Abstract
Aims
Exercise training (ET) has been variably associated with body composition changes among persons with type 2 diabetes (T2DM). The degree to which these changes are related to hyperglycemia remains unclear. Our objective was to investigate the relationship of baseline fasting glucose (FG) to the magnitude of muscle gains and fat loss after ET in individuals with T2DM.
Methods
Participants were enrolled in the SHAPE-2 trial, a six month supervised aerobic and resistance training intervention (three days/week), at Johns Hopkins. This was a post-hoc single arm intervention study of participants who completed the exercise intervention (n=50). Participants were aged 40-65 years and had T2DM that was not treated with insulin. Body composition was assessed by DEXA.
Results
After 6 months of ET, total fat mass decreased (−2.1 ± 3.1 kg) and total lean body mass (LBM) increased (0.5 ± 2.0 kg) overall, but there was variability among individual participants. There was an increase in % total LBM (1.4 ± 1.9%) and decrease in % total body fat mass (−1.5±2.0%) after ET. Interestingly, each standard deviation (SD) increase in baseline FG (mean=135.5 mg/dl; SD= 39.0 mg/dl) was related to a significant increase in % total LBM (0.54±0.26%, p=0.048) and decrease in % total body fat (−0.57±0.27%, p=0.04) after ET among individual participants.
Conclusions
Our data demonstrate that muscle gains and fat loss after ET are positively related to baseline hyperglycemia. Further studies are needed to characterize differences in metabolic response following ET among persons with diabetes.
Key terms: diabetes, muscle function, lean body mass, exercise training
1. Introduction
The favorable effects of aerobic and resistance training in individuals with type 2 diabetes have been well-described. 1-4 Exercise training yields favorable changes in body composition such as loss of fat and increase in lean body mass with improvements in muscle strength and quality. 5 Increase in lean body mass, in particular, after exercise training may be subsequently related to reduced risk of functional limitations and disability. 6 Current evidence-based consensus guidelines from societies such as the American Diabetes Association and the American College of Sports Medicine 7 thus recommend regular physical exercise for persons with type 2 diabetes. However, not all persons with diabetes observe positive benefits following exercise; in fact, “exercise resistance” has been reported in 15-20% of persons with diabetes for reasons that remain unclear. Exercise resistance is characterized by lack of metabolic improvement after exercise, including in oxidative capacity and mitochondrial content, postulated to be related to genetic and epigenetic differences at the level of the skeletal muscle. 8
We have previously reported that hyperglycemia is related to decreased muscle function in persons with type 2 diabetes over time. 9 Specifically, hyperglycemia, independent of associated comorbidities, is related to the loss of muscle mass and strength, and contributes to functional impairment in older persons with type 2 diabetes. 10,11 However, the reversibility of muscle loss after exercise training in persons with diabetes remains unclear. If the mechanism by which diabetes relates to accelerated muscle loss is mediated through hyperglycemia and/or insulin resistance as these previous studies suggest, 12 then persons with the most severe hyperglycemia at the onset of exercise training might be expected to have the lowest muscle mass to begin with and have the potential for the greatest gains in lean body mass. On the other hand, if muscle mass loss is partially or incompletely irreversible or if the adverse effects of persistent hyperglycemia attenuates observed gains in muscle mass after exercise training, these persons may be less likely to achieve increases in lean body mass. The extent to which the magnitude of hyperglycemia in persons with type 2 diabetes is related to favorable changes in body composition after exercise training has not been previously described, yet may have implications for the prescription of exercise programs.
Using data from the Sugar, Hypertension and Physical Exercise (SHAPE) 2 trial, a six month randomized controlled exercise trial in persons with type 2 diabetes, we examined the following hypotheses: 1) exercise training will lead to variable changes in body composition for individual persons with type 2 diabetes but, overall, result in decreased fat mass and increased lean body mass among study participants; and 2) the degree of hyperglycemia at the beginning of the study will be related to the magnitude of fat loss and muscle mass gain observed for individual participants after the exercise intervention.
2. Research Design and Methods
2.1 Setting and Participants
SHAPE-2 was designed to determine the efficacy of supervised exercise on blood pressure, the primary outcome. Secondary outcomes included glycemic control, body composition, and aerobic and strength fitness.
