Abstract
MB12066 is a molecule derived from β-lapachone that shown effects on obesity in previous studies. The present studies were conducted to evaluate the tolerability and pharmacokinetics (PK) of MB12066 after the oral administration of single and multiple doses to healthy volunteers. The study comprised 2 independent, randomized, double-blind, placebo-controlled, combined single and multiple ascending dose first-in-human clinical trials to evaluate the safety, tolerability and PK of MB12066 in healthy Korean volunteers. Subjects were randomly assigned to receive a single 10, 30, 100, 150, 200, 300 or 400 mg of MB12066 and multiple 100 or 200 mg of MB12066. The subjects’ vital signs, 12-lead electrocardiograms, clinical laboratory tests, adverse event statuses, and physical examinations were assessed during the study. Blood and urine samples were collected to determine the concentration of MB12066 from predose to 72 hours after the single administration and from predose to 96 hours postdose of day 7 after the multiple administration. NADH:quinone oxidoreductase 1 genotyping was performed to analyze the association between genetic polymorphisms and PK. MB12066 was well tolerated after oral administration of single and multiple doses. The systemic exposure to MB12066 after a single administration tended to increase in a dose-dependent manner in the dose range of 30–200 mg. The overall fraction of MB12066 excreted unchanged in urine was <1% of the administered dose. A significant relationship was observed between NADH:quinone oxidoreductase 1 polymorphisms and exposure to MB12066 after multiple administrations, but the result was not conclusive because of the small number of subjects. A single dose of MB12066 within the dose range of 10–400 mg and multiple doses of 100 and 200 mg of MB12066 were safe and tolerated in healthy subjects. Additionally, MB12066 was mainly eliminated through metabolism in humans.
Keywords: pharmacokinetics, Phase I, first-in-human, NQO1, pharmacogenomics
Introduction
Metabolic syndrome is associated with central obesity, lipid disorders, inflammation, insulin resistance or diabetes, and an increased risk of developing cardiovascular disease. Additionally, obesity is strongly related to metabolic syndrome.1–5 Although various forms and definitions of metabolic syndrome and obesity have been proposed, controversy regarding the risks related to metabolic syndrome does not exist.2,4,6,7 Furthermore, obese individuals are stigmatized and face multiple forms of prejudice and discrimination regarding their weight.8,9 However, the prevalence of metabolic syndrome and obesity has increased recently and is expected to increase continuously. Moreover, obesity is emerging as a substantial clinical and public health burden worldwide.1,7,9,10 Although metabolic syndrome arises due to a complex interaction between genetic and environmental factors, the main issue in metabolic syndrome is a positive energy balance.5,11–14 Physical activity and calorie restriction have been shown to reverse the phenotypes of metabolic syndrome and obesity by activating proteins that regulate metabolism and are related to mitochondrial functions.15–19
Nicotinamide adenine dinucleotides (NAD+ and NADH) function as fundamental mediators of cellular energy metabolism, and an increased NAD+-to-NADH ratio induces obesity, which is implicated in metabolic syndrome.20–22 NAD(P)H:quinone oxidoreductase 1 (NQO1) is a cytosolic antioxidant flavoprotein that catalyzes the reduction of reactive quinone metabolites.23 NQO1-mediated production of hydroquinone, which is readily conjugated and excreted from the cell, constitutes a protective mechanism against these types of damage.24 According to the results from in vitro and in vivo studies, NQO1 activity is important in regulating the intracellular redox levels by oxidizing NAD(P)H, and the loss of NQO1 alters pyridine levels and the intracellular redox status.25,26 Additionally, NQO1 is expressed at high levels in human adipocytes, and positive correlations between NQO1 expression and adipocyte size, as well as metabolic complications of human obesity have been observed.26
β-Lapachone is a naturally occurring o-naphthoquinone present in the bark of the Lapacho tree (Tabebuia avellanedae) native to South America. As shown in the study by Pink et al, NQO1 is an intracellular target for β-lapachone,27 and Hwang et al reported that β-lapachone ameliorates metabolic syndrome in rodent models.21 MB12066 (3,4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b]pyran-5,6-dione) is a molecule derived from β-lapachone that exerts effects on obesity in vitro and in vivo.28 In toxicology studies administering single or multiple doses (for 4 weeks), a 25 mg/kg no-observed-adverse-effect level (NOAEL) was confirmed in rats and the NOAEL in beagle dogs was 30 mg/kg. Additionally, the minimal effective dose (MED) of MB12066 in a diet-induced obese (DIO) model rat was 5 mg/kg/day. The objective of the Phase I studies was to evaluate the pharmacokinetics (PK), safety and tolerability profile of MB12066 and the effect of NQO1 genetic polymorphisms on the PK of MB12066 after oral administration of single and multiple doses to healthy volunteers.
Methods
An overview of the two independent, randomized, double-blind, placebo-controlled, combined single and multiple ascending dose first-in-human clinical trials designed to evaluate the safety, tolerability and PK of MB12066 in healthy Korean volunteers is provided in Table 1. The protocols of both studies (ClinicalTrials.gov Identifier: NCT01285388 and NCT01444677) were reviewed and approved by the Ministry of Food and Drug Safety of Korea and the institutional review board of Seoul National University Hospital (SNUH, Seoul, South Korea). All study procedures were conducted in accordance with the Declaration of Helsinki and Korean Good Clinical Practices.
