Abstract
Objective
Amphotericin B (AMB), a potent antifungal agent, has been employed as topical and systemic therapy for sinonasal fungal infections. A novel formulation of nanodisc (ND) containing super aggregated AMB (ND-AMB) for the treatment of fungal infections has been recently developed to provide greater protection from AMB toxicity than current, clinically approved lipid-based formulations. The objective of the current study was to evaluate the safety and potency of ND-AMB for sinonasal delivery using an in vitro model.
Methods
Human sinonasal tissue was harvested during endoscopic sinus surgery and grown at air–liquid interface until well-differentiated. Cultures were exposed to ND-AMB vs AMB and changes in K+ permeability and resistance were measured and recorded via Ussing chamber assay. Ciliary beat frequency (CBF) was analyzed in parallel as well as cytotoxic assay. Potency was assessed using real-time PCR measurement of the Aspergillus fumigatus 18S rRNA.
Results
Ussing chamber studies revealed K+ currents that increased rapidly within 30 s of adding AMB (10 μg/mL) to the apical side, indicating apical membranes had become permeable to K+ ions. In contrast, negligible induction of K+ current was obtained following addition of ND-AMB [AMB = (107.7 ± 15.9) μA/cm2 AMB vs ND-AMB = (2.3 ± 0.7) μA/cm2 ND-AMB; P = 0.005]. ND-AMB also protected nasal epithelial cells from cytotoxicity of AMB (P < 0.05). There was no difference in ciliary beat frequency between the two groups (P = 0.96). The expression of A. fumigatus 18S rRNA with exposure of lower dose of ND-AMB was significantly lower compared to that with AMB (P < 0.05).
Conclusions
Data from the present study suggests ND-AMB protects human nasal epithelia membranes from AMB toxicity by protecting against apical cell K+ permeability while maintaining uncompromised antifungal property compared to AMB. ND-AMB could provide a novel topical therapy for sinonasal fungal diseases.
Keywords: Chronic rhinosinusitis, Amphotericin, Drug delivery, Transepithelial ion transport, Sinusitis, Sinus epithelium, Mucociliary clearance, Ciliary beat frequency
Introduction
Since fungi are present throughout the environment, human exposure is inevitable and normal respiration routinely deposits fungal elements within the nose and paranasal sinuses.1 In most instances, the presence of fungal elements in the nose has no consequences. However, fungi can contribute to the pathogenesis of rhinosinusitis (fungal rhinosinusitis) in tissue-invasive or noninvasive conditions. Regional variation in incidence has been reported, with the southern part of United States particularly endemic.2 Fungal rhinosinusitis (FRS) has been categorized primarily based on whether the fungus invades local tissues or not, a characteristic intimately associated with the status of the host's immune system. Patients who are immunocompromised are highly susceptible to invasive fungal sinusitis (IFS), and despite prophylactic treatment, mortality due to fungal infections remains high. With the use of newer and more potent chemotherapeutic agents and regimens, more patients are now susceptible to fatal invasive fungal sinusitis.3
Amphotericin B (AMB) is a potent antifungal agent and topical delivery of AMB may have significant advantages over systemic intake (either intravenous or oral) including the avoidance of systemic and infusion-related toxicities. However, AMB affects the integrity of apical cell membranes in human nasal epithelial cells and can be toxic at higher doses.4, 5 Oda et al6, 7, 8 have developed a novel formulation of NanoDisk (ND) containing super aggregated AMB for the treatment of fungal infections. NanoDisk amphotericin B (ND-AMB) provided greater protection from AMB toxicity than current clinically approved lipid-based formulations of AMB in pulmonary tissue and highly efficacious treatment for invasive candidiasis in a mouse model. This drug also has important applications in topical or inhaled therapy for fungal-related respiratory diseases. The objective of the present study is to determine whether a novel nano-particle delivery platform for AMB, ND-AMB, is less disruptive to human nasal epithelium than existing formulations of AMB and maintains similar antifungal property. We hypothesized that AMB would cause minimal damage to the nasal epithelium with similar potency when formulated within the ND.
Methods
Amphotericin
Amphotericin (AMB; Sigma-Aldrich, St. Louis, MO) was prepared according to manufacturer's instructions. ND-AMB was provided as a gift from the Oda laboratory and preparation of ND-AMB has been described previously by Michael Oda.8
Primary cell culture
Institutional Review Board and Institutional Animal Care and Use Committee approval were obtained prior to initiating these studies. Human septonasal epithelial cells (HSNE) were harvested, grown on Costar 6.5-mm diameter permeable filter supports (Corning Life Sciences, Lowell, MA), and submerged in culture medium as previously described.9, 10, 11, 12 Media was removed from the monolayers on day 4 after the epithelium reached confluence, and cells fed via the basal chamber. Differentiation and ciliogenesis occurred in all cultures within 10–14 days. Cultures were used for experiments when well-differentiated with widespread ciliogenesis with transepithelial resistances (Rt) > 100 Ω cm2.