Participants were recruited from the greater Baltimore area from 2004 to 2010, primarily through newspaper advertisements. Eligibility criteria included age 40 to 65 years and type 2 diabetes. Type 2 diabetes was defined by the 2003 ADA diagnostic criteria and verified with the subjects’ health care providers. Patients could be treated with diet or oral medications, but insulin use was an exclusion criterion. Other exclusion criteria included fasting blood glucose >400 mg/dl, HbA1c >11% (97 mmol/mol), history of myocardial infarction, prior coronary artery bypass grafting or coronary angioplasty, chronic heart failure, self-reported substance abuse, co-morbid conditions that would limit the ability to exercise, high degree heart block, smoking, and regular participation in moderate to vigorous exercise for >90 minutes per week. Since the primary outcome of the initial SHAPE-2 trial was blood pressure control, participants needed to have untreated suboptimal blood pressure (systolic blood pressure [SBP] 120-159 mmHg or diastolic blood pressure [DBP] 85-99 mmHg) or treated hypertension with SBP ≤159 mmHg and DBP ≤99 mmHg with no lower limit for BP if being treated. A screening maximal graded stress test was also performed. Subjects with >1 mm ST-T wave depression, high-grade ventricular arrhythmias, or cardiac symptoms were excluded and referred to their health care provider for follow-up. 13 The Institutional Review Board at the Johns Hopkins School of Medicine approved the study and all participants gave informed consent.
2.2 Randomization and Intervention
Eligible participants were randomized to either a supervised exercise intervention or a usual care control group in the SHAPE2 trial. Participant eligibility and enrollment in the study has previously been described. 13 Participants in the intervention group were scheduled to attend three supervised exercise sessions/week which included resistance and aerobic components based on guidelines for diabetes and hypertension. 7 A total of 78 sessions were prescribed (3 days per week for 6 months). If participants did not attend at least 62 sessions (80% compliance) over the 6 months, an additional month was allowed to reach this goal. Resistance training consisted of 2 sets of seven exercises (latissimus dorsi pull down, leg extension, leg curl, bench press, leg press, shoulder press, and seated mid-rowing) at 12 to 15 repetitions per exercise and at 50% of 1-repetition maximum on a multi-station machine (Hoist 6000; Hoist Fitness, San Diego, CA) per American College of Sports Medicine guidelines. When the participant could complete 15 repetitions of an exercise with little difficulty, the weight was increased. Aerobic exercise lasted 45 minutes with a 10-15 minute warm up and cool down. Participants were allowed to use a treadmill, stationary cycle, or stairstepper. The target heart rate was 60-90% of maximum and was assessed using heart monitors (Polar Inc., Lake Success, NY). As fitness improved, the workload was increased to maintain target heart rate levels.
Of the 70 participants randomized to the exercise intervention, 17 participants dropped out and 2 other participants were withdrawn due to new onset illness not related to the study (cancer, cardiovascular disease with stent placement). One additional participant did not have complete follow-up testing. Thus, a total of 50 exercisers completed 6 month testing and were analyzed in the present study. No additional follow-up data are available for those who dropped out or were withdrawn. The mean length of time for those who dropped out was 2.74±2.78 months. Exercisers who completed the 6 months of training attended a mean 72 of their prescribed 78 sessions (92 %). 13 Factors predictive of dropout have previously been described. 14 The following study is a post-hoc single arm analysis of participants who completed the exercise intervention in the SHAPE-2 trial.
All potential participants during screening were given “Exercise: A Guide from the National Institute on Aging” (NIH, Bethesda MD). They were also given American Heart Association dietary guidelines from and asked to maintain their usual caloric intake throughout the trial. No additional dietary advice was provided. Participants returned for monthly BP monitoring visits.
2.3 Body Composition and Muscle Strength
Absolute fat mass, absolute lean mass, percentage lean body mass and percentage body fat were assessed using dual energy x-ray absorptiometry (GE Lunar Prodigy, Software Version 13 GE Medical Systems, Milwaukee, WI). Muscle strength was assessed by one repetition maximum on each of four upper body and three lower body exercises on the Hoist multistation machine. One repetition maximum is the highest weight lifted following methods described previously. 15 Total strength was the sum of the weights of these seven exercises. Lower extremity muscle quality was defined as knee extensor strength/leg lean mass as in prior studies. 9
The Johns Hopkins General Clinical Research Center Core Laboratory analyzed fasting blood samples. Glucose was measured using an assay that had a sensitivity of 4 μM and that utilized the glucose oxidase-peroxide reaction for the determination of glucose concentrations (Eton Biosciences, Inc.). The interassay coefficient of variation was <3%. Standard methods for lipids (Alere Cholestech LDX system, Alere Inc.), insulin (radioimmunoassay, Linco Research, Inc.), and HbA1c (A1CNow Blood Analyzer, Bayer Healthcare, LLC) were used. Inflammatory markers were assessed by enzyme-linked immunosorbent assay including IL-6 (R&D Systems, Inc.) and TNF-α (R&D Systems, Inc.). The quantitative insulin sensitivity check index (QUICKI) was calculated to estimate insulin sensitivity using the formula QUICKI = 1/[log fasting insulin × log fasting glucose].
2.4 Statistical Analysis
Participant characteristics are presented as means (standard deviation) or as percentages and are compared across treatment groups using independent t-tests for continuous variables and chi-squared tests for categorical variables. Only trial completers were included in the final analysis.
