Abstract
Prior to adaptation of endoscopic approaches for sinonasal pathology, patients regularly endured significant morbidity from open approaches to the sinonasal cavity that were often fraught with failure. With improvements in transnasal endoscopy, functional endoscopic sinus surgery subsequently emerged from the work of Messerklinger and other pioneers in the field. The popularity of endoscopic sinus surgery quickly escalated and expanded to pathology other than inflammation. Here, we discuss the evolution of endoscopic sinus surgery as it relates to improvements in understanding disease pathogenesis, improvements in instrumentation and expansion of indications.
Keywords: Chronic sinusitis, Endoscopic sinus surgery, Skull base surgery
History of endoscopic procedures
The first attempt at nasal endoscopy is largely credited to Hirschman in 1901. In this early work, a modified cystoscope was used to examine the sinonasal cavity.1 Subsequently, Reichert performed what would be regarded as the first endoscopic procedure; rudimentary maxillary sinus manipulations with a 7 mm endoscope through an oroantral fistula.2 In 1925, Maltz promoted use of nasal endoscopes for diagnostic evaluation of the sinonasal cavity and coined the term ‘sinuscopy’.3 The creation of the Hopkins rod system in the 1960s was perhaps the major turning point in the field of sinonasal endoscopy.
Professor Harold H. Hopkins developed the rod optic endoscope system as well as several other inventions such as the zoom camera lens and fiberoptic gastroscope. The new telescope design resulted in markedly enhanced light delivery and superior optical quality allowing exceptional detailing of the sinonasal cavity.4 Using this new innovation, Messerklinger subsequently composed a landmark book in 1978 on diagnostic endoscopy of the nose from his work studying mucociliary clearance in fresh cadavers.5 Given the frequent failures of Caldwell-Luc surgery, the morbidity of frontal sinus osteoplasty and difficulties of performing headlight intranasal ethmoidectomy, there was a strong rationale for trying to improve surgical techniques for chronic sinusitis (CRS).
The relevance of the ostiomeatal complex (OMC) had been proposed by Naumann Proctor and Drettner, but it had previously not been adequately visualized, either on rhinoscopy or by plane film imaging. Messerklinger detailed the endoscopic anatomy and pathology of this region and also started to utilize polytomography to improve visualization of the anatomy and pathology. With improvements in imaging and endoscopic assessment, increasing emphasis was placed on anatomical aspects of the ostiomeatal complex and their potential impact on the pathogenesis of chronic rhinosinusitis. As scientific support for the importance of this region increased, several surgeons began performing select endoscopic procedures.
After having had the opportunity to meet Messerklinger during a conference in Dubrovnic, the senior author became convinced that endoscopic surgical diagnosis, more accurate imaging of the ostiomeatal complex and more functional surgery for CRS was truly an important step forward in its management. Given the very high radiation dose involved with polytomography, it was clear that new imaging methodologies needed to be developed. Zinreich et al6 devised parameters which provided superior visualization of the OMC with computed tomography at lower radiation dosage. After gaining experience with endoscopic surgical techniques, the first endoscopic surgical course was held at Johns Hopkins Medical Center in 1985. Amid growth of the techniques followed the publication of landmark papers delineating the theory, diagnostic evaluation and technique of functional endoscopic sinus surgery (FESS) and subsequent animal experiments demonstrating the validity of the concept.7, 8, 9
Evolution in understanding the pathogenesis of sinonasal disease
These and other early publications rekindled interest in sinus disease and its management and subsequently resulted in the broad adoption of endoscopic diagnostic and surgical techniques worldwide. The focus of surgical intervention was on anatomic and inflammatory issues in the ostiomeatal complex and the re-establishment of mucociliary clearance and ventilation. However, we also recognized the importance of environmental factors, general host factors, such as immunodeficiency and certain genetic diseases, even during the early years of FESS (Table 1).
Table 1.
Factors associated with chronic sinusitis.
| Environmental factors |
| Bacteria, viruses, fungi |
| Pollution, smoking |
| Allergens, chemical exposures |
| Host factors |
| Atopy |
| Immune deficiency |
| Genetic – Cystic fibrosis, ciliary dyskinesia, etc. |
| Innate immunity – bitter taste receptors |
| Local factors |
| Anatomic abnormalities |
| Inflammation of underlying bone |
| Obstructing tumors |
| Chronic mucosal inflammation |
Since the introduction of FESS, we have learned significantly more about the underlying pathophysiologies of CRS but we still await a complete classification of this syndrome of disorders. Currently utilized classifications such as CRS with and without polyps are unsatisfactory in terms of fully classifying this broad spectrum of diseases. This makes detailed therapeutic recommendations or treatment outcome comparisons difficult. Classification systems are now moving into an era where genetic markers and cytokine profiles allow for more precise grouping of this syndrome of disorders. Recent evidence has shown high levels of endothelin-1 (ET-1), thymus and activation-regulated chemokine (TARC/CCL17), and alpha-defensins in CRS with nasal polyps. Neopterin levels have been found at higher concentration in patients with CRS without nasal polyps.10, 11 Additional literature has shown increased eosinophil production of prostaglandin D2 in aspirin-exacerbated respiratory disease.12 The pathophysiologic roles of these biomarkers in CRS are still yet to be determined.
