Abstract
Evidence supporting physical activity, diet, and weight management for cancer survivors has grown, leading to the development of guidelines and interventions. The next step is to identify necessary practice and policy changes, and a research agenda, to inform how interventions can be delivered to survivors most effectively and efficiently in both healthcare settings and by community-based organizations. We propose an agenda for research, practice, and policy that incorporates recommendations for a range of programming options, a patient-centered, tailored screening and referral approach, and training needs for survivorship care providers and providers of exercise, nutrition, and weight management services. Research needs to focus on sustainability, dissemination, and implementation. We present needed policy changes as well as opportunities to leverage current health care policies.
Keywords: cancer survivor, obesity, physical activity, nutrition, policy
Introduction
There is increasing evidence that weight management, physical activity, and diet are related to prognosis and survival after cancer and that addressing these health needs improves survivors’ functioning and quality of life. Observational studies indicate that insufficient physical activity and obesity are associated with disease-related outcomes (1–8), including recurrence risk, death from cancer, and overall mortality, as well as the risk of subsequent malignancies (9–12). Furthermore, for many cancer survivors, cardiovascular disease is a significant cause of mortality (13–16) for which obesity, physical inactivity, and poor diet quality are established risk factors (17). Diabetes mellitus also appears to be associated with an elevated risk for additional cancer events (18). Evidence for these health behavior effects on cancer prognosis are difficult to test in randomized controlled trials using survival endpoints because of the extensive length of follow-up needed to detect mortality differences. However, substantial evidence from randomized trials support the potential for physical activity, diet, and weight management interventions to reduce cancer-related symptoms and improve quality of life, including functional health outcomes (19–24).
Given growing evidence suggesting the benefits of physical activity, high diet quality, and weight management (referred to collectively as lifestyle behaviors) for cancer survivors, the American Cancer Society (ACS) has published guidelines on nutrition and physical activity for cancer survivors (Figure 1) (25), as have other national organizations such as the National Comprehensive Cancer Network and the American College of Sports Medicine (ACSM) (26, 27). Many survivors have limited awareness of these guidelines and most do not achieve the recommended lifestyle goals (28–33). Clinicians in both oncology and primary care have limited knowledge of the guidelines and are frequently unprepared for counseling patients in these areas (34). A lack of education, as well as misinformation available on the internet and within the community, may confuse survivors and their healthcare providers about the best evidence-based lifestyle programs for a given survivor. Further, in the U.S., effective programs and services to help survivors adopt recommended behaviors are not widely available in survivorship care settings or the community. Despite irrefutable evidence of health benefits, these interventions are rarely reimbursed by health insurance. The purpose of this report is to propose an agenda for research, practice, and policy to move the field toward comprehensive, systematic support for addressing lifestyle behaviors in cancer survivorship wherein programs are available to all survivors, from diagnosis onward, in order to optimize health. We use the phrase “cancer survivor”, adhering to the National Cancer Institute definition of any person who has been diagnosed with cancer “from the time of diagnosis, through the balance of his or her life” (35).
Figure 1.

American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Survivors (25)
Our goal is to translate the existing research regarding the benefits of exercise, diet quality, and weight management for cancer survivors into actionable and evidence-based practices (36). With the goal of increasing the availability, accessibility, and uptake of cancer survivor lifestyle behavior change programs, as well as long-term adherence to guidelines, we propose the following action areas:
Expand the availability of a range of evidence-based options for weight management, nutrition counseling, and physical activity programs for cancer survivors
Improve screening and referral of survivors to exercise, nutrition, and weight management services
Improve health care providers’ capability and capacity to screen/assess and refer survivors to weight management, diet, and exercise information, programs, and services
Increase and support the oncology-specific training and certification of dietitians, exercise professionals, physical therapists and physiatrists to increase the competency of the workforce needed to appropriately deliver services to cancer survivors
Expand dissemination and implementation research to test models for service delivery of evidence-based interventions
Advocate for and leverage healthcare policy changes that support availability, access, affordability, and uptake of services
1. Expand the availability of a range of evidence-based options for weight management, nutrition counseling, and physical activity programs for cancer survivors
Programs to help cancer survivors address lifestyle behaviors must reflect the diversity of their needs, interests, goals, preferences, and resources. Programs can be delivered as supervised, monitored, or unsupervised, each suggesting different roles for professionals. While some survivors may be able to proceed with exercise or weight management independently, others may experience barriers that make lifestyle change challenging or even unsafe in unsupervised settings and thus need to be part of a structured cancer rehabilitation program before proceeding with home or community-based programs (37). For reasons of resources, logistics, or preference, survivors may prefer programs offered through a cancer treatment center, while others are interested in community-based or self-led programs. Survivors also differ in their motivation for pursuing lifestyle programming. Some seek health promotion that targets the prevention of future health problems, while others need to remediate impairments and activity limitations through medical rehabilitation. Mobile health intervention strategies (e.g., mobile apps, wearables) may be useful across the range of programs, either to provide information and self-monitoring support to survivors in supervised programs, or as free standing interventions for survivors who are interested in a self-directed program. Optimal design of such interventions to maximize engagement and effectiveness is a critical research need (38). Regardless of program type or delivery characteristics, all programs need to be evidence-based, with demonstrated efficacy, effectiveness and safety. Table 1 provides a summary of existing types of programs and resources that cancer survivors may be able to access for assistance with lifestyle behavior change.
Table 1.
Types of programs addressing physical activity, diet, or weight management for cancer survivors.
| Program type | Description/examples | Cost coverage | Advantages | Limitations |
|---|---|---|---|---|
| Medically-based programs | ||||
| Cancer rehabilitation | Services provided by a therapist, physiatrist, or interdisciplinary team with the goal of maintaining or restoring function, reducing symptom burden, maximizing independence, and improving quality of life | Covered as an essential health benefit under the Affordable Care Act, out-of-pocket cost sharing (i.e., co-pays) and other medical management often apply (65) | Highly personalized, matched to survivors’ needs and adapted to their health conditions | Availability and accessibility may be limited by costs, location, insurance plan, and time; likely not reimbursable for survivors without diagnosable impairments (e.g., those interested in health promotion); insufficient workforce of providers with cancer-specific training |
| Oncology dietitian services | Nutrition assessment and counseling from a registered dietitian nutritionist (RDN), including certified specialists in oncology (CSO) | Outpatient oncology dietitian services not routinely covered by insurance, may be offered at no charge to the patient in some cancer treatment settings | Highly personalized, matched to survivors’ needs and adapted to their health conditions | Limited accessibility, referral-based model requires health care provider to recognize a need (84), insufficient workforce of RDNs with cancer-specific training, outside of major cancer centers survivors may be required to pay for services |
| Weight loss, lifestyle change in non-oncology settings | Primary care provider obesity screening, weight loss counseling, reduction of behavioral cardiovascular risk factors; medical weight loss programs (which can include counseling, meal replacements, medication); bariatric surgery | Primary care obesity screening and counseling covered at no cost to the patient in most marketplace and employer-based plans and in Medicare for those with BMI ≥30, dietary and physical activity counseling covered at no cost to the patient in most marketplace and employer-based plans and in Medicare for patients with cardiovascular disease risk factors; some but not all costs of medical weight loss programs may be covered by insurance; Medicare and many Medicaid and private insurance plans cover bariatric surgery for patients with severe obesity or obesity-related co-morbidities | Primary care counseling is convenient and accessible to patients, integrated into health care. Bariatric surgery can produce large weight losses. | Services are not cancer-specific; few primary care providers have sufficient time and training and thus do not adequately implement evidence-based guidelines for weight loss counseling (34, 85) |
| Community-based programs | ||||
