Summary
Background
People who inject drugs (PWID) are a key population affected by the global HIV and hepatitis C virus (HCV) epidemics. HIV and HCV prevention interventions for PWID include needle and syringe programmes (NSP), opioid substitution therapy (OST), HIV counselling and testing, HIV antiretroviral therapy (ART), and condom distribution programmes. We aimed to produce country-level, regional, and global estimates of coverage of NSP, OST, HIV testing, ART, and condom programmes for PWID.
Methods
We completed searches of peer-reviewed (MEDLINE, Embase, and PsycINFO), internet, and grey literature databases, and disseminated data requests via social media and targeted emails to international experts. Programme and survey data on each of the named interventions were collected. Programme data were used to derive country-level estimates of the coverage of interventions in accordance with indicators defined by WHO, UNAIDS, and the UN Office on Drugs and Crime. Regional and global estimates of NSP, OST, and HIV testing coverage were also calculated. The protocol was registered on PROSPERO, number CRD42017056558.
Findings
In 2017, of 179 countries with evidence of injecting drug use, some level of NSP services were available in 93 countries, and there were 86 countries with evidence of OST implementation. Data to estimate NSP coverage were available for 57 countries, and for 60 countries to estimate OST coverage. Coverage varied widely between countries, but was most often low according to WHO indicators (<100 needle-syringes distributed per PWID per year; <20 OST recipients per PWID per year). Data on HIV testing were sparser than for NSP and OST, and very few data were available to estimate ART access among PWID living with HIV. Globally, we estimate that there are 33 (uncertainty interval [UI] 21–50) needle-syringes distributed via NSP per PWID annually, and 16 (10–24) OST recipients per 100 PWID. Less than 1% of PWID live in countries with high coverage of both NSP and OST (>200 needle-syringes distributed per PWID and >40 OST recipients per 100 PWID).
Interpretation
Coverage of HIV and HCV prevention interventions for PWID remains poor and is likely to be insufficient to effectively prevent HIV and HCV transmission. Scaling up of interventions for PWID remains a crucial priority for halting the HIV and HCV epidemics.
Funding
Open Society Foundations, The Global Fund, WHO, UNAIDS, United Nations Office on Drugs and Crime, Australian National Drug and Alcohol Research Centre, University of New South Wales Sydney.
Introduction
We recently estimated that 15·6 million (uncertainty interval [UI] 10·2–23·7 million) people inject drugs.1 The estimated global prevalence of HIV infection among people who currently inject drugs is 18% (UI 11–25), and more than half are hepatitis C virus (HCV) antibody positive (52%, UI 42–62).1 Recent years have seen HIV outbreaks and persistently high HCV incidence among people who inject drugs (PWID) in a range of settings.2, 3, 4
WHO, UNAIDS, and the UN Office on Drugs and Crime (UNODC) supports a comprehensive package of interventions for the prevention and treatment of HIV and HCV infections among PWID. Core interventions include needle and syringe programmes (NSP) to prevent shared use of injecting equipment; opioid substitution therapy (OST) to reduce the frequency of and encourage cessation of opioid injecting; HIV testing and counselling as a gateway to HIV treatment and care; HIV antiretroviral therapy (ART) to reduce community viral load; and condom distribution programmes to prevent viral transmission to sexual partners.5
The presence of interventions alone is not sufficient; the greatest prevention benefits are reported when NSP and OST are implemented at high coverage and in combination.6, 7, 8 WHO emphasises that in countries where injecting drug use occurs, priority should be given to immediate implementation of NSP and OST,9 and recommends that countries aim to distribute in excess of 200 needle-syringes per PWID annually, and to provide OST to more than 40 people per 100 PWID.5 In 2010, however, a comprehensive review reported very low coverage of all of the above interventions among PWID globally, noting that there were few countries where coverage was considered sufficient to prevent viral transmission.10
Research in context.
Evidence before this study
In 2010, a systematic review estimated country, regional, and global coverage of interventions for the prevention and treatment of HIV among people who inject drugs (PWID), including needle and syringe programmes (NSP), opioid substitution therapy (OST), HIV testing and counselling, HIV antiretroviral therapy (ART), and condom distribution programmes. The review reported coverage of services for PWID to be poor across countries, with few exceptions. However, lack of data and poor quality data on injecting prevalence and service provision were notable barriers to accurate ascertainment of service coverage. Since 2010, estimates of intervention coverage have been reported by government and intergovernmental agencies. However, estimates typically come from a limited number of countries (eg, the European Drug Report published by the European Monitoring Centre on Drugs and Drug Addiction) or derive from member state reporting (eg, country progress reports submitted to UNAIDS). Estimates might not always comprise objective measurement of service provision or the size of the population of PWID nor reflect evidence from all available sources. Harm Reduction International produces a biennial report in which harm reduction services are summarised, but no estimates of service coverage are made. No global, systematic review of data to generate estimates of intervention coverage for PWID has been undertaken since the 2010 review.
Added value of this study
The previous review indicated that the current scale of coverage of the reviewed interventions was unlikely to prevent, halt, or reverse HIV epidemics among this population. In the intervening period, capacity to capture and disseminate information regarding access to services has been enhanced, particularly in low-income and middle-income countries. Furthermore, international public health agencies have established goals for reductions in prevalence, and even elimination, of HIV and viral hepatitis. Updated estimates of service coverage are crucial to monitor progress towards these targets. This Article presents updated estimates of national, regional, and global coverage of five interventions (NSP, OST, HIV testing, ART, and condom programmes) for the prevention and treatment of HIV and hepatitis C virus infections among PWID. Estimates were derived from a comprehensive systematic review of peer-reviewed and grey literature, data reported by national and intergovernmental agencies, and expert consultation. It presents the first global picture of combination coverage of the highest priority of these interventions, NSP and OST.
Implications of all the available evidence
Coverage of these interventions remains poor at the global level, and is insufficient to prevent, halt, or reverse HIV and HCV epidemics among PWID. Greater investment in HIV and HCV prevention and treatment is urgently needed for the 16 million PWID globally.
In 2015, the UN General Assembly adopted Sustainable Development Goals (SDGs) relevant to HIV and HCV,11 including access to drug dependence treatment; targets for reductions in HIV and viral hepatitis incidence and prevalence; and access to essential medicines, including medicines used in OST (namely, methadone and buprenorphine).11, 12 Additionally, the WHO Global Health Sector Strategy on Viral Hepatitis 2016–2021 includes harm reduction for PWID and HCV treatment as core interventions to eliminate viral hepatitis.13 There is clear recognition of the need to monitor progress towards these targets.11
Despite recognition of the need to monitor coverage, there is no comprehensive, regularly updated system for collating, critiquing, and synthesising data on coverage of PWID populations with these interventions. Harm Reduction International produces a biennial report documenting the existence of NSP, OST, and ART services for PWID;14 some data on service provision are presented, but not estimates of service coverage. Annual reporting to UNAIDS on service provision includes data on HIV testing and ART for PWID, but indicators relating to PWID are the most poorly reported of all key population indicators, and missing data are common.15 As a result, the most recent source for global coverage data remains the review published in 2010,10 meaning that the data informing decisions about country-level responses to HIV and HCV among PWID are in many cases over a decade old.
