Abstract
Background
The association of genes XRCC1, TP53 and MDM2 with breast cancer (BC) has never been tested in Kyrgyz population. We, therefore, aimed to identify an association of alleles and genotypes of polymorphic markers Arg399Gln of gene XRCC1, Arg72Pro of gene TP53, and T309G of gene MDM2 with the risk of BC in Kyrgyz women.
Methods
This was a case-control study of 219 women of Kyrgyz origin with morphologically verified BC (N = 117) and 102 controls, age-matched with BC cases. The mean age of subjects in this study was 52.2 ± 10.8 years. We extracted DNA from the venous blood and genotyped polymorphic markers Arg399Gln of gene XRCC1, Arg72Pro of gene TP53 and T309G of gene MDM2 using polymerase chain reaction and the method of restriction fragment polymorphism.
Results
Allele 399Gln (OR 1.57; 95% CI 1.05–2.35), Arg399Gln of gene XRCC1 heterozygous genotype (OR 2.77; 95% CI 1.60–4.80), the combination of Arg399Gln/Arg72Pro of genes XRCC1/TP53 heterozygous genotype (OR 3.98; 95% CI 1.57–10.09), Arg399Gln/T309G of genes XRCC1/MDM2 (OR 3.0; 95% CI 1.18–7.56), as well as Arg399Gln/Arg72Pro/T309G of genes XRCC1/TP53/MDM2 (OR 6.40; 95% CI 1.18–34.63) were associated with BC in Kyrgyz women.
Conclusions
This is the first study to identify the inter-loci interaction and to find molecular markers of individual risk of BC in Kyrgyz women.
Keywords: Breast cancer, XRCC1, TP53, MDM2, Kyrgyz population
Background
In Kyrgyzstan, breast cancer (BC) appears to be one of the leading cancer localizations in females, remaining the second most prevalent and the third fatal type of cancer. The advanced disease is diagnosed in 40% of new cases, hampering both treatment and cure [1]. Therefore, molecular markers of predisposition to BC may be a cornerstone strategy for early detection and primary prevention of this malignant disease.
BC is known to develop from a combined effect of environmental and genetic predictors, whose interplay will determine the individual susceptibility to the negative impact of the environment. To date, series of candidate gene are under study to test the genetic component of such predisposition. Those genes regulating cellular cycle and facilitating DNA reparation as well as inducing apoptosis may have the greatest potential for that [2, 3].
XRCC1 (X-ray repair cross-complementing group) is one of the leading proteins associated with DNA reparation and coded with XRCC1 gene of the 19th chromosome in 19q13.2 locus [4]. A polymorphic marker Arg399Gln is located in exon 10 of this gene and has been ben tested for an association with a few malignancies, including BC [5–7]. Moreover, BC is known to be linked with apoptosis. Protein p53 is a main driver of apoptosis after cellular genome injury, coded by TP53 gene, located on a short arm of the 17th chromosome [8]. This gene TP53 is known to contain polymorphic marker Arg72Pro in exon 4 and may play role in carcinogenesis. This marker codes arginine- and proline-containing p53 protein, differing in their capacity to activate transcription of TP53 target genes and promote p53-mediated apoptosis [9]. MDM2 protein controls the overall amount of p53 in the cell and performs as a natural p53 inhibitor, coded by gene MDM2, located on a long arm of the 12th chromosome in locus 12q14.3-12q15 [10]. The first intron of MDM2 gene contains mononucleotide polymorphism T309G, associated with BC in selected ethnic groups [11–13]. The association of genes XRCC1, TP53 and MDM2 with BC has never been tested in Kyrgyz population. We, therefore, aimed to identify an association of alleles and genotypes of polymorphic markers Arg399Gln of gene XRCC1, Arg72Pro of gene TP53, and T309G of gene MDM2 with the risk of BC in Kyrgyz women.
Methods
Study design and patients
This was a case-control study of 219 women of Kyrgyz origin. There were 117 cases of patients with a diagnosis of BC, verified with morphological methods and treated in the inpatient department of the National Centre of Oncology in Bishkek from 2015 until 2016. The National Centre of Oncology in Bishkek approved the use of the third party data for this study. We also enrolled 102 controls with no diagnosis of BC, age-matched with BC cases. The mean age of subjects in this study was 52.2 ± 10.8 years. Almost half of both cases and controls lived in the city (Table 1). Most cases showed infiltrating ductal carcinoma, but other histological cancer types were also present. Most tumors included in this analysis were moderately differentiated. Each patient signed informed consent prior to biological material withdrawal, whereas the study followed the basic ethical principles and Declaration of Helsinki.
