Abstract
The principles of engineering and physics have been applied to oncology for nearly 50 years. Engineers and physical scientists have made contributions to all aspects of cancer biology, from quantitative understanding of tumour growth and progression to improved detection and treatment of cancer. Many early efforts focused on experimental and computational modelling of drug distribution, cell cycle kinetics and tumour growth dynamics. In the past decade, we have witnessed exponential growth at the interface of engineering, physics and oncology that has been fuelled by advances in fields including materials science, microfabrication, nanomedicine, microfluidics, imaging, and catalysed by new programmes at the National Institutes of Health (NIH), including the National Institute of Biomedical Imaging and Bioengineering (NIBIB), Physical Sciences in Oncology, and the National Cancer Institute (NCI) Alliance for Nanotechnology. Here, we review the advances made at the interface of engineering and physical sciences and oncology in four important areas: the physical microenvironment of the tumour and technological advances in drug delivery; cellular and molecular imaging; and microfluidics and microfabrication. We discussthe research advances, opportunities and challenges for integrating engineering and physical sciences with oncology to develop new methods to study, detect and treat cancer, and we also describe the future outlook for these emerging areas.
In addition to biochemical and genetic abnormalities, tumours generate and exert physical forces during growth, progression, and metastasis1,2. These physical forces compress blood and lymphatic vessels, thereby reducing perfusion rates and generating hypoxia. In turn, these conditions promote tumour progression and metastasis, contribute to immune evasion and reduce the efficacy of therapeutics1. In combination with a stiffened extracellular matrix (ECM), physical forces generated by tumours act to increase invasive and metastatic potential3. Both malignant and non-malignant cells in the surrounding stroma proliferate and pull on the structural components of the tumour microenvironment (TME) to alter gene expression and cellular signalling1,4. Tumour vessels that nourish tumours are leaky and disorganized in part due to these forces, which further decrease perfusion5. Vessel leakiness and lymphatic compression together elevate interstitial fluid pressure (IFP) in tumours1. These structural and functional abnormalities hinder delivery of systemically administered targeted therapies and nanotherapeutics and lower the efficacy of chemotherapeutic agents, radiotherapy and immunotherapies5,6. Additionally, shear forces exerted by flowing blood and interstitial fluids modulate the behaviour of tumour cells and the surrounding TME1,7. By detecting and quantifying these physical abnormalities, physical scientists and engineers in collaboration with cancer biologists and oncologists are identifying new therapeutic strategies for cancer5,6.
Progress in cancer treatment relies on the development of new technologies originating from engineering and the physical sciences. The first researcher to coin the term ‘chemotherapy’ was a German chemist, Paul Ehrlich, who in 1908 first demonstrated the efficacy of animal models to screen chemicals for their activity against disease8. His accomplishments had major ramifications for the development of cancer chemotherapeutic agents, which now rely on collaborations between oncologists and medicinal chemists. In radiation therapy, oncologists work closely with physicists to ensure that patients receive prescribed radiation doses and dose distributions within acceptable degrees of accuracy that spare essential normal tissues. Radiation therapy has been continuously evolving with the development of new radiation techniques and advanced imaging modalities developed by physicists and oncologists in a collaborative effort9. In addition to traditional forms of treatment, novel targeted therapies are being developed by engineers to improve drug formulation and delivery, such as those that adapt to, exploit or ‘normalize’ the TME and have the potential to improve the outcome of radiation, chemotherapy, and immunotherapy6,10– 12. Specifically, chemotherapy has improved, and molecularly targeted therapeutics that rely heavily on advances in engineering are now being used in the clinic owing to new delivery formulations with reduced toxicity13,14. Additionally, high-throughput microfabricated drug screening platforms are being developed to identify biomarkers and to test drug responses during the course of personalized therapy15. Engineers and mathematicians are also using these technologies to develop pharmacokinetic models to predict drug distribution and efficacy16.
In this Review, we provide recent examples to illustrate how engineering and the physical sciences have contributed to the improved detection, treatment and fundamental understanding of cancer in four key areas. These areas are: the physical microenvironment of the tumour; drug delivery; cellular and molecular imaging; and microfluidics and microfabrication specifically applied to cancer.
Physical microenvironment of the tumour
Engineers and physical scientists have pioneered research into our understanding that cancer is more than simply malignant cells with genetic mutations but can instead be viewed as aberrant organs composed of cancer cells and their surrounding stroma, referred to as the TME3,6,17–19. Many aspects of the TME are abnormal, fuelling tumour progression and treatment resistance6,20–22.
Vascular and interstitial barriers
Despite the development of many cancer therapeutics in recent years, physical barriers in the TME limit drug delivery13,23. A meta-analysis of 117 studies of nanomedicine delivery showed that only 0.7% (median) of administered nanoparticle dosages reached tumour sites24. Nanomedicine delivery to tumours is thought to rely on the enhanced permeability and retention (EPR) effect25,26. Studies since these initial descriptions of the EPR effect have further elucidated EPR mechanisms in animal models, including imbalances between proangiogenic and antiangiogenic signalling6,27, impaired recruitment of pericytes28 and collapsed tumour lymphatics29. While similar EPR pathophysiology is observed in humans, its benefits remain unclear, as most nanotherapies have not demonstrated substantial benefits over conventional chemotherapy30. Evidence suggests that EPR is active in cancer patients, but physiological barriers exist that counteract it13,31. In fact, larger nanoparticles (~100 nm diameter) can extravasate from leaky tumour vessels but cannot penetrate through dense ECM, resulting in perivascular localization32. Nanoparticles may remain there, but chemotherapeutic agents released from these perivascular ‘depots’ either return to the circulation or advance minimally into tumour tissue, as they largely bind to the first cells they encounter23.
Recent work using advanced in vivo imaging, computational modelling and animal models has identified barriers in the TME that hinder therapeutic delivery and promote tumour progression1,5. These approaches have shown that leaky, disorganized vessels contribute to increased IFP and reduced blood supply to tumours6. Increased IFP abolished convective transport of drugs across vessel walls and tumour tissue, limiting penetration of drugs to diffusion alone33,6 (FIG. 1). To improve therapeutic delivery and slow tumour progression, strategies to normalize vasculature and lower IFP have been investigated. Antiangiogenic therapies such as blocking antibodies against vascular endothelial growth factor receptors (VEGFRs) prune immature tumour vessels and reduce leakiness of the remaining vasculature to improve integrity and function34. Specifically, treatment of tumour vasculature with VEGFR2 blocking antibodies improved smaller (~10 nm diameter) nanoparticle delivery, with no improvement for larger (~100 nm diameter) nanoparticles14. Mathematical models showed that reductions in vessel wall pore size through vascular normalization with VEGFR2 antibody treatment reduced IFP and improved small nanoparticle penetration14. In several clinical trials, vascular normalization with agents that target VEGF or its receptors in combination with chemotherapy and/or radiation therapy is associated with the survival of patients with various tumour types5, including glioblastoma35, non-small-cell lung cancer (NSCLC)36, and breast cancer37; the extended patient survival observed in these clinical trials is potentially due to improved vascular function as determined through measurement of vascular parameters by immunohistochemistry, magnetic resonance imaging (MRI), and/or computed tomography (CT) (NCT00662506; NCT00642759)38,39. In fact, vascular normalization contributes to improved survival with more than a dozen approved cancer drugs that block VEGF signalling, including the VEGFA blocking antibody bevacizumab (Avastin; Genentech)5.
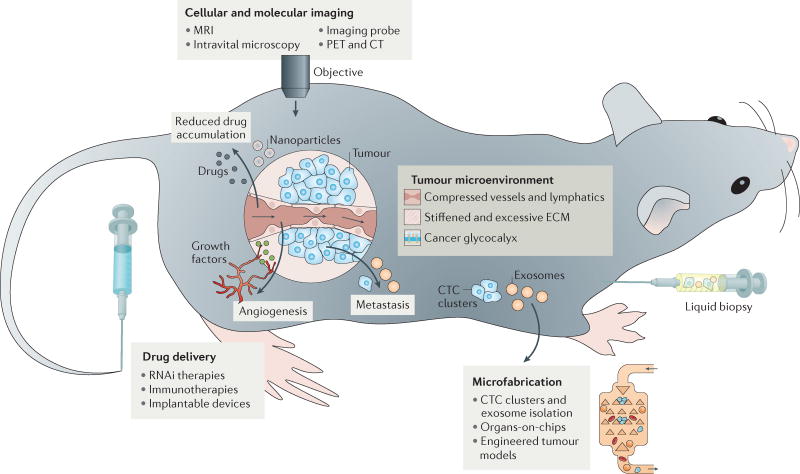
Figure 1. An overview of engineering and physical sciences in oncology.
Physical abnormalities of the tumour microenvironment (TME) have been identified using tools and concepts from engineering and the physical sciences. These include blood vessel and lymphatic compression, stiffened and excessive extracellular matrix (ECM), and the cancer cell glycocalyx. Collapsed blood vessels and increased solid stresses lead to reduced accumulation and limited delivery of drugs to tumour tissues. Steep pressure gradients in the periphery push fluid leaking from blood vessels located in the tumour margin into the surrounding normal tissues, facilitating the transport of growth factors and cancer cells into normal tissue and thus fuelling tumour growth, angiogenesis and metastasis. Pressure gradients also reduce the retention time of drugs and inhibit their homogeneous distribution inside the tumour. Advances in imaging, drug delivery and microfabrication have all been used to detect, manipulate and therapeutically target various aspects of this microenvironment. CT, computed tomography; CTC, circulating tumour cell; MRI, magnetic resonance imaging; PET, positron emission tomography.
Normalized vasculature and improved therapeutic delivery can also be achieved by targeting other components of the TME. Alterations in the ECM together with proliferating tumour and stromal cells leads to accumulation of solid stresses independent of IFP, which compress vessels29,40,41. These forces have been quantified using mathematical modelling and tumour deformation assays41,42. Depletion of tumour cells, cancer-associated fibroblasts (CAFs), or ECM showed that all three components contribute to solid stress accumulation in tumours in mice40,41. Obesity also contributes to vessel compression and poor vascular perfusion in pancreatic ductal adenocarcinoma (PDAC), as pancreatic tumours in obese mice have increased numbers of neutrophils, pancreatic stellate cells and adipocytes, creating a denser microenvironment43. Treatment with losartan, a clinically approved angiotensin II receptor antagonist shown to reduce desmoplasia6, also reduced solid stresses and pancreatic tumour progression in mice43. Moreover, angiotensin system inhibitors including losartan can also activate both innate and adaptive immune pathways in patients with PDAC44. The feasibility of using losartan to treat patients with pancreatic cancer was demonstrated in a phase II clinical trial (NCT01821729)45,46.
ECM stiffening
Human tumour tissue is often stiffer than normal tissue due to numerous factors, including desmoplasia and ECM reorganization and crosslinking. Cells including CAFs47 alter key parameters that affect ECM stiffness including density48, crosslinking4,49, and architectural and component changes50–52. Enhanced ECM density and rigidity facilitates liver and breast cancer detection via ultrasonography, and may indicate risk factors for tumour progression and therapeutic response53,54. ECM changes alter mechanotransduction55,56 and promote malignant behaviour by disrupting epithelial morphogenesis57, growth factor secretion and signalling58, invasive phenotype59,60 and stem cell differentiation61, as well as angiogenesis and vessel permeability62. As such, efforts have focused on characterizing altered ECM and how it contributes to tumour progression, with the goal of normalizing ECM for therapy3,63 (FIG. 2). Studies applying physical and biological approaches in vitro and in vivo, combined with patient samples, have demonstrated that ECM stiffening promotes the progression of many cancers, including cancers of the breast, pancreas, brain, lung and skin64–66.
