Abstract
Human retinoblastomas are malignant intraocular tumors and have a high incidence in children. Chemotherapy combined with local therapy is the principal means of retinoblastoma treatment, the application of which has saved the eye of many children and avoided external irradiation. UNBS5162, a naphthalimide, has broad prospects as a tumor treatment, with fewer toxic side effects and higher cancer-suppression efficiency. However, the efficacy of UNBS5162 in human retinoblastomas is still not clear. In the present study, we investigated the specific mechanism of UNBS5162 in the human retinoblastoma cell lines WERIRb1 and Y79. Compared with a negative-control (NC) group, UNBS5162 treatment for 72 hours significantly decreased cell proliferation; meanwhile, more apoptotic cells were observed in the UNBS5162-treated group (27.1% in WERIRb1, 20.83% in Y79) than in the NC group (11.59% in WERIRb1, 12.89% in Y79). We also found caspase 3 p17 and Bax expression to be upregulated and Bcl2 downregulated significantly in UNBS5162-treated WERIRb1 and Y79 cells. The effects of UNBS5162 on human retinoblastoma cells may be regulated by the Akt–mTOR pathway. We found expression of the Akt pathway and key proliferation-related genes – those for p-Akt, p-mTOR, p70, and cyclin D1 – were downregulated significantly in the UNBS5162-treated group compared with the NC group in WERIRb1 and Y79. Therefore, for the first time, we demonstrated that UNBS5162 can inhibit proliferation and promote apoptosis of human retinoblastoma cells by regulating activity of the Akt–mTOR pathway in vitro, suggesting the potential value of UNBS5162 in treatment for human retinoblastoma.
Keywords: UNBS5162, human retinoblastoma, cell apoptosis, WERIRb1, CXCL
Introduction
Human retinoblastomas are malignant intraocular tumors and have a high incidence in children. Retinoblastoma treatment is based primarily on surgery and chemotherapy. Although the curative effect and survival rate of children have been considerably improved, retinoblastoma is still a serious threat to the health of children worldwide.1 At the same time, the therapeutic principle of retinoblastoma is not only confined to save life and keep the eye but also to save the effective eyesight of the children as much as possible. Chemotherapy combined with local treatment is the principal means of treatment of retinoblastoma, and its application has saved many children’s eyeballs and avoided external irradiation.2,3 However, chemotherapy drugs have more limitations, due to toxicity, side effects, drug resistance, changes in the disease spectrum, and the emergence of drug-induced diseases. New antitumor drugs provide a new approach for the treatment of human retinoblastoma.4
Naphthalimides, as a class of DNA intercalating agent, have been used widely in antitumor pharmaceutical research. At present, the naphthalimide compounds amonafide, mitonafide, UNBS5162, elinafide (LU79553), and bisnafide (DMP840) have been evaluated clinically.5–7 Previous studies have shown that UNBS5162 has broad prospects as a tumor treatment with less toxic side effects and higher cancer-suppression efficiency.8 However, the efficacy of UNBS5162 in retinoblastoma remains unknown.
In the present study, we demonstrated that UNBS5162 inhibited proliferation and promoted apoptosis of human retinoblastoma cells. The aim of the current study was to confirm the overall mechanism of action of UNBS5162 in human retinoblastoma in vitro, emphasizing the potential of UNBS5162 to combat human retinoblastoma.
Materials and methods
Cell lines and cell cultures
The human retinoblastoma cell lines WERIRb1 and Y79 were purchased from the type-culture collection of the Chinese Academy of Sciences, Shanghai, China. WERIRb1 cells were maintained in DMEM supplemented with 10% fetal bovine serum, penicillin (100 units/mL), and streptomycin (100 units/mL; Thermo Fisher Scientific, Waltham, MA, USA) at 37°C in a humidified atmosphere with 5% CO2.
Compounds and antibodies
UNBS5162 was obtained from MedChemExpress (Princeton, NJ, USA). All experiments were performed using 10 μM UNBS5162 as the work concentration. Reference antibodies were obtained: rabbit antihuman Akt, p-Akt, mTOR, p-mTOR (Cell Signaling Technology, Danvers, MA, USA), rabbit antihuman p70, Bcl2, Bax, caspase 3 p17, mouse antihuman cyclin D1, and GAPDH (ProteinTech Wuhan, China). The electrochemiluminescence kit was also purchased from ProteinTech.
