Abstract
Introduction:
The 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) is a popular recreational drug and a major source of substance abuse, which ultimately leads to sensations of well-being, elation and euphoria, moderate derealization/depersonalization, and cognitive disruptions, as well as intense sensory awareness. The mechanisms involved in memory impairment induced by MDMA are not completely understood.
Methods:
The current study used 40 Sprague-Dawley rats, weighted 200 to 250 g. Experiments were performed in four groups, each containing 10 rats. The first group of rats was used as the control, treated with dimethyl sulfoxide (DMSO). The second group was treated with MDMA. The third group was treated with MDMA and CGS (the adenosine A2A receptor agonist, 2-[p-(2-carboxyethyl) phenethylamino]-5′-N-ethylcarboxamidoadenosine) (CGS 21680) and the fourth group was treated with MDMA and SCH (the A2A receptor antagonist [7-(2-phenylethyl)-5-amino-2-(2-furyl-) pyrazolo-[4, 3-e]-1, 2, 4 triazolo [1,5-] pyrimidine]) (SCH 58261). The drugs in all groups were administrated intraperitoneally (i.p.) once a day for 7 days. In 5 rats of each group, following perfusion, samples were taken from hippocampi to investigate apoptosis. Accordingly, the samples were stained using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay kit, and studied by light microscopy. In other rats, fresh tissue was also removed to study the expression of bax and bcl-2 by Western blotting technique.
Results:
It was observed that the coadministration of MDMA with CGS reduced bax expression and prevented apoptosis of hippocampal cells. The coadministration of MDMA and SCH increased bax expression, and also increased the frequency of hippocampal cell apoptosis.
Conclusion:
The results of the current study showed that administration of CGS with MDMA decreased the common side effects associated with MDMA.
Keywords: Ecstasy or MDMA, Neurotoxicity, Adenosine receptor, Agonist of A2A receptor, Antagonist of A2A receptor
1. Introduction
Ecstasy (3,4-methylenedioxymethamphetamine; MDMA) is a psychoactive hallucinogenic compound considered as a source of substance abuse worldwide. It is a derivative of amphetamine related to the hallucinogenic compound, and the United Nations estimated that worldwide use of ecstasy affects 9 million people aged 15 to 64 years. Currently, there are more than 3 million ecstasy users in Europe, which represent 36% of ecstasy users worldwide (Capela, et al., 2009). MDMA produces feeling of well-being, comfort, and elation. The use of this drug also leads to a sense of moderate derealization/depersonalization, and cognitive distortions, as well as intense sensory awareness (Liechti, 2000).
Several studies reported that MDMA has the capacity to induce toxicity in neurons. Despite researches in this context, precise mechanisms of MDMA are not obvious. MDMA induces neuronal damage in several brain areas such as the hippocampus, striatum, and cortex (Battaglia, Yeh, & DeSouza, 1988; Riezzo, et al., 2010). Several factors contributed to MDMA-induced neurotoxicity, specifically hyperthermia, monoamine oxidase metabolism of dopamine and serotonin, mitochondrial dysfunction, dopamine oxidation, serotonin transporter action, formation of peroxinitrite, glutamate excitotoxicity, and importantly deficits in serotonergic biochemical markers (Broening, Bowyer, & Slikker, 1995; Fumagalli, et al., 1999; Pu, Broening, & Vorhees, 1996; Deng & Cadet, 1999; Sheng, Cerruti, Ali, & Cadet, 1996).
The hippocampus is a brain area particularly susceptible to the neurotoxic effects of MDMA. The hippocampus is critical for learning and memory (Kesner, 2007). The most complaint of MDMA is impairment in short-term memory. MDMA users show significant deficiency in delayed memory tasks, which directly associate with the increase in 5-HT receptor binding ratios. Significantly, those who take MDMA have longer reaction time to visual and auditory stimuli, lower visual recall, and lower working memory scores (Green, Mechan, Elliott, O’Shea, & Colado, 2003). Deficiency in spatial learning and memory is obvious in rats treated with MDMA (Sheng, et al., 1996; Gibb, Johnson, & Hanson, 1990; Steranka & Rhind, 1987).