| Not-for-profit programs | Group or individual programming with exercise specialist, RDN, or health educator; Example: LIVESTRONG at the YMCA, 12-week lifestyle change program, shown to increase physical activity and improve quality of life and fitness (86) | Historically offered free of charge or at low cost to survivors | Cancer-specific focus, community-based locations may be more convenient for survivors, provide in-person assistance for survivors who may need in-person guidance in how to do exercises, change diet, etc. | Not available in all communities, lack of sustainable funding model for operational expenses, and lack of program standards/accreditation process, which may make it difficult for survivors and their providers to judge program quality, safety, and appropriateness |
| Worksite programs | Employers may include programs for cancer survivors as part of their health and wellness programs; Example: the World Bank’s global headquarters provided a 6-week lifestyle program “Back to Life”, which included consultation with an exercise specialist, and individual exercise plan, group sessions twice a week focusing on lifestyle change strategies, strength training and balance, and stress relief. Half of participants reported a decline in fatigue and 65% demonstrated reduction in waist circumference (87), cancer survivors may also participate in health and wellness programs provided for the general employee population in their worksite | May be offered at no cost or with cost sharing | Convenient for participants, participating with co-workers may provide built-in social support (88), cancer-specific programs responsive to the needs of survivors | Cancer-specific worksite programs are not widely available, worksite wellness programs may be less available/accessible to low-income workers and small business, large proportion of cancer survivors are retired so unable to access worksite programs |
| Commercial programs | Examples include Weight Watchers, Curves | Survivor generally pays for the program or membership; some insurance or Medicare Advantage plans may cover the cost of gym memberships | Convenient for participants, studies with survivors have shown that both Weight Watchers and Curves were effective for cancer survivors when combined with some cancer-specific content (41, 89) | Not known if they are effective without cancer-specific content, costly for participants, some programs are reputable, but there are many commercial weight loss programs that do not use evidence-based methods |
| Home-based programs | ||||
| Cancer-specific programs | Provides information, resources and guidance through print materials, web-based or mobile apps, telephone coaching peer support and equipment/devices to support behavior change, e.g., activity monitors, food scales. Example: RENEW, a program for older cancer survivors to increase physical activity, improve diet quality, and promote modest weight loss, shown in research to benefit physical functioning and quality of life (21, 90) | To date such programs have been available only through research studies, at no cost to participants. No current insurance coverage for this type of program | Strong research support for effectiveness of these programs on lifestyle behavior, functioning, and quality of life (72, 73, 91–94), convenient for participants, fewer geographic, time, and physical barriers to participation than with in-person programs | Dissemination of these programs has been limited, no sustainable model for intervention delivery has been identified, may not be appropriate for survivors with more serious impairments/comorbidities |
| Non-cancer specific | Websites, mobile apps, print information available to the general public; Example: ChooseMyPlate.gov, My Fitness Pal | Some sources are free, while other have fees that are not reimbursed by insurance | Convenient for participants, fewer geographic, time, and physical barriers to participation than with in-person programs | Minimal cancer specific information, not all products use evidence-based methods (95), survivors must be motivated to seek out and use these resources |
Are cancer survivor-specific programs needed?
The question remains whether lifestyle intervention programs must be specific to cancer survivors or whether survivors should use programs available for the general population. The answer depends on the health of the survivor, the risk level associated with activity, and the comfort level of the survivor. A cancer-specific program that provides appropriate guidance and supervision to minimize risk is appropriate for survivors who experience or are at risk for significant treatment side effects (e.g., survivors at risk of lymphedema, undernutrition, or health problems exacerbated by prevalent co-morbidities). Some survivors, even those with relatively few cancer sequelae, may lack self-efficacy for lifestyle behavior change after cancer (39, 40), and be more comfortable with a program that can address their concerns as a survivor, as well as reinforce the survivor-specific benefits of improved lifestyle factors. These survivors also may benefit from programs tailored for cancer survivors (41).
Clearly cancer survivors need wider availability of a range of programs. Medically-based programs may be limited to people receiving care at major centers, and access is also limited by cost, availability of transportation, and time away from work that may be required. Program standards are needed for community programs to assure survivors and providers that offered services are safe and evidence-based. To better connect survivors with appropriate programs, we need to highlight a variety of evidence-based programs on websites of cancer centers and of national non-profit organizations, and ideally in a national registry. Additional research and program evaluation is needed to further bolster program safety and effectiveness, identify which programs are effective for whom, and to test models of program delivery that are efficient, effective, and sustainable.
2. Improve screening and referral of survivors to exercise, nutrition, and weight management services
In identifying the best approach to lifestyle behavior change for a particular cancer survivor, it is clear that “one size does not fit all.” Cancer and treatment-related adverse effects frequently lead to loss of normal body structure and physiologic dysfunction, which in turn may lead to difficulties in executing needed or desired activities (42). The nature and degree of impairments and limitations cannot always be predicted based on cancer type and treatment. Personal factors, such as demographic characteristics and co-morbid health problems, may be more predictive of functional health status than cancer type, treatment or disease stage, and therefore need to be factored into decision making when referring patients to programs (43).
Because of the interaction between cancer sequelae and personal factors, some cancer survivors may have relatively few limitations, some experience lingering or late effects of treatment such as lymphedema or peripheral neuropathy, and still others may experience serious ongoing symptoms like cardiomyopathy or severe, ongoing fatigue. Other survivors have few ongoing problems related to their cancer or its treatment, but have co-morbidities such as diabetes, cardiovascular disease or hypertension that affect their functioning and quality of life. Obesity is a risk factor for several types of cancer, including endometrial, post-menopausal breast, esophageal, liver, colorectal, kidney, gall bladder, gastric cardia, meningioma, ovarian, thyroid, multiple myeloma, and pancreas (44–47), thus many survivors will be overweight or obese prior to diagnosis and will remain so after treatment, increasing the risk of other cardiometabolic disease (48). Cancer and cancer treatment also may result in weight gain for some survivors, particularly those entering treatment with normal body mass index, and weight loss for others, particularly those with advanced disease or experiencing multiple therapies.
A patient-centered tailored approach is needed to identify appropriate lifestyle behavior change and rehabilitation services from self-led approaches to inpatient treatment (49). Alfano et al. (50) describe approaches to matching the impairment with the appropriate levels of rehabilitation. At level I are survivors who are deconditioned but not experiencing any cancer-specific impairments or complicating comorbidities. Level II refers to individuals who lack specific cancer-related impairments but have co-morbid or other conditions that may warrant a supervised approach. Level III includes survivors with cancer- or treatment-related impairments, but who do not have systemic health concerns, such as cardiomyopathy. Level IV includes survivors with more severe, possibly systemic symptoms (e.g., persistent severe fatigue) or refractory impairments.
A survivor’s goals and preferences should also be considered in determining an appropriate referral. While it is desirable from a guidelines perspective to encourage all survivors to follow the guidelines completely (aerobic exercise, resistance training, healthy eating and weight management practices), research establishing the efficacy of multiple behavior change interventions within cancer survivors is limited (51). Multiple health behavior change strategies may work for some survivors, but others may experience difficulties making changes in several behaviors simultaneously. Some survivors might be more interested in the safety or potential benefits of more holistic approaches, such as yoga, and may not be ready for a more comprehensive exercise regimen. Likewise, survivors might be interested in learning how to improve the healthfulness of their diets, but not be ready to engage in more structured weight loss efforts. Indeed, research and theory on behavior change indicates that making incremental changes in behavior is more easily achieved than major changes in lifestyle, and builds self-efficacy, which in turn fosters sustained change (52). To create a patient-centered process for screening and referring survivors to lifestyle programs, survivor preferences must be integrated with information about their impairments, functioning, comorbidities and access.