Drug-sensitivity assay
Cells were harvested in the exponential phase. Single-cell suspensions were prepared and dispersed in 96-well plates at 2,000 cells/well. Nine gradients of UNBS5162 were prepared: 0.625, 1.25, 2.5, 5, 8, 10, 25, 50, and 100 μM. Three duplicates were used for each determination. After incubation with UNBS5162 for 48 hours, 10 μL of CCK-8 solution was added to each well, the plates were incubated at 37°C for 2 hours, and absorbance of each well was measured at 450 nm using a multimode reader. The IC50 value was defined as the concentration of drugs required for a 50% inhibition rate relative to the controls.
Cell-proliferation assay
Cellular proliferation was confirmed by CCK-8 assay. WERIRb1 cells were plated in a 96-well plate at a concentration of 1,000 cells per well. The cells were divided into two groups: negative control (NC) group and UNBS5162. The cell-proliferation assay was performed: 10 μL CCK-8 solution was added to each well, and the plate was then incubated at 37°C for 2 hours. Absorbance was measured at a wave length of 450 nm using an enzyme-labeling instrument.
Protein extraction and Western blotting
Cells were washed once with PBS and lysed in radioimmunoprecipitation-assay buffer. Total protein (44 μg) from each sample was resolved by 15% sodium dodecyl sulfate polyacrylamide-gel electrophoresis, transferred to a polyvinylidene difluoride membrane, and analyzed by Western blotting. The membrane was blocked by 5% fat-free milk in TBS containing 0.1% Tween 20 for 1 hour at room temperature and then incubated with primary antibodies at a dilution of 1:1,000 for 2 hours with secondary antibodies at a dilution of 1:5,000 for 1 hour. The assay was repeated three times. Quantification results were analyzed using ImageJ. The relative expression levels of proteins were calculated by determining a ratio between the amount of target protein and GAPDH.
Flow-cytometry analysis for apoptosis determination
After incubation with UNBS5162 for 24 hours, WERIRb1 cells were maintained in serum-free medium to starve for 24 hours under normal conditions. Cells were made into a suspension with binding buffer at 1–5×106 cells/mL. Annexin V–fluorescein isothiocyanate/propidium iodide was added to cell suspensions, followed by incubation in the dark for 5 minutes at room temperature. After 10 μL dye liquor had been added, samples were detected by flow cytometry and analyzed with FlowJo software.
Statistical analyses
Date are expressed as means ± SD and analyzed using SPSS version 17 (SPSS, Chicago, IL, USA). Statistical analyses were performed using Student’s t-test to analyze changes in experimental results between the groups. Differences with P<0.05 were considered significant.
Results
UNBS5162 inhibited proliferation of human retinoblastoma cells
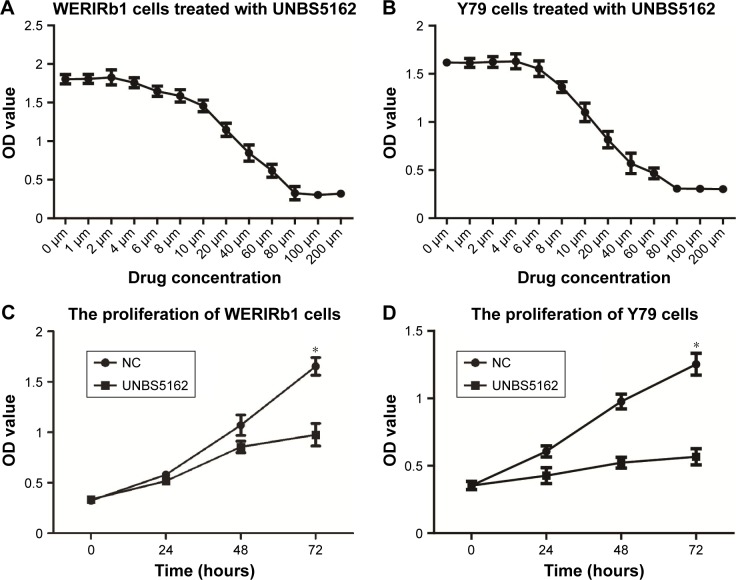
Concentrations of UNBS5162 treatment were determined by drug-sensitivity assays. As shown in Figure 1A and B, with increased UNBS5162 concentration, WERIRb1 and Y79 cell proliferation gradually decreased. IC50 values were analyzed using SPSS, and 16.5 μM was determined to be the IC50 of UNBS5162 treatment.