There are also reasons to be concerned for the future of HIV and HCV service provision for PWID. The June, 2016, UN High Level meeting on AIDS made only very brief mention of insufficient coverage of services for this population. Funding to UNAIDS is decreasing, and in July, 2016, UNAIDS ceased funding for HIV activities to UNODC, the lead UN agency for HIV services among PWID. Civil society organisations have raised concerns regarding sustainability of global funding for harm reduction services.16, 17 The Global Fund is a leading source of financial support for harm reduction services internationally, but has shifted way from funding activities in middle-income countries.17 National government investment following Global Fund withdrawal from countries has often been insufficient, leading to declining budgets and service provision.18, 19, 20 To monitor the ongoing impacts of these changes to the funding environment, it is crucial to understand current levels of intervention coverage.
We aimed to: identify current availability and estimate national, regional, and global coverage of five interventions (NSP, OST, HIV testing, ART, and condom programmes) for the prevention and treatment of HIV and HCV infections among PWID; determine the extent to which countries are meeting WHO targets for implementation of these interventions; and determine the extent to which high-coverage NSP and OST are implemented in combination.
Methods
Reporting rationale
Reporting of this study is in accordance with PRISMA21 and GATHER22 guidelines (appendix p 3). The protocol was registered on PROSPERO, number CRD42017056558.23
Search strategy and data extraction
The methods used for literature searching, screening, and extracting data were consistent with those adopted in a previous global review of intervention coverage.10 We searched for data on 17 indicators relating to NSP, OST, HIV testing, ART, and condom distribution programmes, selected from the WHO/UNODC/UNAIDS target setting guide (table 1).5 Our approach included multiple stages of searches of the peer-reviewed and grey literature to identify relevant data sources published from 2010 (the year of the most recent global systematic review of coverage data10) onwards, and international expert consultation to seek additional data or confirm data that had been collected. Searches and data verification commenced in September, 2016, with data verification ongoing until Aug 14, 2017.
Table 1.
Core HIV and hepatitis C virus prevention and treatment interventions for people who inject drugs and indicator data collected to develop estimates of intervention coverage
| Indicators | Definition used for coverage estimate | Coverage levels* | |
|---|---|---|---|
| Needle and syringe programmes | Number of sites; number of clients or occasions of service in a given time period; percentage of PWID accessing NSP in a given time period; number of needle-syringes distributed in a given time period | Number of needle-syringes distributed per PWID per year | Low: <100; moderate: 100 to <199; high: ≥200 |
| Opioid substitution therapy | Number of sites and forms of OST provided (methadone, buprenorphine, heroin, or other); percentage of PWID accessing OST at a given point in time; number of OST clients at a given point in time | Number of OST clients per 100 PWID; number of OST clients per 100 primary opioid injectors† | Low: <20; moderate: 20 to <39; high: ≥40 |
| HIV testing and counselling | Evidence of HIV testing programmes targeted to PWID; percentage of PWID receiving an HIV test in the previous 12 months (who know the result); number of PWID receiving an HIV test in the previous 12 months (who know the result) | Number of PWID receiving an HIV test in the past 12 months per 100 PWID | Low: <40; moderate: 40 to <74; high: ≥75 |
| HIV ART | Presence of policy restrictions limiting access to ART on the basis of injecting drug use history or status; percentage of HIV-positive PWID receiving ART; number of HIV-positive PWID receiving ART at a given point in time | Number of PWID receiving ART per 100 HIV-positive PWID | Low: <25; moderate: 25 to <74; high: ≥75 |
| Condom programmes | Number of sites distributing condoms to PWID; number of PWID receiving condoms from targeted programmes; percentage of PWID receiving condoms from targeted programmes; number of condoms distributed by programmes targeting PWID | Number of condoms distributed by PWID-targeted services per PWID | Low: <50; moderate: 50 to <99; high: ≥100 |
PWID=people who inject drugs. OST=opioid substitution therapy. NSP=needle and syringe programmes. ART=HIV antiretroviral therapy.
Suggested targets developed by WHO, UNAIDS, and the UN Office on Drugs and Crime to assess intervention coverage.5
Prevalence of primary opioid injecting estimated in Degenhardt and colleagues.1
We searched MEDLINE, Embase, and PsycINFO using terms developed in consultation with a librarian specialising in drug and alcohol research (appendix p 12). Searches were done in September, 2016, and limited to sources published from Jan 1, 2010, onwards. No other restrictions were applied to the search; citations for papers in languages other than English were included.
Websites identified as sources of information on injecting drug use, HIV, and viral hepatitis were searched via their own search function or Google advanced search between October, 2016, and February, 2017. Methods to identify and search these sources were updated for this review24 (appendix p 15).
Reports from relevant international agencies were searched, including UNAIDS (country progress reports), UNODC World Drug Report, the Global Fund to Fight AIDS, Tuberculosis and Malaria (grant reports), and the European Monitoring Centre on Drugs and Drug Addiction (EMCDDA; annual country reports and the European Drug Report). Additionally, data were requested via an email distribution process and social media posts. This comprised initial emails sent to experts or organisations globally, including contacts in regional and country offices of WHO, UNAIDS, and UNODC, with the request to forward emails to relevant contacts (appendix p 81). One member of the research team (SL) posted a request for data via Twitter, which was delivered to 5525 individual feeds.
Documents identified as potentially relevant were catalogued using Endnote (version X.8). Document screening to determine eligibility was undertaken by a multilingual team; members of the team could read English, French, Spanish, Portuguese, Hebrew, Russian, Ukrainian, Chinese, and Bahasa Malaysian. Assistance with translation of documents in other languages was provided by personal contacts of the authors. Google Translate was used to translate documents where no contacts could be identified
Peer-reviewed articles and grey literature were screened by title (and abstract where available) by one reviewer. The full-text of those sources deemed likely to contain data relevant to the indicators was then screened by two reviewers, with disagreements resolved by consensus. All sources identified from supplementary searches were reviewed in full for eligibility.
Sources identified as containing information relevant to the indicators of interest were categorised by country. All UN Member States were included, as were countries or territories where injecting drug use has been reported,1 or where we identified evidence that an intervention for PWID was being implemented. Research staff reviewed all data sources for a country, and the single most recent national data across these sources was identified for each indicator. In the absence of national data, the most recent subnational data were identified (see panel and appendix p 83 for decision rules). Data were entered into a Microsoft Excel 2016 database. Data extraction was reviewed for accuracy by LD, SL, or AP and any discrepancies were resolved by consensus.
Beginning in March, 2017, country-specific reports were generated listing all extracted data. These reports were emailed to key experts and organisations globally, with a request for any additional, more recent or more complete data (an example email and report is shown in the appendix p 90). These emails were frequently forwarded on to additional recipients. Revised or new indicator data provided in response to these requests were reviewed in full, and extracted where decision rules for inclusion were satisfied. New data were accepted for inclusion until Aug 14, 2017.
Data analysis and reporting
We collected data that identified availability of services (eg, any implementation of NSP, OST, HIV testing and treatment of PWID, and condom distribution programmes targeted towards PWID); programme data that described service delivery (eg, number of needle-syringes distributed by NSP in a country in a year) to estimate coverage; and survey data on contact with services (eg, proportion of a sample of PWID that reported accessing NSP in a specified time period) to provide additional indicators of coverage. All unadjusted data are presented in the appendix (pp 93–153).