Ussing chamber analysis
Transwell inserts (Costar) were mounted in Ussing chambers to investigate pharmacologic manipulation of vectorial ion transport as previously described.13, 14, 15 Transepithelial current measurements were performed with Easy Mount Ussing chambers (Physiologic Instruments, San Diego, CA) with an apical-to-basolateral directed gradient for K+.6, 16 High K+ buffer in the apical reservoir was (in mmol/L): 120 KCl, 20 NaHCO3, 5 KHCO3, 1.2 NaH2PO4, 5.6 glucose, 2.5 CaCl2, and 1.2 MgSO4. The basolateral reservoir buffer was (in mmol/L): 120 NaCl, 20 NaHCO3, 5 KHCO3, 1.2 NaH2PO4, 5.6 glucose, 2.5 CaCl2, and 1.2 MgSO4. All experiments were conducted at 37 °C and solutions were continuously gassed with 95% air, 5% CO2 resulting in pH 7.4. Trans-epithelial voltage was clamped to 0 mV using a standard four electrode voltage clamp and gradient-driven K+ current was recorded at 5 Hz by an analog-to-digital board (DATAQ Instruments, Inc. Akron, OH) connected to a personal computer. Positive currents were defined as cation movement from apical to basal reservoir. AMB or ND-AMB was introduced to the apical reservoir of the Ussing chamber and transepithelial K+ was allowed to reach equilibrium.
Lactate dehydrogenase (LDH) assay
To determine the cytotoxicity of cells after exposure to AMB or ND-AMB (18 h), culture medium was tested for the presence of released lactate dehydrogenase (LDH). A 96-well LDH Cytotoxicity Assay Kit (Cayman Chemical Company, Ann Arbor, MI) was used, with the following modifications: on the day of the assay, 100 μL of culture medium was removed and added to 100 μL of Reaction Solution. Absorbance at 490 nm was obtained with Epx Precision Microplate Reader (Molecular Devices, Sunnyvale, CA). We measured the LDH levels 30 min after exposure. Triton X-100 (10%) was used as a positive control (maximum release of LDH) and cell medium (without phenol) was used as a negative control (spontaneous release of LDH). % Cytotoxicity of test sample was calculated based on below formula:
Ciliary beat frequency
Images were visualized using a 20× objective on an inverted scope (Fisher Scientific, Pittsburgh, PA). Data was captured using a Model A602f-2 Basler area scan high-speed monochromatic digital video camera (Basler AG, Ahrensburg, Germany) at a sampling rate of 100 frames per second and a resolution of 640 × 480 pixels. Images were analyzed using the Sisson-Ammons Video Analysis (SAVA) system version 2.16 and virtual instrumentation software for CBF monitoring. All recordings were made at 200× magnification. Experiments were all performed at ambient temperature (23 °C). A baseline recording of CBF was conducted for each cell monolayer prior to apical administration of test solution [AMB (75 μg/ml) vs ND-AMB (75 μg/ml)] and compared to the corresponding control using vehicle alone. Whole field analysis was performed with each point measured representing one cilia and analysis was normalized to fold-change over baseline.
Preparation of Aspergillus fumigatus conidia and exposure to AMB or ND-AMB
A. fumigatus isolate 13073 (ATCC, Manassas, Virginia, United States) was used in all experiments. The frozen stocks were thawed at room temperature prior to the experiments and used within 24 h. After being washed in sterile saline and counted under a microscope using a hemocytometer, conidium suspension of 1 × 104 colony forming unit (CFU)/ml was inoculated into potato dextrose medium, followed by incubation at 37 °C and shaking at 200 rpm for 16 h. The cultures were then treated with the antifungal drugs at different doses for 4 h, and the same amount of DMSO and PBS were added to the no-drug control. The final drug concentrations in the mycelium cultures were 10 μg/ml and 50 μg/ml for AMB and 10 μg/ml and 50 μg/ml for ND-AMB. Total RNA was extracted from 0.1 ml of mycelium cultures using a MasterPure yeast RNA purification kit (Epicentre Biotechnologies, Madison, WI), which includes a DNase treatment step to eliminate genomic DNA.17 Total RNA was also extracted from serial 1:10 dilutions of live A. fumigatus conidia (101–109) and DNase treated to form a standard curve. A. fumigatus burden was analyzed with real-time PCR measurement of the A. fumigatus 18S rRNA (GenBank accession number AB008401) and quantified using a standard curve of A. fumigatus conidia.17, 18
Statistical analysis
Statistical analyses were conducted using Excel 2010 and GraphPad Prism 6.0 software (La Jolla, Ca) with significance set at P < 0.05. Statistical evaluation utilized unpaired Student t tests for electrophysiology data and analysis of variance followed by Tukey–Kramer multiple comparison test if necessary for LDH, CBF and RNA assay. Data is expressed +/− standard error of the mean.