Figure 1.
UNBS5162 inhibited cell proliferation of human retinoblastoma cell lines.
Notes: (A, B) Concentration of UNBS5162 treatment was determined by drug sensitivity assay; 16.5 μM was the IC50 value of UNBS5162 treatment. (C, D) Proliferation of WERIRb1 and Y79 examined by CCK8 assay. Compared with the negative-control (NC) group, cell proliferation in the UNBS5162-treated group decreased and showed significant differences at 72 hours in these two cell lines (*P<0.05).
To investigate the effect of UNBS5162 on cellular proliferation, WERIRb1 and Y79 cells were treated with NUBS5162 for 72 hours and cell proliferation measured by CCK-8 assays. The viability of WERIRb1 and Y79 cells treated with UNBS5162 decreased significantly compared to the NC group. After 72 hours, the OD value of the UNBS5162-treated group was 0.93±0.08, significantly decreased compared with the NC group (1.68±0.05) in WERIRb1. Meanwhile, in Y79 cells the OD value in the UNBS5162-treated group (0.45±0.05) also decreased significantly compared with the NC group (1.22±0.05) (Figure 1B). These results suggested that UNBS5162 significantly prevented proliferation of human retinoblastoma cells.
UNBS5162 promoted apoptosis of human retinoblastoma cells
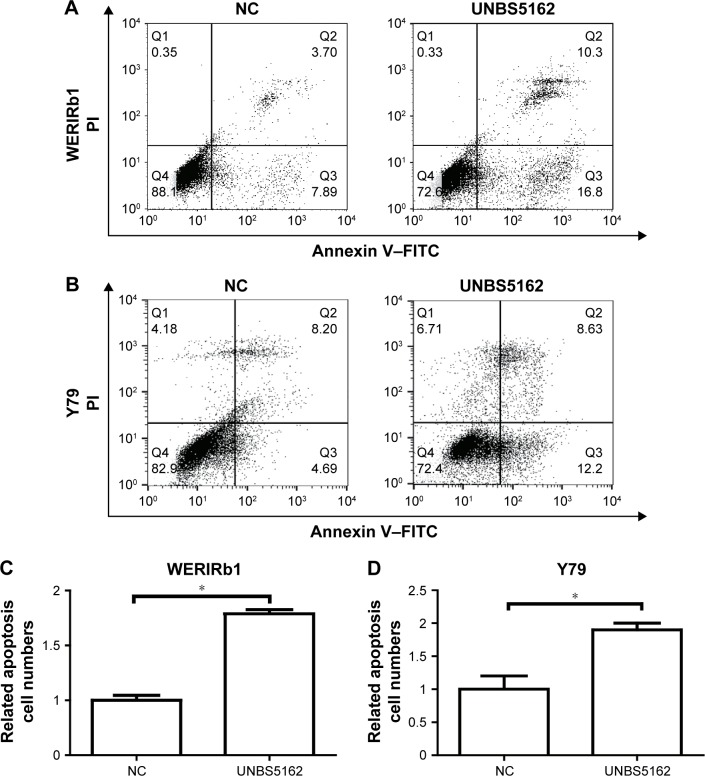
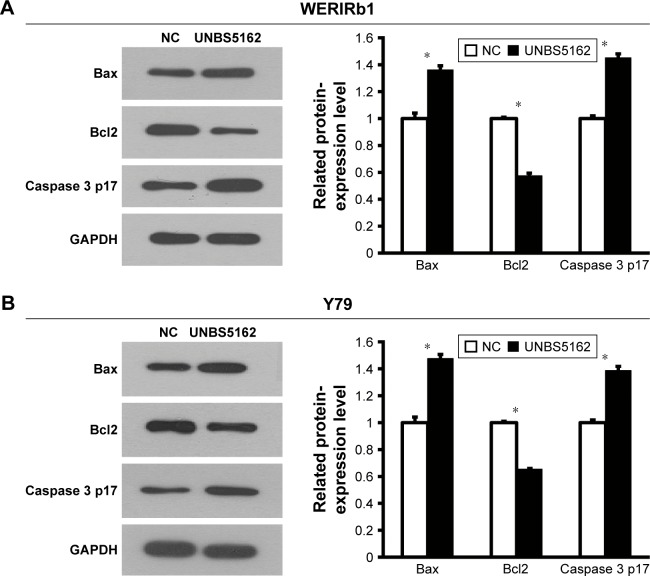
To corroborate the role of UNBS5162 on apoptosis of human retinoblastoma cells, WERIRb1 and Y79 cells were treated with UNBS5162 and apoptotic cells in the NC and UNBS5162 groups examined by flow cytometry. The results demonstrated that there were more apoptotic cells in the UNBS5162-treated group (27.1% in WERIRb1, 20.83% in Y79) than in the NC group (11.59% in WERIRb1, 12.89% in Y79) (Figure 2). To confirm the effect of UNBS5162 on cell apoptosis of retinoblastoma cells further, we investigated the expression of apoptosis related genes – those for Bax, Bcl2, and caspase 3 p17 – in WERIRb1 and Y79. As shown in Figure 3, compared with the NC group, caspase 3 p17 and Bax expression were both upregulated and the apoptosis suppressor Bcl2 was downregulated significantly in WERIRb1 and Y79 (Figure 3). These results suggested that UNBS5162 induced apoptosis of human retinoblastoma cells.