Table 1.
Overview of the study design
| Single ascending dose | Multiple ascending dose | |
|---|---|---|
| Study 001 (Single ascending-dose study, ClinicalTrials.gov identifier: NCT01285388) | 10, 30, 100, 150 and 200 mg | |
| Study 002 (Single and multiple ascending-dose study, ClinicalTrials.gov identifier: NCT01444677) | 300 and 400 mg | 100 and 200 mg |
Study design
Healthy male adults aged 20–45 years with a body mass index of 18.5–25 kg/m2 were eligible. Major exclusion criteria were smokers; the use of other medications, including vitamins and herbal products; a history of major illness; a history of alcohol/drug abuse; positive status for HIV or hepatitis B or C; and clinically abnormal laboratory and 12-lead electrocardiography (ECG) safety parameters. Prior to enrollment and initiation of any study-related procedures, all subjects provided written informed consent.
The single-dose study comprised 2 randomized, double-blind, placebo-controlled, single ascending dose clinical trials in healthy volunteers. Subjects were randomly assigned to receive a single MB12066 dose of 10, 30, 100, 150, 200, 300 or 400 mg; each group contained 8 subjects, 2 of whom were randomly allocated to receive the placebo. In the single-dose study, 10 mg of MB12066 enteric-coated tablet (lot no 10001; manufactured in April 2010; KT&G Life Sciences Corporation, Seoul, South Korea) and 100 mg of MB12066 enteric-coated tablet (lot no 10001; manufactured in April 2010; KT&G Life Sciences Corporation) were administered. Subjects were admitted on the morning of day −1 (1 day before study drug administration) for baseline evaluations. On the next day (day 1), subjects were orally administered the scheduled dosage with 240 mL of water at ~9 AM after an overnight fast of at least 10 hours. Subjects were discharged on the morning of day 4 and attended post-treatment evaluations on day 8 or 10.
In the multiple-dose study, subjects were randomly assigned to receive 100 or 200 mg of MB12066; each group comprised 10 subjects, 2 of whom received the placebo. In the multiple-dose study, 100 mg of MB12066 enteric-coated tablet (lot no 10001; manufactured in April 2010; KT&G Life Sciences Corporation) were administered. The subjects were subjected to an identical schedule to the single-dose study for days −1 and 1. From days 2 through 7, subjects were administered MB12066 or placebo with 240 mL of water once a day at the same time as on day 1. The subjects were discharged on the morning of day 11 and attended posttreatment evaluations on day 15 or 17.
Tolerability assessments
All adverse events (AEs) were collected from the subjects’ spontaneous reports or investigators’ questionnaires. Additionally, AEs were monitored using vital sign measurements, 12-lead ECGs and clinical laboratory tests throughout the study period. All AEs were recorded, regardless of the suspected relationship to the study drug. The investigators assessed all AEs with respect to severity, course, outcome, seriousness and relationship to the study drug. Clinical laboratory tests were performed by the Department of Laboratory Medicine at SNUH, and quality control was conducted according to the internal standard (IS) operating procedure of SNUH.
Pharmacokinetic assessments
In these studies, blood samples (5 mL) used for PK assessments were collected in heparinized tubes via either an intravenous cannula or venipuncture. In the single-dose study, blood samples were collected at the scheduled times: before dosing (0 hour) and at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 16, 24, 36, 48 and 72 hours after drug administration. For the multiple-dose study, blood samples were collected before dosing and at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 16 and 24 hours after dosing on day 1, immediately before dosing on days 3, 4, 5, 6 and 7, and at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 16, 24, 36, 48, 72 and 96 hours after dosing on day 7. The blood samples were centrifuged (2,100 g, 4°C) for 7 minutes, and the plasma was separated and stored at −70°C until analysis.
Urine samples used for the PK analysis were collected before dosing and at predefined intervals of 0–6, 6–12, 12–24, 24–48 and 48–72 hours after dosing in the single-dose study. Additionally, urine samples were collected before dosing, 0–6, 6–12 and 12–24 hours after dosing on day 1 and 0–6, 6–12 and 12–24 hours after dosing on day 7 in the multiple-dose study.
Determination of MB12066 concentrations
Both plasma and urine samples for the PK analysis were assessed using high-performance liquid chromatography (Agilent 1100 series; Agilent Technologies, Santa Clara, CA, USA) coupled with a mass spectrometer (API4000 Quadrupole; Applied Biosystems, Waltham, MA, USA). The reference standards MB12066 and the IS, MB12066_d6, were provided by Mazence Inc. (Suwon, Korea), and protein precipitation method using acetonitrile was applied as sample preparation. Chromatography was performed using a Luna 5 μm C18 (100×2.0 mm) column (Phenomenex, Torrance, CA, USA), and the mobile phase consisted of 20 mM ammonium formate (mobile phase A) and 100% acetonitrile (mobile phase B). The flow rate was 0.5 mL/min. The lower limit of quantification (LLOQ) for plasma samples was 0.5 ng/mL and the LLOQ of urine samples was 1.0 ng/mL. The calibration range of plasma and urine samples was 0.5–200 ng/mL and 1.0–1,000 ng/mL, respectively. The intrabatch and interbatch coefficients of variation (CV) for plasma samples were <2.677% and 5.016%, respectively. Likewise, the intrabatch and interbatch accuracy values for plasma samples ranged from 96.92% to 101.2% and from 99.28% to 101.9%, respectively. The intrabatch and interbatch CV values for urine samples were <4.732% and 5.449%, respectively. Likewise, the intrabatch and interbatch accuracy values for urine samples ranged from 93.70% to 106.3% and from 92.42% to 109.9%, respectively.
