. We note, however, that the best fit density value depends on the assumed emission beam filling factor5 for each molecule. Lower densities (by a factor of up to ~10) would still be consistent with observations if one assumes a semi-extended emission source (i.e. more compact than the beam-size at each observed frequency; see Sect. 5.4).
Higher-angular-resolution maps are needed to spatially resolve the structures producing the molecular emission (Goicoechea et al. 2016). Either way, CH3OH and HC3N seem to arise from a different, cooler component where the FUV flux has been attenuated. This scenario agrees with the different H2CO and CH3OH spatial distributions observed by Leurini et al. (2010) with the IRAM 30 m telescope and the PdB interferometer. In particular, Leurini et al. found that both species show different spatial distributions, with CH3OH only tracing the denser and cooler clumps seen deeper inside the Bar, whereas H2CO also traces the warmer extended (interclump) gas directly exposed to the strong FUV-field. Consistent with previous works (Goicoechea et al. 2011, 2016; Nagy et al. 2013), we derive very high thermal gas pressures at the PDR edge (Pth/k = nH TK ≳ 108 K cm−3).
5.3. Subthermal excitation effects on the rotational diagrams of symmetric- and asymmetric-top molecules
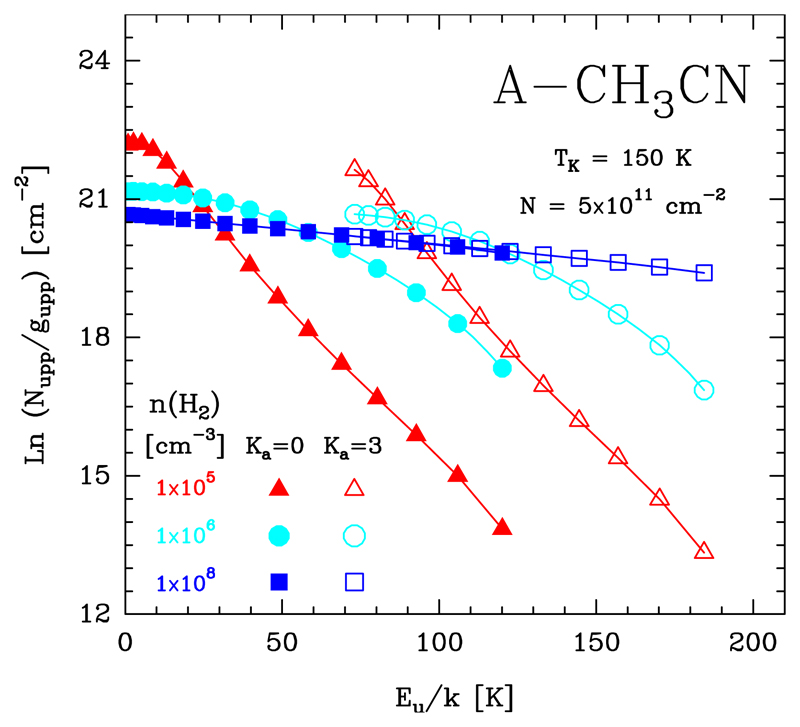
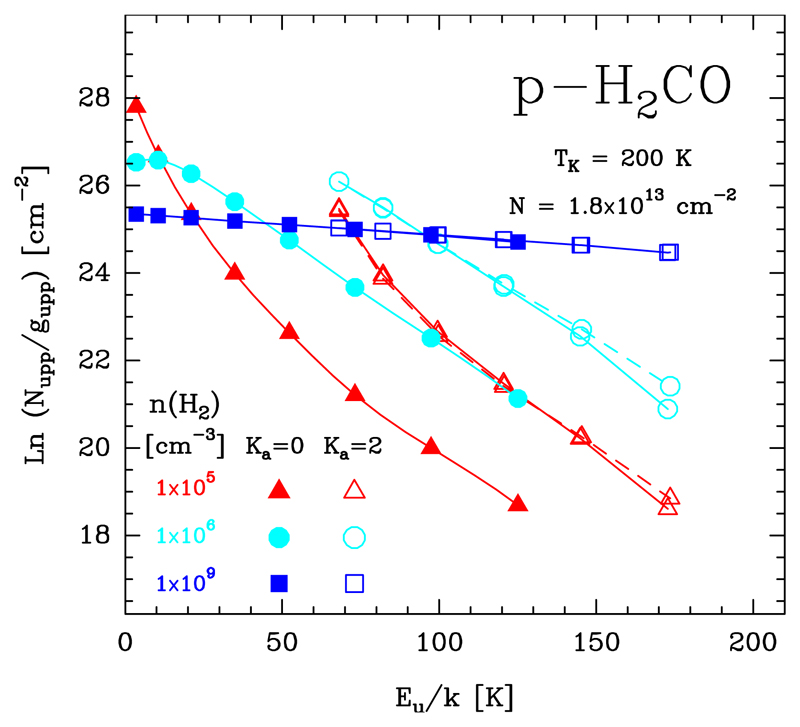
In this Section we show how subthermal excitation effects modify the resulting rotational population diagram of symmetric- and asymmetric-top molecules such as CH3CN and H2CO, respectively. In particular we run a grid of LVG models for varying gas densities, but keeping the same gas temperature and column density. Figures 16 and 17 show model results in the form of population diagrams for A-CH3CN transitions in the Ka = 0 and 3 rotational ladders (Tk = 150 K and Ntot = 5 × 1011 cm−2), and for p-H2CO in the Ka = 0 and 2 rotational ladders (Tk = 200 K and Ntot = 1.8 × 1013 cm−2). Only at very high gas densities, higher than the critical density for collisional excitation (n » ncr), do inelastic collisions drive the level populations close to LTE (Trot ≃ Tk). In a rotational diagram, the Ka ladders merge in a single straight line with a slope equal to −1/Tk. Owing to the high gas densities, this characteristic straight diagram is typically seen towards hot cores (e.g. Bisschop et al. 2007). As the gas density decreases, the excitation becomes subthermal (Trot < Tk), and the rotational population diagram starts to show separate rotational ladders for each set of transitions with the same Ka quantum number. For very polar molecules such as CH3CN and H2CO (thus high ncr), subthermal excitation happens at relatively high densities. Therefore, accurate column densities and rotational temperatures from rotational diagrams can only be obtained if the individual column densities for each rotational ladder are computed independently. This likely explains the discrepancy with the high Trot obtained by Nagy et al. (2017) in the rotational diagram of their submm o-H2CO lines observed with Herschel/HIFI towards the Bar.
Fig. 16.
Rotational population diagrams for A-CH3CN computed with a non-LTE excitation code. All models adopt the same gas temperature (Tk = 150 K) and column density (Ntot = 5 × 1011 cm−2), but three different n(H2) values. For simplicity, only rotational transitions in the Ka = 0 (filled symbols) and 3 (empty symbols) ladders are shown.
Fig. 17.
Rotational population diagrams for p-H2CO computed with a non-LTE excitation code. All models adopt the same gas temperature (Tk = 200 K) and column density (Ntot = 1.8 × 1013 cm−2), but three different n(H2) values. For simplicity, only rotational transitions in the Ka = 0 (filled symbols) and 2 (empty symbols) ladders are shown. We note that at densities <108 cm−3, the set of transitions with the Ka = 2 number are also split into two components: (i) transitions with Kc = J − 1 (empty symbols connected with solid lines) and (ii) transitions with Kc = J − 2 (empty symbols connected with dashed lines).
5.4. Rotational diagrams and column-density determination
We determined the beam-averaged column density (N) and the rotational temperature (Trot) for each molecule found by building rotational diagrams (e.g. Goldsmith & Langer 1999; Cuadrado et al. 2015). The large number of detected lines allows us to accurately determine rotational temperatures and column densities. Optically thin emission is assumed (consistent with our best LVG models of H2CO, HC3N, CH3CN, and CH3OH). We note that owing to their specific spectroscopy and prevailing excitation conditions at the edge of the Bar (subthermal excitation), a correct determination of the rotational temperatures and column densities for species such as H2CO, H2CS, H2CCO, CH3OH, and CH3CN requires building specific diagrams for each rotational ladder (see Sect. 5.3). For the molecules with known collisional rates, we determine NLVG ≈ NRD (within a factor of 2). This comparison shows that the NRD computed for the other molecules are accurate.
The rotational diagrams were built considering two limiting cases: (i) That the detected emission is extended (i.e. uniform beam filling), with ηbf = 1; and (ii) that the emission is semi-extended, assuming that θs = 9″ (the typical beam of the IRAM 30 m telescope at ~1 mm)6. We only considered lines not blended with other transitions and detected above a 3σ level. The resulting rotational diagrams are shown in Fig. 18. Rotational temperatures and column densities obtained by linear least squares fits are listed in Table 4. The uncertainties shown in Table 4 indicate the uncertainty obtained from the least squares fit to the rotational diagram. The uncertainty obtained in the determination of the line parameters with the Gaussian fitting programme are included in the uncertainty bars at each point of the rotational diagram.