National estimates of intervention coverage were constructed according to the approach set out in the WHO/UNODC/UNAIDS Technical Guide for assessing access to HIV prevention interventions among PWID (table 1).5 Programme data (eg, number of needle-syringes distributed per PWID per year; and number of people receiving OST at a specific point in time) were used for numerators. Adjustments made to programme data to ensure consistency in measurement between countries are shown in the panel, with full details provided in the appendix (p 88). Denominators for national estimates of NSP, OST, and HIV testing coverage were 2015 PWID population sizes reported by us elsewhere.1 Denominators for national estimates of ART coverage were the number of PWID living with HIV infection.1 National estimates for intervention coverage were compared with targets suggested by WHO, UNODC, and UNAIDS (table 1).5
To determine regional estimates of NSP coverage, we calculated the average number of needle-syringes distributed per region, weighted by country PWID population size. Denominators included the estimated population size of PWID for all countries in the region where injecting drug use has been reported.1 For countries where NSP was known to be implemented, but the number of needle-syringes distributed was not known, we assumed the same level of coverage as in other countries in the region. The global estimate of NSP coverage was developed following a similar approach to the regional estimates, weighting the regional estimates by the size of the regional population of PWID.
We calculated regional and global OST coverage with reference to two denominators: the number of PWID (as in the endorsed indicators published by WHO/UNAIDS/UNODC),5 and the number of PWID whose main drug injected is opioids (hereafter primary opioid injectors).1 Examining OST coverage specifically among primary opioid injectors provides additional information to contextualise the estimate among PWID.
The process of determining regional and global estimates of OST coverage among all PWID proceeded as for the regional and global estimates of NSP coverage. We determined the average number of people receiving OST per 100 PWID in each region, weighted by country PWID population size. We assumed the same level of coverage as in other countries in the region in those countries where OST is known to be implemented but no coverage estimate was available. The global estimate was determined by weighting the regional estimates by the size of the regional population of PWID.
To determine regional and global estimates of OST coverage among primary opioid injectors, we used data from our review on the epidemiology of injecting drug use.1 In that review, we presented results on the estimated proportion of primary opioid injectors among PWID in a country. To estimate the number of primary opioid injectors in this review, we multiplied the country population prevalence of injecting drug use by the proportion of primary opioid injectors among PWID. We multiplied this by the country population size, then 95% UIs were estimated using Monte Carlo simulation taking 100 000 draws. We used a binomial distribution because the parameters of interest were proportions. Sample sizes were derived based on the 95% CIs and SEs of estimates in each country. The simulated UIs incorporated the uncertainty of both the injecting drug use prevalence and opioid injecting proportion estimates. The process of determining regional and global estimates of OST coverage among primary opioid injectors thereafter was as above for OST coverage estimates among PWID.
Regional estimates for HIV testing coverage among PWID were calculated as for NSP and OST. Where no data were available for a region, we did not calculate a regional estimate. Given the scarcity of data for this indicator, we did not calculate a global estimate. Regional and global estimates of ART and condom distribution programme coverage were not calculated due to the sparseness of the identified data.
Among countries with a coverage estimate for both NSP and OST, we plotted coverage levels for both interventions to assess the extent to which combination high-level coverage of OST and NSP is being implemented. Low, moderate, and high coverage were defined in accordance with targets recommended by UN agencies.5
Role of the funding source
The funders of the study had no role in the study design, conduct, analysis, or interpretation of findings. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
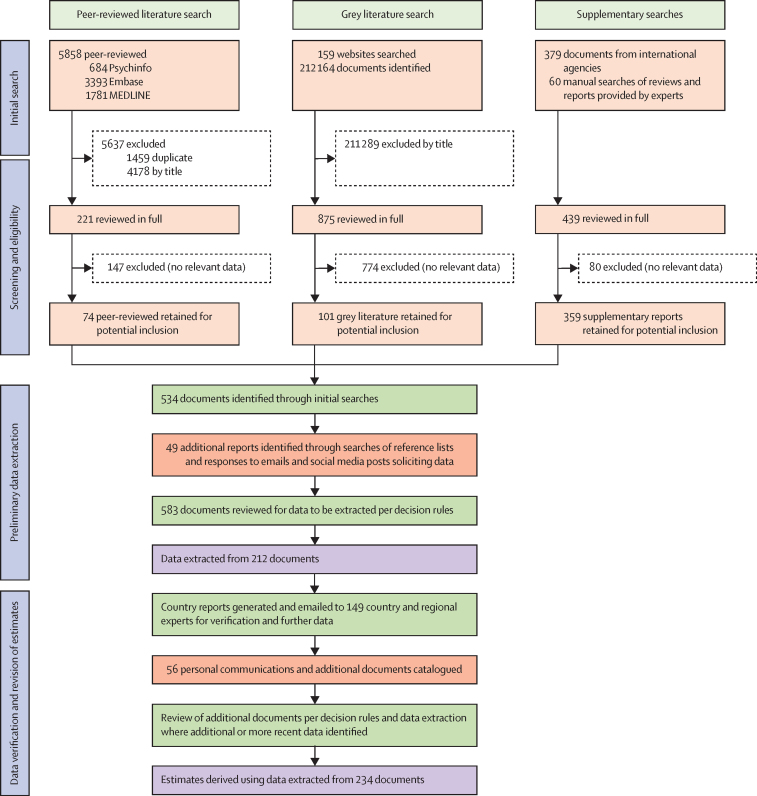
Figure 1 presents an overview of the process of reviewing and selecting data for inclusion in the analysis. We screened 218 510 documents for relevant data (5858 peer-reviewed publications, 212 164 grey literature reports, 379 documents from international agencies, 60 reviews and other documents provided by experts, and 49 additional documents identified during preliminary data extraction). Data for the 17 indicators of interest were extracted from 212 documents to produce country-level reports that were disseminated to experts for verification. An additional 56 reports and personal communications containing data were received following expert consultation; ultimately, 234 documents provided datapoints that were included in the analysis.
Figure 1.
Study selection
The diagram details searches undertaken to determine numerators used to develop estimates. For searches and methods used to determine population sizes (denominators), see Degenhardt and colleagues.4
We identified evidence of NSP operating in 93 of the 179 countries and territories where injecting drug use is known to occur (ie, in 52% of countries where injecting drug use is reported; Table 2, Table 3). NSP was confirmed to be absent in 83 countries where injecting drug use occurs; we were unable to confirm the presence or absence of NSP in three countries where injecting drug use is thought to occur.
Table 2.
