The title compound is a salt containing a protonated endo-3-aminotropane cage and a novel anionic copper(II) complex, [CuCl3(NO3)(H2O)]2−.
Keywords: crystal structure, tropane, nitrogen heterocycle, copper(II)complex, isomer separation
Abstract
The structure of a salt of diprotonated endo-3-aminotropane crystallized with a copper(II) anionic cluster is reported, viz. (C8H18N2)[CuCl3(NO3)(H2O)]. Neither ion in the salt has been structurally characterized previously. In the crystal, the ions pack together to form a three-dimensional structure held together by a network of intermolecular N—H⋯O, O—H⋯Cl and N—H⋯Cl hydrogen-bonding interactions. Selective crystallization of the title compound can be considered as a simple method for the separation of the exo and endo isomers of 3-aminotropane.
Chemical context
The bicyclic ring of tropane [(1R,5S)-8-methyl-8-azabicyclo[3.2.1]octane] is the fuctional core of pharmaceutically important alkaloids, such as atropine, hyoscyamine, scopolamine, cocaine and their semisynthetic derivatives (Pollini et al., 2006 ▸; Kim et al., 2016 ▸). As a consequence, there have been a large number of structural studies devoted to tropane-based compounds. It is surprising, however, that some of the simplest derivatives of tropane, such as 3-aminotropane, have not been structurally characterized in their unsubstituted forms. The structures of other simple and well-known bicyclic organic compounds have been reported only very recently, including 1,4-diazabicyclo[3.2.1]octane (Britvin et al., 2017 ▸) and 7-azabicyclo[2.2.1]heptane (7-azanorbornane) (Britvin & Rumyantsev, 2017 ▸). In the course of our ongoing studies of cage-like heterocyclic amines (Britvin & Lotnyk, 2015 ▸; Britvin et al., 2016 ▸), we report herein for the first time the molecular structure of the endo isomer of 3-aminotropane in its protonated form (see Scheme). In the title compound, (1R,5S)-endo-(8-methyl-8-azoniabicyclo[3.2.1]oct-3-yl)ammonium aquatrichloridonitratocopper(II), 1, the protonated endo-3-aminotropane skeleton (Fig. 1 ▸) is charge-balanced by the [CuCl3(NO3)(H2O)]2− anion. The anion (Fig. 2 ▸) is the first example of a complex in which a copper(II) centre is coordinated to both nitrate and chloride ligands (as well as water). It is noteworthy that the synthesized compound 1 contains the pure endo-3-aminotropane isomer, whereas the starting material, 3-aminotropane dihydrochloride, comprised a mixture of exo and endo isomers. Therefore, selective crystallization of 1 reported herein can be recommended as a simple and effective method for the separation of the exo and endo isomers of 3-aminotropane.
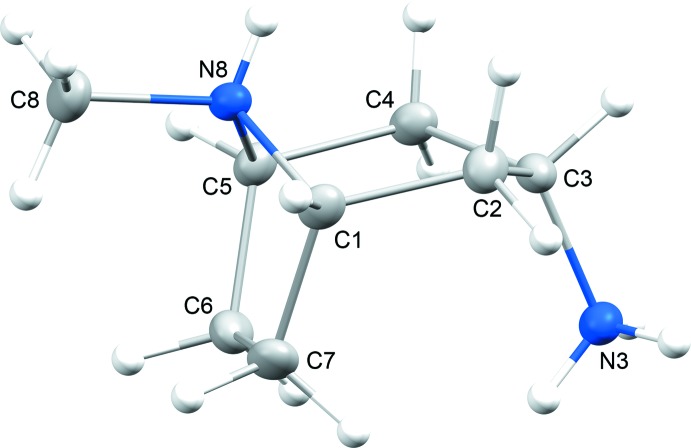
Figure 1.
The endo-3-aminotropane skeleton in the crystal structure of 1. The atomic numbering scheme of the tropane cage is given in accordance with IUPAC nomenclature (Pollini et al., 2006 ▸; Kim et al., 2016 ▸). Displacement ellipsoids are drawn at the 30% probability level. H atoms are shown as fixed-size spheres of 0.15 Å radius.
Figure 2.
The molecular structure of the novel copper(II) anionic complex, [CuCl3(NO3)(H2O)]2−, in 1. Displacement ellipsoids are drawn at the 30% probability level. H atoms are shown as fixed-size spheres of 0.15 Å radius.