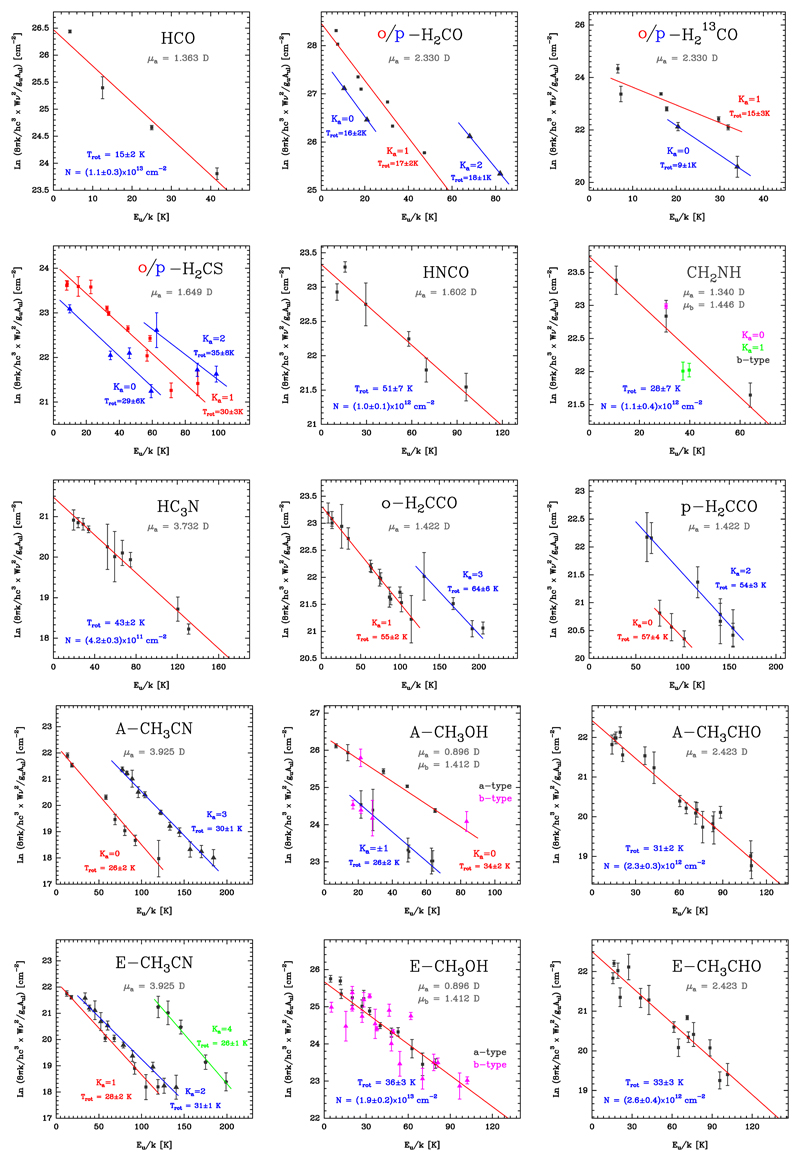
Fig. 18.
Rotational diagrams of the detected complex molecules in the Orion Bar PDR (assuming uniform beam filling). Fitted values of the rotational temperature, Trot, column density, N, and their respective uncertainties are also indicated for each molecule.
Table 4.
Rotational temperatures (Trot), column densities (N), and abundances with respect to hydrogen nuclei inferred in the Orion Bar PDR.
| Uniform beam filling |
Semi-extended source |
|||||
|---|---|---|---|---|---|---|
| Trot [K] | N(X) [cm−2] | Trot [K] | N(X) [cm−2] | Abundance * | Notes | |
| HCO | 15 ± 2 | (1.1 ± 0.3) × 1013 | 9 ± 2 | (5.3 ± 3.5) × 1013 | (1.7 − 8.4) × 10−10 | a |
| o-H2CO Ka = 1 | 17 ± 2 | (3.7 ± 0.6) × 1013 | 12 ± 1 | (1.1 ± 0.3) × 1014 | a | |
| o-H2CO Ka = 3 | 17 | (6.9 ± 1.0) × 1012 | 12 | (1.1 ± 0.2) × 1013 | b,c | |
| p-H2CO Ka = 0 | 16 ± 2 | (9.8 ± 1.4) × 1012 | 9 ± 1 | (3.9 ± 0.6) × 1013 | a,c | |
| p-H2CO Ka = 2 | 18 ± 1 | (6.2 ± 0.2) × 1012 | 13 ± 1 | (1.3 ± 0.1) × 1013 | a,c | |
| [(o+p)-H2CO] | — | (6.0 ± 0.6) × 1013 | — | (1.7 ± 0.3) × 1014 | (0.9 ± 2.7) × 10−9 | d |
| o-CO | 15 ± 3 | (7.3 ± 2.4) × 1011 | 10 ± 2 | (3.0 ± 1.1) × 1012 | a | |
| p-CO | 9 ± 1 | (2.3 ± 0.4) × 1011 | 7 ± 1 | (8.0 ± 1.2) × 1011 | a,c | |
| [(o+p)-CO] | — | (9.6 ± 2.4) × 1011 | — | (3.8 ± 1.1) × 1012 | (1.5 − 6.0) × 10−11 | d |
| o-H2CS Ka = 1 | 30 ± 3 | (3.1 ± 0.5) × 1012 | 19 ± 1 | (1.3 ± 0.2) × 1013 | a | |
| o-H2CS Ka = 3 | 30 | (6.7 ± 1.9) × 1011 | 19 | (2.7 ± 0.4) × 1012 | e,c | |
| p-H2CS Ka = 0 | 29 ± 6 | (5.0 ± 1.4) × 1011 | 16 ± 2 | (2.6 ± 1.0) × 1012 | a | |
| p-H2CS Ka = 2 | 35 ± 8 | (7.8 ± 4.4) × 1011 | 15 ± 3 | (2.6 ± 1.9) × 1012 | a | |
| [(o+p)-H2CS] | — | (5.0 ± 0.7) × 1012 | — | (2.1 ± 0.3) × 1013 | (0.8 − 3.3) × 10−10 | d |
| HNCO | 51 ± 7 | (1.0 ± 0.1) × 1012 | 26 ± 3 | (5.6 ± 1.3) × 1012 | (1.6 ± 8.9) × 10−11 | a |
| CH2NH | 28 ± 7 | (1.1 ± 0.4) × 1012 | 27 ± 7 | (2.4 ± 1.0) × 1012 | (1.7 − 3.8) × 10−11 | a |
| o-H2CCO Ka = 1 | 55 ± 2 | (3.0 ± 0.1) × 1012 | 30 ± 2 | (1.3 ± 0.2) × 1013 | a | |
| o-H2CCO Ka = 3 | 64 ± 6 | (1.3 ± 0.3) × 1012 | 34 ± 1 | (4.2 ± 0.7) × 1012 | a | |
| p-H2CCO Ka = 0 | 57 ± 4 | (4.8 ± 0.6) × 1011 | 42 ± 3 | (1.3 ± 0.2) × 1012 | a | |
| p-H2CCO Ka = 2 | 54 ± 3 | (1.1 ± 0.1) × 1012 | 29 ± 2 | (6.1 ± 1.8) × 1012 | a | |
| [(o+p)-H2CCO] | — | (5.9 ± 0.3) × 1012 | — | (2.5 ± 0.3) × 1013 | (0.9 − 4.0) × 10−10 | d |
| HC3N | 43 ± 2 | (4.2 ± 0.3) × 1011 | 27 ± 1 | (3.2 ± 0.3) × 1012 | (0.7 − 5.1) × 10−11 | a |
| cis-HCOOH | 23 ± 4 | (4.6 ± 0.7) × 1011 | 21 ± 4 | (4.2 ± 0.6) × 1012 | f | |
| trans-HCOOH Ka = 0 | 12 ± 2 | (3.5 ± 0.5) × 1011 | 6 ± 1 | (4.1 ± 0.6) × 1012 | f | |
| trans-HCOOH Ka = 1 | 12 ± 3 | (3.3 ± 1.3) × 1011 | 6 ± 1 | (3.6 ± 2.1) × 1012 | f | |
| trans-HCOOH Ka = 2 | 13 ± 3 | (6.3 ± 2.8) × 1011 | 7 ± 1 | (5.0 ± 2.4) × 1012 | f | |
| [(cis+trans)-HCOOH] | — | (1.8 ± 0.3) × 1012 | — | (1.7 ± 0.3) × 1013 | (0.3 − 2.7) × 10−10 | g |
| A-CH3CN Ka = 0 | 26 ± 2 | (2.8 ± 0.5) × 1011 | 19 ± 1 | (1.9 ± 0.6) × 1012 | a | |
| A-CH3CN Ka = 3 | 30 ± 1 | (3.0 ± 0.6) × 1011 | 21 ± 1 | (1.5 ± 0.6) × 1012 | a | |
| E-CH3CN Ka = 1 | 28 ± 2 | (2.9 ± 0.5) × 1011 | 20 ± 1 | (1.8 ± 0.4) × 1012 | a | |
| E-CH3CN Ka = 2 | 31 ± 1 | (1.7 ± 0.2) × 1011 | 22 ± 1 | (1.0 ± 0.3) × 1012 | a | |
| E-CH3CN Ka = 4 | 26 ± 1 | (1.1 ± 0.4) × 1011 | 18 ± 1 | (6.7 ± 3.2) × 1011 | a | |
| [(A+E)-CH3CN] | — | (1.2 ± 0.1) × 1012 | — | (6.9 ± 1.0) × 1012 | (0.2 − 1.1) × 10−10 | h |
| A-CH3OH Ka = 0 | 34 ± 2 | (8.0 ± 0.6) × 1012 | 18 ± 2 | (5.3 ± 1.1) × 1013 | a | |
| A-CH3OH Ka = ± 1 | 26 ± 2 | (3.5 ± 0.9) × 1012 | 14 ± 1 | (2.8 ± 0.7) × 1013 | a | |
| E-CH3OH | 36 ± 3 | (1.9 ± 0.2) × 1013 | 26 ± 3 | (6.5 ± 1.4) × 1013 | a | |
| [(A+E)-CH3OH] | — | (3.1 ± 0.2) × 1013 | — | (1.5 ± 0.2) − 1014 | (0.5 − 2.4) × 10−9 | h |
| A-CH3CHO | 31 ± 2 | (2.3 ± 0.3) × 1012 | 20 ± 1 | (1.2 ± 0.2) × 1013 | a | |
| E-CH3CHO | 33 ± 3 | (2.6 ± 0.4) × 1012 | 21 ± 1 | (1.2 ± 0.2) × 1013 | a | |
| [(A+E)-CH3CHO] | — | (4.9 ± 0.5) × 1012 | — | (2.4 ± 0.3) × 1013 | (0.8 − 3.8) × 10−10 | h |
Notes.
