Abstract
Background
The special features of nonsteroidal anti-inflammatory drugs (NSAIDs) enteropathy were partially clarified by single-balloon endoscopy(SBE).
We aimed to investigate the characteristics of NSAIDs injuries that were differ from other ulcer diseases and efficacy of SBE compared with capsule endoscopy(CE).
Material/Methods
1,644 symptomatic patients (221 patients taking NSAIDs) hospitalized between January 2006 and March 2016 were recruited and underwent SBE and/or CE.
Results
NSAIDs damages were identified in 110 patients (49.77%). The special features of NSAIDs lesions included: variform, superficial, multiple and irregular arrangement; <1 cm in diameter (67.27%); the location in jejunum and ileum was similar; ileocecal valve was rarely influenced (20.91%). The specificity and positive predictive value of SBE for diagnosing NSAIDs breaks were higher than CE (95.74% vs. 80.00%; 95.45% vs. 81.63%, p<0.05). There were no differences in the detection rate and the diagnostic accuracy rate of small bowel diseases between SBE and CE in the NSAIDs group (69.4% vs. 66.3% and 83.58% vs. 80.65%, p>0.05 respectively). The consistency in diagnosing NSAIDs breaks for the 2 methods was 82.61%. More tiny lesions at the distal ileum were detected by SBE. Four patients misdiagnosed by CE got accurate diagnose through biopsy by SBE. Three patients with active bleeding caused by NSAIDs-induced ulcers underwent hemostasis successfully by SBE.
Conclusions
NSAIDs injuries might be distinguished from other diseases by endoscopic features and biopsy through SBE, which appeared to be an effective method for diagnosis and treatment.
MeSH Keywords: Anti-Inflammatory Agents, Non-Steroidal; Capsule Endoscopy; Endoscopy, Gastrointestinal; Intestine, Small
Background
NSAIDs have been used in clinical settings for their antipyretic, analgesic and anti-inflammatory effects in many chronic diseases. NSAIDs are well known causative agents of gastroduodenal mucosal lesions as an adverse effect [1,2]. Recently, a number of capsule endoscopy (CE) studies have demonstrated that the high incidence of small intestine damage is 50–80% in long-term users [3–6]. Historically, this has been given little clinical attention since NSAIDs-induced intestinal disease is usually asymptomatic and is difficult to detect until typical features such as bleeding, perforation, stricture and protein-losing enteropathy occur. Capsule endoscopy has been verified to provide significant value from clinical data world-wide, while it has limitations of possible retention and lack of biopsy and therapeutic functions. Single-balloon endoscopy (SBE) is complementary to CE for the identification of small intestinal pathology of various types, and the procedure supplies clear visualization and interventions by means of a conventional endoscope [7,8].
However, there have been only a few case descriptions about the special characteristics of NSAIDs-induced damage different from other intestinal ulcer diseases and the diagnostic value of SBE compared with CE so far. In this study, we aimed to retrospectively investigate prevalence and features of NSAIDs injuries and evaluate the efficacy of SBE.
Material and Methods
Patients
A total of 1,644 symptomatic patients undergoing SBE and/or CE at Binzhou, Yantai and Weihai affiliated hospitals of Binzhou Medical University and Yantai Yuhuangding affiliated hospital of Qingdao Medical University between January 2006 and March 2016 were recruited. We registered the medical history, endoscopic findings and clinical features of all patients. Indications for the enteroscopy included obscure gastrointestinal bleeding (OGIB), obstruction, abdominal pain, diarrhea, distention, emaciation, and anepithymia, etc. Patients taking NSAIDs due to osteoarthritis (OA), rheumatoid arthritis (RA), coronary artery disease, hypertension, cerebrovascular disease and metabolic diseases (diabetes and hyperlipemia) were verified as having been given NSAIDs at least 3 times per week for longer than 4 weeks prior to enteroscopy. Patients not taking NSAIDs were identified as non-NSAIDs group. Patients who did not take NSAIDs combined with any of the chronic diseases such as cardiac-cerebral vascular diseases or metabolic disease were identified as the control group. NSAIDs included traditional NSAIDs, selective COX-2 inhibitors and aspirin. Informed consent for endoscopy/treatment was obtained from all of the patients. This study was approved by the Institutional Review Board of the Binzhou Medical University and Qingdao Medical University.
The definition of NSAIDs-induced small-intestine injury
The inclusion criteria for NSAIDs-induced small bowel injury included the following [9,10]: 1) having a history of NSAIDs use; 2) endoscopic findings of erosion and/or ulcer and/or typical diaphragm-like stricture and no specific pathological findings observed from tissue biopsies; and 3) improvement in clinical findings (signs and symptoms) and/or endoscopic findings by cessation of NSAIDs, except for diaphragm disease. Malignant tumors, inflammatory bowel disease, Behcet’s disease, or infectious diseases were excluded. Patients with cardiopulmonary insufficiency, liver cirrhosis, or acute kidney failure were eliminated.