Framework for referral to appropriate lifestyle behavior services
Because of the diversity in survivor goals and interests, better systems are needed in survivorship care to evaluate and triage survivors to exercise, diet and weight management programs that align with their health conditions, needs and preferences. In Figures 2a and 2b, we propose a framework for referring cancer survivors to appropriate services based on both their physical condition and their preferences and goals. The framework identifies the “least restrictive alternative” in terms of medical screening and/or supervision for the activity. It does not imply that more intensive or supervised services would not be beneficial for a survivor, but that it can be reasonably assumed that the specified level of intervention represents a safe starting place. Figure 2a identifies the appropriate starting point for exercise or physical activity depending on the survivor’s health characteristics and the type of activity. For example, many survivors can decrease sedentary behavior by increasing short bouts of light and moderate intensity lifestyle activity, without the need for medical screening or a supervised program, but those with significant adverse treatment-related effects, cancer-related symptoms or global functional health compromises should consult with a physician. For survivors without the presence of any of these and well-managed co-morbidities (e.g., well-controlled hypertension), self-directed moderate intensity aerobic exercise can be engaged in at home or in a community facility or program. Survivors with unmanaged comorbidities, or those who want to engage in exercise that involves more risk, should undergo more intensive medical screening and supervision of activity initially, with consideration of a structured cancer rehabilitation program.
Figure 2.
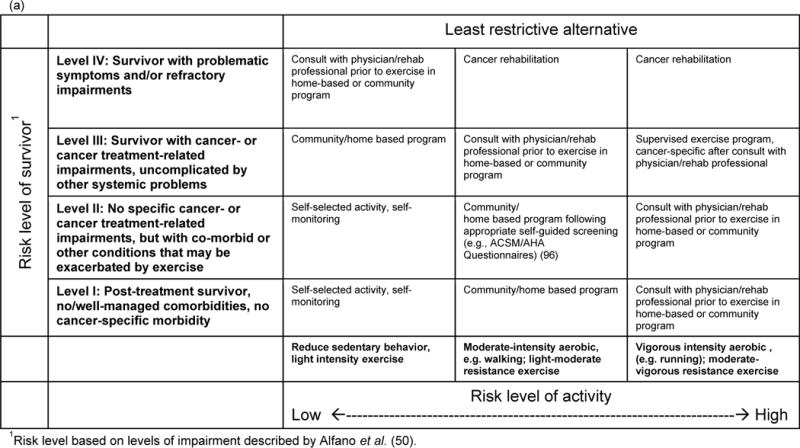
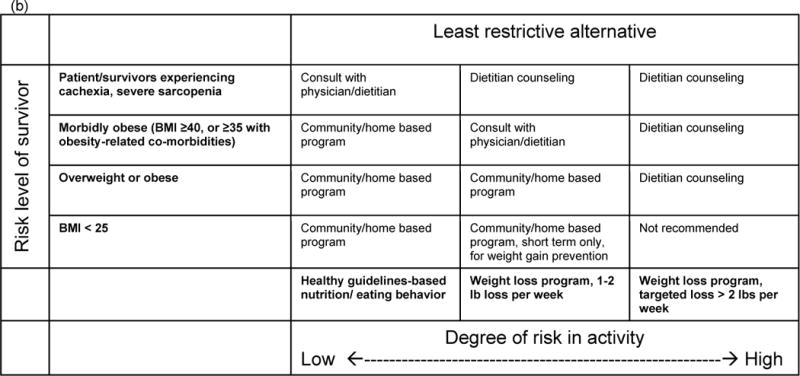
Patient-centered, tailored framework for identifying appropriate (a) physical activity/exercise and (b) weight management programming for cancer survivors. Framework takes into account both survivor health condition (risk level) and goals and preferences. Programming types identified represent a reasonably safe starting place for survivors, although it may be possible that more intensive services could be beneficial for the survivor.
A similar framework has relevance for diet and weight management. Survivors interested in developing healthy eating habits may do so on their own or in community programs that are not medically supervised. In contrast, survivors who are experiencing unintended weight loss should consult with a physician and registered dietitian nutritionist (RDN) for professional advice to guide health behavior change. Survivors with severe obesity or related co-morbidities should also seek out an RDN for weight loss assistance, which can include counseling, aiding in food selection, and instruction in food preparation.
Implementation of screening strategies using this framework can help connect survivors with services tailored to their physical needs and personal goals, without producing additional barriers (e.g., needing to attend a specific program at a designated time and place) that might limit participation.
3. Improve health care providers’ capability and capacity to screen/assess and refer survivors to weight management, diet, and exercise information, programs, and services
The importance of healthcare providers assessing lifestyle factors and directing cancer survivors to appropriate weight management, physical activity, and nutrition resources cannot be overstated. Cancer survivors indicate strong preferences for receiving information about physical activity, diet, and weight management from their oncology providers (53) and studies indicate that such discussions can be influential (54, 55). There is evidence that physician referrals are associated with increased physical activity among cancer survivors (56, 57). Recognizing this need, guidelines for survivorship care specify that healthcare providers should assess lifestyle behaviors and refer survivors for these programs (58–61) and the American Society of Clinical Oncology (ASCO) has issued a statement on the importance of oncology providers addressing these behaviors in their patients (62). However, survivors generally are not receiving recommendations about health behavior change from their providers (54, 63, 64). For example, a study of 15,254 colorectal cancer survivors found that those who recalled receiving physical activity advice from a provider were more likely to be physically active, but only 31% received such advice (55). Health care providers often feel they do not have the adequate training or time to provide screening, advice or referral to connect cancer patients with lifestyle counseling or programming (65) and their general knowledge of diet, physical activity and weight management is limited (66). Without this training and knowledge acquisition, referrals to support services are unlikely to occur. Efforts to enhance medical education in relation to lifestyle are being implemented, including recent efforts by the ASCO (62). A comprehensive evidence-based guide to obesity treatment for the general population have been published by The Obesity Society, American Heart Association, and the American College of Cardiology (67) that can be useful to survivorship care providers as well. However, more emphasis is needed in health care provider training on how to consistently address lifestyle behaviors in cancer care, and to do so in a culturally competent manner, and research is needed to understand the type of information providers need in order to increase their comfort with screening and referring patients.
Additionally, effectively enabling providers to address this issue will require changes in workflow and routines, provision of simple and time-efficient screening tools, resources, and reminders for providers to assess and refer to services. Implementation models like the 5As (Ask, Advise, Assess, Assist, Arrange) are widely used and highly effective for tobacco cessation support (68). For efficiency, clinics have abbreviated this approach to an Ask, Advise, Connect resulting in similar uptake of tobacco cessation for patients. These approaches could be considered as a starting point for helping providers address lifestyle behavior change with cancer survivors under their care.
4. Increase training and certification of dietitians, exercise professionals, physical therapists, occupational therapists and speech therapists, and physiatrists to increase the workforce needed to appropriately handle referrals
Of the more than 15 million cancer survivors in the U.S., there is likely a small percentage who require no additional assistance to adopt healthier eating and increased physical activity. For the remainder, some form of professional support is likely to be useful. Thus, there is a need for exercise and nutrition professionals with appropriate training to provide support to cancer survivors. Entry-level RDNs receive sparse training specific to oncology, but there is a specialty board certification available in oncology nutrition from the Academy of Nutrition and Dietetics. This professional certification requires RDNs to have demonstrated 2000+ hours of documented practice in an oncology nutrition setting, current licensure as a Registered Dietitian/Nutritionist and passage of an oncology certification exam. More than 600 RDNs are Certified Specialists in Oncology (CSO). Likewise, the ACSM has a specialty certification called the Cancer Exercise Trainer (CET) that was developed in collaboration with the ACS. This program recognizes the need for exercise professionals with specialty training to provide appropriate consultation that addresses the unique needs of cancer survivors. As of June 2017, there are 465 ACSM CETs in the U.S. For survivors who are less physically able, for whom outpatient rehabilitation is likely the first step toward physical function recovery after cancer, there also is a need for outpatient rehabilitation clinical professionals with specialty training in oncology. In the majority of community oncology settings, this will be a physical therapist. While there are more than 200,000 licensed physical therapists in the U.S., not all have expertise with cancer survivors. The American Physical Therapy Association is creating a specialty board certification in the field of oncology, and the first exams will be held in 2019. Having board certification in oncology will help maintain standards of care and allow better estimates of the size of physical therapy workforce with this expertise. Likewise, while there are more than 10,000 board certified physiatrists in the US, few are fellowship trained in or practice cancer rehabilitation. Although the American Academy of Physical Medicine and Rehabilitation is working with cancer rehabilitation physiatrists to develop cancer-specific training, education and research programs, no specialty certification is yet available.