One important breakthrough has been the discovery of a new local immune system at least one element of which follows Mendelian genetics. Bitter taste receptors are expressed in the airway, where they appear to play several important roles in innate immune defense.13, 14 They are located on motile cilia in the upper airway and in response to bitter compounds and gram negative bacteria have been observed in sinonasal epithelium to result in increased nitric oxide production with potent bactericidal action.13 Acyl-homoserine lactones (AHLs) are a subclass of lactones that can stimulate bitter taste receptors. AHLs are secreted by gram-negative bacteria and serve as biofilm quorum-sensing molecule.15 Thus, once sufficient AHLs are produced in a localized environment, a biofilm may be produced. There is evidence that bitter taste receptors, through their lactone sensing mechanism, act as an adaptive response to detect biofilm quorum-sensing molecules and preemptively obviate biofilm formation and chronic inflammation. As such, it is postulated that host genetic defects in bitter taste receptors may predispose to CRS and early clinical studies support this concept. Knowledge surrounding this newly identified defense mechanism continues to evolve, with identification of the inhibitory action of sweet taste receptors and the potential for novel therapeutic targets. Defects in other components of innate immunity, such as mucociliary clearance, antimicrobial peptides and toll-like receptors have also been implicated in refractory chronic inflammation.16, 17, 18
While there is no argument that bone becomes involved in the inflammatory process of CRS, there is still debate with regard to the role of bone inflammation in both spread and persistence of chronic inflammation. While it has been demonstrated that inflammation does spread through the bone and is associated with more difficult to treat disease, the extent to which this is an active and important clinical process in patients still requires further elucidation.19
Understanding the native microbial community of the paranasal sinuses is the newest frontier in defining the pathogenesis of CRS. Paralleling pioneering research in the gastrointestinal tract, the microbiome of the paranasal sinuses likely contributes to maintaining a healthful state of the sinonasal mucosa. Disruption of this community, known as dysbiosis, may contribute to inflammation through a number of mechanisms.20 Extensive study of the gut epithelium in allergic and inflammatory disorders has shown that commensal microbiota promotes immunologic development and maturation, direct immune homeostasis, and influence susceptibility to inflammatory disease.21 Hence, it is postulated that maintenance of a healthy assortment of commensal organisms provides beneficial functions as well as exclude overabundance of pathogenic microbes. A negative correlation between Staphylococcus epidermidis and S. aureus has been observed in the nasal cavity supporting the concept of direct competitive inhibition.22 Additional work has shown that Corynebacterium pseudodiphtheriticum acts as a negative predictor of S. aureus in the sinonasal cavity and demonstrated an antagonistic effect in vitro.23 Several studies have reported no difference in overall quantity of bacteria present in CRS versus healthy patients.24, 25 Therefore, a shift in microbial community is postulated to occur resulting in a greater portion of pathogenic organisms. Not surprisingly, reduced species richness and diversity is found in CRS. In particular, anaerobes and S. aureus are often found to be significantly more prevalent in CRS versus healthy controls.26, 27 The field of sinonasal microbiome research is still in its infancy and understanding how the degree of dysbiosis that arises in an individual's sinonasal cavities impacts disease severity and outcomes has yet to be fully elucidated.
Therapeutic evolution
As our knowledge of CRS pathogenesis has continued to evolve, it has further confirmed that CRS is rarely a simple disorder that responds completely to restoration of mucociliary clearance and ventilation. The importance of controlling environmental and inflammatory components cannot be overemphasized, and one important goal of surgical intervention is to increase access of the inflamed mucosa to anti-inflammatory topical therapies. Given the increased emphasis on addressing host inflammation, surgical principles have generally evolved away from more focused procedures within the ostiomeatal complex to more complete frontoethmoidectomies with maxillary antrostomies and, when indicated, sphenoidotomy. With this approach, all diseased bony partitions are removed skeletonizing the orbit and skull base, while at the same time preserving the mucoperiosteum and yielding a unified sinonasal cavity optimized for topical steroid irrigation. The success of high volume/high dose topical budesonide irrigations in treating patients with persistent inflammation has only rendered more support for this surgical principle.28 This principle is further supported by newer evidence demonstrating that patients with severe inflammatory load benefit from extended sinus procedures such as the Draf 3 frontal sinusotomy. A recent study by Wormald et al has shown that in patients with AERD and CRS with nasal polyps, complete sphenoethmoidectomy, maxillary antrostomy and Draf 3 frontal sinusotomy is successful in the majority of patients with low complication rate and facilitates ongoing medical management.29
Advances in instrumentation
Early surgical intervention was primarily performed with grasping instruments with little regard to mucosal preservation. Endoscopic follow up often revealed denuded bone that healed poorly. These regions of stripped mucosa often resulted in scarring, chronic inflammation, neo-osteogenesis and occasionally mucocele formation. For this reason, intranasal, fine, through-cutting instruments were developed. Such instrumentation allowed fine cutting of bone and mucosa without mucosal stripping.30
Early in the endoscopic era, sphenoidotomy was performed by infracturing the anterior wall of the sphenoid sinus, a procedure with the potential for carotid artery or intracranial entry. The development of through cutting instruments, such as the straight mushroom punch, allowed widening of the natural os of the sphenoid after removal of the inferior third of the superior turbinate. Typically, it is our preference to perform a wide sphenoidotomy, extending to the skull base and medial orbital wall. As with the removal of other bony partitions, this can be achieved safely by first feeling behind the bony partitions prior to bone removal.
