The retromer acts as the gatekeeper blocking signaling mediated by beta-arrestin 1 from internalized cannabinoid 2 receptors. This work provides further confirmation of the relevance and prevalence of signaling from internalized receptors at endosomal compartments after ligand-induced endocytosis.
Abstract
G protein–coupled receptors mediate their complex functions through activation of signaling cascades from receptors localized at the cell surface and endosomal compartments. These signaling pathways are modulated by heterotrimeric G proteins and the scaffold proteins beta-arrestin 1 and 2. However, in contrast to the events occurring at the cell surface, our knowledge of the mechanisms controlling signaling from receptors localized at intracellular compartments is still very limited. Here we sought to investigate the intracellular signaling from cannabinoid 2 receptor (CB2R). First, we show that receptor internalization is required for agonist-induced phosphorylation of extracellular signal-regulated protein kinases 1 and 2 (ERK1/2). Then we demonstrate that ERK1/2 activation is mediated by beta-arrestin 1 from receptors localized exclusively at Rab4/5 compartments. Finally, we identify the retromer complex as a gatekeeper, terminating beta-arrestin 1–mediated ERK phosphorylation. These findings extend our understanding of the events controlling signaling from endocytosed receptors and identify the retromer as a modulator of beta-arrestin–mediated signaling from CB2R.
INTRODUCTION
The endocannabinoid system is composed of two major receptors (CB1R and CB2R) together with the enzymes that produce and degrade their ligands (Pertwee et al., 2010; Kendall and Yudowski, 2017). Both receptors are members of the G protein–coupled receptor family (GPCR) and are mainly coupled to Gi/o proteins. CB2Rs are highly expressed in immune cells such as macrophages and microglia, but they are also expressed at much lower levels in the CNS (Benito et al., 2008; Atwood and Mackie, 2010; Dhopeshwarkar and Mackie, 2014). Owing to their localization and roles during immune response, CB2R have been proposed as a potential therapeutic target to alleviate pain, tissue injury, and inflammatory diseases among other pathological states (Pertwee, 2001; Benito et al., 2008; Dhopeshwarkar and Mackie, 2014). However, its therapeutic modulation has been hampered by the intricate pharmacology and our limited understanding of this receptor. Complexity is further increased by their functional selectivity and biased signaling and their ability to activate or inhibit different subsets of signaling pathways with variable potencies and efficacies depending on the type of ligands, receptor species and localization (Urban et al., 2007; Atwood et al., 2012; Soethoudt et al., 2017).
Activation of CB2R results in the modulation of signaling cascades from receptors localized at the cell surface and in endosomal compartments (Brailoiu et al., 2014; Zhang et al., 2016). These cascades include reduction of cAMP levels, activation of ion channels, and beta-arrestin recruitment among others (Felder et al., 1995; Bouaboula et al., 1996; Shoemaker et al., 2005; Soethoudt et al., 2017). Like other GPCRs, ligand binding can initiate CB2R endocytic trafficking. Receptors later recycle to the cell surface via Rab4-, Rab5-, and Rab11-dependent pathways (Grimsey et al., 2011; Atwood et al., 2012; Chen et al., 2014), but Rab11-independent recycling has also been proposed (Leterrier et al., 2004). CB2Rs localized at the cell surface and at endosomal compartment can also modulate calcium levels (Brailoiu et al., 2014). However, our knowledge of the mechanism and signaling events elicited from CB2R traversing the endosomal compartment is very limited.
RESULTS
CB2R-mediated activation of ERK occurs after receptor internalization
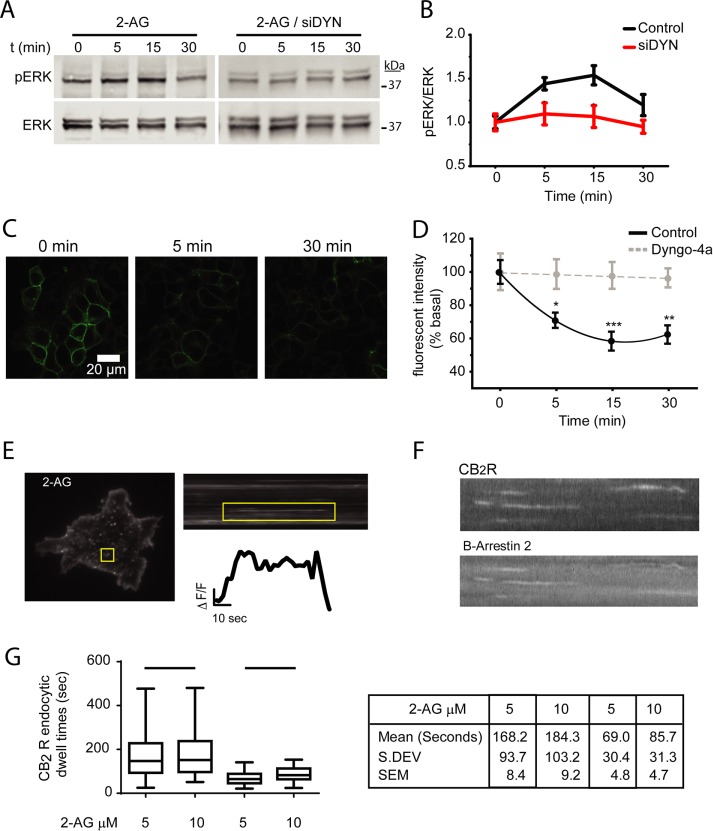
Phosphorylation of ERK1/2 downstream from CB1Rs is mediated by beta-arrestin 1 and begins during the endocytic process and continues into endosomal compartments (Ahn et al., 2013; Delgado-Peraza et al., 2016b). Activation of CB2R can also result in ERK1/2 phosphorylation in heterologous and native tissues such as immune cells and microglia, but the location and mechanisms driving phosphorylation have not been defined. To test whether activation of ERK1/2 by CB2R occurs from receptors located at the cell surface or from internalized receptors, we exposed HEK293 cells stably expressing human CB2Rs to the endocannabinoid 2-AG. Maximal ERK1/2 phosphorylation was observed within 5 min of agonist addition and was maintained at 15 min before returning to basal levels at 30 min (Figure 1, A and B). To determine whether receptors located at the cell surface or at intracellular compartments mediate the phosphorylation of ERK1/2, we inhibited receptor internalization by silencing the ubiquitous dynamin 2. Cells were transfected with small interfering RNA (siRNA) against human dynamin 2 and were incubated with 10 µM 2-AG. The effectiveness of siRNA treatments was confirmed by the reduction of dynamin 2 expression levels (Supplemental Figure 1). Silencing dynamin 2 resulted in a marked inhibition of ERK1/2 phosphorylation, indicating that ERK1/2 phosphorylation is mediated from internalized CB2R (Figure 1, A and B). To verify that dynamin is involved in the agonist-induced removal of CB2R, we investigated CB2R endocytosis at the cell population level. Internalization of CB2R was observed within 5 min of 10 µM 2-AG addition to the incubation media, reaching a constant steady state level within 15 min (Figure 1, C and D). These results are similar to previously published data utilizing rat CB2R (Atwood et al., 2012). Pharmacological inhibition of dynamin by Dyngo 4a, completely prevented decreases in CB2R surface fluorescence, supporting the notion that receptor endocytosis is mediated by dynamin (Figure 1D). Our previous work on the CB1R demonstrated that the time receptors are clustered into endocytic pits, defined as receptor dwell times, is proportional to ERK1/2 activation (Flores-Otero et al., 2014). Because the activation of ERK1/2 by CB2R occurs after receptors are internalized, we wondered how individual CB2R dwell times compare to those from CB1R. We used total internal reflection microscopy (TIRFM) to directly visualize individual endocytic events of SEP-CB2R induced by 10 µM 2-AG. CB2R endocytic pits were clearly visible as diffraction-limited spots and their fluorescence kinetics were similar to the clathrin-mediated endocytic events described previously for the CB1R and several other GPCRs (Figure 1E). Beta-arrestin 2 coexpressed in these cells and was rapidly recruited to individual endocytic events as previously observed for other receptors (Figure 1F and Supplemental Video 1). Analysis of single endocytic events demonstrated that CB2R remain clustered in individual endocytic pits ∼85 s before their removal, a much more rapid kinetics than CB1R and other GPCRs under similar conditions (Figure 1G). Overall, these results indicate that 2-AG induces rapid CB2R internalization via dynamin dependent mechanism and that ERK1/2-induced phosphorylation occurs after receptor internalization.