Evidence of interventions targeted towards PWID, regionally and globally
| Number of countries |
Number of countries with evidence of injecting drug use |
Needle and syringe programmes | Opioid substitution therapy | HIV testing among PWID | Countries with programme data identifying antiretroviral therapy among PWID | Condom distribution programmes for PWID | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Countries implementing |
Countries with programme data |
Countries implementing |
Countries with programme data |
Countries with evidence of targeted programmes |
Countries with programme data identifying PWID |
Countries with evidence of targeted programmes |
Countries with programme data |
||||||||||||||
| 2007* | 2017† | 2010‡ | 2017 | 2010‡ | 2017 | 2010‡ | 2017 | 2010‡ | 2017 | 2010‡ | 2017 | 2010‡ | 2017 | 2010‡ | 2017 | 2010‡ | 2017 | 2010‡ | 2017 | ||
| Eastern Europe | 17 | 18 | 17 | 17 | 17 | 16 | 17 | 15 | 16 | 15 | 16 | 5 | 9 | 8 | 13 | 14 | 4 | 16 | 11 | 5 | 7 |
| Western Europe | 33§ | 28 | 31 | 23 | 29 | 21 | 22 | 26 | 30 | 24 | 27 | 6 | 15 | 2 | 7 | 14 | 4 | 12 | 10 | 2 | 3 |
| East and southeast Asia | 17 | 16 | 16 | 10 | 10 | 10 | 6 | 7 | 8 | 7 | 8 | 3 | 0 | 4 | 3 | 5 | 1 | 10 | 4 | 4 | 1 |
| South Asia | 9 | 9 | 9 | 6 | 6 | 6 | 4 | 5 | 6 | 4 | 5 | 4 | 2 | 3 | 4 | 3 | 3 | 6 | 3 | 3 | 2 |
| Central Asia | 5 | 5 | 5 | 5 | 4 | 4 | 4 | 2 | 3 | 2 | 3 | 3 | 1 | 1 | 3 | 4 | 3 | 5 | 1 | 4 | 2 |
| Caribbean | 15 | 6 | 6 | 1 | 2 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Latin America | 20 | 18 | 19 | 5 | 6 | 2 | 1 | 2 | 3 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 3 | 1 | 1 | 0 |
| North America | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 0 |
| Australasia | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| Pacific Islands | 17 | 11 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Middle East and north Africa | 22 | 21 | 21 | 8 | 8 | 5 | 8 | 4 | 7 | 3 | 5 | 2 | 3 | 0 | 6 | 0 | 0 | 3 | 5 | 2 | 4 |
| Sub-Saharan Africa | 47 | 13 | 36 | 2 | 7 | 2 | 7 | 4 | 8 | 2 | 3 | 2 | 3 | 2 | 3 | 2 | 1 | 4 | 6 | 1 | 2 |
| Global | 206§ | 148 | 179 | 81 | 93 | 70 | 75 | 70 | 86 | 61 | 72 | 26 | 34 | 20 | 40 | 44 | 17 | 62 | 42 | 23 | 22 |
“Countries with programme data” does not equate to countries with estimates for that indicator as country-level estimates were not reported where no estimate of the number of people who inject drugs was available. PWID=people who inject drugs.
From Mathers and colleagues.25
From Degenhardt and colleagues.1
From Mathers and colleagues.10
The number of countries in western Europe and globally differs from previous reviews: the UK (presented as one jurisdiction in the previous review) is now presented separately as England, Northern Ireland, Scotland, and Wales; Croatia is now included in western Europe; Greenland is included in western Europe; Northern Mariana Islands is included in Pacific Island states and territories; and South Sudan is presented for the first time.
Table 3.
Regional and global estimates of coverage of needle and syringe programmes, opioid substitution therapy, and HIV testing for PWID
| Estimated number of PWID (UI) |
Needle and syringe programmes |
Opioid substitution therapy |
HIV testing |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Countries implementing (% ERPP) | Countries with data (% ERPP) | Needle-syringes per PWID per year (UI) | Countries implementing (% ERPP) | Countries with data (% ERPP) | Clients per 100 PWID (UI) | Clients per 100 primary opioid injectors (UI) | Countries with data (% ERPP) | Number of PWID receiving HIV tests per 100 PWID (UI) | ||
| Eastern Europe | 3 020 000 (1 653 500–5 008 000) | 17 (100%) | 17 (100%) | 15 (9–27) | 16 (41%) | 17 (100%) | 1 (<1–2) | 1 (1–3) | 13 (98%) | 15 (9–27) |
| Western Europe | 1 009 500 (686 500–1 386 500) | 29 (100%) | 22 (26%) | 166 (118–243) | 30 (100%) | 29 (100%) | 64 (46–95) | 94 (67–GTP) | 7 (7%) | 15 (11–22) |
| East and southeast Asia | 3 989 000 (3 041 000–4 955 000) | 10 (88%) | 12 (92%) | 16 (13–21) | 9 (87%) | 16 (100%) | 8 (7–11) | 9 (7–11) | 3 (10%) | 40 (33–53) |
| South Asia | 1 023 500 (783 500–1 263 000) | 6 (100%) | 7 (85%) | 43 (35–56) | 6 (56%) | 8 (93%) | 82 (67–GTP) | 91 (73–GTP) | 4 (18%) | 7 (6–10) |
| Central Asia | 281 500 (189 500–416 500) | 4 (92%) | 5 (100%) | 115 (78–172) | 3 (58%) | 5 (100%) | 1 (<1–1) | 1 (1–2) | 3 (58%) | 41 (28–61) |
| Caribbean | 79 500 (53 000–118 000) | 2 (63%) | 6 (100%) | 6 (4–8) | 1 (35%) | 5 (65%) | 8 (6–13) | 11 (7–18) | 0 (0%) | NK |
| Latin America | 1 823 000 (1 392 000–2 380 000) | 6 (75%) | 13 (33%) | 6 (5–8) | 3 (21%) | 17 (87%) | 3 (2–4) | 3 (3–5) | 1 (8%) | 2 (1–2) |
| North America | 2 557 000 (1 498 500–4 428 000) | 2 (100%) | 2 (100%) | 39 (22–66) | 2 (100%) | 2 (100%) | 20 (11–34) | 27 (16–61) | 0 (0%) | NK |
| Australasia | 115 500 (83 000–148 000) | 2 (100%) | 2 (100%) | 396 (309–550) | 2 (100%) | 2 (100%) | 46 (36–64) | 73 (56–GTP) | 0 (0%) | NK |
| Pacific Islands | 22 500 (15 000–33 500) | 0 (0%) | 15 (100%) | 0 (0–0) | 0 (0%) | 15 (100%) | 0 (0–0) | 0 (0–0) | 0 (0%) | NK |
| Middle East and north Africa | 349 500 (177 500–521 500) | 8 (36%) | 21 (100%) | 2 (1–4) | 7 (33%) | 19 (98%) | 6 (4–12) | 6 (4–11) | 6 (41%) | 2 (2–4) |
| Sub-Saharan Africa | 1 378 000 (645 500–3 080 000) | 7 (38%) | 36 (100%) | 2 (<1–4) | 8 (37%) | 31 (91%) | 1 (<1–2) | 1 (<1–4) | 3 (31%) | 1 (<1–3) |
| Global | 15 648 000 (10 219 000–23 737 500) | 93 (86%) | 158 (82%) | 33 (21–50) | 87 (64%) | 166 (97%) | 16 (11–25) | 19 (13–31) | .. | .. |
Estimated number of people who inject drugs from Degenhardt and colleagues.1 Number of countries with data includes countries where injecting is confirmed to occur but intervention is known to not be implemented (ie, coverage estimate is zero); therefore number of countries with data for an intervention can exceed number of countries implementing an intervention. UI=uncertainty interval. PWID=people who inject drugs. NSP=needle and syringe programmes. OST=opioid substitution therapy. ERPP=estimated regional PWID population. GTP=estimate greater than parity. NK=not known.
OST was confirmed to be available in 86 countries where injecting drug use is known to occur (48% of countries where injecting drug use is reported; Table 2, Table 3), confirmed to be absent in 92 countries where injecting occurs, and we were unable to confirm the presence or absence of NSP in one country where injecting occurs. Methadone was the most frequently available medication used in OST, prescribed in 81 countries. Buprenorphine was prescribed for OST in 56 countries (of which 52 also prescribed methadone), and diamorphine was prescribed in seven countries (all of which also prescribed methadone and buprenorphine). Other forms of OST (eg, tincture of opium, slow-release morphine) were prescribed in 12 countries. There were 79 countries implementing both NSP and OST (44% of countries where injecting drug use is reported).
