Abstract
Objective
To evaluate exercise as a treatment for stimulant use disorders.
Method
The STimulant Reduction Intervention using Dosed Exercise study was a randomized clinical trial conducted in nine residential addiction treatment programs across the United States from July 2010 to February 2013. Of 497 adults referred to the study, 302 met all eligibility criteria, including DSM-IV criteria for stimulant abuse and/or dependence, and were randomized to either a dosed exercise intervention (Exericse) or a health education intervention (Health Education) control, both augmenting treatment as usual and conducted thrice weekly for 12 weeks. The primary outcome of percent stimulant abstinent days during study weeks 4–12 was estimated using a novel algorithm adjustment incorporating self-reported Timeline Follow Back (TLFB) stimulant use and urine drug screen (UDS) data.
Results
Percent abstinent days based on TLFB was 90.8% (SD=16.4) for Exercise and 91.6% (SD=14.7) for Health Education participants. Percent abstinent days using the ELCON algorithm was 75.6% (SD=27.4) for Exercise and 77.3% (SD=25.1) for HEI. The primary intent-to-treat analysis, using a mixed model controlling for site and the ELCON algorithm, produced no treatment effect (p=0.60). In post hoc analyses controlling for treatment adherence and baseline stimulant use, Exercise participants had a 4.8% higher abstinence rate (78.7%) compared to HEI participants (73.9%) (p=0.03, number needed to treat=7.2).
Conclusions
The primary analysis indicated no significant difference between exercise and health education. Adjustment for intervention adherence showed modestly but significantly higher percent abstinent days in the exercise group, suggesting that exercise may improve outcomes for stimulant users with better adherence to an exercise dose.
Introduction
Suboptimal outcomes in the treatment of stimulant use disorders suggest a need for innovative treatments. Randomized trials of pharmacological and non-pharmacological interventions have shown significant variability in abstinence rates and none of these studies have produced highly effective treatment options for this difficult to treat population1, 2. These findings clearly indicate a need for new treatments for stimulant use disorders.
Previous studies suggest that exercise could be a promising treatment for stimulant use disorders. Animal studies support the use of exercise for stimulant use disorders, as several trials have demonstrated reduced cocaine-seeking following wheel running in rats and mice3–6. Previous human studies indicate that exercise is associated with reduced use, increased abstinence, and longer duration of abstinence from alcohol, marijuana and other substances in both adults and adolescents7–10. Exercise has been associated with improvements in smoking outcomes, with greater support for reduced craving and withdrawal, and more limited support for smoking cessation, particularly with respect to long-term outcomes11 However, methodological issues, such as insufficient exercise intensity, issues with respect to adherence to exercise and the timing of exercise implementation (e.g., post-quit status), and small sample sizes have been major limitations Exercise has also been shown to improve cognition12, 13 and mood,14, 15,16 both of which may be altered in stimulant using populations. Finally, several plausible biological mechanisms, including alterations in dopaminergic, serotonergic, glutamatergic and adrenergic functioning, as well as epigenetic regulation of the brain-derived neurotrophic factor gene, have been proposed to support the effects of exercise on substance use.17, 18 And yet, there have been few well-controlled trials designed to examine the efficacy of exercise, particularly as augmentation to treatment as usual, in this population.
This paper reports primary outcome results for the STimulant Reduction Intervention using Dosed Exercise (STRIDE) study. STRIDE was implemented through the National Drug Abuse Treatment Clinical Trials Network (CTN) at nine residential substance abuse treatment programs across the U.S. from July 2010 to February 2013. The STRIDE trial aimed to examine the efficacy of an aerobic exercise intervention in reducing stimulant use by recruiting patients in a residential treatment facility but followed in outpatient treatment settings. The hypothesis was that exercise would result in greater percent abstinent days compared to a health education control condition, both of which were added to treatment as usual (TAU), during the 12-week acute phase of the study.
Methods
The design and methodology of STRIDE have been described elsewhere19–23. An overview of the study design relevant to the reported outcomes is presented below. The study was approved by the Institutional Review Boards associated with each of the participating residential treatment programs. Written informed consent was obtained and the study was registered at Clinicaltrials.gov (identifier: NCT01141608).
Participants
Adult stimulant users, aged 18–65, in residential substance abuse treatment were recruited and met the following inclusion criteria: 1) ability and willingness to provide informed consent and contact information, 2) agreement to complete residential treatment, 3) self- reported stimulant use (cocaine, methamphetamine, amphetamine, or other stimulant, excluding caffeine and nicotine) in the 30 days prior to treatment admission, 4) met past year DSM-IV criteria for stimulant abuse or dependence, 5) cleared to exercise via a protocol-defined stress test (in accordance with American College of Sports Medicine guidelines), 6) body mass index (BMI) ≤ 40 kg/m2, or BMI > 40 kg/m2 and medically cleared to exercise, and 7) ability to comprehend and communicate in English. Exclusion criteria included: 1) evidence of a general medical condition or other abnormality contraindicating exercise, 2) past year opioid dependence, 3) considered a high risk for suicide and/or study non-completion due to the need for psychiatric hospitalization, 4) current psychotic disorder, 5) pregnancy, 6) aerobically exercising more than 3 times per week for 20 minutes or more, consistently for the three months prior to study enrollment, 7) prescribed beta blockers or any opioid replacement therapies, and 8) anticipated circumstances making study completion unlikely or hazardous.