Linear regression models were created to explore the relationship of baseline fasting glucose levels (per standard deviation increase in fasting glucose) with changes in lean body mass (total, arm, and leg), total fat mass, percentage lean body mass (total, arm, and leg), percentage total fat mass, muscle strength (total, upper, and lower), and lower extremity muscle quality, between 0 and 6 months. The following models sequentially included covariates of interest: model 1 was adjusted for age, race, sex, baseline muscle measure, baseline weight and height; model 2 was adjusted for variables included in model 2 as well as log interleukin-6 (IL-6) and log tumor necrosis factor-α (TNF-α). We chose to adjust for height and weight separately in our models rather than BMI, as increases in BMI may occur due to an increase in total lean mass, similar to prior studies suggesting both height and weight were independently related to muscle outcomes.9
In secondary analyses, the relationship of other glucose biomarkers such as hemoglobin A1c (HbA1c) and QUICKI with muscle outcomes was explored. Sensitivity analyses were performed to exclude any potential outliers.
A two-tailed P value < 0.05 was used to indicate statistical significance. Analyses were completed using Stata version 11.1 (StataCorp, College Station, TX).
3. Results
The mean age of participants was 58 years (95% CI 52.9 to 63.1 years; p=0.03) and 66% were male A total of 60% of the cohort identified as non-Hispanic white, 36%, non-Hispanic black, and 4% Hispanic (Table 1).
Table 1.
Demographics, clinical characteristics, and laboratory measures of participants at baseline and 6 month after exercise training (n=50).
| Demographics | |||
|---|---|---|---|
| Age(years) | 58±5.1 | -- | - |
|
| |||
| Race/Ethnicity (%) | |||
|
| |||
| Non-Hispanic White | 60 | -- | -- |
|
| |||
| Non-Hispanic Black | 36 | -- | -- |
|
| |||
| Hispanic | 4 | -- | -- |
|
| |||
| Other | 0 | -- | -- |
|
| |||
| Male (%) | 66 | -- | -- |
| Clinical Characteristics | −2.1±4.7 | ||
|
| |||
| Weight (kg) | 97.5±17.4 | 95.3±17.3 | −0.1±0.4 |
|
| |||
| Height (cm) | 173.3±9.0 | 173.2±9.0 | |
| BMI (kg/m2) | 32.6±5.4 | 31.8±5.0 | −0.8±1.7 |
| Systolic BP (mm Hg) | 127±13.1 | 125±14.5 | −1±10.2 |
| Diastolic BP (mm Hg) | 71±8.0 | 69±9.1 | −2±5.8 |
| Laboratory Measures | 135.5±39.0 | ||
|
| |||
| Fasting glucose (mg/dL) | 21.5±19.9 | 134.4±34.5 | −2.1±25.7 |
|
| |||
| Fasting insulin (mcIU/mL) | 6.6±1.5 (49±5) | 19.6±12.2 | −2.3±22.0 |
|
| |||
| HbA1c (%) (mmol/mol) | 0.3±0.03 | 6.4±1.2 (46±5) | −0.2±1.2 (3±5) |
|
| |||
| QUICKI | 0.3±0.2 | 0.3±0.02 | 0.002±0.02 |
|
| |||
| Log IL-6 (pg/mL) | 0.2±0.2 | 0.2±0.2 | −0.01±0.3 |
|
| |||
| Log TNF-alpha (pg/mL) | 0.2±0.2 | −0.01±0.2 | |
At the end of the six months of exercise training, participants lost on average 2.1 kg (95% CI −6.8 to 2.7 kg) of total body weight (Table 1). Changes in systolic blood pressure (SBP), diastolic blood pressure (DBP), and lipids are outlined in Table 1. Baseline fasting glucose and HbA1c were 135.5±39.0 mg/dl and 6.6±1.5% (49±5 mmol/mol) respectively without a significant change at the end of the 6 month trial (Table 1). There were no significant changes in log IL-6, or log TNF-α levels at the end of 6 months (Table 1). Differences in metabolic parameters as compared to control subjects in the primary SHAPE-2 trial were previously described. 13
The changes in lean body and fat mass after six months of exercise training are outlined in Table 2. Overall, a wide range of changes in muscle mass, muscle strength and muscle quality were observed in participants. The exercise group had a non-significant gain in absolute (0.5±2.0 kg) and percentage total lean mass (1.4±1.9 kg). Non-significant gains were also seen in leg (0.2±1.2 kg), arm (0.1±0.7 kg) and trunk lean mass (0.2±1.8 kg). The exercise group had increased leg strength (73.5 ± 80.1 kg), arm strength (60.1 ± 44.7 kg), total strength (123.1±133.6 kg), and muscle quality (0.8±1.6) after 6 months. Differences in lean mass and muscle strength in the exercise intervention versus the control group have been described previously. 13
Table 2.
