Summary
Background
Sharing of equipment used for injecting drug use (IDU) is a substantial cause of disease burden and a contributor to blood-borne virus transmission. We did a global multistage systematic review to identify the prevalence of IDU among people aged 15–64 years; sociodemographic characteristics of and risk factors for people who inject drugs (PWID); and the prevalence of HIV, hepatitis C virus (HCV), and hepatitis B virus (HBV) among PWID.
Methods
Consistent with the GATHER and PRISMA guidelines and without language restrictions, we systematically searched peer-reviewed databases (MEDLINE, Embase, and PsycINFO; articles published since 2008, latest searches in June, 2017), searched the grey literature (websites and databases, searches between April and August, 2016), and disseminated data requests to international experts and agencies (requests sent in October, 2016). We searched for data on IDU prevalence, characteristics of PWID, including gender, age, and sociodemographic and risk characteristics, and the prevalence of HIV, HCV, and HBV among PWID. Eligible data on prevalence of IDU, HIV antibody, HBsAg, and HCV antibody among PWID were selected and, where multiple estimates were available, pooled for each country via random effects meta-analysis. So too were eligible data on percentage of PWID who were female; younger than 25 years; recently homeless; ever arrested; ever incarcerated; who had recently engaged in sex work, sexual risk, or injecting risk; and whose main drugs injected were opioids or stimulants. We generated regional and global estimates in line with previous global reviews.
Findings
We reviewed 55 671 papers and reports, and extracted data from 1147 eligible records. Evidence of IDU was recorded in 179 of 206 countries or territories, which cover 99% of the population aged 15–64 years, an increase of 31 countries (mostly in sub-Saharan Africa and the Pacific Islands) since a review in 2008. IDU prevalence estimates were identified in 83 countries. We estimate that there are 15·6 million (95% uncertainty interval [UI] 10·2–23·7 million) PWID aged 15–64 years globally, with 3·2 million (1·6–5·1 million) women and 12·5 million (7·5–18·4 million) men. Gender composition varied by location: women were estimated to comprise 30·0% (95% UI 28·5–31·5) of PWID in North America and 33·4% (31·0–35·6) in Australasia, compared with 3·1% (2·1–4·1) in south Asia. Globally, we estimate that 17·8% (10·8–24·8) of PWID are living with HIV, 52·3% (42·4–62·1) are HCV-antibody positive, and 9·0% (5·1–13·2) are HBV surface antigen positive; there is substantial geographic variation in these levels. Globally, we estimate 82·9% (76·6–88·9) of PWID mainly inject opioids and 33·0% (24·3–42·0) mainly inject stimulants. We estimate that 27·9% (20·9–36·8) of PWID globally are younger than 25 years, 21·7% (15·8–27·9) had recently (within the past year) experienced homelessness or unstable housing, and 57·9% (50·5–65·2) had a history of incarceration.
Interpretation
We identified evidence of IDU in more countries than in 2008, with the new countries largely consisting of low-income and middle-income countries in Africa. Across all countries, a substantial number of PWID are living with HIV and HCV and are exposed to multiple adverse risk environments that increase health harms.
Funding
Australian National Drug and Alcohol Research Centre, Australian National Health and Medical Research Council, Open Society Foundation, World Health Organization, the Global Fund, and UNAIDS.
Introduction
Sharing of equipment used for injecting drug use (IDU) causes substantial disease burden. Transmission via contaminated injection paraphernalia of blood-borne viruses, including HIV, hepatitis C virus (HCV), and hepatitis B virus (HBV), is a leading contributor to morbidity and mortality as a consequence of IDU.1 Quantification of the size of the population of people who inject drugs (PWID), their demographic characteristics, and the extent of their exposure to risk behaviours and environments is essential to enable effective health policy planning.2, 3, 4, 5
In 20086 and, for hepatitis, 2011,7 we did systematic reviews to estimate the prevalence of IDU globally and the prevalence of HIV, HCV, and HBV among PWID. These reviews generated new evidence, documented an increase in the number of countries where IDU had been recorded relative to a review 10 years earlier,8 and identified gaps and shortcomings in the available data (for example, we identified many countries where no studies had been done with PWID).
Research in context.
Evidence before this study
In 2008 and 2011, global systematic reviews were done to estimate the prevalence of injecting drug use (IDU) globally, as well as the prevalence of HIV, hepatitis C virus (HCV), and hepatitis B virus (HBV) among people who inject drugs (PWID). These reviews noted an increase in the number of countries where IDU had been identified relative to a review from 1998. Although annual updates are produced by agencies such as the UN Office on Drugs and Crime (UNODC) and the European Monitoring Centre on Drugs and Drug Addiction (EMCDDA), these focus on a limited number of countries (EMCDDA), rely on member state reporting (UNODC), and do not involve systematic reviews of evidence. These estimates do not adhere to the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER). Studies suggest that experience of homelessness, arrest, imprisonment, and sex work can increase exposure of PWID to HIV, HCV, and HBV, and increase risks of health harm. Age, gender, and the types of drugs injected affect exposure to blood-borne virus risk and might require quite different treatment and harm reduction responses. To our knowledge, there has never been a global review of these issues among PWID.
Added value of this study
In the past decade, surveillance capacity has been enhanced across many low-income and middle-income countries. Targets for reductions in HIV and viral hepatitis due to IDU have been developed, and drug dependence treatment coverage has been listed as one of the UN Sustainable Development Goals, making updates of previous estimates crucially important. This Article updates estimates of the number of PWID at the country level, regional level, and global level by use of a multi-stage systematic review of peer-reviewed and grey literature, data reported by government and other agencies, and expert consultation. To our knowledge, our work represents the first global review of sociodemographic characteristics of and risk factors for PWID, and we present the first estimates of IDU by gender at the country, regional, and global level. We also summarise evidence on PWID, including their experience of incarceration, sex work, and homelessness.
Implications of all the available evidence
IDU has now been documented in most countries and territories in the world, and HIV and HCV infection are prevalent in many populations of PWID, representing a substantial challenge to public health. There is a clear mandate to invest in blood-borne virus prevention activities such as needle and syringe programmes and opioid substitution therapy, and to provide treatment and care for those who are living with HIV and HCV. Importantly, our results highlight the consistently high levels of exposure to significant risk factors faced by PWID across countries. These risk factors include those about which there might be less capacity for individual control, emphasising the clear need to address structural and environmental drivers of vulnerability, risk, and harm.
This Article updates these previous estimates. During the past decade, surveillance capacity has been enhanced in many countries, including Global Fund support for studies of and services for PWID across multiple low-income and middle-income countries. Patterns of drug use have shifted globally, including increasing pharmaceutical opioid use and injection in some countries and changes in amphetamine and coca derivatives in some regions.9 Targets for reductions in HIV and viral hepatitis infection have been developed,10, 11 and drug dependence treatment coverage has been listed as one of the UN's Sustainable Development Goals.12 All of these factors make updating of previous estimates crucially important.
Experience of homelessness,13 arrest,14 incarceration,15, 16 and sex work17 can increase exposure of PWID to HIV, HCV, and HBV, and increase their risks of physical and mental health harms. Age,18 gender,19 and the types of drugs injected20, 21, 22 are associated with blood-borne virus risk among PWID, and could require quite different treatment and harm reduction responses.
We aimed to update the estimates of the number of PWID at country, regional, and global levels. This Article also represents the first global review of sociodemographic characteristics of and risk factors for PWID. At the country, regional, and global level, we examined the number of countries with evidence of IDU and estimates of the number of PWID; the prevalence of HIV, HBV, and HCV among PWID; and the characteristics of PWID and patterns of drug use and risk history, including exposure to homelessness, arrest, incarceration, and sex work.
Methods
Search strategy and selection criteria
This study was a global, multistage systematic review of peer-reviewed and grey literature. The methods used were consistent with previous global reviews6, 7 and in accordance with the PRISMA23 and GATHER24 guidelines (appendix pp 3–5). The protocols were registered on PROSPERO, numbers CRD42016052858 and CRD42016052853. Our search strategy had several stages, as in previous reviews.6, 7 We did not limit the searches by language.
We searched electronic peer-reviewed literature databases (MEDLINE, Embase, and PsycINFO) using a comprehensive set of search terms developed in consultation with a specialist drug and alcohol librarian (appendix pp 6–10). We limited the searches to studies published since Jan 1, 2008, or since Jan 1, 2011 for hepatitis (ie, from the year of the previously published reviews). We did the searches in April, 2016, and updated them in June, 2017. Any systematic reviews with potentially relevant sources that were identified were hand-searched for relevant papers or reports.
We searched grey literature and online databases that we had identified as sources of papers or reports on IDU and blood-borne viruses25 via their own search function or Google advanced search between April and June, 2016, limiting our search to records published since Jan 1, 2008 (or Jan 1, 2011, for hepatitis). These sources included the websites of drug surveillance systems, regional harm reduction networks, and country-specific ministries of health. We updated our methods to identify and systematically search grey literature for this review25 and the sites searched are detailed in the appendix (pp 11–60).
Between April and August, 2016, we searched key documents by relevant international agencies, including the UN Office on Drugs and Crime's (UNODC) World Drug Reports,9 Harm Reduction International's Global State of Harm Reduction reports,26 and reports from the European Monitoring Centre on Drugs and Drug Addiction (EMCDDA), WHO, UNAIDS, and Global Fund. We updated the searches between May and June, 2017. We contacted members of these organisations directly to obtain data when additional information was required.
We also sought data via expert requests. We requested data on the epidemiology of IDU and blood-borne viruses in October, 2016, via an email distribution process and social media. This process consisted of initial emails sent to more than 2000 key experts and organisations, including contacts in the global, regional, and country offices of WHO, UNAIDS, Global Fund, and UNODC (appendix p 61). Staff in those agencies also forwarded the request to their colleagues and other relevant contacts. One member of the research team (SL) posted a request for data on Twitter, which was delivered to 5525 individual feeds (appendix p 62).