Genotyping of NQO1 single-nucleotide polymorphisms
Since polymorphisms in the NQO1 gene may influence the pharmacological action of MB12066, and for future genotyping needs, blood samples were collected for genotyping. Blood sample 2 mL was collected using an EDTA tube and stored at −70°C until analysis. Genotyping of the polymorphisms of primary interest (NQO1 609C > A [NQO1*2]) was accomplished using the ABI Prism 7500 Sequence Detection System (Thermo Fisher Scientific, Waltham, MA, USA). TaqMan assays were used to genotype the C609T NQO1 polymorphism (rs1800566; assay ID C_2091255_30).
PK and statistical analyses
The noncompartmental PK analysis of MB12066 was performed by WinNonlin (Version 6.3.0; Pharsight Corporation, Mountain View, CA, USA). The actual times of blood collection were used in the PK analysis. The maximum plasma concentrations (Cmax), steady-state Cmax (Cmax,ss), the time to reach Cmax (Tmax) and steady-state Tmax (Tmax,ss) were determined directly from the observed data. The terminal elimination rate constant (λz) was estimated using a linear regression analysis of the log-linear regression of the individual plasma concentration–time data. The terminal half-life (T1/2) and steady-state T1/2 (T1/2,ss) were calculated for each individual as ln(2)/λz. The individual area under the time–concentration curve (AUC) from time 0 to the last measurable time (AUClast) and AUC during a dosing interval (AUCτ) was calculated using the linear trapezoidal rule, and the AUC extrapolated to infinity (AUCinf) was calculated as AUClast + Clast/λz (Clast: the last measurable concentration). The apparent clearance (CL/F) was calculated as dose/AUCinf. The individual renal clearance (CLR) was calculated as Ae (amount excreted)/AUClast. The accumulation index was calculated as AUCτ,ss (day 7, from 144 to 168 hours)/AUCτ (day 1, from 0 to 24 hours) in the multiple-dose study.
Statistical analyses were performed using SAS (Version 9.4; SAS Institute, Cary, NC, USA). Arithmetic means and SDs, medians, and the minimum and maximum values for continuous data, as well as the absolute and relative frequencies for categorical data, were calculated. Analysis of variance (ANOVA) was performed on dose-normalized Cmax and AUClast between the dose groups to examine the dose-independent linear PK properties. Additionally, linear regression analyses of the log-transformed dose and log-transformed Cmax or AUClast were performed. In the multiple-dose study, predose concentrations of MB12066 on days 5, 6 and 7 were compared using ANOVA to confirm the steady state after multiple administration of MB12066, and P-values <0.05 were considered statistically significant. ANOVA was used to compare the Cmax and AUClast values of MB12066 between the variant genotypes, and P-values <0.05 were considered statistically significant.
Results
Demographics
Ninety-one Korean male subjects were enrolled in the study, with 70 subjects in the single ascending-dose study and 21 in the multiple ascending-dose study. All subjects, except 1 in the 100-mg dose group of the multiple ascending-dose study who withdrew, completed the study in accordance with the protocol. The safety populations comprised 70 subjects in the single ascending-dose study and 21 in the multiple ascending-dose study. The PK population comprised 56 and 16 subjects in the single and multiple ascending-dose studies, respectively. Additionally, valid genotyping data were generated for 56 and 15 subjects in the single and multiple ascending-dose studies.
Safety and tolerability
Single-dose study
Forty-one treatment-emergent AEs (TEAEs) were reported from 20 subjects (29%) in the single ascending-dose study. All TEAEs in the single ascending-dose study were of mild intensity, and the most common TEAE was diarrhea. The number of gastrointestinal symptoms, including diarrhea, nausea, vomiting, abdominal distension, pain and discomfort, was 24 of 41 TEAEs (59%). More than half of the gastro-intestinal symptoms were observed in the 300- and 400-mg dose groups (58%). A moderately intense serious AE (SAE), atrioventricular block, was observed in a subject in the 150-mg dose group on the day on which the study drug was administered. The subject was closely monitored without any intervention or treatment, and 24-hour Holter ECG monitoring was conducted after MB12066 elimination. A follow-up cardiology consultation concluded that the subject had no identifiable structural cardiac pathology or cardiovascular risk factors, based on a workup. Additionally, the cardiologist did not consider this event related to the study drug. Based on the opinion of the cardiologist and pharmacology and nonclinical data of MB12066, the investigator considered that this event was unlikely to be related to the study drug. No trends were observed for changes in any of the clinical laboratory parameters, vital signs or 12-lead ECG parameters caused by the single administration of MB12066.
Multiple-dose study
Sixteen TEAEs were reported from 9 subjects (43%) in the multiple ascending-dose study. All TEAEs in the multiple ascending-dose study were of mild intensity. Abdominal pain, diarrhea and epistaxis were the most common TEAEs. Gastrointestinal symptoms, including abdominal pain and diarrhea, accounted for 6 of 15 TEAEs (40%). No SAE was reported in the multiple ascending-dose study. No trends were observed for changes in any of the clinical laboratory parameters, vital signs or 12-lead ECG parameters caused by multiple administrations of MB12066.