In human abusers of MDMA, the most consistent finding is the impairments in short-term memory (Bolla, McCann, & Ricaurte, 1998; Vorhees, Reed, Skelton, & Williams, 2004; Able, Gudelsky, Vorhees, & Williams, 2006). It is shown that the multiple-time administration of MDMA produces persistent deficiency in biochemical markers of 5-HT axon terminals in some brain areas such as hippocampus (Green, et al., 2003; Able, et al., 2006).
Despite the persistent effects of MDMA on 5-HT axon terminals, there is evidence that MDMA produces neuronal degeneration within the hippocampus (Kermanian, et al., 2012; Warren, et al., 2007). The mechanism of MDMA-induced depletion of the central nervous system (CNS) serotonin (5-hydroxytryptamine, 5-HT) is believed to involve the generation of Reactive Oxygen Species (ROS) (Broening, et al., 1995; Kermanian, et al., 2012; Warren, et al., 2007). The process of apoptosis is controlled by a diverse range of cell signals, which may originate either extracellularly via extrinsic inducers, or intracellularly via intrinsic inducers. The involvement of ROS at different phases of the neuronal apoptotic pathways is clearly established (Maycotte, Guemez-Gamboa, & Moran, 2012).
Extracellular adenosine acts via receptors coupled with G-protein (adenosine receptor subtypes A1, A2A, A2B, and A3) and exerts diverse physiological effects (Fredholm, Jacobson, Klotz, & Linden, 2001). A number of cellular components regulate apoptosis. The Bcl-2 protein can inhibit apoptosis by direct action, while Bax and/or Bak promote apoptosis (Kuwana & Newmeyer, 2003). The current study already found that A2A agonist and CGS treatment may protect against MDMA induced apoptosis in striatum (Soleimani, Katebi, Alizadeh, Mohammadzadeh, & Mehdizadeh, 2012). The current study aimed at investigating the interaction between A2A receptor and MDMA treatment at the molecular level in hippocampus.
2. Methods
2.1. Drugs and chemicals
MDMA hydrochloride, and other reagents used in the present experiment were purchased from Sigma Chemical (Sigma, LaJola, CA, USA.); the adenosine A2A receptor agonist, (2-[p-(2-carboxyethyl) phenethylamino]-5′-N-ethylcarboxamidoadenosine) (CGS 21680), and the A2A receptor antagonist [7-(2-phenylethyl)-5-amino-2-(2-furyl-) pyrazolo-[4,3-e]-1,2,4 triazolo [1,5-] pyrimidine] (SCH58261) were purchased from Tocris Cookson (Ball-win, MO, USA). CGS and SCH in a dose of 0.03 mg/kg body weight dissolved in 10% dimethylsulfoxide (DMSO) were administered intraperitoneally (i.p.) in animals.
2.2. Animals
A total of 40 adult male Sprague-Dawley rats (Pasture Institute, Tehran, Iran), weighted 200 to 500 g were used in the current study. Rats were maintained at the animal house under standard conditions (food and water ad libitum, 12:12 hours light/dark cycle, 21±3°C). All experiments involving rats were approved by the Animal Care Committee of Iran University of Medical Sciences, Tehran, Iran. Rats were randomly assigned into 4 experimental groups (n=10): Control: 10% DMSO 1 mL/kg, i.p., once a day for seven days. Treatment I: MDMA 10 mg/kg, i.p., once a day for seven days. Treatment II: MDMA+CGS, i.p., once a day for seven days. Treatment III: MDMA+SCH, i.p., once a day for seven days.
At the end of the seventh day, 5 rats in each group were decapitated and perfused. Their brains were removed for terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) test. TUNEL is a widely used method to detect apoptotic cells in tissue sections. Five rats in each group were killed and their brains were removed for the Western blotting study.
2.3. Tissue preparation
2.3.1. TUNEL staining
Rats were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and, then, an incision was made on the skin to expose heart. Another incision was made on left ventricle to enter the perfusion tube; 10 to 150 mL of normal saline with 0.1 mL of heparin perfused to remove blood from vessels, followed by 150 to 200 mL of paraformaldehyde 4% in 0.1 M/L phosphate buffer (pH 7.4) as fixative solution. Then, whole brains were extracted. Tissue was processed for paraffin embedding and sagittally sectioned at 5 μm. The sections were deparaffinized and dehydrated by heating at 60°C in oven for 60 minutes and, then, rehydrated by xylol and graded ethanol solution, respectively.