Compared with NSP and OST, far fewer data were identified on HIV-testing programmes for PWID. There were 34 countries with evidence of such programmes (table 2) and 17 countries that confirmed no targeted HIV-testing sites, but data were not identified for this indicator for 125 countries. No formal policy restrictions preventing ART access to PWID were identified, although several of the reviewed publications reported that PWID have poor access to ART due to discrimination and fear of criminal justice sanctions.14, 26, 27 Although ART is not formally restricted, data on ART access among PWID were not available for most (n=162) countries. There were 42 countries that reported the existence of condom distribution programmes for PWID (table 2), and six countries with reports stating that condoms were not distributed in a targeted manner to PWID; data were not available for 131 countries for this indicator.
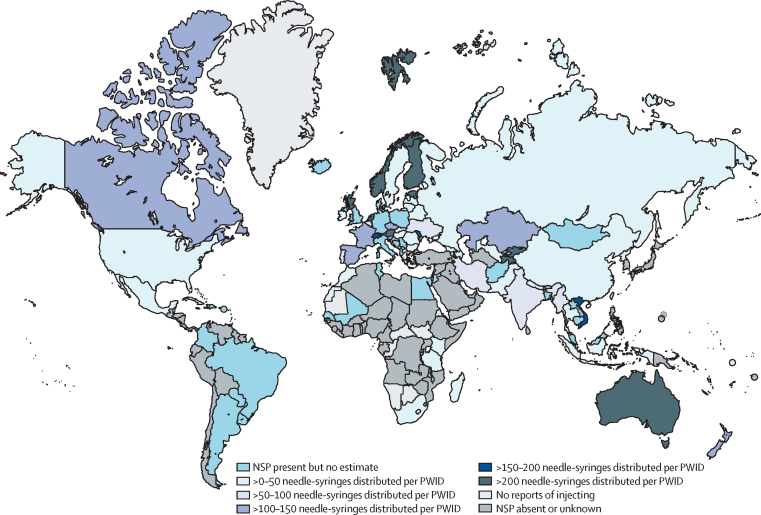
Of the 93 countries confirmed to implement NSP, data on the number of needle-syringes distributed per PWID per year was available for 75 countries, and denominator data were available to estimate NSP coverage in 57 of these countries (appendix pp 93–102). Only nine countries (216 500 [1%] of the global PWID population) had high NSP coverage (ie, distributed >200 needle-syringes per PWID per year; figure 2). Coverage was in the moderate range in 12 countries (784 000 [5%] of the global PWID population) and low range in 36 countries (9 434 500 [60%] of the global PWID population).
Figure 2.
Global coverage of needle and syringe programmes among people who inject drugs
NSP=needle and syringe programmes. PWID=people who inject drugs.
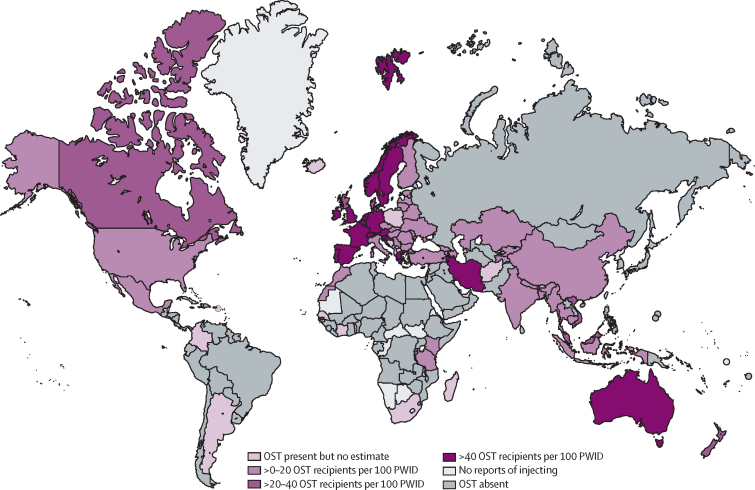
Data on the number of people receiving OST in a country were available for 72 countries, and it was possible to derive coverage estimates for 60 of these countries (appendix pp 93–102). With reference to PWID, 20 countries (814 500 [5%] of the global PWID population) were implementing high-coverage OST (figure 3). OST coverage was in the moderate range in six countries (806 000 [5%] of the global PWID population), and low in 34 countries (7 389 000 [47%] of the global PWID population).
Figure 3.
Global coverage of opioid substitution therapy among people who inject drugs
OST=opioid substitution therapy. PWID=people who inject drugs.
Programme data on the number of PWID receiving an HIV test annually were available for 41 countries, and it was possible to estimate HIV testing coverage for 29 of these (appendix pp 93–102). Coverage was low in 22 of these countries (3 408 000 [22%] of the global PWID population [15 648 000]), and high in only four countries (194 500 [1%] of the global PWID population). Survey data reporting the proportion of PWID being tested for HIV were more common than programme data (49 countries; appendix p 125–31); however, representativeness of survey samples was often unclear. There were 13 countries where both programme and survey data were available; in eight of these, programme data reported lower (often substantially lower) proportions tested compared with survey data (appendix pp 93–102).
Programme data on the number of PWID accessing ART was missing for most countries, being available for only 17 countries (appendix pp 93–102). Completeness of the available data was often unclear, and estimates ranged from less than one ART recipient per 100 HIV-positive PWID to greater than parity (ie, the number of PWID in ART was greater than the estimated number of current PWID). There were just seven countries with survey data on ART access (appendix pp 137–43), ranging from 5% (95% CI 4–6) of HIV-positive PWID receiving ART in Malaysia up to 67% (63–71) in the USA. For the two countries with both programme and survey data, coverage based on programme data was lower than that based on survey data (12% vs 25% in Ukraine and 12% vs 67% in the USA).
22 countries reported data on the number of condoms distributed by programmes targeting PWID (usually operating through NSP), from which 15 estimates of the number of condoms distributed per PWID per year could be derived. In these countries, estimates of the number of condoms distributed annually per PWID ranged from <1 (UI 0–1) to 52 (35–77; appendix pp 144–53).
Regionally, NSP coverage was highest in Australasia (396 needle-syringes per PWID per year, UI 309–550) and western Europe (166, 118–243), and lowest in the Pacific Islands (no NSP), and the Middle East and north Africa (2, 1–4), and sub-Saharan Africa (2, 1–4; table 3). Globally, we estimate that there are 33 (UI 21–50) needle-syringes distributed per PWID per year.
At the regional level, coverage of OST among PWID was highest in south Asia (82 OST clients per 100 PWID, UI 67–greater than parity) and western Europe (64, 46–95; table 3). Globally, there are 16 (UI 11–25) OST recipients per 100 PWID. Coverage increased somewhat using the denominator of primary opioid injectors; we estimated that there are 19 (UI 13–31) OST recipients per 100 primary opioid injectors (table 3).