Wellness professions need to expand oncology-specific training to ensure a sufficient workforce to provide lifestyle behavior interventions to meet the needs of more than 15 million cancer survivors. Further, efforts are needed to identify and train other professionals who could provide lifestyle behavioral support and information. For example, there may be a role for patient navigators, health educators, and community health workers to help motivate survivors to access recommended services. Motivating survivors, particularly those who are reluctant to access services or consider lifestyle behavior change, requires skills like motivational interviewing that are distinct from the skills required to develop exercise recommendations or provide weight loss coaching.
5. Expand dissemination and implementation research to test models for service delivery of evidence-based interventions
There is a need to conduct dissemination and implementation (D&I) research to assure that the results of research in areas of weight management, nutrition, and physical activity among cancer survivors are translated into programs that are available to all survivors who need them. However, according to a recent portfolio review of National Cancer Institute grants on lifestyle interventions in cancer survivors, very little D&I research in this area is being conducted (69). Models of effective screening and referral for physical activity and weight management programs are needed for survivorship care, and effective and disseminable program models need to be refined and tested.
The efficacy studies that have been conducted in lifestyle behaviors and cancer survivorship have focused on internal validity, with little attention paid to how the program could be implemented in a real world setting. Frameworks such as RE-AIM (which assesses program Reach, Efficacy/Effectiveness, Adoption, Implementation, and Maintenance) can be used to study the population impact of different implementation models (70). An example from the field of smoking cessation provides an illustration of the use of RE-AIM to assess the effects of two implementation models for encouraging uptake of smoking cessation treatment. Vidrine and colleagues compared an “Ask, Advise, Refer” model (AAR, Refer: providing information about how to contact the quitline) among smokers in primary care and “Ask, Advise, Connect” model (AAC, Connect: asking for permission to provide patient contact information to the quitline personnel, who then contacted the patient for an appointment). Primary care practices in a safety net healthcare setting were randomly assigned to AAR or AAC. AAC had superior impact, with 14.7% of identified smokers enrolling in treatment, compared to 0.5% in the AAR clinics. (71). A similar proactive model could be useful in referring survivors to lifestyle behavior intervention programs.
Pinto, Stein, and Dunsiger tested a community-based implementation model of an evidence-based program to help breast cancer survivors be more physically active (72). In this study, 18 peer volunteers (breast cancer survivors) with the ACS’s Reach to Recovery program were trained and supervised to deliver a 12-week physical activity program by telephone to 76 breast cancer survivors. The peer mentors effectively increased participants’ physical activity compared to a contact control condition (73). This is an illustration of scaling up an intervention beyond the research setting and potentially increasing the reach of an intervention by collaborating with a well-established community-based organization.
Researchers have begun D&I research to evaluate diet and physical activity interventions for cancer survivors outside of research settings (65, 74). But more systematic efforts are needed to achieve the goal of widespread dissemination of active lifestyle and healthy nutrition to promote cancer recovery. D&I researchers and community-/clinic-based partners interested in this area can find evidence-based interventions on national websites (e.g., Cancer Control P.L.A.N.E.T and the Research-tested Intervention Programs), and we encourage researchers to post their interventions to add to this resource. Other resources for implementation of research into practice include the Agency for Healthcare Research and Quality’s ACTION (Accelerating Change and Transformation in Organizations and Networks). In addition to a need for D&I of existing interventions, we recommend that researchers planning new studies on lifestyle behavior among cancer survivors consider external validity, dissemination, and the potential for sustainability, developing programs that can be generalized to a broader cancer survivor population. There is also value to more deeply exploring the context of community oncology clinics, and of survivorship care provided in primary care settings, to better understand how programs can be implemented and sustained in these practice settings.
6. Advocate for and leverage policy changes that support availability, access, affordability, and uptake of services
Public policy – whether at the federal, state, or local levels – can influence affordability of, access to, and utilization of evidence-based nutrition, physical activity, and weight management programs and services for the general population and for cancer survivors.
Insurance coverage for lifestyle behavior programs for cancer survivors
Requirements or incentives for health insurance coverage is one way public policy affects access to nutrition, physical activity, and weight management services. For individuals with health insurance, reducing cost-sharing and other medical management further removes barriers to accessing health care programs and services that improve quality of life, reduce treatment-related complications or comorbidities, and potentially reduce overall health care costs and enhance disease-free and overall survival (75).
With respect to nutrition, physical activity, and weight management programs and services, coverage varies considerably depending factors that include type of service, reason for treatment, provider, treatment setting, type of payer, and the individual’s specific health insurance plan. Coverage for oncology nutrition services varies greatly across institutions and healthcare systems, and no health insurance routinely covers outpatient oncology nutrition services provided during treatment. Some payment models include offering the service to patients at no charge as a benefit of seeking cancer care at a particular institution and reimbursing the provider through the cancer center’s overhead or as part of a bundled payment, billing patients directly, or contracting with a third party organization that provides funding for oncology nutrition services. While these models provide some cancer patients with access to nutrition services at no cost, others do not have access to nutrition support unless they are able to pay for the service out-of-pocket. Unfortunately, this means that where individuals seek cancer care and their ability to pay determines their level of access to nutrition services. Given that many cancer patients already experience financial concerns related to their treatment (76), most are unable to afford nutrition services or prioritize this service among other costs. In addition, when oncology nutrition services are not covered by a patient’s insurance policy, the money the patient spends on those services does not count toward an individual’s out-of-pocket maximum, adding financial strain.
Coverage for physical activity interventions also varies widely based on the type of intervention. The Affordable Care Act1 requires coverage of rehabilitative services as Essential Health Benefits. While this means physical therapy for cancer survivors is covered by most private insurance plans, barriers to access still exist, including no requirement for coverage in grandfathered plans, cost sharing, and other forms of medical management. A study of a physical therapist-led group exercise intervention for breast cancer survivors reported that of patients who sought insurance coverage for the program, none were denied (65), but some were charged co-payments as high as $80 per session, presenting a barrier to access. Medicare Part B also covers medically necessary outpatient physical and occupational therapy, although coverage is subject to co-payments, deductibles, and payment limits (77). It also covers medically necessary outpatient physiatry care, subject to co-payments and deductibles, but in general no payment limits.
We are not aware of any systematic assessment of private insurance coverage for cancer survivor physical activity interventions that do not meet the definition of physical therapy and expect that coverage for this type of service is very limited. The Medicare program does not cover any type of cancer survivor-specific physical activity program. However, insurance coverage for community-based lifestyle change programs for the prevention and management of other chronic diseases may help to pave the way for coverage of similar programs for cancer survivors. For example, in March 2016, HHS announced that its independent Office of the Actuary in Centers for Medicare & Medicaid Services (CMS) certified that expansion of the Diabetes Prevention Program, a model funded through the Centers for Medicare and Medicaid Innovation, would reduce net Medicare spending and improve patient care (78). An evaluation of the program found that Medicare beneficiaries who attended at least four weekly sessions lost an average of 4.73 percent of their body weight, resulting in a savings of $2,650 per enrollee over a 15-month period (78). In November 2016, CMS released the final rule on the Medicare Diabetes Prevention Program Expanded Model, allowing for Medicare to reimburse for providing the program to eligible beneficiaries beginning in 2018 (79). Several private insurers have covered the program since 2010 either directly with community-based providers like the YMCA or as part of a cooperative agreement with the Centers for Disease Control and Prevention (80).