The principle of mucosal preservation is underscored in the narrow corridor of the frontal recess. Due to the anatomic constraints of the frontal recess, mucosal stripping frequently resulted in the development of stenosis, osteoneogenesis and mucocele formation. Complete frontal recess dissection necessitates the removal of all obstructing cells and bony partitions from the frontal recess in a mucosal sparing manner. Therefore, curved, fine, through cutting instruments were developed for frontal recess dissection (Fig. 1). Other key frontal instrumentation such as the forward cutting (cobra) forceps and Hosemann punch allowed for controlled removal of bone (Fig. 2).
Fig. 1.
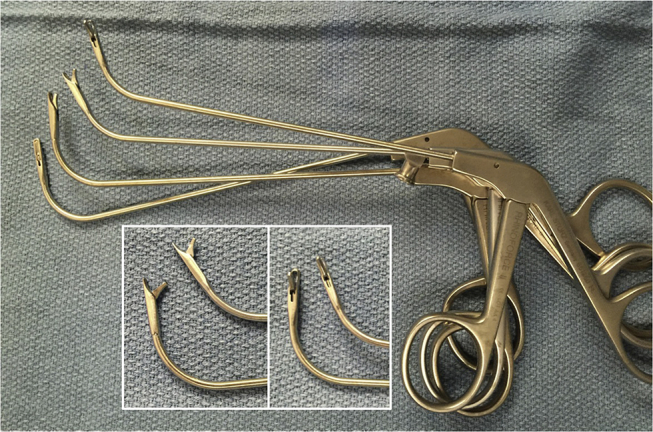
Through cutting frontal instrumentation.
Fig. 2.
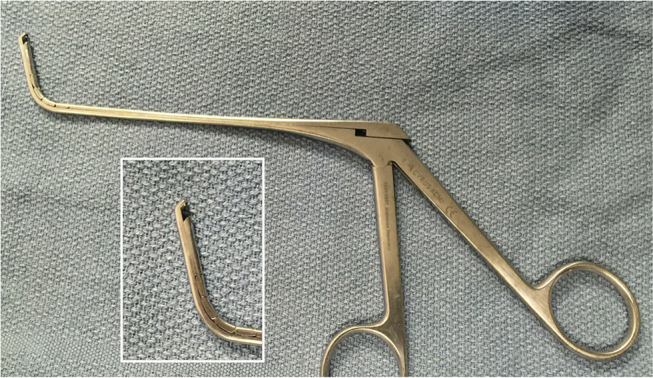
The Bachert forceps allows for atraumatic removal of bone in the frontal recess.
The microdebrider was originally developed for orthopedic cartilage removal during small joint arthroscopy. Setliff and Parsons introduced the instrument for endonasal surgery.31, 32 Early microdebriders were slow and frequently obstructed, but newer versions provided dramatic improvements in blade cutting ability, suction, speed, size and angulation allowing expedient removal of diseased tissue and polyps in an atraumatic, mucosal preserving fashion. Although fast and efficient, the lack of tactile feedback and speed of tissue removal created the potential for markedly more severe orbital and intracranial complications. In one such report, a complete enucleation of the orbit was sustained and was only appreciated by the surgeon after marked enophthalmos was noted.33
Further advancements in powered instrumentation followed with the creation of straight and curved suction-irrigating drills. The curved suction-irrigating drills have allowed for removal of osteitic bone in patients with chronic sinusitis particularly in extended frontal sinus procedures. Fine, higher speed drills designed for intranasal usage have also allowed for efficient skull base osteotomy to access the anterior cranial fossa for intracranial tumor removal.
Originally, the telescopes most frequently utilized during endoscopic sinus surgery were the 0, 30 and 70°, 4 mm scopes, with the 2.7 mm endoscopes reserved for pediatric cases. More recently, the addition of a wide-angle 45-degree scope with improved illumination in comparison to the 70-degree scope has, in the practice of the senior author, largely replaced the 30 and 70-degree endoscopes for many procedures. The 2.7 mm telescope, although fragile, has been increasingly utilized for in office procedures because of its improved patient comfort. Image quality and light sensitivity has dramatically improved from single chip cameras to high definition cameras, and most recently 4k cameras, providing impeccable imaging quality. The newest high definition technology utilizes image enhancement algorithms automatically enhancing brightness, minimizing reflection and overexposure, and enhancing tissue contrast. Although multiple companies have introduced 3D endoscopes, none to date have been widely adopted. This is due to the tendency for the technology to induce dizziness and headaches with prolonged use, as well as the issue of cost. The introduction of endoscope lens washing sheaths, initially utilized to wash the lens in conjunction with pulsed holmium laser use, has made a made a major difference in terms of maintaining visualization during routine endoscopic sinus and skull base procedures.