Structural commentary
In the structure of 1, the bicyclic skeleton of 3-aminotropane has a boat-like conformation with the 3-amino group located in the endo position (see Scheme and Fig. 1 ▸). Only five examples of structurally characterized endo isomers of 3-aminotropane have been reported previously (Fludzinski et al., 1987 ▸; Bradley et al., 1992 ▸; Collin et al., 1995 ▸; Omae et al., 2002 ▸), all of which are N-3-substituted derivatives. The detailed description of the geometry of the endo-3-aminotropane skeleton in 1 can be found in the supporting information. The 3-aminotropane unit has two chiral centres located at the C1 (R) and C5 (S) C atoms. The packing of the 3-aminotropane molecules in the crystal generates an inversion centre establishing the chiral balance between the alternating 3-aminotropane units. The anionic moiety, [CuCl3(NO3)(H2O)]2−, in the structure of 1 (Fig. 2 ▸) is interesting because it is the first reported example of a copper(II) complex coordinated by both chloride and nitrate ligands, in addition to water. The coordination of the CuII atom by nitrate and water or ammonia ligands is well documented [see, for example, the structures of Cu(NH3)4(NO3)2 (Morosin, 1976 ▸; Chukanov et al., 2015 ▸) and Cu(NO3)2(H2O)2.5 (Garaj & Gazo, 1969 ▸)]. In addition, a limited number of isolated chloride–aqua and chlorate–aqua complexes of CuII have been reported as both neutral clusters, e.g. [Cu(H2O)2Cl2] (Matkovic et al., 1969 ▸; Bhakay-Tamhane et al., 1980 ▸) and [Cu(H2O)4(ClO3)2] (Blackburn et al., 1991 ▸), and anionic complexes, e.g. [Cu(H2O)2Cl4]2− (Begley et al., 1988 ▸) and [Cu(H2O)2Cl3]− (Wei & Willett, 1996 ▸). Therefore, the new complex anion, viz. [CuCl3(NO3)(H2O)]2−, can be considered as a valuable contribution to the aqueous coordination chemistry of copper(II). The geometry of this unusual cluster (Fig. 2 ▸) can be described as a severely distorted octahedron, with three Cu—Cl bonds [Cu1—Cl1 = 2.3019 (3), Cu1—Cl2 = 2.5856 (4) and Cu1–Cl3 = 2.2499 (3) Å], one Cu—OH2 bond [Cu1—OW1 = 2.0646 (10) Å] and two Cu—O bonds from the asymmetrically bonded NO3 ligand [Cu1—O1 = 1.9923 (9) Å and the very weak Cu1—O2 = 2.609 (1) Å]. Similar bonding of an NO3 group to a CuII centre, with two distinct bond lengths, has been reported, for example, in Cu(NO3)2(H2O)2.5 (Garaj & Gazo, 1969 ▸), anhydrous β-Cu(NO3)2 (Troyanov et al., 1995 ▸) and (NH4)3[Cu(NO3)4](NO3) (Morozov et al., 1998 ▸).
Supramolecular features
The overall integrity of the crystal structure of 1 is achieved via a complex three-dimensional network of intermolecular hydrogen bonds (Fig. 3 ▸). Three types of hydrogen bonding are observed: (i) N—H⋯O interactions between the protonated N atom, N8, and the water molecule coordinated to the CuII atom, (ii) O—H⋯Cl interactions involving the same water molecule located between two chloride ions and (iii) N—H⋯Cl interactions between the protonated amino group NH3 + and chloride ions Cl1 and Cl3 (Table 1 ▸).
Figure 3.
A network of hydrogen bonds maintains the structural integrity of 1. The bond lengths are given in Table 1 ▸.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N8—H8⋯OW1i | 0.783 (17) | 2.236 (17) | 2.9600 (15) | 154.0 (15) |
| OW1—HW1A⋯Cl1ii | 0.79 (2) | 2.33 (2) | 3.1145 (11) | 172.4 (19) |
| OW1—HW1B⋯Cl2iii | 0.79 (2) | 2.30 (2) | 3.0851 (11) | 179 (2) |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Database survey
Among the 204 structures containing the tropane core in the Cambridge Structural Database (CSD, Version 5.38, latest update May 2017; Groom et al., 2016 ▸), 11 entries contain 3-aminotropane derivatives, all of which are substituted at the 3-amino group. There are five structures in the CSD and nine in the ICSD (ICSD, 2017 ▸), which contain isolated chloro–aqua complexes of copper(II) (Matkovic et al., 1969 ▸; Bhakay-Tamhane et al., 1980 ▸; Begley et al., 1988 ▸; Wei & Willett, 1996 ▸).