Lesions except NSAIDs damages were final diagnosed by pathology, treatment outcomes, and follow-up results.
Endoscopy procedures
Capsule endoscopy was recommended to the patients for whom an invasive test would be too risky. The other patients underwent capsule endoscopy or single-balloon endoscopy, according to the clinical conditions.
SIF-Q260 single-balloon endoscopy (Olympus, Tokyo, Japan) was used. SIF-Q260 has an accessory channel that is 2.8 mm in diameter, allowing the use of a variety of therapeutic devices. Biopsy specimens were reviewed histologically only if the patients had active bleeding or serious coagulant diseases.
Antegrade and retrograde enteroscopy were performed carefully according to the procedure described by Yamamoto [11]. When the location of the lesion could be predicted in advance by the color of the feces or other examination findings, an insertion approach close to the lesion was selected, either oral or anal. When the location could not be predicted, the retrograde approach was selected first. When the responsible lesion was identified by the first approach and the location was limited, the other approach was not performed. However, when no lesion was found or the responsible lesion was not found by the first approach, the other approach was performed. All procedures were performed under intravenous anesthesia.
CE was performed using a PillCam SB (Given Imaging) device. Patients fasted for 12 h before capsule ingestion. Drinking clear fluids was allowed at 2 h and eating light meals was allowed at 4 h after ingestion of the capsule. The data recorder was removed 10 h later, and the patients were subsequently discharged. The recorded digital information was downloaded from the recorder to the computer, and the images were analyzed using the proprietary RAPID software.
Endoscopic pictures of all patients were reviewed by 2 gastroenterologists with more than 5 years of experience with SBE and CE.
Statistical analyses
Statistical analyses were performed using SPSS 17.0. Continuous variables are expressed as the mean ± standard deviation, whereas categorical variables are expressed as percentages and numbers. Variables were compared between the 2 groups using the t test and χ2 test. P<0.05 was considered statistically significant.
Results
Comparison of baseline characteristics between the NSAIDs group and control group
A total of 221 patients (163 with and 58 without small bowel injury) taking NSAIDs were enrolled in this study between January 2006 and March 2016. Of these patients, 95 patients underwent SBE, 89 patients underwent CE, and 37 patients underwent both examinations. We chose 299 patients who had not been treated with NSAIDs as the control group. The baseline characteristics of these patients are shown in Table 1.
Table 1.
Comparison between the clinical features of the NSAIDs group and the control group.
| NSAIDs group | Control group | NSAIDs group vs. Control group | |||
|---|---|---|---|---|---|
| SBE | CE | CE + SBE | |||
| No. of patients | 95 | 89 | 37 | 299 | |
| Male/Famale | 49/46 | 45/44 | 17/20 | 158/141 | P=0.555 |
| Age (years) | 64.02±7.86 | 63.27±7.87 | 63.66±8.36 | 62.27±8.84 | P=0.065 |
| Indications for endocsopy no. (%) | |||||
| OGIB | 77 (81.05%) | 67 (75.28%) | 22 (59.46%) | 213 (71.24%) | P=0.058 |
| SBO | 4 (4.21%) | 1 (1.12%) | 1 (2.70%) | 61 (20.40%) | P=0.000 |
| Other | 14 (14.74%) | 21 (24.72%) | 14 (37.84%) | 25 (8.36%) | P=0.000 |
| Indications for NSAIDs use no. (%) | |||||
| Cardiac-cerebral vascular disease | 12 (12.63%)* | 49 (60.67%)* | 11 (39.73%)* | ||
| Metabolic diseases | 31 (32.63%) | 15 (16.85%) | 9 (24.32%) | ||
| Osteoarticular diseases | 52 (54.74%)# | 25 (28.09%)# | 17 (45.95%) | ||
| Species of NSAIDs | |||||
| Aspirin | 43 | 64 | 20 | ||
| Diclofenac | 13 | 5 | 7 | ||
| Meloxicam | 15 | 9 | 5 | ||
| Celecoxib | 6 | 3 | 1 | ||
| Others | 18 | 8 | 4 | ||
| Hemoglobin (g/l )(mean ±SD) | 119.37±17.05 | 115.96±15.13 | 115.03±16.09 | 118.21±12.45 | P=0.084 |
SBE – single balloon endoscopy; CE – capsule endoscopy; OGIB – obscure gastrointestinal bleeding; SBO – small bowel obstruction; metabolic diseases include diabetes and hyperlipemia; Other indications included abdomen pain, diarrhea, distention, emaciation, anepithymia etc.
There was significant difference in the proportion of patients with cardiac-cerebral vascular disease within three enteroscopy group in the NSAIDs group (P<0.001).
The proportion of patients with osteoarticular diseases was higher in SBE group than in CE group (P=0.018). P<0.05 was considered statistically significant.