Screening and extraction
We created an Endnote (version X.8) library to catalogue papers and reports and remove duplicates. References were screened by three researchers (LD, SL, and AP), assisted by researchers at the National Drug and Alcohol Research Centre, University of New South Wales (UNSW); the Kirby Institute UNSW; the University of Queensland; the University of Bristol; and the Ukrainian Institute on Public Health Policy. The research team had members proficient in reading sources written in English, French, Spanish, Portuguese, Hebrew, Bahasa Malaysian, Russian, Ukrainian, and Chinese languages. Other non-English language data sources were read via Google Translate or the Microsoft Word translate function.
Initial screening of title and abstract was done independently by one reviewer with a random 10% check by another (LD, SL, or AP), with no discrepancies found. Screened references were selected for full-text review if the title or abstract suggested that the document might contain relevant information (panel; appendix pp 63–68). Full-text review was also independently done by two authors (LD, SL, or AP), with discrepancies resolved by consensus except for fewer than 30 records, for which a third reviewer was consulted (MH; consensus was reached in all instances). 67 authors were contacted to request full-text where that was unavailable (eg, if only an abstract was presented) or to provide details of the methods or results.
Panel. Decision rules for data extraction and estimation processes.
Overall
-
•
Estimates with sample sizes ≤40 PWID were excluded
-
•
Samples which represented a subpopulation (eg, prisoners, all HIV positive or HIV negative) were excluded, as were those with 15% or more missing data (eg, due to incomplete responding or attrition in follow-up)
-
•
Where multiple sources were identified with data from the same sample, the sources with the most complete data regarding the various indicators of interest were included
-
•
Where possible, if calculation or typesetting errors were detected in reported estimates, these were recalculated or clarified with authors
Prevalence of injecting drug use
-
•
Where multiple estimates were available, higher grade estimates (grading classifications are detailed in the appendix p 62) were selected in preference to other grades
-
•
Geographic coverage was preferred over recency of an estimate—an older national estimate was used in preference to a newer estimate that focused only on one city or region; this rule recognises that, especially in large countries (eg, India), there can be considerable regional variation in IDU prevalence
-
•
Estimates of current PWID defined as those who had injected in the past 12 months were selected in preference to estimates for PWID defined by other criteria; other estimates were included in the absence of the preferred definition; the definition of injecting drug use for each estimate is noted in the findings
-
•
When deriving the prevalence of injecting drug use, it was also assumed that PWID were aged between 15 and 64 years of age
HIV, hepatitis C, and hepatitis B among PWID
-
•
City, subnational, and national estimates (grade A–C) published within the last 4 years of the most recently available estimate were pooled where multiple estimates were available for a country
-
•
For sentinel surveillance, if no details were provided on whether a single or multiple sample types were used, it was assumed that only a single sample type was used and graded C
-
•
Estimates based on case notifications, self-report, or unspecified methodologies were excluded
-
•
Studies that excluded PWID according to sex (eg, those actively excluding female PWID) were not included if mixed gender studies were available
Characteristics of PWID
-
•
All eligible data for each country were pooled where multiple estimates were available
-
•
Estimates were excluded if the sample inclusion or exclusion criteria reflected the characteristic of interest (eg, samples where participation was restricted to male PWID were excluded from meta-analyses of the percentage female)
-
•
Where categorical data for age were available, we calculated the percentage who were young, defined where possible as ≤24 years
-
•
In the case of time-varying indicators (eg, injecting risk behaviours), estimates for recent timeframes (ie, ≤12 months) were pooled
-
•
A range of risk behaviours could occur around injecting drug use; we chose to extract data on receptive needle-syringe sharing (ie, using a needle after someone else)
-
•
Similarly, a range of behaviours could confer risk during sexual activity; we focused on extracting estimates of unprotected sex (ie, sex without a condom)
-
•
We also extracted data from surveys of PWID who reported what the main drug was that they injected; in some instances we could not locate any studies assessing this, but surveys in a country reported “last drug injected”; or if not last drug injected, whether they had recently injected—in these instances (appendix) we used those estimates
Full details are available in the appendix (pp 67, 68). PWID=people who inject drugs.
Data from eligible studies were extracted into a purpose-built database in Microsoft Access 2016. We extracted data at all levels reported in the study, including city, subnational, and country. Data were then checked for accuracy against the original source by one of three authors (LD, SL, or AP). All extracted data were categorised by country: we included all UN Member States, as well as countries or territories for which IDU has been reported, or where we identified evidence that an intervention for PWID was being implemented.27 We extracted data on studies estimating the prevalence of IDU. From eligible studies of PWID, we extracted data on sociodemographic characteristics and risk variables (gender, age, unstable housing or homelessness, recent incarceration, arrest, and recent involvement in sex work), patterns of drug use and risk (recent injecting risk, sexual risk, and types of drugs injected), HIV-antibody prevalence, HCV-antibody prevalence (previous exposure) and reports of HCV-RNA prevalence (active infection), and HBV surface antigen (HBsAg) prevalence (active infection).
Data analysis
Our data analysis approach was informed by methods used in earlier reviews6, 7 (panel; appendix pp 63–68). On the basis of the extracted data, we made initial calculations of country-level prevalence estimates in accordance with a classification system and a set of decision rules (panel; appendix pp 63–71). Estimates were generated by one member of the research team (SC or LD), and independently reviewed by at least two others (LD, AP, JL, or SL); any discrepancies were resolved with discussion and consultation (SC, LD, AP, SL, JL). External checks were made with specific requests to experts in countries where additional data or clarification of identified data were needed. JG did a third independent check once estimates had been selected and pooled. All authors also finally reviewed all selected estimates.
Estimates of the prevalence of IDU were graded by quality (appendix pp 63, 64), and higher-grade estimates were selected over lower-grade estimates; we also aimed to maximise geographic coverage of estimates within a country. If two or more estimates of the same quality grade were identified, these were pooled via random-effects meta-analysis. The proportions were pooled across studies within a given country via random-effects meta-analyses in Stata version 14 by use of the metaprop command. Metaprop allows meta-analyses of proportions for binomial data. The CIs were calculated with the exact method based on the binomial distribution. If no estimate of IDU prevalence was located of the same or higher quality since the previous review,6 the estimate from the previous review was used again.
Eligible data on the prevalence of HIV antibody, HBsAg, and HCV antibody among PWID were selected and, where multiple estimates were available, pooled for each country (decision rules around selection of estimates are shown in the appendix pp 65, 66). On the basis of these extracted data, we made initial calculations of country-level prevalence estimates in accordance with agreed decision rules around the selection of estimates, approaches to pooling estimates within-country (panel), and determination of uncertainty intervals (UIs) around estimates. Estimates were pooled via random-effects models. To estimate the number of PWID with blood-borne virus infections (HIV antibody, HBsAg, and HCV antibody) among the country population sizes, we multiplied IDU prevalence by the proportion of each blood-borne virus variable among PWID. We then multiplied this product by the size of the country population aged 15–64 years to obtain number of PWID with blood-borne viruses. 95% UIs were estimated with Monte Carlo simulation taking 100 000 draws. We used a binomial distribution because our parameters of interest were proportions (product of IDU proportion among population and blood-borne virus proportion among PWID). Estimated sample sizes were derived on the basis of the 95% CIs and standard errors of the proportion estimates in each country. The simulated UIs incorporated the uncertainty of both IDU and blood-borne virus estimates.
Following the collation of country-specific estimates, regional and global IDU, HIV, and viral hepatitis estimates were derived. Regional groupings were based on those used by UNAIDS, WHO, and UNODC. We made region-specific, weighted estimates of the prevalence of HIV, HCV, HBV, and IDU using all the observed estimates and 95% CIs of estimates in each country within that region and deriving a weighted estimate and UIs, taking into account country population size. We used UN Population Division estimates of country population size (age 15–64 years).28 We then used regional estimates to estimate the global prevalence (appendix pp 69, 70).
Although few prevalence estimates of IDU present gender-specific estimates, this information is crucial for service planning. We estimated the number of women (and men) who inject drugs by extracting all available data in each country on the percentage of PWID samples who were female. We pooled these proportions across studies within a given country via random-effects meta-analyses in Stata version 14 using the metaprop command. To estimate the number of female PWID, we multiplied the proportion of women among PWID by the prevalence of IDU, then by the population size of the country. We calculated population-level prevalence of IDU for women and men using UN population estimates for men and women aged 15–64 years in each country.28
For pooling percentages of PWID across studies with data for sociodemographic characteristics and risk factors, we pooled all eligible estimates of each characteristic for a country via random-effects meta-analyses in Stata version 14 using the metaprop command. We calculated the CIs using the exact method based on the binomial distribution. We report pooled estimates of the percentage of PWID who were young (age <25 years at the time of interview), had unstable housing or were homeless (current or past year), had a lifetime experience of police arrest, had a lifetime history of incarceration, and had recently engaged in sex work (current or past year among all PWID in a sample, not by gender). We also report pooled estimates of the percentage of PWID who had recently engaged in injecting risk behaviour (predominantly receptive needle sharing, typically in the past month) and pooled estimates of the percentage of PWID who recently engaged in sexual risk behaviour (predominantly no or inconsistent condom use with casual partner, typically within the past month). We also extracted data on the reported main drug for injection; we report the percentage of PWID across countries whose main drug for injection was either an opioid (heroin or other opioid) or stimulant (amphetamine or cocaine). Further details of this process, including definitions and decision rules around selection of estimates for inclusion in these pooled estimates, are provided in the panel and appendix (pp 67, 68).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
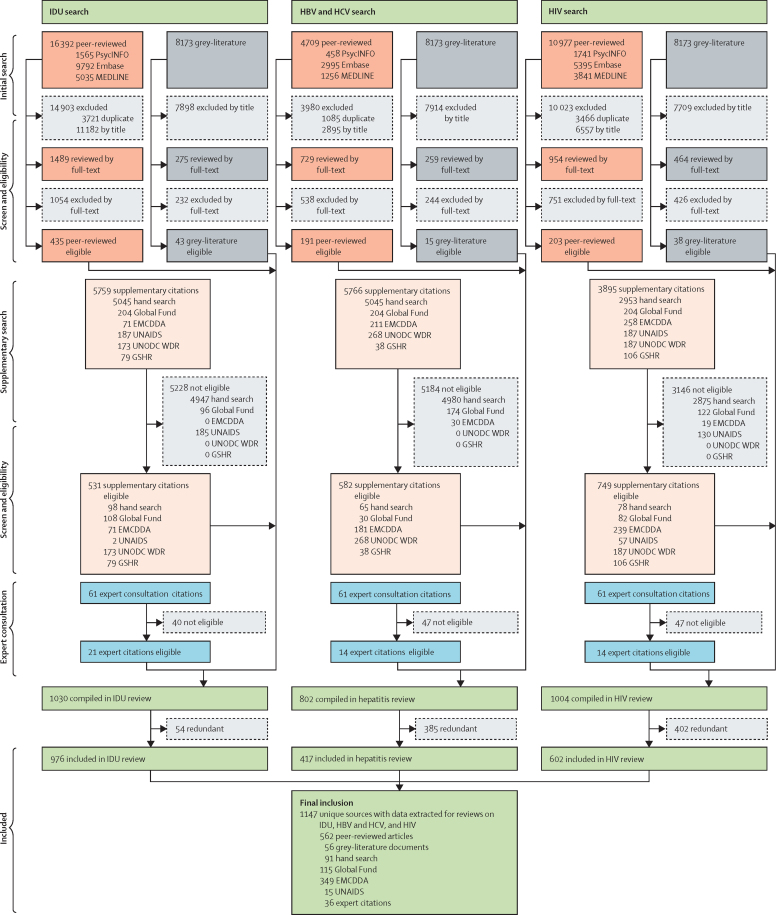
We screened 55 671 records, consisting of 32 078 peer-reviewed publications across the three searches (IDU, HBV and HCV, and HIV), 8173 grey literature reports from websites listed in the appendix (pp 11–60), and, in our supplementary search, 15 420 papers or reports from international organisations or from hand searches of 85 reviews that we identified as relevant. We received an additional 61 papers or reports from experts. Across these search stages, 1147 records were ultimately eligible for at least one aspect of our review (figure 1).