Screening
Interested persons, identified early in residential treatment as potential participants, were briefly pre-screened by study personnel. The study was described as a health intervention to aid in the treatment of stimulant abuse or dependence. Those who provided written informed consent were screened for eligibility. Substance use disorders were diagnosed using the World Health Organization Composite International Diagnostic Interview v2.124. Psychiatric disorders were diagnosed using the Mini International Neuropsychiatric Interview25. The Timeline Follow Back (TLFB) was used to assess stimulant use. A study-trained physician provided medical clearance to exercise following a physical evaluation and maximal exercise test.
Treatment Assignment
Randomization was stratified by site and within each site by presence of depressive symptoms defined as a score of ≥11 on the Quick Inventory of Depressive Symptomatology [QIDS] – Clinician rated [16-item]26 and by severity of stimulant use (≤ 18 days or > 18 days of use prior to admission). A permuted-block randomization procedure was implemented via the electronic data capture system.
Study Interventions
Eligible participants were randomized to one of two treatment arms that augmented TAU: 1) Exercise or 2) Health Education. Both groups received substance use disorder TAU, first in a residential setting and then typically continued in an outpatient treatment program. Professional attention was controlled for across the two groups. Participants received 12 weeks of acute phase intervention followed by an additional 24 weeks of intervention with supervision once per week.
Exercise Intervention
Participants randomized to Exercise20, 21 completed supervised exercise sessions 3 times per week during the 12-week acute phase. Exercise was prescribed at a dose of 12 kcal/kg/week (KKW), with intensity ranging from 70–85% of maximal heart rate (HRmax). This dose is similar to those used in several studies of exercise interventions27, 28, including in efficacy studies with smokers29, 30 and is equivalent to ≥150 minutes of moderate exercise per week (i.e., approximately 30–50 minutes, 3–5 days per week). Exercise dose and intensity were gradually increased during the first 3 weeks (Week 1: 4KKW at 50–60% HRmax; Week 2: 8 KKW at 60–70% HRmax; Week 3–12: 12 KKW at 70–85% HRmax). For most participants, the maximum intensity was equivalent to walking at a moderate speed and incline (3.0 mph at 5% incline) for approximately 150 minutes per week. Additional sessions could be completed for those needing more to achieve the target dose. Supervised sessions were conducted as one-on-one sessions.
Health Education Intervention
Participants randomized to Health Education22 also completed 3 visits per week during the 12-week acute phase. Health Education consisted of one-on-one sessions in which information on health-related topics (e.g., cancer, heart disease, mental health) was distributed via didactics, websites, audio, video, and written materials. Exercise was not an included topic.
Outcome Measures
Stimulant use outcomes were assessed at the assessment visits which were conducted three times per week. Days of self-reported drug use were assessed by the TLFB, a semi- structured interview that uses a calendar to retrospectively assess daily drug use since the last assessment. The TLFB was originally developed to assess alcohol use31, but has been adapted to acquire information for other substances, including cocaine and other stimulants32. The TLFB has high test-retest reliability (intraclass correlation coefficient values from 0.70 to 0.94, with all p<0.001), good convergent and discriminant validity, and acceptable agreement with urine drug screens33.
Urine drug screens (UDS) measured stimulant use (cocaine, amphetamine, methamphetamine), as well as opiates, marijuana, benzodiazepines, barbiturates, methadone, methylenedioxymethamphetamine (MDMA, ecstasy), and oxycodone. UDS was collected to augment the veracity of TLFB.
Statistical Analyses
The primary outcome measure was the percent of stimulant abstinent days during days 22–84 (weeks 4–12) of the acute phase of the study. Outcome measurement began at day 22 because it was anticipated a priori that most individuals would be in residential treatment during the first 21 days of the study and, therefore, would have little opportunity to use illicit substances (i.e., the groups would not likely differ during this time period).
Stimulant abstinent days were based on TLFB. In order to estimate the number of days of use when either there were missing UDS data or the thrice weekly urine drug screens showed discrepancy with TLFB, the Eliminate Contradiction (ELCON) algorithm34 was used. First, all missing TLFB days and UDS results, including any missing data due to participants discontinuing before the end of the study, were imputed as positive for stimulant use. The ELCON algorithm was then implemented by comparing the TLFB to UDS day by day. For any comparison in which the UDS was positive and the 3 TLFB days prior to the UDS were negative, the TLFB for the last day in the comparison period was changed from negative to positive in order to eliminate the contradiction between the self-report and objective data. Once the ELCON algorithm was applied, the number of abstinence days was summed, the percent of stimulant abstinent days was calculated, and this was used in all planned and post-hoc analyses.
The primary analysis compared the percent of stimulant abstinence days between the two treatments taking into account variability in the overall level of abstinence among sites. A linear mixed-effects model was used with site as a random effect and treatment group as a fixed effect. As specified in the analysis plan, this model was used three more times with the addition of each of three covariates: gender, race, and ethnicity, along with their interactions with treatment group. All participants’ data were utilized for the primary analysis and post hoc analyses regardless of their adherence to the interventions in accordance with the intent to treat principle.
Because a large between-group difference in adherence (number of intervention sessions attended/number of sessions required) was observed, post hoc analyses were performed in which the treatment effect was evaluated by including adherence as a covariate. Days of stimulant use in the 30 days prior to residential treatment was also included as a covariate. Thus, treatment adherence and prior use were covariates in an adjusted linear mixed- effects model. The interaction of each covariate with treatment group was tested and any interaction terms that were not significant and were removed from the model.