Table 1.
Baseline demographic characteristics of cases and controls with histological attributes types of cancer cases
| Indicator | Cases | Controls |
|---|---|---|
| Kyrgyz ethnicity, N (%) | 117 (100) | 102 (100) |
| Age, mean ± SD | 53.8 ± 9.3 | 45.8 ± 8.7a |
| Urban residents, N (%) | 54 (46) | 62 (61)a |
| Tumor morphology | ||
| Infiltrating ductal carcinoma | 54 (46) | – |
| Lobular carcinoma | 40 (34) | – |
| Less prevalent types, including solid carcinoma, medullar carcinoma, fibrosarcoma, tubular adenocarcinoma | 23 (20) | – |
| Degree of differentiation | ||
| Highly differentiated | 5 (4) | – |
| Moderately differentiated | 103 (88) | – |
| Low differentiated | 9 (8) | – |
SD standard deviation
asignificant difference between groups using either t-test or 2*2 χ2 test where appropriate
DNA extraction and genotyping analysis
Venous blood sample (5 ml) was drawn from each subject’s cubital vein for the subsequent DNA extraction. We extracted DNA from the venous blood using a conventional technique of phenol-chlorophorm extraction with subsequent DNA precipitation with 96% ethanol [14]. We genotyped polymorphic markers Arg399Gln of gene XRCC1, Arg72Pro of gene TP53 and T309G of gene MDM2 using polymerase chain reaction (PCR) and the method of restriction fragment polymorphism (RFP).
We used the following primers for the amplification of Arg399Gln locus of gene XRCC1: direct 5′-TGCTTTCTCTGTGTCCA-3′, and reverse 5′-TCCAGCCTTTTCTGATA-3′. After PCR-products were restricted with MspI endonuclease, we identified alleles of Arg399Gln of gene XRCC1 polymorphism via electrophoresis in 3% agarose gel. DNA fragments with the length of 615, 374, and 241 base pairs corresponded to 399Gln allele, whereas the ones with the length of 374 and 241 base pairs were attributed to Arg399 allele (Fig. 1) [15].
Fig. 1.
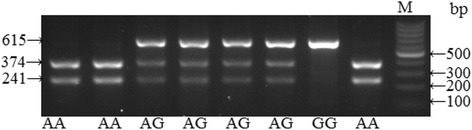
Electrophoretic separation of Arg399Gln polymorphic locus of XRCC1 gene in 3% agarose gel. М – molecular scales marker with 100 bp step. Arg/Gln genotype are fragments 615 + 374 + 241 bp wide; Gln/Gln genotype 615 bp wide; Arg/Arg genotype 374 + 241 bp wide
We used the following primers for the amplification of Arg72Pro locus of gene TP53: direct 5` TTGCCGTCCCAAGCAATGGATGA – 3`, and reverse 5` TCTGGGAAGGGACAGAAGATGAC– 3`. We used BstUI endonuclease to split PCR-products after amplification. Following restriction, we obtained DNA fragments with the length of 113 and 86 base pairs, corresponding to Arg allele, as well as fragments with the length of 199, 113, and 86 base pairs for Pro allele (Fig. 2) [16].
Fig. 2.
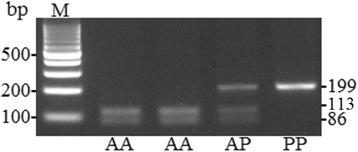
Arg72Pro polymorphic locus of Р53 gene genotypes identification in 3% agarose gel after processing with BstUI endonuclease. Pro/Pro is 199 bp long; Arg/Pro is 199 + 113 + 86 bp long; Arg/Arg is 113 + 86 bp long. М – molecular scales marker with 100 bp step
Finally, PCR for MDM2 (T309G) gene was done using the primers: direct 5`- CGGGAGTTCAGGGTAAAGGT -3`, and reverse 5` AGCAAGTCGGTGCTTACCTG-3`. In this case, MspaII endonuclease was used split PCR products. We obtained DNA fragments with the length of 233, 187, 88, 46, and 31 base pairs corresponding to G allele and 233, 88, and 31 base pairs for T allele [17] using electrophoresis (Fig. 3). Fragments 46 and 31 bp long cannot be seen because of low molecular weight.