PK properties
Single-dose study
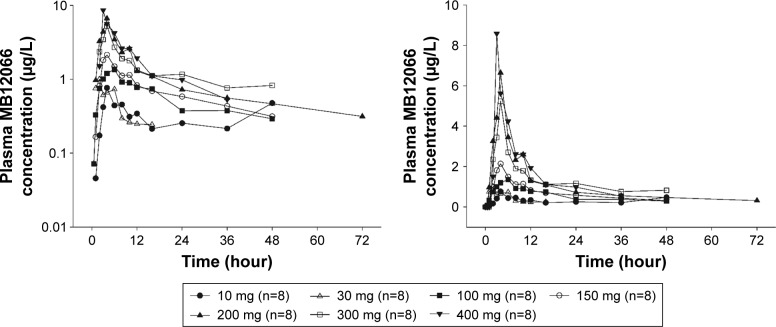
The mean plasma MB12066 concentrations detected after the administration of a single dose of MB12066 during the study are presented in Figure 1, and PK parameters observed after the administration of a single dose of MB12066 are presented in Table 2. The PK parameters of MB12066 were highly variable between subjects. The investigational drug was absorbed with a median Tmax of 4.0 hours (range: 0.5–10.0 hours) after administration. MB12066 was eliminated with a mean T1/2 of 10.4–24.6 hours among all dose groups. The overall fe of MB12066 was <1%.
Figure 1.
Mean plasma concentration–time profiles after the oral administration of a single dose of MB12066 from predose to 72 hours postdose (left panel: log-linear scale; right panel: linear scale).
Table 2.
Summary of the PK parameters after the oral administration of a single MB12066 dose
| PK parameters | Treatment
|
||||||
|---|---|---|---|---|---|---|---|
| 10 mg (n=8) | 30 mg (n=8) | 100 mg (n=8) | 150 mg (n=8) | 200 mg (n=8) | 300 mg (n=8) | 400 mg (n=8) | |
| Tmax (hour)a | 3.5 (1.0–4.0) | 2.5 (1.0–6.0) | 5.0 (1.0–10.0) | 4.0 (3.0–10.0) | 4.0 (2.0–10.0) | 4.0 (2.0–10.0) | 3.0 (3.0–8.0) |
| Cmax (μg/L) | 0.88±0.59 | 1.73±1.72 | 1.56±0.50 | 2.33±1.11 | 8.90±7.85 | 5.70±4.58 | 9.54±6.64 |
| AUClast (μg·hour/L) | 5.15±5.51 | 5.49±3.37 | 19.74±11.92 | 31.52±9.52 | 60.81±25.87 | 44.80±35.41 | 53.39±32.25 |
| AUCinf (μg·hour/L) | 10.62±11.70b | 12.04±13.35c | 26.98±16.92 | 43.22±16.06 | 72.97±30.55 | 64.27±38.52b | 74.07±23.57b |
| T1/2 (hour) | 10.4±9.8b | 18.7±36.3c | 16.4±12.6 | 24.6±16.3 | 22.7±10.5 | 19.2±15.5b | 13.4±4.3b |
| Vd/F (L) | 15,560.9±8,862.3b | 44,004.4±33,321.9c | 84,798.5±20,192.7 | 122,603.6±56,617.4 | 97,757.1±38,356.8 | 147,885.8±100,396.5 | 114,983.3±56,232.9b |
| CL/F (L/hour) | 1,959.9±1,549.5b | 5,225.7±4,455.0c | 7,736.5±10,096.4 | 3,893.2±1,400.7 | 4,280.1±4,859.2 | 6,507.8±3,821.1 | 5,754.6±1,343.9 |
| Ae (μg) | 73.8±22.0 | 116.9±69.9 | 285.1±137.0 | 541.8±163.7d | 769.9±399.9d | 425.4±336.9 | 992.5±637.5 |
| fe | 0.007±0.002 | 0.004±0.002 | 0.003±0.001 | 0.004±0.001d | 0.004±0.002d | 0.001±0.001 | 0.004±0.002 |
| CLR (L/hour) | 13.74±9.49b | 18.10±15.58c | 15.79±11.32 | 15.00±6.02d | 12.47±5.05d | 8.72±6.57b | 16.45±10.30b |
Notes: Values are presented as means ± SD.
Values are presented as medians (minimum–maximum).
N=7,
n=6 from unestimated terminal elimination constants.
N=7 from urine loss.
Abbreviations: Ae, cumulative amount of unchanged drug excreted into the urine; AUCinf, area under the plasma concentration curve extrapolated to infinity; AUClast, area under the plasma concentration curve from time 0 to the last detectable time point; Cmax, maximum plasma concentration; CL/F, apparent clearance; CLR, renal clearance; fe, fraction of drug excreted unchanged; PK, pharmacokinetics; T1/2, elimination half-life; Tmax, time to reach the maximum plasma concentration; Vd/F, apparent volume of distribution.
The Cmax and AUClast appeared to increase as the dose increased, but the increase was not linear with the dose within the dose range tested (Figure 2). However, the dose-normalized Cmax (Cmax/D) and AUClast (AUClast/D) were significantly different among the dose groups (P=0.001 and 0.023, respectively). The power model exponents (95% CI) for Cmax and AUClast were 0.60 (0.43–0.78) and 0.77 (0.54–0.99), respectively. Based on an additional analysis, the exposure to MB12066 appeared to increase in a dose-proportional manner within the 30–200-mg dose range. The Cmax/D and AUClast/D values were not significantly different among the dose groups (P=0.051 and 0.135, respectively), and the power model exponents (95% CI) for Cmax and AUClast were 0.71 (0.33–1.08) and 1.24 (0.90–1.58), respectively.