Figure 2.
UNBS5162 promoted cell apoptosis of human retinoblastoma cell lines.
Notes: (A, B) Apoptotic cell numbers in negative-control (NC) and UNBS5162-treated groups were examined by flow cytometry. (C, D) Numbers of apoptotic cells in the UNBS5162-treated group (27.1% in WERIRb1, 20.83% in Y79) were significantly higher than in the NC group (11.59% in WERIRb1, 12.89% in Y79; *P<0.05).
Abbreviations: FITC, fluorescein isothiocyanate; PI, propidium iodide.
Figure 3.
UNBS5162 affected the expression of apoptosis-related genes in human retinoblastoma cell lines.
Notes: Western blot was used to examine the expression of apoptosis-related genes in the negative-control (NC) and UNBS5162-treated groups. As a result, compared with the NC group, the expression of Caspase 3 p17 and Bax were upregulated and Bcl2 downregulated significantly in WERIRb1 (A) and Y79 (B) cells (*P<0.05).
UNBS5162 inhibited proliferation of human retinoblastoma cells through Akt–mTOR pathway
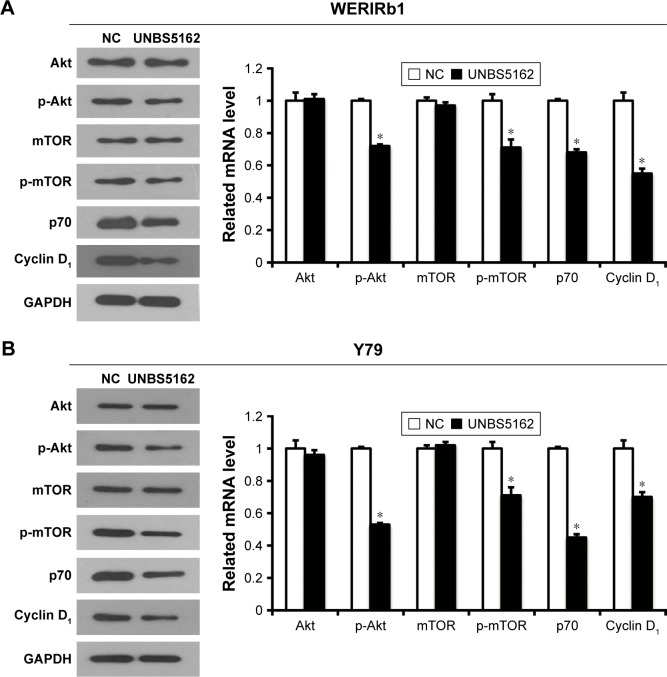
The Akt–mTOR pathway has been shown to be involved in the regulation of numerous cellular functions, including proliferation and apoptosis; however, the effect of UNBS5162 on this pathway in human retinoblastoma has not been reported previously. To corroborate the effect of UNBS5162 on the Akt–mTOR pathway further, we investigated the expression level of related genes by Western blotting in WERIRb1 and Y79 cells. The results demonstrated that p-Akt and p-mTOR were downregulated significantly in the UNBS5162-treated group compared with those in the NC group, while Akt- and mTOR-expression levels were identical. Meanwhile, we detected the expression level of cellular proliferation related genes – those for p70 and cyclin D1 – in the UNBS5162 and NC groups. As shown in Figure 4, p70 and cyclin D1 expression were decreased significantly in the UNBS5162 group compared with the NC group in both WERIRb1 and Y79 cells. These results suggested that UNBS5162 may influence the cell viability of human retinoblastoma cells through the Akt–mTOR pathway.