Fig. 3.
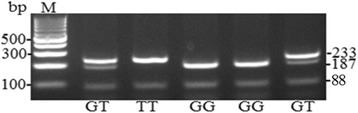
Electrophoretic separation of T309G polymorphic locus of MDM2 gene. DNA fragments with the length of 233, 187, 88, 46, and 31 bp corresponding to G allele; and 233, 88, and 31 bp for T allele. М is a marker of DNA molecular mass
Statistical analysis
First, we tested the distribution of genotypes in the studied sample to fit Hardy-Weinberg equilibrium using χ2 and the critical value of p < 0.05 to reject the null hypothesis assuming the absence of such equilibrium. In the main analysis, we primarily report the associations of genotypes and alleles with BC, which were the primary endpoints. However, we also studied the secondary points, such as the association of genotypes and alleles with histologic types, tumor size, N or M stage and degree of differentiation. We compared the frequencies of alleles and genotypes in the groups of patients and healthy subjects using χ2 with Yates’s correction. The effect measure of the association between the allele or genotype with BC was odds ratio (OR) with the corresponding 95% confidence interval, which we tested using unadjusted regression models and, therefore, report crude OR values with their corresponding 95% CI. We ran all tests in STATISTICA 8.0. (StatSoft) and GraphPad Prism 5.0.
Results
Allele and genotype distribution
Table 2 shows the distribution of Arg399Gln of gene XRCC1, Arg72Pro of gene TP53, and T309G of gene MDM2 in the groups of subjects with and without BC. We found that genotype frequency in the control sample corresponded to the expected one with Hardy-Weinberg principle with regard to all the tested markers.
Table 2.
The distribution of genotypes and alleles of Arg399Gln of gene XRCC1, Arg72Pro of gene TP53 and T309G of gene MDM2 in BC patients of Kyrgyz ethnicity compared to healthy controls
| Markers | Alleles and genotypes | Cases, n (%) | Controls, n (%) | χ2 | р | OR | CI 95% |
|---|---|---|---|---|---|---|---|
|
Arg399Gln
gene XRCC1 rs25487 |
Allele Arg399 | 144 (62) | 146 (72) | 4.46 | 0.034 | 0.64 | 0.42–0.95 |
| Allele 399Gln | 90 (38) | 58 (28) | 1.57 | 1.05–2.35 | |||
| Arg399Arg | 38 (32) | 56 (55) | 13.86 | 0.0010 | 0.39 | 0.22–0.68 | |
| Arg399Gln | 68 (58) | 34 (33) | 2.77 | 1.60–4.80 | |||
| Gln399Gln | 11 (10) | 12 (12) | 0.77 | 0.32–1.84 | |||
| HWE χ2 /р | 6.07/0.01 | 3.33/0.06 | |||||
|
Arg72Pro
gene TP53 rs1042522 |
Allele Arg72 | 164 (70) | 142 (70) | 0.011 | 0.913 | 1.02 | 0.68–1.54 |
| Allele 72Pro | 70 (30) | 62 (30) | 0.98 | 0.65–1.47 | |||
| Arg72Arg | 57(49) | 53 (52) | 1.80 | 0.41 | 0.88 | 0.52–1.49 | |
| Arg72Pro | 50 (43) | 36 (35) | 1.37 | 0.79–2.36 | |||
| Pro 72Pro | 10 (8) | 13 (13) | 0.64 | 0.27–1.53 | |||
| HWE χ2 /р | 0.04/0.83 | 2.80/0.09 | |||||
|
T309G
gene MDM2 rs2279744 |
Allele T309 | 120 (49) | 111(46) | 0.31 | 0.58 | 0.88 | 0.60–1.28 |
| Allele 309G | 114 (51) | 93 (54) | 1.13 | 0.77–1.65 | |||
| G309G | 29 (24) | 28 (27) | 0.500 | 0.77 | 0.87 | 0.47–1.59 | |
| T309G | 62 (53) | 55 (54) | 0.96 | 0.56–1.64 | |||
| T309 T | 26 (22) | 19 (18) | 1.24 | 0.64–2.42 | |||
| HWE χ2 /р | 0.42/0.51 | 0.77/0.38 | |||||
OR odds ratio, CI confidence interval, HWE Hardy-Weinberg equilibrium
Heterozygous genotype Arg399Gln and 399Gln allele of gene XRCC1 were associated with BC when compared to controls. This genotype Arg399Gln resulted in almost 3-fold increase of BC probability (OR 2.77 (95% CI 1.60–4.80)), whereas the 399Gln allele was a marker of BC risk (OR 1.57 (95% CI 1.05–2.35)). With regard to Arg399 allele, we found its protective effect for BC (OR 0.64 (95% CI 0.42–0.95)) (Table 1).
