Highlights
-
•
Elipse™ IGB is a novel procedureless device for weight loss.
-
•
We represent a successful laparoscopic management of a small bowel obstruction caused by Elipse™ IGB migration.
-
•
This is the first reported adverse event in literature regarding Elipse™ IGB device.
Abbreviations: BIB, bio-enteric gastric balloon; GI, gastrointestinal; IGB, intragastric balloon; OD, once daily; PCFNA, percutaneous fine needle aspiration; PO, per oral; SBO, small bowel obstruction
Keywords: Elipse, Intragastric balloon, Capsule, Obesity, Case report
Abstract
Introduction
The Elipse™ intragastric balloon (IGB) for weight loss is a swallowable capsule that is filled with 550 mL of fluid and resides in the stomach for four months before being excreted from the gastrointestinal tract. Although initial data showed that use of this device is safe and free from serious complications, we report for the first time the successful management of an Elipse™ IGB-related adverse event.
Presentation of case
A 41-year-old woman presented to our emergency department following two days of abdominal pain, vomiting, and constipation. Her medical history included four caesarean sections and insertion of the Elipse™ IGB 16 weeks prior to presentation. The patient was vitally stable at presentation and abdominal examination revealed a mildly distended abdomen. Plain X-ray revealed a small bowel obstruction (SBO), and a double contrast computed tomography scan showed a dilated small bowel with mild free fluid proximal to a transition zone at the distal jejunum. Laparoscopic enterotomy was performed just proximal to the obstruction site, and the balloon was visualized and extracted after it had been incised and emptied. The enterotomy incision was closed with an intracorporeal continuous absorbable suture. The patient’s recovery was uneventful and she was discharged on postoperative day 4.
Discussion
We discuss the possible etiologies of SBO following Elipse™ IGB insertion, and present a brief literature review regarding surgical and nonsurgical management options for such cases.
Conclusion
Although initial data showed the Elipse™ IGB to be safe, complications can occur and be managed successfully.
1. Introduction
For those who fall into the overweight or class I obesity body mass index categories, weight loss options are limited if diet attempts fail. Thus, intragastric balloon (IGB) insertion, which has been shown to be safe and effective for weight loss [1], has become the most demanded procedure among such patients [2]. The first IGB approved for weight loss was the Garren-Edwards gastric bubble in 1985 [3]. Although it was withdrawn due to serious adverse effects, advances to its design led to more effective and safer endoscopic IGBs [3], [4]. At present, the United States Food and Drug Administration has approved three new IGBs [5], with newer less invasive IGBs also being recently launched [6], [7].
The Elipse™ (Allurion Technologies, Wellesley, Massachusetts, USA) is a new IGB that was recently introduced in Kuwait. A swallowable capsule, it is filled with 550 mL of pH-titrated fluid through a catheter and converted to a balloon after plain X-ray confirmation of being in the stomach [7] (Fig. 1). Thus, it does not require endoscopy or anesthesia during insertion or removal, and is now commonly used due to being ‘procedureless’ [6]. Although its mode of delivery is novel, it acts similarly to previous conventional IGBs by reducing stomach volume to limit food intake and facilitate weight loss [6], [7]. After four months, a self-releasing valve is opened and the thin wall of the balloon gradually deflates and is excreted through the gastrointestinal (GI) tract [6], [7].
Fig. 1.
The Elipse™ IGB. The figure shows the capsule attached to a catheter and the balloon after filling.
Although initial data showed the device to be safe, effective, and free from serious complications [6], [7], we provide a novel case report (reported in line with the SCARE criteria [8]) of the successful laparoscopic management of a small bowel obstruction (SBO) secondary to Elipse™ IGB migration.
2. Case report
A 41-year-old woman presented to our emergency department after two days of generalized colicky abdominal pain and multiple episodes of vomiting, and one day of constipation. While her medical history did not include abdominal distension, fever, or other similar complaints, she had undergone an uneventful Elipse™ IGB insertion at another center four months prior to presentation. She also had a history of four caesarian sections and paroxysmal atrial fibrillation that was being managed with Bisoprolol (Concor®, 5 mg PO, OD).