Figure 4.
UNBS5162 inhibited proliferation of human retinoblastoma cells through the Akt–mTOR pathway.
Notes: Following Western blot, expression of Akt-pathway and key proliferation-related genes – those for p-Akt, p-mTOR, p70, and cyclin D1 – was found to be downregulated significantly in the UNBS5162-treated group compared with the negative-control (NC) group in WERIRb1 (A) and Y79 (B) cells (*P<0.05).
Discussion
In recent years, with rapid development in modern biotechnology, life science has entered the era of molecular biology, leading to the development of related disciplines.9 Because of the difference between the DNA in cancer and normal cells, DNA has been an ideal target for antitumor drugs. DNA-embedding agents play an important role in tumor treatment, and the application of naphthalimides has been a focus of tumor research in recent years.10 Braña et al synthesized a series of naphthalimide derivatives. These compounds have been proved to be effective in embedding DNA, increasing DNA length, and unwinding superhelix DNA.11,12 Among naphthalimide derivatives, representative drugs have entered clinical trials, including mitonafide, amonafide, and UNBS5162.13–15 These compounds can be inserted into DNA to inhibit the synthesis of DNA, RNA, and topoisomerase II activity.16 However, in clinical trials, mitonafide and amonafide have significant side effects in the central nervous system, and these two drugs have limited clinical efficacy for solid tumors. Notably, UNBS5162 works better and has less toxic side effects according to clinical trials, while less research has been performed in human retinoblastoma tumors.
For the first time, we demonstrated that UNBS5162 inhibited proliferation and promoted apoptosis of human retinoblastoma cells by regulating the activity of the Akt–mTOR pathway. UNBS5162 is a novel naphthalimide, and can decrease CXCL chemokine expression in cancers.17–19 In a recent report, a Phase I study of UNBS5162 was conducted to establish pharmacokinetics, maximum tolerated dose, dose-limiting toxicity, safety, and antitumor activity in patients with advanced solid tumors or lymphoma. A total of 24 patients with metastatic carcinoma and one with lymphoma were treated with UNBS5162 at eight dose levels (18–234 mg/mL). Grade 3 toxicities were observed in three cases, and QTc prolongation was observed in six cases.20 In vivo UNBS5162 after repeat administration significantly increased survival rate in orthotopic human prostate cancer models, and in vitro exposure of the prostate cancer cell line PC3 to UNBS5162 dramatically decreased the expression of proangiogenic CXCL chemokines.8 Chemokines are proinflammatory cytokines, which are chemotactic for immune cells and operate as cytokines through binding to transmembrane G-protein-coupled receptors.21 The interaction of chemokines and their receptors can induce chemotactic migration and cytoskeletal rearrangement of target cells and enhance the adhesion ability of endothelial cells.22–24 Previous studies have shown that CXCL is involved in many biological functions, such as cell growth, apoptosis, and angiogenesis, and plays an important role in the development and progression of tumors.25–27 Chemokines can also regulate expression of the Rb protein, the gene for which is critically mutated in the pathogenesis of retinoblastoma. Totonchy et al found a novel mechanism for CXCR7-mediated proliferation via proteasomal degradation of Rb.28 Khan et al found that CXCL12 could increase Rb protein and RNA levels in rat cortical neurons and stimulate Rb activity as a transcription repressor.29