We failed to find similar associations of polymorphic loci Arg72Pro of gene TP53 and T309G of gene MDM2, and the prevalence of these genotypes and alleles in the group of BC patients did not differ from healthy controls (р > 0.05). Therefore, taken separately, polymorphic loci Arg72Pro of gene TP53 and T309G of gene MDM2 were not associated with BC in the population of Kyrgyz women.
Because BC phenotype results from a combination of genotypes and alleles of various genes, rather than one gene only, making BC a genetically heterogeneous disease, we performed the analysis of intergenic (XRCC1/TP53/MDM2) interactions in order to identify the most meaningful gene-gene combinations, which can result in BC in Kyrgyz women.
Gene-gene interaction between XRCC1 and TP53 polymorphisms
When we tested gene-gene interactions of polymorphic loci of Arg399Gln and Arg72Pro, we found statistically significant 2-loci combinations of genotypes XRCC1/TP53 (Arg399Gln/Arg72Pro), which results in a significant increase of BC probability in Kyrgyz women. Thus, Arg72Pro heterozygous variant of gene TP53 combined with Arg399Gln heterozygous genotype of gene XRCC1 was associated with almost 4-fold increase in BC probability in the studied sample (OR 3.98 (95% CI 1.57–10.09)) (Table 3).
Table 3.
The distribution of combinations of Arg399Gln of gene XRCC1 and Arg72Pro of gene TP53 polymorphic markers in Kyrgyz women with BC and controls
| XRCC1/TP53 genotypes | Cases, n (%) | Controls, n (%) | OR (95% CI) | χ2/р |
|---|---|---|---|---|
| Arg399Arg/Arg72Arg | 19 (16) | 27 (26) | Ref. | |
| Arg399Arg/Arg72Pro | 18 (15) | 21 (21) | 1.22 (0.52–2.88) | 0.20/0.65 |
| Arg399Arg/Pro72Pro | 1 (1) | 8 (8) | 0.18 (0.02–1.54) | 2.97/0.085 |
| Arg399Gln/Arg72Arg | 32 (27) | 20 (20) | 2.27 (1.01–5.11) | 3.23/0.07 |
| Arg399Gln/Arg72Pro | 28 (24) | 10 (9) | 3.98 (1.57–10.09) | 7.58/0.0059 |
| Arg399Gln/Pro72Pro | 8 (7) | 4 (4) | 2.84 (0.75–10.81) | 2.46/0.116 |
| Gln399Gln/Arg72Arg | 6 (5) | 6 (6) | 1.42 (0.40–5.09) | 0.29/0.588 |
| Gln399Gln/Arg72Pro | 4 (3) | 5 (5) | 1.14 (0.27–4.80) | 0.03/0.861 |
| Gln399Gln/Pro72Pro | 1 (1) | 1 (1) | 1.42 (0.08–24.18) | 0.06/0.807 |
OR odds ratio, CI confidence interval
Gene-gene interaction between XRCC1 and MDM2 polymorphisms
Compared to controls (18%), BC women had statistically significant greater prevalence of Arg399Gln/T309G (38%) genotype (Table 3). The combination of T309G of gene MDM2 heterozygous genotype with Arg399Gln of gene XRCC1 heterozygous genotype was associated with a 3-fold increase of BC probability (OR 3.0 (95% CI 1.18–7.56)) (Table 4), which makes this combination of haplotypes a genetic risk factor of BC in Kyrgyz women.
Table 4.