Figure 1.
Flowchart presenting number of sources from identification to inclusion
UNODC WDR=UN Office on Drugs and Crime's World Drug Report. GSHR=HRI's Global State of Harm Reduction. EMCDDA=European Monitoring Centre on Drugs and Drug Addiction. HBV=hepatitis B virus. HCV=hepatitis C virus. IDU=injecting drug use.
As of June 5, 2017, evidence of IDU was reported in 179 of 206 countries or territories; these countries hold 99% of the world's population aged 15–64 years (table 1). This is an increase of 31 countries since the previous review of IDU prevalence.6 The additional countries were mostly in sub-Saharan Africa (n=23) and four Pacific Island States and Territories. The number of studies estimating IDU prevalence also increased, with an additional 22 countries now having an estimate of IDU prevalence since the previous review (nine of these in sub-Saharan Africa; table 1), such that 83 countries (containing 82% of world population aged 15–64 years) now have an estimate of IDU prevalence.
Table 1.
Countries with evidence on injecting drug use and HIV, anti-HCV, and HBsAg prevalence among people who inject drugs
| Number of countries | Number of people aged 15–64 years (millions)* |
People who inject drugs |
HIV among people who inject drugs |
HCV among people who inject drugs |
HBV among people who inject drugs |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Countries with evidence of IDU |
Population with evidence of IDU* |
Countries with IDU prevalence estimates |
Population with IDU prevalence estimates* |
Countries with prevalence of HIV |
Population of PWID with HIV prevalence estimates† |
Countries with prevalence of anti-HCV |
Population of PWID with HCV prevalence estimates† |
Countries with prevalence of HBsAg |
Population of PWID with HBsAg prevalence estimates† |
|||||||||||||
| 20076 | 2017 | 20076 | 2017 | 20076 | 2017 | 20076 | 2017 | 20076 | 2017 | 20076 | 2017 | 20117 | 2017 | 20117 | 2017 | 20117 | 2017 | 20117 | 2017 | |||
| Eastern Europe | 17 | 232·51 | 18 | 17 | 100% | 100% | 13 | 15 | 79% | 87% | 17 | 17 | 99% | 100% | 14 | 17 | 92% | 100% | 11 | 16 | 89% | 99% |
| Western Europe | 33‡ | 293·76 | 27 | 31 | >99% | >99% | 17 | 21 | 94% | 97% | 19 | 24 | 96% | 98% | 22 | 27 | 99% | >99% | 17 | 20 | 93% | 84% |
| East and southeast Asia | 17 | 1592·68 | 16 | 16 | 98% | 99% | 8 | 10 | 91% | 95% | 9 | 11 | 90% | 94% | 11 | 11 | 98% | 98% | 8 | 8 | 90% | 82% |
| South Asia | 9 | 1185·79 | 9 | 9 | 100% | 100% | 6 | 8 | 99% | >99% | 6 | 8 | 99% | >99% | 6 | 7 | 99% | 99% | 6 | 7 | 99% | 99% |
| Central Asia | 5 | 44·43 | 5 | 5 | 100% | 100% | 4 | 4 | 92% | 92% | 4 | 4 | 92% | 92% | 3 | 4 | 83% | 92% | 1 | 1 | 28% | 26% |
| Caribbean | 15 | 27·44 | 6 | 6 | 63% | 65% | 1 | 1 | 10% | 9% | 1 | 1 | 10% | 9% | 0 | 1 | 0% | 9% | 0 | 0 | 0% | 0% |
| Latin America | 20 | 394·46 | 18 | 19 | >99% | >99% | 3 | 5 | 48% | 68% | 7 | 8 | 81% | 81% | 5 | 6 | 67% | 75% | 3 | 3 | 45% | 44% |
| North America | 2 | 242·20 | 2 | 2 | 100% | 100% | 2 | 2 | 100% | 100% | 2 | 2 | 100% | 100% | 2 | 2 | 100% | 100% | 1 | 1 | 90% | 90% |
| Pacific Island States and Territories | 17‡ | 6·82 | 11 | 15 | 97% | >99% | 0 | 0 | 0% | 0% | 3 | 3 | 8% | 7% | 0 | 0 | 0% | 0% | 0 | 0 | 0% | 0% |
| Australasia | 2 | 19·66 | 2 | 2 | 100% | 100% | 2 | 2 | 100% | 100% | 2 | 2 | 100% | 100% | 2 | 2 | 100% | 100% | 2 | 2 | 100% | 100% |
| Sub-Saharan Africa | 47 | 511·52 | 13 | 36 | 50% | 97% | 3 | 12 | 12% | 36% | 1 | 13 | 5% | 56% | 4 | 10 | 13% | 42% | 3 | 7 | 10% | 35% |
| Middle East and north Africa | 22‡ | 301·07 | 21 | 21 | 98% | 98% | 2 | 3 | 2% | 9% | 11 | 15 | 67% | 80% | 8 | 11 | 53% | 63% | 7 | 9 | 48% | 54% |
| Global | 206‡ | 4852·36 | 148 | 179 | 94% | 99% | 61 | 83 | 76% | 82% | 82 | 108 | 83% | 90% | 77 | 98 | 84% | 88% | 59 | 74 | 77% | 76% |
IDU=injecting drug use. HCV=hepatitis C virus. HBV=hepatitis B virus. Anti-HCV=HCV antibodies. HBsAg=hepatitis B surface antigen.
Percentage of general population aged 15–64 years for whom an eligible estimate of the prevalence of injecting drug use had been identified in that region or globally.
Percentage of the estimated population of people who inject drugs in that region or globally for whom an eligible HIV, HCV, or HBV prevalence estimate was available.
The number of countries in western Europe and the global total differs from that of the earlier reviews6, 7 in that the UK (previously presented as one jurisdiction) is now presented separately as England, Northern Ireland, Scotland, and Wales; Croatia and Greenland are included in western Europe; the Northern Mariana Islands are included in the Pacific Island States and Territories; and South Sudan is presented as a separate country.
We also noted increases in the number of countries with studies quantifying the prevalence of HIV, HCV, and HBV infection among PWID (table 1). The region with the largest number of countries with new data was sub-Saharan Africa, which had new studies of HIV in 12 countries, HCV in six countries, and HBV in four countries. The Middle East and north Africa also had increases in the number of countries with studies (four on HIV, three on HCV, and two on HBV).
Globally, in 2015 an estimated 15·6 million people (95% UI 10·2–23·7 million) injected drugs, amounting to approximately 0·33% (0·21–0·49) of those aged 15–64 years (table 2; detailed country estimates are given in the appendix (pp 72–78). We estimated that 3·2 million (1·6–5·1 million) women inject drugs globally.
Table 2.