Then, the histological samples were incubated in proteinase K (15 μg/mL) for half an hour. After that, the sections were quenched in 3% hydrogen peroxide/methanol for 10 minutes, in dark at room temperature. After 3 washes in Tris wash buffer (each 5 minutes), the sections were incubated with TUNEL reaction mixture for 1 hour at 37°C. Sections were washed in Tris wash buffer 3 times for 5 minutes each and, then, incubated with POD for 15 minutes at 37°C. Again, sections were washed in Tris wash buffer 3 times for 5 minutes each and, then, color development was performed in the dark room with DAB for 15 minutes. Then, hematoxylin solution was used as counter stain. After washing in Tris wash buffer 3 times for 5 minutes, the number of TUNEL positive CA1 neurons per mm length of the medial CA1 pyramidal cell layer was counted carefully in 5 sections per animal. Cell counts from the hippocampus on each of the 5 sections were averaged to provide the mean value.
2.3.3. Western blot analysis
After anesthesia, craniotomy was performed and the brain was removed and placed on ice. The meninges were removed and hippocampus was separated from the hemispheres, snapped frozen in liquid nitrogen, and stored at −70°C. Collected tissues were homogenized in an ice-cold homogenizing buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), and 0.5 mM Triton X-100, pH 7.4) and protease inhibitor cocktail tablets (Roche, Germany) for 1 hour and, then, were centrifuged (Eppendorf, Hamburg, Germany) at 12 000 g for 20 minutes at 4°C. The supernatant was removed and the protein concentration was determined with a Bio-Rad assay system (Bio-Rad, San Francisco, CA, USA). The protein extracts (10 μg) were run on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE), and electroblotted on to nitrocellulose membranes (Millipore, USA).
The membranes were, then, stained with washable Ponceau S solution to confirm equal protein loading. After washing the membranes with distilled water, they were blocked with Tris-buffered saline containing 0.02% Tween-20 and 5% of nonfat milk. Antibodies for Bax (mouse monoclonal, 1:1000 dilution; Beyotime Biotech) and Bcl-2 (mouse monoclonal, 1:1000 dilution; Beyotime Biotech) were applied at 4°C. The blots were, then, washed and incubated with respective alkaline phosphatase-coupled secondary antibodies (Bio-Rad) at 1:10000 dilutions. After extensive washing, the protein bands detected by the antibodies were analyzed. Values were compared using densitometric measurements using an image analysis system (UVI doc, Houston, Texas, USA) and explain by optical density (OD) ratio.
2.4. Statistical analysis
Data were shown as mean±structural equation modeling (SEM). All statistical analyses were conducted by SPSS software version 15. Differences between the groups were performed using one-way ANOVA and the Tukey test. A value of P<0.05 was considered statistically significant.
3. Results
3.1. CGS decreased and SCH increased the cell apoptosis induced by MDMA
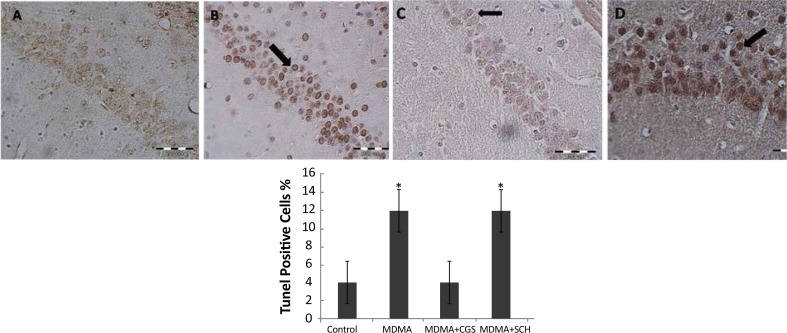
The number of TUNEL-positive cells in MDMA treated rats were significantly higher than those of the control group (P<0.05). Interestingly, the number of TUNEL-positive cells significantly reduced in CGS group compared to MDMA group (Figure 1). The number of TUNEL-positive cells in rats with antagonist treatment (SCH) significantly increased when compared to that of MDMA group (P<0.05) (Figure 2).
Figure 1.