The distribution of combinations of Arg399Gln of gene XRCC1 and T309G of gene MDM2 polymorphic markers in Kyrgyz women with BC and controls
| XRCC1/MDM2 genotypes | Cases, n (%) | Controls, n (%) | OR (95% CI) | χ2/р |
|---|---|---|---|---|
| Arg399Arg/G309G | 12 (10) | 17 (17) | Reference | |
| Arg399Arg/T309G | 18 (15) | 31 (30) | 0.82 (0.32–2.11) | 0.17/0.684 |
| Arg399Arg/T309T | 8 (7) | 8 (8) | 1.42 (0.42–4.84) | 0.31/0.578 |
| Arg399Gln/ G309G | 14 (12) | 8 (8) | 2.48 (0.79–7.76) | 2.48/0.115 |
| Arg399Gln/ T309G | 38 (32) | 18 (18) | 3.00 (1.18–7.56) | 4.49/0.034 |
| Arg399Gln/ T309T | 16 (14) | 8 (8) | 2.83 (0.92–8.73) | 3.37/0.066 |
| Gln399Gln/ G309G | 3 (3) | 3 (3) | 1.42 (0.24–8.26) | 0.15/0.697 |
| Gln399Gln/ T309G | 6 (5) | 6 (5) | 1.42 (0.37–5.48) | 0.26/0.613 |
| Gln399Gln/ T309T | 2 (2) | 3 (3) | 1.42 (0.17–11.51) | 0.11/0.74 |
OR odds ratio, CI confidence interval
Gene-gene interaction between TP53 and MDM2 polymorphisms
When comparing genotype distribution of Arg72Pro polymorphic loci of TP53 gene and T309G of MDM2 gene, no statistical differences between BC and control groups were identified (Table 5). Of note, Pro72Pro/G309G genotype combination was only found in control group, but not in BC group.
Table 5.
The distribution of combinations of Arg72Pro of gene TP53 and T309G of gene MDM2 polymorphic markers in Kyrgyz women with BC and controls
| TP53/MDM2 genotypes | Cases, n (%) | Controls, n (%) | OR (95% CI) | χ2/р |
|---|---|---|---|---|
| Arg72Arg/G309G | 8 (7) | 11(11) | Reference | |
| Arg72Arg/G309T | 36 (31) | 31 (30) | 1.60 (0.57–4.47) | 0.80/0.37 |
| Arg72Arg/T309T | 13 (11) | 11 (11) | 1.63 (0.48–5.47) | 0.62/0.43 |
| Arg72Pro/ G309G | 21 (18) | 11 (11) | 2.63 (0.82–8.43) | 2.69/0.10 |
| Arg72Pro/ G309T | 18 (15) | 19 (19) | 1.30 (0.43–3.98) | 0.22/0.64 |
| Arg72Pro/ T309T | 11 (9) | 6 (6) | 2.52 (0.65–9.71) | 1.84/0.18 |
| Pro72Pro/ G309G | 0 (0) | 6 (6) | 0.10 (0.005–2.11) | 3.72/0.05 |
| Pro72Pro / G309T | 8 (7) | 5 (5) | 2.20 (0.52–9.30) | 1.17/0.28 |
| Pro72Pro / T309T | 2 (2) | 2(2) | 1.38 (0.16–11.94) | 0.08/0.77 |
OR odds ratio, CI confidence interval
Gene-gene interaction between XRCC1, TP53 and MDM2 polymorphisms
We tested 27 different combinations (XRCC1, TP53, MDM2) and found that the interaction of Arg399Gln/Arg72Pro/T309G of genes XRCC1/TP53/MDM2 heterozygous genotypes was associated with BC (χ2 = 5.04; р = 0.025) and increased its likelihood with an OR of 6.40 (95% CI 1.18–34.63). Additionally, we tested whether the selected polymorphic loci were associated with cancer histologic type, tumor size, N or M stage or even degree of differentiation. We found no association of these markers with any of these attributes of cancer in our patients.
Discussion
In this case-control study, we have identified a number of genetic associations with BC in Kyrgyz women. These exposures included allele 399Gln (OR 1.57; p = 0.034), Arg399Gln of gene XRCC1 heterozygous genotype (OR 2.77; p = 0.001), as well as the combination of Arg399Gln/Arg72Pro of genes XRCC1/TP53 heterozygous genotype (OR 3.98; p = 0.0059), Arg399Gln/T309G of genes XRCC1/MDM2 (OR 3.0; p = 0.034), and Arg399Gln/Arg72Pro/T309G of genes XRCC1/TP53/MDM2 (OR 6.40; p = 0.025).
Arg399Gln polymorphism of gene XRCC1, Arg72Pro of TP53 gene and T309G of MDM2 gene, coding enzyme synthesis with a variety of reparative and apoptosis activity, may shift the balance of reparation and injury both ways. Our findings confirm the association of heterozygous genotypes of XRCC1/TP53/MDM2 genes with the elevated risk of BC. The strongest association of heterozygous carriage of XRCC1/TP53/MDM2 genes with the disease calls for further analysis and more studies.