Cohen’s d35 was computed as a standardized measure of the unadjusted and adjusted mean difference between treatments. Number needed to treat (NNT)36 was also computed. For both measures of effect size positive effect sizes favor Exercise and negative effect sizes favor Health Education. P-values less than 0.05 were considered statistically significant.
Results
Participant Characteristics
Four hundred and ninety seven participants were screened, resulting in 302 randomized (Exercise, n = 152; Health Education, n = 150) participants. A CONSORT diagram (Figure 1) presents data on participants who were screened, reasons for exclusion, and reasons enrolled participants discontinued participation during the acute phase. Baseline demographic and clinical information is presented in Table 1. The two treatment groups did not differ statistically on any demographic or baseline characteristic. Few participants scored ≥11 on the QIDS scale for depression (16 total: 12 in Exercise, 4 in Health Education,). Average days of stimulant use prior to treatment entry was 12.9 (SD=8.8) and 13.2 (SD=9.5) for the Exercise and Health Education groups, respectively, and were not significantly different (t=0.3, df=300, p=0.77). The average duration of residential treatment was 18.3 days (SD=11) in the Exercise group and 17.9 (SD=10) in the Health Education group.
Figure 1. CONSORT Diagram.
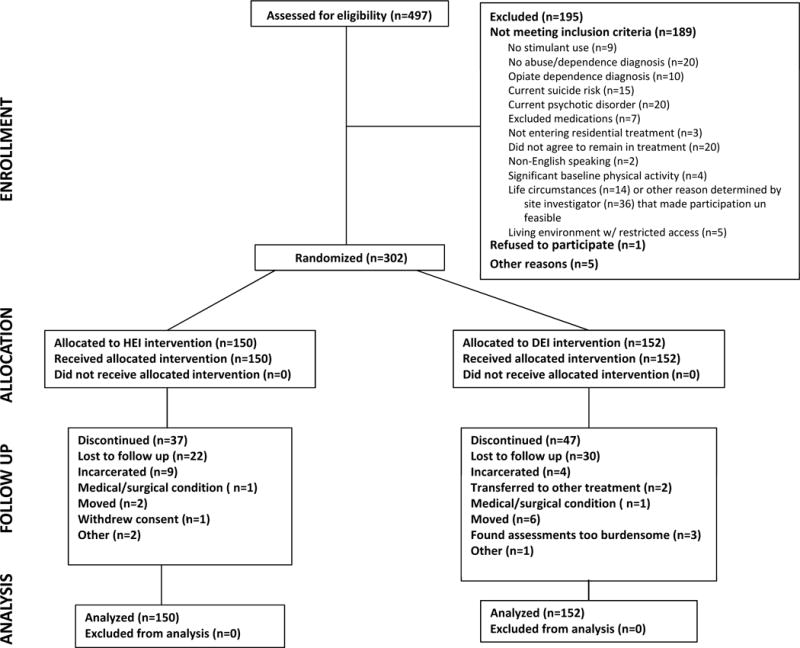
Note. In the Allocation row, “received allocated intervention” refers to the fact that all eligible participants were assigned to an intervention; however, this does not account for non- adherence. Because the analyses were intent to treat, they were conducted on all participants who were randomized to an intervention, regardless of their adherence to the intervention.
Table 1.
Baseline Demographic, Drug Use, and Other Clinical Characteristics
| Demographic | Total (N=302) |
HEI (n=150) |
DEI (n=152) |
|---|---|---|---|
| Gender | |||
| Male | 181 (60%) | 92 (61%) | 89 (59%) |
| Female | 121 (40%) | 58 (39%) | 63 (41%) |
| Age, mean (SD) | 39.0 (11) | 39.5 (11) | 38.5 (10) |
| Race | |||
| Black/not Hispanic | 130 (43%) | 75 (50%) | 55 (36%) |
| White/not Hispanic | 137 (45%) | 63 (42%) | 74 (49%) |
| Othera/not Hispanic | 12 (4%) | 6 (4%) | 6 (4%) |
| Hispanic ethnicity | 31 (10%) | 12 (8%) | 19 (13%) |
| Education in years, mean (SD) | 12.4 (2) | 12.3 (2) | 12.4 (2) |
| Marital status | |||
| Married | 40 (13%) | 17 (11%) | 23 (15%) |
| Divorced/Separated/Widowed | 101 (33%) | 46 (31%) | 55 (36%) |
| Never married | 161 (53%) | 87 (58%) | 74 (49%) |
| Employment status | |||
| Full time | 133 (44%) | 70 (47%) | 63 (41%) |
| Part time | 53 (18%) | 30 (20%) | 23 (15%) |
| Unemployed | 92 (30% | 37 (25%) | 55 (36%) |
| Other | 24 (8%) | 13 (9%) | 11 (7%) |
| Drug Use/Treatment | |||
| Days in residential treatment, mean (SD) | 18.1 (10) | 17.9 (10) | 18.3 (11) |
| Days of stimulant use in 30 days prior to treatment admission, mean (SD) | 13.1 (9) | 13.2 (10) | 12.9 (9) |
| Cocaine | 9.1 (9) | 8.7 (10) | 9.5 (9) |
| Methamphetamine | 3.7 (8) | 4.1 (8) | 3.3 (8) |
| Other stimulant | 0.5 (3) | 0.6 (3) | 0.4 (3) |
| Dependence diagnoses | |||
| Cocaine | 253 (84%) | 117 (78%) | 136 (90%) |
| Other stimulant | 114 (38%) | 58 (39%) | 56 (37%) |
| Alcohol | 152 (50%) | 71 (47%) | 81 (53%) |
| Marijuana | 96 (32%) | 47 (31%) | 49 (32%) |
| Other illicit drugs | 53 (18%) | 29 (19%) | 24 (16%) |
| Fagerström Nicotine Dependence | 3.4 (2) | 3.7 (2) | 3.2 (2) |
| Clinical | |||
| QIDS score, mean (SD) | 5.4 (3) | 4.8 (3) | 5.9 (3) |
| Body Mass Index, mean (SD) | 27.8 (6) | 27.6 (6) | 28.0 (6) |
Note: Designations of “American Indian or Alaska Native”, “Asian”, “Native Hawaiian or Pacific Islander”, “Other”, “Multiracial”, “Unknown”, and “Participant chose not to answer” were collapsed into one new category of “Other” due to the small numbers of participants in these groups.