FIGURE 1:
Internalization is required for ERK1/2 activation. (A) Time course showing ERK1/2 phosphorylation in HEK293 cells stably expressing human SEP-CB2R exposed to 10 µM 2-AG and in cells transfected with dynamin2 siRNA. Cell lysates were analyzed using Western blots with phospho-ERK1/2 (top panel) and total ERK1/2 (bottom panel). (B) Multiple experiments were quantified and normalized to total ERK1/2 levels. Data represents the mean ± SEM at each point (n = 9–10 independent experiments). (C) HEK293 cells expressing human SEP-CB2Rs were exposed to 10 µM 2-AG and sequential confocal images were acquired. (D) Total fluorescence time-course analysis indicates there is a ligand-induced decrease in fluorescence associated with receptor internalization. The dynamin inhibitor Dyngo 4a prevented internalization (n > 5 independent experiments). (E) Total internal reflection image showing HEK293 cells expressing SEP-CB2Rs in the presence of 10 µM 2-AG. Individual endocytic events appear as bright dots (yellow box). Kymographs were obtained from sequential images and individual endocytic events were easily identifiable (yellow rectangle). (F) Fluorescence intensity measurements from individual endocytic events depicting event formation, maturation, and removal from the cell surface. (G) Analysis of multiple endocytic dwell times from SEB-CB1R and SEP-CB2R elicited by 2-AG at the indicated concentrations. Results are depicted as box-and-whiskers plots representing maximum and minimum data and adjacent table (n > 120 events, five to seven cells/analysis).
ERK1/2 phosphorylation is mediated by beta-arrestin 1
GPCRs can activate ERK1/2 via heterotrimeric G proteins or beta-arrestins (Gurevich and Gurevich, 2004; DeWire et al., 2007). Activation of rat CB2R by the agonists CP55,940 or WIN55,212-2 results in strong recruitment of beta-arrestin 2 to the cell surface in HEK293 cells (Atwood et al., 2012). Our results also indicate that beta-arrestin 2 is rapidly recruited to the cell surface into CB2R endocytic pits (Figure 1F and Supplemental Video 1). However, it is currently unknown whether beta-arrestins can modulate ERK1/2 phosphorylation downstream of CB2R activation. To answer these questions, we first tested whether pertussis toxin (PTX)-sensitive Gi/o proteins were responsible for ERK1/2 phosphorylation. Overnight PTX preincubation (10 ng/ml) resulted in no significant changes in the phosphorylation kinetics of ERK1/2 induced by 2-AG (Figure 2, A and B). PTX functionality was simultaneously confirmed by inhibiting PTX-sensitive signaling from CB1R in the same conditions as previously reported (Supplemental Figure 2) (Delgado-Peraza et al., 2016a). Next, we utilized silencing technology to reduce the protein expression levels of beta-arrestin 1 and beta-arrestin 2 in HEK293 cells stably expressing CB2R. Cells were incubated with 10 µM 2-AG and ERK1/2 phosphorylation was analyzed. Strikingly, reduction of beta-arrestin 1 levels resulted in a significant decrease in ERK1/2 activation at the 5-min time point (Figure 2, C and D). However, signaling at 15 min was not affected (Figure 2, C and D). Interestingly, removal of beta-arrestin 2 had no effect on ERK1/2 phosphorylation (Figure 2, E and F). The effectiveness of siRNAs was confirmed in all experiments by Western blot (Supplemental Figure 1). This suggest that early ERK1/2 phosphorylation (∼5 min) is mediated by beta-arrestin 1 after receptors are rapidly endocytosed.
FIGURE 2:
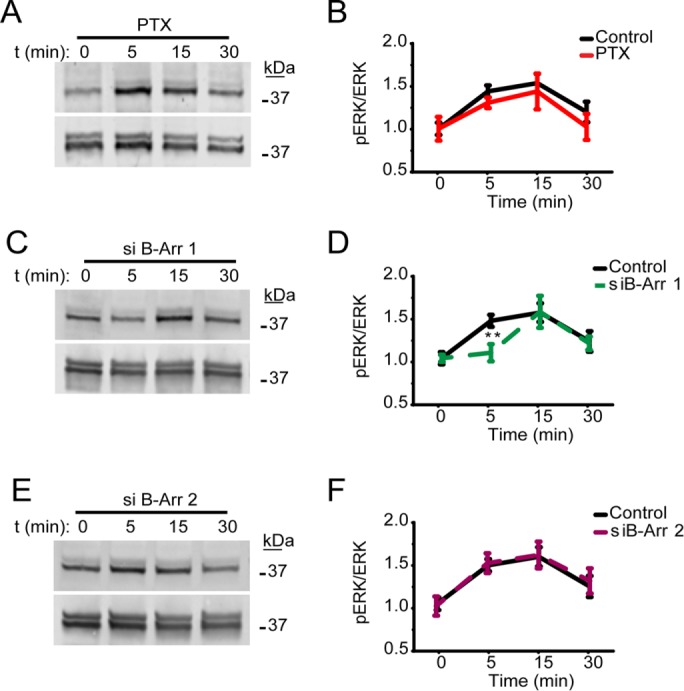
Activation of ERK1/2 is mediated by beta-arrestin 1 at 5 min. (A) HEK293 cells stably expressing human CB2Rs and preincubated overnight with 10 ng/ml PTX were incubated with 10 µM 2-AG. Cell lysates were analyzed by Western blots with antibodies against phospho-ERK1/2 (top panel) and total ERK1/2 (bottom panel). (B) Time course represents quantification of multiple experiments normalized to total ERK1/2 levels. Data represent the mean ± SEM at each point (n > 5 independent experiments). (C) HEK293 cells expressing human CB2R and transfected with beta-arrestin 1 siRNA were incubated with 10 µM 2-AG and compared with mock-transfected controls. (D) Multiple experiments were quantified and normalized to total ERK1/2 levels. Data indicate a significant reduction in ERK1/2 phosphorylation at the 5-min time point in cells transfected with beta-arrestin 1 siRNA (mean ± SEM at each point (n = 6). (E) HEK293 cells expressing CB2R and transfected with beta-arrestin 2 siRNA or mock controls were incubated with 10 µM 2-AG as indicated. (F) Plotted data from multiple experiments indicate no significant effects of beta-arrestin 2 siRNA (n = 6).
