Abstract
Objective
The aim of this study was to investigate the regeneration process of the nasal mucosa after a surgically created mucosal defect in the rabbit nasal septum, and to evaluate the effects of different interventions.
Methods
A 7 mm-diameter circular mucosal defect was made in the septum of forty New Zealand white rabbits. The rabbits were divided into four groups (ten rabbits in each group) according to the type of intervention; no treatment (control), silastic sheet (SS), hyaluronic acid (HA), and silastic sheet and hyaluronic acid (SS + HA) group. The diameter of the defect, mucosal thickness, epithelial thickness, and ciliated cell count were evaluated every week for five weeks.
Results
The average diameter of the defect in the control group were 5.1, 3.65, 1.2, 0.75, and 0.05 mm at postoperative 1, 2, 3, 4, and 5 weeks. In the SS group, the diameter decreased to 4.35, 2.1, 0.35, 0.15, and 0 mm at postoperative 1, 2, 3, 4, and 5 weeks, respectively, in which the mean diameter of the postoperative week 2 was significantly smaller compared to control (3.65 mm vs. 2.1 mm, P = 0.039). For the HA group and SS + HA group, the diameter of the defect did not show a significant difference from the control group during the five weeks. The mucosal thickness, epithelial thickness, and ciliated cell count of the regenerated mucosa were not significantly different among the groups.
Conclusion
The regeneration process of the nasal septal mucosa was identified using a novel rabbit model. Mucosal regeneration can be accelerated by applying silastic sheets.
Keywords: Nasal septum, Nasal mucosa, Mucous membrane, Hyaluronic acid, Rabbits
Introduction
Mucosa of the nasal septum can be injured in a number of rhinologic surgeries. With the recent advances in endoscopic skull base surgery and the use of the nasoseptal flap for reconstruction, the number and extent of iatrogenic injury to the septal mucosahas increased. However, a comprehensive understanding of the process involved in the healing of the septal mucosa is lacking and the factors that influence its regeneration have yet to be clarified.
The process of wound repair has been extensively studied in tissues such as the gingiva and the skin.1 Although the healing process of the sinonasal cavity has been reported using different animal models,2, 3, 4, 5, 6 there has been no studies evaluating wound healing after injury to the nasal septal mucosa.
Silastic sheet is commonly used after nasal surgery to promote mucosal healing. It is thought to accelerate the mucosal healing process by moistening and humidifying the wound.7, 8 Although there have been a number of reports on clinical outcome with a silastic sheet after septoplasty, the effect of this material on septal mucosa has not been clearly documented. Moreover, there are relatively few studies describing such effects at a histological level. Hyaluronic acid has shown to bring about a shorter epithelialization time in patients undergoing sinus surgery,9 but its effect on promoting healing of the septal mucosa has not been proven.
The objective of this study was to investigate the regeneration process of the nasal mucosa in a surgically created defect in the rabbit nasal septum, and to evaluate the effects of different interventions that can promote mucosal wound healing.
Materials
Septal mucosa wound healing model in rabbit
The experiment was performed in the Seoul National University Hospital Biomedical Research Institute in Seoul, Korea. Approval from Institutional Animal Care and Use Committee in Seoul National University was obtained before initiation of the study (No. 13–0085). The study was conducted in accordance with the principles of the Helsinki Declaration on the use of laboratory animals. Forty adult New Zealand white rabbits with a mean body weight of 3,700 g (3000 to 4200 g) were used as experimental animals. They were randomly assigned to one of four groups; control group, silastic sheet group (SS), hyaluronic acid group (HA), and both silastic sheet and hyaluronic acid group (SS + HA).