There were no differences in sex, age, or hemoglobin between the NSAIDs group and the control group. There were no significant differences in clinical features among the 3 enteroscopy subgroups in the NSAIDs group.
The indications for enteroscopy were different between the NSAIDs group and the control group (p=0.000, χ2=48.145). There were no significant differences in the proportion of patients with OGIB between the 2 groups (p=0.058, χ2=3.583); whereas patients with obstruction comprised only 2.84% in the NSAIDs group, such patients accounted for 20.40% in the control group (p=0.000, χ2=48.164). In contrast, non-typical symptoms were more frequent in the NSAIDs group than in the control group (23.22% vs. 8.36%, p=0.000, χ2=22.026). There were no significant differences in the proportion of symptoms within the 3 enteroscopy groups in the NSAIDs group (p>0.05).
There were significant differences in the proportion of patient indications for NSAIDs use within the 3 enteroscopy groups. Patients with cardiac-cerebral vascular disease comprised 60.67%, 12.63%, and 39.73% of the CE, SBE, and CE combined SBE groups, respectively (p=0.000, χ2=19.274). The proportion of patients with osteoarticular diseases was higher in the SBE group than in the CE group (54.74% vs. 28.09%, p=0.018, χ2=5.580). There were no significant differences in the proportion of patients with metabolic disease within the 3 groups (p=0.157, χ2=3.705).
Final results of the NSAIDs group and the non-NSAIDs group
NSAIDs-induced small bowel injuries were mainly nonspecific lesions, which included red spot, erosion, and morphologic ulcer and diaphragm. Endoscopic views of these injuries are shown in Figure 1. These injuries comprised 49.77% of the NSAIDs group. In contrast, small bowel tumor (20.24%) was the most frequent in the non-NSAIDs group, followed by vascular lesions (1.88%), other miscellaneous diseases (8.71%), nonspecific lesions (7.59%), Crohn’s disease (6.47%), congenital anomalies (diverticulum/repeat deformity) (5.69%), Behcet’s or related disease (2.67%), and infectious disease (2.6%). The final results of the NSAIDs group and the non-NSAIDs group are shown in Table 2.
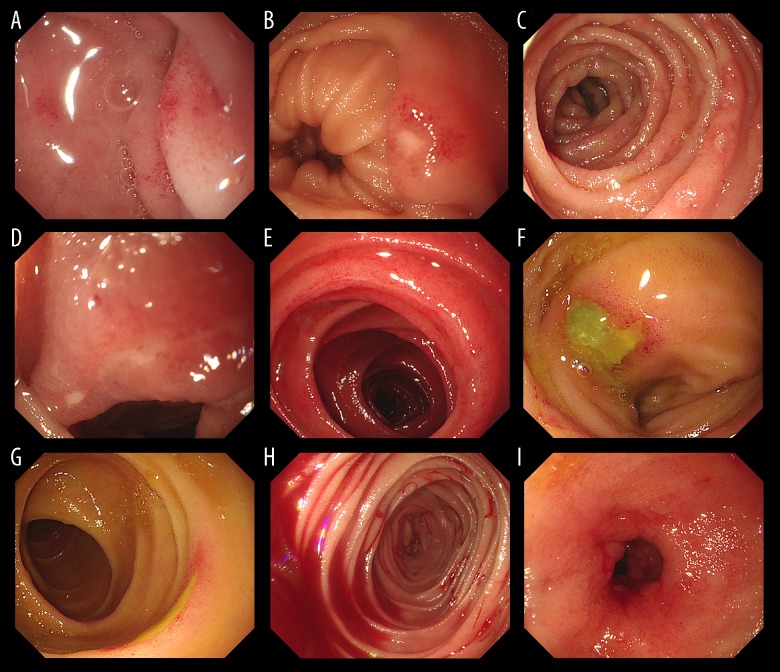
Figure 1.
Endoscopic views of the small-bowel injury induced by NSIADs. (A) Red spot; (B) erosion; (C) hemorrhagic spot and erosion; (D) linear scar; (E) annular scar; (F) round ulcer; (G) annular ulcer; (H) active bleeding; (I) diaphragm stricture.
Table 2.