Advances in imaging and technology
The adaptation of CT imaging to rhinology revolutionized ones ability to better understand sinonasal disease. The subsequent advancement toward high-resolution CT imaging and rapid scanning times further facilitated accurate assessment of disease severity and provided anatomical detail critical for preoperative planning and preparation. Key anatomic details provided by CT imaging include: osteitis representing the chronicity of infection, the slope and integrity of the ethmoid roof, dehiscence of the medial orbital wall, anatomic variants such as spheno-ethmoidal (Onodi) and infraorbital ethmoid (Haller) cells, positions of the anterior ethmoid arteries, and pneumatization of the sphenoid sinus. One of the most significant advances has been the ability to detail the frontal recess anatomy and conceptualize the frontal sinus drainage pathway using triplanar imaging and thereby dramatically reduce frontal recess trauma. In-office, cone beam reduced-dose, CT scanners have now been developed allowing high resolution imaging in minutes. The role of MRI is supplemental to CT and provides soft tissue characterization as well as delineation of the intracranial interface. MRI becomes essential in assessment of sinonasal tumors and meningoencephaloceles and is recommended whenever there is soft tissue opacification adjacent to a skull base erosion.
Because of the rigid bony framework of the sinonasal cavity, endoscopic sinus surgery became an ideal candidate for computer-aided image-guided surgery. Initial attempts at image-guided technology occurred in the 1980s by Zinreich and were based on rigid servo arms and joints.34 Early systems were difficult to setup and required head fixation. Early electromagnetic systems were heavily inaccurate and distorted easily due to dental fillings, operative tables and other minimal magnetic fields.34
Modern day image-guidance systems are less intrusive, allow for rapid registration and offer a wide range of trackable instrumentation, including fine malleable probes, which can be introduced to confirm the frontal sinus drainage pathway prior to instrumentation. Today's system also allow CT imaging to be fused with MRI providing the superior bone detail of CT and improved soft tissue visualization of MR, a feature particularly useful for tumor and skull base surgeries. Although there is no definitive evidence supporting reduced complications with the use of image-guidance, it is generally accepted to be helpful in select cases with significant mucosal disease, altered anatomy, revision surgery, and disease processes abutting critical structures. The AAO-HNS has published recommendations regarding appropriate indications for image-guided surgery.35
Future endeavors in image-guided surgery include augmented reality. Currently in its infancy, augmented reality fuses preoperative imaging data and intraoperative images. Using augmented reality, surgeons may annotate images preoperatively and then visualize information as a projection that is displayed over the anatomy in the surgeon's view.36, 37 This technology has been used in a cadaver model to annotate images with frontal recess anatomy, the position of the sphenoid os and optic nerve.38
The focus on surgery as an adjunct to medical therapy in CRS
Previously, recurrence of nasal polyposis after multiple surgeries was frequently anticipated. Currently, endoscopic surgery combined with medical therapy is aimed at long-term disease control and eventual resolution. It is now recognized that all patients with significant CRS have persistent inflammation post-surgery, although it is frequently asymptomatic. Control of this persistent inflammation requires prolonged medical management, local debridement and endoscopic surveillance. The introduction of high-dose, high-volume topical nasal steroid irrigations (0.5 mg of budesonide or 0.6 mg of mometasone in 240 ml of normal saline) once or twice daily has become routine in eosinophilic disease. As such, adequate access for postoperative steroid irrigation to the areas of inflammation has become one of the goals of surgery. In the early postoperative period, oral steroid therapy is also frequently required, and when there is a significant neutrophilic or bacterial component, or bone exposure within the ethmoid cavity, prolonged oral antibiotics is required. Whereas in chronic skin wounds topical antibiotics based upon microbiome analysis (16S pyrosequencing) have been shown to significantly improve wound healing, the efficacy of such therapy in the postoperative CRS patients remains to be fully evaluated.39
Long-term CRS management frequently also requires environmental considerations including allergy evaluation and management. Evaluation for immunodeficiency and other host factors predisposing towards disease, such as Churg-Strauss syndrome, also need to be considered in refractory disease. Whereas control of CRS has been clearly demonstrated to improve asthma, the presence of asthma also creates additional therapeutic opportunities for CRS control with the introduction of monoclonal antibodies. In patients with severe persistent nasal polyposis and asthma, the introduction of omalizumab, mepolizumab and reslizumab create new, albeit very expensive therapeutic options based upon polyp cytokine profile. Dupilumab, in particular, is demonstrating therapeutic promise in eosinophilic polypoid disease with significant reductions in symptomatic chronic sinusitis and nasal polyposis refractory to intranasal steroids.40 A recent study by Chandra et al has shown that omalizumab therapy is associated with decrease in overall antibiotic use and steroid dependence in patients with asthmatic CRS.41 A double blind, randomized control trial has shown that patients with severe nasal polyposis showed a significant reduction in nasal polyp size in comparison to control in patients treated with mepolizumab, an IL-5 monoclonal antibody.42
With the advent of endoscopic surveillance and additional medical therapeutic options, the focus has shifted towards long-term disease control and resolution, rather than acceptance of recurrent surgical intervention. It has previously been demonstrated that once the ethmoid cavity has returned to completely healthy mucosa following surgical intervention, the risk of long-term disease recurrence requiring further surgical intervention is essentially eliminated and the long-term medical therapy requirement for both asthma and CRS is reduced.43 The ability to return a postoperative cavity to normal has been further enhanced by the introduction of steroid eluting implants.44
Tumor, skull base and orbital surgery
As endoscopic instrumentation and proficiency advanced, the indications for endoscopic approaches have expanded to include sinonasal tumors, skull base and orbital pathology. Beginning in the 1980s, surgeons had begun to perform endoscopic tumor resections, orbital decompressions, malignant tumor resections and pituitary procedures. The ability to perform skull base surgery was markedly advanced when the success of endoscopic CSF leak and skull base defect closure was demonstrated.45, 46
In the early 1990s, the first series of endoscopic pituitary surgery was published by Jankowski.47 From then on, interest grew and the procedure was further popularized by Jho and Carrau in 1996.48 With the subsequent adaptation of the Haddad flap for skull base reconstruction, the majority of pituitary surgeries are performed endoscopically today with reduced patient morbidity. Additionally, indications now extend to more extensive pathology such as meningiomas, craniopharyngioms and chordomas.