Figure 2.
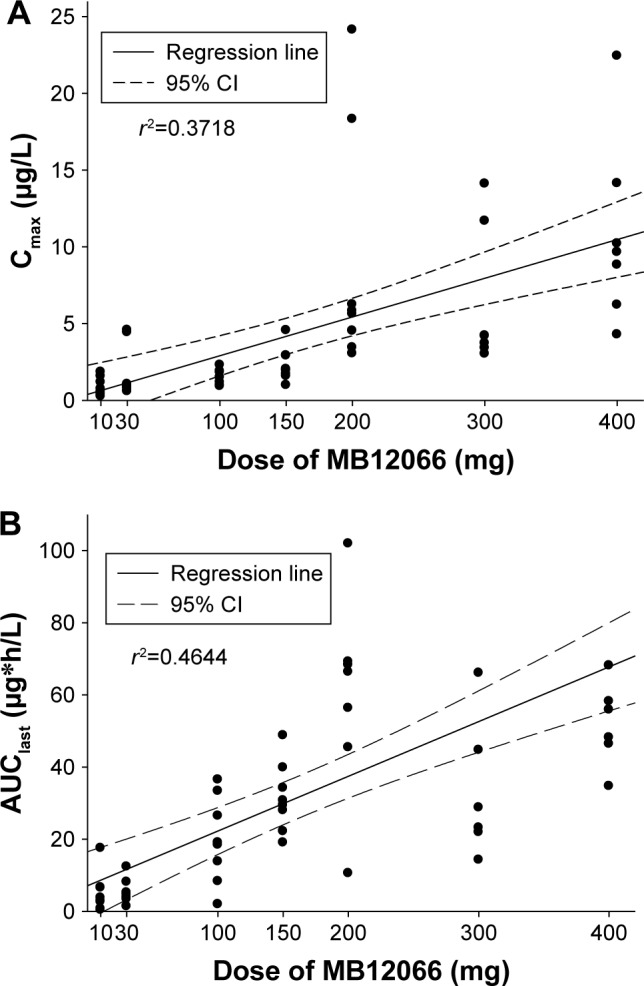
Relationship between individual (A) Cmax or (B) AUClast and doses after a single administration of MB12066.
Abbreviations: AUClast, area under the plasma concentration curve from time 0 to the last detectable time point; Cmax, maximum plasma concentration.
Multiple-dose study
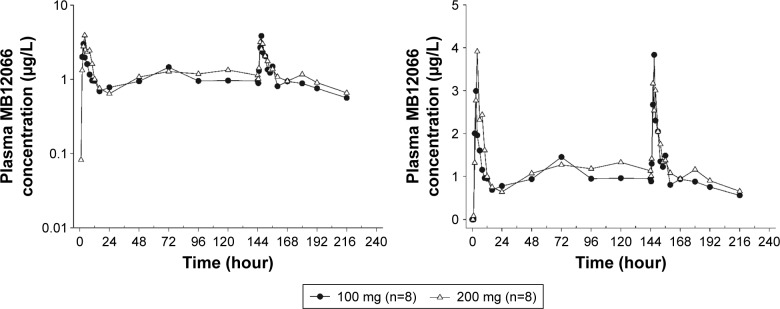
The mean plasma MB12066 concentrations detected following the repeated administration of 100 and 200 mg of MB12066 during the study are shown in Figure 3. Additionally, Table 3 summarizes the PK of MB12066 following the repeated administration of 2 different doses of MB12066. The steady-state concentration was reached between days 3 and 4 in both dose groups. Similar Tmax values were achieved on days 1 and 7. The T1/2,ss values of MB12066 were 17.35±16.91 (mean ± SD) hours and 13.74±11.40 hours for the 100- and 200-mg dose groups, respectively, which were within the range of T1/2 values in the single ascending-dose study. The steady-state exposure to MB12066 was similar in both dose groups. The Cmax,ss values of MB12066 were 3.87±2.76 and 3.82±2.29 μg/L, and the AUCτ,ss values were 29.92±19.10 and 33.13±15.40 μg·hour/L in the 100- and 200-mg dose groups, respectively. Accumulation indices were 1.67±0.96 and 1.48±0.61 in the 100- and 200-mg dose groups, respectively.
Figure 3.
Mean plasma concentration–time profiles after the oral administration of multiple doses of MB12066 from predose to 96 hours after the last dose (left panel: log-linear scale; right panel: linear scale).
Table 3.