Opportunities to Increase Access
Recent shifts in the health care delivery system provide promise for increasing access to evidence-based lifestyle behavior programs and services. Health care systems and payers are increasingly focused on value-driven care, which rewards improved care quality and reduced costs in place of the more traditional fee-for-service system which incentivizes increased care volume (81). Further, as the U.S. Department of Health and Human Services continues its work to transform payment and delivery (82), we believe it should consider incentivizing health care systems and providers to deliver nutrition, exercise and/or weight management services to patients as a way to improve patients’ exercise, nutritional status and overall quality of life and reduce cancer risk or recurrence. CMS should also conduct additional studies incentivizing patient navigation and appropriate care transitions between oncology and primary care for post-treatment cancer survivors. Models tested should address access and navigation to an appropriate program or service that will facilitate healthy lifestyle behavior change (83).
Many new payment and delivery models tie incentive payments to outcomes. Quality measures focused on outcomes, rather than process, incentivize provision of high-quality care. While the National Quality Forum (NQF) has endorsed 54 cancer-related quality measures, none of them directly addresses diet, physical activity or weight management for cancer survivors.2 Future quality measures focused on diet, physical activity, and weight status outcomes could help to monitor progress and incentivize providers to offer or refer to evidence-based behavioral interventions to improve lifestyle behaviors. In addition, researchers should consider including existing quality measures as outcomes in studies of lifestyle behavior interventions, so results provide a rationale for covering services.
Public and private payers are recognizing the long-term benefits and potential cost savings of coverage of cancer survivor lifestyle change programs and services. Additional research is needed to increase the evidence base about the effectiveness and cost effectiveness of various lifestyle change programs and services for cancer survivors, including discerning which programs and services are most effective for whom, and how insurance coverage and employer provision of programs and services affects utilization and outcomes. Engagement of payers is needed in the formulation of research questions so that research results can better inform their decisions about benefit design and coverage.
Sustainable funding for lifestyle change programs and services by third party payers should be a long-term goal. In the interim, existing innovative service delivery and payment models implemented by cancer centers, employers, and community-based organizations to provide lifestyle change programs to cancer survivors and keep them affordable should continue. Scaling programs for broader reach to a larger number of cancer survivors is an additional programming goal. All stakeholders must play a role in ensuring that cancer survivors have the support needed to engage in healthy dietary, physically active, and weight management behaviors on a long-term basis.
Conclusion
Many factors and components must be addressed to ensure that cancer survivors’ needs for nutrition counseling, physical activity and weight management are met, as summarized in Figure 3. Research gaps must be addressed with added D&I research, with the goal to optimize care and health outcomes for the full population of cancer survivors (see Table 2). A critical component of assuring access to quality lifestyle behavior support is the need to train healthcare providers and to develop programs and systems to accommodate the routine delivery of this care. The advent of value-based health care is expected to support this process, as weight loss in other populations has demonstrated significant cost-benefit within a 15-month period. Indeed, with such data in hand, it is time to promote health for all cancer survivors by providing each survivor with the comprehensive lifestyle behavior care necessary to ensure their journey of cancer survivorship is long and marked by optimal health.
Figure 3.
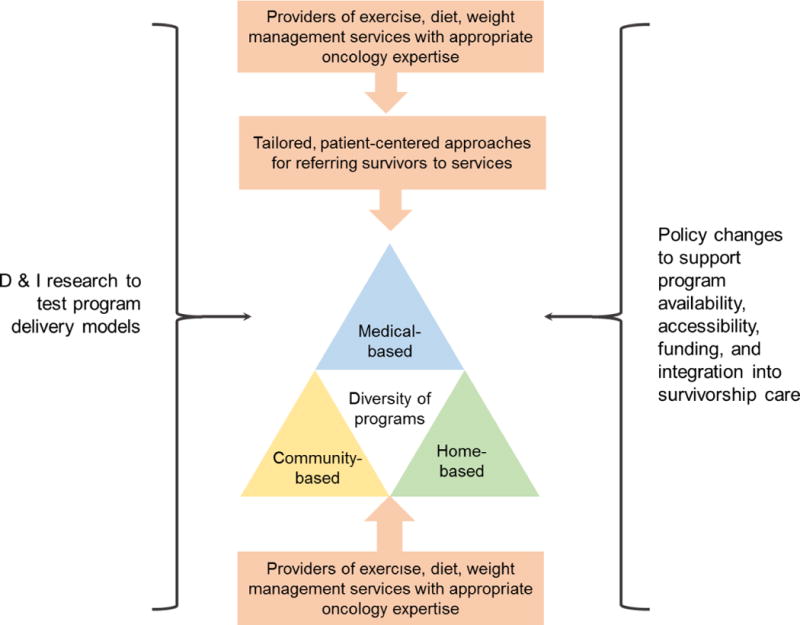
Summary of agenda for translating physical activity/exercise, diet, and weight management programs to cancer survivorship care.
Table 2.
Research agenda summary
| Priority research topics | Relevant agenda items1 | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Determining effectiveness and safety of lifestyle interventions in real-world settings with populations that are diverse with regard to race and ethnicity, age, socioeconomic status, and comorbidities (agenda item 1) | X | |||||
| Developing and refining personalized lifestyle interventions: what works best for whom? (agenda items 1, 2) | X | X | ||||
| Investigating provider- and system-level barriers to screening, brief counseling, and referral to lifestyle intervention programs (agenda items 2, 3, 5) | X | X | X | |||
| Developing and testing models of screening, brief counseling, and referral (agenda items 2, 5), including testing navigation to rehabilitation or lifestyle programs as part of CMS novel payment delivery pilots (agenda item 6) | X | X | X | |||
| Developing and testing models of program delivery in both clinical and community settings (agenda items 1, 3, 4, 5) | X | X | X | X | ||
| Determining the effects of lifestyle interventions for cancer survivors on health care utilization, quality indicators (NQF), and costs (agenda item 6) | X | |||||
| Cross-cutting methodological and design considerations | ||||||
| ||||||
Agenda items: (1) Expand the availability of a range of evidence-based options for weight management, nutrition counseling, and physical activity programs for cancer survivors; (2) Improve screening and referral of survivors to exercise, nutrition, and weight management services; (3) Improve health care providers’ capability and capacity to screen/assess and refer survivors to weight management, diet, and exercise information, programs, and services; (4) Increase and support the oncology-specific training and certification of dietitians, exercise professionals, physical therapists and physiatrists to increase the competency of the workforce needed to appropriately deliver services to cancer survivors; (5) Expand dissemination and implementation research to test models for service delivery; (6) Advocate for and leverage healthcare policy changes that support availability, access, affordability, and uptake of services
What is already known about this subject?
In cancer survivors weight management, high diet quality, and physical activity/exercise are associated with reduced comorbidity and improved physical functioning, metabolic health, and quality of life.
Uptake of these behaviors among cancer survivors is limited, and there are insufficient programs accessible to cancer survivors or systems to refer survivors to existing programs.
What does this review add?
This review identifies necessary practice and policy changes, along with a research agenda, to support effective and efficient delivery of lifestyle interventions to survivors in health care, home-based, and community-based settings.