Abbreviations: QIDS: Quick Inventory for Depressive Symptomatology, SD: Standard Deviation.
Study Retention and Primary Outcome Availability
Two hundred eighteen participants (72%) completed the Week 13 assessment; 105 (69%) in Exercise and 113 (75%) participants in HEI. The most frequent reason for not completing the Week 13 assessment was being lost to follow-up (n=52), followed by incarceration (n=13) and moving from the area (and did not complete phone or off-site assessments) (n=8). Availability of data during the primary outcome period was excellent, with 92% of TLFB data available (92% in Exercise, 93% in Health Education,) and 67% of UDS data available (63% in Exercise, 70% in Health Education).
Primary Analysis
Group analyses of self-reported TLFB data produced a non-significant difference (Exercise: 90.8%, SD=16.4, Health Education: 91.6%, SD=14.7, d=−0.05, NNT=−34.1, p=0.67) as did an analysis using only UDS data (Exercise: 80.2%, SD=29.8, Health Education: 74.6%, SD=32.7, d=0.18, NNT=10.0, p=0.14). After imputing missing TLFB days and missing UDS results as positive and applying the ELCON algorithm, the percent of stimulant abstinent days was 76.4% (SD=26.2) for all participants, 75.6% (SD=27.4) for Exercise participants, and 77.3% (SD=25.1) for Health Education participants (d=−0.06, NNT=−27.4) (see Table 2). After adjustment for random site effects, the difference between groups was not significantly different (f=0.3, df=1, 292, p = 0.60). Adjustment for site and site × treatment interactions also indicated no statistically significant difference in percent days abstinent between the two intervention groups. Because the days in residential treatment were less than anticipated, we conducted a secondary analysis using all days post-residential treatment. This analysis yielded similar results; percent days abstinent was not significantly different between Exercise (76.2%, SD=26.4) and Health Education (77.9%, SD=24.1, d=−0.07; p=0.59, NNT=−26.4).
Table 2.
Percent stimulant abstinent days based on Timeline Follow Back (TLFB) and Eliminate Contradiction (ELCON) algorithm adjustment
| Outcome | N | All Mean (SD) |
n | DEI Mean (SD) |
n | HEI Mean (SD) |
|---|---|---|---|---|---|---|
| TLFB | 291 | 91.2 (15.6) | 145 | 90.8 (16.4) | 146 | 91.6 (14.7) |
| ELCON algorithm | 302 | 76.4 (26.3) | 152 | 75.5 (27.4) | 150 | 77.2 (25.1) |
Note. Mean percent stimulant abstinent days are for days 22 to 84. Eleven participants had no TLFB data: their data are missing when there is no imputation (TLFB row); their data are present when there is imputation and all data are imputed as stimulant use days (ELCON algorithm row).
Subgroup Analyses
Tests for interaction of treatment revealed a marginally significant interaction between treatment and ethnicity (Hispanic and non-Hispanic) (p=0.051), such that Hispanic participants had 83.1% (SD=16.5) abstinent days when assigned to Exercise and 66.8% (SD=30.2) in HEI (d=0.72, NNT=2.6), whereas non-Hispanic participants had 74.5% (SD=28.5) abstinent days when assigned to Exercise and 78.2% (SD=24.5) in Health Education (d=−0.14, NNT=−12.6). Analysis yielded no statistically significant interactions by gender or race.
Adherence and Associated Post Hoc Analysis
Participants in Exercise attended 64.0% (SD=30.4) of the 36 (3 visits/week for 12 weeks) expected intervention visits, compared to 74.7% (SD=28.7) in Health Education, and this difference was significant (t=3.2, df=300, p=0.002). Participants in Exercise completed a median 8.3 KKW per week or 69.2% of the prescribed exercise dose (i.e., approximately 79 minutes per week). Table 3a shows estimates and tests for all effects in the full post-hoc model. The interactions of treatment group with the covariates were not significant. After removing these interactions, ignificant effects were found for percent of sessions attended (p<0.001) and for treatment group (Table 3b). The adjusted proportion of abstinent days was 78.7% (SE=0.02) for Exercise participants and 73.9% (SE=0.02) for Health Education participants (d=0.25, NNT=7.2, f=4.7, df=1,290, p=0.03). Figure 2 shows linear regression lines fit for each group independently and illustrates approximately 5% improvement in days abstinent in Exercise over Health Education. Note that the pronounced upward slope of the line was a result of assigning missing data as days of use.
Table 3a.