Body composition measures at baseline and the change after 6 months of exercise training
| Total lean mass (kg) | 57.4±11.1 | 57.9±11.3 | 0.5±2.0 |
| Leg lean mass (kg) | 19.5±4.1 | 19.7±4.2 | 0.2±1.2 |
| Arm lean mass (kg) | 6.5±1.8 | 6.6±1.9 | 0.1±0.7 |
| Total trunk mass (kg) | 31.7±8.4 | 31.3±7.5 | 0.2±1.8 |
| Total body fat (kg) | 36.1±11.3 | 34.1±10.4 | −2.1±3.1 |
| Total lean mass (%) | 59.6±7.6 | 61±7.1 | 1.4±1.9 |
| Total fat mass (%) | 36.9±7.9 | 35.4±7.3 | −1.5±2.0 |
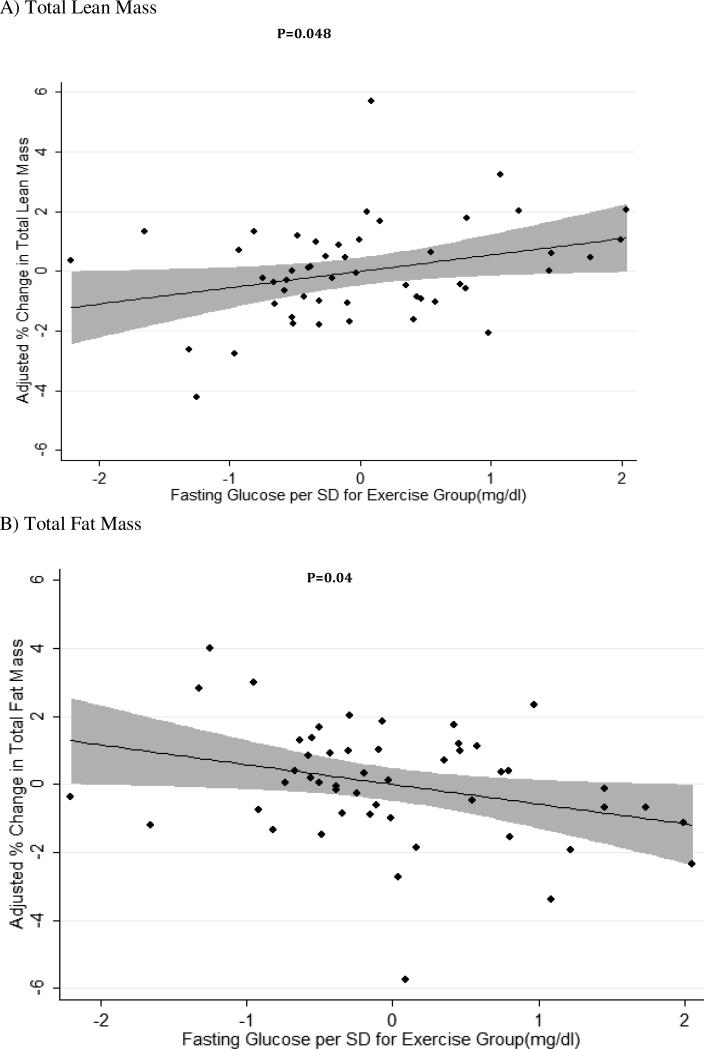
Each standard deviation (SD) higher level of baseline fast- ing glucose (mean = 135.5 mg/dl; SD = 39.0 mg/dl) was related to a significant increase in percentage of total lean body mass gain after 6 months of exercise training (0.54 ± 0.26% per SD of glucose (mg/dl), p = 0.048), controlling for age, race, sex, baseline muscle mass, weight, and height (Fig. 1A). Further, each SD higher level of fasting glucose at baseline was also associated with significantly greater loss in the percentage of total body fat among exercisers after six months (−0.57±0.27%, p=0.04) (Figure 1B). After further accounting for log IL-6 and log TNF-α levels in regression models (model 2), the significance of the relationship between fasting glucose and percentage gain in lean mass (0.55±0.28%, p=0.05) and percentage decline in fat mass (−0.58±0.29%, p=0.05) remained significant.
Figure 1. Adjusted % change in total lean body and fat mass, per increase in standard deviation of baseline fasting glucose, after exercise training.
Figure 1A: Adjusted change in % total lean mass body mass after exercise per change in standard deviation of baseline fasting blood glucose from the mean (mean =135.48 mg/dl; SD=39.0 mg/dl) (beta coefficient = 0.54 ± 0.26 % per SD of glucose (mg/dL), p = 0.048).
Figure 1B: Adjusted change in % total body fat after exercise per change in standard deviation of baseline fasting blood glucose from the mean (mean =135.48 mg/dl; SD=39.0 mg/dl) (beta coefficient = -0.57 ± 0.27 % per SD of glucose (mg/dL) , p = 0.04).