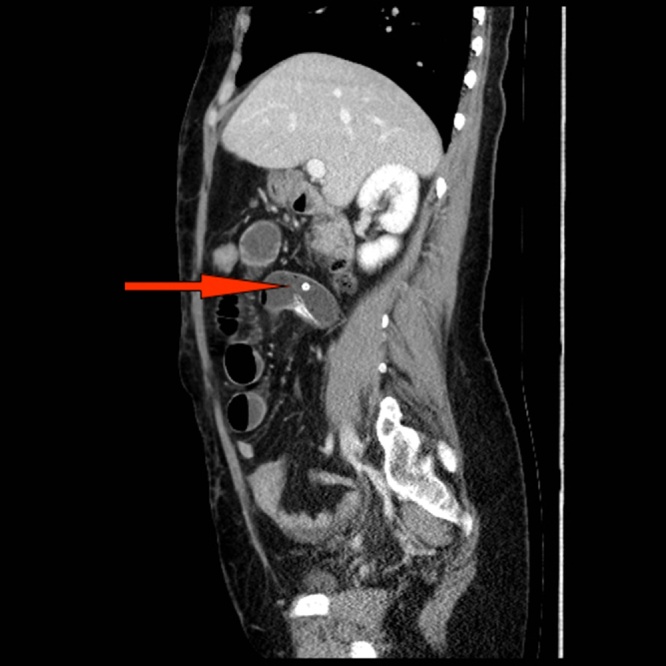
Upon arrival, the patient was lethargic with the following vital signs: pulse, 80 beats/min; blood pressure, 130/80 mmHg; respiratory rate, 20/min; body temperature, 37.2 °C; and oxygen saturation, 98% on room air. Clinical examination revealed a slightly distended abdomen with no signs of herniation at her Pfannenstiel scar, mild tenderness over the peri-umbilical region with no rebound or rigidity, and hyperactive bowel sounds. Chest examination revealed equal bilateral air entry. Initial laboratory results were as follows: white blood cell count, 11.9 × 109/L (neutrophilic); blood urea nitrogen, 3.7 mmol/L; creatinine, 56 umol/L; lactic acid, 0.9 mmol/L; and normal liver function, amylase, and lipase. An abdominal radiograph demonstrated multiple air-fluid levels in a stepladder configuration. Double contrast-enhanced computed tomography of the abdomen and pelvis revealed dilated small bowel loops with a dense transition zone mostly representing the balloon seen at the level of the proximal ilium (Fig. 2). Beyond this, the ileal loops were fully collapsed. Mild free fluid was also noted between the bowel loops and in the pelvis (Fig. 2).
Fig. 2.
Double contrast computed tomography scan with a sagittal view of the abdomen and pelvis. A transitional zone containing the balloon marker (red arrow) can be seen, with dilated small bowel loops located proximally and the section of fully collapsed bowel located distally.
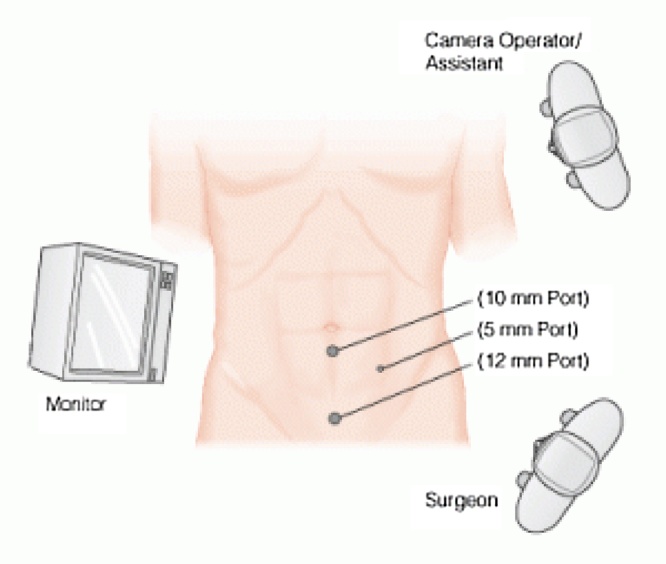
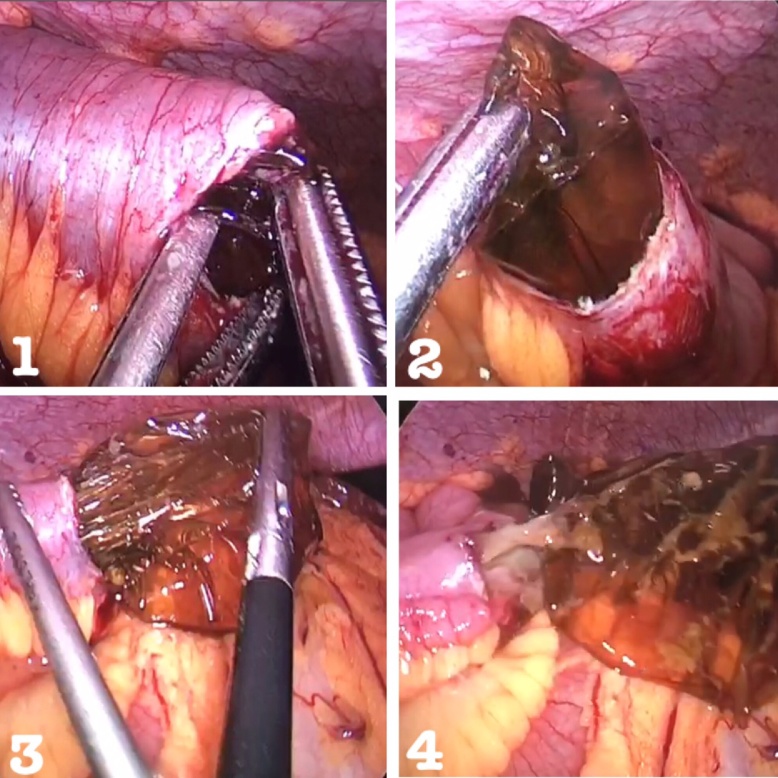
Resuscitation was commenced with immediate nasogastric decompression, intravenous hydration and analgesia, administration of proton-pump inhibitors, and electrolyte replacement. After consenting to surgery, the patient underwent general anesthesia and was placed in a supine position. The open Hasson technique was performed through a supraumbilical incision to achieve pneumoperitoneum, and two additional working ports were inserted (Fig. 3). Inspection of the peritoneal cavity revealed a dilated small bowel up to the level of the distal jejunum with a moderate amount of serous fluid. Dense adhesions at the site of previous caesarean sections were also seen. The small bowel was then inspected from the terminal ileum to the level of the obstruction, around 130 cm from the ileocecal valve. A 3-cm transverse enterotomy was performed with a monopolar diathermy hook proximal to the level of the obstruction. The balloon was identified and opened using endoscissors, causing fluid to be immediately expelled and aspirated (Fig. 4). The balloon then collapsed and was extracted, and the enterotomy site received primary closure with an intracorporeal continuous 2-0 coated Vicryl® (polyglactin 910) suture. The balloon remnant was subsequently removed from the abdomen via the 12-mm port, after which the intraabdominal fluid was aspirated, and a large surgical vacuum drain and nasogastric tube were inserted. Intraveneous analgesia was started immediately after the procedure.
