Abstract
We report the successful use of ceftolozane/tazobactam (C/T) to treat a pulmonary exacerbation in a 35 year old female, post lung transplant, with cystic fibrosis (CF), malnutrition, chronic kidney disease, and multi-drug resistant Pseudomonas aeruginosa infection (MDR PSA). Given the complexity of the clinical profile, we measured drug levels of C/T during treatment of her current exacerbation to determine pharmacokinetics. The patient achieved an estimated ceftolozane peak of 174.1 μg/mL and trough of 9.2 μg/mL. Serum half-life was found to be slightly shorter than previously reported in normal subjects, (2.3 hr. vs. 2.6 hr.) despite the presence of renal insufficiency. Treatment resulted in improvement in serum inflammatory markers and symptoms and was well-tolerated.
Keywords: Ceftolozane, Tazobactam, Cystic fibrosis, Renal insufficiency
1. Introduction
Cystic fibrosis (CF) is a genetic lung disease that is typically characterized by chronic lower airway infection and development of progressive bronchiectasis. In the 2015 United States CF Patient Registry, 9.2% of adult CF patients in 2015 were infected with MDR PSA [1]. Treatment of pulmonary exacerbations in affected individuals is currently problematic due to limited antimicrobial choices.
Ceftolozane/tazobactam (C/T) is a novel second-generation β-lactam/β-lactamase inhibitor that was recently approved by the Food and Drug Administration for treatment of complicated intra-abdominal and urinary tract infections. Ceftolozane contains a pyrazole side chain, increasing stability to AmpC β-lactamase, an inducible enzyme that confers drug resistance in PSA [2]; as a result of this enhanced activity, C/T is an important new therapeutic option for MDR PSA infections [3]. However, information about C/T use in CF is limited [4]. We report the successful employment of this agent in a CF patient with multiple comorbid conditions and characterize the pharmacokinetics of C/T during treatment.
2. Case presentation
A 35 year-old female with CF (homozygous for the F508del mutation) and chronic MDR PSA infection presented with a pulmonary exacerbation. She had a history of living donor lung transplant 17 years prior, CF related diabetes, calcineurin-related chronic kidney disease (estimated creatinine clearance of 40–50 mL/min calculated using the Cockroft-Gault equation), and hypertension. She previously received 9 days of intravenous ciprofloxacin 400 mg every 8 hours and piperacillin-tazobactam 4.5 g every 8 hours infused over 4 hours without clinical improvement and was subsequently transitioned to 14 days of meropenem 2 g every 8 hours via 3 hour infusion. While symptoms improved briefly on meropenem, measured pulmonary function was unchanged with FEV1 of 0.77 L (27% of predicted), and she returned four weeks later with increased dyspnea, productive cough, and fatigue. Akron pulmonary exacerbation score was 13 on current admission, after having been 9 at last admission, and 6-min walk distance was lower at 1020 feet, after having been 1400 feet prior to discharge from her last hospitalization [5]. CRP on current admission was 62 mg/L, increased from 3.0 mg/L prior to discharge from her previous admission.
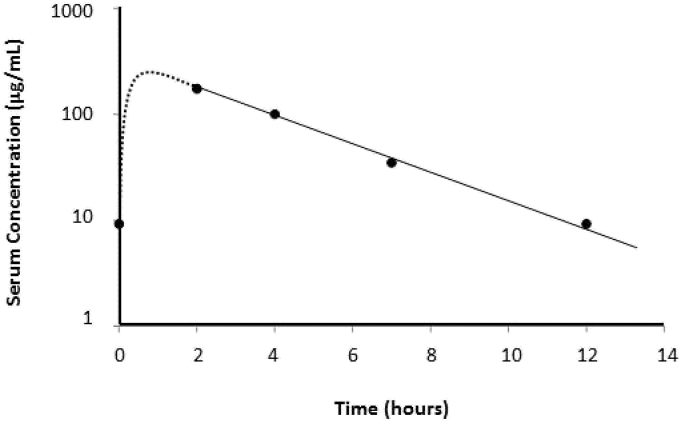
After reviewing the medication list for agents that may affect the clearance of C/T, we administered C/T 2000 mg/1000 mg intravenously every 12 hours. The selected dose was adopted from a clinical trial (NCT02070757) involving C/T for nosocomial pneumonia in mechanically ventilated patients. We obtained blood samples to assess the steady state concentrations of C/T. Serum concentrations were determined utilizing a validated high-performance liquid chromatography assay [6]. The steady state 1-h post infusion peak concentration (Cmax,ss) was 174.1 μg/mL for ceftolozane (Fig. 1). The ceftolozane steady-state volume of distribution (Vss), half-life (t1/2), and minimum concentration (Cmin,ss) were 11.5 L, 2.3 hours, and 9.2 μg/mL, respectively. Tazobactam concentrations could only be measured at two time points due to interference from other substances in the samples. However, tazobactam t1/2, calculated from available samples, was 2.1 hours.