Tunel assay of hippocampi. A: control, B: MDMA, C: MDMA+CGS, D: MDMA+SCH groups. Black arrow shows apoptotic cells. *: P>0.05
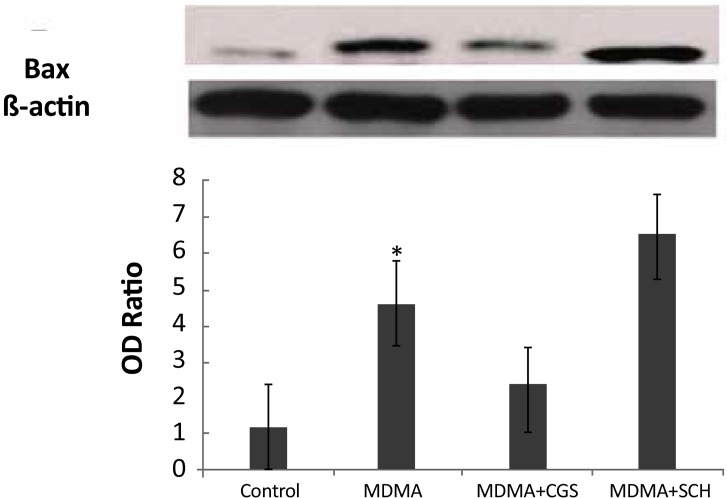
Figure 2.
Western blot analysis of BAX expression in the rat hippocampi. *: P>0.05
3.2. Involvement of Bcl-2 and Bax Proteins in the cell apoptosis-induced MDMA
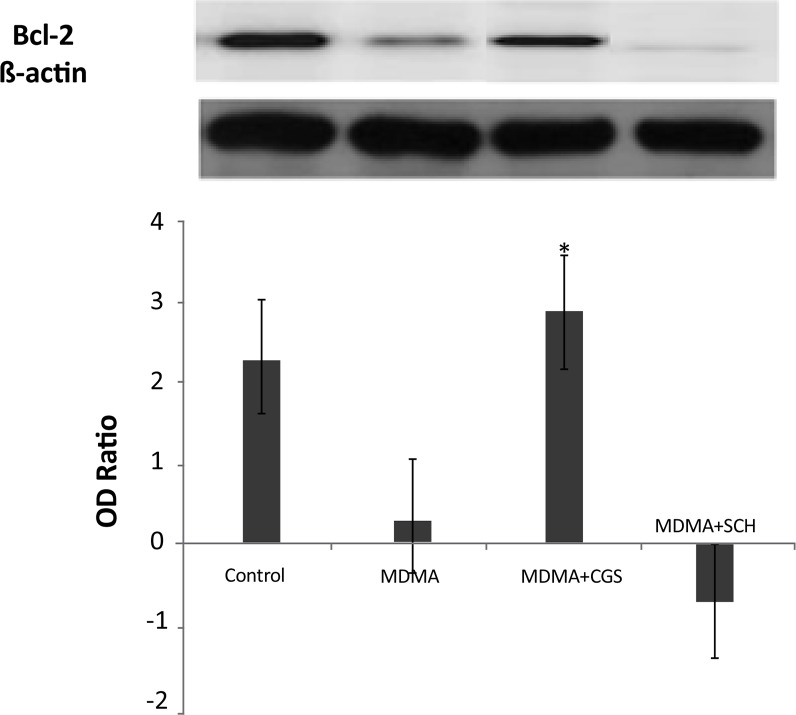
The expression of bcl-2 significantly increased in MDMA+CGS group, compared with MDMA group (P<0.05). The expression of bcl-2 significantly decreased and bax expression significantly increased in MDMA group, compared with the control group (P<0.05). The bax expression increased in rats with antagonist treatment (SCH), compared with MDMA group (Figure 3).
Figure 3.
Western blot analysis of BCL-2 expression in the rat hippocampi. *: P>0.05
The data are shown as means±SEM. The number of rats in each group was 5. The data were analyzed by oneway ANOVA, followed by the Tukey Test (P<0.05).