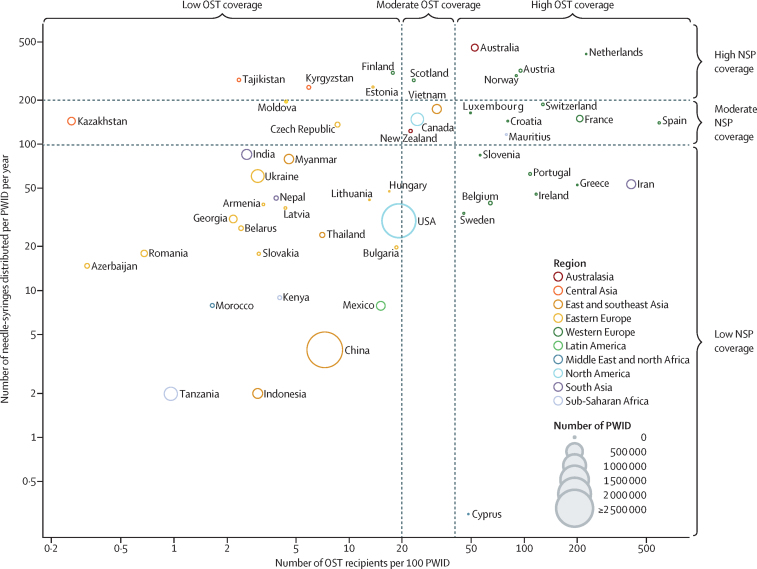
There were 51 countries for which a non-zero estimate of the coverage of both NSP and OST were available (figure 4; appendix p 154), 22 of which had low coverage across both interventions. High coverage of both NSP and OST was found in only four countries (Australia, Austria, the Netherlands, and Norway); these countries collectively include just 123 000 (1%) of the estimated global population of PWID (15 648 000). An additional ten countries (Canada, Croatia, France, Luxembourg, Mauritius, New Zealand, Scotland, Spain, Switzerland, and Vietnam) had either moderate or high coverage of both NSP and OST (figure 4; appendix p 154). Together, the 14 countries that provide at least moderate coverage of both NSP and OST are home to 752 000 (5%) of the global population of PWID.
Figure 4.
Combination coverage of needle and syringe programmes and opioid substitution therapy for people who inject drugs
Includes only countries with a non-zero estimate of both NSP and OST coverage. Circle area indicates national estimate of population size of PWID. PWID=people who inject drugs. NSP=needle and syringe programmes. OST=opioid substitution therapy.
Discussion
Implementation of core interventions to prevent and treat HIV and HCV infections among PWID is occurring in a greater number of countries than in previous years,10 but information on intervention coverage is often not available, and where available, suggests that coverage remains poor in many countries. NSP and OST are identified by WHO and UNAIDS as the highest priority of the core interventions,9 but on the basis of available data, only ten and 19 countries, respectively, are meeting suggested NSP and OST high-coverage targets. Less than 1% of all PWID live in countries with high coverage of both NSP and OST. Compared with the other interventions reviewed, there were considerably fewer data available on HIV testing and ART access among PWID. However, where data were available, coverage was typically low in comparison with suggested targets.5
The three regions with the largest populations of PWID—east and southeast Asia, eastern Europe, and North America—were all estimated to have poor coverage of NSP and OST. These are all regions where injecting drug use is well established. HIV prevalence in these regions is estimated to range from 9% (UI 7–11) in North America to 25% (16–34) in eastern Europe, and HCV antibody prevalence from 50% (38–63) in east and southeast Asia to 65% (57–73) in eastern Europe.1 Several countries in these regions have experienced recent HIV outbreaks as well as persistently high HCV prevalence among PWID,4, 28, 29 highlighting the need for better access to prevention interventions. Of particular concern, Russia (with an estimated 1·9 million PWID [UI 1·0–3·1], 30% [18–43] of whom are estimated to be living with HIV, and 69% [60–78] with HCV antibodies)1 is the only country in the eastern European region that does not provide OST, and access to NSP is very limited.
Rapid scale-up of NSP and OST is urgently needed in regions where injecting drug use is an emerging issue, such as sub-Saharan Africa. Of the 37 countries in that region where injecting drug use has now been reported, only seven offer NSP, and eight OST. Coverage of these interventions is very low at the regional level: just two needle-syringes distributed per PWID per year (UI <1–4), and one person receiving OST per 100 PWID (UI <1–2). HIV prevalence among PWID in the region is estimated at 18% (UI 11–25), similar to the global estimate of 18% (11–25);1 however, HCV antibody prevalence is 22% (18–27), much lower than the global estimate of 52% (42–62).1 Increased intervention coverage will contribute to reduced HIV transmission and prevent further escalation of the HCV epidemic among PWID in sub-Saharan Africa.
There is often considerable uncertainty around estimates of injecting drug use prevalence, which translates into uncertainty in estimates of intervention coverage. Methods and definitions used to calculate estimates of injecting drug use differ across countries and over time. We have avoided directly comparing these estimates to previously derived estimates, as differences in methodology and data sources might explain some apparent changes over time. The estimates reported here might differ from those of some national or regional reporting bodies due to differences in methodology and data sources included. To facilitate transparency in reporting, we have provided references for all datapoints used to generate these estimates in the appendices, as well as online interactive presentations of these data. We encourage feedback regarding the estimates via email.
Much of the indicator data reported here were derived from reports produced by government, intergovernmental, and non-government organisations. The quality of such data depends largely on existing monitoring systems and capacity within a country to collect data in a timely and representative manner. Establishing and maintaining monitoring systems requires dedicated resources. Without these, reporting against defined indicators can be poor. Some reports provided an estimate of intervention coverage without the data used to inform the estimate (eg, an estimate of the number of needle-syringes distributed per PWID per year, but without the population size and numerator used for this calculation). We did not use such estimates in this review, as we were unable to assess their validity. Lack of transparency in reporting can easily be remedied by providing the data used to generate estimates (ie, denominator and numerator), as well as the estimates themselves.
Compared with NSP and OST, data for HIV testing, ART, and condom distribution programmes specifically for PWID appear less likely to be systematically and routinely collected and reported. This is in part due to the interventions being applicable to the broader population, but also because HIV testing and treatment programmes might not collect or report data on risk exposure due to concerns regarding stigma or criminal justice implications for PWID. Given the potential for negative consequences for the patient, WHO guidelines on ART programme monitoring recommend against recording data related to patient risk behaviours in ART registers (as distinct from case surveillance data).30 Without such data, though, it is difficult to assess the extent to which particularly vulnerable populations are accessing these interventions.
Survey data might go some way towards addressing this problem, but to do so, surveys must employ recruitment methods that minimise risk of selection bias in findings. Recruitment of PWID from multiple community settings (not solely treatment or other services), such as through respondent-driven sampling or structured chain referral methods, can minimise selection bias.31, 32, 33 In presenting survey data, basic parameters including the precise indicator that was measured, sample size responding to the survey item, and numerator, should be presented in addition to the calculated proportion and associated uncertainty.
Clinic exit surveys are an alternative approach to estimating coverage of ART and other service use among PWID (and other key populations) being considered. Clients attending clinics providing ART (or other interventions) could complete a short survey via a tablet with results being immediately transmitted to a secure server separate from the clinic. This could involve a small number of questions including services received, viral load, mode of transmission, and current behaviours (including injecting drug use). If undertaken at large clinics across countries, this approach would permit estimates of the percentage of ART clients who inject drugs, which could be used to generate the number of PWID clients at the country level.
Although we did a comprehensive, multilingual search for relevant data, some data might have been overlooked. Grey literature, such as reports from organisations providing services or by government departments, can be difficult to locate, even when published online. To address this, we worked directly with staff from WHO, UNAIDS, the Global Fund, UNODC, and EMCDDA, and contacted experts around the world for assistance in identifying and verifying data. Uncertainty can occur in interpreting indicator data, as they are sometimes reported without contextual details (eg, the time frame to which data referred), or such details are unclear. All extracted data were checked multiple times by senior team members, and we sought clarification from people familiar with the data or context (eg, local researchers, service providers, and staff of intergovernmental organisations) if needed. Data searching and verification through contact with in-country experts continued until August, 2017, to ensure we included the most recent programme data possible.
We have provided regional estimates of NSP, OST, and, for some regions, HIV testing coverage. For countries where an intervention was known to be in place, but no programme data were available, we assumed that coverage in that country was the same as in other countries in the same region. For some countries, this might have resulted in overestimation or underestimation of true coverage, affecting the validity of the regional estimates. We suggest caution in interpreting the regional estimates as precise indications of intervention coverage; rather, we provide them to highlight regional differences and areas for particular focus for investment of resources.