Results
AMB vs ND-AMB induced trans-epithelial K+ currents in vitro
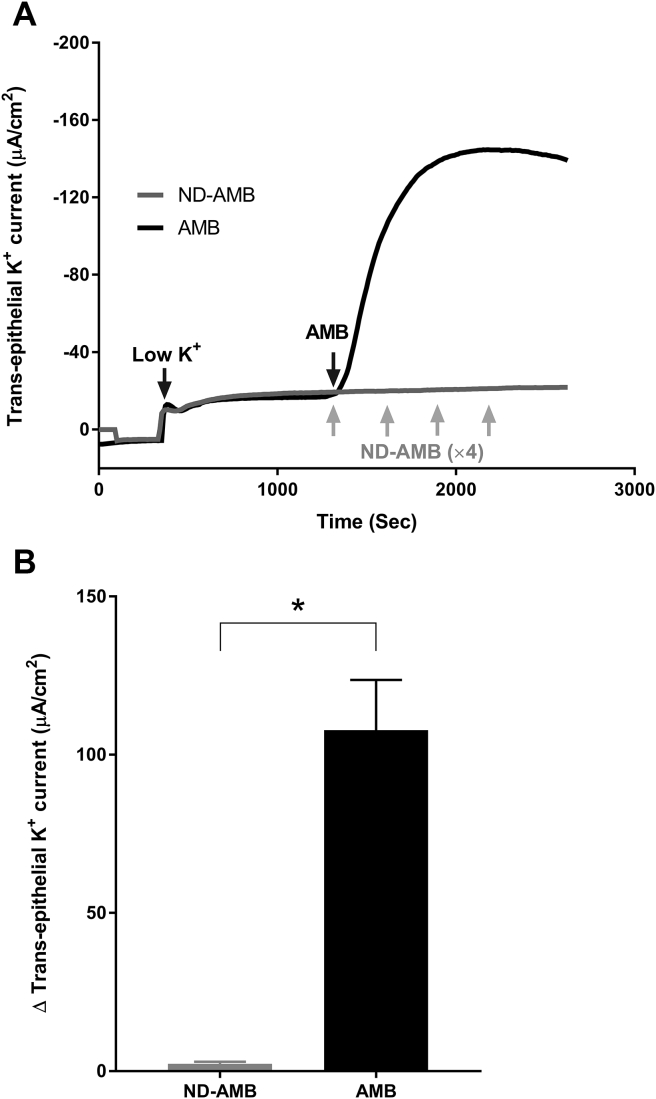
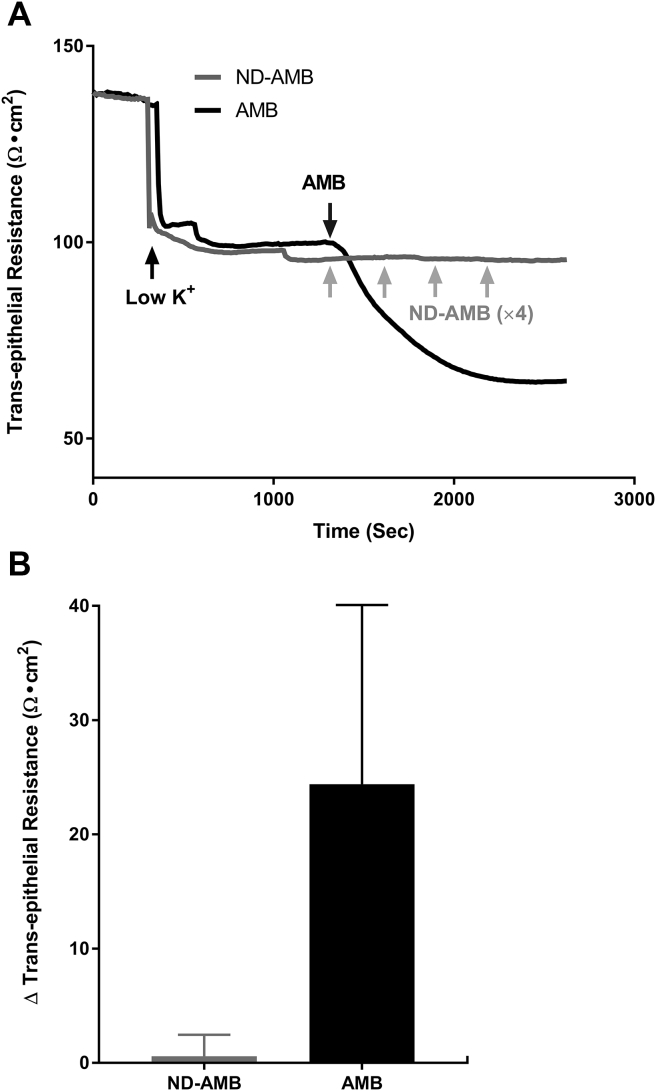
Ussing chamber tracings were used to evaluate the AMB vs ND-AMB induced trans-epithelial K+ currents across the HSNE (Fig. 1A). Once the AMB (10 μg/ml) was exposed to the apical side, K+ currents increased immediately, demonstrating the apical membrane of HNSE had become permeable to K+ ions. In contrast, adding increasing concentrations of ND-AMB (10 μg/ml × 4 every 5 min = total 40 μg/ml) resulted in negligible inductions of K+ current in HNSE. Fig. 1B represents the summary of AMB vs ND-AMB induced trans-epithelial K+ current changes (n = 4, per condition). Statistically significant difference in K+ current changes was observed between the two groups [AMB = (107.7 ± 15.9) μA/cm2 AMB (n = 4) vs ND-AMB = (2.3 ± 0.7) μA/cm2 ND-AMB (n = 4); P = 0.005]. Resistance was also traced with induction of AMB vs ND-AMB. With exposing AMB (10 μg/ml) to apical side, transepithelial resistance decreased immediately, representing the K+ currents across the HSNE (Fig. 2A). In contrast, negligible change in transepithelial resistance was noticed after exposing increasing concentrations of ND-AMB (10 μg/ml × 4 every 5 min). Fig. 2B represents the summary of AMB vs ND-AMB induced trans-epithelial resistance changes (n = 4, per condition). ΔR (changes in transepithelial resistance, Ω cm2) following application of AMB was significantly higher than those cells exposed to ND-AMB [ΔR with AMB (n = 4) = (24.4 ± 15.7) Ω cm2; ΔR with AMB (n = 4) = (0.6 ± 1.9) Ω cm2], although this did not achieve statistical significance.