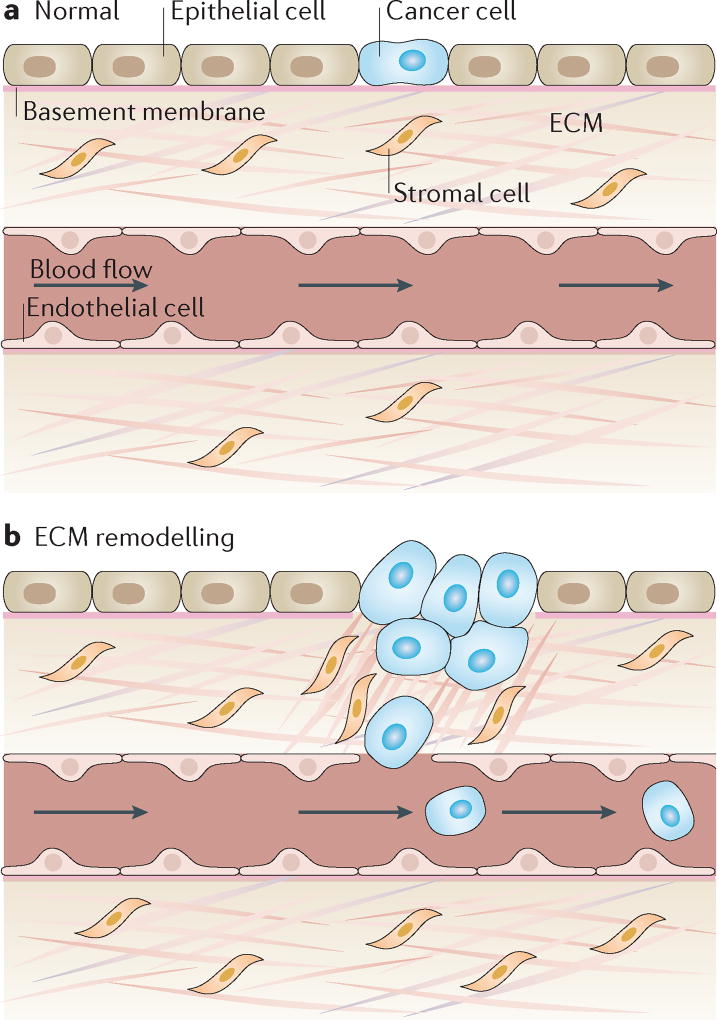
Figure 2. Extracellular matrix stiffening promotes cancer progression.
a | Under homeostatic conditions, the extracellular matrix (ECM) maintains tissue integrity and blocks rare tumour-prone cells from malignant progression by maintaining an overall healthy microenvironment. b | Under pathological conditions, ECM remodelling leads to collagen fibre alignment, bundling, and stiffening, which in turn alter interactions between the matrix and stromal and tumour cells to enhance pro-angiogenic secretion from a range of cells in the microenvironment as well as the migration of cancer cells. This process consequently promotes both the invasion of tumour cells from the primary site into the circulation and the recruitment of endothelial cells for vascularization of the tumour to initiate tumour growth, invasion into the surrounding stroma and, finally, metastasis3.
Traditionally considered independent risk factors, ECM stiffness and obesity were recently linked in the context of breast cancer67. Obesity-induced fibrotic remodelling of adipose tissue was previously shown to promote mammary tumour progression68. However, a multidisciplinary approach applying conformational fluorescence resonance energy transfer (FRET)-based ECM sensors, second-harmonic generation (SHG) ECM imaging, indentation-based mechanical measurements of ECM, combined with mouse models of obesity showed that mammary fat pads from obese mice have increased numbers of myofibroblasts that deposit ECM, which is associated with enhanced stiffness and increased breast cancer cell growth67. Interestingly, caloric restriction in this mouse model reduced levels of α-smooth muscle actin (α-SMA), a myofibroblast marker, indicating that fibrosis and ECM can be normalized to enhance cancer therapy67.
Recently, ECM stiffening in PDAC was linked to tumour progression69. Elevated epithelial tumour cell signal transducer and activator of transcription 3 (STAT3) signalling via genetic perturbations in transforming growth factor-β (TGFβ) signalling induced stiff, matricellular-enriched fibrosis associated with altered collagen fibre structure, increased epithelial tension and shortened survival in a mouse model of PDAC. In addition to this change in tumour genotype affecting ECM stiffness, earlier work showed that sonic hedgehog (SHH) signalling by the stroma contributes to ECM stiffening70, and SHH inhibition reduced fibrosis and enhanced penetration of chemotherapeutic agents in mouse models of pancreatic cancer71. By contrast, recent work using SHH knockouts and ablation of activated stromal cells showed that complete abrogation of the desmoplastic response increases pancreatic cancer metastasis and reduces survival in vivo72,73.
As an alternative means to reprogramme the stroma, another recent study showed that the vitamin D receptor (VDR) is expressed on pancreatic stellate cells, and activation of VDR with the ligand calcipotriol reduced both inflammation and fibrosis in pancreatitis and human tumour stroma74. This was also associated with increased chemotherapeutic retention, reduced tumour volume and increased survival in mice. Collectively, recent findings indicate that the normalization and reprogramming of tumour stroma, rather than destruction, can improve PDAC treatment. Several molecules that normalize these stromal components, including vitamin D, hyaluronidase, and losartan, are being assessed in clinical trials, and some have shown promising outcomes (NCT01821729)6,45,46.
Cancer glycocalyx
The sugar-rich glycocalyx coating on the surface of cancer cells (FIG. 3) facilitates tumour progression by enhancing angiogenesis, tumour growth and invasion75. The glycocalyx on epithelial and endothelial cells consists of proteoglycans and glycosaminoglycans, as well as matrix proteins including collagen76. Cell and matrix adhesion molecules are also embedded within the glycocalyx77. Mucin 1, a glycocalyx component that is overexpressed on cancer cells, mediates signal transduction to promote malignancy78. Hyaluronan, another glycocalyx component overexpressed on cancer cells, increases the tumorigenicity and metastatic potential of many cancers79. Anti-VEGF treatment can also increase hyaluronan in tumours in mice and patients and thereby confer treatment resistance80.
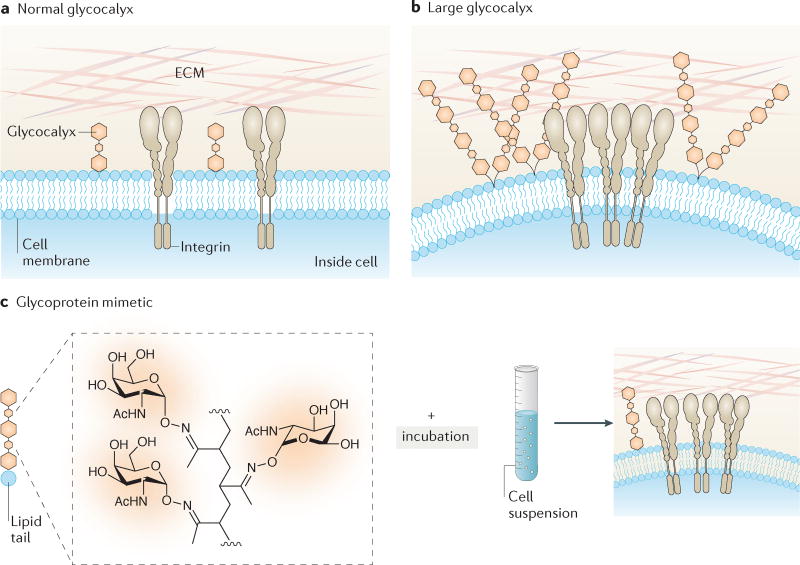
Figure 3. Role of the cancer cell glycocalyx in cancer progression.
a | Normal cells with a short glycocalyx have a uniform distribution of glycoproteins and adhesion molecules (integrins) across the cell membrane, which is close to the surrounding extracellular matrix (ECM). b | Cells with a larger glycocalyx, such as tumour cells81, exhibit extended gaps between the membrane and ECM, clustering of integrins, the exclusion of glycopolymers from regions of integrin adhesion, and membrane bending. These physical effects can alter cell signalling and promote tumour survival. c | Engineering the cancer cell glycocalyx via incorporation of synthetic glycoprotein mimetics with lipid insertion domains into living cell membranes81. The glycopolymers consist of a long-chain polymer backbone, pendant glycan chains mimicking natural mucin O-glycans, a phospholipid insertion domain and a fluorophore for imaging incorporation into cells. The approach enables synthetic mucin glycoprotein mimetics of a range of lengths to be rapidly incorporated into plasma membranes, where they project perpendicular to the cell surface. Synthetic glycoprotein mimetics have been used to study how the physical properties of the glycocalyx coating regulate cell survival during tumour invasion. Parts a and b are from REF. 209, Macmillan Publishers Limited.
A recent interdisciplinary study using scanning angle interference microscopy, polymeric glycomimetics, FRET probes and mathematical modelling showed that glycocalyx physical properties can regulate tumour cell growth, survival and metastasis81. Expression of bulky glycoproteins in the cancer cell glycocalyx facilitated integrin clustering and membrane blebbing, which enhanced survival through alterations in MEK, PI3K, and focal adhesion kinase (FAK) signalling pathways. Circulating tumour cells (CTCs) from patient blood also showed high surface expression of glycoproteins, suggesting that a bulky glycocalyx could promote tumour cell invasion and metastasis81.
The glycocalyx has also been implicated in mechanistic studies of immune evasion82,83. Increased expression of sialylated glycans on the cancer cell glycocalyx inhibited the activation of natural killer (NK) cells via engagement of inhibitory receptors on the NK cell surface82. Using glycopolymers functionalized with phospholipids on their ends, synthetically defined glycans were systematically introduced into the tumour cell membrane (FIG. 3) to probe the NK cell response82. Increased numbers of glycans reduced NK cell cytotoxicity in many tumour types, suggesting that glycocalyx sialylation offers a survival advantage to tumour cells under immunosurveillance. Approaches are now being explored for targeted removal of glycocalyx components to enhance NK cell-mediated tumour killing. For example, therapeutic conjugates consisting of the HER2-specific antibody trastuzumab fused to a recombinant sialidase removed sialylated glycans from tumour cells and induced potent NK cell cytotoxic activity84.
As engineers and physical scientists continue to study the TME, future efforts should increasingly focus on identifying safe and well-tolerated ‘normalizing’ therapeutics, which can be used in combination with immunotherapies, radiation or chemotherapies in the clinic. Such approaches hold great clinical promise, as some drugs that normalize the microenvironment are clinically approved for cancer and/or are being used in the clinic for other diseases6. The importance of the glycocalyx in cancer is only beginning to be recognized, and future detailed studies of its involvement in cancer will potentially lead to novel therapeutic strategies. For example, drugs that suppress the synthesis and/or assembly of the glycocalyx could be exploited for use in cancer therapy.
Drug delivery
Delivery materials for dosage and/or spatiotemporally controlled drug release were pioneered in the 1970s85–87. Drug delivery systems offer a means to deliver therapeutics to malignant cells in a safe and targeted manner compared with standard treatments that rely on radiation and chemotherapy, which can kill normal cells and induce toxicity in patients88. These systems consist of a variety of soft (that is, polymers and lipids) and hard (inorganic) materials at the microscale and nanoscale, and they offer advantages for therapy including the improved delivery of poorly soluble agents, protection of molecules from harsh microenvironments, targeted delivery to cells and tissues, controlled release at precise dosages, and reduced toxicity89. Early work led to the first drug delivery technologies for cancer therapy, including matrix-type systems such as Gliadel (Eisai), which are implantable polymeric wafers for the treatment of brain tumours90, and Lupron depot (AbbVie), which are polymeric microspheres that release a hormone therapy for prostate cancer treatment91. At the nanoscale, the first liposome-encapsulated chemotherapeutic agent, a doxorubicin delivery system (Doxil; Janssen), has improved chemotherapy in the clinic by reducing patient cardiotoxicity92.
Immunotherapy
One promising form of immunotherapy is the use of therapeutic cancer vaccines, which target tumours by mimicking immune mechanisms used against viral infections93. One major challenge is the delivery of vaccine antigens to lymph nodes, where much of the antitumour immune response is orchestrated94. Inspired by the ability of sentinel lymph node dyes used for biopsies to ‘hitchhike’ to serum albumin, lymph node-targeting amphiphilic vaccines were developed whereby an antigen or adjuvant is linked to a lipophilic albumin-binding tail using polymeric linkers95. Amphiphilic vaccines efficiently drained along with albumin into lymph nodes, induced a 30-fold increase in T cell priming and enhanced in vivo tumour cell killing while reducing systemic toxicity95. Biomaterial scaffolds can also be used as cancer vaccines and have been reviewed in detail93.
Nanoparticles have also garnered interest in immunotherapy, due to their ability to enhance delivery to target sites30. Nanoparticles targeting tumour-draining lymph nodes induced stronger immune and antitumour responses compared with targeting non-tumour-draining lymph nodes, possibly due to relief of immunosuppression and the boost of resident T cells that already had a high affinity for tumour-associated antigens96,97. In an alternative approach, mimicking pathogens in blood, nanoparticles containing tumour-derived RNA were engineered to systemically target dendritic cells, which initiate immune responses in lymphoid tissues in the presence of pathogens and foreign antigens98 (FIG. 4). Without targeting ligands, nanoparticle surface charge was optimized via alterations in lipid:RNA ratios to enable delivery to dendritic cells in the spleen and lymphoid tissues. These nanoparticles enabled dendritic cells to translate tumour RNA and consequently to express tumour antigens, present them to T cells and prime an antitumour immune response. This approach is in clinical trials for melanoma therapy and has shown promising immune responses in patients (NCT02410733)98,99. Looking forward, nanoparticle systems to deliver replicon mRNA can be used to amplify antitumour immunity100.