To help define the roles of beta-arrestin during receptor endocytosis and signaling, we analyzed the recruitment kinetics of beta-arrestins to the cell surface utilizing TIRFM. HEK293 cells stably expressing CB2R were transfected with either beta-arrestin 1 or beta-arrestin 2 tagged with RFP. Beta-arrestin recruitment analysis was performed as described before (Flores-Otero et al., 2014). As reported with the rat CB2R treated with CP55,940 and WIN55.212 (Atwood et al., 2012) activation of the human CB2R by 2-AG resulted in significant recruitment of beta-arrestin 2 to the cell surface (Figure 3, B, C, and D). In contrast, recruitment of beta-arrestin 1, which mediates intracellular phosphorylation of ERK1/2 (Figure 2), was not observed (Figure 3, A, C, and D). Interestingly, the fluorescence intensity from beta-arrestin 1 was decreased in the TIRF field, indicating a departure from the cell surface and suggesting a later engagement with CB2R (Figure 3, C and D). Because beta-arrestin 1 mediates signaling from the CB1R, and the decreased in fluorescence observed in beta-arrestin 1 in TIRF, we sought to investigate whether CB2R colocalize with beta-arrestin 1 after internalization. To answer this, we performed live-cell confocal microscopy of HEK293 cells coexpressing CB2R and beta-arrestin 1 before and after agonist incubation. In these cells, we observed a high degree of colocalization between receptors and beta-arrestin 1 in intracellular compartments only at the 5-min time point (Figure 3, E and F). These data together with ERK activation kinetics suggest that beta-arrestin 1–mediated ERK phosphorylation occurs after receptor are internalized and might occur at endosomal compartments.
FIGURE 3:
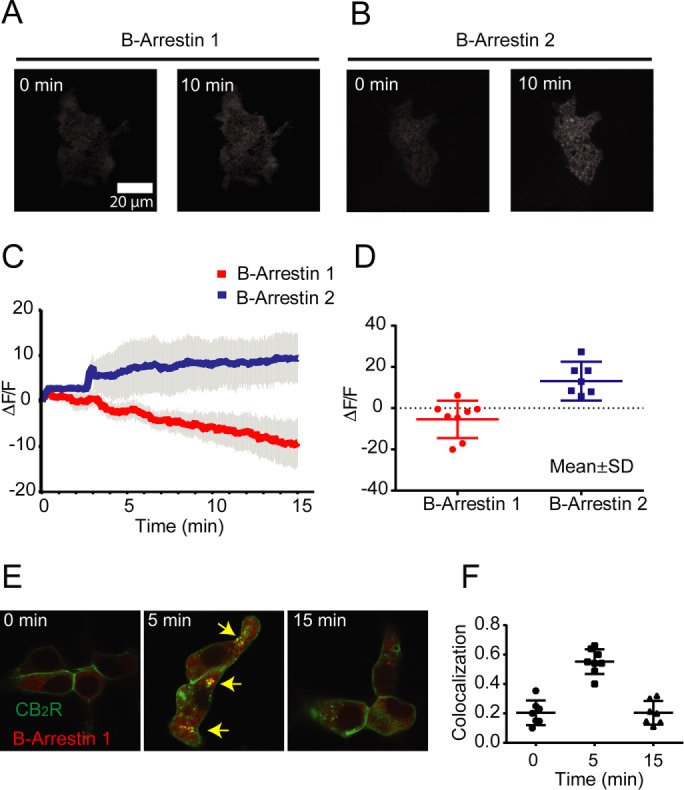
2-AG induces beta-arrestin 2 recruitment to the plasma membrane. (A) TIRFM images showing HEK293 cells stably expressing SEP-CB2R coexpressing beta-arrestin 1-mRFP before and after 10-min incubation with 10 µM 2-AG. (B) TIRFM images showing HEK293 cells stably expressing SEP-CB2R coexpressing beta-arrestin 2-mRFP before and after 10-min incubation with 10 µM 2-AG. (C) Changes in total plasma membrane fluorescence from the red channel (beta-arrestins) were continuously analyzed before and after agonist incubation by TIRM (n = 8–10 cells). (D) Quantification of multiple beta-arrestin recruitment experiments at 5 min after 10 µM 2-AG (n = 8–10 cells). (E) Confocal images of HEK293 cells expressing CB2R (green) and beta-arrestin 1 (red) before and after 2-AG incubations. (F) Pearson’s colocalization coefficients for internalized receptors with beta-arrestin 1 (individual replicates are represented, n = 7–10).