In conclusion, UNBS5162 inhibited proliferation and promoted apoptosis of human retinoblastoma cells by regulating activity of the Akt–mTOR pathway. The present results suggest that UNBS5162 is a potential therapeutic agent in retinoblastoma that requires further experimental study to validate its curative effect.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Benavente CA, Dyer MA. Genetics and epigenetics of human retinoblastoma. Annu Rev Pathol. 2015;10:547–562. doi: 10.1146/annurev-pathol-012414-040259. [DOI] [PubMed] [Google Scholar]
- 2.Abramson DH, Shields CL, Munier FL, Chantada GL. Treatment of retinoblastoma in 2015: agreement and disagreement. JAMA Ophthalmol. 2015;133(11):1341–1347. doi: 10.1001/jamaophthalmol.2015.3108. [DOI] [PubMed] [Google Scholar]
- 3.Hemminki K, Chen B. Familial risks for eye melanoma and retinoblastoma: results from the Swedish Family-Cancer Database. Melanoma Res. 2015;16(2):191–195. doi: 10.1097/01.cmr.0000198453.11580.7b. [DOI] [PubMed] [Google Scholar]
- 4.Seregard S, Singh AD. Retinoblastoma: direct chemotherapeutic drug delivery into the vitreous cavity. Br J Ophthalmol. 2012;96(4):473–474. doi: 10.1136/bjophthalmol-2012-301528. [DOI] [PubMed] [Google Scholar]
- 5.Ingrassia L, Lefranc F, Kiss R, Mijatovic T. Naphthalimides and azonafides as promising anti-cancer agents. Curr Med Chem. 2009;16(10):1192–1213. doi: 10.2174/092986709787846659. [DOI] [PubMed] [Google Scholar]
- 6.Braña MF, Cacho M, García MA, et al. New analogues of amonafide and elinafide, containing aromatic heterocycles: synthesis, antitumor activity, molecular modeling, and DNA binding properties. J Med Chem. 2004;47(6):1391–1399. doi: 10.1021/jm0308850. [DOI] [PubMed] [Google Scholar]
- 7.Zhang G, An Y, Lu X, et al. A novel naphthalimide compound restores p53 function in non-small-cell lung cancer by reorganizing the Bak-Bcl-xl complex and triggering transcriptional regulation. J Biol Chem. 2016;291(8):4211–4225. doi: 10.1074/jbc.M115.669978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mijatovic T, Mahieu T, Bruyère C, et al. UNBS5162, a novel naphthalimide that decreases CXCL chemokine expression in experimental prostate cancers. Neoplasia. 2008;10(6):573–586. doi: 10.1593/neo.08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q, Deady LW, Baguley BC, Denny WA. Electron-deficient DNA intercalating agents as antitumor drugs: Aza analogs of the experimental clinical agent N-[2-(dimethylamino)ethyl]acridine-4-carboxamide. J Med Chem. 1994;37(5):593–597. doi: 10.1021/jm00031a008. [DOI] [PubMed] [Google Scholar]
- 10.Gellerman G. Recent developments in the synthesis and applications of anticancer amonafide derivatives: a mini review. Lett Drug Des Discov. 2016;13(1):47–63. [Google Scholar]
- 11.Braña MF, Castellano JM, Roldán CM, Santos A, Vázquez D, Jiménez A. Synthesis and mode(s) of action of a new series of imide derivatives of 3-nitro-1,8 naphthalic acid. Cancer Chemother Pharmacol. 1980;4(1):61–66. doi: 10.1007/BF00255461. [DOI] [PubMed] [Google Scholar]
- 12.Waring MJ, González A, Jiménez A, Vázquez D. Intercalative binding to DNA of antitumour drugs derived from 3-nitro-1,8-naphthalic acid. Nucleic Acids Res. 1979;7(1):217–230. doi: 10.1093/nar/7.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casado A, Rosell R, García-Gómez R, et al. Phase II study of mitonafide in non-small cell lung cancer (NSCLC) Invest New Drugs. 1996;14(4):415–417. doi: 10.1007/BF00180820. [DOI] [PubMed] [Google Scholar]