After excluding an outlier in sensitivity analysis for lean body mass who had an adjusted change in lean body mass of 5.7% after six months of exercise training, the results were unchanged (−0.51±0.23%, p=0.03). After excluding the same individual who was also an outlier in the total fat analysis with an adjusted change in total fat of −5.7%, the results also remained significant (−0.54±0.24, p=0.03).
No significant relationship was observed between baseline fasting glucose and lower muscle strength (0.02±12.19 kg, p= 0.5) or muscle quality (0.20±0.24 kg/kg, p= 0.4) in adjusted models. Similarly, the relationship of baseline fasting glucose with other muscle outcomes demonstrated no significant relationship in secondary analysis. Secondary analyses that examined the relationship between HbA1c and QUICKI with muscle outcomes were also not significant.
4. Conclusions
Our findings demonstrate that after six months of supervised exercise training, persons with type 2 diabetes generally had a trend towards a reduction in percentage fat mass, along with a corresponding trend towards an increase in percentage total lean body mass, but this was not observed in all participants. We further demonstrate that the relative magnitude of lean body mass gains and fat mass losses in individuals with type 2 diabetes after exercise training is significantly and positively related to baseline glycemic control, a novel finding. These results extend previously published studies demonstrating the favorable effects of exercise training on body composition in individuals with type 2 diabetes and suggest that the presence of elevated baseline fasting glucose does not appear to attenuate the exercise training response but, instead, may be related to more favorable body composition changes. 16-22
The ability of persons with diabetes to have favorable changes in body composition after exercise training versus those without diabetes has been previously examined in other observational and longitudinal studies with conflicting results. 16,18-20,22-26 Geirsdottir et al. (2012) compared healthy Icelandic men to those with prediabetes and type 2 diabetes, and demonstrated that all three groups were able to achieve significant improvements in muscle strength and physical function even without changes in glycemic control after a 12 week program of resistance training. Their results suggest that diabetes status does not impair the capacity for muscle gains. 16 Our study further extends these findings by demonstrating that progressive elevations in fasting glucose, specifically, does not negatively impact muscle mass gains after exercise. In contrast, Ibañez et al. (2007) reported that a 16-week progressive resistance training program yielded lesser gains in maximal arm and leg muscle strength in persons with diabetes as compared to non-diabetic controls. These results suggested that individuals with diabetes can achieve positive body composition changes, but may have a lesser capacity to do so as compared to non-diabetic persons. 23 Similarly, Ekman et al. (2015) demonstrated that insulin-sensitive individuals with a family history of diabetes did not have changes in VO2, weight, or height related to exercise volume as was observed in those without a history of diabetes. Unlike the group without a family history of diabetes, no changes in expression of genes involved in metabolism, oxidative phosphorylation and cellular respiration after exercise was seen. Overall, these results suggest that genetics may predispose to a lesser ability for individuals with diabetes to achieve favorable body composition changes.27 In contrast, our study, which examined the effects of a combination aerobic and resistance exercise intervention, detected greater gains in those with higher glucose levels at baseline. Interestingly, significant relationships were observed between fasting blood glucose, but not baseline HbA1c, with body composition measures in our study. This may be related to increased short-term variability in fasting glucose as compared to HbA1c, which is a relatively longer-term marker of glycemic status and may not change as rapidly during a self-limited exercise intervention. 28
There are several potential mechanisms that may underlie the favorable changes in body composition after exercise training in individuals with diabetes. Hyperglycemia and the presence of insulin resistance may increase autophagy, muscle protein degradation and mitochondrial dysfunction, which may negatively impact skeletal muscle function. 12 Exercise training may improve muscle function through improvements in hyperglycemia and insulin sensitivity, and also in part due to increased GLUT4-mediated glucose transport and increased tissue expression of insulin receptor substrate (IRS)-1, an insulin signaling intermediate. 22,29,30 Insulin-independent mechanisms may also be important. 8 Chronic inflammation has been associated with obesity and type 2 diabetes. Exercise training, in turn, has been shown to attenuate both inflammatory profiles and inducible nitric oxide synthase (iNOS) protein content. 31 However, our findings were independent of inflammatory markers. Interestingly, approximately 15-20% of individuals with type 2 diabetes fail to achieve improvements in HbA1c, muscle mitochondrial content, muscle substrate metabolism, percentage body fat, and BMI despite adherence with an exercise training program. Several mechanisms have been postulated, and further characterizing those persons with type 2 diabetes who may benefit most from regular exercise warrants future investigation. 8
The long-term sustainability of improvements in body composition after intensive lifestyle changes remains unclear. 32 Individuals with diabetes who detrain after a period of exercise training may not maintain improvements in body composition. 17,18 These findings thus underscore the need for ongoing regular exercise, consistent with present guidelines. 7 Although long-term maintenance of lean body mass improvements after exercise training was not specifically examined in our study, this should be explored in future studies.