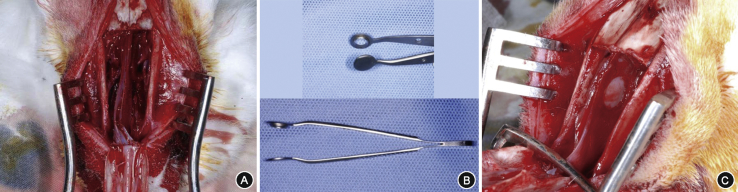
Prior to surgery, each animal received an intramuscular injections consisting of Zoletil® 10 mg/kg (tiletamine 125 mg/ml, zolazepam 125 mg/ml) and Rumpun® (2% xylazine) at a ratio of 1:2 for general anesthesia. Areas over the nose (snout) were shaved and draped with povidone-iodine solution. After infiltration with a mixture of 1% lidocaine and 1:100,000 epinephrine, a 5 cm-long midline nasal dorsum skin incision was made through the periosteum with a #10 blade. The laterally-based periosteal flaps were raised bilaterally, fully exposing the nasal bone. Nasal osteotomy was performed in a rectangular shape using a 4 mm straight osteotome gaining access to the nasal septum. The septum was fully exposed by separating the upper lateral cartilages from the septum and performing bilateral partial inferior turbinectomy (Fig. 1A). A circular mucosal incision was made with a diameter of 7 mm on the concave side of the septum,10 at an intersection point 3 mm below the septal roof and 3 mm caudal to the end of the middle turbinate, using a circular punch that we had manufactured (Fig. 1B). The mucosa of the circular lesion was elevated and stripped off together with the perichondrium using a blunt duckbill elevator, exposing the underlying septal cartilage (Fig. 1C). Bleeding control was achieved with bosmin-soaked gauze. Sixteen rabbits had the left septum used as the intervention side, while the right side was used in 24 rabbits.
Fig. 1.
Rabbit model of septal mucosal injury. Nasal bone is elevated in a rectangular shape, and the septum is exposed after separation of upper lateral cartilages and bilateral partial inferior turbinectomy (A). A custom made circular punch was used to make the mucosal defect (B), and a 7 mm-diameter circular full thickness mucosal has been removed from the left nasal septum (C).
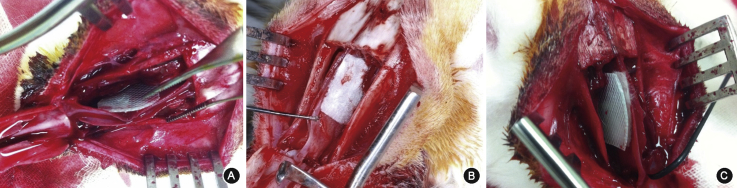
For the control group, the elevated nasal bone was put back in place after the mucosal removal, followed by skin closure. For the SS group, a 1 cmX 1 cm square piece of a silastic sheet (Medtronic Xomed, Jacksonville, FL) was placed on the septum covering the defect, and anchored to the septum superiorly using a 5-0 Vicryl (Ethicon, Inc., Somerville, NJ) (Fig. 2A). For the HA group, a piece of MeroGel® (Ethicon, Inc., Somerville, NJ) was cut to a 1 cmX 1 cm-sized square piece and placed on the injured septum (Fig. 2B), then it was hydrated with 1 ml of sterile normal saline according to the manufacturer's instructions. Lastly, for the SS + HA group, the silastic sheet was applied over the HA (Fig. 2C). Intramuscular procaine penicillin (40,000 IU) was administered on a prophylactic basis for three consecutive days postoperatively, and fentanyl (0.02 mg/kg) was injected subcutaneously for pain control. All surgical procedures were performed by one investigator.
Fig. 2.
Representative photos of the experimental groups. Silastic sheet (A), hyaluronic acid (B), silastic sheet and hyaluronic acid (C) were applied to septal defect.
In order to evaluate the process of mucosal healing, two rabbits in each group were sacrificed using a phenobarbital overdose after 1, 2, 3, 4, and 5 weeks. The entire cartilaginous septum was harvested for evaluation.