Final results of the NSAIDs group and the non- NSAIDs group.
| NSAIDs group | NSAIDs group total | Non-NSAIDs group | |||
|---|---|---|---|---|---|
| SBE | CE | CE + SBE | |||
| NSAIDs injuries/Non-specific mucosal break(s) | 48 (50.5%) | 44 (46.4%) | 18 (48.65%) | 110 (49.77%) | 108 (7.59%) |
| Crohn’s disease | 5 (5.26%) | 4 (4.50%) | 2 (5.40%) | 11 (4.98%) | 92 (6.47%) |
| Behcet’s disease/related disease | 4 (4.21%) | 4 (4.50%) | 1 (2.70%) | 9 (4.07%) | 38 (2.67%) |
| Intestinal tuberculosis | 1 (1.05%) | 0 | 0 | 1 (0.452%) | 18 (1.26%) |
| Eosinophilic gastroenteritis | 0 | 1 (1.12%) | 2 (5.40%) | 3 (1.36%) | 21 (1.48%) |
| Allergic purpura | 2 (2.11%) | 2 (2.25%) | 1 (2.70%) | 5 (2.26%) | 29 (2.04%) |
| Infective disease | 0 | 1 (1.12%) | 2 (5.40%) | 3 (1.36%) | 37 (2.60%) |
| Small intestinal tumor/polyp | 2 (2.11%) | 2 (2.25%) | 2 (5.40%) | 6 (2.71%) | 288 (20.24%) |
| Vascular lesions | 3 (3.16%) | 2 (2.25%) | 1 (2.70%) | 6 (2.71%) | 169 (11.88%) |
| Congenital anomalies | 2 (2.11%) | 1 (1.12%) | 2 (5.40%) | 5 (2.26%) | 81 (5.69%) |
| Other miscellaneous diseases | 0 | 1 (1.12%) | 3 (8.11%) | 4 (1.80%) | 124 (8.71%) |
| Negative result | 28 (29.47%) | 27 (30.34%) | 3 (8.11%) | 58 (26.24%) | 418 (29.37%) |
| Total | 95 | 89 | 37 | 221 | 1423 |
SBE – single balloon endoscopy; CE – capsule endoscopy.
Comparison between NSAIDs injuries and other ulcerous diseases
Small bowel ulcerous diseases mainly included Crohn’s disease, Behcet’s/related disease, and intestinal tuberculosis in the non-NSAIDs group. The differences between these injuries and NSAIDs injuries are shown in Table 3.
Table 3.
Comparison of NSAIDs breaks and other ulcerous diseases (Crohn’s disease, Behcet’s disease and Intestinal tuberculosis).
| NSAIDs-injury (n=110) | Crohn’s disease (n=103) | Behcet’s disease (n=47) | Intestinal tuberculosis (n=19) | NSAIDs vs. Crohn’s disease | NSAIDs vs. Behcet’s disease | NSAIDs vs. intestinal tuberculosis | |
|---|---|---|---|---|---|---|---|
| Erosion/ulcer/both | 27/37/36 | 19/78/6 | 5/39/3 | 3/14/2 | P<0.001 | P<0.001 | P=0.011 |
| Location (jejunum/ileum/both) | 33/38/29 | 6/78/19 | 3/37/7 | 1/16/2 | P<0.001 | P<0.001 | P=0.001 |
| Lesion <1 cm | 74 (67.27%) | 38 (36.89%) | 15 (31.91%) | 4 (21.50%) | P<0.001 | P<0.001 | P<0.001 |
| Single/Multiple | 26/84 | 21/82 | 21/26 | 2/17 | P=0.568 | P=0.008 | P=0.201 |
| Ileocecal valve involved | 23 (20.91%) | 37 (35.92%) | 22 (46.81%) | 11 (57.89%) | P=0.015 | P=0.003 | P=0.002 |
| Deepth of ulcer | Superfical | Deep | Deep | Deep | |||
| Arrangement | Irregular | Longitudinal | Irregular | Annular | |||
| Relation to mesentery | Irregular | Mesenteric | Antimesenteric | Irregular | |||
| Stricture | Diaphragm-like | Longitudinal/Eccentric | Irregular | Annular/ concentric | |||
| Morphology | Round/annular/Longitudinal | Longitudinal/Irregular/Aphthous ulcers | Round/ irregular | Annular/ irregular |
P<0.05 was considered statistically significant.
Both erosion and ulcer were prevalent in NSAIDs injuries, while ulcer was more frequent in Crohn’s disease, Behcet’s/related disease, and intestinal tuberculosis (p<0.001, χ2=37.401; p<0.001, χ2=27.577; p=0.011, χ2=9.054 respectively). The risk of NSAIDs injuries in the jejunum and ileum was similar, while lesions in the other 3 diseases were observed mainly in the ileum (p<0.001, χ2=34.532; p<0.001, χ2=22.24; p=0.001, χ2=13.904, respectively). NSAIDs injuries were mostly superficial, with irregular arrangement, and no relation to the mesentery.
Multiple lesions were more prevalent than single lesions in NSAIDs injuries, Crohn’s disease, and intestinal tuberculosis, whereas single lesions occurred more frequently in Behcet’s/related disease. Lesions less than 1 cm were more frequent in NSAIDs injuries than in the other 3 diseases (p<0.001, χ2=19.689; p<0.001; p<0.001, χ2=16.767; p<0.001, χ2=14.479). The proportion of ileocecal valves involved in NSAIDs injuries (20.91%) was less than with Crohn’s disease (35.92%), Behcet’s/related disease (46.81%), and intestinal tuberculosis (57.89%) (p=0.015, χ2=5.925; p=0.003, χ2=8.532; p=0.002, χ2=9.526, respectively).