In general, favorable changes in body composition such as a decline in fat mass and increase in lean body mass have been associated with improvements in physical function 33 and potentially a decline in mortality. 34 Even a small gain in lean body mass may be an important benefit of exercise training as body weight is often lost in exercise. Persons with diabetes have an increased risk of functional limitations with up to 70% of patients having difficulty with routine physical tasks. 35,36 Thus, the favorable body composition changes observed in our study may have implications in ultimately reducing the burden of disability in persons with diabetes and this needs to be investigated in future studies.
Strengths of our study include the duration of exercising training intervention, which is comparable, and in some instances, longer, than other studies in type 2 diabetes, and the inclusion of both upper and lower body exercises and aerobic and resistance training in the exercise training intervention. 3-5,16,17,19,21,37,38 In addition, the comprehensive testing in the SHAPE-2 trial allowed us to assess multiple measures of body composition and strength testing. Further, the randomized controlled trial study design minimized potential confounders.
Limitations of the study may include generalizability. The mean HbA1c at baseline among our participants was on average relatively well-controlled and persons with severe hyperglycemia or using insulin therapy were excluded from enrollment in SHAPE-2. Yet, we were still able to detect significant relationships. As noted, there were dropouts among the exercisers. However, body composition parameters were not a significant predictor of study dropout in the SHAPE2 trial as previously reported 14 and thus would not have been expected to significantly impact our findings. We chose to analyze only those participants who completed the trial, similar to previous studies of exercise interventions, 39,40 as our primary focus was to examine body composition changes after a full six months of training. The focus of the present study was to conduct post-hoc analyses among persons with diabetes following exercise training; previous studies have reported significant differences between exercisers and controls in the SHAPE-2 trial particularly with regards to changes in body composition changes. 13 Although the sample size of our study is relatively small, it is within the range of other exercise trials. Additionally, we identified robust relationships, even with exclusion of outliers. We also did not find significant relationships of surrogate markers of insulin sensitivity with changes in body composition; however, euglycemic insulin clamp would have provided more definitive information. Given that changes in fat and muscle mass were observed in parallel over the 6 months, we were unable to characterize the temporality of these respective changes without interval assessments. Also, the focus of our study was to examine the relationship of hyperglycemia to changes in fat and muscle mass after exercise training and not necessarily the degree to which these changes were independent of changes in body weight, but this should be explored in the future. However, we noted significant relationships of fasting hyperglycemia to relative (percentage) measures of lean and fat body mass which also account for total body weight, compared to absolute measures of fat and lean body mass alone. Although the goal of our study was not to examine the clinical implications of such findings in terms of physical function, this should be examined in future studies. Additionally, duration of diabetes and the presence of complications may contribute to the observed changes in body composition; this information was not collected in our study. 24 Peripheral neuropathy may relate to decreased muscle function and was an exclusion criterion for participants in our study. 9 It is also unclear if use of specific glucose-lowering therapies may have impacted the observed relationships and this should be examined in other studies. 41 Lastly, it has been reported that statin-associated myalgias may be worsened by exercise, potentially limiting exercise potential. 42 We do not have data on statin-induced myalgias, nor duration of statin therapy in our study. However, we anticipate that the inability to reach maximal exercise potential would have resulted in more conservative estimates of changes in lean body and fat mass in our study, yet we still detected significant findings.
Overall, our findings demonstrate that the magnitude of lean body mass gains and fat mass losses in persons with type 2 diabetes after exercise training is related to baseline glycemic control. Further studies evaluating the impact of these body composition changes from exercise training on functional status could ultimately help inform clinical practice. The results of our study support the importance of physical activity for all persons with type 2 diabetes, but suggest that these benefits relate to the degree of hyperglycemia, and may ultimately inform future targeted prevention and treatment strategies for those who have the greatest potential to gain from regular exercise.
Acknowledgements
Funding. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-62368 and K23-DK-093583); and by the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research (UL1-RR-025005).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: D.V. is a consultant for Consumable Science, Inc. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.Y., K.S., S.G., A.D., D.B., D.V., B.K. and R.K. were responsible for study concept and design, analysis and interpretation of the data, and drafting and critical revision of the manuscript for important intellectual content. N.J. was responsible for study concept and design, acquisition of data, analysis and interpretation of data. K.S. and N.J. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Duality of Interest. D.V. is a consultant for Consumable Science, Inc. No other potential conflicts of interest relevant to this article were reported.
Prior Presentation. Parts of this study were presented in abstract form at the 75th Scientific Sessions of the American Diabetes Association in Boston, Massachusetts, June 5-9, 2015.