Analysis of mucosal regeneration
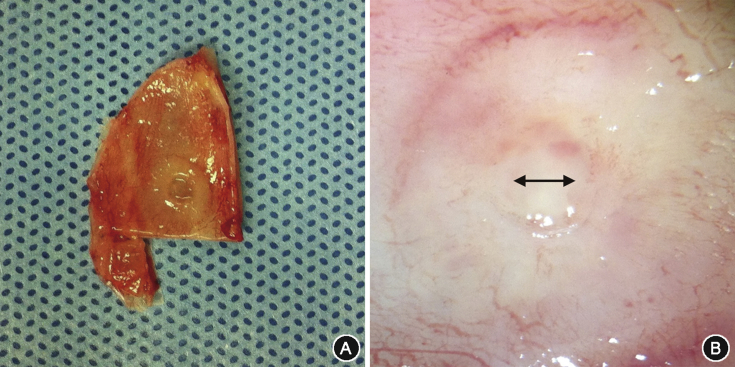
Harvested septum was washed three times in phosphate buffered saline (Sigma–Aldrich, St. Louis, MO) and digital photographs of the wound site were obtained (Fig. 3A). Remaining blood clot, foreign body, and granulation tissue formed on the septum were all removed with gentle suction and irrigation. The diameter of the remaining defect was analyzed by measuring the shortest distance in the gross specimen with the micrometer on the light microscope (Eclipse E600 Nikon, Tokyo, Japan) (Fig. 3B). A diameter of less than 0.01 mm was considered complete healing.
Fig. 3.
Measurement of the defect size. The whole cartilaginous septum was harvested after sacrifice (A), and the size of the defect was measured under light microscopy (double-sided arrow) (B).
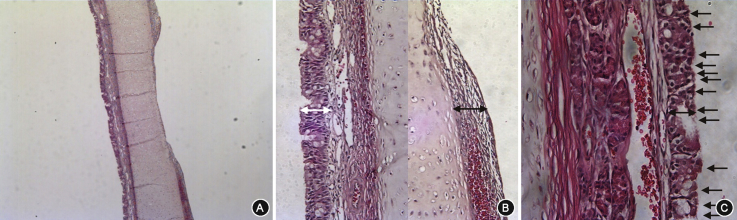
The specimen was then fixated for 24 h in 10% buffered formaldehyde and embedded in paraffin in a coronal orientation. Four serial 4 μm thick sections of the nasal septum incorporating the defect site were taken from each block, and stained with hematoxylin and eosin. Slides were evaluated under light microscopy, and histological analysis of the regenerated septal mucosa was performed (Fig. 4A). Mucosal thickness was measured at the transition zone from the defect to normal mucosa from the cartilage (Fig. 4B). Mucosal thickness index (MTI) was calculated as the ratio of the amount of regenerated mucosa to the intact contralateral side. The epithelial thickness index (ETI) of the regenerated mucosa was also calculated in the same manner from the thickness measured from the basement membrane (×400 magnification) (Fig. 4C).6 The ciliated cell index (CCI) was calculated as the ratio of the newly formed ciliated cell count on the regenerated side to the ciliated cell count on the contralateral side, in the same section as internal control (×400 magnification) (Fig. 4C).6 Measurement was performed by two investigators blinded to the objectives of the study and the average value was used.
Fig. 4.
Histological analysis of the regenerated septal mucosa. Harvested septum was observed under light microscopy in coronal section (A) (hematoxylin and eosin stain; original magnification ×40). Mucosal thickness was measured vertically at the transition zone from the defect to normal mucosa (black double-sided arrow) at ×200 magnification, which was compared with the contralateral mucosa (white double-sided arrow) (B). Epithelial thickness (double-sided arrow) and ciliated epithelial cells (arrows) were evaluated under ×400 magnification (C).
Data analysis
Data analysis was performed using IBM SPSS Statistics version 20 (IBM Corporation, Armonk, NY, USA) and were expressed as mean ± SEM. Results in different groups were compared using the nonparametric Kruskall–Wallis test followed by post-hoc testing using Dunn's multiple comparison of means. P values < 0.05 were considered statistically significant.