Estimates of the prevalence of injecting drug use and number of people who inject drugs, by gender
|
All |
Women |
Men |
|||||
|---|---|---|---|---|---|---|---|
| Population prevalence of IDU (95% UI) | Estimated number of PWID (95% UI) | PWID who are women* (95% UI) | Population prevalence of IDU (95% UI) | Estimated number of PWID (95% UI) | Population prevalence of IDU (95% UI) | Estimated number of PWID (95% UI) | |
| Eastern Europe | 1·30% (0·71–2·15) | 3 020 000 (1 653 500–5 008 000) | 25·4% (22·0–28·6) | 0·64% (0·31–1·04) | 768 000 (369 000–1 242 000) | 2·00% (0·98–3·20) | 2 252 000 (1 100 000–3 597 000) |
| Western Europe | 0·34% (0·23–0·47) | 1 009 500 (686 500–1 386 500) | 28·6% (12·6–44·3) | 0·20% (0·11–0·31) | 289 000 (154 500–455 500) | 0·49% (0·31–0·70) | 720 500 (458 500–1 032 500) |
| East and southeast Asia | 0·25% (0·19–0·31) | 3 989 000 (3 041 000–4 955 000) | 20·8% (16·1–25·4) | 0·10% (0·07–0·14) | 828 000 (578 000–1 119 000) | 0·40% (0·31–0·51) | 3 161 000 (2 409 500–3 978 500) |
| South Asia | 0·09% (0·07–0·11) | 1 023 500 (783 500–1 263 000) | 3·1% (2·1–4·1) | 0·01% (0·00–0·01) | 32 000 (18 500–49 000) | 0·16% (0·13–0·20) | 991 000 (767 500–1 230 000) |
| Central Asia | 0·63% (0·43–0·94) | 281 500 (189 500–416 500) | 12·6% (9·7–15·6) | 0·16% (0·09–0·24) | 35 500 (20 000–54 500) | 1·13% (0·70–1·61) | 246 000 (152 500–350 500) |
| Caribbean | 0·44% (0·30–0·66) | 79 500 (53 000–118 000) | 11·1% (8·2–14·0) | 0·16% (0·09–0·24) | 14 500 (8500–21 500) | 0·74% (0·45–1·05) | 65 000 (40 000–93 000) |
| Latin America | 0·46% (0·35–0·60) | 1 823 000 (1 392 000–2 380 000) | 13·0% (5·0–21·3) | 0·12% (0·03–0·23) | 236 500 (60 500–457 500) | 0·81% (0·58–1·07) | 1 586 500 (1 135 500–2 083 000) |
| North America | 1·06% (0·62–1·83) | 2 557 000 (1 498 500–4 428 000) | 30·0% (28·5–31·5) | 0·63% (0·30–1·02) | 766 000 (361 500–1 238 000) | 1·49% (0·70–2·40) | 1 790 500 (838 500–2 898 500) |
| Pacific Island States and Territories† | 0·33% (0·22–0·49) | 22 500 (15 000–33 500) | 22·1% (17·8–26·3) | 0·15% (0·09–0·22) | 5000 (3000–7500) | 0·51% (0·31–0·73) | 17 500 (11 000–25 000) |
| Australasia | 0·59% (0·42–0·75) | 115 500 (83 000–148 000) | 33·4% (31·0–35·6) | 0·39% (0·28–0·51) | 38 500 (28 000–50 000) | 0·78% (0·57–1·01) | 77 000 (56 000–99 500) |
| Sub-Saharan Africa | 0·28% (0·13–0·62) | 1 378 000 (645 500–3 080 000) | 11·6% (7·8–15·6) | 0·06% (0·02–0·14) | 160 000 (43 000–350 500) | 0·49% (0·14–1·00) | 1 218 000 (350 500–2 472 500) |
| Middle East and north Africa | 0·12% (0·06–0·18) | 349 500 (177 500–521 500) | 3·5% (2·5–5·2) | 0·01% (0·00–0·02) | 12 500 (5000–22 500) | 0·22% (0·12–0·34) | 337 000 (189 000–513 500) |
| Global | 0·33% (0·21–0·49) | 15 648 000 (10 219 000–23 737 500) | 22·1% (17·8–26·3) | 0·13 (0·07–0·21) | 3 185 500 (1 650 000–5 067 500) | 0·52% (0·31–0·76) | 12 462 500 (7 509 000–18 373 000) |
Numbers are rounded to the nearest 500. Estimates are for people aged 15–64 years. Country-level estimates of IDU prevalence are available in the appendix. IDU=injecting drug use. PWID=people who inject drugs. UI=uncertainty interval.
The country-level pooled estimates of the percentage of PWID who are women, which informed these regional estimates, are presented in the appendix.
No estimates of the prevalence of injecting drug use could be located for the Pacific Island States and Territories, so we used the weighted observed global prevalence here; we also did not locate any eligible studies of people who inject drugs in these countries, so the observed global weighted percentage of PWID who are women was used in estimating the number of male and female PWID—considerable caution should be used in the interpretation of these estimates.
At a regional level, prevalence varied from 0·09% (95% UI 0·07–0·11) in south Asia to 1·30% (0·71–2·15) in eastern Europe (table 2). The largest populations of PWID were in east and southeast Asia (4·0 million, 3·0–5·0 million), eastern Europe (3·0 million, 1·7–5·0 million), and North America (2·6 million, 1·5–4·4 million).
The percentage of PWID who were women varied substantially across regions (table 2). We estimated that women represented 30·0% (95% UI 28·5–31·5) of PWID in North America and 33·4% (31·0–35·6) in Australasia, compared with 3·1% (2·1–4·1) among PWID in south Asia. The prevalence of IDU among men was far higher than in women in all regions.
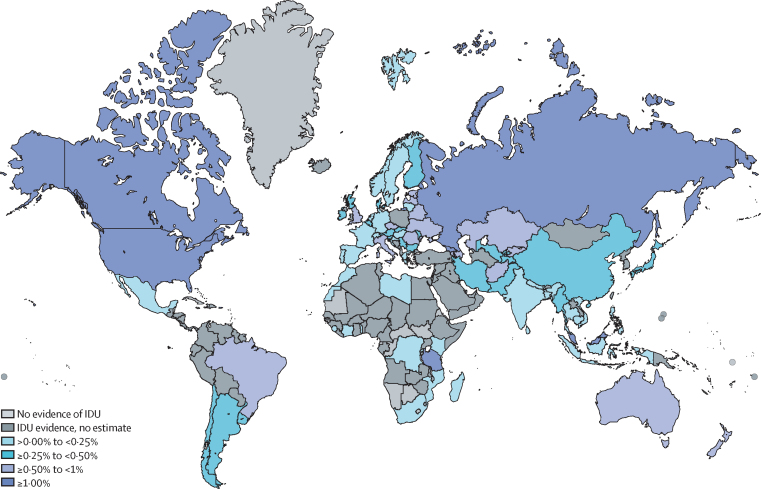
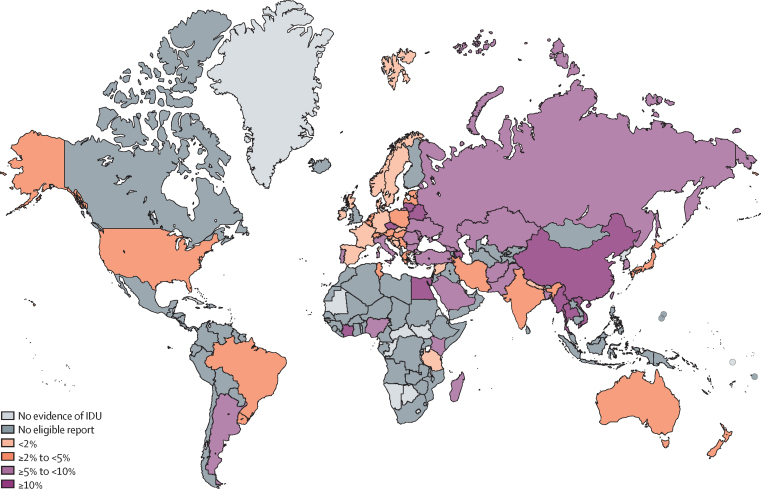
We noted substantial variation in the estimated country-level prevalence of IDU (figure 2); country estimates and further details are available in the appendix (pp 72–78). Georgia and Seychelles had the highest estimates for IDU prevalence; however, Russia, the USA, and China contributed the largest proportions of the total IDU population. We estimated much lower prevalence for countries in Asia and sub-Saharan Africa than in other regions, with some exceptions, for example Seychelles and Malaysia.
Figure 2.
Estimated prevalence of injecting drug use by country
IDU=injecting drug use.
Globally, we estimate that 2·8 million (95% UI 1·5–4·5 million) PWID are living with HIV, amounting to 17·8% (10·8–24·8) of PWID (table 3). HIV prevalence among PWID varied substantially across geographical regions, from 1·1% (0·8–1·4) in Australasia, 3·6% (1·5–6·2) in the Middle East and north Africa, and 4·5% (3·2–6·0) in western Europe, to 24·7% (15·6–33·9) in eastern Europe and 35·7% (15·0–56·6) in Latin America. We estimate that eastern Europe and Latin America have the largest numbers of PWID living with HIV.
Table 3.
Regional and global estimates of people who inject drugs who are HIV positive, anti-HBC positive, and HBsAg positive
|
HIV |
HCV |
HBV |
||||
|---|---|---|---|---|---|---|
| Prevalence among PWID (95% UI) | Estimated number of PWID living with HIV (95% UI) | Prevalence among PWID (95% UI) | Estimated number of PWID who are HCV-antibody positive (95% UI) | Prevalence among PWID (95% UI) | Estimated number of PWID who are HBsAg positive (95% UI) | |
| Eastern Europe | 24·7% (15·6–33·9) | 747 000 (313 500–1 331 500) | 64·7% (56·6–72·9) | 1 955 500 (927 000–3 171 000) | 7·9% (5·7–10·0) | 238 000 (107 500–405 500) |
| Western Europe | 4·5% (3·2–6·0) | 46 000 (24 500–73 000) | 53·2% (48·4–57·9) | 537 000 (339 500–777 000) | 3·2% (0·9–5·6) | 32 000 (11 500–60 500) |
| East and southeast Asia | 15·2% (9·9–20·4) | 605 000 (375 000–879 500) | 50·3% (37·7–62·8) | 2 007 500 (1 337 500–2 783 500) | 19·8% (9·8–30·0) | 791 500 (405 500–1 249 000) |
| South Asia | 19·4% (15·0–23·8) | 198 500 (141 500–264 500) | 38·6% (17·2–62·4) | 395 000 (239 500–573 500) | 5·7% (4·1–7·3) | 58 000 (38 500–82 000) |
| Central Asia | 10·5% (8·6–12·5) | 29 500 (17 500–44 000) | 54·0% (49·4–58·4) | 152 000 (93 000–218 000) | 9·3% (5·5–13·1) | 26 000 (15 000–39 500) |
| Caribbean | 13·5% (8·3–19·1) | 11 000 (6000–16 500) | 63·6% (54·3–72·6) | 50 500 (31 000–73 000) | 10·2% (5·4–15·2) | 8 000 (4500–12 500) |
| Latin America | 35·7% (15·0–56·6) | 651 000 (417 000–926 000) | 61·9% (58·9–64·9) | 1 128 000 (823 500–1 458 000) | 2·8% (1·7–4·0) | 51 000 (27 000–81 500) |
| North America | 9·0% (7·0–11·1) | 230 500 (105 000–389 000) | 55·2% (40·8–67·7) | 1 411 000 (667 000–2 388 500) | 4·8% (3·0–7·2) | 122 500 (47 500–226 500) |
| Pacific Island States and Territories* | 16·3% (10·0–22·6) | 3 500 (2000–5500) | 55·5% (43·8–67·0) | 12 500 (7500–18 000) | 10·2% (5·4–15·2) | 2 000 (1500–3500) |
| Australasia | 1·1% (0·8–1·4) | 1 000 (1000–2000) | 57·1% (52·7–61·5) | 66 000 (47 500–86 000) | 3·6% (2·2–5·1) | 4 000 (2500–6500) |
| Sub-Saharan Africa | 18·3% (11·3–25·4) | 251 500 (75 000–508 500) | 21·8% (17·6–26·5) | 300 000 (90 500–608 000) | 3·7% (2·3–5·9) | 51 000 (9500–123 000) |
| Middle East and north Africa | 3·6% (1·5–6·2) | 12 500 (4500–24 500) | 48·1% (39·2–57·1) | 168 000 (88 000–263 500) | 8·1% (6·1–10·3) | 28 000 (14 000–46 500) |
| Global | 17·8% (10·8–24·8) | 2 787 000 (1 482 500–4 464 000) | 52·3% (42·4–62·1) | 8 182 500 (4 691 500–12 418 000) | 9·0 % (5·1–13·2) | 1 412 500 (683 500–2 336 500) |
Country-level estimates of IDU prevalence are available in the appendix (pp 71–76; details of country-level estimates of HIV, HBV, and HCV are in the appendix pp 77–109). Numbers are rounded to the nearest 500. IDU=injecting drug use. PWID=people who inject drugs. HCV=hepatitis C virus. HBV=hepatitis B virus. Anti-HCV=HCV antibodies. HBsAg=hepatitis B surface antigen.