Results for full ad-hoc mixed-effects model
| Effect | Estimate | Std Err | Num DF | Den DF | F Stat | p-val |
|---|---|---|---|---|---|---|
| Intercept | 0.3856 | |||||
| Treatment Group | −0.0439 | 0.1 | 1 | 288 | 0.4 | 0.510 |
| Days of Use for 30 Days Prior to RTP | −0.0012 | 0.0 | 1 | 288 | 2.2 | 0.138 |
| Percent Sessions Attended | 0.0060 | 0.0 | 1 | 288 | 259.3 | <0.001 |
| Days Prior Use by Treatment Group | −0.0013 | 0.0 | 1 | 288 | 0.3 | 0.581 |
| Percent Sessions Attended by Treatment Group | 0.0002 | 0.0 | 1 | 288 | 0.1 | 0.795 |
Table 3b.
Results for ad-hoc mixed-effects model after removing non-significant Interaction terms
| Effect | Estimate | Std Err | Num DF | Den DF | F Stat | p-val |
|---|---|---|---|---|---|---|
| Intercept | 0.3889 | |||||
| Treatment Group | −0.0477 | 0.0 | 1 | 290 | 4.7 | 0.032 |
| Days of Use for 30 Days Prior to RTP | −0.0019 | 0.0 | 1 | 290 | 2.3 | 0.127 |
| Percent Sessions Attended | 0.0061 | 0.0 | 1 | 290 | 261.0 | <0.001 |
Abbreviations: Std Err-Standard Error, Num-Numerator, DF-Degrees of Freedom, Den- Denominator.
Figure 2. Adherence Adjusted Analyses.
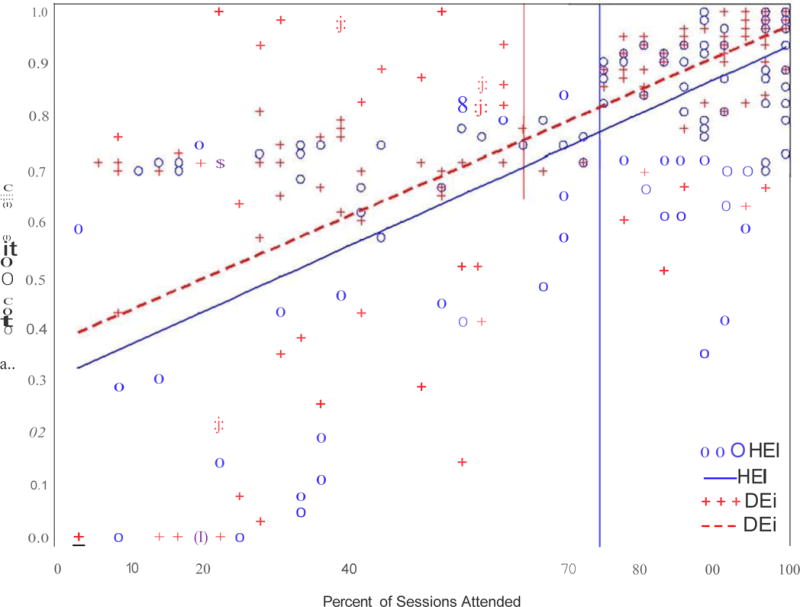
Independent simple regressions of percent of abstinent days on adherence as defined by percent of intervention sessions attended. Vertical reference lines mark the significantly different means of percent adherence in the two treatment groups.
Adverse Events
Of the 192 total post-randomization adverse events (AEs) that occurred, 76 were deemed not related to study procedures, while 116 were considered related or possibly related to study procedures. Seventy-nine (52%) of participants in the Exercise group had an AE, compared to 28 (19%) in the Health Education group. Sixty-five percent (125/192) of AEs were classified as mild or moderate, with the majority of those occurring in the Exercise group (96%). The most common AEs were classified as musculoskeletal and connective tissue disorders, and they occurred primarily in the Exercise group (32% of Exercise participants reported 49 of the 50 [98%] AEs in that category). Specific AEs in this category that occurred in over 5% of Exercise participants include arthralgia, back pain, muscle spasms, myalgia, and pain in extremity. Dizziness was the only other specific AE that occurred in over 5% of Exercise participants (5.3%) but not Health Education participants (0%).
Fifty AEs met designated criteria for serious adverse events (SAEs). No SAEs were determined to be related to study procedures. The occurrence of SAEs was comparable across interventions, with 26 in the Exercise group and 24 in the Health Education group. SAEs included 42 inpatient hospital admissions and one death.
Discussion
STRIDE is the first large-scale study evaluating the efficacy of exercise training compared to health education, both added to TAU, as potential treatments for stimulant use disorders. STRIDE is also the first clinical trial utilizing the novel ELCON algorithm to reconcile results from Timeline Follow Back and urine drug screen.
The primary analysis using the ELCON algorithm in this study did not find a statistically significant difference in the percent of abstinent days between the Exercise and the Health Education groups. Overall, the mean days of use for the 30 days prior to RTP was 13.1 (SD=9) and the abstinence rates across groups following treatments were extremely high – over 90% via self-report (TLFB), but around 75% by the ELCON algorithm-corrected analyses when missing data were assigned as days of use.
Participants in the Exercise group attended significantly more intervention sessions than those in the Health Education group (64.0% [SD=30.4] vs. 74.7% [SD=28.7], respectively. A post hoc analysis adjusting for intervention adherence and stimulant use prior to treatment entry suggested a positive treatment effect for exercise, albeit modest. This analysis revealed a significant difference between groups, with an approximately 5% greater percent of days abstinent in the Exercise condition versus those in Health Education, suggesting exercise may improve outcomes for stimulant users with good adherence to an exercise training program. Subjects in the exercise group completed approximately 8KKW of the 12KKW dose. Previous research suggests this is likely a suboptimal dose of exercise. In a study of aerobic exercise dose on depression outcomes, a 7KKW dose was less effective in reducing depression outcomes compared to a 17.5 KKW dose37. Similarly, in a study of post-menopausal women, an 8KKW dose resulted in significantly less improvement in cardiorespiratory fitness compared to a 12 KKW dose27.