REFERENCES
- 1.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diabetes Prevention Program Research Group. Knowler WC, Fowler SE, et al. 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigal RJ, Kenny GP, Boule NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: A randomized trial. Ann Intern Med. 2007;147(6):357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z, Scott CA, Mao C, Tang J, Farmer AJ. Resistance exercise versus aerobic exercise for type 2 diabetes: A systematic review and meta-analysis. Sports Med. 2014;44(4):487–499. doi: 10.1007/s40279-013-0128-8. [DOI] [PubMed] [Google Scholar]
- 5.Tomas-Carus P, Ortega-Alonso A, Pietilainen KH, et al. A randomized controlled trial on the effects of combined aerobic-resistance exercise on muscle strength and fatigue, glycemic control and health-related quality of life of type 2 diabetes patients. J Sports Med Phys Fitness. 2015 [PubMed] [Google Scholar]
- 6.Miller CT, Fraser SF, Levinger I, et al. The effects of exercise training in addition to energy restriction on functional capacities and body composition in obese adults during weight loss: A systematic review. PLoS One. 2013;8(11):e81692. doi: 10.1371/journal.pone.0081692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: The american college of sports medicine and the american diabetes association: Joint position statement. Diabetes Care. 2010;33(12):e147–67. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephens NA, Sparks LM. Resistance to the beneficial effects of exercise in type 2 diabetes: Are some individuals programmed to fail? J Clin Endocrinol Metab. 2015;100(1):43–52. doi: 10.1210/jc.2014-2545. [DOI] [PubMed] [Google Scholar]
- 9.Kalyani RR, Metter EJ, Egan J, Golden SH, Ferrucci L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care. 2015;38(1):82–90. doi: 10.2337/dc14-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpato S, Bianchi L, Lauretani F, et al. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care. 2012;35(8):1672–1679. doi: 10.2337/dc11-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalyani RR, Tian J, Xue QL, et al. Hyperglycemia and incidence of frailty and lower extremity mobility limitations in older women. J Am Geriatr Soc. 2012;60(9):1701–1707. doi: 10.1111/j.1532-5415.2012.04099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2(10):819–829. doi: 10.1016/S2213-8587(14)70034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobrosielski DA, Gibbs BB, Ouyang P, et al. Effect of exercise on blood pressure in type 2 diabetes: A randomized controlled trial. J Gen Intern Med. 2012;27(11):1453–1459. doi: 10.1007/s11606-012-2103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam S, Dobrosielski DA, Stewart KJ. Predictors of exercise intervention dropout in sedentary individuals with type 2 diabetes. J Cardiopulm Rehabil Prev. 2012;32(6):370–378. doi: 10.1097/HCR.0b013e31826be485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart KJ, Deregis JR, Turner KL, et al. Fitness, fatness and activity as predictors of bone mineral density in older persons. J Intern Med. 2002;252(5):381–388. doi: 10.1046/j.1365-2796.2002.01050.x. [DOI] [PubMed] [Google Scholar]
- 16.Geirsdottir OG, Arnarson A, Briem K, Ramel A, Jonsson PV, Thorsdottir I. Effect of 12-week resistance exercise program on body composition, muscle strength, physical function, and glucose metabolism in healthy, insulin-resistant, and diabetic elderly icelanders. J Gerontol A Biol Sci Med Sci. 2012;67(11):1259–1265. doi: 10.1093/gerona/gls096. [DOI] [PubMed] [Google Scholar]
- 17.Park SY, Lee IH. Effects on training and detraining on physical function, control of diabetes and anthropometrics in type 2 diabetes; a randomized controlled trial. Physiother Theory Pract. 2015;31(2):83–88. doi: 10.3109/09593985.2014.958265. [DOI] [PubMed] [Google Scholar]
- 18.Tokmakidis SP, Touvra AM, Douda HT, Smilios I, Kotsa K, Volaklis KA. Training, detraining, and retraining effects on glycemic control and physical fitness in women with type 2 diabetes. Horm Metab Res. 2014;46(13):974–979. doi: 10.1055/s-0034-1390483. [DOI] [PubMed] [Google Scholar]
- 19.Egger A, Niederseer D, Diem G, et al. Different types of resistance training in type 2 diabetes mellitus: Effects on glycaemic control, muscle mass and strength. Eur J Prev Cardiol. 2013;20(6):1051–1060. doi: 10.1177/2047487312450132. [DOI] [PubMed] [Google Scholar]