The abundance of each species with respect to H nuclei is given by with N(H2) ≃ 3 × 1022 cm−2 and N(H) ≃ 3 × 1021 cm−2 (van der Werf et al. 2013).
Trot and N from rotational diagram analysis.
Only two lines detected with the same Eu/k. N calculated assuming the same Trot that o-H2CO Ka = 1.
ΔN estimated assuming a 15% of the calculated N.
Total N calculated as the sum of the ortho and para species.
Only two lines detected with upper level energies of 138.3 K and 149.8 K. They are too similar for an accurate estimation of Trot and N from a rotational diagram analysis. N calculated assuming the same Trot that o-H2CS Ka = 1.
From Cuadrado et al. (2016).
Total N calculated as the sum of the cis and trans species.
Total N calculated as the sum of the A and E species.
For H2CO, H2CS, H2CCO, A-CH3OH, A-CH3CN, and E-CH3CN we have built specific rotational diagrams for different sets of transitions with the same Ka quantum number value. The total column density of the molecule is obtained by adding the column density of each rotational ladder. For the other molecules, it is possible to fit the observed transitions by a single Trot and N, independently of the Ka value. The cis- and trans-HCOOH rotational diagrams are shown in Cuadrado et al. (2016).
For molecules with hyperfine structure, like that of HCO, each rotational transition was described without splits, only with a single rotational number N. For this purpose, the integrated intensity, W, level degeneracy, g, and line strength, S , of each transition was calculated as the sum of all allowed hyperfine components of each N+1 → N transition. The characteristic frequency, ν, was determined using the weighted average with the relative strength of each line as weight, and the Einstein coefficient, A, was calculated using the weighted values in the usual relation.
Comparing the resulting rotational diagrams, we see that: (i) H2CO and CH3OH are, by far, the most abundant organic molecules (by a factor of ≳5), followed by HCO, H2CCO, CH3CHO, H2CS, HCOOH, CH3CN, CH2NH, HNCO, and HC3N (in decreasing order of abundance); (ii) the ortho-to-para (OTP) ratios obtained from the H2CO, H2CS, H2CCO column densities are ~3, as expected at high gas temperature; (iii) we obtain similar rotational temperatures for ortho-para and A-E species; (iv) and the inferred ratio is 63 ± 3, similar to the 12C/13C ≈ 67 isotopic ratio in Orion (Langer & Penzias 1990). Hence, this confirms that even lines from the most abundant organic species studied in this work, are optically thin.
In order to confirm the correct line identification and possible line blendings, we have modelled the spectrum of each molecule using the MADEX radiative transfer model assuming a Boltzmann distribution and a single excitation temperature for the rotational levels population (we recall that for the observed set of lines/ladders of a given molecule, we infer a single temperature component from the rotational diagrams). Figures 2, 5, 6, 7, 8, and 13 show the observational spectra (black histograms) and the modelled spectra (red lines) of each molecule. The obtained fits agree very well with the observations and rotational diagrams.
5.5. Abundances
In order to determine molecular abundances with respect to hydrogen nuclei6 towards the line survey position, we derived the beam-averaged H2 column density from our observations of the optically thin C18O lines (J = 1 → 0, 2 → 1, and 3 → 2 transitions) assuming 16O/18O ≈ 500 (Wilson & Rood 1994) and a [CO]/[H2] abundance of ~10−4 (lower than the canonical value due to photodissociation). The resulting H2 column density, N(H2) ≃ 3 × 1022 cm−2, is in good agreement with previous estimations of N(H2) close to the dissociation front (see e.g. Hogerheijde et al. 1995). A hydrogen atom column density, N(H), of ~3 × 1021 cm−2 has been inferred from HI observations towards the edge of the Orion Bar (van der Werf et al. 2013). Molecular abundances with respect to hydrogen nuclei are listed in Table 4. The abundances of the detected species range from 10−9 to 10−11.
5.6. Undetected complex organic molecules and precursors
The broadband frequency coverage of the survey allowed us to obtain upper limits for other chemically interesting organic molecules that have not been detected towards the edge of the PDR, but could have been expected. In particular, we searched for HDCO, CH3O, CH3NC, CH3CCH, CH3OCH3, HCOOCH3, CH3CH2OH, CH2CHCN, and CH3CH2CN, because they have been detected in other PDRs and star-forming regions (e.g. Horsehead, Orion KL, Barnard 1-b, or IRAS 16293-2422; see Table 6 for references). The CH3O radical is thought to be an important intermediary for the H2CO and CH3OH chemistry but so far, has only been detected in cold and dense gas (Cernicharo et al. 2012; Bacmann & Faure 2016). HDCO has been detected by Parise et al. (2009) towards the lukewarm, dense and more FUV-shielded clump #3 of Lis & Schilke (2003), but it is not detected towards the warmer and high FUV-illuminated line survey position, near the dissociation front.
Table 6.
Abundances relative to H2 in different environments (in units of 10−10).
| Molecule | Orion Bar1* | Horsehead |
Orion KL |
L1157-B17 | IRAS 16293-24228 | B1-b9 | ||
|---|---|---|---|---|---|---|---|---|
| PDR | PDR2* | Core3* | HC4 | CR5 | Outflow | Hot corino | Dense core | |
| HCO | 1.7 | 8.4b | < 0.8b | — | < 0.36,g | — | < 2.0 | 0.2n |
| HNCO | 0.2 | — | — | 780h | — | 605k | 1.7 | 0.7p |
| H2CO | 9.0 | 2.9c | 2.0c | 1200h | 440h | 4000l | 7.0 | 4.6q |
| H2CS | 0.8 | — | — | 150h | 74h | 1100l | — | 0.9q |
| t-HCOOH† | 0.2a | 0.5d | 0.1d | 800i | — | — | < 3.0 | 0.1n |
| CH2NH | 0.2 | — | — | 42h | — | — | < 5.0 | — |
| H2CCO | 0.9 | 1.5d | 0.5d | — | 51h | — | 1.8 | 0.2n |
| HC3N | 0.07 | 0.06e | 0.08e | 81h | — | 100l | 0.3 | 3.1r |
| CH3OH | 5.0 | 1.2f | 2.3f | 22000h | 12000h | 115000l | 44 | 31r,s |
| CH3CN | 0.2 | 2.5e | 0.08e | 300h | 120h | — | 1.5 | 0.4r |
| CH3CHO | 0.8 | 0.7d | 0.2d | 9.5i | — | 250m | 1.0 | 0.1n |
| CH3CCH | < 1.0 | 4.4d | 3.0d | 24i | 1336,i | — | 6.5 | 5.0r |
Notes.
Abundances with respect to total hydrogen nuclei.
To date, the cis conformer of HCOOH has only been detected towards the Orion Bar PDR (Cuadrado et al. 2016), therefore, we only provide the abundances of the trans conformer.
This work. Abundances calculated assuming uniform beam filling.