Synthesis and crystallization
106.6 mg (0.5 mmol) of 3-aminotropane dihydrochloride (a mixture of the 3-exo and 3-endo isomers, Sigma–Aldrich) was dissolved in 1 ml of deionized water. 60.4 mg (0.25 mmol) of Cu(NO3)2·3H2O (reagent grade) was dissolved in another 1 ml aliquot of water. On mixing the two solutions, a transparent pale-yellow–green solution was formed. Light-green needles of 1 were grown by slow evaporation of the solution at room temperature.
Refinement
H atoms at the protonated N8 and N9 atoms and water molecule OW1 were refined freely, whereas H atoms on C atoms were refined based on a riding model. Crystal data, data collection and structure refinement details are summarized in Table 2 ▸.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | (C8H18N2)[CuCl3(NO3)(H2O)] |
| M r | 392.16 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 150 |
| a, b, c (Å) | 6.2464 (3), 13.5674 (6), 17.4584 (8) |
| β (°) | 100.128 (1) |
| V (Å3) | 1456.50 (12) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 2.06 |
| Crystal size (mm) | 0.25 × 0.20 × 0.15 |
| Data collection | |
| Diffractometer | Bruker APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Sheldrick, 2015 ▸) |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 16933, 3523, 3382 |
| R int | 0.012 |
| (sin θ/λ)max (Å−1) | 0.661 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.018, 0.049, 1.05 |
| No. of reflections | 3523 |
| No. of parameters | 197 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.43, −0.31 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989017014633/cq2021sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989017014633/cq2021Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989017014633/cq2021Isup3.mol
CCDC reference: 1571888
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors thank the X-ray Diffraction Center of Saint Petersburg State University for providing instrumental resources.
supplementary crystallographic information
Crystal data
| (C8H18N2)[CuCl3(NO3)(H2O)] | F(000) = 804 |
| Mr = 392.16 | Dx = 1.788 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 6.2464 (3) Å | Cell parameters from 9865 reflections |
| b = 13.5674 (6) Å | θ = 2.8–35.0° |
| c = 17.4584 (8) Å | µ = 2.06 mm−1 |
| β = 100.128 (1)° | T = 150 K |
| V = 1456.50 (12) Å3 | Block, green |
| Z = 4 | 0.25 × 0.20 × 0.15 mm |
Data collection
| Bruker APEX-II CCD diffractometer | 3523 independent reflections |
| Radiation source: fine-focus sealed tube | 3382 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.012 |
| φ and ω scans | θmax = 28.0°, θmin = 1.9° |
| Absorption correction: multi-scan SADABS (Sheldrick, 2015) | h = −8→8 |
| k = −17→17 | |