- 20.Senechal M, Swift DL, Johannsen NM, et al. Changes in body fat distribution and fitness are associated with changes in hemoglobin A1c after 9 months of exercise training: Results from the HART-D study. Diabetes Care. 2013;36(9):2843–2849. doi: 10.2337/dc12-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hameed UA, Manzar D, Raza S, Shareef MY, Hussain ME. Resistance training leads to clinically meaningful improvements in control of glycemia and muscular strength in untrained middle-aged patients with type 2 diabetes mellitus. N Am J Med Sci. 2012;4(8):336–343. doi: 10.4103/1947-2714.99507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorge ML, de Oliveira VN, Resende NM, et al. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism. 2011;60(9):1244–1252. doi: 10.1016/j.metabol.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Ibanez J, Gorostiaga EM, Alonso AM, et al. Lower muscle strength gains in older men with type 2 diabetes after resistance training. J Diabetes Complications. 2008;22(2):112–118. doi: 10.1016/j.jdiacomp.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes. 2006;55(6):1813–1818. doi: 10.2337/db05-1183. [DOI] [PubMed] [Google Scholar]
- 25.Cauza E, Strehblow C, Metz-Schimmerl S, et al. Effects of progressive strength training on muscle mass in type 2 diabetes mellitus patients determined by computed tomography. Wien Med Wochenschr. 2009;159(5-6):141–147. doi: 10.1007/s10354-009-0641-4. [DOI] [PubMed] [Google Scholar]
- 26.Praet SF, Jonkers RA, Schep G, et al. Long-standing, insulin-treated type 2 diabetes patients with complications respond well to short-term resistance and interval exercise training. Eur J Endocrinol. 2008;158(2):163–172. doi: 10.1530/EJE-07-0169. [DOI] [PubMed] [Google Scholar]
- 27.Ekman C, Elgzyri T, Strom K, et al. Less pronounced response to exercise in healthy relatives to type 2 diabetics compared to controls. J Appl Physiol (1985) 2015:jap.01067.2014. doi: 10.1152/japplphysiol.01067.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007;167(14):1545–1551. doi: 10.1001/archinte.167.14.1545. [DOI] [PubMed] [Google Scholar]
- 29.Frosig C, Richter EA. Improved insulin sensitivity after exercise: Focus on insulin signaling. Obesity (Silver Spring) 2009;17(Suppl 3):S15–20. doi: 10.1038/oby.2009.383. [DOI] [PubMed] [Google Scholar]
- 30.Lund S, Holman GD, Schmitz O, Pedersen O. Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci U S A. 1995;92(13):5817–5821. doi: 10.1073/pnas.92.13.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eghbalzadeh K, Brixius K, Bloch W, Brinkmann C. Skeletal muscle nitric oxide (NO) synthases and NO-signaling in “diabesity”--what about the relevance of exercise training interventions? Nitric Oxide. 2014;37:28–40. doi: 10.1016/j.niox.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Pownall HJ, Bray GA, Wagenknecht LE, et al. Changes in body composition over 8 years in a randomized trial of a lifestyle intervention: The look AHEAD study. Obesity (Silver Spring) 2015;23(3):565–572. doi: 10.1002/oby.21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sternfeld B, Ngo L, Satariano WA, Tager IB. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol. 2002;156(2):110–121. doi: 10.1093/aje/kwf023. [DOI] [PubMed] [Google Scholar]
- 34.Church TS, Cheng YJ, Earnest CP, et al. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care. 2004;27(1):83–88. doi: 10.2337/diacare.27.1.83. [DOI] [PubMed] [Google Scholar]
- 35.de Rekeneire N, Volpato S. Physical function and disability in older adults with diabetes. Clin Geriatr Med. 2015;31(1):51–65, viii. doi: 10.1016/j.cger.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Kalyani RR, Saudek CD, Brancati FL, Selvin E. Association of diabetes, comorbidities, and A1C with functional disability in older adults: Results from the national health and nutrition examination survey (NHANES), 1999-2006. Diabetes Care. 2010;33(5):1055–1060. doi: 10.2337/dc09-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: A systematic review and meta-analysis. JAMA. 2011;305(17):1790–1799. doi: 10.1001/jama.2011.576. [DOI] [PubMed] [Google Scholar]
- 38.Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. JAMA. 2001;286(10):1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 39.Kadoglou NP, Iliadis F, Angelopoulou N, et al. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil. 2007;14(6):837–843. doi: 10.1097/HJR.0b013e3282efaf50. [DOI] [PubMed] [Google Scholar]
- 40.Barone Gibbs B, Dobrosielski DA, Althouse AD, Stewart KJ. The effect of exercise training on ankle-brachial index in type 2 diabetes. Atherosclerosis. 2013;230(1):125–130. doi: 10.1016/j.atherosclerosis.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mensink M, Hesselink MK, Russell AP, Schaart G, Sels JP, Schrauwen P. Improved skeletal muscle oxidative enzyme activity and restoration of PGC-1 alpha and PPAR beta/delta gene expression upon rosiglitazone treatment in obese patients with type 2 diabetes mellitus. Int J Obes (Lond) 2007;31(8):1302–1310. doi: 10.1038/sj.ijo.0803567. [DOI] [PubMed] [Google Scholar]
- 42.Parker BA, Thompson PD. Effect of statins on skeletal muscle: Exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40(4):188–194. doi: 10.1097/JES.0b013e31826c169e. [DOI] [PMC free article] [PubMed] [Google Scholar]