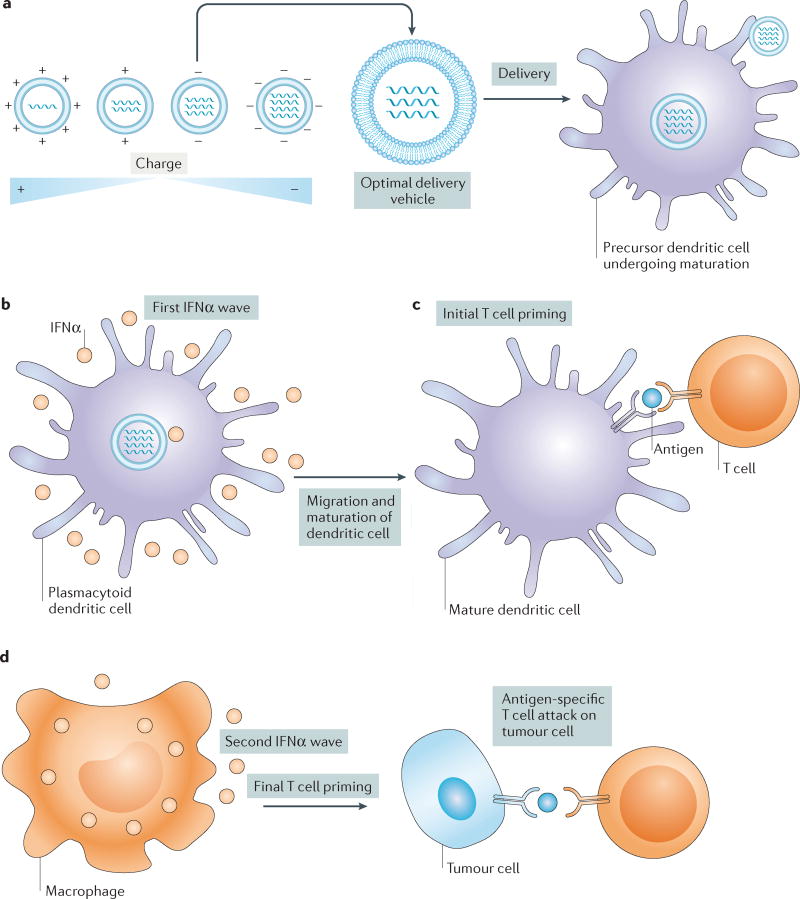
Figure 4. Drug delivery vehicles to enhance cancer immunotherapy.
a | A nanoparticle library is engineered to have varying surface charge in the absence of targeting ligands. Nanoparticle charge is altered by tailoring the ratio of the lipid delivery material to the amount of encapsulated RNA. These materials are then screened in mice for efficient delivery and transfection of dendritic cells in the spleen and other lymphoid organs. The top candidate with a slightly negative charge is delivered into mice via a nanoparticle RNA vaccine to target precursor dendritic cells, which causes them to develop into mature antigen-presenting dendritic cells that migrate to the T cells in lymph nodes. b | Uptake by plasmacytoid dendritic cells promotes secretion of an initial wave of interferon-α (IFNα) production that helps to prime initial T cell activation in lymph nodes98. c | Mature dendritic cells express tumour antigens derived from RNA, adjuvant or antigen in delivery vehicles and present them to T cells in lymph nodes. d | Uptake of delivery vehicles by macrophages leads to a second wave of IFNα release, fully priming T cells against specific antigens. Primed T cells then migrate to tumour sites, attacking and killing tumour cells. Figure from REF. 210, Macmillan Publishers Limited.
The reinfusion of ex vivo-expanded immune cells into patients has also been used as a form of immunotherapy, as the first chimeric antigen receptor (CAR) T cell gene therapy, Kymriah (Novartis), was recently approved by the US Food and Drug Administration (FDA) for the treatment of certain paediatric and young patients with a form of acute lymphoblastic leukaemia. However, adoptive T cell therapies require high systemic dosing of toxic adjuvant drugs101. To overcome this high dosing, the surfaces of immune cells have been functionalized with adjuvant-loaded nanoparticles via chemical conjugation, which enhances the antitumour response of T cells while minimizing systemic toxicity102. Engineering the immune cell surface with nanoparticles containing immunomodulators can also regulate the T cell synapse and antitumour immunity103. A similar approach has also been used to functionalize T cells with chemotherapeutic-containing nanoparticles, which actively targeted lymphoma in lymph nodes in which tumours typically evade systemic therapies104.
As an alternative to ex vivo approaches, strategies have been developed to bind therapeutics to immune cells in the bloodstream105,106. Inspired by NK cell cytotoxic activity, liposomal systems functionalized with a cell adhesion receptor, E-selectin, and an immune cytokine, tumour necrosis factor-related apoptosis-inducing ligand (TRAIL, also known as TNFSF10), that binds to death receptors on cancer cells to trigger apoptosis107 have been developed to tether to immune cells in the circulation105. In mice, this approach improved the circulation half-life of TRAIL, killed tumour cells in the bloodstream and prevented the spontaneous metastasis of prostate cancer108.
RNAi delivery systems
RNAi has the potential to efficiently silence ‘undruggable’ genes that contribute to disease progression. The most advanced RNAi-based drug to date is a lipid nanoparticle RNAi drug, patisiran, developed by Alnylam Pharmaceuticals, which has successfully met its primary efficacy end point and all secondary end points in a recent phase III clinical trial; it inhibits hepatic production of transthyretin as a form of transthyretin amyloidosis therapy (NCT01960348)109,110. In oncology, several early-phase trials of small interfering RNA (siRNA) therapeutics for solid cancers are ongoing or have been completed (NCT00938574; NCT01591356)109,111–113. Improvements in RNAi-based therapeutics are still needed, with a challenge being the safe and efficient delivery of siRNA to tumours in vivo. Nanoparticles as non-viral delivery vectors can address these needs by preventing nucleic acid degradation, evading immune detection, avoiding renal clearance and mediating cell entry and endosomal escape114 (FIG. 5).
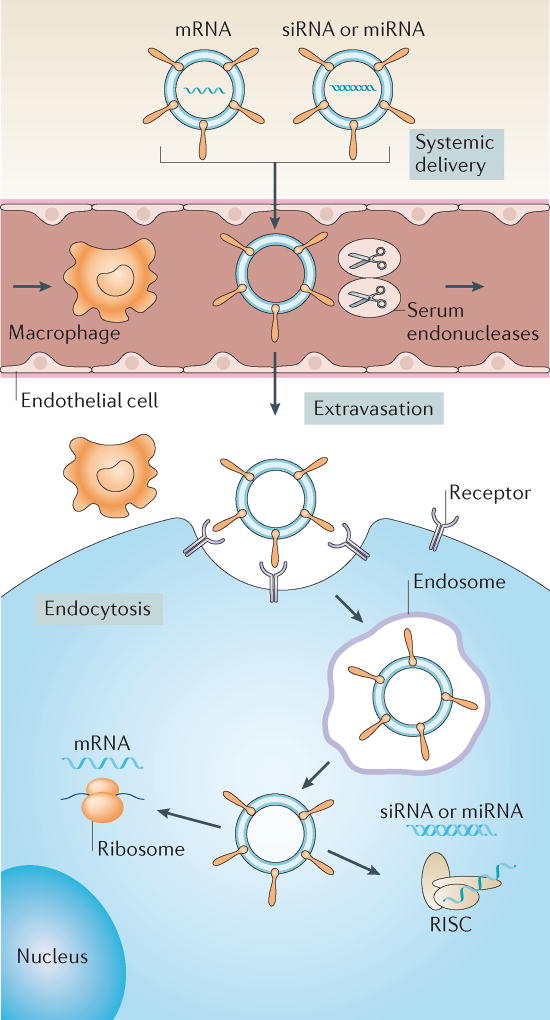
Figure 5. Non-viral delivery vectors for RNA-based therapies.
Various non-viral vectors, such as nanoparticles, can be used to deliver mRNA, small interfering RNA (siRNA) or microRNA (miRNA) therapeutics to target cells in vivo. These vectors prevent degradation of nucleic acids by serum endonucleases and evade immune detection. For effective delivery, these vehicles need to avoid renal clearance from the circulation and prevent nonspecific interactions with cells and proteins. When delivered intravenously, these vectors need to (i) extravasate from the bloodstream to reach target tissues, owing to nanoparticle characteristics and/or targeting ligands, (ii) enter target cells via the plasma membrane and (iii) induce endosomal escape into the cytosol. siRNA and miRNA must be loaded into the RNA-induced silencing complex (RISC) to initiate RNAi, whereas mRNA binds to translational machinery for subsequent protein expression. Figure from REF. 114, Macmillan Publishers Limited.
Recently, nanoparticles consisting of a cationic lipid–siRNA complex-containing poly(d,l-lactideco-glycolide) (PLGA) polymer core and a lipid-polyethylene glycol (PEG) shell extended the circulation half-life of systemically administered siRNA to ~8 hours, compared with naked siRNA, which rapidly clears from blood within 30 min, with high accumulation in tumours and effective gene silencing in vivo115. Prohibitin, a protein associated with drug resistance that is upregulated in several cancers and lacks effective inhibitors, was silenced by RNAi nanoparticle delivery and shown to inhibit tumour growth in a mouse model of NSCLC115. In addition to enhancing biodistribution, siRNA delivery vehicles have been designed to improve tumour penetration, through the use of tandem peptides capable of both tumour homing and penetration along with siRNA delivery across cell membranes116. The peptides were electrostatically bound to siRNA to form nanoparticles, and they effectively silenced the oncogenic inhibitor of DNA binding 4 (Id4), suppressing the growth of ovarian cancer xenografts and extending survival in mice116. siRNA delivery to the brain and endothelium has also been demonstrated using nanoparticles. Spherical nucleic acid (SNA) nanoparticles, consisting of gold nanoparticles covalently functionalized with densely packed siRNA duplexes, were engineered for the treatment of glioblastoma117. In a process dependent on the activity of scavenger receptors, SNAs penetrated the blood–brain and blood–tumour barriers to reduce glioma progression in a xenograft mouse model117. Beyond the brain, nanoparticles that induce potent gene silencing in endothelium were discovered through systematic screening of a combinatorial chemical library of ~2,400 formulations118. Nanoparticles consisting of low-molecular-weight polyamines and lipids preferentially delivered siRNA to endothelium in many organs in vivo without significantly reducing gene expression in liver and immune cells118. In a model of metastatic Lewis lung carcinoma, silencing of angiogenesis regulators reduced primary tumour growth and lung surface metastases in vivo118. The mechanism of endothelium targeting remains unclear, but it likely involves nanoparticle–serum protein interactions that enhance delivery119. Similar nanoparticle formulations combining both siRNA and microRNA (miRNA) have also been used to treat genetically engineered mouse models of lung cancer that have not shown durable responses to chemotherapy120. Other recent nanoparticle delivery systems have been engineered to co-deliver siRNA and chemotherapies via layer-by-layer self-assembly121 and to encapsulate siRNA within materials that have intrinsic cytotoxic effects toward tumour cells122.
Implantable delivery devices
The ability to predict and execute cancer therapeutic regimens remains an unmet need, owing to a failure to identify optimal drug choices to treat a patient’s tumour and the development of drug resistance over time, even when the optimal therapy is initially administered123. These factors remain challenging due to a lack of methods to screen the responses of a patient to a range of drugs in vivo. Recently, implantable devices have been engineered to administer drugs directly into tumours in mice in situ124,125. The devices deliver small doses of drugs simultaneously within the tumour, after which cytotoxic effects are assessed (FIG. 6). In one example, a cylindrical, microscale implantable device was fabricated from medical-grade Delrin acetal resin blocks with 16 separate drug reservoirs124. These devices were implanted into tumours using a biopsy needle, and they released microdoses of drugs into spatially distinct tumour regions. Small samples of the surrounding tissue and the device were removed via a standard biopsy procedure and showed correlations between apoptosis and the administered drug concentration from the device124. The cell death response to drugs delivered from the device was comparable to that of conventional systemic delivery, demonstrating that the device mimics the effects of systemic administration.
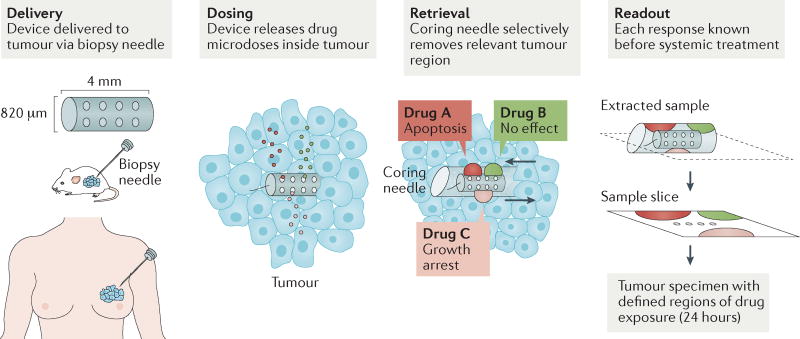
Figure 6. Implantable drug delivery devices for simultaneous screening of many drugs in tumours.