The abstinence rates observed in our study are significantly higher than those commonly seen in other trials examining combined pharmacological and behavioral treatments for stimulant use, with three recent studies reporting percent days of abstinence of approximately 48–58% among active treatment groups38–40 with one yielding a higher range of approximately 60–73%41. The relatively higher rate of abstinence observed in both groups from the current study may be related to either a modest rate of pre-treatment days of drug use in our sample or the continuing effect of the residential treatment prior to randomization. These results could either suggest that both Exercise and Health Education are ineffective in decreasing stimulant use or, given the high abstinence rates observed, it is possible that both interventions were effective in decreasing stimulant use. Participants in both groups received considerable contact with study personnel, which could have impacted stimulant use. Unfortunately, without a study arm of participants receiving only TAU, we are unable to assess the impact of this increased professional contact.
Studies have routinely emphasized the importance of exercise adherence in interpreting the results of studies with exercise interventions. Brown et al.42 noted in their recent pilot study that future research may need to better identify expectations and preferences in drug abusing populations, as well as identify and troubleshoot barriers that prohibit adequate adherence. Similarly, Williams et al.43 commented on the fact that several studies examining exercise for smoking cessation have had poor adherence rates, and they assert that this may be the primary reason that those trials did not yield significant findings, again stressing the importance of adequate and sustained adherence in such interventions. The importance of adherence in exercise trials is not specific to substance abuse outcomes. Adjustment for adherence is often necessary in efficacy studies examining the effects of exercise in patients with other chronic illnesses, such as depression37 and type 2 diabetes28. It is important to note that exercise was generally well-tolerated in this population, with the majority of AEs in the Exercise group being classified as mild or moderate, and expected in association with exercise (e.g., muscle spasms). However, further consideration to tolerability in the evaluation of both adherence and efficacy of exercise in this population is warranted.
In addition to better evaluating the role of adherence to exercise on stimulant use outcomes, it is important to evaluate potential mediators and moderators of exercise that may impact its efficacy. It is conceivable that exercise is only effective in a subset of individuals, due either (or both) to certain baseline behavioral characteristics (e.g. severity of illness factors, such as years of drug use and past treatment history; poor response inhibition; a particular BDNF polymorphism [e.g., rs6265])44 or mediators (e.g., improved mood, withdrawal, craving, and cognition; changes in BDNF and dopamine, etc.). Further investigation will be important to ascertain what behavioral and biological characteristics and/or changes are associated with the efficacy of exercise in individuals with stimulant use disorders.
STRIDE was a hybrid efficacy-effectiveness study with specific eligibility criteria that excluded at-risk individuals with physical or psychiatric conditions that might contraindicate exercise. In addition, the study had fewer participants than expected in the stratum of greater stimulant use at baseline (i.e., >18 days in the 30 days prior to residential treatment entry), or those with significant depressive symptoms (QIDS-SR >10), and therefore may have enrolled a less severe group of individuals who use stimulants compared to other individuals in residential treatment. Finally, differential adherence rates in the treatment arms, although not unusual in studies of this sort, are a further limitation.
Despite the above limitations, the study had several notable strengths—the use of geographically diverse sites, adequate intervention adherence rates in a population that had significant attendance and participation barriers (e.g., transportation, relapse to drug use), and a well-received comparative condition. Furthermore, this study demonstrated it is possible to conduct intensive interventions with this population. Because of the unusually high abstinence rates in both intervention groups, as well as the post hoc adjustment for adherence yielding a significant effect of exercise, we believe it is important to continue research in this area to better understand whether exercise may benefit individuals with stimulant use disorders. Additionally, subsequent research should investigate more appealing strategies to encourage exercise (e.g., leader-led groups with music, buddy system, use of electronic systems, etc.). Future trials should evaluate the influence of adherence on outcomes and aim to improve adherence to exercise interventions.
Clinical Points.
-
-
Novel treatment approaches for stimulant use disorders are needed and preliminary evidence suggests exercise may be effective in this population, but this intervention has not been sufficiently studied.
-
-
Dosed exercise augmentation was not superior to health education augmentation in reducing stimulant use days, with both groups showing greater than 75% of stimulant abstinent days; however, post hoc analyses that considered the differential adherence rates between groups showed a modest, but significant difference of approximately 5% greater percent of days abstinent with exercise.
-
-
Exercise augmentation to treatment as usual may be considered for individuals with stimulant use disorders, particularly when adherence to exercise is good.
Acknowledgments
The authors would like to sincerely thank all those who assisted with this project. The authors also recognize, with great appreciation, all the study participants who contributed to this project.
Funding/Support:
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Numbers U10 DA020024 and UG1DA020024 (PI: Trivedi), U10 DA013727 (PI: Brady), U10 DA013035 (PIs: Nunes & Rotrosen), U10 DA013720 (PIs: Szapocznik & Metsch), U10 DA013732 (PI: Winhusen), HHSN271200900034C and HHSN271200900034C (EMMES Corporation). Additional grant support provided by NIDA K24 DA022412 (PI: Nunes) and NIMH K01 MH097847 (PI: Rethorst). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Role of Funder/Sponsor:
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Potential Conflicts of Interest:
Dr Trivedi has been an advisor/consultant and received fees from: Alkermes, AstraZeneca, Cerecor, Eli Lilly & Company, Lundbeck, Naurex, Neuronetics, Otsuka Pharmaceuticals, Pamlab, Pfizer Inc., SHIRE Development and Takeda. In addition, Dr. Trivedi has received grants/research support from: National Institute of Mental Health (NIMH) and National Institute on Drug Abuse (NIDA).