ERK1/2 phosphorylation occurs at Rab4/5 compartments
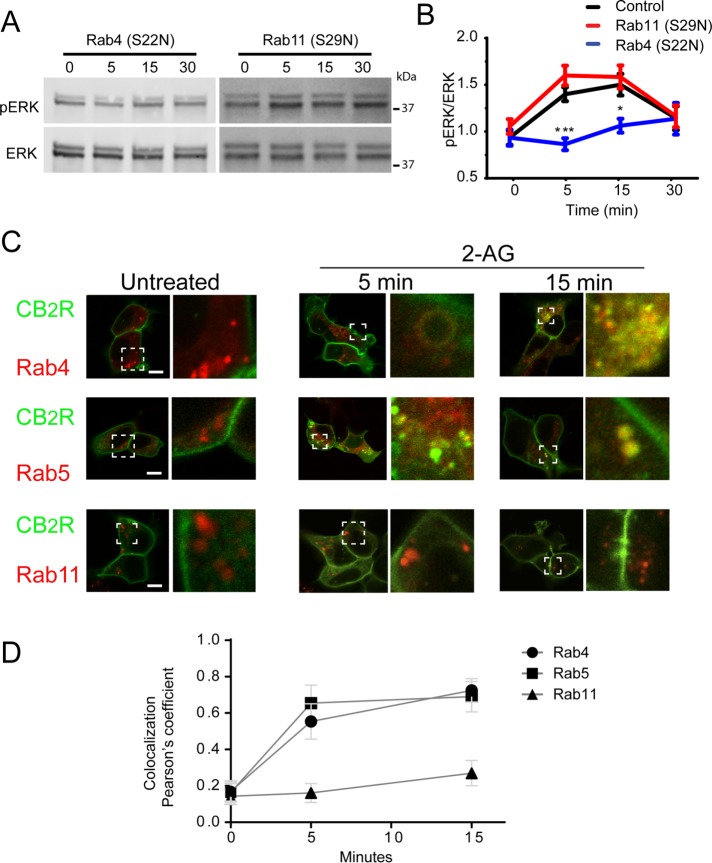
Rab GTPases are members of a large family of small GTPases involved in coordinating and mediating intracellular membrane trafficking events such as the endocytic and endo/lysosomal trafficking of surface receptors among others (Stenmark, 2009). To identify the intracellular compartment where CB2R activates ERK1/2, we transfected HEK293 cells stably expressing CB2R with dominant negative variants of Rab4 (S22N) or Rab11 (S29N) to specifically disrupt these intracellular compartments (Roman-Vendrell et al., 2012). Cells expressing CB2R and either Rab4 (S22N) or Rab11 (S29N) were treated with 2-AG and ERK1/2 phosphorylation was analyzed by Western blot (Figure 4A). Cells expressing Rab4 (S22N) significantly impaired ERK1/2 activation by 2-AG. However, Rab11 (S29N) had no effect on ERK1/2 activation kinetics or magnitude when compared with controls (Figure 4, A and B). Next, confocal microscopy was utilized to verify the intracellular localization of receptors after agonist treatment. Cells stably expressing CB2R were transfected with either wild-type Rab4, Rab5, or Rab11 constructs tagged with mCherry and treated with 2-AG (Figure 4C). As signaling data from Figure 4A and imaging from Figure 3, E and F, suggested, internalized CB2R was highly localized with Rab4 and Rab5 labeled compartments within 5 min of agonist exposure and continued to colocalize at 15 min. Analysis of multiple cells in independent experiments indicated a high degree of colocalization between internalized receptors with Rab4/5 and only limited colocalization was observed with Rab11 as measured by pearson’s colocalization coefficient (Figure 4D). Taken together, these data indicate that ERK1/2 phosphorylation occurs early during the endocytic trafficking of the CB2Rs at Rab4/5 compartments.
FIGURE 4:
ERK1/2 phosphorylation occurs from CB2R localized at Rab4/5 endosomes. (A) Time course showing ERK1/2 phosphorylation in HEK293 cells stably expressing human CB2R and cotransfected with either Rab4(S22N) or Rab11(S29N) exposed to 10 µM 2-AG. Cell lysates were analyzed using western blots against phospho-ERK1/2 (top panel) and total ERK1/2 (bottom panel). (B) Multiple experiments were quantified and normalized to total ERK1/2 levels. Significant reduction of ERK1/2 phosphorylation was observed in cells expressing Rab4(S22N). Data represent the mean ± SEM at each point (n = 9–10). (C) Confocal imaging from HEK293 cells stably expressing SEP-CB2R and cotransfected with Rab4, Rab5, or Rab11 before and after incubations with 10 µM 2-AG. Each image has a white square depicting a selected region expanded on the right side (size bar = 20 µm). (D) Pearson’s colocalization coefficients for internalized receptors with Rab4, Rab5, and Rab11, were obtained from confocal images at selected time points before and after 10 µM 2-AG addition to the imaging media. (n > 7 cells/colocalization point)
Beta-arrestin 1–mediated ERK1/2 phosphorylation is terminated by the retromer
The retromer is a key component in the intracellular trafficking of transmembrane receptors, controlling their transport from the endosomes to the trans-Golgi network and to the plasma membrane in heterologous and primary cultures (Temkin et al., 2011; Choy et al., 2014; Small and Petsko, 2015). Interestingly, the retromer can also terminate signaling from internalized receptors (Feinstein et al., 2011, 2013). To investigate whether the retromer can terminate signaling mediated by beta-arrestins, we sought to disrupt the expression of VPS29, an essential component of the cargo recognition core in the retromer, by overexpression and separately by silencing technology (Cullen and Korswagen, 2012; Klinger et al., 2015; Small and Petsko, 2015). Cells expressing CB2R were either transfected with siRNA against human VPS29 or with wild-type VPS29 tagged with mCherry. Reduction of VPS29 expression was confirmed by Western blots (Supplemental Figure 1) and resulted in increased ERK1/2 phosphorylation when compared with control cells (Figure 5, A and B). On the contrary, overexpression of VPS29 resulted in a complete inhibition of ERK1/2 phosphorylation (Figure 5, A and B).
FIGURE 5:
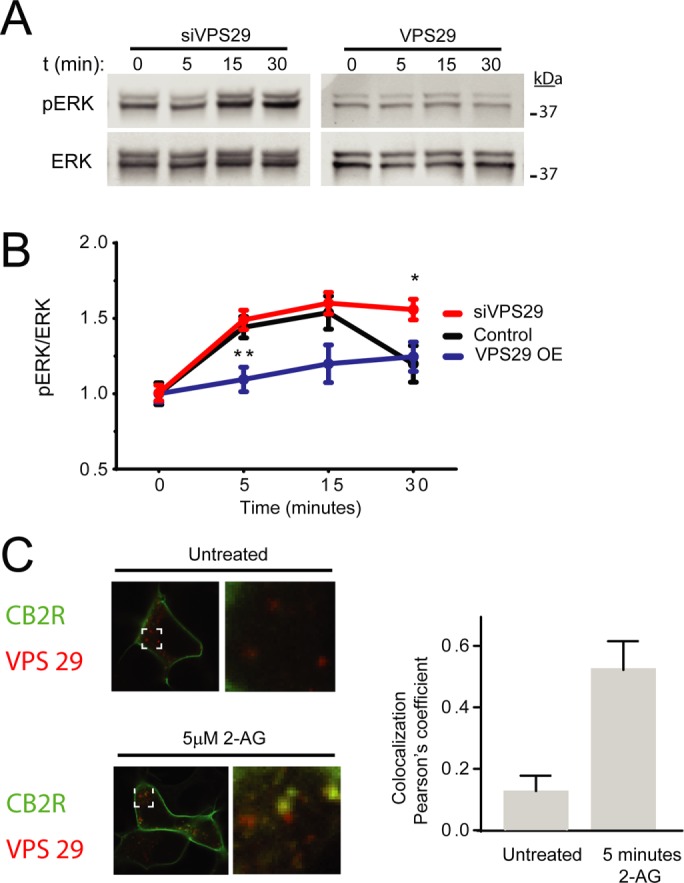
VPS29 impacts beta-arrestin–mediated ERK1/2 activation. (A) Time course showing ERK1/2 phosphorylation in HEK293 cells stably expressing CB2R and cotransfected with either siRNA against VPS29 or wild-type VPS29 in the presence of 10 µM 2-AG. Cell lysates were analyzed by Western blots against phospho-ERK1/2 (top panel) and total ERK1/2 (bottom panel). (B) Quantification of multiple time courses revealed significant effects of VPS29 siRNA and VPS29 overexpression in ERK1/2 phosphorylation. Data represent the mean ± SEM at each point (n = 7–10 independent experiments). (C) Confocal imaging of HEK293 cells stably expressing CB2Rs and transfected with VPS29 mCherry before and after 5 min 10 µM 2-AG. Pearson’s colocalization coefficient was calculated from several independent experiments at 5 min.