Diagnostic value of SBE in NSAIDs-induced injuries compared with CE
All patients in the NSAIDs group successfully underwent the SBE examination. Adverse reactions mainly included transient throat discomfort, abdominal distension, and abdominal pain. There were no serious complications. Patients in the CE group finished all small bowel examinations except for 1 patient with retention in the ileum.
Fifteen patients underwent SBE by the oral approach only and 34 patients by the anal approach only. Both approaches were carried out in 46 patients. The whole small intestine was examined in 22 patients (23.19%). The detection rate of SBE for small bowel diseases in the NSAIDs group was 69.47% (66/95) and the diagnostic accuracy rate was 83.58% (56/67). NSAIDs-induced injuries were identified in 48 patients (50.53%, 48/95). There were 8 mistakes made by SBE: 6 cases of NSAIDs injuries were misdiagnosed as 4 cases of Crohn’s disease and 2 cases of Behcet’s or related disease; and 1 case of Crohn’s disease and 1 case of Behcet’s or related disease were misdiagnosed as NSAIDs breaks. Two patients with active bleeding caused by NSAIDs-induced ulcers at the duodenal horizontal section and jejunum had hemostasis successfully performed with electrocoagulation and titanium clamping by SBE, respectively. One patient with active bleeding was diagnosed with hemangioma during surgery, while SBE could not find the lesion because of blurred vision.
The detection rate of CE for small bowel diseases in the NSAIDs group was 66.29% (59/89), and the diagnostic accuracy rate was 80.65% (50/62). There were 44 patients (49.44%, 44/89) verified to have NSAIDs breaks. There were 13 mistakes made by CE: 4 cases of NSAIDs injuries were misdiagnosed as 3 cases of Crohn’s disease and 1 case of Behcet’s or related disease; and 1 case of eosinophilic gastroenteritis, 1 case of allergic purpura, 1 case of infectious disease, and 1 case of vasculitis were all misdiagnosed as NSAIDs breaks. Three patients with active bleeding and no identifiable cause were finally diagnosed with vascular malformation, diverticulum, and mesenchymoma during the surgery. One patient with CE retention because of the diaphragm accepted surgical intervention.
The detection rate and diagnostic accuracy rate of SBE were similar with that of CE (69.47% vs. 66.29%, p=0.644, χ2=0.214; 83.58% vs. 80.65%, p=0.663, χ2=0.190, respectively) in the NSAIDs group.
The specificity and positive predictive value of SBE for diagnosis of NSAIDs breaks were higher than those with CE (95.74% vs. 80.00%, p=0.02, χ2=5.414; 95.45% vs. 81.63%, p=0.039, χ2=4.247, respectively), while there were no differences in the sensitivity and negative predictive value between the 2 methods (87.50% vs. 90.91%, p=0.6, χ2=0.275; 88.24% vs. 90.00%, p=0.789, χ2=0.071, respectively).
Thirty-three patients underwent CE examination followed by SBE, and 4 patients underwent examinations by the other approach. Antegrade SBE was carried out in 6 patients and retrograde SBE was performed in 21 patients, and both of the approaches were performed in 10 patients. The diagnostic accuracy rate was 72.97% (27/37) by only 1 examination approach.
The detection rate of CE combined with SBE for small bowel diseases in the NSAIDs group was 91.8% (34/37), the diagnostic accuracy rate was 88.24% (30/34), and the diagnostic accuracy rate for NSAIDs breaks was 94.4% (17/18). The consistency in diagnosing lesions for CE and SBE was 67.57% (25/37) and the final diagnoses were not consistent in 12 cases. The consistency of diagnosis in NSAIDs breaks for CE and SBE was 82.61% (19/23). Inspection results of CE combined with SBE in the NSAIDs group are shown in Table 4. One patient using diclofenac sodium with active bleeding caused by an ulcer at the distal ileum had hemostasis successfully performed with injection of lauromacrogol by SBE.
Table 4.
Inspection results of capsule endoscopy combined with single balloon endoscopy in the NSAIDs group.