Our results highlight the role of Arg399Gln polymorphic locus of gene XRCC1 in BC origin in Kyrgyz women. Our findings confirm the earlier data from Chinese [4], Polish [6], American [5], and Egyptian [7] populations, which altogether showed that 399Gln allele and Arg399Gln genotype carriers had a greater BC risk compared to Arg399 allele and Arg399Arg genotype. The association of 399Gln allele and Arg399Gln genotype with BC may sound plausible, because published reports have shown that XRCC1 protein, having glutamine in its 399th position, has a smaller potency to repair damaged DNA, and that results in the accumulation of genetically unstable cells and may promote malignancy [2].
Arg72Pro of gene TP53 polymorphic marker is located in the high proline concentration domain [8], and this domain is responsible for apoptotic functioning of р53 protein. After mutation, р53 is no more capable of activating transcription of pro-apoptotic genes, resulting in disrupted apoptosis, which altogether leads to a greater number of cells of various DNA alterations with subsequent cellular proliferation. Arginine-containing variant of р53 (Аrg72) protein is more potent to induce apoptosis that it’s proline-containing variant (Pro72) [10].
Literature data on the association of Arg72Pro of gene TP53 polymorphic versions with BC are not homogenous and are somewhat contrasting. Some studies have demonstrated that 72Pro of gene TP53 allele has a significant association with BC [18, 19]. Other studies, in contrast, have confirmed Arg72 allele may be more relevant [20, 21] to promote BC. Moreover, in newer studies and even meta-analyses, the associations of Arg72Pro of gene TP53 marker with BC was not statistically significant [22, 23]. Such contradicting findings are likely explained by the ethnic differences in the molecular and genetic mechanisms of BC initiation and progression.
The concentration and activity of р53 cancer suppressing protein in a cell is controlled by MDM2 protein, which inactivates and accelerates degrading of р53 [10] cancer suppressing protein, thus, hampering DNA reparation and, therefore, promotes, carcinogenesis.
With regard to T309G of gene MDM2 polymorphic marker, we failed to demonstrate its statistically significant association with BC in a group of BC females compared to controls. However, Arg72Pro heterozygous variant in combination with Arg399Gln of gene XRCC1 heterozygous genotype was associated with a 4-fold increase in the probability of BC (OR 3.98 (95% CI 1.57–10.09)).
A combination of risk genotypes of a number of candidate genes, producing additive effect, may result in simultaneous DNA reparation disorder and apoptosis, leaving some potential for a new phenotype formation [11].
The latest meta-analysis [24] of 19 publications with a total of 9788 BC cases and 11,195 controls has shown that T309G of gene MDM2 polymorphic locus is associated with BC both in Asian and Caucasian populations. The magnitude of such association was most pronounced when T309G of gene MDM2 heterozygous genotype was present with the greatest effect in Asians (OR 1.21 (95% CI 1.03–1.41)); p = 0.02), compared to Caucasians (OR 1.09 (95% CI 1.00–1.18); p = 0.04). Of note, GG genotype of polymorphic locus of MDM2 gene is considered a risk factor for BC in Taiwanese women (OR 3.05 (95% CI 1.04–8.95)); p = 0.04) [12].
Therefore, combined with the findings of other cohorts, our data confirmed the individual susceptibility to BC resulting from polymorphic markers of DNA repair genes (XRCC1), apoptosis genes (TP53), as well as of apoptosis inhibition genes (MDM2).
Conclusions
Our study has enabled to identify the inter-loci interaction and to find molecular markers of individual risk of BC in Kyrgyz women. The list of potential risk factors for BC in Kyrgyz females may include 399Gln allele and Arg399Gln of gene XRCC1 heterozygous genotype, as well a combination of heterozygous genotypes of Arg399Gln/Arg72Pro of genes XRCC1/TP53, Arg399Gln/T309G of genes XRCC1/MDM2 and Arg399Gln/Arg72Pro/T309G of genes XRCC1/TP53/MDM2.
Identification of risk combinations of genes XRCC1, TP53 and MDM2 with BC may increase the study validity and determine groups of women with high individual risk of BC, which may help in the prevention, early detection and effective cure of this condition.
Acknowledgements
We thank all patients for their participation in this study. We would also like to express our gratitude to the National Centre of Oncology (Bishkek, Kyrgyz Republic) doctor, Kiyal Makieva for her help in collecting biosamples and clinical data.