Fig. 1.
Reduced trans-epithelial K+ currents after exposure to ND-AMB compared to AMB. A: Representative Ussing chamber tracings reveal K+ currents after exposure to either ND-AMB or AMB. By convention, a positive deflection in the tracing represents movement of a cation (i.e. K+) in the mucosal to serosal direction. Once the AMB (10 μg/ml) was exposed to the apical side, K+ currents increased immediately but negligible induction of K+ current was observed when exposed to ND-AMB. As there was no response to the initial 10 μg/ml of ND-AMB, repeated ND-AMB was given every 5 min (10 μg/ml × 4). B: Results were summarized relative to trans-epithelial K+ current elicited by AMB vs ND-AMB exposure. * Statistical significance (P < 0.05).
Fig. 2.
Reduced trans-epithelial resistance (Ω cm2) after exposure to AMB compared to ND-AMB A: Representative Ussing chamber tracings reveal changes of trans-epithelial resistance after exposure to either ND-AMB or AMB. A negative deflection in the tracing represents decrease in trans-epithelial resistance (Ω cm2). With exposing AMB (10 μg/ml) to apical side, trans-epithelial resistance decreased immediately, representing the K+ currents. As there was no response to initial 10 μg/ml of ND-AMB, repeated ND-AMB was given every 5 min (10 μg/ml × 4). Negligible change was noticed after exposing ND-AMB. B: Summary representation of changes (Δ) in trans-epithelial resistance (Ω cm2) (n = 4, per condition). ΔR (changes in trans-epithelial resistance, Ω cm2) following application of AMB was significantly higher than those cells exposed to ND-AMB (P > 0.05).