Dr Greer has received honoraria, speakers or advisory boards and/or consultant fees from H. Lundbeck A/S.
Dr Warden currently owns stock in Pfizer, Inc. and has owned stock in Bristol-Myers Squibb Company within the last five years.
Dr Nunes has received medication from Alkermes (vivitrol) for a NIDA funded research study on treatment of opioid dependence in recent years, and has served as an unpaid consultant to Alkermes at an advisory meeting about planning a study on treatment of opioid dependence.
Dr Rethorst, Dr Carmody, Mr Bruce Grannemann, Dr Walker, Dr Shores-Wilson, Dr Stoutenberg, Dr Oden, Dr Silverstein, Dr Hodgkins, Dr Love, Dr Seamans, Dr Stotts, Dr Causey, Dr Reed, Dr Rinaldi, Dr Myrick, Michele Straus, Dr Liu, Dr Lindblad, Dr Church and Dr Blair report no disclosures and conflicting interests.
Previous Presentation: Portions of these data were presented at the American Academy of Addiction Psychiatry Annual Meeting, December 4–7, 2014, Aventura, FL.
References
- 1.de Lima MS, de Oliveira Soares BG, Reisser AA, Farrell M. Pharmacological treatment of cocaine dependence: a systematic review. Addiction (Abingdon, England) 2002 Aug;97(8):931–949. doi: 10.1046/j.1360-0443.2002.00209.x. [DOI] [PubMed] [Google Scholar]
- 2.Knapp WP, Soares BG, Farrel M, de Lima MS. Psychosocial interventions for cocaine and psychostimulant amphetamines related disorders. The Cochrane database of systematic reviews. 2007;(3):Cd003023. doi: 10.1002/14651858.CD003023.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Mustroph ML, Stobaugh DJ, Miller DS, DeYoung EK, Rhodes JS. Wheel running can accelerate or delay extinction of conditioned place preference for cocaine in male C57BL/6J mice, depending on timing of wheel access. Eur J Neurosci. 2011 Oct;34(7):1161–1169. doi: 10.1111/j.1460-9568.2011.07828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thanos PK, Tucci A, Stamos J, et al. Chronic forced exercise during adolescence decreases cocaine conditioned place preference in Lewis rats. Behav Brain Res. 2010 Dec 20;215(1):77–82. doi: 10.1016/j.bbr.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiel KJ, Engelhardt B, Hood LE, Peartree NA, Neisewander JL. The interactive effects of environmental enrichment and extinction interventions in attenuating cue-elicited cocaine- seeking behavior in rats. Pharmacol Biochem Behav. 2011 Jan;97(3):595–602. doi: 10.1016/j.pbb.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch WJ, Piehl KB, Acosta G, Peterson AB, Hemby SE. Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biol Psychiatry. 2010 Oct 15;68(8):774–777. doi: 10.1016/j.biopsych.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinyor D, Brown T, Rostant L, Seraganian P. The role of a physical fitness program in the treatment of alcoholism. Journal of studies on alcohol. 1982 Mar;43(3):380–386. doi: 10.15288/jsa.1982.43.380. [DOI] [PubMed] [Google Scholar]
- 8.Weinstock J, Barry D, Petry NM. Exercise-related activities are associated with positive outcome in contingency management treatment for substance use disorders. Addictive behaviors. 2008 Aug;33(8):1072–1075. doi: 10.1016/j.addbeh.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collingwood TR, Reynolds R, Kohl HW, Smith W, Sloan S. Physical fitness effects on substance abuse risk factors and use patterns. Journal of drug education. 1991;21(1):73–84. doi: 10.2190/HV5J-4EYN-GPP7-Y3QG. [DOI] [PubMed] [Google Scholar]
- 10.Buchowski MS, Meade NN, Charboneau E, et al. Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. PloS one. 2011;6(3):e17465. doi: 10.1371/journal.pone.0017465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ussher MH, Taylor AH, Faulkner GE. Exercise interventions for smoking cessation. The Cochrane database of systematic reviews. 2014;8:Cd002295. doi: 10.1002/14651858.CD002295.pub5. [DOI] [PubMed] [Google Scholar]
- 12.Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol (1985) 2006 Oct;101(4):1237–1242. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- 13.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008 Jan;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 14.Wipfli BM, Rethorst CD, Landers DM. The anxiolytic effects of exercise: a meta-analysis of randomized trials and dose-response analysis. J Sport Exerc Psychol. 2008 Aug;30(4):392–410. doi: 10.1123/jsep.30.4.392. [DOI] [PubMed] [Google Scholar]
- 15.Rethorst CD, Wipfli BM, Landers DM. The antidepressive effects of exercise: a meta- analysis of randomized trials. Sports medicine (Auckland, NZ) 2009;39(6):491–511. doi: 10.2165/00007256-200939060-00004. [DOI] [PubMed] [Google Scholar]