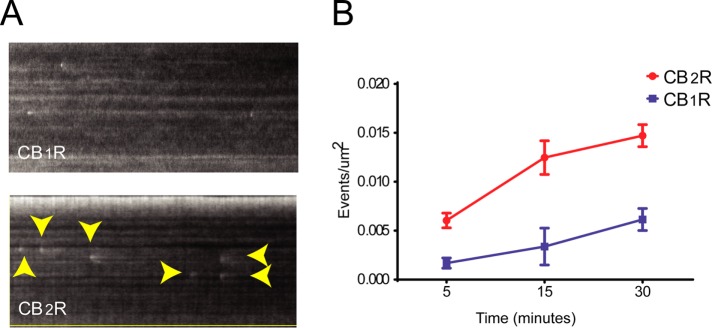
Further support of a VPS29 role during CB2R trafficking was obtained by confocal microscopy. HEK293 cells expressing CB2R were transfected with VPS29 mCherry and incubated with 2-AG. Colocalization between VPS29 and CB2Rs was analyzed by Pearson’s correlation coefficient analysis from untreated and 5-min incubations. Significant increase in colocalization was observed in cells exposed to 2-AG when compared with nontreated (Figure 5C). We noticed that the effect of beta-arrestin siRNA is limited to only 5 min, while dynamin chemical inhibition affects the entire time course. This suggests that other signaling pathways that are independent of beta-arrestins, most likely endosomal G protein signaling, are eliciting ERK phosphorylation. We sought to test this hypothesis by chemically inhibiting Gβγ (Gue1654), Gi, or Go (substance P–related peptide) and Gq/11 (D-Trp7,9,10)-substance P. However, we did not achieve any significant reduction of the observed signaling (Supplemental Figure 3). Finally, we followed CB2R trafficking and investigated if CB2R, after internalization, recycles back to the cell surface. We performed TIRFM to look for individual receptor recycling events as previously described (Flores-Otero et al., 2014) and observed a significant number of CB2R recycling events when compared with CB1R (Figure 6), suggesting a rapid turnover or receptors through the endocytic and recycling pathway back into the cell surface after ligand-induced endocytosis.
FIGURE 6:
CB2R recycling to the cell surface. (A) HEK cells stably expressing SEP-CB2R or SEP-CB1R were incubated with 10 µM 2-AG and imaged under TIRFM at time intervals as described under Materials and Methods. Kymographs depict single cells over time, where individual exocytic events are visible (arrowheads). (B) Individual exocytic events were quantified on multiple individual experiments and normalized to surface area (n = 10–12 cells).
DISCUSSION
GPCR can signal in multiple waves and from different cellular compartments (Irannejad and von Zastrow, 2014; Nogueras-Ortiz and Yudowski, 2016). The β2 adrenergic receptor, parathyroid hormone receptor and luteinizing hormone receptor are only a small example of a growing list of receptors that can signal from the endolysosomal compartment after their ligand-induced internalization (Feinstein et al., 2011; Irannejad et al., 2013; Inda et al., 2016; Lyga et al., 2016). These signaling pathways can be mediated by heterotrimeric G proteins, or by the scaffold beta-arrestins, regulating the activation of kinases and gene expression (Maudsley et al., 2015; Delgado-Peraza et al., 2016a). However, our understanding of the roles and mechanisms controlling the inactivation of these signaling events is currently very limited. Our data show that activation of CB2R by 2-AG leads to the phosphorylation of ERK1/2 via beta-arrestin 1 after receptor internalization at the 5-min time point (Figures 1 and 2). Interestingly, preincubation with PTX or silencing beta-arrestin 2 did not prevent ERK1/2 activation indicating complex signaling events toward ERK1/2 at later time points (10–15 min), possibly mediated by PTX-insensitive G proteins (Figure 7).
FIGURE 7:
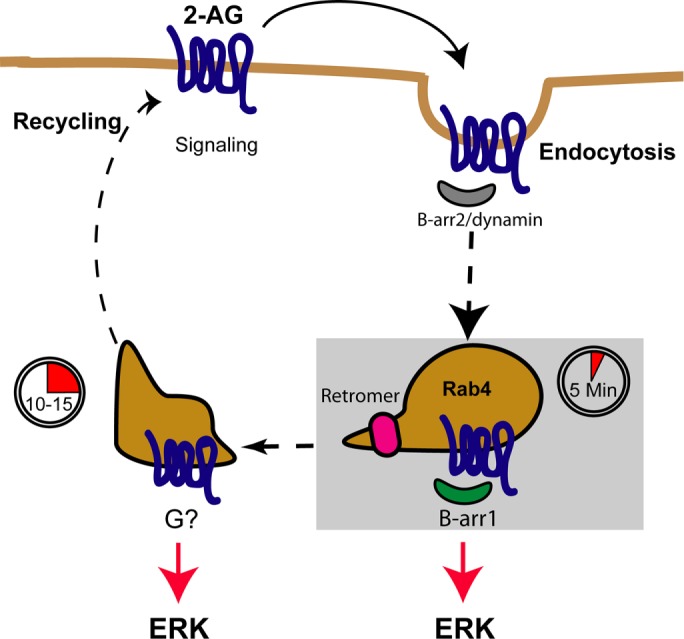
CB2R trafficking and signaling model. Cartoon depicting CB2R signaling at the cell surface. After agonist addition, receptors signal via G proteins and are rapidly recruited into endocytic pits together with beta-arrestin 2 for internalization. Receptor removal from the cell surface by endocytosis requires dynamin and occurs more rapidly than CB1R. After internalization, CB2R colocalizes to Rab4/5 endosomes within 5 min of ligand exposure, resulting in beta-arrestin 1 recruitment and signaling. Beta-arrestin 1–mediated signaling is terminated when receptors traverse the retromer into other endosomal compartment, most likely on their way toward the cell surface for recycling. In this undefined compartment, ERK1/2 is also activated via an unknown mechanism.
Beta-arrestin 2 was robustly recruited to the plasma membrane on receptor activation, likely to mediate receptor desensitization and endocytosis (Figures 1 and 3). Interestingly, beta-arrestin 1 was not recruited to the plasma membrane. In fact, the fluorescence associated with beta-arrestin 1 was decreased on agonist addition, suggesting an active removal of this protein from the plasma membrane. Confocal imaging showed that CB2R colocalized with beta-arrestin 1 at likely Rab4 endosomal compartments (Figures 3 and 4). Taken together, our data support the idea of divergent roles of beta-arrestins as previously observed with CB1R and several other GPCRs (Ahn et al., 2013; Srivastava et al., 2015; Delgado-Peraza et al., 2016a). Endocytosis of the CB2R occurs rapidly as measured by receptor population approaches such as total surface expression. At the single receptor level, endocytosis and recycling of individual CB2R was also extremely fast, especially when compared with other GPCRs (Figures 1G and 6), indicating a fast endocytic trafficking in these cells. Differences in receptor dwell times at individual endocytic pits might reflect different events occurring during the internalization process and not necessarily correlate with endocytic rates. Fast endocytic events (50–100 s) could reflect simple endocytosis while slow events (100–180 s) could reflect not only the removal or receptors but also the interaction of receptors with signaling molecules at the endocytic pits, likely regulating G protein independent signaling pathways.