| CE (n) | SBE (n) | Final diagnosis (n) | |
|---|---|---|---|
| Consistency of diagnosis (22) | NSAIDs (17) | NSAIDs (17) | NSAIDs (17) |
| NSAIDs (2) | NSAIDs (2) | Simple ulcer (1) Crohn’s disease (1) |
|
| Crohn’s disease (1) | Crohn’s disease (1) | NSAIDs (1) | |
| Radiation enteritis (1) Allergic purpura (1) |
Radiation enteritis (1) Allergic purpura (1) |
Radiation enteritis (1) Allergic purpura (1) |
|
| Non-consistency of diagnosis (5) | NSAIDs (4) | Infectious disease (1) Ischemic enteritis (1) Eosinophilic gastroenteritis (2) |
Infectious disease (1) Ischemic enteritis (1) Eosinophilic gastroenteritis (2) |
| Submucosal lesion (1) | Amyloidosis (1) | Amyloidosis (1) | |
| CE negative result (5) | 5 | Diverticulum with ulcer (2) Parasite (1) Small intestinal lymphangiectasia (1) Vascular malformation (1) |
Diverticulum with ulcer (2) Parasite (1) Small intestinal lymphangiectasia (1) Vascular malformation (1) |
| SBE negative result (2) | Mesenchymoma (1) Polyp (1) | 2 | Mesenchymoma (1) Polyp (1) |
| CE+SBE negative result (3) | 3 | 3 | 3 |
SBE – single balloon endoscopy; CE – capsule endoscopy.
SBE detected 35 lesions that were missed by CE in 5 patients. One patient with 5 erosions and 3 ulcers in the upper jejunum found by CE had 9 erosions and 5 ulcers detected by SBE. SBE detected 4 red spots, 33 erosions, and 44 ulcers at the ileum in 4 patients who were diagnosed with 1 red spots, 22 erosions, and 29 ulcers by CE. The lesions missed by CE were mainly 0.2–0.5 cm, and 25 lesions were located at the distal ileum. The missed ulcers by CE included 12 annular ulcers/scars and 5 round ulcers.
Discussion
NSAIDs-induced gastric and duodenal breaks have been recognized for a long time, while small-intestine injuries have not been emphasized until the advent of VCE and deep enteroscopy in recent years [12–14]. NSAIDs-induced intestinal damage includes the development of increased mucosal permeability, inflammation, erosion, ulcer, malabsorption, stricture, perforation, and intestinal bleeding [15–17]. Obscure gastrointestinal (GI) bleeding was the most frequent clinical manifestation (75.11%), followed by nonspecific symptoms (21.72%) and obstruction (3.17%) in the NSAIDs group in the present study [18,19]. The diagnostic accuracy rates for NSAIDs injuries was 49.77%, which agrees with results of other studies [20–22]. Previous VCE studies have indicated NSAIDs-induced lesions in 50–71% of NSAIDs users [23–25]. These studies analyzed healthy subjects or asymptomatic patients and suggested that most lesions were not clinically pathogenic; however, patients without typical symptoms and confusing enteroscopy examination were excluded from our research, and other ulcerative diseases such as Crohn’s disease, infectious disease, and eosinophilic gastroenteritis that were misdiagnosed as NSAIDs injuries by CE were ultimately verified by SBE.
We investigated the clinical features of the NSAIDs group compared with the control group. There were no significant differences in sex, age, and hemoglobin between the 2 groups. It was shown that there were significant differences in the proportion of clinical manifestations between the 2 groups. OGIB was the most frequent in both groups. Obstruction was less frequent in the NSAIDs group than in the control group because diaphragm-like stricture was a relatively rare NSAIDs-induced complication, whereas small-bowel tumors, vascular lesions, and other miscellaneous diseases were more frequent in the non-NSAIDs group [26]. It was reported that in 2% of patients taking conventional NSAIDs on a long-term basis, small-bowel diaphragm disease developed [27]. Wang YZ analyzed 72 papers about NSAIDs-induced diaphragm disease in their research and reported that a majority (59.7%) of diaphragm disease was seen in the small bowel, and were mainly located in the ileum (57.9%); multiple diaphragms were found in 80% of patients, and nearly 75% of patients with diaphragm disease underwent surgery; endoscopic balloon dilation was performed in 22 patients, and NSAIDs were withdrawn in 53 patients [28]. Diaphragm-like stricture was detected in only 3 patients in our research because there were few patients taking long-term NSAIDs, and a few patients with serious intestinal obstruction underwent surgery and had neither SBE nor CE examination. As CE has the risk of retention, it is necessary to carefully choose CE examination for patients with obstruction. Due to the high anesthesia risk and low tolerance of balloon endoscopy for patients with cardiac-cerebral vascular disease, the proportion of these patients (60.67%) in the CE group was higher than that in the SBE group (12.63%). It was suggested that doctors were inclined to recommend CE for patients with cardiac-cerebral vascular disease and performed SBE for other patients with osteoarthropathy, metabolic disease, or connective tissue diseases.
The enteroscopic features of NSAIDs breaks mainly included red spot, erosion and morphologic ulcerations such as round/longitudinal/annular ulcer, and linear ulcer/scar, but diaphragm stricture was rarely detected [9,10,29]. Red spot, erosion, and ulcerations can co-exist in the same patient [30]. Because of multiple lesions in NSAIDs breaks, there was no difference in the detection rate and sensitivity between CE and SBE. However, the specificity and positive predictive value of SBE for diagnosis of NSAIDs breaks were higher than those with CE (95.74% vs. 80.00%; 95.45% vs. 81.63%). Annular or linear scar and less than 0.5-cm-diameter ulcers were more easily missed by CE in the present research. We determined that SBE can be used for precise biopsy and to observe the lesion carefully and repeatedly. Although the pathological examination of NSAIDs injuries was nonspecific, it has a certain value for identification of infectious disease, eosinophilic gastroenteritis, and allergic purpura.