LDH release after exposure to AMB vs ND-AMB
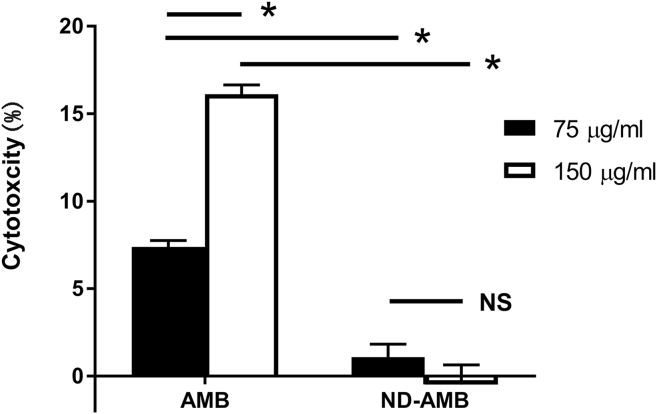
To quantify the release of lactate dehydrogenase (LDH), a marker of cell toxicity, cell culture media was collected after exposure (18 h) to AMB and ND-AMB. By using the positive (Triton X-100) and negative (cell culture media) controls, % cytotoxicity was measure (Fig. 3). Epithelial cells (n = 6) were exposed to toxic concentrations of AMB (75 μg/ml and 150 μg/ml) and same concentration of ND-AMB (75 μg/ml and 150 μg/ml) for 18 h. Dosages were chosen based on commercially available concentrations of AMB. For those cells incubated with AMB, LDH release was significantly increased compared to cells incubated with ND-AMB at same concentration. At 75 μg/ml, ND-AMB protected epithelial cells from the cytotoxicity of AMB, as determined by almost 85% reduction in LDH levels [AMB = (7.40 ± 0.36) % cytotoxicity, ND-AMB = (1.09 ± 0.74) % cytotoxicity, P = 0.001]. And at higher concentration (150 μg/ml) of AMB, significantly higher LDH release was noticed compared to the LDH release at 75 μg/ml [75 μg/ml of AMB = (7.40 ± 0.36) % cytotoxicity, 150 μg/ml of AMB = (16.10 ± 0.53) % cytotoxicity, P = 0.0004], which indicated dose-dependence. However, there was no increase in LDH release when cells were incubated with a higher concentration (150 μg/ml) of ND-AMB [75 μg/ml of ND-AMB = (1.09 ± 0.74) % cytotoxicity, 150 μg/ml of ND-AMB = (−0.48 ± 1.12) % cytotoxicity, P = 0.29].
Fig. 3.
ND-AMB protected airway epithelial cells from AMB mediated cytotoxicity for those cells incubated with AMB, LDH release was significantly increased compared to cells incubated with ND-AMB at same concentration. Dosage was chosen based on commercially available concentration of AMB. At 75 μg/ml, ND-AMB protected epithelial cells from the cytotoxicity of AMB, as determined by almost 85% reduction in lactate dehydrogenase (LDH) levels. At higher concentration (150 μg/ml) of AMB, significantly higher LDH release was noticed compared to LDH release at 75 μg/ml (P = 0.0004). NS – no statistical significance; * Statistical significance (P < 0.05).
CBF in vitro
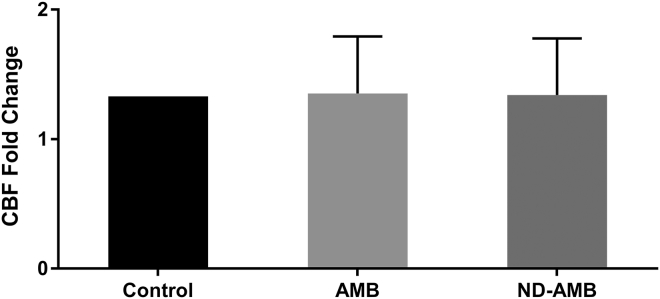
CBF is an important component of mucociliary clearance and to determine whether AMB or ND-AMB deteriorate CBF, the drug was applied to the apical membranes and compared to corresponding vehicle controls. CBF was measured about 15 min after exposure to 75 μg/ml of AMB or ND-AMB. There was no difference in CBF among three groups [Control = (1.33 ± 0.04) CBF fold changes; AMB = (1.35 ± 0.44) CBF fold changes; ND-AMB = (1.34 ± 0.44) CBF fold changes, P = 0.96] (Fig. 4).
Fig. 4.
No changes in ciliary beat frequencies (CBF). There was no difference in CBF between the two groups [Control = (1.33 ± 0.04) CBF fold changes, AMB = (1.35 ± 0.44) CBF fold changes, ND-AMB = (1.34 ± 0.44) CBF fold changes, P = 0.96).