After internalization, receptors clustered within minutes with beta-arrestin 1 and Rab4/5 endosomes, as analyzed in confocal images (Figures 3 and 4) and as described before for CB2Rs and other GPCRs (Grimsey et al., 2011; Roman-Vendrell et al., 2012). Disruption of these compartments by dominant negative approaches resulted in impaired ERK1/2 phosphorylation by CB2R (Figure 4) likely by the disturbance of normal vesicular functions.
How is beta-arrestin–mediated signaling terminated? Reduction or overexpression of VPS29, a key element in the receptor recognition core at the retromer complex, resulted in increased and impaired signaling respectively (Figure 5). Increase phosphorylation by siRNA against VPS29, might be explained by a prolonged receptor retention at Rab4/5 compartments, increasing signaling from these compartments. Furthermore, reduced ERK1/2 phosphorylation by VPS29 overexpression might result from accelerated expulsion of CB2R from these intracellular compartments toward the plasma membrane or other compartments, where signaling is no longer promoted.
The roles of the retromer complex on GPCR trafficking include the delivery of internalized receptors to the cell surface and the termination of cAMP production (Feinstein et al., 2011; Temkin et al., 2011; Choy et al., 2014; McGarvey et al., 2016). Interestingly, in the case of the parathyroid hormone receptor, results suggested that a complex between beta-arrestin 1 and Gβ1γ2 subunits might regulate the intracellular production of cAMP levels by multiple rounds of G protein activation/inactivation or by stabilizing a sustained coupling to active Gαs. Further studies focusing on the dynamic interplay between receptors and signaling molecules at intracellular compartments should answer these and other key questions of the molecular mechanism underlying intracellular signaling. This work extends the functional roles of the retromer to blunting signaling mediated by beta-arrestins from CB2R and identifies a G protein-dependent ERK activation that also requires receptor internalization, further strengthening the notion of endosomal signaling from GPCRs (Irannejad and von Zastrow, 2014) (Figure 7). Further work should focus on identifying the mechanisms sustaining ERK activation after beta-arrestin dissociation and the intracellular signaling pathways in a more physiological cellular context.
MATERIALS AND METHODS
SEP-CB2R construction and saturation binding assays
Super-ecliptic (GenBnk: AY533296.1) human CB2R (GenBank ID:1269/NM_001841.2) was synthetized by Genescript with a linker sequence (CCCATACGATGTTCCAGATTACGCT) and subcloned into pCNA3.1(+) at the Kpnl and BamHI sites. To determine the Kd, a saturation binding assay was performed on a membrane preparation of HEK293 cells transfected with human SEP-CB2R essentially as described (D’Antona et al., 2006). Total binding involved using nine different radiolabeled [3H] CP55,940 concentrations (0 nM–20 nM). Nonspecific binding was determined with 10 μM unlabeled CP55,940. Binding was terminated by subsequent filtration through Whatman GF/C filter paper with a Brandel cell harvester and radioactivity was measured. Each saturation assay was carried out in duplicate, and three independent experiments were performed. Data were analyzed by nonlinear regression using Prism 6.0 (Graphpad Software, San Diego, CA). The value determined, Kd = 2.9 ± 1.3 nM is comparable to the wild-type CB2R (Pertwee et al., 2010).
Cell culture and generation of a stable SEP-CB2R HEK293 cell line and other reagents
Human embryonic kidney cells (HEK293 cells) were obtained from the American Type Culture Collection (Manassas, VA) and maintained at 37°C and 5% CO2 in complete DMEM, supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, 2 mM glutamine, and 1 mM sodium pyruvate (Thermo Fisher Scientific, Waltham, MA). HEK293 cells were transfected with SEP-hCB2R_pCDNA3.1(+) using Effectene (Qiagen, Valencia, CA) and harvested under G418 selection (Thermo Fisher Scientific) according to manufacturers’ instructions. Selection was assessed using the Cellometer Vision CBA Image Cytometer (Nexcelom Bioscience, Lawrence, MA). Rab4, Rab11, and WT-VPS29 constructs were gifts from Mark von Zastrow (University of California, San Francisco). siRNA against dynamin2 (GS1785), VPS29 (GS51699), beta-arrestin 1 (GS408), and 2 (GS409) were purchased from Qiagen. Transfection of mammalian plasmids and siRNAs was carried out using Lipofectamine (Thermo Fisher Scientific) according to the manufacturer’s instructions. Gene silencing was confirmed using the following antibodies: anti-dynamin (cat. no. 17807; Santa Cruz Biotechnology, Santa Cruz, CA), anti-VPS29 (cat. no. NBP1-85288; Novus Biologicals, Littleton, CO), anti-β-arrestin 1 (cat. no. NB110-55485; Novus Biologicals), anti-β-arrestin 2 (cat. no. NBP2-24569; Novus Biologicals), and anti-GAPDH (cat. no. 32233; Santa Cruz Biotechnology) as loading control.
ERK 1/2 phosphorylation assay
Immunoblots were carried out as previously described with modifications detailed bellow (Delgado-Peraza et al., 2016a). SEP-hCB2R HEK293 cells with a passage number of 3–25 were plated for 2 d prior to serum deprivation carried over night. To observe the effects of PTX, overnight serum starvation was carried out using serum-free complete media supplemented with 10 ng/ml PTX. Afterward, cells were subjected to treatment with 10 µM 2-arachidonoylglycerol (2-AG; Tocris Bioscience, Minneapolis, MN) as indicated in the figures. Prior to treatment with 2-AG, cells were incubated with either 5 µM of a Gi/o/s antagonist peptide (Tocris; cat. no. 143675-79-0), 25 µM of the Gβλ inhibitor Gue 1654 (Tocris; cat. no. 397190-30-1) or 25 µM of the Gq inhibitor [D-Trp7,9,10]-Substance P (Tocris; cat. no. 89430-38-6) for 1 or 24 h for experiments depicted on Supplemental Figure 3. To stop CB2R activation, medium was aspirated and replaced with 100 µl of ice-cold radioimmunoprecipitation assay buffer (RIPA; Sigma-Aldrich, St. Louis, MO) supplemented with 5 mM sodium pyrophosphate, 30 mM β-glycerol phosphate, 30 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride (all organic compounds were obtained from Sigma-Aldrich), and 1× protease/phosphatase inhibitor cocktail (Cell Signaling Technology, Danvers, MA). Protein were resolved in 10% polyacrylamide gels prior to transference to nitrocellulose membranes. Antibody nonspecific binding was blocked with Odyssey Blocking Buffer (Licor, Lincoln, NE). Membranes were incubated overnight at room temperature with a 1:500 dilution of phosphoERK1/2 (pERK) antibody (cat. no. 9101; Cell Signaling Technology) in blocking solution. pERK bands were visualized using the Odyssey Imaging System (Licor). Membrane were stripped and reprobed using a 1:500 dilution of ERK antibody for total ERK (cat. no. 4695; Cell Signaling Technology). ERK activation was assessed by normalizing the optical density of pERK bands to that of total ERK (Image Studio, Licor). Data are expressed as a fold change over the basal level of phosphorylation.