Fig. 3.
Diagram showing the placement of ports in the patient’s abdomen. The surgeon and assistant are to the left of the patient and the laparoscopic tower is to the right.
Fig. 4.
The collapsed balloon extracted from the enterotomy site after it was incised with laparoscopic scissors.
Following the operation, the patient was kept fasting on intravenous hydration. The next day, she was gradually weaned and started on sips of water, before oral intake was increased until discharge on postoperative day four. She was followed up at our clinic two weeks post-operation and subsequently returned to daily life activities.
3. Discussion
While initial data showed that the Elipse™ IGB is safe and free from serious adverse events [6], [7], early deflation and balloon emesis can occur [6]. However, there have been no reports of SBO caused by Elipse™ IGB migration, though this was identified as a possible complication of the device. Patient selection criteria were therefore established to minimize the possibility of adverse events occurring during Elipse™ IGB insertion or excretion [6] (Fig. 5). Contraindications include any GI tract pathology that can interfere with the deflation and excretion of the balloon, including intraabdominal adhesions due to multiple previous surgeries [6], [7].
Fig. 5.
Patient selection criteria for Elipse™ IGB insertion.
Our patient’s previous four cesarean sections prior to Elipse™ insertion may therefore have caused SBO. Other possible contributors to early balloon migration and GI obstruction include incomplete balloon filling during insertion, early catheter detachment during balloon filling, false release of the balloon valve during excretion, and balloon leak. For example, the Orbera™ IGB shows a migration rate of 1.4% and a 0.3% SBO prevalence rate [9], with a systematic review finding that migration rates are particularly high in Orbera™ IGBs with lower volumes [10]. Although this device is a bio-enteric intragastric balloon (BIB), the insertion procedure for which differs from that of the Elipse™, these studies suggest that incomplete filling may have caused migration in our case. However, this cannot be confirmed as the balloon was inserted at another center. Larger studies are needed to further elucidate the possible etiologies of migration causing SBO in cases such as ours.