The first reported series of endoscopic orbital surgery was published in 1990.49 Initial applications were employed primarily for orbital decompression in Graves' orbitopathy. Indications have subsequently been extended to optic nerve decompression and removal of orbital tumors. A recent multi-institutional series demonstrated the success of endoscopic removal of orbital cavernous hemangiomas through a fully endoscopic approach. Maintenance of orthotropia and symmetric orbital appearance was achieved in the majority of patients.50
Endoscopic oncologic surgery was initiated in the late 1980s. As initial opposition was encountered as a result of the piecemeal resection of tumors, many series today support endoscopic resection with comparable survival and recurrence rates to more classic open approaches.51, 52, 53, 54, 55, 56, 57 The general principle of endoscopic tumor resection involves tumor debulking with meticulous care to identify the point of tumor attachment. An oncologic resection of the tumor attachment is then performed with wide negative margin. A dural margin is taken if disease abuts the skull base. It is essential that the oncologic margin is not compromised for the sake of an endoscopic resection. As instrumentation and expertise improves, endoscopic approaches can now be used successfully for tumors with intracranial extension (Fig. 3).
Fig. 3.
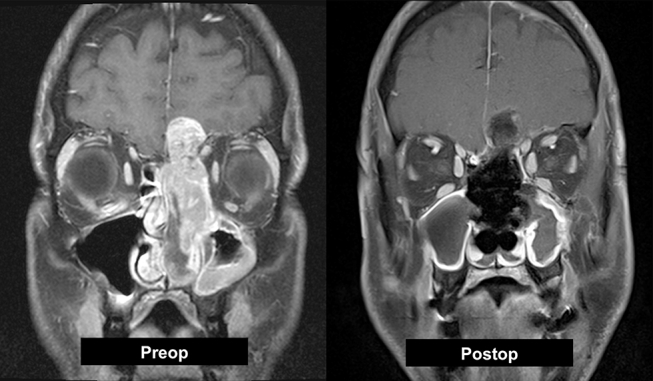
Preoperative MRI shows a sinonasal undifferentiated malignancy. Intraoperatively, the orbit and brain were not grossly invaded and a gross total resection was achieved purely via endoscopic approach.
New frontiers in endoscopic tumor and skull base surgery are being reached as technology continues to advance. In particular, intraoperative near-infrared imaging technology is currently being developed to aid in tumor detection and margin assessment. Early work in this technology utilized an FDA-approved radiolabeled dye that accumulates in cancerous tissue.58 Given the dependence of endoscopic procedures on imaging, it was only natural that this technology would be applied to endoscopic endonasal pathology. Novel radiolabeled-dyes are now being tested in varied pathologies from benign to malignant. Preliminary data supports successful delineation of cancerous tissue from normal (Fig. 4).
Fig. 4.
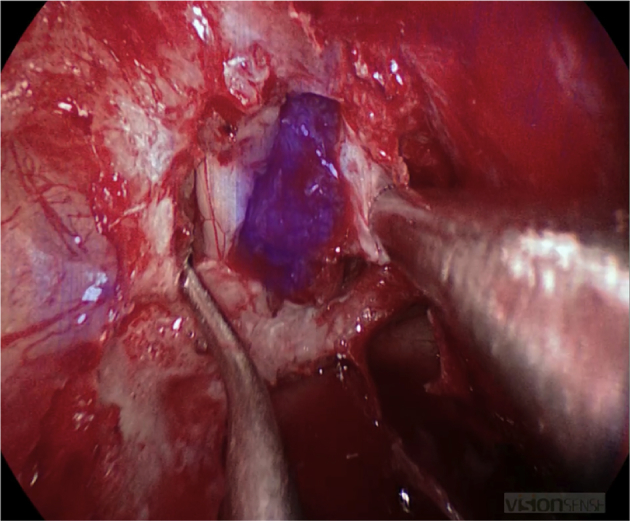
Intraoperative view using high definition, real-time infrared fluorescence imaging. A novel radiolabeled compound was injected preoperatively in a patient with a planum meningioma. Intraoperative view after skull base osteotomy shows uptake from the tumor with surrounding brain and sinonasal tissue excluded.