4. Discussion
The current study investigated the influence of adenosine receptor agonist and antagonist on the MDMA induced apoptosis in hippocampus. A major finding of is the current study was that MDMA can cause apoptosis intra hippocampus mediated by A2A adenosine receptors via mitochondrial pathways. Schmued and Bowyer (1997) showed that in 70% of the mice, degeneration of neurons occurred in the different species within 5 days after methamphetamine administration, which occasionally took place in the hippocampus and cortex (Schmued & Bowyer, 1997). In fact, it was shown that methamphetamine (METH)-induced increases in TUNEL-positive cells, and caspase-3 activation, and poly (ADP-ribose) polymerase (PARP) cleavage are all attenuated in Cu/Zn-SOD mice (Deng & Cadet, 2000).
The current study found similar results showing that the number of TUNEL-positive cells in MDMA-treated rats significantly increased. It is known that bcl-2 family plays a key role in the apoptosis induced by amphetamines (Montiel-Duarte, et al., 2002; Capela, et al., 2007). MDMA induces modifications in the expression of the splice variants of the bcl-x gene in neurons (Stumm, et al., 1999). The fact was that MDMA diminished Bcl-XL protein levels pointed out to mitochondria as a target for its pro-apoptotic effect, since Bcl-2 family proteins modulate the permeabilization of mitochondrial membranes and the subsequent liberation of pro-apoptotic factors such as cytochrome C (Tsujimoto, Shimizu, Narita, & Tsujimoto, 1999). Bcl-2 inhibits the release of the cytochrome C from mitochondria and cause inhibition of caspase activation and apoptosis (He, Xu, Yang, Zhang, & Li, 2004).
It was previously reported that METH induces significant increases in the pro-death bcl-2 family genes bad, bax and bid, and decreases in the anti-death genes bcl-2 and bcl-xl (Cadet, Jayanthi, & Deng, 2005; Jayanthi, Deng, Bordelon, Mccoy, & Cadet, 2001). Moreover, an increase caspase-3 activity was reported in the hippocampus of rats followed by a neurotoxic does of MDMA (Tamburini, 2006). The current study results suggested that MDMA administration might cause increases in the pro-death/anti-death ratio of the bcl-2 family of genes leading to apoptosis in hippocampus.
In agreement to the current study results, it was already reported that METH treatment downregulated bcl-2 in the striatum in mice (Imam, et al., 2001). Authors’ previous study described that coadministration of MDMA with A2A adenosine antagonist, SCH produces a proapoptotic effect, increasing the expression of bax mRNA in rat striatum (Cadet, Ordonez, & Ordonez, 1997). The current study found that the number of TUNEL-positive cells in CGS and MDMA treated group significantly reduced, compared to that of MDMA-treated group. Decrement of apoptosis after the use of A2A receptor agonists are related to the changes of bax and bcl-2 expression that protect neurons after ischemia (Rosin, Hettinger, Lee, & Linden, 2003; Zamani, et al., 2013; Soleimani, et al., 2012; Zamani, Katebi, Mehdizadeh, Mohamadzadeh, & Soleimani, 2012) It was already reported that increased expression of bcl-2 can prevent apoptosis of immortalized neuron cells by methamphetamine (Rosin, et al., 2003).
Jayanthi et al. (2001) reported that injection of methamphetamine causes the activation of apoptotic pathways such as upregulation of bax and downregulation of bcl-2. It was in agreement with the current study results describing that MDMA treatment cause upregulation of bax and downregulation of bcl-2. The presented results significantly reversed by adenosine receptor agonists and intensified by adenosine receptor antagonists. Kermanian et al. (2012) also indicated that A2A agonist can protect against MDMA neurotoxic effects. The current study results suggested that MDMA induced apoptosis intra hippocampus was partly mediated via the mitochondrial pathway and was significantly associated with the adenosine A2A receptors. These data might suggest that adenosine agonist could be used as an agent to treat MDMA induced neurotoxic effects.
The current study investigated the involvement of the selective adenosine A2A receptor agonists in the development of apoptosis associated with acute MDMA injection. The results indicated that stimulation of the adenosine A2A receptor plays a certain role in modulating the neuroadaptive changes appearing during MDMA treatment, and that the adenosine A2A receptor agonists may serve as useful drugs to protect MDMA injury. Hence, the current investigation introduced adenosine A2A agonists as possible vehicles for pharmacotherapy of MDMA dependence.
Acknowledgements
Both Iran University of Medical Sciences and Hormozgan University of Medical Sciences are the financial supporters of the present study.