Implantable drug delivery devices were recently developed to enable in vivo drug sensitivity testing and biomarker analysis in patient tumours. One such device that can be implanted directly into tumours via biopsy needle is shown. It can be used to administer and subsequently evaluate the effects of up to 16 different drugs simultaneously via drug-releasing microwells. Three drugs released from microwells are depicted here for simplicity. The drugs diffuse from the microwells into confined regions of the tumour. The tumour tissue is then biopsied using a second coring needle that retrieves the device itself and a small column of tissue adjacent to the device. This tissue contains regions exposed to the drugs and is used to evaluate drug effects, such as apoptosis or growth arrest124. Another delivery device, the CIVO platform (not shown in this figure), microinjects up to six different drugs into tumours as they are being withdrawn, leaving a 6 mm track of both the drug and inert tracking dye. Tumour cell death in response to drugs is assessed 24–72 hours after injection, via tumour resection. Evaluation of pharmacological and pharmacodynamic markers in these studies, such as cleaved caspase 3 as a marker of tumour cell apoptosis, demonstrated that device outputs were similar to the effects of systemic in vivo therapy125. These devices offer a possible alternative to the traditional way of using cancer drugs that has become accepted practice for clinical trials and animal research. Both devices potentially offer a personalized system for assessing drug sensitivity in vivo and tailoring therapy accordingly. Additionally, both devices provide ease of testing several drug combinations directly within tumours, along with probing inter-tumour and intra-tumour heterogeneity in their response to drugs. Figure from REF. 124: Jonas, O. et al. An implantable microdevice to perform high-throughput in vivo drug sensitivity testing in tumors. Science Translational Medicine 7, 284ra57 (2015). Reprinted with permission from AAAS.
Another device consisting of an array of six micro-needles was developed to microinject drugs into mouse tumours125 (FIG. 6). The needles inject a 6 mm drug microtrack within the tumour, which is then excised and analysed for drug efficacy. The device predicted responses to systemically delivered drugs in mice, and it determined both drug resistance and unexpected sensitivity to cyclophosphamide in multidrug-resistant lymphomas. The device has moved into clinical testing, delivering microinjections of drugs into canines and human patients while remaining well tolerated (NCT01831505 and NCT03056599)125–127. Collectively, this class of devices offers personalized drug assessment in vivo to tailor patient therapies.
Despite advances in drug delivery, unmet clinical challenges remain. The development of novel biologics will require advanced delivery systems that protect these therapeutics in the body. For patients who fail to adhere to daily dosing regimens, devices that release drugs over weeks and months within the body will be needed to improve responses to therapies. Gene editing technologies, such as CRISPR–Cas9, zinc-finger nucleases and transcription activator-like effector nucleases, have the potential to enable permanent genetic modifications in diseased cells that contribute to cancer and will require new systems that efficiently deliver a combination of proteins, small RNAs, and/or mRNAs into target cells128.
Cellular and molecular imaging
Imaging biomarkers
Quantifying biomarkers is essential for basic cancer research, clinical diagnosis, early detection, and mapping disease severity129,130. One standard quantification technique, immunohistochemistry, is limited to detecting one or two biomarkers in samples because of spectral and spatial overlap constraints131. Recently, a method termed multiplexed ion beam imaging has been developed to expand the detection of biomarkers by staining tissues and cells with antibodies carrying isotopically pure elemental metal reporters132,133 (FIG. 7). Secondary ion mass spectrometry is then used to detect secondary ions released from metal-tagged antibodies, thereby producing images of the spatial features of molecular expression within tissues132. In comparison to fluorescence-based methods, this technique is capable of analysing up to 100 metal-tagged targets simultaneously and was shown to detect ten labels in formalin-fixed, paraffin-embedded human breast tumour tissue sections132. In theory, this method could also be applied to fresh samples of tumour tissue.
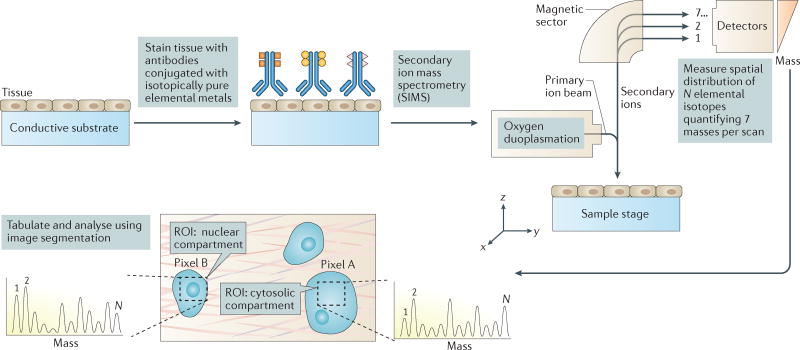
Figure 7. Multiplexed ion beam imaging for detecting as many as 100 targets simultaneously in tumour tissue samples.
Cell and tissue samples are immobilized on a conductive substrate and subsequently stained with antibodies conjugated to unique, isotopically pure elemental metal reporters. Samples are then dried and loaded under vacuum for multiplexed ion beam imaging (MIBI) analysis, in which the surface is rasterized with an oxygen primary ion beam that sputters the antibody-specific metal reporters native to the sample surface as secondary ions. Metal-conjugated antibodies are quantified via replicate scans of the same field of view, and regions of interest (ROIs) demarcating nuclear and cytosolic compartments of cells within the sample are integrated, tabulated and categorized. From these expression data, composite images composed of pseudocoloured categorical features, and quantitative three-colour overlays are then constructed. MIBI is capable of detecting up to 100 unique isotope-labelled antibodies and has been used to analyse paraffin-embedded human breast cancer tissue samples stained simultaneously with ten isotope-labelled antibodies to detect features such as nuclear and cytosolic compartments of cells, providing new insights into disease pathogenesis for basic research and clinical diagnostics. N = number of unique elemental reporters. Figure from REF. 31, Macmillan Publishers Limited.
In vivo imaging of biomarkers is essential for assessing tumours in organs not readily biopsied, including the brain. Brain tumours are typically characterized by genetic abnormalities that are difficult to detect in vivo134. Recently, noninvasive detection of 2-hydroxyglutarate (2HG), a metabolite associated with isocitrate dehydrogenase (IDH)-mutated gliomas in patients, was demonstrated using proton magnetic resonance spectroscopy (MRS)135. MRS measures high concentrations of metabolites in tissues via MRI135. Using MRS, concentrations of 2HG in 30 patients with glioma were estimated and correlated with IDH mutations135. Given that MRI is the primary modality for clinical evaluation of patients with glioma, 2HG detection could have important implications in the diagnosis, prognosis and stratification of brain tumours.
Novel molecular imaging probes are also being developed by engineers to enable tumour detection deep within tissues using modalities including MRI and photoacoustic imaging136. Photoacoustic imaging has translated rapidly to the clinic for many cancer applications, in part owing to this type of imaging being able to provide information via endogenous chromophores such as oxy- and deoxyhaemoglobin136. As not all cancers provide sufficient endogenous contrast to exploit photoacoustic imaging, contrast agents have been engineered to produce a photoacoustic signal high enough to be detected even at low concentrations, while being able to target diseased tissues. Gold nanorods and single-walled carbon nanotubes, which possess a sufficiently large optical absorption cross section to maximize the photoacoustic signal of the imaging probe while being small enough to be eliminated from the body, have improved photoacoustic imaging of cancer in mice137,138. Nanoparticles have also been engineered to be exploited in photoacoustic imaging to delineate the margins of brain tumours and map lymph nodes in mice139,140. Beyond photoacoustic imaging, novel probes are being developed for MRI. When targeting tumours using MRI, conventional ligand-targeted imaging probes typically fail to deliver sufficient numbers of magnetic nanoparticles for image contrast141–143. To increase the number of nanoparticles delivered to tumours per targeting ligand and hence improve contrast, bacteriophages have been used as scaffolds to display nanoparticles and targeting ligands for tumour imaging via MRI in mice144. While novel probes can impact diagnosis and surgical interventions at earlier stages of cancer, it is important to note that such agents add considerable cost to imaging exams, and new agents could require lengthy FDA approval processes.
Imaging therapeutic delivery
Imaging is playing an increasingly important role in drug development, clinical trial design and enhancing the delivery and monitoring of therapies145. For example, positron emission tomography (PET) imaging in the clinic can guide tumour biopsies, deliver drugs via image guidance, and detect therapeutic response in the absence of tumour shrinkage129,146. In research settings, intravital microscopy is used to study drug pharmacokinetics and pharmacodynamics using fluorescent companion imaging drugs147–149, combined with imaging of orthotopic tumour xenograft mouse models and methods to study drug targeting150. For example, precursor compounds have been conjugated to small, cell-permeable fluorophores to synthesize therapeutically active fluorescent companion imaging drugs147. Through the use of an implanted window chamber or medial skin incision at the tumour site, in vivo microscopy has enabled the detection of companion imaging drugs in orthotopic tumours with subcellular resolution and frame rates of several seconds147. These advances enable fundamental understanding of drug behaviour in vivo and provide critical insight into predicting drug efficacy. These techniques provide numerous advantages over the conventional study of therapeutic mechanisms in cell culture, which does not provide insight into delivery and whether or not the assumed mechanism of drug action occurs in vivo.
Imaging techniques have recently been developed to quantify drug concentrations inside cellular compartments and the uptake of drugs within tumour cell populations, as well as to determine the spatiotemporal dynamics of tumour drug resistance148. A combination of intravital imaging and automated single-cell tracking identified a new therapeutic mechanism of action for eribulin, an FDA-approved cytotoxic agent that inhibits microtubule growth148; intravital imaging showed that drug accumulation depended on drug efflux mediated by multidrug resistance 1 (MDR1, also known as ABCB1) and on tumour vascular architecture148, a mechanism that likely would have not been identified in the absence of in vivo imaging148. Using in vivo single-cell imaging in tumour-bearing mice, another recent study showed that polymeric nanoparticle encapsulation of a fluorescent platinum (IV) pro-drug affected both drug pharmacokinetics and drug uptake and response147,151. Intravital imaging showed high accumulation of nanoparticles within tumour-associated macrophages (TAMs), which then released drug to neighbouring tumour cells over time, implicating macrophages as slow release depots of drugs151.
Pairing MRI and intravital imaging can be used to identify tumours with a higher likelihood of nanoparticle accumulation152. To identify tumours with optimal EPR characteristics, magnetic nanoparticles 30 nm in diameter were used to predict colocalization of therapeutic nanoparticles via MRI. Mice with the highest magnetic nanoparticle intratumoural accumulation detected via MRI also showed the highest fluorescent therapeutic nanoparticle accumulation via intravital imaging. These mice showed the greatest reduction in tumour volume when subsequently treated with a paclitaxel-encapsulated nanoparticle152. Given that magnetic nanoparticles such as Ferumoxytol (AMAG Pharmaceuticals) are FDA-approved for other applications153, this approach has potential to select patients with high EPR characteristics and most likely to be responsive to nanoparticle-delivered therapies.
Cancer cell invasion and metastasis
Intravital imaging in animals has revealed aspects of cancer cell invasion and metastasis that cannot be quantified via genetic studies or tumour biopsies20,154. Confocal and multiphoton microscopy can be used to characterize tumour and immune cell behaviour in the microenvironment through fluorescent mouse models154,155. Parameters including cell migration mode, cell velocity and the number of migrating cells, along with tumour stroma–vessel interactions, can be quantified156. In particular, intravital imaging has shown that macrophage interactions with tumour cells promote tumour cell invasion and metastasis157,158. More specifically, in vivo imaging showed that breast cancer cell subpopulations migrate together with macrophages towards vessels in response to paracrine chemotactic signalling159 and intravasate at sites enriched with macrophages158,160. In a mouse model of breast cancer, colony-stimulating factor 1 receptor (CSF1R) antibodies administered via intraperitoneal injection depleted TAMs and dendritic cells, which delayed tumour growth, reduced vascularity and decreased lung metastasis. However, blocking the CSF1 pathway can have adverse effects on tumour progression with some tumour types, as CSF1 blockade significantly reduced the survival benefit of a dual cediranib (a pan-VEGFR tyrosine kinase inhibitor) and MEDI3617 (an angiopoietin 2 neutralizing antibody) therapy in a mouse glioblastoma model161. In addition, intravital imaging of mouse mammary tumours with MMPSense, a fluorescent probe for the activity of matrix metalloproteinases (MMPs) 2, 3, 7, 9 and 13 showed that TAMs and dendritic cells possess substantial MMP activity, which promotes invasion158.
Metastatic cancer cells that migrate towards blood and lymphatic vessels can cross ECM barriers via collagen network remodelling162. Advances in SHG and third-harmonic generation (THG) microscopy enable noninvasive imaging of collagen remodelling in vitro and in vivo without exogenous labelling of collagen162,163. SHG results when two photons with the same frequency interact with one nonlinear material to create one photon of half the wavelength and is typically elicited by collagen fibres in interstitial tissues163. THG results when three simultaneously arriving photons combine to make one of triple the frequency of an individual photon, making it ideal for detecting water–lipid and water–protein interfaces, including cellular membranes and tissue discontinuities along blood and lymph vessels65. In the clinic, SHG is used to classify collagen alignment in biopsied tissue sections from patients with cancer, and it has shown potential in predicting breast cancer survival164. SHG and THG provide minimal photodamage, enhanced optical penetration and quantitative information on collagen remodelling165. Combining SHG and THG with fluorescence microscopy provides insight into tumour tissue organization, cell–matrix interactions, blood flow dynamics, and the dissemination of microvesicles65,166. More specifically, the combination of all three imaging platforms enabled tracking of melanoma invasion into the dermis ~600 µm deep, along with reconstruction of the tumour cell invasion mode and tissue tracks to determine the invasion routes and outcome65. Differential forms of cancer cell invasion and migration were identified, as cancer cells invaded in a collective manner in pre-existing spaces along the interfaces of collagen bundles, whereas discontinuous collagen-rich stroma supported the dissemination of single tumour cells65,167.