In vitro potency of ND-AMB
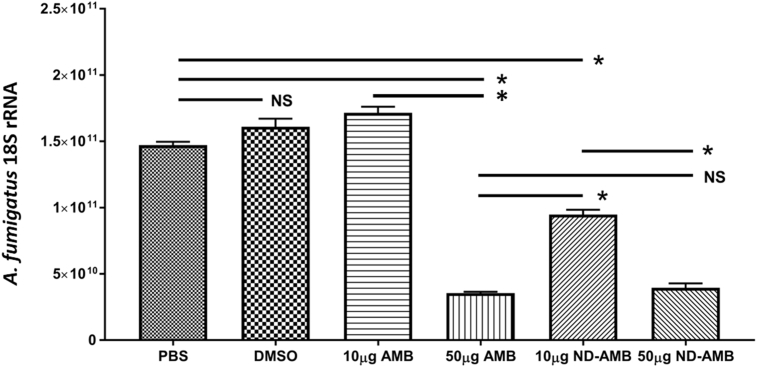
Dramatically decreased expression of A. fumigatus 18S rRNA was noted with 4 h exposure of 50 μg/ml of AMB and 10 μg/ml and 50 μg/ml of ND-AMB compared to controls (PBS and DMSO) and 10 μg/ml of AMB (Fig. 5) (P < 0.0001). The dose-dependent rRNA expression profiles for both AMB (10 μg/ml vs 50 μg/ml) and ND-AMB (10 μg/ml vs 50 μg/ml) were observed, reflected the killing effect of the drugs. At lower concentration (the dose of 10 μg/ml), much smaller expression profiles of A. fumigatus 18S rRNA were observed from ND-AMB compared to AMB (P < 0.0001): ND-AMB was significantly more potent than AMB at lower dose. At higher concentration (the dose of 50 μg/ml), there was no statistical significance in RNA expression profiles between AMB and ND-AMB: ND-AMB was as potent as AMB at higher dose (P < 0.0001).
Fig. 5.
Antifungal property of ND-AMB against A. fumigatus At the dose of 10 μg/ml, much smaller expression profiles of A. fumigatus 18S rRNA were observed from ND-AMB (n = 3) compared to AMB (n = 3) (P < 0.0001): ND-AMB was significantly more potent than AMB at lower dose. At higher concentration (the dose of 50 μg/ml), there was no statistical significance in RNA expression profiles between AMB (n = 3) and ND-AMB (n = 3): ND-AMB was as potent as AMB at higher dose (P < 0.0001). NS – no statistical significance; * Statistical significance (P < 0.05).
Discussion
An estimated 1.5 million fungal species inhabit Earth, with the vast majority poorly described or undiscovered.1 Fungal rhinosinusitis (FRS) has emerged as a major challenge and the incidence of mycotic infections, number, and diversity of pathogenic fungi involved in the disease have increased dramatically in recent years with the use of newer and more potent chemotherapeutic agents and regimens.3, 19, 20 It has been stated that quality of life is significantly inferior in patients with fungal rhinosinusitis as compared with the general population and is even poorer in patients with extensive and recurrent disease with multiple disabilities and high distress in day-to-day lives.21 For individuals with invasive fungal infection, AMB remains a clinically imperative treatment option even after 60 years on the market.7 This is because AMB has several crucial advantages over other classes of antifungal agents: 1) broad-spectrum activity against a wide range of medically relevant fungal species, and 2) virtually no resistant pathological strains have developed. In contrast, resistance to azole and echinocandin antifungal drugs is now a serious concern making AMB an ideal therapy for patients with invasive fungal infections.22, 23, 24 However, AMB exhibits significant side effects such as nephrotoxicity, electrolyte abnormalities, and infusion reactions.
Oda et al8 have developed a novel formulation of ND containing super aggregated AMB for the treatment of fungal infections. The ND drug-delivery vehicle is a complex consisting of a scaffold protein, apolipoprotein A-I (ApoA-I), a phospholipid bilayer (PL), and AMB. Prior work has demonstrated that drugs incorporated into the ND delivery vehicle are fully solubilized and retain bioactivity compared to other forms of the drug. In those studies, ND-AMB loaded with AMB had high antifungal potency and was a highly efficacious treatment for invasive candidiasis in mice.7 Topical delivery of AMB into the sinuses may have significant advantages over intravenous infusion including the avoidance of adverse effects. However, safety remains an important consideration in topical drug delivery into the sinuses, particularly as it relates to the integrity of the epithelium and its ion transport properties, which plays an important role in host defense. Introducing a novel therapeutic to the treatment of FRS that does minimal damage to the human nasal epithelium would vastly expand the current treatment options. The goal of the present study was to determine in vitro whether ND-AMB was less disruptive to sinonasal epithelium than existing AMB with maintaining antifungal property. We observed that ND-AMB protected nasal epithelial cells from the cytotoxicity of AMB, as determined by reduction in cell membrane permeability and almost 85% reduction in lactate dehydrogenase (LDH) levels and maintained antifungal property in this in vitro model.