Footnotes
Conflict of Interest
The authors declared no conflicts of interest.
References
- Able J. A., Gudelsky G. A., Vorhees C. V., Williams M. T. (2006). 3,4-Methylenedioxymethamphetamine in adult rats produces deficits in path integration and spatial reference memory. Biological Psychiatry, 59(12), 1219–26. doi: 10.1016/j.biopsych.2005.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G., Yeh S. Y., De Souza E. B. (1988). MDMA-induced neurotoxicity: Parameters of degeneration and recovery of brain serotonin neurons. Pharmacology Biochemistry and Behavior, 29(2), 269–74. doi: 10.1016/0091-3057(88)90155-4 [DOI] [PubMed] [Google Scholar]
- Bolla K. I., McCann U. D., Ricaurte G. A. (1998). Memory impairment in abstinent MDMA (“Ecstasy”) users. Neurology, 51(6), 1532–7. doi: 10.1212/wnl.51.6.1532 [DOI] [PubMed] [Google Scholar]
- Broening H. W., Bowyer J. F., Slikker J. (1995). Age-dependent sensitivity of rats to the long-term effects of the serotonergic neurotoxicant (+/−) 3,4-methylenedioxymethamphetamine (MDMA) correlates with the magnitude of the MDMA-induced thermal response. Journal of Pharmacology and Experimental Therapeutics, 275(1), 325–33. PMID: [PubMed] [Google Scholar]
- Cadet J. L., Jayanthi S., Deng X. (2005). Methamphetamine-induced neuronal apoptosis involves the activation of multiple death pathways. Review. Neurotoxicity Research, 8(3–4), 199–206. doi: 10.1007/bf03033973 [DOI] [PubMed] [Google Scholar]
- Cadet J. L., Ordonez S. V., Ordonez J. V. (1997). Methamphetamine induces apoptosis in immortalized neural cells: Protection by the proto-oncogene Bcl2. Synapse 25(2), 176–84. doi: [DOI] [PubMed] [Google Scholar]
- Capela J. P., Carmo H., Remião F., Bastos M. L., Meisel A., Carvalho F. (2009). Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: An overview. Molecular Neurobiology, 39(3), 210–71. doi: 10.1007/s12035-009-8064-1 [DOI] [PubMed] [Google Scholar]
- Capela J. P., Fernandes E., Remião F., Bastos M. L., Meisel A., Carvalho F. (2007). Ecstasy induces apoptosis via 5-HT2A-receptor stimulation in cortical neurons. NeuroToxicology, 28(4), 868–75. doi: 10.1016/j.neuro.2007.04.005 [DOI] [PubMed] [Google Scholar]
- Deng X., Cadet J. L. (1999). Methamphetamine administration causes overexpression of nNOS in the mouse striatum. Brain Research, 851(1–2), 254–57. doi: 10.1016/s0006-8993(99)02087-9 [DOI] [PubMed] [Google Scholar]
- Deng X., Cadet J. L. (2000). Methamphetamine-induced apoptosis is attenuated in the striata of copper–zinc superoxide dismutase transgenic mice. Molecular Brain Research, 83(1–2), 121–4. doi: 10.1016/s0169-328x(00)00169-8 [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Jacobson K. A., Klotz K. N., Linden J. (2001). International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacological Reviews 53(4), 527–552. PMID: [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F., Gainetdinov R. R., Wang Y. M., Valenzano K. J., Miller G. W., Caron M. G. (1999). Increased methamphetamine neurotoxicity in heterozygous vesicular monoaminetransporter 2 knock-out mice. Journal of Neuroscience, 19(7), 2424–31. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb J. W., Johnson M., Hanson G. R. (1990). Neurochemical basis of neurotoxicity. Neurotoxicology, 11(2), 317–22. PMID: [PubMed] [Google Scholar]
- Green A. R., Mechan A. O., Elliott J. M., O’Shea E., Colado M. I. (2003). The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacological Reviews, 55(3), 463–508. doi: 10.1124/pr.55.3.3 [DOI] [PubMed] [Google Scholar]