Results
Defect size of the septal mucosa
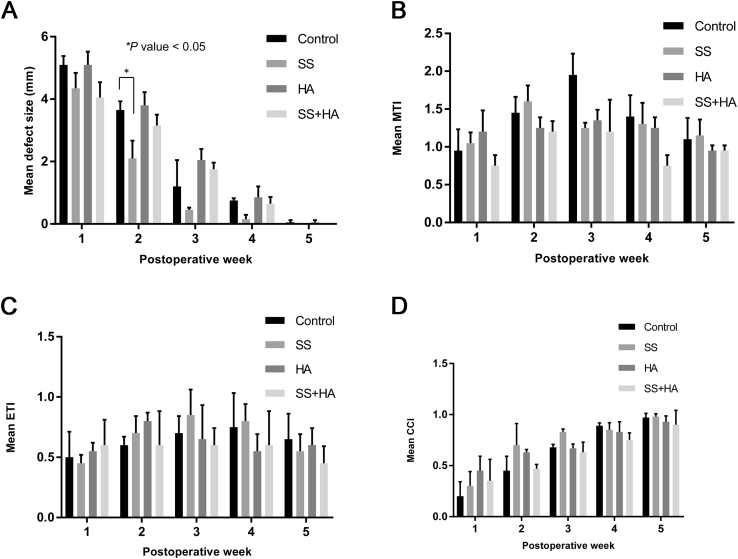
No animals were lost during the perioperative period, and specimens were obtained from all 40 rabbits. Merogel® applied in rabbit septum completely resolved between postoperative 1 and 3 weeks, regardless of the presence of silastic sheet. The mean size of the defect of the septal mucosa according to the time course are depicted in Fig. 5A. For the control group, the average wound size were 5.1, 3.65, 1.2, 0.75, and 0.05 mm at postoperative 1, 2, 3, 4, and 5 weeks, respectively. The defect completely healed after postoperative 5 weeks (0.1 mm and 0 mm). The average defect size was 4.35, 2.1, 0.35, 0.15, and 0 mm in the SS group, 5.1, 3.8, 2.05, 0.85, and 0.05 mm in the HA group and 4.05, 3.15, 1.75, 0.65, and 0 mm in the SS + HA group, at postoperative 1, 2, 3, 4, and 5 weeks, respectively. Complete mucosal healing was observed in all groups after 5 weeks. Complete wound healing occurred one week earlier in the SS group compared to control. The mean size of the defect was also significantly smaller in the SS group at postoperative 2 weeks (3.65 mm vs. 2.1 mm, P = 0.039). The size of the defect in the other groups did not show significant difference from the control group in all periods.
Fig. 5.
Size of the septal mucosal defect and histologic character of the regenerated mucosa according to type of intervention. The mean defect size of the septal mucosa (A), ∗p value < 0.05, mucosal thickness index (MTI) (B), epithelial thickness index (ETI) (C), and ciliated cell index (CCI) (D) according to the postoperative time point.
Mucosal thickness and epithelial thickness
Loss of normal respiratory ciliated epithelium and subepithelium were observed in the early postoperative period. The thickness of the whole mucosa and epithelium increased with time. The average contralateral mucosal thickness and epithelial thickness, used for control, were 167 μm and 29 μm, respectively. The average MTI were 0.95, 1.45, 1.95, 1.4, and 1.1 in the control group, 1.05, 1.6, 1.25, 1.3, and 1.15 in the SS group, 1.2, 1.25, 1.35, 1.25, and 0.95 in the HA group, and 0.75, 1.2, 1.2, 0.75, and 0.95 in the SS + HA group at postoperative 1, 2, 3, 4, and 5 weeks, respectively (Fig. 5B). There was no significant difference in the MTI among the intervention groups.
The average ETI of the four groups were 0.5, 0.45, 0.55, and 0.6, at postoperative 1 week, 0.6, 0.7, 0.8, and 0.6 at postoperative 2 weeks, 0.7, 0.85, 0.65, and 0.6 at postoperative 3 weeks, 0.75, 0.8, 0.55, and 0.6 at postoperative 4 weeks, and 0.65, 0.55, 0.6, and 0.45 at postoperative 5 weeks, respectively (Fig. 5C). The epithelial thickness in the regenerated mucosa did not fully recover after five weeks in all groups (ETI < 1.0). There was no significant difference in the ETI among the intervention groups in any time points.