- 16.Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. The Cochrane database of systematic reviews. 2013;9:Cd004366. doi: 10.1002/14651858.CD004366.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neuroscience and biobehavioral reviews. 2013 Sep;37(8):1622–1644. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koob GF. Neurobiology of addiction. Toward the development of new therapies. Annals of the New York Academy of Sciences. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- 19.Warden D, Trivedi MH, Greer TL, et al. Rationale and methods for site selection for a trial using a novel intervention to treat stimulant abuse. Contemporary clinical trials. 2012 Jan;33(1):29–37. doi: 10.1016/j.cct.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trivedi MH, Greer TL, Grannemann BD, et al. Stimulant reduction intervention using dosed exercise (STRIDE) - CTN 0037: study protocol for a randomized controlled trial. Trials. 2011;12:206. doi: 10.1186/1745-6215-12-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoutenberg M, Rethorst C, Fuzat G, et al. STimulant Reduction Intervention using Dosed Exercise (STRIDE) - Description of the Exercise Intervention and Behavioral Program to Ensure Adherence. Mental health and physical activity. 2012 Dec;5(2):175–182. doi: 10.1016/j.mhpa.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rethorst CD, Greer TL, Grannemann B, Ring KM, Marcus BH, Trivedi MH. A Health Education Intervention as the Control Condition in the CTN-0037 STRIDE multi-site exercise trial: Rationale and Description. Mental health and physical activity. 2014 Mar 1;7(1):37–41. doi: 10.1016/j.mhpa.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greer TL, Ring KM, Warden D, et al. Rationale for using exercise in the treatment of stimulant use disorders. The Journal of Global Drug Policy and Practice. 2012;6(1) [PMC free article] [PubMed] [Google Scholar]
- 24.Robins LN, Wing J, Wittchen HU, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988 Dec;45(12):1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- 25.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur Psychiatry. 1997;12(5):232–241. [Google Scholar]
- 26.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003 Sep 1;54(5):573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 27.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007 May 16;297(19):2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 28.Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010 Nov 24;304(20):2253–2262. doi: 10.1001/jama.2010.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcus BH, Albrecht AE, King TK, et al. The efficacy of exercise as an aid for smoking cessation in women: a randomized controlled trial. Arch Intern Med. 1999 Jun 14;159(11):1229–1234. doi: 10.1001/archinte.159.11.1229. [DOI] [PubMed] [Google Scholar]
- 30.Marcus BH, Albrecht AE, Niaura RS, et al. Exercise enhances the maintenance of smoking cessation in women. Addict Behav. 1995 Jan-Feb;20(1):87–92. doi: 10.1016/0306-4603(94)00048-4. [DOI] [PubMed] [Google Scholar]
- 31.Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. Measuring alcohol consumption: Psychosocial and biochemical methods. 1992:41–72. [Google Scholar]
- 32.Sobell LC, Sobell MB. Timeline Followback user’s guide: A calendar method for assessing alcohol and drug use. Toronto: Addiction Research Foundation; 1996. [Google Scholar]
- 33.Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000 Feb;68(1):134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- 34.Oden NL, VanVeldhuisen PC, Wakim PG, Trivedi MH, Somoza E, Lewis D. Power of automated algorithms for combining time-line follow-back and urine drug screening test results in stimulant-abuse clinical trials. The American journal of drug and alcohol abuse. 2011 Sep;37(5):350–357. doi: 10.3109/00952990.2011.601777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, N.J.: L Erlbaum Associates; 1988. [Google Scholar]
- 36.Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biological psychiatry. 2006 Jun 1;59(11):990–996. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. American journal of preventive medicine. 2005 Jan;28(1):1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Winhusen T, Somoza E, Ciraulo DA, et al. A double-blind, placebo-controlled trial of tiagabine for the treatment of cocaine dependence. Drug Alcohol Depend. 2007 Dec 1;91(2–3):141–148. doi: 10.1016/j.drugalcdep.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 39.Somoza EC, Winship D, Gorodetzky CW, et al. A multisite, double-blind, placebo- controlled clinical trial to evaluate the safety and efficacy of vigabatrin for treating cocaine dependence. JAMA Psychiatry. 2013 Jun;70(6):630–637. doi: 10.1001/jamapsychiatry.2013.872. [DOI] [PubMed] [Google Scholar]
- 40.Winhusen T, Somoza E, Sarid-Segal O, et al. A double-blind, placebo-controlled trial of reserpine for the treatment of cocaine dependence. Drug Alcohol Depend. 2007 Dec 1;91(2–3):205–212. doi: 10.1016/j.drugalcdep.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carroll KM, Nich C, Shi JM, Eagan D, Ball SA. Efficacy of disulfiram and Twelve Step Facilitation in cocaine-dependent individuals maintained on methadone: a randomized placebo- controlled trial. Drug Alcohol Depend. 2012 Nov 1;126(1–2):224–231. doi: 10.1016/j.drugalcdep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown RA, Abrantes AM, Read JP, et al. A Pilot Study of Aerobic Exercise as an Adjunctive Treatment for Drug Dependence. Ment Health Phys Act. 2010 Jun 1;3(1):27–34. doi: 10.1016/j.mhpa.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams DM, Dunsiger S, Whiteley JA, Ussher MH, Ciccolo JT, Jennings EG. Acute effects of moderate intensity aerobic exercise on affective withdrawal symptoms and cravings among women smokers. Addict Behav. 2011 Aug;36(8):894–897. doi: 10.1016/j.addbeh.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haerian BS. BDNF rs6265 polymorphism and drug addiction: a systematic review and meta-analysis. Pharmacogenomics. 2013 Dec;14(16):2055–2065. doi: 10.2217/pgs.13.217. [DOI] [PubMed] [Google Scholar]