Acknowledgments
Funding: These authors received support from the following sources: Basen-Engquist, P30 CA16672; Center for Energy Balance in Cancer Prevention and Survivorship, Duncan Family Institute; Schmitz, NCI U54 155850; Pinto, R01 CA183849; Syrjala, R01 CA215134; Demark-Wahnefried, American Cancer Society, CRP-14-111-01-CPPB
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
Note: Reference to the Affordable Care Act or other federal laws reflect current law in effect as of the date of submission of the paper, July 10, 2017. While Congress is considering legislation to amend or remove some of the Affordable Care Act provisions mentioned in this paper, as of the date of submission, no changes have become law, and the authors decline to speculate on what the impact could be of potential policy changes under consideration by Congress or the Administration.
Based on a search of the National Quality Forum measure database: http://www.qualityforum.org/Qps/QpsTool.aspx# The count includes endorsed measures in the cancer topic area as of June 6, 2017.
Contributor Information
Karen Basen-Engquist, Department of Behavioral Science, The University of Texas MD Anderson Cancer Center.
Catherine M. Alfano, American Cancer Society, Inc.
Melissa Maitin-Shepard, American Cancer Society Cancer Action Network.
Cynthia A. Thomson, Department of Health Promotion Sciences, University of Arizona.
Kathryn H. Schmitz, Department of Public Health Science, Penn State College of Medicine.
Bernardine M. Pinto, College of Nursing, University of South Carolina.
Kevin Stein, Behavior Sciences and Health Education, Emory University Rollins School of Public Health.
David S. Zucker, Swedish Cancer Institute.
Karen L. Syrjala, Biobehavioral Sciences, Fred Hutchinson Cancer Research Center.
Elizabeth Fallon, American Cancer Society, Inc.
Colleen Doyle, American Cancer Society, Inc.
Wendy Demark-Wahnefried, Department of Nutrition Sciences, University of Alabama at Birmingham Comprehensive Cancer Center.
References
- 1.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. Journal National Cancer Institute. 2012;104(11):815–40. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierce JP, Stefanick ML, Flatt SW, Natarajan L, Sternfeld B, Madlensky L, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. Journal of Clinical Oncology. 2007;25(17):2345–51. doi: 10.1200/JCO.2006.08.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Lu W, Zheng W, Gu K, Chen Z, Zheng Y, et al. Obesity and weight change in relation to breast cancer survival. Breast cancer research and treatment. 2010;122(3):823–33. doi: 10.1007/s10549-009-0708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ewertz M, Jensen MB, Gunnarsdottir KA, Hojris I, Jakobsen EH, Nielsen D, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29(1):25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 5.Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012;118(23):5937–46. doi: 10.1002/cncr.27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyerhardt JAGE, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–34. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 7.Meyerhardt JAHD, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–41. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 8.Caan BJ, Kwan ML, Shu XO, Pierce JP, Patterson RE, Nechuta SJ, et al. Weight change and survival after breast cancer in the after breast cancer pooling project. Cancer Epidemiology, Biomarkers and Prevention. 2012;21(8):1260–71. doi: 10.1158/1055-9965.EPI-12-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Travis LB, Demark Wahnefried W, Allan JM, Wood ME, Ng AK. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol. 2013;10(5):289–301. doi: 10.1038/nrclinonc.2013.41. [DOI] [PubMed] [Google Scholar]
- 10.Wu W, Guo F, Ye J, Li Y, Shi D, Fang D, et al. Pre- and post-diagnosis physical activity is associated with survival benefits of colorectal cancer patients: a systematic review and meta-analysis. Oncotarget. 2016 doi: 10.18632/oncotarget.10603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Blarigan EL, Meyerhardt JA. Role of physical activity and diet after colorectal cancer diagnosis. J Clin Oncol. 2015;33(16):1825–34. doi: 10.1200/JCO.2014.59.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25(7):1293–311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 13.Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, Rouzier R, Broglio KR, Hortobagyi GN, et al. Overall survival and cause-specific mortality of patients with stage T1a,bN0M0 breast carcinoma. J Clin Oncol. 2007;25(31):4952–60. doi: 10.1200/JCO.2006.08.0499. [DOI] [PubMed] [Google Scholar]
- 14.Ward KK, Shah NR, Saenz CC, McHale MT, Alvarez EA, Plaxe SC. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecologic oncology. 2012;126(2):176–9. doi: 10.1016/j.ygyno.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Gernaat SAM, Ho PJ, Rijnberg N, Emaus MJ, Baak LM, Hartman M, et al. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast cancer research and treatment. 2017 doi: 10.1007/s10549-017-4282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawkes AL, Lynch BM, Owen N, Aitken JF. Lifestyle factors associated concurrently and prospectively with co-morbid cardiovascular disease in a population-based cohort of colorectal cancer survivors. Eur J Cancer. 2011;47(2):267–76. doi: 10.1016/j.ejca.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2960–84. doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Scappaticcio L, Maiorino MI, Bellastella G, Giugliano D, Esposito K. Insights into the relationships between diabetes, prediabetes, and cancer. Endocrine. 2017;56(2):231–9. doi: 10.1007/s12020-016-1216-y. [DOI] [PubMed] [Google Scholar]
- 19.Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8:CD007566. doi: 10.1002/14651858.CD007566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012;8:CD008465. doi: 10.1002/14651858.CD008465.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. Journal of the American Medical Association. 2009;301(18):1883–91. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demark-Wahnefried W, Colditz GA, Rock CL, Sedjo RL, Liu J, Wolin KY, et al. Quality of life outcomes from the Exercise and Nutrition Enhance Recovery and Good Health for You (ENERGY)-randomized weight loss trial among breast cancer survivors. Breast cancer research and treatment. 2015;154(2):329–37. doi: 10.1007/s10549-015-3627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swisher AK, Abraham J, Bonner D, Gilleland D, Hobbs G, Kurian S, et al. Exercise and dietary advice intervention for survivors of triple-negative breast cancer: effects on body fat, physical function, quality of life, and adipokine profile. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2015;23(10):2995–3003. doi: 10.1007/s00520-015-2667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarroll ML, Armbruster S, Frasure HE, Gothard MD, Gil KM, Kavanagh MB, et al. Self-efficacy, quality of life, and weight loss in overweight/obese endometrial cancer survivors (SUCCEED): a randomized controlled trial. Gynecologic oncology. 2014;132(2):397–402. doi: 10.1016/j.ygyno.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 25.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA: a cancer journal for clinicians. 2012;62(4):243–74. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 26.Denlinger CS, Ligibel JA, Are M, Baker KS, Broderick G, Demark-Wahnefried W, et al. NCCN Guidelines Insights: Survivorship, Version 1.2016. J Natl Compr Canc Netw. 2016;14(6):715–24. doi: 10.6004/jnccn.2016.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine & Science in Sports & Exercise. 2010;42(7):1409–26. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 28.Coups EJ, Ostroff JS. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Prev Med. 2005;40(6):702–11. doi: 10.1016/j.ypmed.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. Journal of Clinical Oncology. 2005;23(34):8884–93. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 30.Williams K, Steptoe A, Wardle J. Is a cancer diagnosis a trigger for health behaviour change? Findings from a prospective, population-based study. Br J Cancer. 2013;108(11):2407–12. doi: 10.1038/bjc.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nayak P, Holmes HM, Nguyen HT, Elting LS. Self-reported physical activity among middle-aged cancer survivors in the United States: Behavioral Risk Factor Surveillance System Survey, 2009. Prev Chronic Dis. 2014;11:E156. doi: 10.5888/pcd11.140067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LeMasters TJ, Madhavan SS, Sambamoorthi U, Kurian S. Health behaviors among breast, prostate, and colorectal cancer survivors: a US population-based case-control study, with comparisons by cancer type and gender. J Cancer Surviv. 2014;8(3):336–48. doi: 10.1007/s11764-014-0347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang FF, Liu S, John EM, Must A, Demark-Wahnefried W. Diet quality of cancer survivors and noncancer individuals: Results from a national survey. Cancer. 2015;121(23):4212–21. doi: 10.1002/cncr.29488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steeves JA, Liu B, Willis G, Lee R, Smith AW. Physicians’ personal beliefs about weight-related care and their associations with care delivery: The U.S. National Survey of Energy Balance Related Care among Primary Care Physicians. Obes Res Clin Pract. 2015;9(3):243–55. doi: 10.1016/j.orcp.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Survivorship definitions. 2017 Available from: https://cancercontrol.cancer.gov/ocs/statistics/definitions.html.