CB2R internalization
SEP-CB2R HEK293 cells were exposed to 10 µM 2-AG for 5, 15, and 30 min. At the end of treatment, ice-cold phosphate-buffered saline (PBS; Thermo Fisher Scientific) was added to cells and quickly replaced with 4% paraformaldehyde (PFA; Electron Microscopy Sciences, Hatfield, PA) in PBS. Cells were incubated in PFA for 15 min at room temperature and then washed twice with PBS before confocal fluorescence microscopy analysis using a Nikon A1R Eclipse Ti-E inverted microscope with a N Apo 60× oil immersion objective (Nikon, Melville, NY). The NIS Elements Advanced Research Microscope Imaging Software (Nikon) was used for image acquisition. Fluorescence intensity analysis from the equatorial plane was carried out using the Fiji imaging processing software (ImageJ, University of Wisconsin–Madison, Madison, WI).
Kinetics of CB2R endocytosis and recycling by TIRF.
To assess the kinetic properties of CB2R internalization, SEP-hCB2R HEK293 cells were subjected to total internal reflection fluorescence microscopy (TIRF) as described elsewhere (Yudowski and von Zastrow, 2011; Delgado-Peraza et al., 2016b). Briefly, cells seeded in PDL-coated 35-mm glass-bottom dishes (MatTek, Ashland, MA) were preincubated with Opti-MEM supplemented with 20 mM HEPES. Cells were placed on a stage warmer and agonist was added to the media. Imaging was performed utilizing a motorized Nikon Ti-E inverted microscope coupled to a CFI-Apo 100× 1.49 oil TIRF objective lens with color correction, a motorized stage with perfect focus and an iXonEM + DU897 back illuminated electron-multiplying charge-coupled device (EMCCD) camera (Andor, Belfast, UK). A 488-nm sapphire laser of 50 mW was used as light source (Coherent, Santa Clara, CA). Total time of live-imaging visualization and recording was less than 30 min. Recycling events were imaged at 10-Hz acquisition rate, whereas endocytosis was performed at 0.3 Hz. Analysis of fluorescence intensities was carried out using the Fiji imaging processing software (ImageJ, University of Wisconsin–Madison, Madison, WI).
In vivo colocalization studies.
To assess the localization of intracellular CB2R with respect to vesicle trafficking markers, HEK293 cells were cotransfected with SEP-hCB2R RFP-Rab4, RFP-Rab5, RFP-Rab11, or RFP-VPS29 using Effectene (Qiagen). After 12 h of transfection, cells were seeded in 35-mm glass-bottom dishes (MatTek) coated with 0.5 mg/ml PDL (Sigma-Aldrich). At a time 24–48 h postplating, media were changed to Opti-MEM supplemented with 20 mM HEPES (Thermo Fisher Scientific) and imaged by confocal fluorescence microscopy under a temperature controlled environment at ∼37°C (Stable “Z” Micro-Environmental Warming System; Bioptechs, Butler, PA). After 2 min of in vivo fluorescence assessment under control conditions, cells were treated with either vehicle or 5 μM 2-AG for 5 and 15 min followed membrane permeabilization with 0.01% Triton X-100 (Sigma-Aldrich) in order to reveal intracellular SEP-hCB2R. Fluorescence colocalization between SEP and RFP fluorophores was assessed using the Fiji imaging processing software by selecting intracellular ROI (ImageJ, University of Wisconsin–Madison, Madison, WI).
Statistical analyses
Creation of graphs and statistical analyses were done using GraphPad Prism (GraphPad Software, San Diego, CA). Student’s t test was used to assess statistical differences among compared groups. Results were labeled *p < 0.005; **p < 0.01; ***p < 0.001.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH) to G.A.Y. (DA03792) and to D.A.K. and G.A.Y. (DA040920), National Institute of Minority Health and Health Disparities 8G12-MD007600 (RCMI), NIH National Institute of General Medical Sciences 1P20GM103642, and National Science Foundation DBI-1337284 to G.A.Y. and C.N.O., and NIH MBRS-RISE GM061838 to C.R.V.
Abbreviations used:
- 2-AG
2-arachidonoylglycerol
- CB2R
cannabinoid 2 receptor
- EMCCD
electron-multiplying charge-coupled device
- ERK1/2
extracellular signal-regulated protein kinases 1 and 2
- GPCR
G protein–coupled receptor
- PDL
poly-d-lysine
- PTX
pertussis toxin
- RFP
monomeric red fluorescent protein
- RIPA
radioimmunoprecipitation assay buffer
- SEP
super-ecliptic phluorin
- ∆9-THC
∆9-tetrahydrocannabinol.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E17-03-0198) on September 27, 2017.