| 16933 measured reflections | l = −23→22 |
Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.018 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.049 | w = 1/[σ2(Fo2) + (0.0249P)2 + 0.617P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.05 | (Δ/σ)max = 0.001 |
| 3523 reflections | Δρmax = 0.43 e Å−3 |
| 197 parameters | Δρmin = −0.31 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.3006 (2) | 0.09472 (10) | 0.65317 (7) | 0.0272 (3) | |
| H1 | 0.1897 | 0.0452 | 0.6581 | 0.033* | |
| C2 | 0.2156 (2) | 0.19808 (10) | 0.66403 (8) | 0.0296 (3) | |
| H2A | 0.1662 | 0.2008 | 0.7136 | 0.036* | |
| H2B | 0.0906 | 0.2101 | 0.6236 | 0.036* | |
| C3 | 0.3811 (2) | 0.28074 (9) | 0.66170 (7) | 0.0255 (2) | |
| H3 | 0.3426 | 0.3338 | 0.6949 | 0.031* | |
| C4 | 0.6143 (2) | 0.24969 (10) | 0.69599 (7) | 0.0273 (2) | |
| H4A | 0.7142 | 0.2906 | 0.6733 | 0.033* | |
| H4B | 0.6397 | 0.2624 | 0.7515 | 0.033* | |
| C5 | 0.66665 (19) | 0.14187 (10) | 0.68295 (7) | 0.0253 (2) | |
| H5 | 0.8146 | 0.1257 | 0.7089 | 0.030* | |
| C6 | 0.6311 (2) | 0.11169 (11) | 0.59713 (8) | 0.0323 (3) | |
| H6A | 0.7268 | 0.0578 | 0.5892 | 0.039* | |
| H6B | 0.6579 | 0.1668 | 0.5647 | 0.039* | |
| C7 | 0.3908 (3) | 0.07923 (11) | 0.57794 (8) | 0.0347 (3) | |
| H7A | 0.3115 | 0.1189 | 0.5361 | 0.042* | |
| H7B | 0.3798 | 0.0105 | 0.5624 | 0.042* | |
| N8 | 0.50100 (18) | 0.07935 (8) | 0.71434 (6) | 0.0239 (2) | |
| C8 | 0.5664 (3) | −0.02653 (10) | 0.72557 (9) | 0.0375 (3) | |
| H8A | 0.4508 | −0.0632 | 0.7418 | 0.056* | |
| H8B | 0.6950 | −0.0313 | 0.7646 | 0.056* | |
| H8C | 0.5955 | −0.0530 | 0.6774 | 0.056* | |
| N3 | 0.3612 (2) | 0.32304 (9) | 0.58084 (7) | 0.0285 (2) | |
| Cu1 | 0.77174 (2) | 0.33409 (2) | 0.41227 (2) | 0.02287 (5) | |
| Cl1 | 0.45571 (5) | 0.24722 (2) | 0.41328 (2) | 0.03028 (7) | |
| Cl2 | 0.84844 (5) | 0.40007 (2) | 0.55338 (2) | 0.03060 (7) | |
| Cl3 | 0.60443 (6) | 0.45734 (2) | 0.33880 (2) | 0.03453 (8) | |
| N1 | 0.94979 (18) | 0.15675 (8) | 0.40161 (7) | 0.0286 (2) | |
| O1 | 0.94479 (15) | 0.21792 (7) | 0.45717 (5) | 0.02895 (19) | |
| O2 | 0.8758 (2) | 0.18232 (10) | 0.33464 (7) | 0.0465 (3) | |
| O3 | 1.0312 (2) | 0.07521 (9) | 0.41756 (10) | 0.0614 (4) | |
| OW1 | 1.05693 (16) | 0.38867 (8) | 0.38441 (6) | 0.02756 (19) | |
| H8 | 0.484 (3) | 0.1001 (12) | 0.7547 (10) | 0.024 (4)* | |
| H3A | 0.374 (3) | 0.2818 (15) | 0.5454 (11) | 0.040 (5)* | |
| HW1A | 1.164 (3) | 0.3573 (15) | 0.3938 (11) | 0.042 (5)* | |
| H3B | 0.234 (4) | 0.3451 (15) | 0.5664 (12) | 0.050 (6)* | |
| HW1B | 1.080 (3) | 0.4426 (17) | 0.4001 (12) | 0.046 (5)* | |
| H3C | 0.451 (4) | 0.3683 (17) | 0.5810 (12) | 0.052 (6)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0225 (6) | 0.0289 (6) | 0.0282 (6) | −0.0064 (5) | −0.0009 (5) | 0.0005 (5) |