Looking forward, physical scientists must address the challenge of detecting small tumours (that is, <1 mm3) deep within tissues to improve patient outcome. Single-cell imaging techniques that reach beyond current depth limits (that is, ~500 µm), with increased multiplexing (that is, >10 targets imaged simultaneously) are needed to study cancer at the molecular level. A novel class of quantum dots that emit in the short-wave infrared region (SWIR; 1000–2000 nm), where large organisms are rendered translucent for fluorescence imaging168, may also become invaluable in the fundamental understanding and treatment of cancer. Improved features of this imaging technique include a lack of autofluorescence, low light absorption by blood and tissue, and reduced scattering. Super-resolution ultrasound imaging, which goes beyond the sub-millimetre resolution of clinical ultrasound imaging by imaging at ultrasound frame rates, now enables noninvasive whole-organ mapping of microvasculature in vivo169. Additionally, novel techniques based on MRI, such as hyperpolarized 13C MRI and chemical exchange saturation transfer (CEST), hold potential for noninvasive imaging of tumour location and metabolism over time to improve both initial diagnosis and monitoring therapy170,171.
Microfluidics and microfabrication
In vitro models of migration and mechanotransduction
The mechanisms behind metastasis remain elusive, despite the fact that it contributes to more than 90% of cancer-related deaths167. The small degree of insight into tumour cell migration during metastasis is partially a reflection of the lack of in vitro models that recapitulate the process. Early models incorporating physiological fluid flow led to the discovery of autologous chemotaxis, a mechanism of tumour cell migration and homing to lymphatics17,172. More recent studies have used microfluidics to study the effects of interstitial flow on tumour cell migration; a microfluidic device comprising a region containing single cells suspended in collagen separating two microfluidic channels173 provided further evidence for autologous chemotaxis along with a competing mechanism whereby tumour cells migrate against the direction of interstitial flow.
In addition to fluid flow, microfabricated models are used to study tumour cell migration through confined microenvironments174,175. One migration process that can be modelled is the track-like structures in ECM used for in vivo tumour cell migration, which can be both naturally occurring and generated by cell–ECM remodelling through secretion of MMPs176. Using micromoulding technology, collagen microtracks were fabricated that mimic those generated by proteolytically active cancer cells in vivo177,178. These microtracks enabled migration of cells that cannot invade 3D collagen matrices, enhanced motility, and revealed that microtrack migration can occur in the absence of MMPs177. Microtracks substantially reduced tumour cell traction force generation, matrix remodelling and deformation processes necessary for tumour cell migration through matrices178.
Beyond microtracks, in vitro models have been developed to study tumour invasion where matrix stiffness and channel width can be altered independently179. The approach combines the polymerization and gelation of polyacrylamide hydrogels of tuneable stiffness onto silicon micromoulds to create microchannels of defined stiffness and geometry. Tumour cells were shown to migrate faster in narrow channels compared with wider ones at a given stiffness, while physical confinement increased migration speed with increasing ECM stiffness179. Cellular traction polarization was essential to this response, as inhibition of non-muscle myosin II dissipated polarization and rendered the relationship between migration and ECM stiffness less sensitive to confinement. Models of confinement have also identified tumour cell migration mechanisms dependent on both cell-volume regulation and water permeation, where water and ions flow in through the leading edge and out from the trailing edge of the cell180. Using microfabricated constrictions of varying dimensions, the size and mechanical properties of the tumour cell nucleus were found to be essential to migration181–184. Constrictions below a threshold cross-sectional area increased nuclear envelope rupture of tumour cells as they migrated through the confined space. However, these nuclear envelope ruptures were temporary, as endosomal sorting complex machinery was recruited to reseal the nuclear membrane, ensuring cell viability after migration through tight restrictions181,182.
Organs-on-chips
‘Organs-on-chips’ are microfabricated devices containing cells and tissues, which are organized to mimic organ-level functions15. These devices recreate physiological functions not possible using traditional 2D and 3D systems, including tissue–tissue interfaces, physicochemical cues and microenvironments, and blood vessel perfusion. Real-time, high-resolution imaging of tissues, along with analysis of cellular biochemical, genetic and metabolic activity can be quantified185. These systems can advance the screening and validation of cancer therapeutic agents, their mechanisms of action, and toxicity testing185.
Compartmental microfluidic models of endothelium have been developed for region-specific activation of endothelial cells under physiological flow, to identify vessel drug targets for breast cancer metastasis not previously identified in 2D culture186. Increased complexity and clinical relevance can be incorporated into such systems, as devices have been developed to mimic interactions between CTCs, endothelium and bone microenvironments as a model of metastasis to bone187. This system recapitulated CTC transendothelial migration and tumour formation in bone tissue in vitro, detected via real-time microscopy. The model identified C-X-C chemokine receptor 2 (CXCR2) on CTCs and C-X-C chemokine ligand 5 (CXCL5) secreted by osteo-differentiated bone marrow-derived mesenchymal stem cells (MSCs) as major signalling mediators in the extravasation process, implicating both as therapeutic targets for metastasis187.
To mimic the pharmacokinetics and pharmacodynamics of drugs in humans, ‘body-on-chip’ systems consisting of interconnected, compartmentalized tissues representing the human body have been developed16 (FIG. 8). A multi-organ model of interconnected microchambers representing colon cancer, liver, and bone marrow was able to reproduce the in vivo metabolism of a chemotherapeutic pro-drug of 5-fluorouracil (5-FU), tegafur, into 5-FU in the liver188. The model also reproduced tumour death by 5-FU, which was not observed in static culture188. Furthermore, these models have also been linked with computational pharmacokinetics and pharmacodynamics to improve drug predictability189.
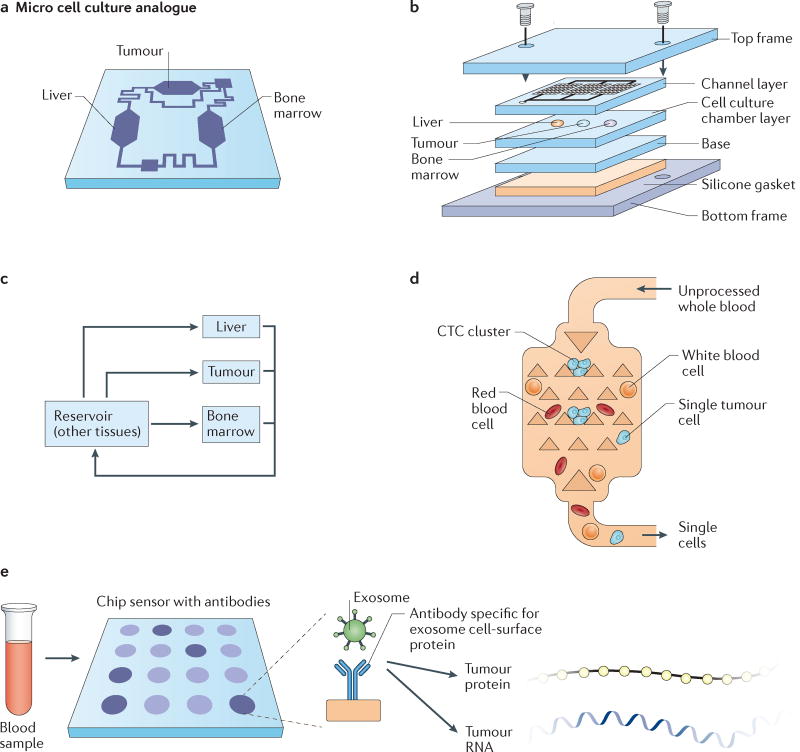
Figure 8. Microfluidics and microfabricated devices for ‘organs-on-chip’ tumour models and cancer diagnostics.
a | A microfluidic device containing interconnected, 3D cell culture microchambers that mimic tissues (tumour, bone marrow, liver) to develop a multi-organ model that simulates absorption, metabolism and activity of chemotherapeutic agents. b | Schematic of the microfabrication of the three-chamber organs-on-chip that is linked via microfluidics. c | Flow diagram of the connections between the tumour, liver and bone marrow compartments of the microfluidic organs-on-chip model. Drugs are added into the culture medium, which is recirculated in a controlled manner through three inline chambers and an external reservoir, to mimic physiological blood flow rates and blood residence times in each organ. Mathematical models are also utilized for fitting to experimental toxicity measurements, and parameter optimization is used to mimic liver, tumour and bone marrow cytotoxicity in vivo189. d | The Cluster-Chip device that captures circulating tumour cell (CTC) clusters from flowing unprocessed whole blood via microfabricated triangular micropillars, while single blood and tumour cells pass through the device200. e | Exosomes can be efficiently captured from blood using a nano-plasmonic exosome sensor, an array of periodic nanoholes patterned in gold film. Exosomes are captured on the sensors via affinity ligands specific for protein markers characteristic of exosomes, such as CD63. Exosome binding to the array changes the local refractive index of the sensor to an extent proportional to the level of the target protein, and can be used to detect the concentration of exosomes as well the abundance of proteins on or within exosomes204. As a result of this chip sensor technique, rare tumour proteins and RNA can then be extracted from exosomes for further analysis. Part a is from REF. 185, Macmillan Publishers Limited. Parts b and c are from REF. 15, Macmillan Publishers Limited. Part e is from REF. 211, Macmillan Publishers Limited.
CTC and exosome isolation
CTCs shed by tumours into the bloodstream are being utilized for detection of metastatic cancer190. As a therapeutic tool, CTCs are used to monitor the appearance of drug-resistant mutations, and they can be cultured ex vivo for personalized testing of drugs191. The use of CTCs in the clinic for prognosis and diagnosis is complicated by lengthy procedures for their isolation from patient blood, resulting in impurities and low yields190. The use of microfluidics has improved CTC isolation by exploiting biological (that is, target antigens) and physical properties (that is, fluid flow, cell size, shape, density and deformity) to separate rare CTCs from billions of contaminating blood cells192. First-generation devices utilized microfluidics containing microposts functionalized with antigens to capture CTCs, while non-target blood cells perfused through the device193. Nanostructures and microstructures have since been developed with additional immuno-based capture markers to improve CTC purity194,195. Given that CTCs are heterogeneous and might not express conventional epithelial biomarkers, devices have been developed for negative selection, where white blood cells are captured while CTCs are isolated via perfusion196. Given that CTC clusters have shown greater metastatic potential than singular CTCs and are associated with poor prognosis197–199, microfluidics have now been engineered to trap CTC clusters through the use of rows of triangular microposts200 (FIG. 8).
Exosomes ~30–100 nm in size are another source of biomarkers secreted in large amounts during carcinogenesis, and they carry proteins and miRNAs associated with metastatic tumours201. Exosome isolation is hampered by time-consuming ultracentrifugation steps and results in low yields (5–25%)202. To overcome these obstacles, microfluidics have also been used for exosome isolation, through incorporation of herringbone grooves within the microchannels to promote exosome mixing, and a channel surface functionalized with antibodies to capture exosomes203. Microfluidics have also been integrated with antibody-functionalized nanoplasmonic exosome sensors, in which surface plasmon resonance is utilized via periodic nanohole arrays on a metal film to detect and identify exosomes from patients with ovarian cancer based on surface protein expression204 (FIG. 8). Similar to CTC isolation, deterministic lateral displacement pillar arrays have been fabricated at the nanoscale to induce size-based displacement and fractioning of exosomes205.
Looking forward, microfabrication and microfluidics in cancer research will require the development of novel materials to enable mass production, along with reductions in the complexity of the experimental setup. Both improvements will provide opportunities for the widespread use of cancer tissue models and early detection (that is, exosomes, CTCs and circulating tumour DNA (ctDNA)) devices in research laboratories and the clinic. Tissue models should be validated with animal and clinical trial data to determine whether human physiology can be fully mimicked and whether they can be predictive of therapy. These systems must be robust and reproducible to be adopted by pharmaceutical companies for drug discovery. CTC isolation devices will likely be most useful for later-stage cancers where sufficient CTCs are detectable. Exosome isolation devices can potentially be used as a preventive-based measure before metastasis, as exosomes are present in measurable levels in blood before metastatic dissemination.