With regard to treatment, there are various management options for SBO secondary to IGB migration. Percutaneous fine needle aspiration (PCFNA) in the bowel has been performed successfully for a variety of indications [11], including migrated intraintestinal balloons that require decompression [12], [13], [14]. Specifically, Frimberger et al. described a water-filled IGB that had migrated into the small bowel and was subsequently punctured percutaneously with a fine needle under ultrasound guidance [12]. Furthermore, Holland et al. reported two patients with migrated IGBs that were successfully treated with a percutaneous transabdominal puncture [13], and Hegade et al. reported a patient who underwent percutaneous deflation of an air-filled balloon under fluoroscopy [14]. A similar strategy could be used to decompress a migrated Elipse™ IGB, though hematoma formation and perforation are rare but possible complications that mandate close monitoring after the procedure [15].
If PCFNA fails to resolve the obstruction, surgical removal of the balloon is required. Others have reported the surgical removal of IGBs using a technique similar to ours, including successful removals of BIBs causing SBO via laparoscopic [16] and open [17] approaches. Furthermore, all cases reported in the literature had excellent outcomes following enterotomy, balloon extraction, and primary closure. After discussing the risks and benefits of surgical and nonsurgical management options with our patient, we also adopted this technique because it has been shown to be safe and is a definitive approach for the removal of IGBs causing SBO.
Another less common and less invasive approach for the management of IGBs lodged in the bowel is enteroscopic balloon removal. Halm et al. reported the successful management of a partially deflated balloon obstructing the jejunum using double-balloon enteroscopy with needle aspiration followed by removal with a polypectomy snare [18]. Vlachou et al. also effectively used the same technique, but instead pushed the balloon to the colon to facilitate its passage with stools [19]. However, perforation and bleeding are possible complications with this technique and close monitoring is important after the procedure [19].
4. Conclusion
Although the Elipse™ IGB has been reported to be safe with no serious adverse effects, we show that SBO secondary to migration of this device can occur. While adherence to the patient selection criteria can minimize the possibility of this event, larger studies are needed to further elucidate its likely etiologies. After discussing the risks and benefits of various management options with our patient, we opted for laparoscopic removal because it is a definitive management procedure with well-established outcomes. However, other management options could also be feasible for cases such as ours, with numerous reports of successful surgical and nonsurgical treatment of SBO caused by IGBs.
Conflicts of interest
The authors declare that they have no conflicts of interest or financial ties to disclose.
Funding
The authors declare that they have no source of funding to disclose.
Ethical approval
Ethical approval and informed consent were obtained and available upon request.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Saud Al-Subaie: Writing the paper and revising it critically for important intellectual content.
Hamad Al-Barjas: Assistant surgeon. Final approval of the version to be submitted.
Salman Al-Sabah: Surgeon in-charge. Revising the paper and Final approval to be submitted.
Saud Al-Helal: Drafting the article and gathering the needed information and material.
Ashraf Alfakharani: Gathering information, and collecting and improving the quality of the figures. Drafting the article.
Salah Termos: Drafting the article and gathering the needed information and material.
Guarantor
Saud Al-Subaie.
Acknowledgements
We acknowledge with deep gratitude the support received from our operating theatre nursing staff at Amiri hospital in providing all the materials needed for this paper. We would also like to thank Editage (www.editage.com) for English language editing.