| C2 | 0.0210 (6) | 0.0344 (7) | 0.0346 (7) | 0.0010 (5) | 0.0081 (5) | 0.0051 (5) |
| C3 | 0.0302 (6) | 0.0244 (6) | 0.0232 (5) | 0.0014 (5) | 0.0080 (5) | 0.0003 (4) |
| C4 | 0.0276 (6) | 0.0269 (6) | 0.0261 (6) | −0.0073 (5) | 0.0010 (5) | 0.0004 (5) |
| C5 | 0.0187 (5) | 0.0295 (6) | 0.0271 (6) | −0.0010 (5) | 0.0021 (4) | 0.0046 (5) |
| C6 | 0.0343 (7) | 0.0362 (7) | 0.0287 (6) | 0.0089 (6) | 0.0116 (5) | 0.0015 (5) |
| C7 | 0.0431 (8) | 0.0345 (7) | 0.0241 (6) | −0.0017 (6) | −0.0006 (5) | −0.0059 (5) |
| N8 | 0.0271 (5) | 0.0236 (5) | 0.0206 (5) | −0.0025 (4) | 0.0031 (4) | 0.0005 (4) |
| C8 | 0.0471 (8) | 0.0242 (6) | 0.0394 (7) | 0.0014 (6) | 0.0029 (6) | 0.0052 (5) |
| N3 | 0.0303 (6) | 0.0289 (6) | 0.0267 (6) | 0.0020 (5) | 0.0059 (5) | 0.0035 (4) |
| Cu1 | 0.01998 (8) | 0.02360 (8) | 0.02488 (8) | 0.00238 (5) | 0.00351 (6) | 0.00206 (5) |
| Cl1 | 0.02233 (14) | 0.03309 (16) | 0.03568 (16) | −0.00169 (11) | 0.00582 (11) | 0.00026 (12) |
| Cl2 | 0.03173 (16) | 0.03230 (16) | 0.02900 (15) | −0.00508 (12) | 0.00873 (12) | −0.00633 (12) |
| Cl3 | 0.03283 (16) | 0.02662 (15) | 0.04061 (18) | 0.00540 (12) | −0.00329 (13) | 0.00498 (13) |
| N1 | 0.0203 (5) | 0.0242 (5) | 0.0404 (6) | −0.0028 (4) | 0.0033 (4) | −0.0055 (4) |
| O1 | 0.0292 (5) | 0.0287 (5) | 0.0280 (4) | 0.0026 (4) | 0.0024 (4) | 0.0006 (4) |
| O2 | 0.0488 (7) | 0.0601 (8) | 0.0312 (5) | −0.0034 (6) | 0.0087 (5) | −0.0084 (5) |
| O3 | 0.0518 (7) | 0.0272 (6) | 0.0952 (11) | 0.0092 (5) | −0.0145 (7) | −0.0147 (6) |
| OW1 | 0.0238 (5) | 0.0249 (5) | 0.0342 (5) | 0.0009 (4) | 0.0057 (4) | 0.0006 (4) |
Geometric parameters (Å, º)
| C1—H1 | 0.9800 | C7—H7B | 0.9700 |
| C1—C2 | 1.5231 (19) | N8—C8 | 1.4970 (17) |
| C1—C7 | 1.5318 (19) | N8—H8 | 0.783 (17) |
| C1—N8 | 1.5103 (16) | C8—H8A | 0.9600 |
| C2—H2A | 0.9700 | C8—H8B | 0.9600 |
| C2—H2B | 0.9700 | C8—H8C | 0.9600 |
| C2—C3 | 1.5305 (18) | N3—H3A | 0.85 (2) |
| C3—H3 | 0.9800 | N3—H3B | 0.85 (2) |
| C3—C4 | 1.5333 (18) | N3—H3C | 0.83 (2) |
| C3—N3 | 1.5086 (16) | Cu1—Cl1 | 2.3019 (3) |
| C4—H4A | 0.9700 | Cu1—Cl2 | 2.5856 (4) |
| C4—H4B | 0.9700 | Cu1—Cl3 | 2.2499 (3) |
| C4—C5 | 1.5247 (18) | Cu1—O1 | 1.9923 (9) |
| C5—H5 | 0.9800 | Cu1—OW1 | 2.0646 (10) |
| C5—C6 | 1.5313 (18) | N1—O1 | 1.2811 (15) |
| C5—N8 | 1.5132 (16) | N1—O2 | 1.2292 (17) |
| C6—H6A | 0.9700 | N1—O3 | 1.2289 (16) |
| C6—H6B | 0.9700 | OW1—HW1A | 0.79 (2) |
| C6—C7 | 1.543 (2) | OW1—HW1B | 0.79 (2) |
| C7—H7A | 0.9700 | ||
| C2—C1—H1 | 110.7 | C6—C7—H7A | 110.7 |
| C2—C1—C7 | 114.95 (11) | C6—C7—H7B | 110.7 |
| C7—C1—H1 | 110.7 | H7A—C7—H7B | 108.8 |
| N8—C1—H1 | 110.7 | C1—N8—C5 | 101.59 (9) |