Summary of the PK parameters after multiple oral administrations of MB12066 (days 1 and 7 [steady state])
| PK parameters | Treatment
|
|
|---|---|---|
| 100 mg (n=8) | 200 mg (n=8) | |
| Day 1 | ||
| Tmax (hour)a | 3.5 (2.0–6.0) | 4.0 (2.0–8.0) |
| Cmax (μg/L) | 3.83±4.04 | 3.70±1.91 |
| AUCτ (μg·hour/L) | 17.71±13.03 | 26.59±17.95 |
| Day 7 | ||
| Tmax,ss (hour)a | 3.5 (3.0–6.0) | 3.99 (2.0–4.0) |
| Cmax,ss (μg/L) | 3.87±2.76 | 3.82±2.29 |
| Ctrough,ss (μg/L) | 0.95±0.61 | 1.13±0.59 |
| AUCτ,ss (μg·hour/L) | 29.92±19.10 | 33.13±15.40 |
| T1/2,ss (hour) | 17.35±16.91 | 13.74±11.40 |
| Accumulation index | 1.67±0.96 | 1.48±0.61 |
| Ae,ss (μg) | 283.3±151.7 | 510.6±401.8 |
| fe,ss | 0.003±0.002 | 0.003±0.002 |
| CLR,ss (L/hour) | 11.31±8.60 | 15.23±10.00 |
Notes: Values are presented as means ± SD.
Values are presented as medians (min−max).
Abbreviations: Ae, cumulative amount of unchanged drug excreted into the urine during the steady state; AUCinf,ss, area under the plasma concentration curve extrapolated to infinity at a steady state; AUClast,ss, area under the plasma concentration curve from time 0 to the last detectable time point at a steady state; AUCτ, area under the plasma concentration curve during a dosage interval; CLR,ss, steady-state renal clearance; Cmax, maximum plasma concentration; Cmax,ss, maximum steady-state plasma concentration; fe,ss, steady-state fraction of drug excreted unchanged; PK, pharmacokinetics; T1/2,ss, steady-state elimination half-life; Tmax, time to reach the maximum plasma concentration; Tmax,ss, time to reach the maximum steady-state plasma concentration.
NQO1 polymorphisms
Since 1 sample from the multiple-dose study was lost to genotyping, 56 and 15 subjects from the single and multiple ascending-dose studies, respectively, were genotypes (Table 4). In the single-dose study, the numbers of subjects with the *1/*1, *1/*2 and *2/*2 NQO1 genotypes were 21, 28 and 7, respectively. In the multiple-dose study, the numbers were 5, 9 and 1, respectively. No significant differences in MB12066 exposure and NQO1 genotypes were observed after the administration of the single dose of MB12066 (Table 4). However, significant differences were observed in the NQO1 genotype and dose-normalized Cmax,ss and AUCτ,ss values after multiple administrations of MB12066 (Table 4). Because only 1 subject in the multiple-dose study carried the NQO1 *2/*2 allele, we compared the steady-state MB12066 exposure between the subjects with *1/*1 or *1/*2 alleles, and the P-values of the dose-normalized Cmax,ss and AUCτ,ss values were 0.014 and 0.004.
Table 4.
Summary of dose-normalized PK parameters stratified by NQO1 genotype
| Single-dose study | *1/*1 (n=21) | *1/*2 (n=28) | *2/*2 (n=7) | P-valuea |
|---|---|---|---|---|
| Cmax/dose (μg/L/mg) | 0.035±0.036 | 0.044±0.051 | 0.023±0.006 | 0.659 |
| AUClast/dose (μg*hour/L/mg) | 0.297±0.369 | 0.202±0.146 | 0.217±0.090 | 0.781 |
|
| ||||
| Multiple-dose study | *1/*1 (n=5) | *1/*2 (n=9) | *2/*2 (n=1) | P-valueb |
|
| ||||
| Cmax,ss/dose (μg/L/mg) | 0.047±0.033 | 0.033±0.031 | 0.056 | 0.036 |
| AUCτ,ss/dose (μg*hour/L/mg) | 0.220±0.111 | 0.119±0.094 | 0.265 | 0.012 |
Note:
ANOVA,
Kruskal–Wallis test.
Abbreviations: AUClast, area under the plasma concentration curve from time 0 to the last detectable time point; AUClast,ss, area under the plasma concentration curve from time 0 to the last detectable time point at the steady state; Cmax, maximum plasma concentration; Cmax,ss, maximum steady-state plasma concentration.
Discussion
The goals of this first-in-human study were to determine the PK characteristics and safety/tolerability of MB12066 after single or multiple administrations and the effect of polymorphisms in the NQO1 gene on the PK of MB12066 in healthy Korean male subjects.
The NOAEL in mice was 25 mg/kg and the value in dogs was 30 mg/kg. Based on a safety factor of 20, this dose corresponded to a maximum recommended starting dose of 12 mg in 60 kg healthy male adults using the NOAEL of mice, the most sensitive species. Additionally, the MED of MB12066 corresponded to a human equivalent dose (in a 60 kg adult) of 48 mg. Based on these data, 10 mg was selected as the starting dose, and 200 mg (4 times the human equivalent MED) was selected as the highest dose in study 001. According to the results of study 001, the administration of a single MB12066 dose was tolerated at up to 200 mg. Therefore, additional 300 and 400 mg single-administration dose groups were selected in study 002. Moreover, 100 and 200 mg multiple-administration groups were selected in study 002 because the human equivalent pharmacological effective dose of MB12066 was 97–290 mg/day from the DIO model rat.