Conclusion
Over the last thirty years, the field of sinus surgery has advanced from open surgical procedures focused on mucosal stripping as standard of care to functional endoscopic procedures using state of the art instrumentation, high definition cameras, and intraoperative stereotactic surgical navigation. The indications have expanded from primarily inflammatory disease to sinonasal tumors, skull base and orbital pathology. As technology continues to improve and our understanding of disease pathogenesis advances, the limitations of our field continue to evolve. Where we will be in the next thirty years has yet to be determined, but the potential is limitless.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Jacobs J.B. 100 years of frontal sinus surgery. Laryngoscope. 1997;107:1–36. doi: 10.1097/00005537-199711001-00001. [DOI] [PubMed] [Google Scholar]
- 2.Pownell P.H., Minoli J.J., Rohrich R.J. Diagnostic nasal endoscopy. Plast Reconstr Surg. 1997;99:1451–1458. doi: 10.1097/00006534-199704001-00042. [DOI] [PubMed] [Google Scholar]
- 3.Cohen N.A., Kennedy D.W. Endoscopic sinus surgery: where we are-and where we're going. Curr Opin Otolaryngol Head Neck Surg. 2005;13:32–38. doi: 10.1097/00020840-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Jennings C.R. Harold Hopkins. Arch Otolaryngol Head Neck Surg. 1998;124:1042. [PubMed] [Google Scholar]
- 5.Messerklinger W. Endoscopy technique of the middle nasal meatus (author's transl) Arch Otorhinolaryngol. 1978;221:297–305. doi: 10.1007/BF00491466. [DOI] [PubMed] [Google Scholar]
- 6.Zinreich S.J., Kennedy D.W., Rosenbaum A.E., Gayler B.W., Kumar A.J., Stammberger H. Paranasal sinuses: CT imaging requirements for endoscopic surgery. Radiology. 1987;163:769–775. doi: 10.1148/radiology.163.3.3575731. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy D.W., Zinreich S.J., Rosenbaum A.E., Johns M.E. Functional endoscopic sinus surgery. Theory and diagnostic evaluation. Arch Otolaryngol. 1985;111:576–582. doi: 10.1001/archotol.1985.00800110054002. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy D.W. Functional endoscopic sinus surgery. Technique. Arch Otolaryngol. 1985;111:643–649. doi: 10.1001/archotol.1985.00800120037003. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy D.W., Zinreich S.J., Shaalan H., Kuhn F., Naclerio R., Loch E. Endoscopic middle meatal antrostomy: theory, technique, and patency. Laryngoscope. 1987;97:1–9. doi: 10.1288/00005537-198708002-00001. [DOI] [PubMed] [Google Scholar]
- 10.Scheckenbach K., Wagenmann M. Cytokine patterns and endotypes in acute and chronic rhinosinusitis. Curr Allergy Asthma Rep. 2016;16:3. doi: 10.1007/s11882-015-0583-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsybikov N.N., Egorova E.V., Kuznik B.I., Fefelova E.V., Magen E. Biomarker assessment in chronic rhinitis and chronic rhinosinusitis: endothelin-1, TARC/CCL17, neopterin, and alpha-defensins. Allergy Asthma Proc. 2016;37:35–42. doi: 10.2500/aap.2016.37.3899. [DOI] [PubMed] [Google Scholar]
- 12.Feng X., Ramsden M.K., Negri J. Eosinophil production of prostaglandin D2 in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;138:1089–1097 e3. doi: 10.1016/j.jaci.2016.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee R.J., Xiong G., Kofonow J.M. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122:4145–4159. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tizzano M., Gulbransen B.D., Vandenbeuch A. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci U S A. 2010;107:3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barraud N., Hassett D.J., Hwang S.H., Rice S.A., Kjelleberg S., Webb J.S. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J Bacteriol. 2006;188:7344–7353. doi: 10.1128/JB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Psaltis A.J., Bruhn M.A., Ooi E.H., Tan L.W., Wormald P.J. Nasal mucosa expression of lactoferrin in patients with chronic rhinosinusitis. Laryngoscope. 2007;117:2030–2035. doi: 10.1097/MLG.0b013e31812e01ab. [DOI] [PubMed] [Google Scholar]
- 17.Galli J., Calò L., Ardito F. Damage to ciliated epithelium in chronic rhinosinusitis: what is the role of bacterial biofilms. Ann Otol Rhinol Laryngol. 2008;117:902–908. doi: 10.1177/000348940811701207. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y., Zhou B., Wang C. Biofilm formation and Toll-like receptor 2, Toll-like receptor 4, and NF-kappaB expression in sinus tissues of patients with chronic rhinosinusitis. Am J Rhinol Allergy. 2012;26:104–109. doi: 10.2500/ajra.2012.26.3718. [DOI] [PubMed] [Google Scholar]