We found that Crohn’s disease, Behcet’s disease, and infectious disease were the main diseases that were easily mistaken as NSAIDs injuries. Identification of these diseases depended on enteroscopic feature, biopsy, and follow-up. There is no relevant report on how to identify such diseases until now. We compared the characteristics of NSAIDs breaks that differ from the other 3 ulcerous diseases as follows: 1) lesions were mostly superficial, multiple, and irregular arrangement; 2) the risk of damage was similar in the jejunum and ileum; 3) lesions of less than 1 cm were more frequent; 4) ileocecal valve involvement was detected less frequently.
The retrospective design of the present study had some limitations. First, we failed to analyze the characteristics of the injuries induced by different dosages and types of NSAIDs. Second, patients without typical clinical features were not enrolled in our research. Large-sample-size and well-designed prospective cohort studies are needed in the future.
Conclusions
In conclusion, NSAIDs-induced small-bowel injuries can be distinguished from other intestinal diseases by certain special endoscopic features and biopsy. SBE appears to be an effective method for diagnosis and treatment of NSAIDs injuries.
Acknowledgments
We thank all members of the department of gastroenterology and laboratory in Binzhou, Yantai and Weihai affiliated hospitals of Binzhou Medical University and Yantai Yuhuangding affiliated hospital of Qingdao Medical University for helpful discussions and comments on the manuscript.
Footnotes
Source of support: This work was supported in part by the Natural Science Foundation of Shandong (ZR2014HM009), the Projects of Medical and Health Technology Development Program of Shandong Province (2015WSB38008), and the Projects of Binzhou Medical University (BK2015KJ41)
Conflict of interest
None.
References
- 1.Sostres C, Gargallo CJ, Lanas A. Nonsteroidalanti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res Ther. 2013;15(Suppl 3):S3. doi: 10.1186/ar4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340:1888–99. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro I, Pinho R, Rodrigues A, et al. Obscure gastrointestinal bleeding: Which factors are associated with positive capsule endoscopy findings? Rev Esp Enferm Dig. 2015;107:334–39. [PubMed] [Google Scholar]
- 4.Endo H, Sakai E, Taniguchi L, et al. Risk factors for small-bowel mucosal breaks in chronic low-dose aspirin users: Data from a prospective multicenter capsule endoscopy registry. Gastrointest Endosc. 2014;80:826–34. doi: 10.1016/j.gie.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Maiden L, Thjodleifsson B, Theodors A, et al. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172–78. doi: 10.1053/j.gastro.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein JL, Eisen GM, Lewis B, et al. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133–41. doi: 10.1016/s1542-3565(04)00619-6. [DOI] [PubMed] [Google Scholar]
- 7.Gill RS, Kaffes AJ. Small bowel stricture characterization and outcomes of dilatation by double-balloon enteroscopy: A single-centre experience. Therap Adv Gastroenterol. 2014;7:108–14. doi: 10.1177/1756283X13513995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Büschel P, Mönkemüller K, von Falkenhausen U, et al. Emergency double balloon enteroscopy: A feasible and promising diagnostic as well as possible therapeutic option in recurrent midgut bleeding. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.06.2010.3068. pii: bcr0620103068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi Y, Yamamoto H, Taguchi H, et al. Nonsteroidal anti-inflammatory drug-induced small-bowel lesions identified by double-balloon endoscopy: Endoscopic features of the lesions and endoscopic treatments for diaphragm disease. J Gastroenterol. 2009;44(Suppl 19):57–63. doi: 10.1007/s00535-008-2277-3. [DOI] [PubMed] [Google Scholar]
- 10.Ishihara M, Ohmiya N, Nakamura M, et al. Risk factors of symptomatic NSAIDs-induced small intestinal injury and diaphragm disease. Aliment Pharmacol Ther. 2014;40:538–47. doi: 10.1111/apt.12858. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto H, Sekine Y, Sato Y, et al. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53(2):216–20. doi: 10.1067/mge.2001.112181. [DOI] [PubMed] [Google Scholar]
- 12.Scarpignato C, Hunt RH. Nonsteroidal antiinflammatory drugrelated injury to the gastrointestinal tract: Clinical picture, pathogenesis, and prevention. Gastroenterol Clin North Am. 2010;39:433–64. doi: 10.1016/j.gtc.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Rostom A, Wells G, Tugwell P, et al. The prevention of chronic NSAID induced upper gastrointestinal toxicity: A Cochrane collaboration meta-analysis of randomized controlled trials. J Rheumatol. 2000;27(9):2203–14. [PubMed] [Google Scholar]