AEs associated with MB12066 were transient, self-limited and mild in severity. MB12066 was not associated with any clinically significant changes, as assessed from the vital signs, clinical laboratory tests or ECG parameters. The most common AEs were gastrointestinal symptoms, including diarrhea, nausea, vomiting, abdominal distension, pain and discomfort after single or multiple administrations of MB12066. In the single and multiple ascending-dose studies, gastrointestinal symptoms accounted for 24 of 41 TEAEs (59%) and 6 of 15 TEAEs (40%), respectively. Additionally, more than half of the gastrointestinal symptoms were observed in the 300- and 400-mg dose groups (58%) in the single ascending-dose study. Additionally, 83% of gastrointestinal symptoms were observed in the 200-mg dose group in the multiple ascending-dose study, although no gastrointestinal symptoms were observed in the 200-mg dose group in the single ascending-dose study. The gastrointestinal (GI) symptoms observed after the administration of a high dose of MB12066 improved without treatment. Therefore, the GI symptoms related to a high dose of MB12066 do not seem to be a major risk. However, since high doses of MB12066 can induce GI symptoms, further evaluations of these AEs and the development of a reporting system for GI symptoms in Phase II clinical trials of MB12066 are warranted.
The PK of MB12066 were characterized by variability. We observed outliers in the analysis of the dose linearity of MB12066 and the relationship between the PK of MB12066 and NQO1 polymorphisms. Additionally, the median Tmax and Tmax,ss values for MB12066 ranged from 3.5 to 5.0 after a single oral administration and a median of 3.5–3.99 hours after multiple oral administration, respectively, but Tmax values extended up to 10 hours in some cases. The T1/2 and T1/2,ss values of MB12066 also varied (Tables 2 and 3). We postulate that as yet unknown genetic factors may be associated with the PK of MB12066. The proportion of MB12066 in the urine was <1% in all subjects. In this study, MB12066 was mainly eliminated through metabolism in human. It is known that β-lapachone is mainly eliminated via NQO1-mediated quinone reduction and subsequent uridine diphosphate-glucuronosyltransferases catalyzed glucuronidation.29,30
Recently, Lee et al presented the PK characteristics of MB12066 after twice daily dosing of 100 mg of MB12066. The AUCτ and accumulation index after twice daily dosing of 100 mg of MB12066 were 50.44±29.68 and 2.72±0.37, respectively.31 The major cause of difference from this study is inter-individual variability of MB12066, and Lee et al also showed considerable interindividual variability.31 It is necessary to confirm this in a study conducted on more subjects.
Since we hypothesized that the NQO1 genotype might be related to the pharmacology of MB12066, we preemptively conducted a genotyping analysis of NQO1 in cases where a genetic analysis was needed later. In this study, the frequencies of the *1/*1, *1/*2, and *2/*2 NQO1 genotypes were 36.6%, 52.1% and 11.3%, respectively, similar to previous reports. Kelsey et al reported that the NQO1 genotype frequencies in different ethnic groups and the frequencies of the *1/*1, *1/*2 and *2/*2 NQO1 genotypes in the Korean population are 33.3%, 47.8% and 18.8%, respectively.32 The PK of MB12066 were affected by the NQO1 genotype in subjects in the multiple-dose study, but not in the single-dose study. MB12066 may have induced NQO1 after multiple administrations, and several studies have examined the induction of NQO1.33,34 However, since the multiple-dose study included a small number of subjects (n=15), we could not conclusively determine whether MB12066 exposure is influenced by the NQO1 genotype. Furthermore, in this study, the PK of MB12066 showed large interindividual variability. Regarding this inconclusive finding, further related investigations are warranted. Additionally, because significant ethnic differences in the allele frequencies of NQO1 have been observed,32 a PK study using subjects of different ethnicities is also recommended.
A single dose of MB12066 ranging from 10 to 400 mg and multiple doses of MB12066 (100 and 200 mg) were safe and well tolerated in healthy Korean male subjects. The PK of MB12066 were characterized by variability. Additionally, the PK of MB12066 were affected by the NQO1 genotype in the multiple-dose study. Based on the safety profiles and the PK characteristics from this study and the human equivalent pharmacological effective dose of MB12066 from the DIO model rat (97–290 mg/day), 100 and 200 mg once daily dose or 100 mg twice daily dose is recommended as a candidate dose for Phase II trial.