Ref.:
Low-UV field Horsehead PDR (χ ≈ 60; NH = 3.8 × 1022 cm−2)
condensation shielded from the UV field (dense core, NH = 6.4 × 1022 cm−2) behind the Horsehead PDR edge.
Ref.:
Orion KL Hot Core (HC)
Orion KL Compact Ridge (CR).
Abundances calculated for the Extended Ridge.
Ref.:
B. Tercero, private communication.
Peak B1 in the blue lobe of the L1157.
Ref.:
Low-mass protostar (hot corino) IRAS 16293-2422. The abundances are computed for a N(H2) = 2 × 1023 cm−2. Abundances averaged over a ~20″ beam (van Dishoeck et al. 1995).
Quiescent dark core Barnard 1-b (B1-b), N(H2) = 1.3 × 1023 cm−2 (see e.g. Hirano et al. 1999; Lis et al. 2002).
Ref.:
N. Marcelino, private communication
First, we estimated 3σ line intensities using the relation
| (1) |
where σ is the rms of the observations per resolution channel [K], δυ is the velocity spectral resolution [km s−1], and Δυ is the assumed line widths (~2 km s−1). Second, we used MADEX to create synthetic models that simulate the line emission to constrain their column densities. The column densities and 3σ upper limit abundances assuming Trot = 20 − 30 K are listed in Table 5. We also provide the following abundance ratio upper limits: [HDCO]/[H2CO] < 0.003 and [CH3NC]/[CH3CN] < 0.1. Parise et al. (2009) estimated an abundance ratio of [HDCO]/[H2CO] > 0.006 towards clump #3, which suggests that the deuteration diminishes from the lukewarm and shielded clumps to the warmer and more FUV-irradiated cloud edge.
Table 5.
Upper limits for undetected COMs.
| Molecule | N(X) [cm−2] | Abundance a |
|---|---|---|
| HDCO b | (1.3 - 1.5) × 1011 | (2.1 - 2.4) × 10−12 |
| CH3O | (2.0 - 4.0) × 1012 | (3.2 - 6.3) × 10−11 |
| CH3NC | (5.0 - 8.0) × 1010 | (0.8 - 1.3) × 10−12 |
| CH3CCH | (5.0 - 8.0) × 1012 | (0.8 - 1.3) × 10−10 |
| CH3OCH3 | (7.0 - 9.0) × 1012 | (1.1 - 1.4) × 10−10 |
| HCOOCH3 | (9.0 - 9.5) × 1012 | (1.4 - 1.5) × 10−10 |
| CH3CH2OH | (1.0 - 1.2) × 1012 | (1.6 - 1.9) × 10−11 |
| CH2CHCN | (9.0 - 9.5) × 1012 | (1.4 - 1.5) × 10−10 |
| CH3CH2CN | (7.0 - 5.0) × 1011 | (1.1 - 0.8) × 10−11 |
Notes.
The abundance of each species with respect to H nuclei (see Sect. 5.5).
Detected by Parise et al. (2009) towards clump #3 (Lis & Schilke 2003) of the Orion Bar.
6. Discussion
In order to shed more light on the possible chemical formation routes of COMs in strongly FUV-irradiated gas from an observational perspective, we compare the observed abundances of several COMs and precursors in different environments (see Table 6). In addition to the Orion Bar, we consider the Horsehead PDR (a low-FUV-flux PDR) and a nearby cold core also in the Horsehead nebula, the Orion KL hot core and compact ridge (warm dense gas at roughly the same distance to the Bar), the L1157 outflow (shocked gas), the low-mass protostar IRAS 16293-2422 (hot corino), and the quiescent dark cloud Barnard 1-b (B1-b).
6.1. COMs in different environments
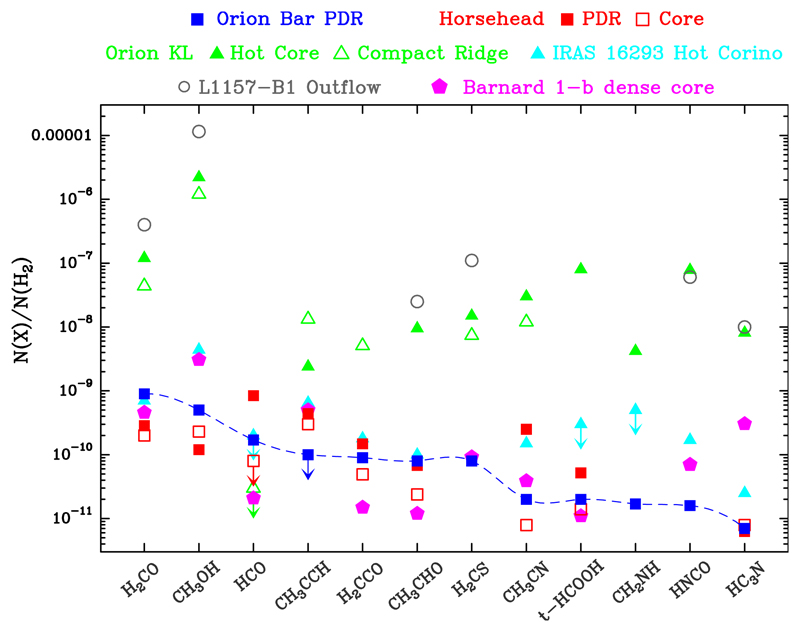
Figure 19 shows a comparison between the abundances of 12 organic molecules detected in the above sources. As expected for widespread interstellar molecules, abundant H2CO and CH3OH are found in all sources (N(X)/N(H2) > 10−10). In diffuse and translucent clouds (n(H2) ≃ 102 − 103 cm−3), only H2CO has been detected so far (Liszt et al. 2006), suggesting that the formation of COMs is more efficient in dense gas.
Fig. 19.
Molecular abundances with respect to H2 in several sources. In the Orion Bar PDR, blue points represent the molecular abundance assuming uniform beam filling.
In general, the abundances of COMs, and CH3OH in particular, are much higher towards Orion KL (hot core and compact ridge) and towards the L1157-B1 outflow. This translates into low [XCOM]/[CH3OH] ≪ 1 abundance ratios in those environments. The Horsehead PDR, however, shows [XCOM]/[CH3OH] > 1 ratios for XCOM = HCO, H2CO, H2CCO, CH3CN, and CH3CCH abundances. The Orion KL hot core and compact ridge show enhanced COM abundances. Owing to the very high dust temperatures (Td > 100 K), ice sublimation and subsequent warm gas-phase chemistry dominates (e.g. Blake et al. 1987). On the other hand, the abundances inferred in the hot corino IRAS 16293-2422 (lower dust temperatures) are more similar to those in the Orion Bar PDR and Horsehead. Interestingly, the L1157 outflow shows the highest abundances of CH3OH, H2CO, H2CS, HC3N, and CH3CHO. This suggests that the combined effects of ice mantle sublimation, grain sputtering, and hot gas-phase chemistry in shocks results in a very efficient COM formation.
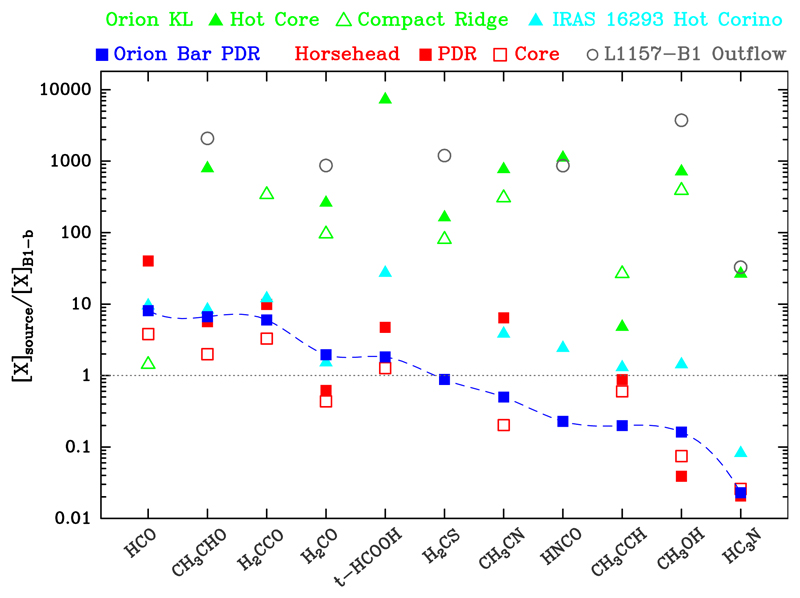
Among the studied species, only the HCO radical and cis-HCOOH (Cuadrado et al. 2016) are more abundant in PDRs. This enhancement is thus a characteristic feature of FUV-illuminated gas. Figure 20 shows normalised abundances with respect to the abundances in B1-b (cold and gas shielded from strong FUV radiation). HCO, CH3CHO, H2CCO, H2CO, and t-HCOOH are a factor of ~2 − 10 more abundant in the PDRs than in the cold core.