Future directions
The integration of engineering, physical sciences, and oncology over the past 50 years has proved to be a powerful approach to cancer research, leading to medical and technological breakthroughs. Looking forward, integration of these disciplines has the potential to enhance early diagnosis of cancer, which will save on expensive later-stage and last-minute treatments of metastatic cancer. The effectiveness of treatments can also be increased using such approaches, including novel delivery vehicles for immunotherapies and vaccines to enable our own bodies to fight cancer. Medical devices developed by engineers can be implanted into the tumours in a minimally invasive manner, which can better predict the efficacy of drugs in vivo and dramatically save on the costs of therapeutics. In terms of fundamental understanding of cancer, convergence of these disciplines will lead to new computational models of complex cancer systems, advanced imaging modalities from the subcellular to the whole-body level, and single-cell analyses with detailed protein, RNA and DNA characterization to expand our understanding of what drives cancer progression.
To fully realize the promise of engineering and physical sciences in oncology, funding from federal agencies should be specifically targeted towards research at the intersection of these disciplines. Indeed, in the US, the National Cancer Institute (NCI) has launched several programmes over the past two decades to catalyse such research, including the Alliance for Nanotechnology in Cancer in 2004, where experts in nanotechnology have worked side by side with oncologists and clinicians to foster new approaches in cancer detection and treatment206. More recently, in 2009, the NCI also launched the Physical Sciences–Oncology Center (PS–OC) Network of 12 interdisciplinary teams, with the goal of incorporating the perspectives of physical scientists that might formulate and approach problems in a distinct way that provides complementary insights into cancer207. In 2015, the NCI continued into phase II of the Physical Sciences–Oncology initiative by building a collaborative network of ten PS–OCs and eight Physical Sciences–Oncology Projects (PS–OPs). In addition to programs at the NCI, the National Institutes of Health (NIH), National Science Foundation (NSF), the US Department of Defense, and the US Department of Energy are now involved in some aspects of research at the integration of physical sciences, engineering and the life sciences — broadly referred to as convergence research208. However, the level of support for convergence research is small, with only 3% of all NIH funding going to principal investigators in physical sciences, engineering and mathematics208 and less going to those focused on integrating their expertise into cancer research.
In the future, engineering and physical sciences in oncology research cannot rely only on special funding programmes but will require dedicated strategic and funding plans from agencies across the world to achieve its full potential. Such dedicated funding mechanisms will enable engineers and physical scientists to collaborate with clinicians and biologists at the earliest stages of research, where the greatest impact in our understanding of the physical, genetic and biochemical properties of cancer can be made. Advances at the interface of these disciplines will supply the innovations that will give physicians and patients the diagnostics, information and therapeutics to eliminate the disease.
Acknowledgments
This work was supported in part by a Cancer Center Support (core) Grant P30-CA14051 from the National Cancer Institute and a grant from the Koch Institute’s Marble Centre for Cancer Nanomedicine (to R.L.) and the National Cancer Institute (P01-CA080124, R01-CA126642, R01-CA115767, R01-CA096915, R01-CA085140, R01-CA098706) and NCI Outstanding Investigator Award (R35-CA197743) (to R.K.J.). M.J.M. was supported by a Burroughs Wellcome Fund Career Award at the Scientific Interface, an NIH F32 fellowship (award number CA200351) and a grant from the Burroughs Wellcome Fund (no. 1015145). The authors thank V. Chauhan, M. Oberli, K. Kozielski, K. Wang, B. R. Seo, D. Fukumura, L. Munn and T. Stylianopoulos for helpful discussions and feedback on the manuscript. The authors thank K. Wang for assisting with conceptualization of figures.
Glossary
- Tumour microenvironment (TME)
The microenvironment surrounding cancer cells, which is composed of blood and lymphatic vessels, fibroblasts, immune cells and other non-malignant host cells, all embedded within extracellular matrix.
- Interstitial fluid pressure (IFP)
Pressure exerted by free interstitial tissue fluid. Increased IFP in tumours pushes fluid, growth factors, administered therapeutic molecules and cells to the peri-tumour tissue, aiding tumour progression.
- Enhanced permeability and retention (EPR)
An effect based on proposed mechanisms for selective tumour delivery of drugs. These mechanisms include the greater permeability of tumour vessels than normal vessels to macromolecules and the retention of macromolecules in tumours due to poor lymphatic clearance.
- Computed tomography (CT)
A diagnostic imaging test used to create images of internal organs, bones, soft tissue and blood vessels. In oncology, cross-sectional CT images are used to confirm the location and size of tumours.
- Solid stresses
Stresses exerted by and accumulated within solid components of tissues (that is, cells and extracellular matrix) during growth and progression. In tumours, solid stress is elevated due to growth and is independent of high interstitial fluid pressure.
- Tumour deformation assays
An assay to quantify stress in tumours. Excised tumours are cut in the middle of the tumour, and stress relaxation is quantified as the extent of tumour opening normalized to the diameter of the tumour.
- Desmoplasia
The formation and growth of fibrous tissue. In cancer, desmoplasia may occur around a neoplasm, causing dense fibrosis around the tumour.
- Ultrasonography
A technique using echoes of ultrasound pulses to delineate objects or areas of different density in the body. In cancer, ultrasonography is used to detect solid tumours.
- Matricellular-enriched fibrosis
The thickening and scarring of tissue surrounding a tumour, composed of dynamically expressed, non-structural proteins that are present in the extracellular matrix.
- Hyaluronidase
An enzyme that catalyses the degradation of hyaluronic acid, a component of the extracellular matrix that contributes to tumour growth.
- Adjuvant
A substance that enhances the body’s immune response to foreign antigens.
- Replicon mRNA
A self-replicating nucleic acid that amplifies production of the encoded protein and prolongs translation.
- Scavenger receptors
A group of receptors that recognize low-density lipoprotein that has been modified by oxidation or acetylation.
- Microdoses
Doses of a drug on the microgram scale, or about one-millionth of the systemic dose of a drug, that are intended to produce a beneficial result while avoiding undesirable side effects.
- Bacteriophages
Long, tubular viruses that infect specific bacteria; they have been used as scaffolds for nanoparticles and targeting ligands for imaging tumours using magnetic resonance imaging (MRI)
- Autologous chemotaxis
A mechanism by which a tumour cell can receive directional cues while at the same time being the source of such cues, enabling dissemination into the lymphatic system.
- Surface plasmon resonance
An optical technique for detecting the interaction of two different molecules or particles, in which one is mobile and one is fixed on a thin gold film.
- Deterministic lateral displacement pillar arrays
Arrays of pillars fabricated from silicon used to sort, separate and enrich microscale particles including parasites, bacteria, blood cells and tumour cells under flow conditions.
Footnotes
Author contributions
M.J.M., R.K.J. and R.L. conceived the ideas, researched the data for the manuscript, discussed the manuscript content and wrote the manuscript. M.J.M. designed the display items. All authors reviewed and edited the article before submission.
Competing interests statement
The authors declare no competing interests.
References
- 1.Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 2014;16:321–346. doi: 10.1146/annurev-bioeng-071813-105259. Comprehensive review of the role of physical forces in tumour progression and therapy for those new to the fields of engineering and physical sciences in oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer. 2011;11:512–522. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tse JM, et al. Mechanical compression drives cancer cells toward invasive phenotype. Proc. Natl Acad. Sci. USA. 2012;109:911–916. doi: 10.1073/pnas.1118910109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell MJ, King MR. Computational and experimental models of cancer cell response to fluid shear stress. Frontiers Oncol. 2013;3:44. doi: 10.3389/fonc.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeVita VT, Chu EA. History of cancer chemotherapy. Cancer Res. 2008;68:8643–8653. doi: 10.1158/0008-5472.CAN-07-6611. [DOI] [PubMed] [Google Scholar]
- 9.Verellen D, et al. Innovations in image-guided radiotherapy. Nat. Rev. Cancer. 2007;7:949–960. doi: 10.1038/nrc2288. [DOI] [PubMed] [Google Scholar]
- 10.Winkler F, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. Seminal study revealing the molecular and physiological mechanisms of vascular normalization, along with how normalization improves the outcome of various therapies. [DOI] [PubMed] [Google Scholar]
- 11.Wong C, et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl Acad. Sci. USA. 2011;108:2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain RK. An indirect way to tame cancer. Scientif. Am. 2014;310:46–53. doi: 10.1038/scientificamerican0214-46. [DOI] [PubMed] [Google Scholar]
- 13.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chauhan VP, et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 2012;7:383–388. doi: 10.1038/nnano.2012.45. First study demonstrating that normalization of leaky, disordered tumour vasculature enhances the delivery of smaller nanoparticle therapeutics to tumours. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat. Biotechnol. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 16.Esch MB, King TL, Shuler ML. The role of body-on-a-chip devices in drug and toxicity studies. Annu. Rev. Biomed. Eng. 2011;13:55–72. doi: 10.1146/annurev-bioeng-071910-124629. [DOI] [PubMed] [Google Scholar]
- 17.Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat. Rev. Cancer. 2012;12:210–219. doi: 10.1038/nrc3186. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain RK, Munn LL, Fukumura D. Dissecting tumour pathophysiology using intravital microscopy. Nat. Rev. Cancer. 2002;2:266–276. doi: 10.1038/nrc778. [DOI] [PubMed] [Google Scholar]
- 21.Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist. Updat. 2012;15:39–49. doi: 10.1016/j.drup.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J. Natl Cancer Inst. 2007;99:1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 23.Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat. Mater. 2013;12:958–962. doi: 10.1038/nmat3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilhelm S, et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1:16014. [Google Scholar]
- 25.Matsumura Y, Maeda HA. New concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 26.Gerlowski LE, Jain RK. Microvascular permeability of normal and neoplastic tissues. Microvasc. Res. 1986;31:288–305. doi: 10.1016/0026-2862(86)90018-x. [DOI] [PubMed] [Google Scholar]
- 27.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 28.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Padera TP, et al. Cancer cells compress intratumour vessels. Nature. 2004;427:695–695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- 30.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koukourakis MI, et al. Liposomal doxorubicin and conventionally fractionated radiotherapy in the treatment of locally advanced non-small-cell lung cancer and head and neck cancer. J. Clin. Oncol. 1999;17:3512–3521. doi: 10.1200/JCO.1999.17.11.3512. [DOI] [PubMed] [Google Scholar]
- 32.Stylianopoulos T, Jain RK. Design considerations for nanotherapeutics in oncology. Nanomedicine. 2015;11:1893–1907. doi: 10.1016/j.nano.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain RK, Baxter LT. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res. 1988;48:7022–7032. Seminal paper on the role of elevated IFP as a barrier to drug delivery. [PubMed] [Google Scholar]
- 34.Tong RT, et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 35.Batchelor TT, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc. Natl Acad. Sci. USA. 2013;110:19059–19064. doi: 10.1073/pnas.1318022110. Clinical evidence that vascular normalization and the resulting increase in perfusion improve survival in cancer patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heist RS, et al. Improved tumor vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer. Proc. Natl Acad. Sci. USA. 2015;112:1547–1552. doi: 10.1073/pnas.1424024112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tolaney SM, et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc. Natl Acad. Sci. USA. 2015;112:14325–14330. doi: 10.1073/pnas.1518808112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.US National Library of Medicine. 2017 doi: 10.1080/15360280801989377. Clinicaltrials.gov https://www.clinicaltrials.gov/ct2/show/NCT00662506. [DOI] [PubMed]
- 39.US National Library of Medicine. 2017 doi: 10.1080/15360280801989377. Clinicaltrials.gov https://www.clinicaltrials.gov/ct2/show/NCT00642759. [DOI] [PubMed]
- 40.Chauhan VP, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stylianopoulos T, et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl Acad. Sci. USA. 2012;109:15101–15108. doi: 10.1073/pnas.1213353109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nia HT, et al. Solid stress and elastic energy as measures of tumour mechanopathology. Nat. Biomed. Eng. 2016;1:0004. doi: 10.1038/s41551-016-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Incio J, et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 2016;6:852–869. doi: 10.1158/2159-8290.CD-15-1177. Study demonstrating the effects of obesity on tumour mechanics, along with potential strategies to overcome these effects using clinically available antifibrotic and inflammatory agents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu H, et al. Use of angiotensin system inhibitors is associated with immune activation and longer survival in non-metastatic pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-17-0256. http://dx.doi.org/10.1158/1078-0432.CCR-17-0256. [DOI] [PMC free article] [PubMed]
- 45.Murphy JE, et al. TGF-B1 inhibition with losartan in combination with FOLFIRINOX (F-NOX) in locally advanced pancreatic cancer (LAPC): preliminary feasibility and R0 resection rates from a prospective phase II study. J. Clin. Oncol. 2017;35:386–386. [Google Scholar]
- 46.US National Library of Medicine. 2017 doi: 10.1080/15360280801989377. Clinicaltrials.gov https://clinicaltrials.gov/show/NCT01821729. [DOI] [PubMed]