| N8—C1—C2 | 107.63 (10) | C1—N8—H8 | 110.9 (12) |
| N8—C1—C7 | 101.74 (10) | C5—N8—H8 | 109.7 (12) |
| C1—C2—H2A | 108.6 | C8—N8—C1 | 113.48 (10) |
| C1—C2—H2B | 108.6 | C8—N8—C5 | 113.39 (11) |
| C1—C2—C3 | 114.80 (10) | C8—N8—H8 | 107.7 (12) |
| H2A—C2—H2B | 107.5 | N8—C8—H8A | 109.5 |
| C3—C2—H2A | 108.6 | N8—C8—H8B | 109.5 |
| C3—C2—H2B | 108.6 | N8—C8—H8C | 109.5 |
| C2—C3—H3 | 106.6 | H8A—C8—H8B | 109.5 |
| C2—C3—C4 | 112.83 (10) | H8A—C8—H8C | 109.5 |
| C4—C3—H3 | 106.6 | H8B—C8—H8C | 109.5 |
| N3—C3—C2 | 111.03 (11) | C3—N3—H3A | 115.4 (13) |
| N3—C3—H3 | 106.6 | C3—N3—H3B | 109.3 (15) |
| N3—C3—C4 | 112.70 (10) | C3—N3—H3C | 109.5 (15) |
| C3—C4—H4A | 108.6 | H3A—N3—H3B | 102.9 (18) |
| C3—C4—H4B | 108.6 | H3A—N3—H3C | 109.8 (19) |
| H4A—C4—H4B | 107.5 | H3B—N3—H3C | 110 (2) |
| C5—C4—C3 | 114.79 (10) | Cl1—Cu1—Cl2 | 100.684 (12) |
| C5—C4—H4A | 108.6 | Cl3—Cu1—Cl1 | 94.122 (14) |
| C5—C4—H4B | 108.6 | Cl3—Cu1—Cl2 | 105.990 (13) |
| C4—C5—H5 | 110.8 | O1—Cu1—Cl1 | 89.92 (3) |
| C4—C5—C6 | 113.86 (11) | O1—Cu1—Cl2 | 84.38 (3) |
| C6—C5—H5 | 110.8 | O1—Cu1—Cl3 | 167.91 (3) |
| N8—C5—C4 | 107.79 (10) | O1—Cu1—OW1 | 86.88 (4) |
| N8—C5—H5 | 110.8 | OW1—Cu1—Cl1 | 164.17 (3) |
| N8—C5—C6 | 102.33 (10) | OW1—Cu1—Cl2 | 94.43 (3) |
| C5—C6—H6A | 110.8 | OW1—Cu1—Cl3 | 86.14 (3) |
| C5—C6—H6B | 110.8 | O2—N1—O1 | 118.83 (11) |
| C5—C6—C7 | 104.90 (11) | O3—N1—O1 | 118.42 (13) |
| H6A—C6—H6B | 108.8 | O3—N1—O2 | 122.75 (13) |
| C7—C6—H6A | 110.8 | N1—O1—Cu1 | 107.35 (8) |
| C7—C6—H6B | 110.8 | Cu1—OW1—HW1A | 119.7 (15) |
| C1—C7—C6 | 105.36 (10) | Cu1—OW1—HW1B | 111.5 (15) |
| C1—C7—H7A | 110.7 | HW1A—OW1—HW1B | 109 (2) |
| C1—C7—H7B | 110.7 | ||
| C1—C2—C3—C4 | −33.81 (15) | C6—C5—N8—C1 | −46.59 (12) |
| C1—C2—C3—N3 | 93.81 (13) | C6—C5—N8—C8 | 75.54 (13) |
| C2—C1—C7—C6 | 86.42 (13) | C7—C1—C2—C3 | −56.77 (15) |
| C2—C1—N8—C5 | −74.06 (12) | C7—C1—N8—C5 | 47.14 (12) |
| C2—C1—N8—C8 | 163.87 (11) | C7—C1—N8—C8 | −74.93 (13) |
| C2—C3—C4—C5 | 33.45 (15) | N8—C1—C2—C3 | 55.76 (14) |
| C3—C4—C5—C6 | 57.78 (14) | N8—C1—C7—C6 | −29.54 (13) |
| C3—C4—C5—N8 | −55.00 (13) | N8—C5—C6—C7 | 27.56 (13) |
| C4—C5—C6—C7 | −88.46 (13) | N3—C3—C4—C5 | −93.28 (13) |
| C4—C5—N8—C1 | 73.74 (12) | O2—N1—O1—Cu1 | 7.68 (14) |
| C4—C5—N8—C8 | −164.13 (11) | O3—N1—O1—Cu1 | −173.23 (11) |
| C5—C6—C7—C1 | 1.22 (14) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N8—H8···OW1i | 0.783 (17) | 2.236 (17) | 2.9600 (15) | 154.0 (15) |
| OW1—HW1A···Cl1ii | 0.79 (2) | 2.33 (2) | 3.1145 (11) | 172.4 (19) |
| OW1—HW1B···Cl2iii | 0.79 (2) | 2.30 (2) | 3.0851 (11) | 179 (2) |
Symmetry codes: (i) x−1/2, −y+1/2, z+1/2; (ii) x+1, y, z; (iii) −x+2, −y+1, −z+1.