Ciliated cell index
The ciliated epithelial cell count of the regenerated mucosa continued to increase with time and the CCI reached almost 1 at postoperative week 5 in all groups. The average CCI was 0.2, 0.45, 0.68, 0.89, and 0.97 in the control group, 0.3, 0.7, 0.83, 0.85, and 0.98 in the SS group, 0.45, 0.63, 0.67, 0.83, and 0.93 mm in the HA group and 0.35, 0.47, 0.63, 0.75, and 0.9 in the SS + HA group, at postoperative 1, 2, 3, 4, and 5 weeks, respectively (Fig. 5D). The silastic sheet and hyaluronic acid resulted in increased CCI in the early postoperative period (postoperative week 1–2) compared to control, but the difference failed to reach statistical significance. The CCI in the late postoperative period were similar regardless of the intervention method.
Discussion
This study is an in vivo experiment that attempted to explore the healing process of the septal mucosa in a rabbit model. Although healing of the nasal mucosal has been investigated, it has not been studied extensively as in other tissues such as the skin and gingiva.1 The sinonasal cavity is lined by pseudostratified columnar ciliated epithelium carrying out important functions such as air conditioning, mucociliary clearance, and major roles in both innate and acquired immunity.3,11, 12, 13 Khalmuratova et al14 reported on the wound healing mechanism of the nasal mucosa in a rat model. Edematous subepithelium and infiltration of neutrophils were noticed at postoperative day 2, followed by infiltration of monocytes and granulation tissue at day 5. Increased subepithelial fibrosis and epithelial thickness were noted at day 14, and goblet cells and ciliated cells began to regenerate from day 14, which restored to near normal at day 28. Weber et al15, 16 reported four phases of wound healing after sinus surgery in humans. Blood clots covered the whole wound in the first phase (postoperative day 7–12), followed by formation of granulation tissue in the second phase (postoperative 2–4 weeks). After the third edematous phase, macroscopic normalization phase appeared at postoperative 12–18 weeks.
Complete regeneration usually occurs after injury in the sinonasal epithelium, which is induced by migration and replication of epithelial cells.17 It has been reported that when the basement membrane is intact after injury, the respiratory epithelium restores to its normal height after several days, but when the basement membrane is damaged, regeneration takes several weeks with the formation of squamous or transitional epithelium.10 The subepithelial glands are not always regenerated, and the lamina propria may be substituted by dense connective tissue.4 A circular wound created in rabbit sinus mucosa decreased in size concentrically with a speed of 20 μm/hour at the initial stage and decreased to 4–5 μm/hour after 7 days.10 However, the regeneration time of a septal mucosal defect, has not been reported in the literature so far.
Manipulation of the rabbit septum has been described by Alkan et al18 who used an open technique with a transcolumellar incision and by Wong et al19 who used a midline nasal osteotomy. However it was not feasible to consistently create a circular defect on the septum through these techniques. Therefore we developed a novel approach via a rectangular shaped osteotomy, temporarily opening the nasal bones which allowed a better surgical view and a larger operation space. The bone flap was replaced in its original position after the intervention minimizing physiologic changes and morbidity related to the surgery. The custom made circular punch helped achieve a reliable and constant mucosal defect and we did not encounter any mortality associated with the procedure. We think that this novel rabbit model for assessing the healing process of the septum can be used in future research to elucidate in depth the involved healing mechanisms and to compare the efficacy of different treatments aimed at enhancing the regeneration process.
Previous reports on ways of improving the mucosal healing process include the use of systemic steroids,5, 6 topical steroids,20, 21 carboxymethylcellulose,22 and chitosan,23 in different animal models. However, none has shown a consistent superiority.2
Silastic sheets or splints are made of polymeric, biologically inert silicone. They have been widely used after septal surgery for various purposes such as septal support, mucosal healing, avoiding adhesion and hematoma collection.24 The role of silastic sheets in obtaining these effects has been debated. While Campbell et al25 reported that a silastic splint was effective in preventing intranasal adhesion, Cook et al26 reported no clear advantage. In a randomized double-blinded controlled trial by Jung et al,27 a silastic splint revealed a significant positive effective in preventing postoperative adhesion after septoplasty. In another experimental study, the silastic sheet did not improve ciliary recovery, but increased collagen deposition.2
Our study has shown that placement of a silastic sheet resulted in a faster time to achieving gross total mucosal healing compared to control. The silastic sheet group showed significantly enhanced healing in the first two weeks. However, the microscopic thickness of the regenerated mucosa or the epithelium did not show significant difference with control. The results from our study can in part elucidate the mechanism for the prevention of postoperative adhesion, and serves as supporting evidence in the clinical use of silastic sheets when there is injury to the septal mucosa. However, the overall healing process will be determined by multiple factors including host factors, size of the defect and duration of placement, to name a few. The mechanism behind the accelerated mucosal regeneration in the silastic group is not clear and we think that there may be a number of factors involved. Moisture can be retained underneath the silastic sheet avoiding desiccation and promoting mucosal wound healing.7, 8 Additionally, the silastic sheet can allow a constant surface tension on the mucosa beneath, making a favorable environment for mucosal growth from the edges of the wound.