- 36.Alfano CM, Smith T, de Moor JS, Glasgow RE, Khoury MJ, Hawkins NA, et al. An action plan for translating cancer survivorship research into care. Journal of the National Cancer Institute. 2014;106(11) doi: 10.1093/jnci/dju287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stull VB, Snyder DC, Demark-Wahnefried W. Lifestyle interventions in cancer survivors: designing programs that meet the needs of this vulnerable and growing population. J Nutr. 2007;137(1 Suppl):243S–8S. doi: 10.1093/jn/137.1.243S. [DOI] [PubMed] [Google Scholar]
- 38.Vandelanotte C, Muller AM, Short CE, Hingle M, Nathan N, Williams SL, et al. Past, Present, and Future of eHealth and mHealth Research to Improve Physical Activity and Dietary Behaviors. J Nutr Educ Behav. 2016;48(3):219–28 e1. doi: 10.1016/j.jneb.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Pinto BM, Rabin C, Dunsiger S. Home-based exercise among cancer survivors: adherence and its predictors. Psychooncology. 2009;18(4):369–76. doi: 10.1002/pon.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes D, Baum G, Jovanovic J, Carmack C, Greisinger A, Basen-Engquist K. An acute exercise session increases self-efficacy in sedentary endometrial cancer survivors and controls. J Phys Act Health. 2010;7(6):784–93. doi: 10.1123/jpah.7.6.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Djuric Z, DiLaura NM, Jenkins I, Darga L, Jen CKL, Mood D, et al. Combining weight-loss counseling with the Weight Watchers plan for obese breast cancer survivors. Obesity Research. 2002;10(7):657–65. doi: 10.1038/oby.2002.89. [DOI] [PubMed] [Google Scholar]
- 42.Ness KK, Wall MM, Oakes JM, Robison LL, Gurney JG. Physical performance limitations and participation restrictions among cancer survivors: a population-based study. Ann Epidemiol. 2006;16(3):197–205. doi: 10.1016/j.annepidem.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Campbell KL, Pusic AL, Zucker DS, McNeely ML, Binkley JM, Cheville AL, et al. A prospective model of care for breast cancer rehabilitation: function. Cancer. 2012;118(8 Suppl):2300–11. doi: 10.1002/cncr.27464. [DOI] [PubMed] [Google Scholar]
- 44.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity and the Prevention of CAncer: A Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 45.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 46.Basen-Engquist K, Chang M. Obesity and cancer risk: recent review and evidence. Curr Oncol Rep. 2011;13(1):71–6. doi: 10.1007/s11912-010-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body Fatness and Cancer–Viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–8. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation. 2016;133(11):1104–14. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheville AL, Mustian K, Winters-Stone K, Zucker DS, Gamble GL, Alfano CM. Cancer Rehabilitation: An Overview of Current Need, Delivery Models, and Levels of Care. Phys Med Rehabil Clin N Am. 2017;28(1):1–17. doi: 10.1016/j.pmr.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Alfano CM, Cheville AL, Mustian K. Developing High-Quality Cancer Rehabilitation Programs: A Timely Need. Am Soc Clin Oncol Educ Book. 2016;35:241–9. doi: 10.1200/EDBK_156164. [DOI] [PubMed] [Google Scholar]
- 51.Green AC, Hayman LL, Cooley ME. Multiple health behavior change in adults with or at risk for cancer: a systematic review. Am J Health Behav. 2015;39(3):380–94. doi: 10.5993/AJHB.39.3.11. [DOI] [PubMed] [Google Scholar]
- 52.Hill JO. Can a small-changes approach help address the obesity epidemic? A report of the Joint Task Force of the American Society for Nutrition, Institute of Food Technologists, and International Food Information Council. Am J Clin Nutr. 2009;89(2):477–84. doi: 10.3945/ajcn.2008.26566. [DOI] [PubMed] [Google Scholar]
- 53.Madsen LT, Cesario S. Dietary resource information for the oncology patient: tips and tools. J Adv Pract Oncol. 2012;3(1):55–8. [PMC free article] [PubMed] [Google Scholar]
- 54.Clark LH, Ko EM, Kernodle A, Harris A, Moore DT, Gehrig PA, et al. Endometrial Cancer Survivors’ Perceptions of Provider Obesity Counseling and Attempted Behavior Change: Are We Seizing the Moment? Int J Gynecol Cancer. 2016;26(2):318–24. doi: 10.1097/IGC.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 55.Fisher A, Williams K, Beeken R, Wardle J. Recall of physical activity advice was associated with higher levels of physical activity in colorectal cancer patients. BMJ Open. 2015;5(4):e006853. doi: 10.1136/bmjopen-2014-006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones LW, Courneya KS, Fairey AS, Mackey JR. Effects of an oncologist’s recommendation to exercise on self-reported exercise behavior in newly diagnosed breast cancer survivors: a single-blind, randomized controlled trial. Ann Behav Med. 2004;28(2):105–13. doi: 10.1207/s15324796abm2802_5. [DOI] [PubMed] [Google Scholar]
- 57.Pinto BM, Papandonatos GD, Goldstein MG. A randomized trial to promote physical activity among breast cancer patients. Health Psychol. 2013;32(6):616–26. doi: 10.1037/a0029886. [DOI] [PubMed] [Google Scholar]
- 58.El-Shami K, Oeffinger KC, Erb NL, Willis A, Bretsch JK, Pratt-Chapman ML, et al. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA: a cancer journal for clinicians. 2015;65(6):428–55. doi: 10.3322/caac.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skolarus TA, Wolf AM, Erb NL, Brooks DD, Rivers BM, Underwood W, 3rd, et al. American Cancer Society prostate cancer survivorship care guidelines. CA: a cancer journal for clinicians. 2014;64(4):225–49. doi: 10.3322/caac.21234. [DOI] [PubMed] [Google Scholar]
- 60.Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016;34(6):611–35. doi: 10.1200/JCO.2015.64.3809. [DOI] [PubMed] [Google Scholar]
- 61.Cohen EE, LaMonte SJ, Erb NL, Beckman KL, Sadeghi N, Hutcheson KA, et al. American Cancer Society Head and Neck Cancer Survivorship Care Guideline. CA: a cancer journal for clinicians. 2016;66(3):203–39. doi: 10.3322/caac.21343. [DOI] [PubMed] [Google Scholar]
- 62.Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32(31):3568–74. doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sabatino SA, Coates RJ, Uhler RJ, Pollack LA, Alley LG, Zauderer LJ. Provider counseling about health behaviors among cancer survivors in the United States. J Clin Oncol. 2007;25(15):2100–6. doi: 10.1200/JCO.2006.06.6340. [DOI] [PubMed] [Google Scholar]
- 64.Nyrop KA, Deal AM, Williams GR, Guerard EJ, Pergolotti M, Muss HB. Physical activity communication between oncology providers and patients with early-stage breast, colon, or prostate cancer. Cancer. 2016;122(3):470–6. doi: 10.1002/cncr.29786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beidas RS, Paciotti B, Barg F, Branas AR, Brown JC, Glanz K, et al. A hybrid effectiveness-implementation trial of an evidence-based exercise intervention for breast cancer survivors. J Natl Cancer Inst Monogr. 2014;2014(50):338–45. doi: 10.1093/jncimonographs/lgu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hicks KK, Murano PS. Viewpoint regarding the limited nutrition education opportunities for physicians worldwide. Educ Prim Care. 2016;27(6):439–42. doi: 10.1080/14739879.2016.1197048. [DOI] [PubMed] [Google Scholar]
- 67.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 68.Siu AL, Force USPST Behavioral and Pharmacotherapy Interventions for Tobacco Smoking Cessation in Adults, Including Pregnant Women: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163(8):622–34. doi: 10.7326/M15-2023. [DOI] [PubMed] [Google Scholar]
- 69.Alfano CM, Bluethmann SM, Tesauro G, Perna F, Agurs-Collins T, Elena JW, et al. NCI Funding Trends and Priorities in Physical Activity and Energy Balance Research Among Cancer Survivors. Journal of the National Cancer Institute. 2016;108(1) doi: 10.1093/jnci/djv285. [DOI] [PubMed] [Google Scholar]
- 70.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–7. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vidrine JI, Shete S, Li Y, Cao Y, Alford MH, Galindo-Talton M, et al. The Ask-Advise-Connect approach for smokers in a safety net healthcare system: a group-randomized trial. Am J Prev Med. 2013;45(6):737–41. doi: 10.1016/j.amepre.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005;23(15):3577–87. doi: 10.1200/JCO.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 73.Pinto B, Stein K, Dunsiger S. Peer mentorship to promote physical activity among cancer survivors: effects on quality of life. Psychooncology. 2015 doi: 10.1002/pon.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pinto BM, Stein K, Dunsiger S. Peers promoting physical activity among breast cancer survivors: A randomized controlled trial. Health Psychol. 2015;34(5):463–72. doi: 10.1037/hea0000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han X, Robin Yabroff K, Guy GP, Jr, Zheng Z, Jemal A. Has recommended preventive service use increased after elimination of cost-sharing as part of the Affordable Care Act in the United States? Prev Med. 2015;78:85–91. doi: 10.1016/j.ypmed.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singleterry J. The Costs of Cancer: American Cancer Society Cancer Action Network. 2017 Available from: https://www.acscan.org/costofcancer.