Fig. 20.
Comparison of the abundance ratios with respect to B1-b in several sources.
Guzmán et al. (2014) observed two positions of the Horsehead, the moderately warm PDR (Tk ≃ 60 K) and a cold core behind the PDR and shielded from stellar FUV field. Interestingly, they found enhanced COM abundances in the PDR compared to the core. Given the low-FUV field in the Horsehead, dust grains are relatively cold even at the PDR edge (Td ≲ 30 K, Goicoechea et al. 2009). These dust temperatures are significantly below the sublimation temperatures of abundant interstellar ices (Gibb et al. 2000) such as H2O (~100 K), CH3OH (~100 K), and even H2CO (~40 K). Hence, grains must be coated with mantles, even in the Horsehead PDR, and photodesorption can be efficient, either desorbing specific COMs or their gas-phase precursors (Guzmán et al. 2014). Indeed, PDR models adapted to the Horsehead and including grain surface reactions and ice photodesorption were invoked to explain the observed gas-phase CH3OH and H2CO abundances (Guzmán et al. 2013). In addition, the low H2CO ortho-to-para ratio of two in the PDR (Guzmán et al. 2011) might support a cold surface origin for H2CO. However, those Horsehead models required relatively high photodesorption yields (several 10−4 to 10−3 molecules per photon) that are apparently not supported by recent laboratory experiments of methanol photodesorption. In fact, little CH3OH is actually seen to desorb in CH3OH ice irradiation experiments (Bertin et al. 2016; Cruz-Diaz et al. 2016).
Also in the Horsehead, CH3CN is 30 times more abundant in the PDR than in the shielded core (Gratier et al. 2013). CH3CN is also an order of magnitude more abundant in the Horsehead than in the Orion Bar PDR. Given the high binding energy of CH3CN ice (similar to CH3OH, Collings et al. 2004) this result is quite surprising (methanol is more abundant than CH3CN in the Bar). In addition, Gratier et al. (2013) detected CH3NC towards the Horsehead PDR, with an isomeric ratio of [CH3NC]/[CH3CN] = 0.15, higher than our upper limit. These authors suggested that photodesorption triggers the CH3CN abundance in the Horsehead. Therefore, photodesorption seems to enhance the gas-phase CH3CN and CH3NC abundances, but not that of CH3OH. This suggests that photodesorption is a very selective process and thus, should be studied molecule by molecule in representative ISM ice analogs. In addition, gas phase reactions may allow the enhancement of CH3CN with respect to CH3OH.
In the Orion Bar, a much more strongly FUV-irradiated PDR, gas and dust grains are warmer (from Td ≃ 40 to 80 K; Arab et al. 2012). Thus, ice mantle abundances must be small close to the cloud edge. Interestingly, we find higher H2CO and CH3OH abundances in the Bar than in the Horsehead PDR (but not HCO). In addition, H2CO is detected close to the irradiated edge of the Orion Bar and we determine a H2CO ortho-to-para ratio of approximately three (consistent with the high temperature limit). Finally, we determine a HCO/H2CO/CH3OH ≃ 1/5/3 abundance ratio, whereas a ~7/2/1 ratio is found in the Horsehead PDR (Gerin et al. 2009; Guzmán et al. 2013). The ratios typically inferred in cold dense gas are ~1/10/10 (Bacmann & Faure 2016). Although HCO is approximately five times more abundant in the Horsehead, both PDRs show [CH3OH]/[H2CO] < 1 abundance ratios. We note that H2CO is also a product of photodissociation of CH3OH. In warm but FUV-shielded environments (hot cores and shocks), CH3OH is much more abundant than in PDRs and cold cores.
6.2. Limits to steady-state PDR gas-phase chemistry
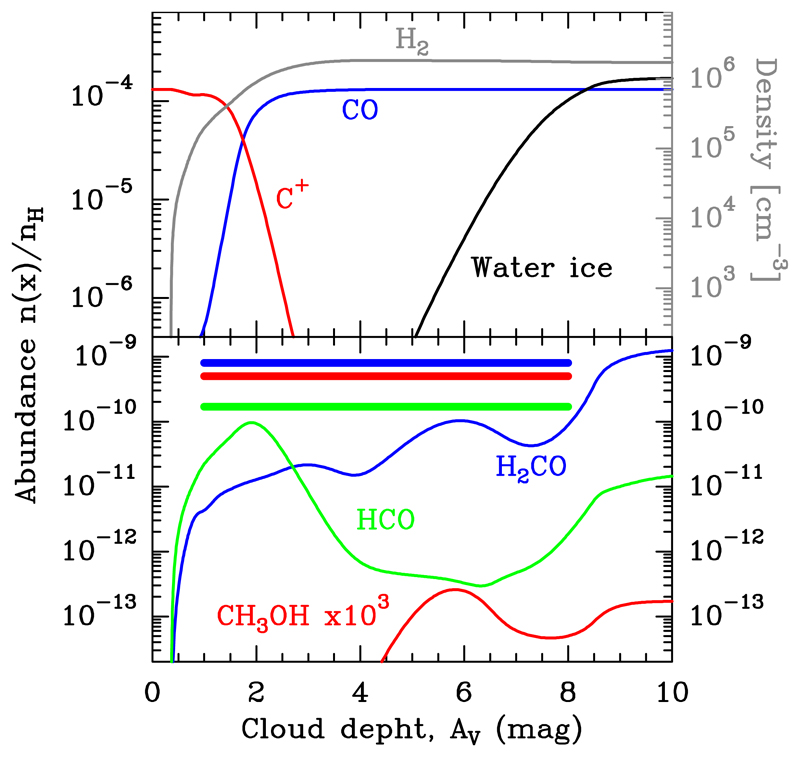
In order to explore the gas-phase production of HCO, H2CO, and CH3OH, we show PDR model results adapted to the illumination conditions in the Bar using the Meudon PDR code (e.g. Le Petit et al. 2006; Goicoechea & Le Bourlot 2007). This is a model of a high gas-pressure isobaric PDR with χ = 2 × 104 and Pth/k = 108 K cm−3. Only H2O, CO, and atoms are allowed to deplete from the gas following their adsorption energies (e.g. Hollenbach et al. 2009). However, we do not include surface chemistry (e.g. water ice only forms through water vapour freeze-out and desorbs thermally or by photodesorption; we refer to Cuadrado et al. 2015 for details). Figure 21 shows the abundance profiles as a function of cloud depth. Although the dust temperature is never low enough to allow CO ice-mantle formation, water ice starts to be very abundant at AV ≳ 5 (a similar behaviour can be expected for CH3OH ice). We note that the typical gas temperature inferred from our observations towards the line survey position (Tk ≈ 150 K) corresponds to AV ≈ 1.5 − 2.0 mag and Td ≈ 60 K in this model. Hence, we expect mostly bare grains at the edge of the PDR, only coated with a few monolayers of very polar ices such as water (with high adsorption energies).
Fig. 21.
Isobaric PDR model for the Orion Bar with Pth/k = 108 K cm−3 and χ = 2 × 104. The thick horizontal bars show the inferred abundances of HCO, H2CO, and CH3OH towards the PDR edge. In this model, these molecules are formed only through gas-phase reactions.
The model shown in Fig. 21 roughly reproduces the HCO abundance enhancement at the PDR edge (driven by reaction O + CH2 → HCO + H), but underpredicts the observed H2CO and CH3OH abundances. In these models, the H2CO formation at AV ≈ 1.5 − 2.0 mag is largely dominated by reaction O + CH3 → H2CO + H. This comparison suggests either the gas-phase model misses important formation routes (or rates) in hot molecular gas, or that (nearly bare) grain surface formation and subsequent desorption is important. Esplugues et al. (2016) have shown PDR models including surface chemistry on grains with different ice content. In their models, chemical/reactive desorption (the surface reaction exothermicity is used to break the adsorbate-surface bond, e.g. Herbst 2015; Minissale et al. 2016), can dominate over photodesorption for some species (e.g. methanol). Nevertheless, the H2CO and CH3OH abundances predicted by Esplugues et al. (2016) in a PDR with high FUV fluxes are still lower than our observed values. Hence, at present, no model seems to reproduce the inferred abundances of molecules such as H2CO and CH3OH towards the Bar.
All these models, however, simulate a static PDR in which steady-state has been reached. A real PDR is likely to be more dynamic (Goicoechea et al. 2016). Time-dependent flows of molecular gas and icy grains might advect from inside the cold molecular cloud to the warm PDR edge. There they can be reprocessed by the combination of high temperatures and the presence of a strong FUV photon flux for some time before photodissociation. Thus, time-dependent desorption and advection of COMs (or gas-phase precursors) from the molecular cloud interior to the PDR may contribute to enhance their abundances.