Funding
This study was supported by the Ministry of Education and Science of Kyrgyz Republic (state register #0007164 of March 13, 2015).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BC
breast cancer
- CI
confidence interval
- MDM2
mouse double minute 2
- OR
odds ratio
- XRCC1
X-ray repair cross-complementing group
- ТР53
tumor protein
Authors’ contributions
JI, NA, and AA conceived and designed the experiments. JI and ET carried out the molecular genetic studies, and performed the statistical analysis. JI and DV wrote the paper. JI and AA undertook data collection, interpretation of results and edited the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The Institutional Review Board of the Scientific and Research Institute of Molecular Biology and Medicine in Bishkek (Protocol #1, January 14, 2015) approved the study protocol. Each patient signed an informed consent to participate.
Consent for publication
Each participant signed an informed consent, including an agreement to anonymously report results.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jainagul Isakova, Phone: +996 (312) 66 38 56, Email: jainagul@mail.ru.
Elnura Talaibekova, Email: elya-1209@mail.ru.
Nazira Aldasheva, Email: med@krsu.edu.kg.
Denis Vinnikov, Email: Denis.Vinnikov@kaznu.kz.
Almaz Aldashev, Email: Aldashev@gmail.com.
References
- 1.Igisinov N, Kokteubaeva N, Kudaibergenova I. Epidemiology of breast cancer in females of reproductive age in Kyrgyzstan. Asian Pac J Cancer Prev. 2005;6:36–39. [PubMed] [Google Scholar]
- 2.Duell EJ, Millikan RC, Pittman GS, Winkel S, Lunn RM, Chiu-Kit JT, et al. Polymorphisms in the DNA repair gene XRCC1 and breast cancer. Cancer Epidemiol. Prev. Biomarkers. 2001;10:217–222. [PubMed] [Google Scholar]
- 3.Lacroix M, Toillon R-A, Leclercq G. p53 and breast cancer, an update. Endocr Relat Cancer. 2006;13:293–325. doi: 10.1677/erc.1.01172. [DOI] [PubMed] [Google Scholar]
- 4.Luo H, Li Z, Qing Y, Zhang S-H, Peng Y, Li Q, et al. Single nucleotide polymorphisms of DNA base-excision repair genes (APE1, OGG1 and XRCC1) associated with breast cancer risk in a Chinese population. Asian Pac J Cancer Prev. 2014;15:1133–1140. doi: 10.7314/APJCP.2014.15.3.1133. [DOI] [PubMed] [Google Scholar]
- 5.Bu T, Liu L, Sun Y, Zhao L, Peng Y, Zhou S, et al. XRCC1 Arg399Gln polymorphism confers risk of breast cancer in American population: a meta-analysis of 10846 cases and 11723 controls. PLoS One. 2014;9:e86086. doi: 10.1371/journal.pone.0086086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romanowicz H, Smolarz B, Baszczyński J, Zadrożny M, Kulig A. Genetics polymorphism in DNA repair genes by base excision repair pathway (XRCC1) and homologous recombination (XRCC2 and RAD51) and the risk of breast carcinoma in the polish population. Pol J Pathol. 2010;61:206–212. [PubMed] [Google Scholar]
- 7.Ramadan RA, Desouky LM, Elnaggar MA, Moaaz M, Elsherif AM. Association of DNA repair genes XRCC1 (Arg399Gln),(Arg194Trp) and XRCC3 (Thr241Met) polymorphisms with the risk of breast cancer: a case–control study in Egypt. Genet Test Mol Biomark. 2014;18:754–760. doi: 10.1089/gtmb.2014.0191. [DOI] [PubMed] [Google Scholar]
- 8.Matlashewski GJ, Tuck S, Pim D, Lamb P, Schneider J, Crawford LV. Primary structure polymorphism at amino acid residue 72 of human p53. Mol Cell Biol. 1987;7:961–963. doi: 10.1128/MCB.7.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumont P, Leu J-J, Della Pietra AC, George DL, Murphy M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet. 2003;33:357–365. doi: 10.1038/ng1093. [DOI] [PubMed] [Google Scholar]