Hyaluronan is a major component of the extracellular matrix that plays an important role in tissue repair.28, 29 Hyaluronic acid and its derivatives have been used in the sinuses to reduce scarring and possibly promote wound healing.24, 29 The composition of hyaluronan can affect wound healing differently.29 MeroGel® is an esterified, fibrillar form of hyaluronic acid. It is one of the customized hyaluronic acid nasal dressing materials that can be used after nasal surgery. Clinical studies have shown better healing with MeroGel® in the sinuses after endoscopic sinus surgery.24, 30 In a randomized, controlled, multicenter clinical trial, decreased synechia formation and improved sinonasal healing were noted in the MeroGel® group compared to a standard non-resorbable nasal dressing after endoscopic sinus surgery.24 Soldati et al31 reported that it reduces crust formation during the first week of wound healing. The effect of MeroGel® on nasal septum has not been evaluated so far. In our study the artificially created septal mucosal defect healed completely regardless of intervention after five weeks. Placement of a hyaluronic acid sheet did not enhance mucosal regeneration. When a silastic sheet was combined with hyaluronic acid to counteract early dislodging of the hyaluronic acid, healing seemed faster compared to hyaluronic acid alone but it did not reach statistical significance. Discrepancies with the beneficial clinical findings may be in part because the hyaluronic acid was not mechanically removed (i.e. nasal irrigation). A prolonged interaction with the wound might have caused a negative impact in the latter stage of wound healing due to a foreign body reaction. However, histologic signs of foreign body reaction or inflammatory cell infiltration were not definite.
One of the limitations of our study is that only the morphology of the regenerated mucosa was evaluated. Morphologic regeneration of the injured mucosa does not guarantee a full functional recovery.32 Furthermore, scanning electron microscopy may still show signs of insufficient recovery in the form of prominent ciliary loss and disorientation.32 Supportive studies are needed incorporating functional recovery of the regenerated mucosa.
Conclusions
We have proposed a novel rabbit nasal septum model for effectively evaluating nasal mucosa healing. Silastic sheet applied to a septal mucosal defect can significantly enhance mucosal regeneration in the early injury period ultimately leading to faster healing.
Acknowledgements
This research was supported by the SNUH Research Fund (30-2012-0180).