No estimates of the prevalence of HIV, anti-HCV or HBsAg could be located for the Pacific Island States and Territories, so we used the weighted observed global prevalence—considerable caution should be used in the interpretation of these estimates.
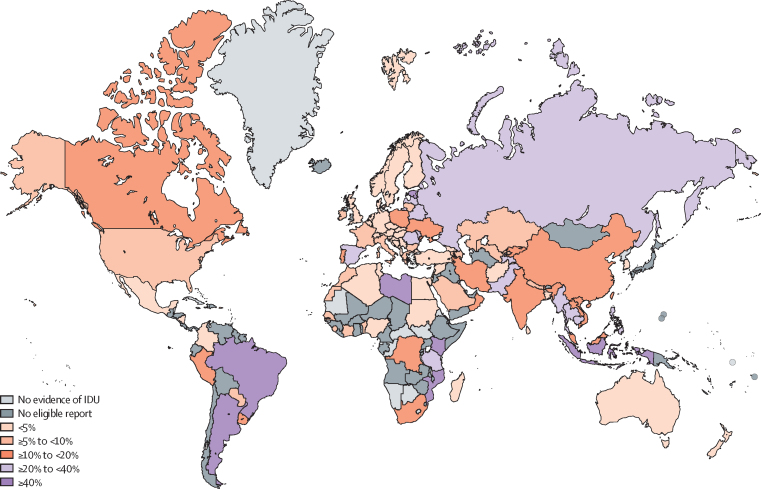
The prevalence of HIV among PWID varied widely across countries even within regions, as shown in figure 3 (country estimates and details are presented in the appendix pp 79–91). For example, in eastern Europe, HIV prevalence estimates ranged from 0·01% in Slovakia to 53·4% in Estonia, while in western Europe, estimates ranged from 0% in Serbia to 32·6% in Spain.
Figure 3.
Estimated HIV prevalence among people who inject drugs by country
IDU=injecting drug use. No eligible report=evidence of IDU located, but no study of HIV prevalence among people who inject drugs that met our eligibility criteria was located.
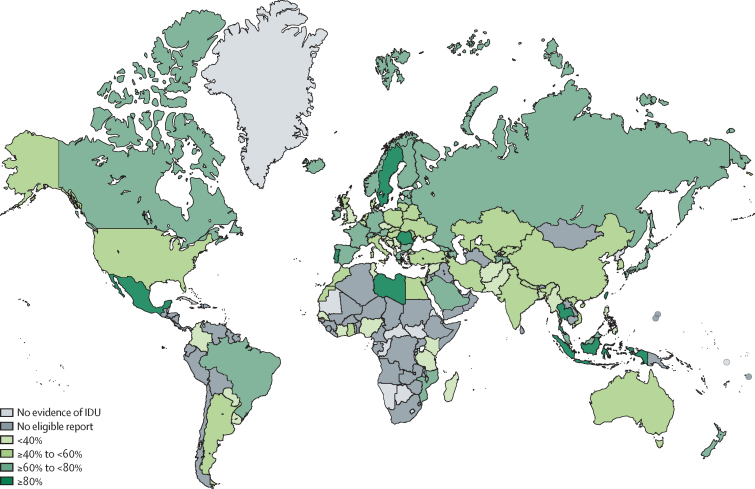
Globally we estimated that 52·3% (95% UI 42·4–62·1) of current PWID have been exposed to hepatitis C (anti-HCV positive), equating to 8·2 million (4·7–12·4 million) people. Only 21 countries had eligible studies with data on HCV-RNA prevalence. In most regions and countries, more than half of PWID have been infected with HCV. We estimated that PWID in sub-Saharan Africa had a lower prevalence of anti-HCV (21·8%, 17·6–26·5) compared with regions where IDU has been established for longer. We estimated higher anti-HCV prevalence in some countries in east and southeast Asia (eg, Indonesia, Taiwan, Thailand), although the regional estimated prevalence was lower, largely because HCV-antibody prevalence among PWID in China was estimated to be lower (figure 4; country estimates and details are provided in the appendix pp 92–103).
Figure 4.
Estimated anti-hepatitis C virus prevalence among people who inject drugs by country
IDU=injecting drug use. No eligible report=evidence of IDU located, but no study of HCV antibody prevalence among people who inject drugs that met our eligibility criteria was located.
We estimated that 9·0% (95% UI 5·1–13·2) of PWID have chronic HBV infection (HBsAg positive), equating to 1·4 million (0·7–2·3 million) people. The region (table 3) with the highest estimated HBsAg prevalence among PWID was east and southeast Asia, although countries with the highest prevalence also included the Czech Republic, Egypt, Belarus, Lithuania, the Côte d'Ivoire, and Azerbaijan (figure 5; country estimates and details are provided in the appendix pp 104–112). We estimated that PWID in east and southeast Asia represent more than half of all HBsAg-positive PWID worldwide (table 3).
Figure 5.
Estimated hepatitis B virus surface antigen prevalence among people who inject drugs by country
IDU=injecting drug use. No eligible report=evidence of IDU located, but no study of hepatitis B surface antigen prevalence among people who inject drugs that met our eligibility criteria was located.
Regional estimates of the characteristics of populations of PWID are presented in table 4 (country-level estimates and source references for all pooled estimates are available in the appendix pp 113–157). We found substantial geographic variation in the age of PWID. The proportion of young PWID (age <25 years) was lower in countries from Australasia (14·9%, range 9·5–20·3) and North America (15·3%, 11·1–27·5), and much higher in countries from Latin America (51·2%, 43·1–59·6%). The lowest proportions of young PWID were in the Caribbean and central Asia, although estimates in these regions were only based on one country each (Puerto Rico and Kyrgyzstan, respectively).
Table 4.
Sociodemographic and risk characteristics of people who inject drugs
| Women | Young people* | Recent homelessness or unstable housing | History of arrest | History of incarceration | Recent sex work | Recent injecting risk† | Recent sexual risk‡ |
Main drug injected§ |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Opioids | Stimulants | |||||||||
| Eastern Europe | 25·4% (22·0–28·6) | 41·8% (32·9–50·7) | 6·7% (4·4–9·2) | 32·7% (27·7–38·0) | 35·7% (31·6–40·0) | 11·7% (6·0–17·5) | 23·7% (15·0–32·5) | 37·7% (33·4–42·0) | 78·3% (69·1–87·5) | 37·5% (34·3–40·8) |
| Western Europe | 28·6% (12·6–44·3) | 29·8% (25·0–34·8) | 21·9% (15·9–27·9) | 66·6% (62·9–70·1) | 36·0% (29·8–41·1) | 5·1% (2·7–7·6) | 10·2% (7·7–12·9) | 38·0% (33·5–42·4) | 69·3% (59·6–79·0) | 19·2% (16·8–21·9) |
| East and southeast Asia | 20·8% (16·1–25·4) | 24·9% (16·6–33·2) | 8·8% (5·9–12·9) | 17·4% (14·8–19·3) | 75·6% (70·9–79·9) | 19·6% (3·3–36·0) | 22·7% (10·8–34·8) | 35·8% (26·9–44·7) | 96·1% (95·0–96·9) | 9·3% (8·7–12·3) |
| South Asia | 3·1% (2·1–4·1) | 30·4% (25·2–35·7) | 27·8% (19·0–36·7) | 80·6% (76·0–84·7) | 55·2% (34·7–75·7) | 15·8% (11·7–19·9) | 31·6% (23·1–40·1) | 36·7% (30·1–43·2) | 90·4% (85·4–94·8) | 3·0% (2·1–4·0) |
| Central Asia | 12·6% (9·7–15·6) | 6·7% (5·2–8·6) | 14·3% (11·7–17·2) | .. | .. | .. | 46·8% (42·7–50·7) | 13·7% (11·5–16·1) | 86·0% (83·5–88·2) | .. |
| Caribbean | 11·1% (8·2–14·0) | 12·2% (6·3–20·8) | 21·5% (17·5–25·5) | .. | 82·4% (79·0–85·7) | 3·3% (1·2–7·1) | 16·3% (13·1–19·6) | 24·0% (11·1–37·0) | 97·6% (95·8–99·4) | 92·8% (88·0–96·1) |
| Latin America | 13·0% (5·0–21·3) | 51·2% (43·1–59·6) | 19·6% (13·0–26·3) | 91·0% (85·0–97·0) | 71·0% (68·2–73·7) | 14·7% (11·5–18·4) | 54·0% (44·5–63·5) | 35·4% (24·3–46·2) | 92·8% (87·5–98·0) | 49·4% (39·3–59·4) |
| North America | 30·0% (28·5–31·5) | 15·3% (11·1–27·5) | 50·3% (39·7–61·0) | 90·3% (88·4–92·3) | 72·2% (61·8–82·6) | 21·3% (11·0–31·6) | 28·0% (21·0–34·8) | 37·9% (23·4–52·3) | 72·7% (64·5–80·9) | 38·7% (18·4–59·1) |
| Pacific Island States and Territories | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. |
| Australasia | 33·4% (31·0–35·6) | 14·9% (9·5–20·3) | 16·5% (11·4–22·1) | 81·6% (72·7–88·5) | 53·6% (47·2–60·0) | 19·4% (12·3–28·4) | 16·0% (12·8–19·1) | 10·7% (6·7–15·1) | 63·9% (58·3–69·6) | 32·6% (25·0–40·2) |
| Sub-Saharan Africa | 11·6% (7·8–15·6) | 19·3% (9·9–28·8) | 26·5% (12·0–41·0) | 75·0% (49·6–93·7) | 37·1% (32·8–41·4) | 14·2% (5·7–22·9) | 24·9% (16·5–33·3) | 47·5% (36·8–58·3) | 78·4% (66·5–90·3) | 50·8% (41·7–59·9) |
| Middle East and north Africa | 3·5% (2·5–5·2) | 38·7% (34·6–43·1) | 9·4% (4·3–14·8) | .. | 80·9% (74·3–87·0) | 11·3% (7·3–16·5) | 26·2% (19·5–33·1) | 40·1% (34·0–46·3) | 96·2% (94·8–97·3) | 14·2% (6·2–22·5) |
| Global | 22·1% (17·8–26·3) | 27·9% (20·9–36·8) | 21·7% (15·8–27·9) | 59·7% (54·7–64·3) | 57·9% (50·5–65·2) | 16·8% (6·9–26·7) | 25·5% (16·7–34·3) | 37·4% (28·6–46·1) | 82·9% (76·6–88·9) | 33·0% (24·3–42·0) |
Data are % (range). ·· shows that although there is evidence that injecting drug use is occurring in this region, we found no eligible studies involving PWID that examined this characteristic. Some countries are not included in our estimates as we did not find documented evidence or reports of injecting drug use for them: Antigua and Barbuda, Barbados, Belize, Botswana, Central African Republic, Comoros, Cuba, North Korea, Dominica, Equatorial Guinea, Eritrea, Greenland, Grenada, Guinea-Bissau, Lesotho, Liechtenstein, Mauritania, Namibia, Nauru, Republic of Congo, Saint Kitts and Nevis, Saint Lucia, São Tomé and Príncipe, Saint Vincent and Grenadines, South Sudan, Trinidad and Tobago, and Tuvalu. Decision rules and data extraction procedures for these characteristics and source references for data used in pooled estimates for each country are available in the appendix.