- 19.Khalid A.N., Hunt J., Perloff J.R., Kennedy D.W. The role of bone in chronic rhinosinusitis. Laryngoscope. 2002;112:1951–1957. doi: 10.1097/00005537-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Frank D.N., Zhu W., Sartor R.B., Li E. Investigating the biological and clinical significance of human dysbiosis. Trends Microbiol. 2011;19:427–434. doi: 10.1016/j.tim.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamada N., Seo S.U., Chen G.Y., Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 22.Frank D.N., Feazel L.M., Bessesen M.T., Price C.S., Janoff E.N., Pace N.R. The human nasal microbiota and Staphylococcus aureus carriage. PLoS One. 2010;5:e10598. doi: 10.1371/journal.pone.0010598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan M., Pamp S.J., Fukuyama J. Nasal microenvironments and interspecific interactions influence nasal microbiota complexity and S. aureus carriage. Cell Host Microbe. 2013;14:631–640. doi: 10.1016/j.chom.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramakrishnan V.R., Feazel L.M., Abrass L.J., Frank D.N. Prevalence and abundance of Staphylococcus aureus in the middle meatus of patients with chronic rhinosinusitis, nasal polyps, and asthma. Int Forum Allergy Rhinol. 2013;3:267–271. doi: 10.1002/alr.21101. [DOI] [PubMed] [Google Scholar]
- 25.Ramakrishnan V.R., Hauser L.J., Feazel L.M., Ir D., Robertson C.E., Frank D.N. Sinus microbiota varies among chronic rhinosinusitis phenotypes and predicts surgical outcome. J Allergy Clin Immunol. 2015;136:334–342.e1. doi: 10.1016/j.jaci.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Feazel L.M., Robertson C.E., Ramakrishnan V.R., Frank D.N. Microbiome complexity and Staphylococcus aureus in chronic rhinosinusitis. Laryngoscope. 2012;122:467–472. doi: 10.1002/lary.22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramakrishnan V.R., Feazel L.M., Gitomer S.A., Ir D., Robertson C.E., Frank D.N. The microbiome of the middle meatus in healthy adults. PLoS One. 2013;8:e85507. doi: 10.1371/journal.pone.0085507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snidvongs K., Pratt E., Chin D., Sacks R., Earls P., Harvey R.J. Corticosteroid nasal irrigations after endoscopic sinus surgery in the management of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2012;2:415–421. doi: 10.1002/alr.21047. [DOI] [PubMed] [Google Scholar]
- 29.Morrissey D.K., Bassiouni A., Psaltis A.J., Naidoo Y., Wormald P.J. Outcomes of modified endoscopic Lothrop in aspirin-exacerbated respiratory disease with nasal polyposis. Int Forum Allergy Rhinol. 2016;6:820–825. doi: 10.1002/alr.21739. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy D.W. Technical innovations and the evolution of endoscopic sinus surgery. Ann Otol Rhinol Laryngol Suppl. 2006;196:3–12. doi: 10.1177/00034894061150s902. [DOI] [PubMed] [Google Scholar]
- 31.Parsons D.S. Rhinologic uses of powered instrumentation in children beyond sinus surgery. Otolaryngol Clin North Am. 1996;29:105–114. [PubMed] [Google Scholar]
- 32.Setliff R.C. The hummer: a remedy for apprehension in functional endoscopic sinus surgery. Otolaryngol Clin North Am. 1996;29:95–104. [PubMed] [Google Scholar]
- 33.Chang J.R., Grant M.P., Merbs S.L. Enucleation as endoscopic sinus surgery complication. JAMA Ophthalmol. 2015;133:850–852. doi: 10.1001/jamaophthalmol.2015.0706. [DOI] [PubMed] [Google Scholar]
- 34.Anon J.B., Klimek L., Mosges R., Zinreich S.J. Computer-assisted endoscopic sinus surgery. An international review. Otolaryngol Clin North Am. 1997;30:389–401. [PubMed] [Google Scholar]
- 35.Huang B.Y., Senior B.A., Castillo M. Current trends in sinonasal imaging. Neuroimaging Clin N Am. 2015;25:507–525. doi: 10.1016/j.nic.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Besharati T.L., Mahvash M. Augmented reality-guided neurosurgery: accuracy and intraoperative application of an image projection technique. J Neurosurg. 2015;123:206–211. doi: 10.3171/2014.9.JNS141001. [DOI] [PubMed] [Google Scholar]
- 37.Winne C., Khan M., Stopp F., Jank E., Keeve E. Overlay visualization in endoscopic ENT surgery. Int J Comput Assist Radiol Surg. 2011;6:401–406. doi: 10.1007/s11548-010-0507-7. [DOI] [PubMed] [Google Scholar]
- 38.Citardi M.J., Agbetoba A., Bigcas J.L., Luong A. Augmented reality for endoscopic sinus surgery with surgical navigation: a cadaver study. Int Forum Allergy Rhinol. 2016;6:523–528. doi: 10.1002/alr.21702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dowd S.E., Wolcott R.D., Kennedy J., Jones C., Cox S.B. Molecular diagnostics and personalised medicine in wound care: assessment of outcomes. J Wound Care. 2011;20(232):234–239. doi: 10.12968/jowc.2011.20.5.232. [DOI] [PubMed] [Google Scholar]