In some cases, estimates for OST, HIV testing, and ART coverage per 100 PWID were greater than parity. This is likely a result of programme data categorising individuals as PWID on the basis of lifetime injecting drug use, whereas our PWID population size data refer to recent injecting drug use. As such, our approach might overestimate service access among recent PWID.
Prescribing of direct-acting antiviral therapies has recently become an important component of HCV prevention and treatment efforts.34 We did not explicitly search for indicators relating to treatment of HCV infection among PWID, such as HCV testing, treatment uptake, or outcomes of treatment, as we judged that at this time, there would be very limited data available for estimating these indicators at the population level. Future iterations of this work should include indicators such as the number of PWID who have received HCV antibody and diagnostic testing, and the number with chronic HCV infection who have been treated and cured.
Prisons and other correctional institutions are important settings for HIV and HCV prevention among people who inject drugs. We did not report on intervention coverage in these settings. Previous work has found that few countries permit NSP in prisons.14 OST is provided in prisons in 52 countries, although programmes often exist on a small scale or in a limited number of institutions.14 Determining coverage of HIV and HCV prevention interventions in prisons is complicated by a lack of data on the number of PWID in prisons. Further work to determine PWID population sizes and coverage of prevention interventions in prisons is needed to understand where gaps in HIV and HCV prevention might exist.
Greater investment in HIV and HCV prevention and treatment is urgently needed for the 16 million PWID globally.1 Effective interventions for HIV and HCV among PWID are delivered at suboptimal levels in almost all countries. Failure to provide interventions to scale ensures ongoing high prevalence and incidence of HIV and HCV infections among PWID, who are being left behind in efforts to end the HIV and HCV epidemics. Concerted international efforts will be required to ensure high coverage of interventions, particularly NSP and OST, is achieved and maintained.
Feedback regarding the estimates can be emailed to global.reviews@unsw.edu.au
Acknowledgments
Acknowledgments
This work was provided with some funding support from the Open Society Foundations, The Global Fund, WHO, UNAIDS, and UN Office on Drugs and Crime. The Australian National Drug and Alcohol Research Centre, UNSW Sydney, provided some funding towards the costs of this systematic review. LD, AP, RPM, and JG are supported by Australian National Health and Medical Research Council Fellowships. EBC is supported by a CanHepC PhD scholarship. MH is an NIHR Senior Investigator and acknowledges support from NIHR Health Protection Research Unit in Evaluation. PV acknowledges support from the Health Protection Research Unit in Blood-borne and Sexually Transmitted Infections and National Institute for Drug Abuse (grant number R01 DA037773-01A1). JS is an NIHR Senior Investigator and is supported by the NIHR Biomedical Research Centre in Mental Health at South London and Maudsley NHS Foundation Trust/Kings College London. The National Drug and Alcohol Research Centre and the Kirby Institute are funded by the Australian Government Department of Health and Ageing. The views expressed in this publication do not necessarily represent the position of the Australian Government. Thanks to the research assistants who assisted with searches for and extraction of data from the eligible papers in this review: Griselda Buckland, Gabrielle Gibson, Inbar Millo, Laura Sergeant, Julia Stadum, Harriet Townsend, and Foong Chin Yong (National Drug and Alcohol Research Centre, UNSW Sydney), and Diana Sergiienko (Ukrainian Institute of Public Health Policy). Thanks to Mary Kumvaj (librarian) who provided specialist advice on our search strategy and search strings for the peer-reviewed literature searches. Thank you also to the individuals who provided encouragement and support in various ways throughout the conduct of this review, including circulating requests for data, provision of contacts in countries and assistance with locating data: Annette Verster (WHO), Daniel Wolfe (Open Society Foundations), Andre Noor (EMCDDA), Eleni Kalamara (EMCDDA), Mauro Guarinieri (Global Fund), Christoforos Mallouris (UNAIDS), Susie McLean, Catherine Cook (Harm Reduction International [HRI]), Maria Phelan (HRI), Katie Stone (HRI), Riku Lehtovuori (UNODC), Keith Sabin (UNAIDS), Jinkou Zhao (Global Fund), and Vladimir Poznyak (WHO). Assistance in sourcing and verifying data was provided by many individuals from government, non-government, and research organisations around the world, for which we are thankful. These individuals are listed in the appendix.
Contributors
LD conceived of the scope of the review with SL, MH, AP, JG, ML, and PV. Screening and review was undertaken by SL, AP, and LD with contributions from LH, KVD, and JG. Data analysis was undertaken by SL, JL, AP, and SC, with input from PG. Figures were generated by EBC with assistance from SC. SL drafted the first iteration of the manuscript. All authors made substantial contributions to critical review, editing, and revision of the manuscript. All authors approved the final version of the manuscript.
Declaration of interests
SL has received investigator-initiated untied educational grants from Indivior. LD has received investigator-initiated untied educational grants for studies of opioid medications in Australia from Reckitt Benckiser, Indivior, Mundipharma, and Seqirus. AP has received investigator-initiated untied educational grants from Mundipharma and Seqirus. JG is a consultant and advisor for, and has received research grants from, Abbvie, Cepheid, Gilead Sciences, and Merck/MSD. MH reports honoraria for speaking at meetings from Gilead, Abbvie, and MSD. JM is supported by research grants from the Department of Health, National Institute for Health Research (NIHR), and the NIHR Biomedical Research Centre for Mental Health at King's Health Partners; he has part-time employment as Senior Academic Advisor for the Alcohol, Drugs and Tobacco Division, Health and Wellbeing Directorate, Public Health England; he declares grant funding for an investigator-led, educational grant from Indivior (administered by Action on Addiction) for a study of adjunctive, personalised psychosocial intervention for non-response to opioid agonist treatment (The ARC Trial), and grant funding at IoPPN and South London and Maudsley NHS Mental Health Foundation Trust from NIHR (Health Technology Assessment) for a trial of extended-release naltrexone (investigational medicinal product supply from iGen). In the past 3 years, JM has received honoraria from Merck Serono (2015; clinical oncology medicine), Martindale Pharma (2017; treatment for opioid use disorder), and Indivior (via PCM Scientific) as co-chair and chair (2015–17) for the Improving Outcomes in Treatment of Opioid Dependence conference; these companies had no role in the design, conduct, interpretation, or publication of findings. JS is supported by the NIHR Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and King's College London and is an NIHR Senior Investigator. JS's employer (King's College London) has received, connected to his work, project grant support, honoraria, and consultancy payments from Department of Health, National Treatment Agency, Public Health England, Home Office, National Institute for Health and Clinical Excellence, and European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) as well as research grants from (last 3 years) the NIHR, Medical Research Council, and Pilgrim Trust. JS has also worked with WHO, United Nations Office on Drugs and Crime, EMCDDA, US Food and Drug Administration, US National Institute on Drug Abuse, and other international government agencies. JS's employer (King's College London) has registered intellectual property on an innovative buccal naloxone with which JS is involved, and JS has been named in a patent registration by a pharmaceutical company as inventor of a potential concentrated naloxone nasal spray. JS's employer (King's College London) has also received, connected to his work, research grant support and payment of honoraria, consultancy payments, and expenses from pharmaceutical companies (including, past 3 years, Martindale, Indivior, MundiPharma, and Braeburn/Camurus) and trial medication supply from iGen and Braeburn and also discussions with various companies about medications potentially applicable in the treatment of addictions and related problems. SC, JL, LH, EBC, RPM, KVD, ML, PG, and PV declare no competing interests.