Edited by Yu-Xin Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Zahm J.M., Chevillard M., Puchelle E. Wound repair of human surface respiratory epithelium. Am J Respir Cell Mol Biol. 1991;5:242–248. doi: 10.1165/ajrcmb/5.3.242. [DOI] [PubMed] [Google Scholar]
- 2.Jain R., Kim R., Waldvogel-Thurlow S., Hwang P., Cornish J., Douglas R. The effects of topical agents on paranasal sinus mucosa healing: a rabbit study. Int Forum Allergy Rhinol. 2015;5:310–317. doi: 10.1002/alr.21470. [DOI] [PubMed] [Google Scholar]
- 3.DePoortere D., Chen B., Cohen N.A. Polyhydrated ionogen with MgBr2 accelerates in vitro respiratory epithelial healing. Am J Rhinol Allergy. 2013;27:333–337. doi: 10.2500/ajra.2013.25.3910. [DOI] [PubMed] [Google Scholar]
- 4.Benninger M.S., Sebek B.A., Levine H.L. Mucosal regeneration of the maxillary sinus after surgery. Otolaryngol Head Neck Surg. 1989;101:33–37. doi: 10.1177/019459988910100107. [DOI] [PubMed] [Google Scholar]
- 5.Beule A.G., Scharf C., Biebler K.E. Effects of topically applied dexamethasone on mucosal wound healing using a drug-releasing stent. Laryngoscope. 2008;118:2073–2077. doi: 10.1097/MLG.0b013e3181820896. [DOI] [PubMed] [Google Scholar]
- 6.Khalmuratova R., Kim D.W., Jeon S.Y. Effect of dexamethasone on wound healing of the septal mucosa in the rat. Am J Rhinol Allergy. 2011;25:112–116. doi: 10.2500/ajra.2011.25.3595. [DOI] [PubMed] [Google Scholar]
- 7.Bhargava D., Raman R. Modifications for silastic sheet use in nasal surgery. Laryngoscope. 2001;111:1113. doi: 10.1097/00005537-200106000-00034. [DOI] [PubMed] [Google Scholar]
- 8.Lee J.Y., Lee S.W. Preventing lateral synechia formation after endoscopic sinus surgery with a silastic sheet. Arch Otolaryngol Head Neck Surg. 2007;133:776–779. doi: 10.1001/archotol.133.8.776. [DOI] [PubMed] [Google Scholar]
- 9.Xu G., Chen H.X., Wen W.P., Shi J.B., Li Y. Clinical evaluation of local application of Merogel after endoscopic sinus surgery. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2003;38:95–97. [PubMed] [Google Scholar]
- 10.Cöloğlu H., Uysal A., Koçer U., Kankaya Y., Oruç M., Uysal S. Rhinoplasty model in rabbit. Plast Reconstr Surg. 2006;117:1851–1859. doi: 10.1097/01.prs.0000221875.24467.2d. [DOI] [PubMed] [Google Scholar]
- 11.Antunes M.B., Gudis D.A., Cohen N.A. Epithelium, cilia, and mucus: their importance in chronic rhinosinusitis. Immunol Allergy Clin North Am. 2009;29:631–643. doi: 10.1016/j.iac.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Watelet J.B., Bachert C., Gevaert P., Van Cauwenberge P. Wound healing of the nasal and paranasal mucosa: a review. Am J Rhinol. 2002;16:77–84. [PubMed] [Google Scholar]
- 13.Schleimer R.P., Kato A., Kern R., Kuperman D., Avila P.C. Epithelium: at the interface of innate and adaptive immune responses. J Allergy ClinImmunol. 2007;120:1279–1284. doi: 10.1016/j.jaci.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khalmuratova R., Jeon S.Y., Kim D.W. Wound healing of nasal mucosa in a rat. Am J Rhinol Allergy. 2009;23:e33–e37. doi: 10.2500/ajra.2009.23.3390. [DOI] [PubMed] [Google Scholar]
- 15.Weber R., Keerl R., Jaspersen D., Huppmann A., Schick B., Draf W. Computer-assisted documentation and analysis of wound healing of the nasal and oesophageal mucosa. J Laryngol Otol. 1996;110:1017–1021. doi: 10.1017/s0022215100135650. [DOI] [PubMed] [Google Scholar]
- 16.Weber R., Keerl R. Healing in the nasal mucosa. J Wound Care. 1998;7:101–102. doi: 10.12968/jowc.1998.7.2.101. [DOI] [PubMed] [Google Scholar]