AMB induced cellular toxicity in the human nasal epithelia in vitro model. At 75 μg/ml of AMB, LDH release was 6.8 time higher than LDH release after exposure to the same concentration of ND-AMB. The commercially available concentration of AMB solution is 100 μg/ml, which is the most commonly recommended concentration for patient use in sinus irrigation. A pilot study by Shirazi et al25 demonstrated that amphotericin B solution (100 μg/ml) was ineffective in killing fungi in vitro over a 6-week period. Concentrations of 300 μg/ml and 200 μg/ml had fungicidal effect on 10 fungi commonly found in patients with fungal-related CRS after 5 and 6 weeks of treatment, respectively. They suggested that higher concentrations of topical amphotericin B need to be tested for efficacy. However, based on our findings, it is highly likely that those concentrations are significantly toxic to airway epithelial cells and thus requires further investigation. Regarding the underlying mechanism, the ratio of AMB to phospholipid bilayer (PL) was identified as an important modulator of protective activity. ND-AMB with a low AMB to PL ratio reduced permeability of the epithelium and therefore it appears that ND extracts AMB from the plasma membrane, rather than simply blocking electrically conductive channels (pores) by vehicles. Burgess et al6 showed the ability of ND-AMB to restore membrane resistance that had been previously compromised by exposure to AMB. We have also demonstrated that an AMB formulation composed of super aggregated AMB contained within the ND drug-delivery bioparticle has potency against A. fumigatus equivalent to the AMB formulation. It seems that ND-AMB is a highly efficacious treatment for FRS.
This novel therapy has important applications in FRS as there are no clinically effective topical fungal therapies currently available, especially in patients with IFS. Further studies should be performed to determine the efficacy of topical ND-AMB irrigation in in vivo animal models with fungal rhinosinusitis, and to measure any advantages of this approach.
Conclusions
ND-AMB protects human nasal epithelia membranes from AMB toxicity by reducing apical cell K+ permeability and LDH release while maintaining uncompromised antifungal property compared to AMB. Topical delivery of ND-AMB into the sinonasal epithelium could expand the current treatment options for FRS as there are no clinically effective topical fungal therapies available.
Research support
This work was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (1 R01 HL133006-01) and National Institute of Diabetes and Digestive and Kidney Diseases (5P30DK072482-04, CF Research Center Pilot Award) to B.A.W., and John W. Kirklin Research and Education Foundation Fellowship Award and UAB Faculty Development Research Award to D.Y.C
Conflict of interest/Financial disclosures
Bradford A. Woodworth, M.D. is a consultant for Olympus and Cook Medical.
Acknowledgement
ND-AMB was received as a gift from Michael Oda PHD at University of California at San Francisco – Children's Hospital at Oakland Research Institute (UCSF-CHORI).
Edited by Jing Li
Footnotes
A portion of submitting manuscript was presented at the American Rhinologic Society Annual Meeting, Dallas, TX, September 26, 2015.
Peer review under responsibility of Chinese Medical Association.
References
- 1.Callejas C.A., Douglas R.G. Fungal rhinosinusitis: what every allergist should know. Clin Exp Allergy. 2013;43:835–849. doi: 10.1111/cea.12118. [DOI] [PubMed] [Google Scholar]
- 2.Schubert M.S. Allergic fungal sinusitis: pathophysiology, diagnosis and management. Med Mycol. 2009;47(suppl 1):S324–S330. doi: 10.1080/13693780802314809. [DOI] [PubMed] [Google Scholar]
- 3.Low C.Y., Rotstein C. Emerging fungal infections in immunocompromised patients. F1000 Med Rep. 2011;3:14. doi: 10.3410/M3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamill R.J. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs. 2013;73:919–934. doi: 10.1007/s40265-013-0069-4. [DOI] [PubMed] [Google Scholar]