- He J., Xu H., Yang Y., Zhang X., Li X. M. (2004). Neuroprotective effects of olanzapine on methamphetamine-induced neurotoxicity are associated with an inhibition of hyperthermia and prevention of Bcl-2 decrease in rats. Brain Research, 1018(2), 186–92. doi: 10.1016/j.brainres.2004.05.060 [DOI] [PubMed] [Google Scholar]
- Imam S. Z., Itzhak Y., Cadet J. L., Islam F., Slikker W., Ali S. F. (2001). Methamphetamine-induced alteration in striatal p53 and bcl-2 expressions in mice. Molecular Brain Research, 91(1–2), 174–8. doi: 10.1016/s0169-328x(01)00139-5 [DOI] [PubMed] [Google Scholar]
- Jayanthi S., Deng X., Bordelon M., Mccoy M. T., Cadet J. L. (2001). Methamphetamine causes differential regulation of pro-death and anti-death Bcl-2 genes in the mouse neocortex. FASEB Journal, 15(10), 1745–52. doi: 10.1096/fj.01-0025com [DOI] [PubMed] [Google Scholar]
- Kermanian F., Mehdizadeh M., Soleimani M., Ebrahimzadeh Bideskan A. R., Asadi-Shekaari M., Kheradmand H., et al. (2012). The role of adenosine receptor agonist and antagonist on Hippocampal MDMA detrimental effects; a structural and behavioral study. Metabolic Brain Disease, 27(4), 459–69. doi: 10.1007/s11011-012-9334-6 [DOI] [PubMed] [Google Scholar]
- Kermanian F., Soleimani M., Ebrahimzadeh A., Haghir H., Mehdizadeh M. (2012). Effects of adenosine A2A receptor agonist and antagonist on hippocampal nuclear factor-kB expression preceded by MDMA toxicity. Metabolic Brain Disease, 28(1), 45–52. doi: 10.1007/s11011-012-9366-y [DOI] [PubMed] [Google Scholar]
- Kesner R. P. (2007). A behavioral analysis of dentate gyrus function. Progress in Brain Research, 163, 567–76. doi: 10.1016/s0079-6123(07)63030-1 [DOI] [PubMed] [Google Scholar]
- Kuwana T., Newmeyer D. D. (2003). Bcl-2-family proteins and the role of mitochondria in apoptosis. Current Opinion in Cell Biology, 15(6), 691–9. doi: 10.1016/j.ceb.2003.10.004 [DOI] [PubMed] [Google Scholar]
- Liechti M. (2000). Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA,”Ecstasy”) are at-tenuated by the serotonin uptake inhibitor citalopram. Neuropsychopharmacol. 22(5), 513–21. doi: 10.1016/s0893-133x(99)00148-7 [DOI] [PubMed] [Google Scholar]
- Maycotte P., Guemez-Gamboa A., Moran J. (2010). Apoptosis and autophagy in rat cerebellar granule neuron death: Role of reactive oxygen species. Journal of Neuroscience Research, 88(1), 73–85. doi: 10.1002/jnr.22168 [DOI] [PubMed] [Google Scholar]
- Montiel-Duarte C., Varela-Rey M., Osés-Prieto J. A., López-Zabalza M. J., Beitia G., Cenarruzabeitia E., Iraburu M. J. (2002). 3,4-Methylenedioxymethamphetamine (“Ecstasy”) induces apoptosis of cultured rat liver cells. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1588(1), 26–32. doi: 10.1016/s0925-4439(02)00112-6 [DOI] [PubMed] [Google Scholar]
- Pu C., Broening H. W., Vorhees C. V. (1996). Effect of methamphetamine on glutamate-positive neurons in the adult and developing rat somatosensory cortex. Synapse, 23(4), 328–34. doi: [DOI] [PubMed] [Google Scholar]
- Riezzo I., Cerretani D., Fiore C., Bello S., Centini F., D’Errico S., et al. (2010). Enzymatic-nonenzymatic cellular antioxidant defense systems response and immunohistochemical detection of MDMA, VMAT2, HSP70, and apoptosis as biomarkers for MDMA (Ecstasy) neurotoxicity. Journal of Neuroscience Research. 88(4), 905–16. doi: 10.1002/jnr.22245 [DOI] [PubMed] [Google Scholar]