- 17.Zahm J.M., Kaplan H., Hérard A.L. Cell migration and proliferation during the in vitro wound repair of the respiratory epithelium. Cell Motil Cytoskeleton. 1997;37:33–43. doi: 10.1002/(SICI)1097-0169(1997)37:1<33::AID-CM4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 18.Alkan S., Dadaş B., Celik D., Coskun B.U., Yilmaz F., Başak T. The efficacy of N-2-butyl cyanoacrylate in the fixation of nasal septum to the anterior nasal spine in rabbits: experimental study. Eur Arch Otorhinolaryngol. 2007;264:1425–1430. doi: 10.1007/s00405-007-0407-9. [DOI] [PubMed] [Google Scholar]
- 19.Wong K.K., Filatov S., Kibblewhite D.J. Septoplasty retards midfacial growth in a rabbit model. Laryngoscope. 2010;120:450–453. doi: 10.1002/lary.20769. [DOI] [PubMed] [Google Scholar]
- 20.Rowe-Jones J.M., Medcalf M., Durham S.R., Richards D.H., Mackay I.S. Functional endoscopic sinus surgery: 5 year follow up and results of a prospective, randomised, stratified, double-blind, placebo controlled study of postoperative fluticasone propionate aqueous nasal spray. Rhinology. 2005;43:2–10. [PubMed] [Google Scholar]
- 21.Jorissen M., Bachert C. Effect of corticosteroids on wound healing after endoscopic sinus surgery. Rhinology. 2009;47:280–286. doi: 10.4193/Rhin08.227. [DOI] [PubMed] [Google Scholar]
- 22.Kastl K.G., Betz C.S., Siedek V., Leunig A. Effect of carboxymethylcellulose nasal packing on wound healing after functional endoscopic sinus surgery. Am J Rhinol Allergy. 2009;23:80–84. doi: 10.2500/ajra.2009.23.3267. [DOI] [PubMed] [Google Scholar]
- 23.Valentine R., Athanasiadis T., Moratti S., Hanton L., Robinson S., Wormald P.J. The efficacy of a novel chitosan gel on hemostasis and wound healing after endoscopic sinus surgery. Am J Rhinol Allergy. 2010;24:70–75. doi: 10.2500/ajra.2010.24.3422. [DOI] [PubMed] [Google Scholar]
- 24.Berlucchi M., Castelnuovo P., Vincenzi A., Morra B., Pasquini E. Endoscopic outcomes of resorbable nasal packing after functional endoscopic sinus surgery: a multicenter prospective randomized controlled study. Eur Arch Otorhinolaryngol. 2009;266:839–845. doi: 10.1007/s00405-008-0841-3. [DOI] [PubMed] [Google Scholar]
- 25.Campbell J.B., Watson M.G., Shenoi P.M. The role of intranasal splints in the prevention of post-operative nasal adhesions. J Laryngol Otol. 1987;101:1140–1143. doi: 10.1017/s0022215100103391. [DOI] [PubMed] [Google Scholar]
- 26.Cook J.A., Murrant N.J., Evans K.L., Lavelle R.J. Intranasal splints and their effects on intranasal adhesions and septal stability. Clin Otolaryngol Allied Sci. 1992;17:24–27. doi: 10.1111/j.1365-2273.1992.tb00982.x. [DOI] [PubMed] [Google Scholar]
- 27.Jung Y.G., Hong J.W., Eun Y.G., Kim M.G. Objective usefulness of thin silasticseptal splints after septal surgery. Am J Rhinol Allergy. 2011;25:182–185. doi: 10.2500/ajra.2011.25.3584. [DOI] [PubMed] [Google Scholar]
- 28.Toole B.P. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001;12:79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 29.Proctor M., Proctor K., Shu X.Z., McGill L.D., Prestwich G.D., Orlandi R.R. Composition of hyaluronan affects wound healing in the rabbit maxillary sinus. Am J Rhinol. 2006;20:206–211. [PubMed] [Google Scholar]
- 30.Franklin J.H., Wright E.D. Randomized, controlled, study of absorbable nasal packing on outcomes of surgical treatment of rhinosinusitis with polyposis. Am J Rhinol. 2007;21:214–217. doi: 10.2500/ajr.2007.21.3011. [DOI] [PubMed] [Google Scholar]
- 31.Soldati D., Rahm F., Pasche P. Mucosal wound healing after nasal surgery. A controlled clinical trial on the efficacy of hyaluronic acid containing cream. Drugs ExpClin Res. 1999;25:253–261. [PubMed] [Google Scholar]
- 32.Kim Y.M., Lee C.H., Won T.B. Functional recovery of rabbit maxillary sinus mucosa in two different experimental injury models. Laryngoscope. 2008;118:541–545. doi: 10.1097/MLG.0b013e31815bf2f3. [DOI] [PubMed] [Google Scholar]