- 47.Kalluri R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 48.Provenzano PP, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naba A, Clauser KR, Lamar JM, Carr SA, Hynes RO. Extracellular matrix signatures of human mammary carcinoma identify novel metastasis promoters. eLife. 2014;3:e01308. doi: 10.7554/eLife.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Provenzano PP, et al. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rivron NC, et al. Tissue deformation spatially modulates VEGF signaling and angiogenesis. Proc. Natl Acad. Sci. USA. 2012;109:6886–6891. doi: 10.1073/pnas.1201626109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schrader J, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53:1192–1205. doi: 10.1002/hep.24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Youk JH, et al. Comparison of strain and shear wave elastography for the differentiation of benign from malignant breast lesions, combined with B-mode ultrasonography: qualitative and quantitative assessments. Ultrasound Med. Biol. 2014;40:2336–2344. doi: 10.1016/j.ultrasmedbio.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 55.Cheng G, Tse J, Jain RK, Munn LL. Microenvironmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing apoptosis in cancer cells. PLoS ONE. 2009;4:e4632. doi: 10.1371/journal.pone.0004632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calvo F, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. Seminal work demonstrating that elevated tissue stiffness promotes malignant behaviour via modulation of integrins. [DOI] [PubMed] [Google Scholar]
- 58.Wang K, et al. Stiffening and unfolding of early deposited-fibronectin increase proangiogenic factor secretion by breast cancer-associated stromal cells. Biomaterials. 2015;54:63–71. doi: 10.1016/j.biomaterials.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Artym VV, et al. Dense fibrillar collagen is a potent inducer of invadopodia via a specific signaling network. J. Cell Biol. 2015;208:331–350. doi: 10.1083/jcb.201405099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doyle AD, Carvajal N, Jin A, Matsumoto K, Yamada KM. Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat. Commun. 2015;6:8720. doi: 10.1038/ncomms9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. Seminal study demonstrating that stem cells that generate specialized cells within the body take cues from tissue stiffness to specify lineage and commit to phenotypes. [DOI] [PubMed] [Google Scholar]
- 62.Bordeleau F, et al. Matrix stiffening promotes a tumor vasculature phenotype. Proc. Natl Acad. Sci. USA. 2017;114:492–497. doi: 10.1073/pnas.1613855114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ulrich TA, De-Juan-Pardo EM, Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009;69:4167–4174. doi: 10.1158/0008-5472.CAN-08-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weigelin B, Bakker G-J, Friedl P. Intravital third harmonic generation microscopy of collective melanoma cell invasion. IntraVital. 2012;1:32–43. doi: 10.4161/intv.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blehm BH, Jiang N, Kotobuki Y, Tanner K. Deconstructing the role of the ECM microenvironment on drug efficacy targeting MAPK signaling in a preclinical platform for cutaneous melanoma. Biomaterials. 2015;56:129–139. doi: 10.1016/j.biomaterials.2015.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seo BR, et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci. Transl Med. 2015;7:301ra130. doi: 10.1126/scitranslmed.3010467. Study linking obesity, fibrotic remodelling and ECM mechanics to breast tumorigenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fukumura D, Incio J, Shankaraiah RC, Jain RK. Obesity and cancer: an angiogenic and inflammatory link. Microcirculation. 2016;23:191–206. doi: 10.1111/micc.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laklai H, et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat. Med. 2016;22:497–505. doi: 10.1038/nm.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bailey JM, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin. Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olive KP, et al. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rhim AD, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Özdemir BC, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sherman MH, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 76.Mitchell MJ, King MR. Physical Biology in Cancer. 3. The role of cell glycocalyx in vascular transport of circulating tumor cells. Am. J. Physiol., Cell Physiol. 2014;306:C89–C97. doi: 10.1152/ajpcell.00285.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 78.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 79.Itano N, Sawai T, Miyaishi O, Kimata K. Relationship between hyaluronan production and metastatic potential of mouse mammary carcinoma cells. Cancer Res. 1999;59:2499–2504. [PubMed] [Google Scholar]
- 80.Rahbari NN, et al. Anti-VEGF therapy induces ECM remodeling and mechanical barriers to therapy in colorectal cancer liver metastases. Sci. Transl Med. 2016;8:360ra135. doi: 10.1126/scitranslmed.aaf5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paszek MJ, et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 2014;511:319–325. doi: 10.1038/nature13535. First study implicating the bulky glycocalyx of tumour cells as a feature that promotes metastasis via mechanically enhanced cell-surface-receptor function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hudak JE, Canham SM, Bertozzi CR. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nature Chem. Biol. 2013;10:69–75. doi: 10.1038/nchembio.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Läubli H, et al. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc. Natl Acad. Sci. USA. 2014;111:14211–14216. doi: 10.1073/pnas.1409580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiao H, Woods EC, Vukojicic P, Bertozzi CR. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc. Natl Acad. Sci. USA. 2016;113:10304–10309. doi: 10.1073/pnas.1608069113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Langer R, Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976;263:797–800. doi: 10.1038/263797a0. First demonstration of the use of polymeric materials for controlled, sustained release of high-molecular-weight compounds for > 100 days. [DOI] [PubMed] [Google Scholar]
- 86.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202:1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 87.Brownlee M, Cerami A. A glucose-controlled insulin-delivery system: semisynthetic insulin bound to lectin. Science. 1979;206:1190–1191. doi: 10.1126/science.505005. [DOI] [PubMed] [Google Scholar]
- 88.Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 2013;48:416–427. doi: 10.1016/j.ejps.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 90.Sampath P, Brem H. Implantable slow-release chemotherapeutic polymers for the treatment of malignant brain tumors. Cancer Control. 1998;5:130–137. doi: 10.1177/107327489800500204. [DOI] [PubMed] [Google Scholar]
- 91.Okada H, Doken Y, Ogawa Y, Toguchi H. Preparation of three-month depot injectable microspheres of leuprorelin acetate using biodegradable polymers. Pharm. Res. 1994;11:1143–1147. doi: 10.1023/a:1018936815654. [DOI] [PubMed] [Google Scholar]
- 92.Gabizon A, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54:987–992. Seminal report showing prolonged circulation time and enhanced tumour accumulation of polyethylene glycol-coated doxorubicin liposome formulations (Doxil) in patients, compared with free doxorubicin. [PubMed] [Google Scholar]
- 93.Gu L, Mooney DJ. Biomaterials and emerging anticancer therapeutics: engineering the microenvironment. Nat. Rev. Cancer. 2016;16:56–66. doi: 10.1038/nrc.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moon JJ, Huang B, Irvine DJ. Engineering nano- and microparticles to tune immunity. Adv. Mater. 2012;24:3724–3746. doi: 10.1002/adma.201200446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu H, et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. Engineering of an amphiphilic cancer vaccine that ‘hitchhikes’ with serum albumin to traffic efficiently to lymph nodes, enabling substantially increased potency and safety of subunit vaccines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jeanbart L, et al. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol. Res. 2014;2:436–447. doi: 10.1158/2326-6066.CIR-14-0019-T. [DOI] [PubMed] [Google Scholar]
- 97.Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the antitumor immune response. Biomaterials. 2014;35:814–824. doi: 10.1016/j.biomaterials.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 98.Kranz LM, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. Synthesis of a nanoparticle RNA vaccine that targets dendritic cells after systemic administration, leading to an antitumour immune response with antiviral features as well as potential efficacy in patients with advanced melanoma. [DOI] [PubMed] [Google Scholar]
- 99.US National Library of Medicine. 2016 doi: 10.1080/15360280801989377. Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT02410733. [DOI] [PubMed]
- 100.Chahal JS, et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl Acad. Sci. USA. 2016;113:E4133–E4142. doi: 10.1073/pnas.1600299113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 2010;16:1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stephan MT, Stephan SB, Bak P, Chen J, Irvine DJ. Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles. Biomaterials. 2012;33:5776–5787. doi: 10.1016/j.biomaterials.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang B, et al. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Sci. Transl Med. 2015;7:291ra94. doi: 10.1126/scitranslmed.aaa5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mitchell MJ, Wayne E, Rana K, Schaffer CB, King MR. TRAIL-coated leukocytes that kill cancer cells in the circulation. Proc. Natl Acad. Sci. USA. 2014;111:930–935. doi: 10.1073/pnas.1316312111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mitchell MJ, King MR. Leukocytes as carriers for targeted cancer drug delivery. Expert Opin. Drug Deliv. 2014;12:375–392. doi: 10.1517/17425247.2015.966684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mitchell MJ, et al. Polymeric mechanical amplifiers of immune cytokine-mediated apoptosis. Nat. Commun. 2017;8:14179. doi: 10.1038/ncomms14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wayne EC, et al. TRAIL-coated leukocytes that prevent the bloodborne metastasis of prostate cancer. J. Control. Release. 2016;223:215–223. doi: 10.1016/j.jconrel.2015.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zuckerman JE, Davis ME. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat. Rev. Drug Discov. 2015;14:843–856. doi: 10.1038/nrd4685. [DOI] [PubMed] [Google Scholar]
- 110.US National Library of Medicine. 2017 doi: 10.1080/15360280801989377. Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT01960348. [DOI] [PubMed]
- 111.Davis ME, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.US National Library of Medicine. 2013 doi: 10.1080/15360280801989377. Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT00938574. [DOI] [PubMed]
- 113.US National Library of Medicine. 2017 doi: 10.1080/15360280801989377. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01591356. [DOI] [PubMed]
- 114.Yin H, et al. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 115.Zhu X, et al. Long-circulating siRNA nanoparticles for validating Prohibitin1-targeted non-small cell lung cancer treatment. Proc. Natl Acad. Sci. USA. 2015;112:7779–7784. doi: 10.1073/pnas.1505629112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ren Y, et al. Targeted tumor-penetrating siRNA nanocomplexes for credentialing the ovarian cancer oncogene ID4. Sci. Transl Med. 2012;4:147ra112. doi: 10.1126/scitranslmed.3003778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jensen SA, et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci. Transl Med. 2013;5:209ra152. doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dahlman JE, et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat. Nanotechnol. 2014;9:648–655. doi: 10.1038/nnano.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Akinc A, et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 2010;18:1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xue W, et al. Small RNA combination therapy for lung cancer. Proc. Natl Acad. Sci. USA. 2014;111:E3553–E3561. doi: 10.1073/pnas.1412686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Deng ZJ, et al. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano. 2013;7:9571–9584. doi: 10.1021/nn4047925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao Y, et al. PolyMetformin combines carrier and anticancer activities for in vivo siRNA delivery. Nat. Commun. 2016;7:11822. doi: 10.1038/ncomms11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Coombes RC. Drug testing in the patient: toward personalized cancer treatment. Sci. Transl Med. 2015;7:284ps10. doi: 10.1126/scitranslmed.aab1214. [DOI] [PubMed] [Google Scholar]
- 124.Jonas O, et al. An implantable microdevice to perform high-throughput in vivo drug sensitivity testing in tumors. Sci. Transl Med. 2015;7:284ra57. doi: 10.1126/scitranslmed.3010564. Development of a minimally invasive microdevice that can be implanted and removed from tumours using a biopsy needle, enabling direct drug sensitivity testing of many compounds within a patient. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Klinghoffer RA, et al. A technology platform to assess multiple cancer agents simultaneously within a patient’s tumor. Sci. Transl Med. 2015;7:284ra58. doi: 10.1126/scitranslmed.aaa7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.US National Library of Medicine. 2017 doi: 10.1080/15360280801989377. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01831505. [DOI] [PubMed]
- 127.US National Library of Medicine. 2017 doi: 10.1080/15360280801989377. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03056599. [DOI] [PubMed]
- 128.Yin H, Kauffman KJ, Anderson DG. Delivery technologies for genome editing. Nat. Rev. Drug Discov. 2017;16:387–399. doi: 10.1038/nrd.2016.280. [DOI] [PubMed] [Google Scholar]
- 129.Weissleder R, Nahrendorf M. Advancing biomedical imaging. Proc. Natl Acad. Sci. USA. 2015;112:14424–14428. doi: 10.1073/pnas.1508524112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bao G, Mitragotri S, Tong S. Multifunctional nanoparticles for drug delivery and molecular imaging. Annu. Rev. Biomed. Eng. 2013;15:253–282. doi: 10.1146/annurev-bioeng-071812-152409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rimm DL. What brown cannot do for you. Nat. Biotechnol. 2006;24:914–916. doi: 10.1038/nbt0806-914. [DOI] [PubMed] [Google Scholar]
- 132.Angelo M, et al. Multiplexed ion beam imaging of human breast tumors. Nat. Med. 2014;20:436–442. doi: 10.1038/nm.3488. Development of a new technique — multiplex ion beam imaging — which utilizes secondary ion mass spectrometry and antibodies labelled with isotopically pure elemental metals to detect up to 100 tumour antigens within a single sample. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bendall SC, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332:687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peet AC, Arvanitis TN, Leach MO, Waldman AD. Functional imaging in adult and paediatric brain tumours. Nat. Rev. Clin. Oncol. 2012;9:700–711. doi: 10.1038/nrclinonc.2012.187. [DOI] [PubMed] [Google Scholar]