- Rosin D. L., Hettinger B. D., Lee A., Linden J. (2003). Anatomy of adenosine A2A receptors in brain: Morphological substrates for integration of striatal function. Neurology, 61, 12–8. doi: 10.1212/01.wnl.0000095205.33940.99 [DOI] [PubMed] [Google Scholar]
- Schmued L. C., Bowyer J. F. (1997). Methamphetamine exposure can produce neuronal degeneration in mouse hippocampal remnants. Brain Research, 759(1), 135–40. doi: 10.1016/s0006-8993(97)00173-x [DOI] [PubMed] [Google Scholar]
- Sheng P., Cerruti C., Ali S., Cadet J. L. (1996). Nitric oxide is a mediator of methamphetamine (METH)-induced neurotoxicity. Annals of the New York Academy of Sciences, 801(1), 174–86. doi: 10.1111/j.1749-6632.1996.tb17440.x [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Shimizu S., Narita M., Tsujimoto Y. (1999). Bcl-2 family proteins regulate the release of apoptogenic cytochrome C by the mitochondrial channel VDAC. Nature, 399(6735), 483–87. doi: 10.1038/20959 [DOI] [PubMed] [Google Scholar]
- Soleimani M., Katebi M., Alizadeh A., Mohammadzadeh F., Mehdizadeh M. (2012). The role of the A2A receptor in cell apoptosis caused by MDMA. Cell Journal. 14(3), 231–6. PMID: [PMC free article] [PubMed] [Google Scholar]
- Steranka L. R., Rhind A. W. (1987). Effect of cysteine on the persistent depletion of brain monoamines by amphetamine, p-chloroamphetamine and MPTP. European Journal of Pharmacology, 133(2), 191–7. doi: 10.1016/0014-2999(87)90150-6. [DOI] [PubMed] [Google Scholar]
- Stone D. M., Hanson G. R., Gibb J. W. (1989). In vitro reactivation of rat cortical tryptophan hydroxylase following in vivo inactivation by methylenedioxymethamphetamine. Journal of Neurochemistry, 53(2), 572–81. doi: 10.1111/j.1471-4159.1989.tb07372.x [DOI] [PubMed] [Google Scholar]
- Stumm G., Schlegel J., Schafer T., Wurz C., Mennel H. D., Krieg J. C., Vedder H. (1999). Amphetamines induce apoptosis and regulation of bcl-x splice variants in neocortical neurons. FASEBJ, 13(19), 1065–72. PMID: [DOI] [PubMed] [Google Scholar]
- Tamburini I., Blandini F., Gesi M., Frenzilli G., Nigro M., Giusiani M., et al. (2006). MDMA induces caspase-3 activation in the limbic system but not in striatum. Annals of the New York Academy of Sciences 1074, 377–81. PMID: [DOI] [PubMed] [Google Scholar]
- Vorhees C. V., Reed T. M., Skelton M. R., Williams M. T. (2004). Exposure to 3,4-methylenedioxymethamphetamine (MDMA) on postnatal days 11–20 induces reference but not working memory deficits in the Morris water maze in rats: Implications of prior learning. International Journal of Developmental Neuroscience, 22(5–6), 247–59. doi: 10.1016/j.ijdevneu.2004.06.003 [DOI] [PubMed] [Google Scholar]
- Warren M. W., Larner S. F., Kobeissy F. H., Brezing C. A., Jeung J. A., Hayes R. L., et al. (2007). Calpain and caspase proteolytic markers co-localize with rat cortical neurons after exposure to methamphetamine and MDMA. Acta Neuropathologica, 114(3), 277–86. doi: 10.1007/s00401-007-0259-9 [DOI] [PubMed] [Google Scholar]
- Zamani M., Katebi M., Mehdizadeh M., Mohamadzadeh F., Soleimani M. (2012). Coenzyme Q10 protects hippocampal neurons against ischemia/reperfusion injury via modulation of BAX/Bcl-2 expression. Basic and Clinical Neuroscience, 3(5), 5–10. [Google Scholar]
- Zamani M., Soleimani M., Golab F., Mohamadzadeh F., Mehdizadeh M., Katebi M. (2013). NeuroProtective effects of adenosine receptor agonist coadministration with ascorbic acid on CA1 hippocampus in a mouse model of ischemia reperfusion injury. Metabolic Brain Disease, 28(3), 367–74. doi: 10.1007/s11011-013-9408-0 [DOI] [PubMed] [Google Scholar]