- 40.Bachert C., Mannent L., Naclerio R.M. Effect of subcutaneous Dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315:469–479. doi: 10.1001/jama.2015.19330. [DOI] [PubMed] [Google Scholar]
- 41.Chandra R.K., Clavenna M., Samuelson M., Tanner S.B., Turner J.H. Impact of omalizumab therapy on medication requirements for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:472–477. doi: 10.1002/alr.21685. [DOI] [PubMed] [Google Scholar]
- 42.Gevaert P., Van Bruaene N., Cattaert T. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128:989–995.e1-8. doi: 10.1016/j.jaci.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 43.Senior B.A., Kennedy D.W., Tanabodee J., Kroger H., Hassab M., Lanza D. Long-term results of functional endoscopic sinus surgery. Laryngoscope. 1998;108:151–157. doi: 10.1097/00005537-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Han J.K., Marple B.F., Smith T.L. Effect of steroid-releasing sinus implants on postoperative medical and surgical interventions: an efficacy meta-analysis. Int Forum Allergy Rhinol. 2012;2:271–279. doi: 10.1002/alr.21044. [DOI] [PubMed] [Google Scholar]
- 45.Mattox D.E., Kennedy D.W. Endoscopic management of cerebrospinal fluid leaks and cephaloceles. Laryngoscope. 1990;100:857–862. doi: 10.1288/00005537-199008000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Papay F.A., Benninger M.S., Levine H.L., Lavertu P. Transnasal transseptal endoscopic repair of sphenoidal cerebral spinal fluid fistula. Otolaryngol Head Neck Surg. 1989;101:595–597. doi: 10.1177/019459988910100517. [DOI] [PubMed] [Google Scholar]
- 47.Jankowski R., Auque J., Simon C., Marchal J.C., Hepner H., Wayoff M. Endoscopic pituitary tumor surgery. Laryngoscope. 1992;102:198–202. doi: 10.1288/00005537-199202000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Carrau R.L., Jho H.D., Ko Y. Transnasal-transsphenoidal endoscopic surgery of the pituitary gland. Laryngoscope. 1996;106:914–918. doi: 10.1097/00005537-199607000-00025. [DOI] [PubMed] [Google Scholar]
- 49.Kennedy D.W., Goodstein M.L., Miller N.R., Zinreich S.J. Endoscopic transnasal orbital decompression. Arch Otolaryngol Head Neck Surg. 1990;116:275–282. doi: 10.1001/archotol.1990.01870030039006. [DOI] [PubMed] [Google Scholar]
- 50.Bleier B.S., Castelnuovo P., Battaglia P. Endoscopic endonasal orbital cavernous hemangioma resection: global experience in techniques and outcomes. Int Forum Allergy Rhinol. 2016;6:156–161. doi: 10.1002/alr.21645. [DOI] [PubMed] [Google Scholar]
- 51.Tajudeen B.A., Arshi A., Suh J.D. Esthesioneuroblastoma: an update on the UCLA experience, 2002–2013. J Neurol Surg B Skull Base. 2015;76(1):43–49. doi: 10.1055/s-0034-1390011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tajudeen B.A., Mundi J., Suh J.D., Bergsneider M., Wang M.B. Endoscopic endonasal surgery for recurrent pituitary tumors: technical challenges to the surgical approach. J Neurol Surg B Skull Base. 2015;76:50–56. doi: 10.1055/s-0034-1383856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eloy J.A., Vivero R.J., Hoang K. Comparison of transnasal endoscopic and open craniofacial resection for malignant tumors of the anterior skull base. Laryngoscope. 2009;119:834–840. doi: 10.1002/lary.20186. [DOI] [PubMed] [Google Scholar]
- 54.Gallia G.L., Reh D.D., Salmasi V., Blitz A.M., Koch W., Ishii M. Endonasal endoscopic resection of esthesioneuroblastoma: the Johns Hopkins Hospital experience and review of the literature. Neurosurg Rev. 2011;34:465–475. doi: 10.1007/s10143-011-0329-2. [DOI] [PubMed] [Google Scholar]
- 55.Patel S.G., Singh B., Stambuk H.E. Craniofacial surgery for esthesioneuroblastoma: report of an international collaborative study. J Neurol Surg B Skull Base. 2012;73:208–220. doi: 10.1055/s-0032-1311754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snyderman C.H., Carrau R.L., Kassam A.B. Endoscopic skull base surgery: principles of endonasal oncological surgery. J Surg Oncol. 2008;97:658–664. doi: 10.1002/jso.21020. [DOI] [PubMed] [Google Scholar]
- 57.Wellman B.J., Traynelis V.C., McCulloch T.M., Funk G.F., Menezes A.H., Hoffman H.T. Midline anterior craniofacial approach for malignancy: results of en bloc versus piecemeal resections. Skull Base Surg. 1999;9:41–46. doi: 10.1055/s-2008-1058171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holt D., Okusanya O., Judy R. Intraoperative near-infrared imaging can distinguish cancer from normal tissue but not inflammation. PLoS One. 2014;9:e103342. doi: 10.1371/journal.pone.0103342. [DOI] [PMC free article] [PubMed] [Google Scholar]