- 135.Choi C, et al. 2-Hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat. Med. 2012;18:624–629. doi: 10.1038/nm.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zackrisson S, van de Ven SMWY, Gambhir SS. Light in and sound out: emerging translational strategies for photoacoustic imaging. Cancer Res. 2014;74:979–1004. doi: 10.1158/0008-5472.CAN-13-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jokerst JV, Cole AJ, Van de Sompel D, Gambhir SS. Gold nanorods for ovarian cancer detection with photoacoustic imaging and resection guidance via Raman imaging in living mice. ACS Nano. 2012;6:10366–10377. doi: 10.1021/nn304347g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.la Zerda de A, et al. Family of enhanced photoacoustic imaging agents for high-sensitivity and multiplexing studies in living mice. ACS Nano. 2012;6:4694–4701. doi: 10.1021/nn204352r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kircher MF, et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 2012;18:829–834. doi: 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pu K, et al. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat. Nanotechnol. 2014;9:233–239. doi: 10.1038/nnano.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine. 2011;6:715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gu F, et al. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc. Natl Acad. Sci. USA. 2008;105:2586–2591. doi: 10.1073/pnas.0711714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 2010;188:759–768. doi: 10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ghosh D, et al. M13-templated magnetic nanoparticles for targeted in vivo imaging of prostate cancer. Nat. Nanotechnology. 2012;7:677–682. doi: 10.1038/nnano.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weissleder R, Schwaiger MC, Gambhir SS, Hricak H. Imaging approaches to optimize molecular therapies. Sci. Transl Med. 2016;8:355ps16. doi: 10.1126/scitranslmed.aaf3936. [DOI] [PubMed] [Google Scholar]
- 146.Miller MA, Weissleder R. Imaging the pharmacology of nanomaterials by intravital microscopy: toward understanding their biological behavior. Adv. Drug Deliv. Rev. 2016;113:61–86. doi: 10.1016/j.addr.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thurber GM, et al. Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo. Nat. Commun. 2013;4:1504. doi: 10.1038/ncomms2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Laughney AM, et al. Single-cell pharmacokinetic imaging reveals a therapeutic strategy to overcome drug resistance to the microtubule inhibitor eribulin. Sci. Transl Med. 2014;6:261ra152. doi: 10.1126/scitranslmed.3009318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miller MA, Askevold B, Yang KS, Kohler RH, Weissleder R. Platinum compounds for high-resolution in vivo cancer imaging. ChemMedChem. 2014;9:1131–1135. doi: 10.1002/cmdc.201300502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dubach JM, et al. In vivo imaging of specific drug-target binding at subcellular resolution. Nat. Commun. 2014;5:3946. doi: 10.1038/ncomms4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Miller MA, et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat. Commun. 2015;6:8692. doi: 10.1038/ncomms9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Miller MA, et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci. Transl Med. 2015;7:314ra183. doi: 10.1126/scitranslmed.aac6522. A combined approach of MRI and intravital imaging, which demonstrated that magnetic nanoparticles can be used to select for tumours in vivo with high EPR and thus are more likely to respond to treatment with therapeutic nanoparticles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Harisinghani MG, et al. A pilot study of lymphotrophic nanoparticle-enhanced magnetic resonance imaging technique in early stage testicular cancer: a new method for noninvasive lymph node evaluation. Urology. 2005;66:1066–1071. doi: 10.1016/j.urology.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 154.Wyckoff J, Gligorijevic B, Entenberg D, Segall J, Condeelis J. High-resolution multiphoton imaging of tumors in vivo. Cold Spring Harb. Protoc. 2011;2011:1167–1184. doi: 10.1101/pdb.top065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jain RK. Normalizing tumor vasculature with antiangiogenic therapy: a new paradigm for combination therapy. Nat. Med. 2001;7:987–989. doi: 10.1038/nm0901-987. Seminal paper to put forward the vascular normalization hypothesis that changed the paradigm in antiangiogenic therapy. [DOI] [PubMed] [Google Scholar]
- 156.Wang W, et al. Coordinated regulation of pathways for enhanced cell motility and chemotaxis is conserved in rat and mouse mammary tumors. Cancer Res. 2007;67:3505–3511. doi: 10.1158/0008-5472.CAN-06-3714. [DOI] [PubMed] [Google Scholar]
- 157.Wyckoff J, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 158.Lohela M, et al. Intravital imaging reveals distinct responses of depleting dynamic tumor-associated macrophage and dendritic cell subpopulations. Proc. Natl Acad. Sci. USA. 2014;111:E5086–E5095. doi: 10.1073/pnas.1419899111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Goswami S, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 160.Wyckoff JB, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 161.Peterson TE, et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc. Natl Acad. Sci. USA. 2016;113:4470–4475. doi: 10.1073/pnas.1525349113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brown E, et al. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat. Med. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 163.Nadiarnykh O, LaComb RB, Brewer MA, Campagnola PJ. Alterations of the extracellular matrix in ovarian cancer studied by Second Harmonic Generation imaging microscopy. BMC Cancer. 2010;10:94. doi: 10.1186/1471-2407-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Conklin MW, et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 2011;178:1221–1232. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gailhouste L, et al. Fibrillar collagen scoring by second harmonic microscopy: a new tool in the assessment of liver fibrosis. J. Hepatol. 2010;52:398–406. doi: 10.1016/j.jhep.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 166.Weigelin B, Bakker G-J, Friedl P. Third harmonic generation microscopy of cells and tissue organization. J. Cell Sci. 2016;129:245–255. doi: 10.1242/jcs.152272. [DOI] [PubMed] [Google Scholar]
- 167.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bruns OT, et al. Next-generation in vivo optical imaging with short-wave infrared quantum dots. Nat. Biomed. Eng. 2017;1:0056. doi: 10.1038/s41551-017-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Errico C, et al. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature. 2015;527:499–502. doi: 10.1038/nature16066. [DOI] [PubMed] [Google Scholar]
- 170.Nelson SJ, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Sci. Transl Med. 2013;5:198ra108. doi: 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Walker-Samuel S, et al. In vivo imaging of glucose uptake and metabolism in tumors. Nat. Med. 2013;19:1067–1072. doi: 10.1038/nm.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shields JD, et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–538. doi: 10.1016/j.ccr.2007.04.020. Seminal study identifying a novel mechanism underlying metastasis through the lymphatic system, named autologous chemotaxis, which directs tumour cell migration via autocrine chemokine gradients induced by interstitial flow. [DOI] [PubMed] [Google Scholar]
- 173.Polacheck WJ, Charest JL, Kamm RD. Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc. Natl Acad. Sci. USA. 2011;108:11115–11120. doi: 10.1073/pnas.1103581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Paul CD, Mistriotis P, Konstantopoulos K. Cancer cell motility: lessons from migration in confined spaces. Nat. Rev. Cancer. 2016;17:131–140. doi: 10.1038/nrc.2016.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mekhdjian AH, et al. Integrin-mediated traction force enhances paxillin molecular associations and adhesion dynamics that increase the invasiveness of tumor cells into a three-dimensional extracellular matrix. Mol. Biol. Cell. 2017;28:1467–1488. doi: 10.1091/mbc.E16-09-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Alexander S, Weigelin B, Winkler F, Friedl P. Preclinical intravital microscopy of the tumour-stroma interface: invasion, metastasis, and therapy response. Curr. Opin. Cell Biol. 2013;25:659–671. doi: 10.1016/j.ceb.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 177.Kraning-Rush CM, Carey SP, Lampi MC. Microfabricated collagen tracks facilitate single cell metastatic invasion in 3D. Integr. Biol. 2013;5:606–616. doi: 10.1039/c3ib20196a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Carey SP, et al. Comparative mechanisms of cancer cell migration through 3D matrix and physiological microtracks. Am. J. Physiol., Cell Physiol. 2015;308:C436–C447. doi: 10.1152/ajpcell.00225.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pathak A, Kumar S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl Acad. Sci. 2012;109:10334–10339. doi: 10.1073/pnas.1118073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stroka KM, et al. Water permeation drives tumor cell migration in confined microenvironments. Cell. 2014;157:611–623. doi: 10.1016/j.cell.2014.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Raab M, et al. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science. 2016;352:359–362. doi: 10.1126/science.aad7611. [DOI] [PubMed] [Google Scholar]
- 182.Denais CM, et al. Nuclear envelope rupture and repair during cancer cell migration. Science. 2016;352:353–358. doi: 10.1126/science.aad7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Irianto J, et al. DNA damage follows repair factor depletion and portends genome variation in cancer cells after pore migration. Curr. Biol. 2017;27:210–223. doi: 10.1016/j.cub.2016.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Irianto J, et al. Nuclear constriction segregates mobile nuclear proteins away from chromatin. Mol. Biol. Cell. 2016;27:4011–4020. doi: 10.1091/mbc.E16-06-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015;14:248–260. doi: 10.1038/nrd4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Song JW, et al. Microfluidic endothelium for studying the intravascular adhesion of metastatic breast cancer cells. PLoS ONE. 2009;4:e5756. doi: 10.1371/journal.pone.0005756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bersini S, et al. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials. 2013;35:2454–2461. doi: 10.1016/j.biomaterials.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sung JH, Shuler ML. A micro cell culture analog (µCCA) with 3D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs. Lab Chip. 2009;9:1385–1394. doi: 10.1039/b901377f. Early work using organs-on-chip models, demonstrating that microfluidic devices combined with 3D hydrogel cell cultures representing individual organs can accurately reproduce anticancer drug effects observed in vivo. [DOI] [PubMed] [Google Scholar]
- 189.Sung JH, Kam C, Shuler ML. A microfluidic device for a pharmacokinetic-pharmacodynamic (PK-PD) model on a chip. Lab Chip. 2010;10:446–455. doi: 10.1039/b917763a. [DOI] [PubMed] [Google Scholar]
- 190.Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J. Cell Biol. 2011;192:373–382. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yu M, et al. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 2014;345:216–220. doi: 10.1126/science.1253533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Murlidhar V, Rivera-Báez L, Nagrath S. Affinity versus label-free isolation of circulating tumor cells: who wins? Small. 2016;12:4450–4463. doi: 10.1002/smll.201601394. [DOI] [PubMed] [Google Scholar]
- 193.Nagrath S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. Seminal study that developed a unique flow-based microfluidic platform containing antibody-coated microposts to isolate rare CTCs from the blood of patients with metastatic cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yoon HJ, et al. Sensitive capture of circulating tumour cells by functionalized graphene oxide nanosheets. Nat. Nanotechnol. 2013;8:735–741. doi: 10.1038/nnano.2013.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gleghorn JP, et al. Capture of circulating tumor cells from whole blood of prostate cancer patients using geometrically enhanced differential immunocapture (GEDI) and a prostate-specific antibody. Lab Chip. 2010;10:27–29. doi: 10.1039/b917959c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ozkumur E, et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci. Transl Med. 2013;5:179ra47. doi: 10.1126/scitranslmed.3005616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hou J-M, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small cell lung cancer. J. Clin. Oncol. 2012;30:525–532. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- 198.Cheung KJ, Ewald AJ. A collective route to metastasis: seeding by tumor cell clusters. Science. 2016;352:167–169. doi: 10.1126/science.aaf6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cheung KJ, et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl Acad. Sci. USA. 2016;113:E854–E863. doi: 10.1073/pnas.1508541113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sarioglu AF, et al. A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat. Methods. 2015;12:685–691. doi: 10.1038/nmeth.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liga A, Vliegenthart A, Oosthuyzen W, Dear JW, Kersaudy-Kerhoas M. Exosome isolation: a microfluidic road-map. Lab Chip. 2015;15:2388–2394. doi: 10.1039/c5lc00240k. [DOI] [PubMed] [Google Scholar]
- 203.Chen C, et al. Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab Chip. 2010;10:505–511. doi: 10.1039/b916199f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Im H, et al. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 2014;32:490–495. doi: 10.1038/nbt.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wunsch BH, et al. Nanoscale lateral displacement arrays for the separation of exosomes and colloids down to 20 nm. Nat. Nanotechnol. 2016;11:936–940. doi: 10.1038/nnano.2016.134. [DOI] [PubMed] [Google Scholar]
- 206.Farrell D, et al. Recent advances from the National Cancer Institute Alliance for Nanotechnology in Cancer. ACS Nano. 2010;4:589–594. doi: 10.1021/nn100073g. [DOI] [PubMed] [Google Scholar]
- 207.Kuhn NZ, Nagahara LA. Integrating physical sciences perspectives in cancer research. Sci. Transl Med. 2013;5:183fs14. doi: 10.1126/scitranslmed.3005804. [DOI] [PubMed] [Google Scholar]
- 208.Sharp P, Hockfield S. Convergence: the future of health. Science. 2017;355:589–589. doi: 10.1126/science.aam8563. [DOI] [PubMed] [Google Scholar]
- 209.Ewald AJ, Egeblad M. Cancer: sugar-coated cell signalling. Nature. 2014;511:298–299. doi: 10.1038/nature13506. [DOI] [PubMed] [Google Scholar]
- 210.De Vries J, Figdor C. Immunotherapy: cancer vaccine triggers antiviral-type defences. Nature. 2016;534:329–331. doi: 10.1038/nature18443. [DOI] [PubMed] [Google Scholar]
- 211.Speicher MR, Pantel K. Tumor signatures in the blood. Nat. Biotechnol. 2014;32:441–443. doi: 10.1038/nbt.2897. [DOI] [PubMed] [Google Scholar]