Supplementary Material
References
- 1.Degenhardt L, Peacock A, Colledge S. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017 doi: 10.1016/S2214-109X(17)30375-3. http://dx.doi.org/S2214-109X(17)30375-3 published online Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folch C, Casabona J, Espelt A. High prevalence and incidence of HIV and HCV among new injecting drug users with a large proportion of migrants—is prevention failing? Subst Use Misuse. 2016;51:250–260. doi: 10.3109/10826084.2015.1092991. [DOI] [PubMed] [Google Scholar]
- 3.Paraskevis D, Nikolopoulos G, Fotiou A. Economic recession and emergence of an HIV-1 outbreak among drug injectors in Athens metropolitan area: a longitudinal study. PLoS One. 2013;8:e78941. doi: 10.1371/journal.pone.0078941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters PJ, Pontones P, Hoover KW. HIV infection linked to injection use of hydromorphone in Indiana, 2014–2015. N Engl J Med. 2016;375:229–239. doi: 10.1056/NEJMoa1515195. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . WHO, UNODC, UNAIDS technical guide for countries to set targets for universal access to HIV prevention, treatment and care for injecting drug users. World Health Organization; Geneva: 2012. [Google Scholar]
- 6.Degenhardt L, Mathers B, Vickerman P, Rhodes T, Latkin C, Hickman M. Prevention of HIV infection for people who inject drugs: why individual, structural and combination approaches are needed. Lancet. 2010;376:285–301. doi: 10.1016/S0140-6736(10)60742-8. [DOI] [PubMed] [Google Scholar]
- 7.Martin NK, Hickman M, Hutchinson S, Goldberg D, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modelling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis. 2013;57(suppl 2):S39–S45. doi: 10.1093/cid/cit296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner KME, Hutchinson S, Vickerman P. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011;106:1978–1988. doi: 10.1111/j.1360-0443.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 9.WHO . Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations—2016 update. World Health Organization; Geneva: 2016. [PubMed] [Google Scholar]
- 10.Mathers B, Degenhardt L, Ali H. HIV prevention, treatment and care services for people who inject drugs: a systematic review of global, regional and national coverage. Lancet. 2010;375:1014–1028. doi: 10.1016/S0140-6736(10)60232-2. [DOI] [PubMed] [Google Scholar]
- 11.UN. A/RES/70/1: Transforming our world: the 2030 Agenda for Sustainable Development. Resolution of the United Nations General Assembly 70th Session of the United Nations General Assembly, September 25, 2015; 2015.
- 12.WHO WHO model list of essential medicines. 2013. http://www.who.int/medicines/publications/essentialmedicines/en/ (accessed Oct 6, 2017).
- 13.WHO . Global health sector strategy on viral hepatitis 2016–2021. World Health Organization; Geneva: 2016. [Google Scholar]
- 14.Harm Reduction International . The global state of harm reduction. Harm Reduction International; London: 2016. [Google Scholar]
- 15.Alfvén T, Erkkola T, Ghys P. Global AIDS reporting— 2001 to 2015: lessons for monitoring the sustainable development goals. AIDS Behav. 2017;21(suppl 1):5–14. doi: 10.1007/s10461-016-1662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook C, Bridge J, McLean S, Phelan M, Barrett D. The funding crisis for harm reduction: Donor retreat, government neglect and the way forward. Harm Reduction International; London: 2014. [Google Scholar]
- 17.Bridge J, Hunter BM, LAlbers E. The Global Fund to Fight AIDS, Tuberculosis and Malaria's investments in harm reduction through the rounds-based funding model. Int J Drug Policy. 2016;27:132–137. doi: 10.1016/j.drugpo.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Open Society Foundations . Undermining the global fight: the disconnect between the Global Fund's strategy and the real-life implications of the new funding model. Open Society Foundations; New York: 2014. [Google Scholar]
- 19.Eurasian Harm Reduction Network . The impact of transition from Global Fund support to governmental funding on the sustainability of harm reduction programs: a case study from Bulgaria, 2015. Eurasian Harm Reduction Network; Vilnius, Lithuania: September, 2015. [Google Scholar]
- 20.Eurasian Harm Reduction Network . The impact of transition from Global Fund support to governmental funding on the sustainability of harm reduction programs: a case study from Serbia. Eurasian Harm Reduction Network; Vilnius: 2015. [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 22.Stevens G, Alkema L, Black R. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet. 2016;388:e19–e23. doi: 10.1016/S0140-6736(16)30388-9. [DOI] [PubMed] [Google Scholar]
- 23.Larney S, Degenhardt L, Peacock A, Leung J, Hines L. Global systematic review on the coverage of HIV prevention and treatment services for people who inject drugs. PROSPERO 2017:CRD42017056558. 2017. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42017056558 (accessed Feb 7, 2017).
- 24.Degenhardt L, Gibson G, Leung J, Kumvaj M, Larney S. Searching the grey literature to access research on illicit drug use, HIV and viral hepatitis: a resource to identify drug-related databases and websites. NDARC technical report number 334. National Drug and Alcohol Research Centre, University of New South Wales; Sydney: 2016. https://ndarc.med.unsw.edu.au/resource/searching-grey-literature-access-research-illicit-drug-use-hiv-and-viral-hepatitis-resource (accessed May 29, 2017). [Google Scholar]
- 25.Mathers B, Degenhardt L, Phillips B. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372:1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 26.European Centre for Disease Prevention and Control . Thematic report: people who inject drugs. European Centre for Disease Prevention and Control; Stockholm: 2015. [Google Scholar]
- 27.East Europe and Central Asia Union of PLWH, Eurasian Harm Reduction Network. Evaluation of program and financial indicators: Russia, 2015.
- 28.Oprea C, Ceausu E, Ruta S. Ongoing outbreak of multiple blood-borne infections in injecting drug users in Romania. Public Health. 2013;127:1048–1050. doi: 10.1016/j.puhe.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Tarján A, Dudás M, Wiessing L. HCV prevalence and risk behaviours among injectors of new psychoactive substances in a risk environment in Hungary—an expanding public health burden. Int J Drug Policy. 2017;41:1–7. doi: 10.1016/j.drugpo.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 30.WHO . Consolidated guidelines on person-centred HIV patient monitoring and case surveillance. World Health Organization; Geneva: 2017. [Google Scholar]
- 31.Deuba K, Ojha B, Shrestha R. Optimizing the implementation of integrated biological and behavioural surveillance surveys of HIV in resource limited settings—lessons from Nepal. Asian Pac J Trop Dis. 2014;4:S605–S615. [Google Scholar]
- 32.Mills HL, Johnson S, Hickman M, Jones NS, Colijn C. Errors in reported degrees and respondent driven sampling: implications for bias. Drug Alcohol Depend. 2014;142:120–126. doi: 10.1016/j.drugalcdep.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocha LEC, Thorson AE, Lambiotte R, Liljeros F. Respondent-driven sampling bias induced by community structure and response rates in social networks. J R Statist Soc A. 2017;180:99–118. [Google Scholar]
- 34.Martin NK, Vickerman P, Dore GJ, Hickman M. The hepatitis C virus epidemics in key populations (including people who inject drugs, prisoners and MSM): the use of direct-acting antivirals as treatment for prevention. Curr Opin HIV AIDS. 2015;10:374–380. doi: 10.1097/COH.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.