Acknowledgments
This study was sponsored by Yungjin Pharmaceutical Co., Ltd., Seoul, Korea. This study was designed and conducted by qualified investigators from the Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital. The sponsor played a minor role in the study design and interpretation.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 2.Eckel RH, Alberti KG, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;375(9710):181–183. doi: 10.1016/S0140-6736(09)61794-3. [DOI] [PubMed] [Google Scholar]
- 3.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM. Adipose tissue and metabolic syndrome: too much, too little or neither. Eur J Clin Invest. 2015;45(11):1209–1217. doi: 10.1111/eci.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reaven GM. The metabolic syndrome: time to get off the merry-go-round? J Intern Med. 2011;269(2):127–136. doi: 10.1111/j.1365-2796.2010.02325.x. [DOI] [PubMed] [Google Scholar]
- 6.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanner RM, Brown TM, Muntner P. Epidemiology of obesity, the metabolic syndrome, and chronic kidney disease. Curr Hypertens Rep. 2012;14(2):152–159. doi: 10.1007/s11906-012-0254-y. [DOI] [PubMed] [Google Scholar]
- 8.Puhl R, Brownell KD. Bias, discrimination, and obesity. Obes Res. 2001;9(12):788–805. doi: 10.1038/oby.2001.108. [DOI] [PubMed] [Google Scholar]
- 9.Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity (Silver Spring) 2009;17(5):941–964. doi: 10.1038/oby.2008.636. [DOI] [PubMed] [Google Scholar]
- 10.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of o¾sity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32(9):1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 11.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.James AM, Collins Y, Logan A, Murphy MP. Mitochondrial oxidative stress and the metabolic syndrome. Trends Endocrinol Metab. 2012;23(9):429–434. doi: 10.1016/j.tem.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Alemany M. Utilization of dietary glucose in the metabolic syndrome. Nutr Metab (Lond) 2011;8(1):74. doi: 10.1186/1743-7075-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thyfault JP, Krogh-Madsen R. Metabolic disruptions induced by reduced ambulatory activity in free-living humans. J Appl Physiol (1985) 2011;111(4):1218–1224. doi: 10.1152/japplphysiol.00478.2011. [DOI] [PubMed] [Google Scholar]
- 15.Guarente L, Picard F. Calorie restriction – the SIR2 connection. Cell. 2005;120(4):473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1(1):15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444(7121):868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- 18.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1(6):361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger F, Ramirez-Hernandez MH, Ziegler M. The new life of a centenarian: signalling functions of NAD(P) Trends Biochem Sci. 2004;29(3):111–118. doi: 10.1016/j.tibs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Hwang JH, Kim DW, Jo EJ, et al. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes. 2009;58(4):965–974. doi: 10.2337/db08-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollak N, Dolle C, Ziegler M. The power to reduce: pyridine nucleotides – small molecules with a multitude of functions. Biochem J. 2007;402(2):205–218. doi: 10.1042/BJ20061638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaiswal AK. Regulation of genes encoding NAD(P)H:quinone oxidoreductases. Free Radic Biol Med. 2000;29(3–4):254–262. doi: 10.1016/s0891-5849(00)00306-3. [DOI] [PubMed] [Google Scholar]
- 24.Ross D, Thor H, Orrenius S, Moldeus P. Interaction of menadione (2-methyl-1,4-naphthoquinone) with glutathione. Chem Biol Interact. 1985;55(1–2):177–184. doi: 10.1016/s0009-2797(85)80126-5. [DOI] [PubMed] [Google Scholar]
- 25.Gaikwad A, Long DJ, 2nd, Stringer JL, Jaiswal AK. In vivo role of NAD(P)H:quinone oxidoreductase 1 (NQO1) in the regulation of intracellular redox state and accumulation of abdominal adipose tissue. J Biol Chem. 2001;276(25):22559–22564. doi: 10.1074/jbc.M101053200. [DOI] [PubMed] [Google Scholar]
- 26.Palming J, Sjoholm K, Jernas M, et al. The expression of NAD(P)H: quinone oxidoreductase 1 is high in human adipose tissue, reduced by weight loss, and correlates with adiposity, insulin sensitivity, and markers of liver dysfunction. J Clin Endocrinol Metab. 2007;92(6):2346–2352. doi: 10.1210/jc.2006-2476. [DOI] [PubMed] [Google Scholar]
- 27.Pink JJ, Planchon SM, Tagliarino C, Varnes ME, Siegel D, Boothman DA. NAD(P)H:Quinone oxidoreductase activity is the principal determinant of beta-lapachone cytotoxicity. J Biol Chem. 2000;275(8):5416–5424. doi: 10.1074/jbc.275.8.5416. [DOI] [PubMed] [Google Scholar]
- 28.Chugh PK, Sharma S. Recent advances in the pathophysiology and pharmacological treatment of obesity. J Clin Pharm Ther. 2012;37(5):525–535. doi: 10.1111/j.1365-2710.2012.01347.x. [DOI] [PubMed] [Google Scholar]
- 29.Liu M, Wang Q, Liu F, et al. UDP-glucuronosyltransferase 1A compromises intracellular accumulation and anti-cancer effect of tanshinone IIA in human colon cancer cells. PLoS One. 2013;8(11):e79172. doi: 10.1371/journal.pone.0079172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng X, Liu F, Yan T, et al. Metabolic profile, enzyme kinetics, and reaction phenotyping of beta-lapachone metabolism in human liver and intestine in vitro. Mol Pharm. 2012;9(12):3476–3485. doi: 10.1021/mp300296m. [DOI] [PubMed] [Google Scholar]
- 31.Lee HW, Seong SJ, Ohk B, et al. Pharmacokinetic and safety evaluation of MB12066, an NQO1 substrate. Drug Des Devel Ther. 2017;11:2719–2725. doi: 10.2147/DDDT.S142339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelsey KT, Ross D, Traver RD, et al. Ethnic variation in the prevalence of a common NAD(P)H quinone oxidoreductase polymorphism and its implications for anti-cancer chemotherapy. Br J Cancer. 1997;76(7):852–854. doi: 10.1038/bjc.1997.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bongard RD, Krenz GS, Gastonguay AJ, Williams CL, Lindemer BJ, Merker MP. Characterization of the threshold for NAD(P)H:quinone oxidoreductase activity in intact sulforaphane-treated pulmonary arterial endothelial cells. Free Radic Biol Med. 2011;50(8):953–962. doi: 10.1016/j.freeradbiomed.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munday R, Zhang Y, Munday CM, Bapardekar MV, Paonessa JD. Structure-activity relationships and organ specificity in the induction of GST and NQO1 by alkyl-aryl isothiocyanates. Pharm Res. 2008;25(9):2164–2170. doi: 10.1007/s11095-008-9595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]