- 5.Yano T., Itoh Y., Kawamura E. Amphotericin B-induced renal tubular cell injury is mediated by Na+ Influx through ion-permeable pores and subsequent activation of mitogen-activated protein kinases and elevation of intracellular Ca2+ concentration. Antimicrob Agents Chemother. 2009;53:1420–1426. doi: 10.1128/AAC.01137-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgess B.L., Cavigiolio G., Fannucchi M.V., Illek B., Forte T.M., Oda M.N. A phospholipid-apolipoprotein A-I nanoparticle containing amphotericin B as a drug delivery platform with cell membrane protective properties. Int J Pharm. 2010;399:148–155. doi: 10.1016/j.ijpharm.2010.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgess B.L., He Y., Baker M.M. NanoDisk containing super aggregated amphotericin B: a high therapeutic index antifungal formulation with enhanced potency. Int J Nanomedicine. 2013;8:4733–4743. doi: 10.2147/IJN.S50113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oda M.N., Hargreaves P.L., Beckstead J.A., Redmond K.A., van Antwerpen R., Ryan R.O. Reconstituted high density lipoprotein enriched with the polyene antibiotic amphotericin B. J Lipid Res. 2006;47:260–267. doi: 10.1194/jlr.D500033-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Bhargave G., Woodworth B.A., Xiong G., Wolfe S.G., Antunes M.B., Cohen N.A. Transient receptor potential vanilloid type 4 channel expression in chronic rhinosinusitis. Am J Rhinol. 2008;22:7–12. doi: 10.2500/ajr.2008.22.3125. [DOI] [PubMed] [Google Scholar]
- 10.Woodworth B.A., Antunes M.B., Bhargave G., Palmer J.N., Cohen N.A. Murine tracheal and nasal septal epithelium for air-liquid interface cultures: a comparative study. Am J Rhinol. 2007;21:533–537. doi: 10.2500/ajr.2007.21.3068. [DOI] [PubMed] [Google Scholar]
- 11.Woodworth B.A., Tamashiro E., Bhargave G., Cohen N.A., Palmer J.N. An in vitro model of Pseudomonas aeruginosa biofilms on viable airway epithelial cell monolayers. Am J Rhinol. 2008;22:235–238. doi: 10.2500/ajr.2008.22.3178. [DOI] [PubMed] [Google Scholar]
- 12.Antunes M.B., Woodworth B.A., Bhargave G. Murine nasal septa for respiratory epithelial air-liquid interface cultures. Biotechniques. 2007;43:195–196. doi: 10.2144/000112531. 198, 200 passim. [DOI] [PubMed] [Google Scholar]
- 13.Alexander N.S., Hatch N., Zhang S. Resveratrol has salutary effects on mucociliary transport and inflammation in sinonasal epithelium. Laryngoscope. 2011;121:1313–1319. doi: 10.1002/lary.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen N.A., Zhang S., Sharp D.B. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope. 2009;119:2269–2274. doi: 10.1002/lary.20223. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S., Skinner D., Hicks S.B. Sinupret activates CFTR and TMEM16A- dependent transepithelial chloride transport and improves indicators of mucociliary clearance. PLoS One. 2014;9:e104090. doi: 10.1371/journal.pone.0104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowley E.A., Linsdell P. Characterization of basolateral K+ channels underlying anion secretion in the human airway cell line Calu-3. J Physiol. 2002;538:747–757. doi: 10.1113/jphysiol.2001.013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattila P.E., Metz A.E., Rapaka R.R., Bauer L.D., Steele C. Dectin-1 Fc targeting of Aspergillus fumigatus beta-glucans augments innate defense against invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2008;52:1171–1172. doi: 10.1128/AAC.01274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowman J.C., Abruzzo G.K., Anderson J.W. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob Agents Chemother. 2001;45:3474–3481. doi: 10.1128/AAC.45.12.3474-3481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clancy C.J., Nguyen M.H. Invasive sinus aspergillosis in apparently immunocompetent hosts. J Infect. 1998;37:229–240. doi: 10.1016/s0163-4453(98)91921-1. [DOI] [PubMed] [Google Scholar]
- 20.Denning D.W., Evans E.G., Kibbler C.C. Guidelines for the investigation of invasive fungal infections in haematological malignancy and solid organ transplantation. British Society for Medical Mycology. Eur J Clin Microbiol Infect Dis. 1997;16:424–436. doi: 10.1007/BF02471906. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava R.M., Rijuneeta, Gupta A.K., Patro S.K., Avasthi A. Quality of life, disability scores, and distress index in fungal rhinosinusitis. Med Mycol. 2014;52:706–714. doi: 10.1093/mmy/myu037. [DOI] [PubMed] [Google Scholar]
- 22.Beyda N.D., Lewis R.E., Garey K.W. Echinocandin resistance in Candida species: mechanisms of reduced susceptibility and therapeutic approaches. Ann Pharmacother. 2012;46:1086–1096. doi: 10.1345/aph.1R020. [DOI] [PubMed] [Google Scholar]
- 23.Verweij P.E., Snelders E., Kema G.H., Mellado E., Melchers W.J. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis. 2009;9:789–795. doi: 10.1016/S1473-3099(09)70265-8. [DOI] [PubMed] [Google Scholar]
- 24.Walker L.A., Gow N.A., Munro C.A. Fungal echinocandin resistance. Fungal Genet Biol. 2010;47:117–126. doi: 10.1016/j.fgb.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirazi M.A., Stankiewicz J.A., Kammeyer P. Activity of nasal amphotericin B irrigation against fungal organisms in vitro. Am J Rhinol. 2007;21:145–148. doi: 10.2500/ajr.2007.21.2988. [DOI] [PubMed] [Google Scholar]