The non-detection of HDCO at the illuminated edge of the Bar ([HDCO]/[H2CO] < 3 × 10−3), and also in the Horsehead PDR (Guzmán et al. 2011), shows that pure gas-phase H2CO deuteration is not efficient at high gas temperatures. However, HDCO has been detected towards the lukewarm (Tk ≲ 70 K),dense and more FUV-shielded clump #3 by Parise et al. (2009). This agrees with specific chemical models in which pure gas-phase deuteration is efficient in gas below Tk ≲ 70 K (Roueff et al. 2007). Time-dependent gas-phase models show that the [HDCO]/[H2CO] abundance ratio is particularly low at early cloud times (Treviño-Morales et al. 2014). In hot cores where high abundances of COMs have been detected, the gas deuteration is very high as well. Both effects are related to the sublimation of ice mantles formed and deuterated in a previous cold cloud stage. In the context of a dynamic PDR, deuterated species could desorb from grain surfaces and advect to the PDR edge as well. The very low [HDCO]/[H2CO] upper limit abundance towards the edge of the Bar suggests that this mechanism is not efficient for deuterated molecules, and that the gas temperature is too high to enhance the deuterium fractionation by pure gas-phase reactions alone.
6.3. CH3CCH non detection
CH3CCH is a widespread hydrocarbon present in the Horsehead PDR (~4.4 × 10−10) and cold core (~3 × 10−10), the extended ridge of Orion KL (~1 × 10−8), the hot corino IRAS 16293-2422 (~7 × 10−10), the Monoceros R2 ultra-compact H II region (~2 × 10−9; Ginard et al. 2012), and even the nucleus of the starburst galaxy M82 (~1 × 10−8; Fuente et al. 2005; Aladro et al. 2011). Unexpectedly, CH3CCH lines are not bright towards the Bar edge. A few 2σ line features seen in the 1 mm range coincide with the expected frequencies of CH3CCH. However, the detection cannot be confirmed with confidence at the sensitivity level of our line survey. Based on the tentative 2σ features, we estimate an upper limit abundance of [CH3CCH] < (0.8 − 1.3) × 10−10. The unattenuated CH3CCH photodissociation rate is high (several 10−9 s−1 for χ = 1) and CH3CCH reacts with C+ ions relatively fast (e.g. Wakelam et al. 2012). Both the FUV photon flux and the C+ column density are particularly high towards the observed position at the edge of the Bar (Ossenkopf et al. 2013; Goicoechea et al. 2015). This combination likely explains the reduced CH3CCH abundance. Indeed, in our PDR models, CH3CCH only reaches detectable abundances at AV > 8 (Cuadrado et al. 2015).
7. Summary and conclusions
We have investigated the presence and abundance of complex organic molecules in the strongly FUV-irradiated edge of the Orion Bar PDR, near the H2 dissociation front. We used the IRAM 30 m telescope to carry out a millimetre line survey, complemented with 8.5″ resolution maps of the molecular line emission at 0.9 mm. Despite being a very harsh environment, our observations show a relatively rich spectrum, with more than 250 lines arising from COMs and related organic precursors with up to seven atoms: H2CO, CH3OH, HCO, H2CCO, CH3CHO, H2CS, HCOOH, CH3CN, CH2NH, HNCO, and HC3N (in decreasing order of abundance). In particular, we obtained the following results:
-
–
The abundance of the detected species range from 10−9 to 10−11; H2CO being the most abundant. The inferred rotational temperatures range from ~10 to ~55 K, significantly lower than the gas temperature and thus consistent with subthermal excitation. and H2CCO have the lowest and the highest rotational temperatures, respectively. We obtain similar rotational temperatures for the ortho-para and A-E species, and ortho-to-para ratios of approximately three.
-
–
For some molecules, we constrain the beam-averaged physical conditions from LVG excitation models. As shown by previous works (e.g. Leurini et al. 2006, 2010), we conclude that not all COMs arise from the same PDR layer. In particular, while CH3OH only arises from dense gas in the more shielded PDR interior (Tk = 40 − 50 K), CH3CN and H2CO also trace warmer gas layers (Tk = 150 − 250 K), more exposed to a strong FUV-field.
-
–
We determine a HCO/H2CO/CH3OH ≃ 1/5/3 abundance ratio. Such relative abundances are not inferred in environments dominated by ice-mantle thermal desorption or grain sputtering. Overall, pure gas-phase models have difficulty reproducing the observed H2CO and especially CH3OH abundances in PDRs. Taking into account the elevated gas and dust temperatures at the edge of the Bar (Td ≃ 60 K), we suggest the following scenarios for the formation of COMs: (i) hot gas reactions not included in current models; (ii) COMs are produced in the warm grain surfaces of nearly bare grains; or (iii) the PDR dynamics is such that COMs or specific precursors formed in cold icy grains deeper inside the molecular cloud desorb and advect into the PDR.
The presence of COMs in the interstellar medium is more widespread that initially expected. It includes very harsh environments such as shocked gas and now strongly FUV-irradiated gas. COM formation reflects the complicated interplay between gas and grain surface chemistry in different environments. However, the specific formation pathways are not fully clear and may even not be the same in different environments. More laboratory experiments to study different grain surface processes and to investigate the products and rates of different desorption mechanisms (photodesorption, sublimation, chemical desorption, etc.) are needed to distinguish between the different possible scenarios. The census of increasingly complex organic molecule detections will obviously increase in the years to come.
Appendix
Acknowledgements
We thank our referee for pointing us towards several missing references in an earlier version of the manuscript. We thank Nuria Marcelino for sharing the column densities of different COMs inferred towards Barnard 1-b. We are very grateful to the IRAM staff for their help during the observations. This work has been partially funded by MINECO grants (CSD2009-00038 and AYA2012-32032). We thank the ERC for support under grant ERC-2013-Syg-610256-NANOCOSMOS.
Footnotes
We used [H2CO–H2] collisional rates for ortho and para H2CO (Green 1991), [A/E-CH3OH–H2] for A- and E-CH3OH (Rabli & Flower 2010), [A/E-CH3 CN–H2] for A- and E-CH3 CN (Green 1986), and [HC3N–H2] for HC3N (Wernli et al. 2007).
Assuming that the emission source has a 2D Gaussian shape, the beam filling factor (ηbf) is equal to with θB the HPBW of the 30 m telescope (in arcsec) and θS the diameter of the Gaussian source (also in arcsec). For the semi-extended emission we assumed that θS = 9″ (see Sect. 5.4).