Young people who inject drugs were defined as younger than 25 years where possible; some countries had studies that used slightly different age groupings.
Recent injecting risk was defined as receptive needle-syringe sharing (ie, using a needle-syringe after someone else); some exceptions to this terminology occurred for some estimates (appendix), but all estimates were for behaviours within the past year.
Recent sexual risk was defined as unprotected sex with a non-regular (casual) sexual partner; some exceptions to this terminology occurred for some estimates (appendix), but all estimates were for behaviours within the past year.
The main drug column estimates are not exactly additive; estimates from different samples of PWID might have been used for each indicator (opioids or stimulants), and, in some countries (eg, Mexico), there was a large proportion of PWID who reported injecting a combination of opioids and stimulants together as their main drug (so-called speedballs), in which case these were counted as both opioids and stimulants being the main drugs injected.
The extent of exposure to risk also varied substantially. Exposure of PWID to recent homelessness or unstable housing ranged from 6·7% (range 4·4–9·2) in eastern Europe to 50·3% (39·7–61·0) in North America. History of incarceration ranged from 35·7% (31·6–40·0) in eastern Europe to 82·4% (79·0–85·7) in Puerto Rico, the only country in the Caribbean for which estimates could be calculated, and recent involvement with sex work ranged from 3·3% (1·2–7·1) in the Caribbean (ie, Puerto Rico) to 21·3% (11·0–31·6) in North America.
The extent of engagement in injecting and sexual risk behaviours varied widely among samples of PWID across countries. The proportion reporting recent injecting risk (using shared needles or syringes) was much higher in Latin America (54·0%, range 44·5–63·5) and central Asia (46·8%, 42·7–50·7), and lowest in western Europe (10·2%, 7·7–12·9). The highest rates of recent sexual risk (ie, unprotected sex) were in sub-Saharan Africa (47·5%, 36·8–58·3), the Middle East and north Africa (40·1%, 34·0–46·3), western Europe (38·0%, 33·5–42·4), and eastern Europe (37·7%, 33·4–42·0), whereas the lowest proportion was in Australasia (10·7%, 6·7–15·1).
Opioids were typically the main drug injected (table 4). The country with the lowest percentage of PWID reporting opioids as their main drug was the Czech Republic (21·6%, range 17·3–26·3; appendix pp 113–57). Higher proportions of PWID had stimulants as their main injected drug in countries in North America, eastern Europe, and Australasia (table 4). The pooled percentages of opioids and stimulants could exceed 100% because different studies could be included in the opioid versus stimulant pooled estimates, and in some countries, PWID reported that a combination of opioid and stimulants (eg, so-called speedballs) was their main drug injected.
The number of estimates and quality grading for each country is shown in the appendix (pp 70–109), for IDU prevalence and HIV, HCV-antibody, and HBsAg prevalence among PWID. Overall, 237 of 343 country-level estimates were based on evidence from grade A or B study methods, and 20 country-level estimates represented pooled data across multiple grade estimates, which included C grade estimates. If we restricted IDU prevalence estimates to grade A or B only (ie, indirect prevalence estimation studies and household surveys only), the global estimated prevalence of PWID would be slightly lower than our main calculation, at 0·27% (95% UI 0·17–0·45). If we restricted the blood-borne virus prevalence studies to A and B grade studies only (ie, studies including varied samples of PWID from varied locations, rather than those with more limited samples or locations), the global prevalence among PWID would be 15·6% (9·8–22·1) for HIV, 51·5% (40·6–62·3) for anti-HCV, and 8·4% (6·4–10·8) for HBsAg. The timeframe for identifying PWID was not clearly specified in 228 (31%) of 735 studies of characteristics and blood-borne viruses.
Discussion
In the years since the last major systematic reviews,6, 7 there has been a marked increase in the amount of evidence documenting injecting drug use (IDU) and the prevalence of HIV, HCV, and HBV infection in PWID. There is now evidence of IDU in 179 countries that contain 99% of the world's population aged 15–64 years, up from 148 countries in 2007, with the increase largely due to low-income and middle-income countries. We estimate the number of PWID globally to be 15·6 million and that roughly one in six are living with HIV, more than half have been exposed to HCV, and one in ten have active HBV. We also estimate that most PWID are exposed to environments that increase their risk of drug-related harm, which, to our knowledge, is the first such global estimate.
There have been improved efforts to understand the epidemiology of IDU and of HIV, HCV, and HBV infection among PWID in many countries. Particularly for blood-borne virus prevalence, we located many new country estimates, as well as greater coverage of countries with prevalence estimates. However, we noted that few countries had eligible studies with estimates of HCV-RNA prevalence among recent PWID, highlighting considerable knowledge gaps that will be important to fill in the future as HCV treatments are rolled out across countries.
Increases have occurred not only in the number of estimates and amount of evidence, but also in the quality of that evidence and strength of data generation. For example, we found an increase in the number of studies using so-called indirect methods to estimate the prevalence of IDU in the general population.29 Indirect methods are regarded as less susceptible to underestimation than general population surveys (ie, direct methods of estimating general population prevalence), which tend to miss populations who inject drugs.29 These indirect methods might involve different sources of data to indirectly estimate the total number of PWID, such as multiplier methods, back-projection, and capture–recapture methods. Nonetheless indirect estimation also can be biased, and needs to be corroborated where possible with other evidence.30
We have not presented our new estimates of IDU and blood-borne virus prevalence alongside those from the previous reviews.6, 7 Even in cases where countries had new estimates since the previous review, direct comparison was often hampered by changes to the methods used (ie, improvements), making it difficult to identify whether any changes in estimates were due to altered methods or changes in epidemiology. The exceptions were some countries, particularly in western and eastern Europe, where similar study designs have been implemented over time, and where the prevalence of injecting seems to have declined (eg, the Netherlands and Spain31). Additionally, the improvements in data in some regions (including region-specific estimates for the first time in the case of sub-Saharan Africa) mean that, compared with the estimates in the previous iteration (which we highlighted in the previous review6 as being very uncertain), the current estimates are now much more robust and arguably more plausible than those in the previous iteration. Furthermore, we improved our methods with respect to the selection and pooling of estimates, and took an approach that favoured better coverage of a country over recency of the data. We used multiple estimates to inform a pooled estimate rather than selecting a single upper and lower estimate, and we took a different approach to the estimation of uncertainty. We feel that this approach better considers the potential geographic heterogeneity within a country than our previous methods (eg, very high IDU prevalence in some areas such as in cities in Germany32 and India33).
Our estimates of demographic and risk characteristics of PWID revealed variation across countries, but also some consistent findings. Although PWID in some countries had lower levels of exposure to risk environments than others, in general, PWID were exposed to adverse risk environments around the world. Compared with the general population, PWID are at greater risk of police arrest, incarceration, sex work, and the experience of homelessness or unstable housing, all of which are associated with increased blood-borne virus transmission. Notably, these experiences were often more common in high-income countries, including those in North America.
We found clear variation in the age and gender profile of PWID, with a tendency for PWID in high-income countries to be older and to include a higher proportion of women than in lower-income countries. There is increasing evidence of ageing populations of PWID in many settings where IDU has been reported for some time, particularly in Europe,34 North America,35 and Australasia.36 Over roughly the past 5 years, increases in IDU and outbreaks of HIV have occurred in the USA, which are related to large-scale prescription of pharmaceutical opioids and subsequent transition to heroin use and IDU.37
Our data have substantial policy importance. There is an imperative to invest in blood-borne virus prevention activities, such as needle and syringe programmes and opioid substitution therapy, and to provide treatment and care for those who are living with HIV38 and HCV.39 In the special session of the UN General Assembly on the world drug problem in 2016, international agencies such as UNODC, WHO, and INCB were tasked to improve the accessibility of such services to people who use drugs, including PWID.40 Access to drug dependence treatment has been explicitly highlighted as one of the Sustainable Development Goals (SDGs) under the 2030 Agenda for Sustainable Development12 and targets for preventing and eliminating HIV and hepatitis have been developed.10, 11 We examined the current coverage levels of these interventions for PWID separately.27
Simultaneously other drivers of vulnerability, risk, and harm among this key population need to be addressed. Our review of existing characteristics of PWID suggests that there is considerable cause for concern across multiple indicators about the level of exposure to high-risk environments that PWID face and the level of engagement in risk behaviours that occurs among PWID in some countries.