REFERENCES
- Ahn KH, Mahmoud MM, Shim J-Y, Kendall DA. Distinct roles of β-arrestin 1 and β-arrestin 2 in ORG27569-induced biased signaling and internalization of the cannabinoid receptor 1 (CB1) J Biol Chem. 2013;288:9790–800. doi: 10.1074/jbc.M112.438804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Wager-Miller J, Haskins C, Straiker A, Mackie K. Functional selectivity in CB2 cannabinoid receptor signaling and regulation: implications for the therapeutic potential of CB2 ligands. Mol Pharmacol. 2012;81:250–263. doi: 10.1124/mol.111.074013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, Tolón RM, Pazos MR, Núñez E, Castillo AI, Romero J. Cannabinoid CB2 receptors in human brain inflammation. Br J Pharmacol. 2008;153:277–285. doi: 10.1038/sj.bjp.0707505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrié B, Rinaldi-Carmona M, Calandra B, Le Fur G, Casellas P. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Eur J Biochem. 1996;237:704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- Brailoiu GC, Deliu E, Marcu J, Hoffman NE, Console-Bram L, Zhao P, Madesh M, Abood ME, Brailoiu E. Differential activation of intracellular versus plasmalemmal CB2 cannabinoid receptors. Biochemistry. 2014;53:4990–4999. doi: 10.1021/bi500632a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zheng C, Qian J, Sutton SW, Wang Z, Lv J, Liu C, Zhou N. Involvement of β-arrestin-2 and clathrin in agonist-mediated internalization of the human cannabinoid CB2 receptor. Curr Mol Pharmacol. 2014;7:67–80. doi: 10.2174/1874467207666140714115824. [DOI] [PubMed] [Google Scholar]
- Choy RWY, Park M, Temkin P, Herring BE, Marley A, Nicoll RA, Von Zastrow M. Retromer mediates a discrete route of local membrane delivery to dendrites. Neuron. 2014;82:55–62. doi: 10.1016/j.neuron.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PJ, Korswagen HC. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat Cell Biol. 2012;14:29–37. doi: 10.1038/ncb2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antona AM, Ahn KH, Wang L, Mierke DF, Lucas-Lenard J, Kendall DA. A cannabinoid receptor 1 mutation proximal to the DRY motif results in constitutive activity and reveals intramolecular interactions involved in receptor activation. Brain Res. 2006;1108:1–11. doi: 10.1016/j.brainres.2006.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Peraza F, Ahn KH, Nogueras-Ortiz C, Mungrue IN, Mackie K, Kendall DA, Yudowski GA. Mechanisms of biased β-arrestin-mediated signaling downstream from the cannabinoid 1 receptor. Mol Pharmacol. 2016a;89:618–629. doi: 10.1124/mol.115.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Peraza F, Nogueras-Ortiz C, Canabal AMAcevedo, Roman-Vendrell C, Yudowski GA. Imaging GPCRs trafficking and signaling with total internal reflection fluorescence microscopy in cultured neurons. Methods Cell Biol. 2016b;132:25–33. doi: 10.1016/bs.mcb.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Dhopeshwarkar A, Mackie K. CB2 cannabinoid receptors as a therapeutic target—what does the future hold. Mol Pharmacol. 2014;86:430–437. doi: 10.1124/mol.114.094649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein TN, Wehbi VL, Ardura JA, Wheeler DS, Ferrandon S, Gardella TJ, Vilardaga J-P. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat Chem Biol. 2011;7:278–284. doi: 10.1038/nchembio.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein TN, Yui N, Webber MJ, Wehbi VL, Stevenson HP, King JD, Hallows KR, Brown D, Bouley R, Vilardaga JP. Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J Biol Chem. 2013;288:27849–27860. doi: 10.1074/jbc.M112.445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- Flores-Otero J, Ahn KH, Delgado-Peraza F, Mackie K, Kendall DA, Yudowski GA. Ligand-specific endocytic dwell times control functional selectivity of the cannabinoid receptor 1. Nat Commun. 2014;5:4589. doi: 10.1038/ncomms5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsey NL, Goodfellow CE, Dragunow M, Glass M. Cannabinoid receptor 2 undergoes Rab5-mediated internalization and recycles via a Rab11-dependent pathway. Biochim Biophys Acta. 2011;1813:1554–1560. doi: 10.1016/j.bbamcr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. The molecular acrobatics of arrestin activation. Trends Pharmacol Sci. 2004;25:105–111. doi: 10.1016/j.tips.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Inda C, Dos Santos Claro PA, Bonfiglio JJ, Senin SA, Maccarrone G, Turck CW, Silberstein S. Different cAMP sources are critically involved in G protein-coupled receptor CRHR1 signaling. J Cell Biol. 2016;214:181–195. doi: 10.1083/jcb.201512075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, Rasmussen SGF, Sunahara RK, El-Samad H, Huang B, von Zastrow M. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R, von Zastrow M. GPCR signaling along the endocytic pathway. Curr Opin Cell Biol. 2014;27:109–116. doi: 10.1016/j.ceb.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall DA, Yudowski GA. Cannabinoid receptors in the central nervous system: their signaling and roles in disease. Front. Cell Neurosci. 2017;10:294. doi: 10.3389/fncel.2016.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger SC, Siupka P, Nielsen MS. Retromer-mediated trafficking of transmembrane receptors and transporters. Membranes (Basel) 2015;5:288–306. doi: 10.3390/membranes5030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier C, Bonnard D, Carrel D, Rossier J, Lenkei Z. Constitutive endocytic cycle of the CB1 cannabinoid receptor. J Biol Chem. 2004;279:36013–3621. doi: 10.1074/jbc.M403990200. [DOI] [PubMed] [Google Scholar]
- Lyga S, Volpe S, Werthmann RC, Götz K, Sungkaworn T, Lohse MJ, Calebiro D. Persistent cAMP signaling by internalized LH receptors in ovarian follicles. Endocrinology. 2016;157:1613–1621. doi: 10.1210/en.2015-1945. [DOI] [PubMed] [Google Scholar]
- Maudsley S, Martin B, Gesty-Palmer D, Cheung H, Johnson C, Patel S, Becker KG, Wood WH, Zhang Y, Lehrmann E, Luttrell LM. Delineation of a conserved arrestin-biased signaling repertoire in vivo. Mol Pharmacol. 2015;87:706–717. doi: 10.1124/mol.114.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarvey JC, Xiao K, Bowman SL, Mamonova T, Zhan Q, Bisello A, Bruce Sneddon W, Ardura JA, Jean-Alphonse F, Vilardaga JP, et al. Actin-sorting nexin 27 (SNX27)-retromer complex mediates rapid parathyroid hormone receptor recycling. J Biol Chem. 2016;291:10986–11002. doi: 10.1074/jbc.M115.697045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueras-Ortiz C, Yudowski GA. The multiple waves of cannabinoid 1 receptor signaling. Mol Pharmacol. 2016;90:620–626. doi: 10.1124/mol.116.104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid receptors and pain. Progr Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SPH, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB 1 and CB 2. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman-Vendrell C, Yu YJJ, Yudowski GA. Fast modulation of μ-opioid receptor (MOR) recycling is mediated by receptor agonists. J Biol Chem. 2012;287:14782–14791. doi: 10.1074/jbc.M111.319616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JL, Ruckle MB, Mayeux PR, Prather PL. Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. J Pharmacol Exp Ther. 2005;315:828–838. doi: 10.1124/jpet.105.089474. [DOI] [PubMed] [Google Scholar]
- Small SA, Petsko GA. Retromer in Alzheimer disease, Parkinson disease and other neurological disorders. Nat Rev Neurosci. 2015;16:126–132. doi: 10.1038/nrn3896. [DOI] [PubMed] [Google Scholar]
- Soethoudt M, Grether U, Fingerle J, Grim TW, Fezza F, de Petrocellis L, Ullmer C, Rothenhäusler B, Perret C, van Gils N, et al. Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat Commun. 2017;8:13958. doi: 10.1038/ncomms13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Gupta B, Gupta C, Shukla AK. Emerging functional divergence of β-arrestin isoforms in GPCR function. Trends Endocrinol Metab. 2015;26:628–642. doi: 10.1016/j.tem.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Temkin P, Lauffer B, Jäger S, Cimermancic P, Krogan NJ, von Zastrow M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol. 2011;13:717–723. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Yudowski GA, von Zastrow M. Investigating G protein-coupled receptor endocytosis and trafficking by TIR-FM. Methods Mol Biol. 2011;756:325–332. doi: 10.1007/978-1-61779-160-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Kolaj M, Renaud LP. Endocannabinoid 2-AG and intracellular cannabinoid receptors modulate a low-threshold calcium spike-induced slow depolarizing afterpotential in rat thalamic paraventricular nucleus neurons. Neuroscience. 2016;322:308–319. doi: 10.1016/j.neuroscience.2016.02.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.