The molecular abundance with respect to hydrogen nuclei is given by
References
- Agúndez M, Cernicharo J, Goicoechea JR. A&A. 2008;483:831. [Google Scholar]
- Aladro R, Martín S, Martín-Pintado J, et al. A&A. 2011;535:A84. [Google Scholar]
- Allegrini M, Johns JWC, McKellar ARW. The Journal of Chemical Physics. 1979;70:2829. [Google Scholar]
- Andree-Labsch S, Ossenkopf-Okada V, Röllig M. A&A. 2017;598:A2. [Google Scholar]
- Anttila R, Horneman VM, Koivusaari M, Paso R. Journal of Molecular Spectroscopy. 1993;157:198. [Google Scholar]
- Arab H, Abergel A, Habart E, et al. A&A. 2012;541:A19. [Google Scholar]
- Austin JA, Levy DH, Gottlieb CA, Radford HE. J Chem Phys. 1974;60:207. [Google Scholar]
- Avery LW, Broten NW, MacLeod JM, Oka T, Kroto HW. ApJ. 1976;205:L173. [Google Scholar]
- Bachiller R, Pérez Gutiérrez M. ApJ. 1997;487:L93. [Google Scholar]
- Bacmann A, Faure A. ArXiv. 2016 e-prints. [Google Scholar]
- Bacmann A, Taquet V, Faure A, Kahane C, Ceccarelli C. A&A. 2012;541:L12. [Google Scholar]
- Batrla W, Wilson TL. A&A. 2003;408:231. [Google Scholar]
- Beers Y, Klein GP, Kirchhoff WH, Johnson DR. Journal of Molecular Spectroscopy. 1972;44:553. [Google Scholar]
- Belloche A, Garrod RT, Müller HSP, Menten KM. Science. 2014;345:1584. doi: 10.1126/science.1256678. [DOI] [PubMed] [Google Scholar]
- Belloche A, Garrod RT, Müller HSP, et al. A&A. 2009;499:215. [Google Scholar]
- Bertin M, Romanzin C, Doronin M, et al. ApJ. 2016;817:L12. [Google Scholar]
- Bisschop SE, Jørgensen JK, van Dishoeck EF, de Wachter EBM. A&A. 2007;465:913. [Google Scholar]
- Blake GA, Sastry KVLN, de Lucia FC. J Chem Phys. 1984;80:95. [Google Scholar]
- Blake GA, Sutton EC, Masson CR, Phillips TG. ApJ. 1987;315:621. [Google Scholar]
- Bockelée-Morvan D, Crovisier J, Mumma MJ, Weaver HA. The composition of cometary volatiles. Comets II (University of Arizona Press); 2004. pp. 391–423. [Google Scholar]
- Bocquet R, Demaison J, Poteau L, et al. Journal of Molecular Spectroscopy. 1996;177:154. doi: 10.1006/jmsp.1996.0264. [DOI] [PubMed] [Google Scholar]
- Bottinelli S, Ceccarelli C, Lefloch B, et al. ApJ. 2004;615:354. [Google Scholar]
- Boucher D, Burie J, Demaison J, et al. Journal of Molecular Spectroscopy. 1977;64:290. [Google Scholar]
- Bowater IC, Brown JM, Carrington A. J Chem Phys. 1971;54:4957. [Google Scholar]
- Brown RD, Crofts JG, Godfrey PD, et al. ApJ. 1975;197:L29. [Google Scholar]
- Brown RD, Godfrey PD, McNaughton D, Pierlot AP, Taylor WH. Journal of Molecular Spectroscopy. 1990;140:340. [Google Scholar]
- Brünken S, Müller HSP, Lewen F, Winnewisser G. Physical Chemistry Chemical Physics. 2003;5 (Incorporating Faraday Transactions) [Google Scholar]
- Burton MG, Hollenbach DJ, Tielens AGGM. ApJ. 1990;365:620. [Google Scholar]
- Caselli P, Ceccarelli C. A&A Rev. 2012;20:56. [Google Scholar]
- Cazaux S, Tielens AGGM, Ceccarelli C, et al. ApJ. 2003;593:L51. [Google Scholar]
- Cazzoli G, Puzzarini C. Journal of Molecular Spectroscopy. 2006;240:153. doi: 10.1006/jmsp.2000.8216. [DOI] [PubMed] [Google Scholar]
- Cernicharo J. EAS Publications Series. Vol. 58. EAS Publications Series; 2012. pp. 251–261. [Google Scholar]
- Cernicharo J, Bachiller R, Duvert G. A&A. 1986;160:181. [Google Scholar]
- Cernicharo J, Guélin M, Kahane C. A&AS. 2000;142:181. [Google Scholar]
- Cernicharo J, Heras AM, Tielens AGGM, et al. ApJ. 2001;546:L123. [Google Scholar]
- Cernicharo J, Kisiel Z, Tercero B, et al. A&A. 2016;587:L4. doi: 10.1051/0004-6361/201527531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernicharo J, Marcelino N, Roueff E, et al. ApJ. 2012;759:L43. [Google Scholar]
- Chen W, Bocquet R, Wlodarczak G, Boucher D. International Journal of Infrared and Millimeter Waves. 1991;12:987. [Google Scholar]
- Codella C, Fontani F, Ceccarelli C, et al. MNRAS. 2015;449:L11. [Google Scholar]
- Collings MP, Anderson MA, Chen R, et al. MNRAS. 2004;354:1133. [Google Scholar]
- Creswell RA, Winnewisser G, Gerry MCL. Journal of Molecular Spectroscopy. 1977;65:420. [Google Scholar]
- Crockett NR, Bergin EA, Neill JL, et al. ApJ. 2014;787:112. [Google Scholar]
- Cronin JR, Chang S. In: NATO Advanced Science Institutes (ASI) Series C. Greenberg JM, Mendoza-Goímez CX, Pirronello V, editors. Vol. 416. NATO Advanced Science Institutes (ASI) Series C; 1993. pp. 209–258. [Google Scholar]
- Cruz-Diaz GA, Martín-Doménech R, Muñoz Caro GM, Chen Y-J. ArXiv. 2016 e-prints. [Google Scholar]
- Cuadrado S, Goicoechea JR, Pilleri P, et al. A&A. 2015;575:A82. [Google Scholar]
- Cuadrado S, Goicoechea JR, Roncero O, et al. A&A. 2016;596:L1. doi: 10.1051/0004-6361/201629913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zafra RL. ApJ. 1971;170:165. [Google Scholar]
- DeLeon RL, Muenter JS. J Chem Phys. 1985;82:1702. [Google Scholar]
- Dickinson DF. Astrophys Lett. 1972;12:235. [Google Scholar]
- Dore L, Bizzocchi L, Degli Esposti C. A&A. 2012;544:A19. [Google Scholar]
- Dore L, Bizzocchi L, Degli Esposti C, Gauss J. Journal of Molecular Spectroscopy. 2010;263:44. doi: 10.1006/jmsp.1998.7541. [DOI] [PubMed] [Google Scholar]
- Draine BT, Roberge WG, Dalgarno A. ApJ. 1983;264:485. [Google Scholar]
- Eliet S, Cuisset A, Guinet M, et al. Journal of Molecular Spectroscopy. 2012;279:12. [Google Scholar]
- Esplugues GB, Cazaux S, Meijerink R, Spaans M, Caselli P. A&A. 2016;591:A52. [Google Scholar]
- Fabricant B, Krieger D, Muenter JS. J Chem Phys. 1977;67:1576. [Google Scholar]
- Fuente A, García-Burillo S, Gerin M, et al. ApJ. 2005;619:L155. [Google Scholar]
- Fuente A, García-Burillo S, Usero A, et al. A&A. 2008;492:675. [Google Scholar]
- Gadhi J, Lahrouni A, Legrand J, Demaison J. Journal de chimie physique. 1995;92:1984. [Google Scholar]
- Gerin M, Goicoechea JR, Pety J, Hily-Blant P. A&A. 2009;494:977. [Google Scholar]
- Gibb EL, Whittet DCB, Schutte WA, et al. ApJ. 2000;536:347. [Google Scholar]
- Ginard D, González-García M, Fuente A, et al. A&A. 2012;543:A27. [Google Scholar]
- Goicoechea JR, Compiègne M, Habart E. ApJ. 2009;699:L165. [Google Scholar]
- Goicoechea JR, Cuadrado S, Pety J, et al. A&A. 2017;601:L9. doi: 10.1051/0004-6361/201730716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goicoechea JR, Joblin C, Contursi A, et al. A&A. 2011;530:L16. [Google Scholar]
- Goicoechea JR, Le Bourlot J. A&A. 2007;467:1. [Google Scholar]
- Goicoechea JR, Pety J, Cuadrado S, et al. Nature. 2016;537:207. doi: 10.1038/nature18957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goicoechea JR, Teyssier D, Etxaluze M, et al. ApJ. 2015;812:75. doi: 10.1088/0004-637X/812/1/75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith PF, Langer WD. ApJ. 1999;517:209. [Google Scholar]
- Gottlieb CA. In: Molecules in the Galactic Environment. Gordon MA, Snyder LE, editors. 1973. p. 181. [Google Scholar]
- Gratier P, Pety J, Guzmán V, et al. A&A. 2013;557:A101. [Google Scholar]
- Green S. ApJ. 1986;309:331. [Google Scholar]
- Green S. ApJS. 1991;76:979. [Google Scholar]
- Greve A, Kramer C, Wild W. A&AS. 1998;133:271. [Google Scholar]
- Guzmán V, Pety J, Goicoechea JR, Gerin M, Roueff E. A&A. 2011;534:A49. [Google Scholar]
- Guzmán VV, Goicoechea JR, Pety J, et al. A&A. 2013;560:A73. [Google Scholar]
- Guzmán VV, Pety J, Goicoechea JR, et al. ApJ. 2015;800:L33. [Google Scholar]
- Guzmán VV, Pety J, Gratier P, et al. Faraday Discussions. 2014;168:103. doi: 10.1039/c3fd00114h. [DOI] [PubMed] [Google Scholar]