We have identified various structural barriers to and opportunities for reducing risks for PWID and improving access to services to prevent and treat HIV and hepatitis. For example, roughly three in five PWID surveyed globally report exposure to incarceration, where high levels of risk often occur both in terms of drug use and other risks to wellbeing.4, 15 Prisons often have limited or no health-care services, an issue of importance given that risk of drug withdrawal, suicide, and overdose following prolonged abstinence can all be elevated in that environment for PWID. Prisons can also serve as places where the health of PWID can be improved and this must be better addressed, such as through ensuring access to drug treatment, blood-borne virus prevention and treatment, and other general health care.41, 42, 43
There are several limitations related to the nature of the data located in this review. IDU is a comparatively rare exposure and PWID are a highly marginalised population, so traditional general survey methods are unlikely to capture the frequency or prevalence of exposure or harm, and we had to rely on a mixture of indirect methods and specific surveys of PWID. This feature could be part of the reason for the poor availability of data and national estimates in some countries. Definitions of IDU also varied by study; the timeframe for identifying PWID was not clearly specified in 31% of studies. Poor operationalisation of key variables also often applied to the literature on sociodemographic and risk characteristics, in that the specific behaviour and timeframe (eg, receptive syringe sharing within the past month) were not consistently detailed. Importantly, we also assumed that studies reported gender (rather than sex) in extracting data on the percentage of PWID who were women; some studies might have assessed sex rather than gender, and people who are transgender or gender diverse could have been misclassified in those studies.
There has been an increase in research involving PWID over the past decade. Nonetheless, there remains substantial scope to continue increasing the quality and geographical coverage of estimates for all of the indicators examined in this study. Until national studies are implemented consistently and in repeated fashion over time, we will be limited in our capacity to reach firm conclusions about changes in population-level IDU prevalence or blood-borne virus prevalence among PWID. As long as national estimates remain absent for some countries, the possibility remains that estimates of IDU and profiles of the characteristics of PWID based on subnational studies might not provide an accurate profile of PWID across the whole country.
Our systematic review was subject to limitations. First, despite the wide scope of our online searches and requests for information from people across many countries, grey literature reports can be difficult to access, especially when they are not formally posted online. Undoubtedly, we will have missed some of these studies. To address this challenge as much as possible, we liaised directly with WHO, the Global Fund, UNODC, EMCDDA, and UNAIDS staff to facilitate contact with people in-country and obtain reports that were not available online, and in the case of the Global Fund, this was an important source of data from their Integrated HIV Bio-behavioural Surveillance surveys.
Second, many documents were reviewed by a small research team in a short period of time, so we might have missed some information in this process. However, internal checks were done by members within this team and we used a process of double and triple checking. Where queries existed around estimates that we could not resolve, we contacted people in-country to request their feedback. Furthermore, the estimates produced were circulated to all potential authors for input, allowing for the identification of missing or incorrect data.
Third, errors could have been made in the data interpretation. To reduce such errors, all sources and data from which the final estimates were derived were double checked by at least two reviewers before inclusion, with a further round of checks by a third reviewer before finalisation. We have online interactive presentations of these data to facilitate transparency and increase the potential for many people to interact with the estimates and results. We encourage feedback by email.
Fourth, in contrast to the previous global review, in this review, we had teams of researchers able to search and screen in multiple languages other than English. Nonetheless, we might have missed documents in languages in which we are not fluent. Again, we encourage anyone with access to data or reports in other languages to contact us.
It is also important to acknowledge a number of features of our approach to synthesis and imputation of estimates, which were driven by the gaps in the data available. Although there has been a clear increase in efforts to quantify the extent of IDU and the prevalence of blood-borne virus infection among PWID, there are still major gaps in data in some regions. In the Caribbean region, Puerto Rico was the only territory from which our review found data available on the prevalence of IDU and blood-borne viruses. In the Pacific Island States and Territories, IDU could be occurring in at least 15 of 17 countries, but no data estimating the prevalence of IDU or blood-borne viruses among PWID were available for any country. As such, estimates for this region were imputed based on the global weighted average. Although this method enables final global estimates that are better than assuming zero prevalence in countries where IDU occurs, greater availability of national estimates will improve precision in the regional and global estimates.
Our current method of calculating regional and global estimates of the prevalence of IDU and blood-borne viruses essentially assumes a correlation of 1 between countries within a given region, which is unlikely. Our midpoint estimates are accurate, but our standard errors are likely to be overestimated, leading to wider uncertainty and confidence intervals. However, without data on the correlation of prevalence between countries within regions, our current method is more conservative than the alternative of assuming zero correlation, which would result in narrower intervals. Future work on the correlation of IDU prevalence between countries within regions would provide data that we can use to improve the accuracy of the lower and upper bounds.
We used a hierarchical grading system to evaluate estimates on the basis of generalisability geographically (eg, from multiple sites) and across various populations of PWID (eg, treatment and non-treatment samples). Exclusion of estimates on the basis of a study's methodology grade was only applied to estimates of IDU and blood-borne virus prevalence. Nonetheless, our new approach, which involved pooling estimates, and our more sophisticated approach to estimating uncertainty around all our estimates, including our method of estimating uncertainty around imputed estimates, are both improvements on previous reviews.
For the purposes of calculating pooled estimates of the characteristics of PWID, we needed to make decisions about which types of data to pool (appendix pp 69–71). For measures of injecting and sexual risk, although we selected estimates of receptive needle-syringe sharing, and unprotected sexual intercourse, there was variation in the specific wording and questions used to assess these behaviours. We also report the percentage of PWID who had recently engaged in sex work, which was typically defined as in the past year; it is likely that levels of sex work varied among men and women, but studies rarely reported this factor disaggregated by gender.
IDU has been documented in most countries and HIV and HCV are prevalent among many populations of PWID, representing a significant challenge to global public health. Through a process of collating and summarising data on a number of characteristics of PWID and their experiences, it is also obvious that PWID experience substantial exposure to risk environments. This study reinforces the importance of IDU to the global disease burden of blood-borne viruses. The improved data availability should allow for more effective programme planning and the allocation of resources at both national and international levels, particularly to address HIV and HCV, which are highly prevalent among PWID in many places of the world. However, considerable gaps in knowledge remain that require further research, especially on the prevalence of blood-borne viruses among populations of PWID. Our data highlight the substantial exposure of PWID to risk environments, suggesting that efforts to reduce harms among PWID also need to address structural and environmental factors that predispose PWID to elevated risks of harm.
To provide feedback contact global.reviews@unsw.edu.au
Acknowledgments
Acknowledgments
The Australian National Drug and Alcohol Research Centre, UNSW Sydney, provided some funding towards the costs of this systematic review. LD is supported by an Australian National Health and Medical Research Council (NHMRC) Principal Research Fellowship. AP is supported by an NHMRC Early Career Fellowship. JL acknowledges funding from the Bill & Melinda Gates Foundation. The Kirby Institute is funded by the Australian Government Department of Health and Ageing. The views expressed in this publication do not necessarily represent the position of the Australian Government. JG is supported by an NHMRC Career Development Fellowship. JS acknowledges funding from a PhD scholarship from the Engineering and Physical Sciences Research Council (EPSRC). EBC acknowledges funding from Canadian Network on Hepatitis C. AT has received PhD funding from the National Institute for Health Research (NIHR). MH and PV acknowledge support from NIHR Health Protection Research Unit (HPRU) in Evaluation of Interventions at University of Bristol. MH is an NIHR senior investigator and acknowledges NIHR School of Public Health Research PV acknowledges support from the NIHR HPRU in Blood Borne and Sexually Transmitted Infections at University College London and National Institute for Drug Abuse (grant number R01 DA037773–01A1). We thank the research assistants who assisted with searches for and extraction of data from the eligible papers in this review: Erin Yong, Gabrielle Gibson, Griselda Buckland, Harriet Townsend, Julia Stadum and Laura Sergeant (NDARC, UNSW), and Diana Sergiienko (Ukrainian Institute of Public Health Policy). We also thank Mary Kumvaj, the librarian who provided specialist advice on our search strategy and search strings for the peer-reviewed literature searches. Finally, we thank the individuals who provided encouragement and support in various ways throughout the conduct of this study, including circulating requests for data, provision of in-country contacts and assistance with locating data: Annette Verster (WHO), Daniel Wolfe (Open Society Foundations), Andre Noor (EMCDDA), Eleni Kalamara (EMCDDA), Mauro Guarinieri (Global Fund), Christoforos Mallouris (UNAIDS), Susie McLean, Catherine Cook (Harm Reduction International [HRI]), Maria Phelan (HRI), Katie Stone (HRI), Riku Lehtovuori (UNODC), Keith Sabin (UNAIDS), Jinkou Zhao (Global Fund), Vladimir Poznyak (WHO), and Gilberto Gerra (UNODC). Assistance in sourcing and verifying data was provided by many individuals from government, non-government, and research organisations around the world, for which we are thankful. These individuals are listed in the appendix (p 154).
Contributors
LD conceived of the scope of the review with SL, MH, AP, JG, and PV. Screening and review was done by LD, AP, SL, JL, and MH. SC, KD, JL, EBC, JG, JS, and AT had additional roles overseeing and conducting the data extraction and verification. The approach to selection and pooling of all data was developed and agreed on by LD, MH, SL, AP, JG, JL, PV, and ML. Data analysis and estimate generation were done by SC, JL, JS, AT, AP, and LD. Maps were generated by EBC with assistance from SC. LD drafted the first iteration of manuscript. All authors made substantial contributions to the critical review, editing, and revision of the manuscript. All authors approved the final version of the manuscript.