References
- 1.Zheng Y., Wang M., He S., Ji G. Short-term effects of intragastric balloon in association with conservative therapy on weight loss: a meta-analysis. J. Transl. Med. 2015;13:246. doi: 10.1186/s12967-015-0607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar N., Sullivan S., Thompson C.C. The role of endoscopic therapy in obesity management: intragastric balloons and aspiration therapy. Diabetes Metab. Syndr. Obes. 2017;10:311–316. doi: 10.2147/DMSO.S95118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gleysteen J.J. A history of intragastric balloons. Surg. Obes. Relat. Dis. 2016;12:430–435. doi: 10.1016/j.soard.2015.10.074. [DOI] [PubMed] [Google Scholar]
- 4.Nunes G.C., Pajecki D., de Melo M.E., Mancini M.C., de Cleva R., Santo M.A. Assessment of weight loss with the intragastric balloon in patients with different degrees of obesity. Surg. Laparosc. Endosc. Percutan. Tech. 2017;27:e83–e86. doi: 10.1097/SLE.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 5.Laing P., Pham T., Taylor L.J., Fang J. Filling the void: a review of intragastric balloons for obesity. Dig. Dis. Sci. 2017;62:1399–1408. doi: 10.1007/s10620-017-4566-2. [DOI] [PubMed] [Google Scholar]
- 6.Machytka E., Gaur S., Chuttani R., Bojkova M., Kupka T., Buzga M. Elipse, the first procedureless gastric balloon for weight loss: a prospective, observational, open-label, multicenter study. Endoscopy. 2017;49:154–160. doi: 10.1055/s-0042-119296. [DOI] [PubMed] [Google Scholar]
- 7.Raftopoulos I., Giannakou A. The Elipse balloon, a swallowable gastric balloon for weight loss not requiring sedation, anesthesia or endoscopy: a pilot study with 12-month outcomes. Surg. Obes. Relat. Dis. 2017;13:1174–1182. doi: 10.1016/j.soard.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., for the SCARE Group The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Kim S.H., Chun H.J., Choi H.S., Kim E.S., Keum B., Jeen Y.T. Current status of intragastric balloon for obesity treatment. World J. Gastroenterol. 2016;22:5495–5504. doi: 10.3748/wjg.v22.i24.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar N., Bazerbachi F., Rustagi T., McCarty T.R., Thompson C.C., Galvao Neto M.P. The influence of the orbera intragastric balloon filling volumes on weight loss, tolerability, and adverse events: a systematic review and meta-analysis. Obes. Surg. 2017;27:2272–2278. doi: 10.1007/s11695-017-2636-3. [DOI] [PubMed] [Google Scholar]
- 11.de Sio I., Funaro A., Vitale L.M., Niosi M., Francica G., Federico A. Ultrasound-guided percutaneous biopsy for diagnosis of gastrointestinal lesions. Dig. Liver Dis. 2013;45:816–819. doi: 10.1016/j.dld.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Frimberger E., Kühner W., Weingart J., Waldthaler A., Ottenjann R. Percutaneous decompression of an intraintestinal balloon-case report. Hepatogastroenterology. 1982;29:38–39. [PubMed] [Google Scholar]
- 13.Holland S., Bach D., Duff J. Balloon therapy for obesity-when the balloon bursts. J. Can. Assoc. Radiol. 1985;36:347–349. [PubMed] [Google Scholar]
- 14.Hegade V.S., Sood R., Douds A.C. Small bowel obstruction induced by spontaneous partial deflation of an intragastric balloon. Ann. R. Coll. Surg. Engl. 2012;94:e171–3. doi: 10.1308/003588412X13171221590539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields S., Libson E. CT-guided aspiration core needle biopsy of gastrointestinal wall lesions. J. Comput. Assist. Tomogr. 2000;24:224–228. doi: 10.1097/00004728-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Di Saverio S., Bianchini Massoni C., Boschi S., Biscardi A., Tugnoli G. Complete small-bowel obstruction from a migrated intra-gastric balloon: emergency laparoscopy for retrieval via enterotomy and intra-corporeal repair. Obes. Surg. 2014;24:1830–1832. doi: 10.1007/s11695-014-1271-5. [DOI] [PubMed] [Google Scholar]
- 17.Mousavi Naeini S.M., Sheikh M. Bowel obstruction due to migration of an intragastric balloon necessitating surgical removal before completion of the recommended 6 months. Case Rep. Med. 2012;2012 doi: 10.1155/2012/414095. Article ID 414095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halm U., Grothoff M., Lamberts R. Gastric balloon causing small bowel obstruction: treatment by double-balloonenteroscopy. Endoscopy. 2013;45:e78–9. doi: 10.1055/s-0032-1326265. [DOI] [PubMed] [Google Scholar]
- 19.Vlachou E., Direkz S., Murino A., Wylie P., Hamilton M.I., Murray C.D. Small bowel obstruction caused by a migrated Obalon gastric bariatric balloon: nonsurgical management by antegrade double-balloon panenteroscopy. Endoscopy. 2016;48:e403–4. doi: 10.1055/s-0042-120289. [DOI] [PubMed] [Google Scholar]