- 10.Manfredi JJ. The Mdm2–p53 relationship evolves: Mdm2 swings both ways as an oncogene and a tumor suppressor. Genes Dev. 2010;24:1580–1589. doi: 10.1101/gad.1941710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leu J-D, Wang C-Y, Tsai H-Y, Lin I, Chen R-C, Lee Y-J. Involvement of p53 R72P polymorphism in the association of MDM2-SNP309 with breast cancer. Oncol Rep. 2011;25:1755–1763. doi: 10.3892/or.2011.1254. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y-F, Leu J-D, Chen S-M, Lin I-F, Lee Y-J. Results based on 124 cases of breast cancer and 97 controls from Taiwan suggest that the single nucleotide polymorphism (SNP309) in the MDM2 gene promoter is associated with earlier onset and increased risk of breast cancer. BMC Cancer. 2009;9:13. doi: 10.1186/1471-2407-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lum SS, Chua HW, Li H, Li W-F, Rao N, Wei J, et al. MDM2 SNP309 G allele increases risk but the T allele is associated with earlier onset age of sporadic breast cancers in the Chinese population. Carcinogenesis. 2008;29:754–761. doi: 10.1093/carcin/bgn024. [DOI] [PubMed] [Google Scholar]
- 14.Mathew CC. The isolation of high molecular weight eukaryotic DNA. Methods Mol Biol. 1984;2:31–34. doi: 10.1385/0-89603-064-4:31. [DOI] [PubMed] [Google Scholar]
- 15.Demokan S, Demir D, Suoglu Y, Kiyak E, Akar U, Dalay N. Polymorphisms of the XRCC1 DNA repair gene in head and neck cancer. Pathol Oncol Res. 2005;11:22–25. doi: 10.1007/BF03032401. [DOI] [PubMed] [Google Scholar]
- 16.Onrat ST, Ellidokuz E, Kupelioglu A, Durhan E. Frequency of TP53 codon72 polymorphism in cases with colon cancer. Turk. J. Cancer. 2009;39:005–010. [Google Scholar]
- 17.Joshi AM, Budhathoki S, Ohnaka K, Mibu R, Tanaka M, Kakeji Y, et al. TP53 R72P and MDM2 SNP309 polymorphisms and colorectal cancer risk: the Fukuoka colorectal cancer study. Jpn J Clin Oncol. 2011;41:232–238. doi: 10.1093/jjco/hyq200. [DOI] [PubMed] [Google Scholar]
- 18.Proestling K, Hebar A, Pruckner N, Marton E, Vinatzer U, Schreiber M. The pro allele of the p53 codon 72 polymorphism is associated with decreased intratumoral expression of BAX and p21, and increased breast cancer risk. PLoS One. 2012;7:e47325. doi: 10.1371/journal.pone.0047325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X-E, Hamajima N, Katsuda N, Matsuo K, Hirose K, Mizutani M, et al. Association ofp53 codon arg72pro andp73 G4C14-to-A4T14 at exon 2 genetic polymorphisms with the risk of japanese breast cancer. Breast Cancer. 2003;10:307–311. doi: 10.1007/BF02967650. [DOI] [PubMed] [Google Scholar]
- 20.Ohayon T, Gershoni-Baruch R, Papa MZ, Menachem TD, Barzilai SE, Friedman E. The R72P P53 mutation is associated with familial breast cancer in Jewish women. Br J Cancer. 2005;92:1144–1148. doi: 10.1038/sj.bjc.6602451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sjalander A, Birgander R, Hallmans G, Cajander S, Lenner P, Athlin L, et al. p53 polymorphisms and haplotypes in breast cancer. Carcinogenesis. 1996;17:1313–1316. doi: 10.1093/carcin/17.6.1313. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y, Yang J, Liu Z, Zhang P, Yang Z, Wang Y, et al. No significant association between the TP53 codon 72 polymorphism and breast cancer risk: a meta-analysis of 21 studies involving 24,063 subjects. Breast Cancer Res Treat. 2011;125:201–205. doi: 10.1007/s10549-010-0920-1. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Wang M, Wu D, Wang M, Tong N, Tian Y, et al. P53 codon 72 polymorphism contributes to breast cancer risk: a meta-analysis based on 39 case–control studies. Breast Cancer Res Treat. 2010;120:509–517. doi: 10.1007/s10549-009-0480-4. [DOI] [PubMed] [Google Scholar]
- 24.Gao J, Kang A-J, Lin S, Dai Z-J, Zhang S-Q, Liu D, et al. Association between MDM2 rs 2279744 polymorphism and breast cancer susceptibility: a meta-analysis based on 9,788 cases and 11,195 controls. Ther Clin Risk Manag. 2014;10:269–277. doi: 10.2147/TCRM.S60680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