- 14.Malviya VK, Liu PY, Alberts DS, Surwit EA, Craig JB, Hannigan EV. Evaluation of amonafide in cervical cancer, phase II: a SWOG study. Am J Clin Oncol. 1992;15(1):41–44. doi: 10.1097/00000421-199202000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Mahieu T, Mijatovic T, Quaquebeke EV, et al. UNBS5162 is a novel naphthalimide derivative that induces autophagy and senescence in human prostate cancer cells. Mol Cancer Ther. 2007;6(12):3373S. [Google Scholar]
- 16.Slunt KM, Grace JM, Macdonald TL, Pearson RD. Effect of mitonafide analogs on topoisomerase II of Leishmania chagasi. Antimicrob Agents Chemother. 1996;40(3):706–709. doi: 10.1128/aac.40.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mijatovic T, Mahieu T, Bruyère C, et al. UNBS5162, CXCL, prostate cancer. Neoplasia. 2008;10(6):573–586. doi: 10.1593/neo.08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dholakia AS, Kumar R, Raman SP, et al. Mapping patterns of local failure after pancreaticoduodenectomy for pancreatic adenocarcinoma: a new approach to adjuvant radiation field design. Int J Radiat Oncol Biol Phys. 2013;87(5):1007–1015. doi: 10.1016/j.ijrobp.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Sun J, Feng Y, Tian X, Wang B, Zhou Y. Oncogenic roles and drug target of CXCR4/CXCL12 axis in lung cancer and cancer stem cell. Tumour Biol. 2016;37(7):8515–8528. doi: 10.1007/s13277-016-5016-z. [DOI] [PubMed] [Google Scholar]
- 20.Mahadevan D, Northfelt DW, Chalasani P, et al. Phase I trial of UNBS5162, a novel naphthalimide in patients with advanced solid tumors or lymphoma. Int J Clin Oncol. 2013;18(5):934–941. doi: 10.1007/s10147-012-0475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 22.Kothur K, Wienholt L, Brilot F, Dale RC. CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: a systematic review. Cytokine. 2016;77:227–237. doi: 10.1016/j.cyto.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Reiland J, Furcht LT, McCarthy JB. CXC-chemokines stimulate invasion and chemotaxis in prostate carcinoma cells through the CXCR2 receptor. Prostate. 2015;41(2):78–88. doi: 10.1002/(sici)1097-0045(19991001)41:2<78::aid-pros2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Kitade H, Ni Y, Ota T. Roles of chemokines and chemokine receptors in obesity-associated insulin resistance and nonalcoholic fatty liver disease. Biomolecules. 2015;5(3):1563–1579. doi: 10.3390/biom5031563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan XH, Fu QC, Shi D, et al. Activation of spinal chemokine receptor CXCR3 mediates bone cancer pain through an Akt-ERK crosstalk pathway in rats. Exp Neurol. 2015;263:39–49. doi: 10.1016/j.expneurol.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Imai H, Sunaga N, Shimizu Y, et al. Clinicopathological and therapeutic significance of CXCL12 expression in lung cancer. Int J Immunopathol Pharmacol. 2015;23(1):153–164. doi: 10.1177/039463201002300114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo F, Wang Y, Liu J, Mok SC, Xue F, Zhang W. CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene. 2016;35(7):816–826. doi: 10.1038/onc.2015.139. [DOI] [PubMed] [Google Scholar]
- 28.Totonchy JE, Osborn JM, Botto S, Clepper L, Moses AV. Aberrant proliferation in CXCR7+ endothelial cells via degradation of the retinoblastoma protein. PLoS One. 2013;8(7):e69828. doi: 10.1371/journal.pone.0069828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan MZ, Brandimarti R, Shimizu S, Nicolai J, Crowe E, Meucci O. The chemokine CXCL12 promotes survival of postmitotic neurons by regulating Rb protein. Cell Death Differ. 2008;15(10):1663–1672. doi: 10.1038/cdd.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]