- 14.Maiden L. Capsule endoscopic diagnosis of nonsteroidal anti-inflammatory drug-induced enteropathy. J Gastroenterol. 2009;44(Suppl 19):64–71. doi: 10.1007/s00535-008-2248-8. [DOI] [PubMed] [Google Scholar]
- 15.Richy F, Bruyere O, Ethgen O, et al. Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: A consensus statement using a meta-analytic approach. Ann Rheum Dis. 2004;63(7):759–66. doi: 10.1136/ard.2003.015925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sidhu R, Brunt LK, Morley SR, et al. Undisclosed use of nonsteroidal anti-inflammatory drugs may underlie small-bowel injury observed by capsule endoscopy. Clin Gastroenterol Hepatol. 2010;8:992–95. doi: 10.1016/j.cgh.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Chan FK, Cryer B, Goldstein JL, et al. A novel composite endpoint to evaluate the gastrointestinal (GI) effects of nonsteroidal antiinflammatory drugs through the entire GI tract. J Rheumatol. 2010;37:167–74. doi: 10.3899/jrheum.090168. [DOI] [PubMed] [Google Scholar]
- 18.Cho KM, Park SY, Chung JO, et al. Risk factors for small bowel bleeding in chronic nonsteroidal anti-inflammatory drug users. J Dig Dis. 2015;16:499–504. doi: 10.1111/1751-2980.12269. [DOI] [PubMed] [Google Scholar]
- 19.Cryer B, Li C, Simon LS, et al. GI-REASONS: A novel 6-month, prospective, randomized, open-label, blinded endpoint (PROBE) trial. Am J Gastroenterol. 2013;108(3):392–400. doi: 10.1038/ajg.2012.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto T, Kudo T, Esaki M, et al. Prevalence of non-steroidal anti-inflammatory drug-induced enteropathy determined by double-balloon endoscopy: A Japanese multicenter study. Scand J Gastroenterol. 2008;43:490–96. doi: 10.1080/00365520701794121. [DOI] [PubMed] [Google Scholar]
- 21.Fujimori S, Hanada R, Hayashida M, et al. Celecoxib monotherapy maintained small intestinal mucosa better compared with loxoprofen plus lansoprazole treatment: A double-blind, randomized, controlled trial. J Clin Gastroenterol. 2016;50(3):218–26. doi: 10.1097/MCG.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 22.Scarpignato C, Dolak W, Lanas A, et al. Rifaximin reduces the number and severity of intestinal lesions associated with use of nonsteroidal anti-inflammatory drugs in humans. Gastroenterology. 2017;152(5):980–82.e3. doi: 10.1053/j.gastro.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein JL, Eisen GM, Lewis B, et al. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133–41. doi: 10.1016/s1542-3565(04)00619-6. [DOI] [PubMed] [Google Scholar]
- 24.Fujimori S, Seo T, Gudis K, et al. Prevention of nonsteroidal anti-inflammatory drug-induced small-intestinal injury by prostaglandin: A pilot randomized controlled trial evaluated by capsule endoscopy. Gastrointest Endosc. 2009;69:1339–46. doi: 10.1016/j.gie.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55–59. doi: 10.1016/s1542-3565(04)00603-2. [DOI] [PubMed] [Google Scholar]
- 26.Slesser AA, Wharton R, Smith GV, Buchanan GN. Systematic review of small bowel diaphragm disease requiring surgery. Colorectal Dis. 2012;14(7):804–13. doi: 10.1111/j.1463-1318.2011.02741.x. [DOI] [PubMed] [Google Scholar]
- 27.Maiden L, Thjodleifsson B, Seigal A, et al. Long-term effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective agents on the small bowel: A cross-sectional capsule enteroscopy study. Clin Gastroenterol Hepatol. 2007;5:1040–45. doi: 10.1016/j.cgh.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 28.Wang YZ, Sun G, Cai FC, Yang YS. Clinical features, diagnosis, and treatment strategies of gastrointestinal diaphragm disease associated with nonsteroidal anti-inflammatory drugs. Gastroenterol Res Pract. 2016;2016:3679741. doi: 10.1155/2016/3679741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe T, Takeuchi T, Handa O, et al. A multicenter, randomized, double-blind, placebo-controlled trial of high-dose rebamipide treatment for low-dose aspirin-induced moderate-to-severe small intestinal damage. PLoS One. 2015;10(4):e0122330. doi: 10.1371/journal.pone.0122330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwamoto J, Mizokami Y, Saito Y, et al. Small-bowel mucosal injuries in low-dose aspirin users with obscure gastrointestinal bleeding. World J Gastroenterol. 2014;20:13133–38. doi: 10.3748/wjg.v20.i36.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]