- Habart E, Dartois E, Abergel A, et al. A&A. 2010;518:L116. [Google Scholar]
- Herbst E. European Physical Journal Web of Conferences. 2015;84 European Physical Journal Web of Conferences, 06002. [Google Scholar]
- Herbst E, van Dishoeck EF. ARA&A. 2009;47:427. [Google Scholar]
- Hirano N, Kamazaki T, Mikami H, Ohashi N, Umemoto T. In: Star Formation 1999. Nakamoto T, editor. 1999. pp. 181–182. [Google Scholar]
- Hocking WH, Gerry MCL, Winnewisser G. Canadian Journal of Physics. 1975;53:1869. [Google Scholar]
- Hogerheijde MR, Jansen DJ, van Dishoeck EF. A&A. 1995;294:792. [Google Scholar]
- Hollenbach D, Kaufman MJ, Bergin EA, Melnick GJ. ApJ. 2009;690:1497. [Google Scholar]
- Ikeda M, Ohishi M, Nummelin A, et al. ApJ. 2001;560:792. [Google Scholar]
- Jansen DJ, Spaans M, Hogerheijde MR, van Dishoeck EF. A&A. 1995;303:541. [Google Scholar]
- Johns JWC, Nemes L, Yamada KMT, et al. Journal of Molecular Spectroscopy. 1992;156:501. [Google Scholar]
- Johnson DR, Powell FX, Kirchhoff WH. Journal of Molecular Spectroscopy. 1971;39:136. [Google Scholar]
- Johnson HR, Strandberg MWP. J Chem Phys. 1952;20:687. [Google Scholar]
- Kirchhoff WH, Johnson DR, Lovas FJ. Journal of Physical and Chemical Reference Data. 1973;2:1. [Google Scholar]
- Kleiner I, Lovas FJ, Godefroid M. Journal of Physical and Chemical Reference Data. 1996;25:1113. [Google Scholar]
- Kukolich S, Nelson A, Yamanashi B. Journal of the American Chemical Society. 1971;93:6769. [Google Scholar]
- Kukolich SG. J Chem Phys. 1982;76:97. [Google Scholar]
- Kukolich SG, Ruben DJ, Wang JHS, Williams JR. J Chem Phys. 1973;58:3155. [Google Scholar]
- Langer WD, Penzias AA. ApJ. 1990;357:477. [Google Scholar]
- Lapinov AV, Golubiatnikov GY, Markov VN, Guarnieri A. Astronomy Letters. 2007;33:121. [Google Scholar]
- Le Petit F, Nehmé C, Le Bourlot J, Roueff E. ApJS. 2006;164:506. [Google Scholar]
- Lees RM, Baker JG. J Chem Phys. 1968;48:5299. [Google Scholar]
- Lees RM, Lovas FJ, Kirchhoff WH, Johnson DR. Journal of Physical and Chemical Reference Data. 1973;2:205. [Google Scholar]
- Leurini S, Parise B, Schilke P, Pety J, Rolffs R. A&A. 2010;511:A82. [Google Scholar]
- Leurini S, Rolffs R, Thorwirth S, et al. A&A. 2006;454:L47. [Google Scholar]
- Lis DC, Roueff E, Gerin M, et al. ApJ. 2002;571:L55. [Google Scholar]
- Lis DC, Schilke P. ApJ. 2003;597:L145. [Google Scholar]
- Liszt HS, Lucas R, Pety J. A&A. 2006;448:253. [Google Scholar]
- Maeda A, Medvedev IR, Winnewisser M, et al. ApJS. 2008;176:543. [Google Scholar]
- Marcelino N, Cernicharo J, Roueff E, Gerin M, Mauersberger R. ApJ. 2005;620:308. [Google Scholar]
- Marcelino N, Cernicharo J, Tercero B, Roueff E. ApJ. 2009;690:L27. [Google Scholar]
- Marconi A, Testi L, Natta A, Walmsley CM. A&A. 1998;330:696. [Google Scholar]
- Mbosei L, Fayt A, Dréan P, Cosléou J. Journal of Molecular Structure. 2000;517:271. [Google Scholar]
- Meier DS, Turner JL. ApJ. 2005;618:259. [Google Scholar]
- Meier DS, Turner JL. ApJ. 2012;755:104. [Google Scholar]
- Mendoza E, Lefloch B, López-Sepulcre A, et al. MNRAS. 2014;445:151. [Google Scholar]
- Menten KM, Reid MJ, Forbrich J, Brunthaler A. A&A. 2007;474:515. [Google Scholar]
- Minissale M, Moudens A, Baouche S, Chaabouni H, Dulieu F. MNRAS. 2016;458:2953. [Google Scholar]
- Müller HSP, Drouin BJ, Pearson JC. A&A. 2009;506:1487. [Google Scholar]
- Müller HSP, Gendriesch R, Margulès L, et al. Physical Chemistry Chemical Physics (Incorporating Faraday Transactions) 2000;2 [Google Scholar]
- Müller HSP, Schlöder F, Stutzki J, Winnewisser G. Journal of Molecular Structure. 2005;742:215. [Google Scholar]
- Müller HSP, Thorwirth S, Roth DA, Winnewisser G. A&A. 2001;370:L49. [Google Scholar]
- Nagy Z, Choi Y, Ossenkopf-Okada V, et al. A&A. 2017;599:A22. [Google Scholar]
- Nagy Z, Van der Tak FFS, Ossenkopf V, et al. A&A. 2013;550:A96. [Google Scholar]
- Öberg KI, Bottinelli S, Jørgensen JK, van Dishoeck EF. ApJ. 2010;716:825. [Google Scholar]
- Ossenkopf V, Röllig M, Neufeld DA, et al. A&A. 2013;550:A57. [Google Scholar]
- Parise B, Leurini S, Schilke P, et al. A&A. 2009;508:737. [Google Scholar]
- Parmar PS, Lacy JH, Achtermann JM. ApJ. 1991;372:L25. [Google Scholar]
- Pavone FS, Zink LR, Prevedelli M, Inguscio M, Fusina L. Journal of Molecular Spectroscopy. 1990;144:45. [Google Scholar]
- Pickett HM, Poynter RL, Cohen EA, et al. J Quant Spec Radiat Transf. 1998;60:883. [Google Scholar]
- Rabli D, Flower DR. MNRAS. 2010;406:95. [Google Scholar]
- Remijan AJ, Hollis JM, Snyder LE, Jewell PR, Lovas FJ. ApJ. 2006;643:L37. [Google Scholar]
- Requena-Torres MA, Martí-Pintado J, Rodríguez-Franco A, et al. A&A. 2006;455:971. [Google Scholar]
- Roueff E, Parise B, Herbst E. A&A. 2007;464:245. [Google Scholar]
- Saito S. ApJ. 1972;178:L95. [Google Scholar]
- Sastry KVLN, Lees RM, Van der Linde J. Journal of Molecular Spectroscopy. 1981;88:228. [Google Scholar]
- Schilke P, Pineau des Forêts G, Walmsley CM, Martí-Pintado J. A&A. 2001;372:291. [Google Scholar]
- Simon R, Stutzki J, Sternberg A, Winnewisser G. A&A. 1997;327:L9. [Google Scholar]
- Snyder LE, Buhl D, Zuckerman B, Palmer P. Physical Review Letters. 1969;22:679. [Google Scholar]
- Stoerzer H, Stutzki J, Sternberg A. A&A. 1995;296:L9. [Google Scholar]
- Tercero B, Cernicharo J, López A, et al. A&A. 2015;582:L1. doi: 10.1051/0004-6361/201526255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tercero B, Cernicharo J, Pardo JR, Goicoechea JR. A&A. 2010;517:A96. [Google Scholar]
- Tercero B, Kleiner I, Cernicharo J, et al. ApJ. 2013;770:L13. [Google Scholar]
- Thorwirth S, Müller HSP, Winnewisser G. Journal of Molecular Spectroscopy. 2000;204:133. doi: 10.1006/jmsp.2000.8209. [DOI] [PubMed] [Google Scholar]
- Tielens AGGM, Hollenbach D. ApJ. 1985a;291:747. [Google Scholar]
- Tielens AGGM, Hollenbach D. ApJ. 1985b;291:722. [Google Scholar]
- Tielens AGGM, Meixner MM, van der Werf PP, et al. Science. 1993;262:86. doi: 10.1126/science.262.5130.86. [DOI] [PubMed] [Google Scholar]
- Treviño-Morales SP, Pilleri P, Fuente A, et al. A&A. 2014;569:A19. [Google Scholar]
- Turner BE. ApJ. 1971;163:L35. [Google Scholar]
- Šimečková M, Urban Š, Fuchs U, et al. Journal of Molecular Spectroscopy. 2004;226:123. [Google Scholar]
- van der Tak FFS, Nagy Z, Ossenkopf V, et al. A&A. 2013;560:A95. [Google Scholar]
- van der Werf PP, Goss WM, O’Dell CR. ApJ. 2013;762:101. [Google Scholar]
- van der Werf PP, Stutzki J, Sternberg A, Krabbe A. A&A. 1996;313:633. [Google Scholar]
- van der Wiel MHD, van der Tak FFS, Ossenkopf V, et al. A&A. 2009;498:161. [Google Scholar]
- van Dishoeck EF, Blake GA, Jansen DJ, Groesbeck TD. ApJ. 1995;447:760. [Google Scholar]
- Wakelam V, Herbst E, Loison J-C, et al. ApJS. 2012;199:21. [Google Scholar]
- Walmsley CM, Natta A, Oliva E, Testi L. A&A. 2000;364:301. [Google Scholar]
- Wernli M, Wiesenfeld L, Faure A, Valiron P. A&A. 2007;464:1147. [Google Scholar]
- Wilson TL, Rood R. ARA&A. 1994;32:191. [Google Scholar]
- Xu L-H, Fisher J, Lees RM, et al. Journal of Molecular Spectroscopy. 2008;251:305. [Google Scholar]
- Yamada KMT, Moravec A, Winnewisser G. Zeitschrift Naturforschung Teil A. 1995;50:1179. [Google Scholar]
- Young Owl RC, Meixner MM, Wolfire M, Tielens AGGM, Tauber J. ApJ. 2000;540:886. [Google Scholar]
- Ziurys LM, McGonagle D. ApJS. 1993;89:155. doi: 10.1086/191842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.