Funding Statement
This work was funded by Saint-Petersburg State University grants 0.37.235.2015 and 3.37.222.2015.
References
- Begley, M. J., Hubberstey, P., Martindale, S. P., Moore, C. H. M. & Price, N. S. (1988). J. Chem. Res. (M), pp. 101–128.
- Bhakay-Tamhane, S. N., Sequeira, A. & Chidambaram, R. (1980). Acta Cryst. B36, 2925–2929.
- Blackburn, A. C., Gallucci, J. C. & Gerkin, R. E. (1991). Acta Cryst. B47, 474–479. [DOI] [PubMed]
- Bradley, G., Ward, T. J., White, J. C., Coleman, J., Taylor, A. & Rhodes, K. F. (1992). J. Med. Chem. 35, 1515–1520. [DOI] [PubMed]
- Britvin, S. N. & Lotnyk, A. (2015). J. Am. Chem. Soc. 137, 5526–5535. [DOI] [PubMed]
- Britvin, S. N. & Rumyantsev, A. M. (2017). Acta Cryst. E73, 1385–1388. [DOI] [PMC free article] [PubMed]
- Britvin, S. N., Rumyantsev, A. M., Zobnina, A. E. & Padkina, M. V. (2016). Chem. Eur. J. pp. 14227–14235. [DOI] [PubMed]
- Britvin, S. N., Rumyantsev, A. M., Zobnina, A. E. & Padkina, M. V. (2017). J. Mol. Struct. 1130, 395–399.
- Bruker (2015). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Chukanov, N. V., Britvin, S. N., Möhn, G., Pekov, I. V., Zubkova, N. V., Nestola, F., Kasatkin, A. V. & Dini, M. (2015). Mineral. Mag. 79, 613–623.
- Collin, S., Moureau, F., Quintero, M. G., Vercauteren, D. P., Evrard, G. & Durant, F. (1995). J. Chem. Soc. Perkin Trans. 2, pp. 77–84.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Fludzinski, P., Evrard, D. A., Bloomquist, W. E., Lacefield, W. B., Pfeifer, W., Jones, N. D., Deeter, J. B. & Cohen, M. L. (1987). J. Med. Chem. 30, 1535–1537. [DOI] [PubMed]
- Garaj, J. & Gazo, J. (1969). Chem. Zvesti, 23, 829–842.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- ICSD (2017). Inorganic Crystal Structure Database. FIZ-Karlsruhe, Germany, and the National Institute of Standards and Technology (NIST), USA. http://www.fiz-karlsruhe.de/ecid/Internet/en/DB/icsd/.
- Kim, N., Estrada, O., Chavez, B., Stewart, C. Jr & D’Auria, J. C. (2016). Molecules, 21. Article No. 1510. [DOI] [PMC free article] [PubMed]
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Matkovic, B., Peterson, S. W. & Willett, R. D. (1969). Croat. Chem. Acta, 41, 65–72.
- Morosin, B. (1976). Acta Cryst. B32, 1237–1240.
- Morozov, I. V., Fedorova, A. A. & Troyanov, S. I. (1998). Z. Anorg. Allg. Chem. 624, 1543–1647.
- Omae, T., Sakurai, M., Ashizawa, K. & Kajima, T. (2002). Anal. Sci. 18, 729–730. [DOI] [PubMed]
- Pollini, G. P., Benetti, S., De Risi, C. & Zanirato, V. (2006). Chem. Rev. 106, 2434–2454. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Troyanov, S. I., Morozov, I. V., Znamenkov, K. O. & Korenev, Yu. M. (1995). Z. Anorg. Allg. Chem. 621, 1261–1265.
- Wei, M. & Willett, R. D. (1996). Inorg. Chem. 35, 6381–6385. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989017014633/cq2021sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989017014633/cq2021Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989017014633/cq2021Isup3.mol
CCDC reference: 1571888
Additional supporting information: crystallographic information; 3D view; checkCIF report