- 77.Centers for Medicare and Medicaid Services. Your Medicare Coverage: Physical therapy/occumpational therapy/speech-language pathology services. 2016 Available from: https://www.medicare.gov/coverage/pt-and-ot-and-speech-language-pathology.html.
- 78.CMS Office of the Actuary. Memo from Paul Spitalnic, Chief Actuary, CMS, reagrding Certification of Medicare Diabetes Prevention Program, March 14, 2016. 2016 Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Research/ActuarialStudies/Downloads/Diabetes-Prevention-Certification-2016-03-14.pdf.
- 79.CMS. Medicare Diabetes Prevention Program (MDPP) Expanded Model. Updated May 18, 2017. 2017 Available from: https://innovation.cms.gov/initiatives/medicare-diabetes-prevention-program/
- 80.America’s Health Insurance Plans. Health Plans Preventing Diabetes and Improving Well-Being. 2017 Available from: https://www.ahip.org/diabetes/
- 81.Alliance of Community Health Care Plans. Rewarding High Quality: Practical Models for Value-Base Physician Payment. 2016 Available from: http://www.achp.org/wp-content/uploads/ACHP-Report_Rewarding-High-Quality_4.20.16.pdf.
- 82.Centers for Medicare and Medicaid Services. Health Care Payment Learning and Action Network. 2017 Available from: https://innovation.cms.gov/initiatives/Health-Care-Payment-Learning-and-Action-Network/
- 83.Rocque GB, Partridge EE, Pisu M, Martin MY, Demark-Wahnefried W, Acemgil A, et al. The Patient Care Connect Program: Transforming Health Care Through Lay Navigation. J Oncol Pract. 2016;12(6):e633–42. doi: 10.1200/JOP.2015.008896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.National Academies of Sciences E, and Medicine. Examining access to nutrition care in outpatient cancer centers: Proceedings of a workshop. Washington, DC: The National Academies Press; 2016. Contract No.: 10.17226/23579. [PubMed] [Google Scholar]
- 85.Antognoli EL, Smith KJ, Mason MJ, Milliner BR, Davis EM, Harris-Haywood S, et al. Direct observation of weight counselling in primary care: alignment with clinical guidelines. Clin Obes. 2014;4(2):69–76. doi: 10.1111/cob.12050. [DOI] [PubMed] [Google Scholar]
- 86.Irwin ML, Cartmel B, Harrigan M, Li F, Sanft T, Shockro L, et al. Effect of the LIVESTRONG at the YMCA exercise program on physical activity, fitness, quality of life, and fatigue in cancer survivors. Cancer. 2017;123(7):1249–58. doi: 10.1002/cncr.30456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Willson R. Presentation; Nutrition and Physical Activity Interventions for Cancer Survivors: A Policy Seminar; 12/10/2015; Washington, DC: American Cancer Society Cancer Action Network, International Health, Racquet, and Sportsclub Association, and Academy of Nutrition and Dietetics; 2015. [Google Scholar]
- 88.Tabak RG, Hipp JA, Marx CM, Brownson RC. Workplace social and organizational environments and healthy-weight behaviors. PLoS One. 2015;10(4):e0125424. doi: 10.1371/journal.pone.0125424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Greenlee HA, Crew KD, Mata JM, McKinley PS, Rundle AG, Zhang W, et al. A pilot randomized controlled trial of a commercial diet and exercise weight loss program in minority breast cancer survivors. Obesity (Silver Spring) 2013;21(1):65–76. doi: 10.1002/oby.20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Demark-Wahnefried W, Morey MC, Sloane R, Snyder DC, Miller PE, Hartman TJ, et al. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J Clin Oncol. 2012;30(19):2354–61. doi: 10.1200/JCO.2011.40.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Demark-Wahnefried W, Clipp EC, Lipkus IM, Lobach D, Snyder DC, Sloane R, et al. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;25(19):2709–18. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- 92.Demark-Wahnefried W, Jones LW, Snyder DC, Sloane RJ, Kimmick GG, Hughes DC, et al. Daughters and Mothers Against Breast Cancer (DAMES): main outcomes of a randomized controlled trial of weight loss in overweight mothers with breast cancer and their overweight daughters. Cancer. 2014;120(16):2522–34. doi: 10.1002/cncr.28761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hayes SC, Rye S, Disipio T, Yates P, Bashford J, Pyke C, et al. Exercise for health: a randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast cancer research and treatment. 2013;137(1):175–86. doi: 10.1007/s10549-012-2331-y. [DOI] [PubMed] [Google Scholar]
- 94.Harrigan M, Cartmel B, Loftfield E, Sanft T, Chagpar AB, Zhou Y, et al. Randomized Trial Comparing Telephone Versus In-Person Weight Loss Counseling on Body Composition and Circulating Biomarkers in Women Treated for Breast Cancer: The Lifestyle, Exercise, and Nutrition (LEAN) Study. J Clin Oncol. 2016;34(7):669–76. doi: 10.1200/JCO.2015.61.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bardus M, van Beurden SB, Smith JR, Abraham C. A review and content analysis of engagement, functionality, aesthetics, information quality, and change techniques in the most popular commercial apps for weight management. Int J Behav Nutr Phys Act. 2016;13:35. doi: 10.1186/s12966-016-0359-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Riebe D, Franklin BA, Thompson PD, Garber CE, Whitfield GP, Magal M, et al. Updating ACSM’s Recommendations for Exercise Preparticipation Health Screening. Med Sci Sports Exerc. 2015;47(11):2473–9. doi: 10.1249/MSS.0000000000000664. [DOI] [PubMed] [Google Scholar]