Declaration of interests
In the past 3 years, LD has received investigator-initiated untied educational grants for studies of opioid medications in Australia from Indivior, Mundipharma, and Seqirus. SL has received investigator-initiated untied educational grants from Indivior. AP has received investigator-initiated untied educational grants from Mundipharma and Seqirus. JG is a consultant and adviser for and has received research grants from Abbvie, Cepheid, Gilead Sciences, and Merck/MSD. EBC received PhD funding from the Canadian Network on Hepatitis C. MH reports personal fees from Gilead, Abbvie, and MSD. JS reports non-financial support from Gilead Sciences. SC, JL, KD, ML, PV, RPM, AT, and PG declare no competing interests.
Supplementary Material
References
- 1.Degenhardt L, Charlson F, Stanaway J. Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: findings from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:1385–1398. doi: 10.1016/S1473-3099(16)30325-5. [DOI] [PubMed] [Google Scholar]
- 2.ECDC. EMCDDA . Prevention and control of infectious diseases among people who inject drugs. European Centre for Disease Control and European Monitoring Centre for Drugs and Drug Addiction; Stockholm: 2011. [Google Scholar]
- 3.Degenhardt L, Mathers B, Vickerman P, Rhodes T, Latkin C, Hickman M. Prevention of HIV infection for people who inject drugs: why individual, structural, and combination approaches are needed. Lancet. 2010;376:285–301. doi: 10.1016/S0140-6736(10)60742-8. [DOI] [PubMed] [Google Scholar]
- 4.Altice FL, Azbel L, Stone J. The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in Eastern Europe and Central Asia. Lancet. 2016;388:1228–1248. doi: 10.1016/S0140-6736(16)30856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC . Guidelines for viral hepatitis surveillance and case management. Centers for Disease Control and Prevention; Atlanta, GA: 2005. [Google Scholar]
- 6.Mathers BM, Degenhardt L, Phillips B. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372:1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 7.Nelson PK, Mathers BM, Cowie B. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ball AL, Rana S, Dehne KL. HIV prevention among injecting drug users: responses in developing and transitional countries. Public Health Rep. 1998;113(suppl 1):170–181. [PMC free article] [PubMed] [Google Scholar]
- 9.UNODC . World Drug Report 2016. United Nations; Vienna: 2016. [Google Scholar]
- 10.WHO Global health sector strategy on viral hepatitis 2016–2021. 2017. http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf?ua=1 (accessed June 5, 2017).
- 11.UNAIDS . 90–90–90 An ambitious treatment target to help end the AIDS epidemic. UNAIDS; Geneva: 2014. [Google Scholar]
- 12.United Nations A/RES/70/1: transforming our world: the 2030 agenda for sustainable development. Resolution of the United Nations General Assembly Paper on September 25th 2015. http://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&ampampLang=E (accessed Sept 24, 2017).
- 13.Eckhardt B, Winkelstein ER, Shu MA. Risk factors for hepatitis C seropositivity among young people who inject drugs in New York City: implications for prevention. PLoS One. 2017;12:e0177341. doi: 10.1371/journal.pone.0177341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood EF, Werb D, Beletsky L. Differential experiences of Mexican policing by people who inject drugs residing in Tijuana and San Diego. Int J Drug Policy. 2017;41:132–139. doi: 10.1016/j.drugpo.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larney S, Kopinski H, Beckwith CG. The incidence and prevalence of hepatitis C in prisons and other closed settings: results of a systematic review and meta-analysis. Hepatology. 2013;58:1215–1224. doi: 10.1002/hep.26387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genberg BL, Astemborski J, Vlahov D, Kirk GD, Mehta SH. Incarceration and injection drug use in Baltimore, Maryland. Addiction. 2015;110:1152–1159. doi: 10.1111/add.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blouin K, Leclerc P, Morissette C. Sex work as an emerging risk factor for human immunodeficiency virus seroconversion among people who inject drugs in the SurvUDI Network. Sex Transm Dis. 2016;43:648–655. doi: 10.1097/OLQ.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 18.Barratt D, Hunt N, Stoicescu C. Injecting drug use among under-18s: a snapshot of available data. Harm Reduction International; London: 2013. [Google Scholar]
- 19.Springer SA, Larney S, Alam-Mehrjerdi Z, Altice FL, Metzger D, Shoptaw S. Drug treatment as HIV prevention among women and girls who inject drugs from a global perspective: progress, gaps, and future directions. J Acquir Immune Defic Syndr. 2015;69(suppl 2):S155–S161. doi: 10.1097/QAI.0000000000000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozlov AP, Shaboltas AV, Toussova OV. HIV incidence and factors associated with HIV acquisition among injection drug users in St Petersburg, Russia. AIDS. 2006;20:901–906. doi: 10.1097/01.aids.0000218555.36661.9c. [DOI] [PubMed] [Google Scholar]
- 21.Zule WA, Desmond DP. An ethnographic comparison of HIV risk behaviors among heroin and methamphetamine injectors. Am J Drug Alcohol Abuse. 1999;25:1–23. doi: 10.1081/ada-100101843. [DOI] [PubMed] [Google Scholar]
- 22.Kaye S, Darke S. A comparison of the harms associated with the injection of heroin and amphetamines. Drug Alcohol Depend. 2000;58:189–195. doi: 10.1016/s0376-8716(99)00102-7. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 24.Stevens G, Alkema L, Black R. Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. Lancet. 2016;388:e19–e23. doi: 10.1016/S0140-6736(16)30388-9. [DOI] [PubMed] [Google Scholar]
- 25.Degenhardt L, Gibson G, Leung J, Kumvaj M, Larney S. Searching the grey literature to access research on illicit drug use, HIV and viral hepatitis: a resource to identify drug-related databases and websites. NDARC Technical Report No. 334. National Drug and Alcohol Research Centre; Sydney: 2016. https://ndarc.med.unsw.edu.au/resource/searching-grey-literature-access-research-illicit-drug-use-hiv-and-viral-hepatitis-resource (accessed May 29, 2017). [Google Scholar]
- 26.Harm Reduction International . The Global State of Harm Reduction. Harm Reduction International; London: 2016. https://www.hri.global/contents/1739 (accessed June 14, 2017). [Google Scholar]
- 27.Larney S. A systematic review to estimate global, regional and national coverage of HIV prevention and treatment services for people who inject drugs. Lancet Glob Health. 2017 doi: 10.1016/S2214-109X(17)30373-X. http://dx.doirg/10.1016/S2214-109X(17)30373-X published online Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.UN Population Division . World population prospects: the 2015 revision. United Nations; New York: 2016. https://esa.un.org/unpd/wpp/Publications/Files/Key_Findings_WPP_2015.pdf (accessed May 29, 2017). [Google Scholar]
- 29.Hickman M, Taylor C, Chatterjee A. Estimating the prevalence of problematic drug use: a review of methods and their application. Bull Narc. 2002;LIV:15–32. [Google Scholar]
- 30.Jones HE, Hickman M, Welton NJ, De Angelis D, Harris RJ, Ades AE. Recapture or precapture? Fallibility of standard capture-recapture methods in the presence of referrals between sources. Am J Epidemiol. 2014;179:1383–1393. doi: 10.1093/aje/kwu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrio Anta G, Oliva J, Bravo MJ, De Mateo S, Domingo-Salvany A. Estimating the prevalence of drug injection using a multiplier method based on a register of new HIV diagnoses. Eur J Public Health. 2011;21:646–648. doi: 10.1093/eurpub/ckq076. [DOI] [PubMed] [Google Scholar]
- 32.Fraser H, Martin NK, Brummer-Korvenkontio H, et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol (in press). [DOI] [PMC free article] [PubMed]
- 33.Medhi GK, Mahanta J, Akoijam BS, Adhikary R. Size estimation of injecting drug users (IDU) using multiplier method in five districts of India. Subst Abuse Treat Prev Policy. 2012;7:9. doi: 10.1186/1747-597X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.EMCDDA . Treatment and care for older drug users. European Monitoring Centre for Drugs and Drug Addiction; Lisbon: 2010. [Google Scholar]
- 35.Armstrong GL. Injection drug users in the united states, 1979–2002: an aging population. Arch Intern Med. 2007;167:166–173. doi: 10.1001/archinte.167.2.166. [DOI] [PubMed] [Google Scholar]
- 36.AIVL . ‘Double jeopardy’: older injecting opioid users in Australia—AIVL Discussion Paper. Australian Injecting and Illicit Drug Users League; Canberra: 2011. http://www.aivl.org.au/resource/double-jeopardy-older-injecting-opioid-users-in-australia/ (accessed June 15, 2017). [Google Scholar]
- 37.Banerjee G, Edelman EJ, Barry DT. Non-medical use of prescription opioids is associated with heroin initiation among US veterans: a prospective cohort study. Addiction. 2016;111:2021–2031. doi: 10.1111/add.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.United Nations . Declaration of Commitment on HIV/AIDS. UN General Assembly Special session on HIV/AIDS. 2 August 2001. United Nations; New York: 2001. [Google Scholar]
- 39.Dore GJ, Feld JJ. Hepatitis C virus therapeutic development: in pursuit of “perfectovir”. Clin Infect Dis. 2015;60:1829–1836. doi: 10.1093/cid/civ197. [DOI] [PubMed] [Google Scholar]
- 40.United Nations . A/RES/S-30/1. Our joint commitment to effectively addressing and countering the world drug problem. Resolution adopted by the General Assembly on 19 April 2016. United Nations; New York: 2016. [Google Scholar]
- 41.WHO . Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. World Health Organization; Geneva: 2014. [PubMed] [Google Scholar]
- 42.Rich JD, Beckwith CG, Macmadu A. Clinical care of incarcerated people with HIV, viral hepatitis, or tuberculosis. Lancet. 2016;388:1103–1114. doi: 10.1016/S0140-6736(16)30379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamarulzaman A, Reid SE, Schwitters A. Prevention of transmission of HIV, hepatitis B virus, hepatitis C virus, and tuberculosis in prisoners. Lancet. 2016;388:1115–1126. doi: 10.1016/S0140-6736(16)30769-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.