Fig. 1.
Pharmacokinetics of ceftolozane. Dotted line represents the distribution phase following drug administration (blood samples were not collected during this period). The solid line represents the elimination phase.
The patient completed 14 days of total therapy with C/T without any significant side effects or complications. She reported significant improvement in sputum production, fatigue and dyspnea. The forced expiratory volume in the first second improved from 25% to 30% of predicted, and serum C-reactive protein declined from 62 to <1.0 mg/L. She did not experience side effects or complications attributable to C/T.
3. Discussion
To our knowledge, this is the second report of successful treatment of a CF pulmonary exacerbation with C/T [7] and the first to describe the pharmacokinetics of the compound in a CF patient with renal insufficiency. Selecting the optimal dose and frequency of administration of C/T in this case was challenging in several respects. It is well recognized that many medications exhibit altered pharmacokinetic-pharmacodynamic (PKPD) profiles in CF [8]. Published C/T PKPD data are currently sparse, and it was unclear how this limited information would apply to a patient with a body mass of only 47 kg, chronic kidney disease, and concomitant use of immunosuppressive medications.
Initial studies of C/T in healthy volunteers displayed dose proportional and linear kinetics. Our patient exhibited a slightly higher Cmax than non-CF patients who have received ceftolozane and C/T [9]. This may be attributable to her lower body mass and Vss. Monogue et al. [8] reported lower central compartment volume in CF patients compared to non-CF adults. Chandorkar et al. [10] characterized pharmacokinetics of C/T in patients with varying degrees of renal dysfunction and found decreased ceftolozane clearance in those with reduced creatinine clearance. Interestingly, despite having impaired renal function, our patient displayed a shorter half-life compared to healthy subjects (∼2.3 vs. 2.6 hours, respectively). Furthermore, our patient displayed a shorter half-life in comparison to the mean half-life reported by Monogue et al. (2.87 hours) [8].
We later performed susceptibility testing on two PSA isolates from a sputum sample collected prior to C/T initiation (research use ETEST® strips, bioMériuex, Inc). The resulting minimum inhibitory concentrations (MICs) were 2/4 and 0.5/0.25 μg/mL. Given the penetration ratios of 0.59 of ceftolozane and tazobactam of 0.38 [11], we estimated the trough tissue concentrations of ceftolozane and tazobactam to be approximately 5.4 and 0.8 μg/mL, respectively. Therefore, optimal time above MIC (estimated 100% time above MIC of ceftolozane achieved against both isolates) was likely attained at the dose and frequency provided in this case.
Given her excellent clinical response to this dosing, as well as the appropriate pharmacokinetic levels obtained we would plan similar dosing for patients with these comorbidities in the future. Indeed, in the past year, this patient has been treated a second time with ceftolozane/tazobactam with the same dosing for another pulmonary exacerbation with similar improvement, though we did not repeat drug levels.
The serum concentrations of ceftolozane obtained from our patient may provide a basis for estimating appropriate dosing regimens for CF patients with similar characteristics. We hope these results stimulate further studies of C/T for treating pulmonary exacerbations in CF patients especially those with complex co-morbidities.
Conflicts of interest
Dr. Nicolau has acted as a consultant, speaker bureau member and research investigator for Merck; however, no funds were received in support of any aspect of this current report. All other authors declare that they have no conflicts of interest regarding the publication of this paper.
Acknowledgements
None.
Contributor Information
Katie Stokem, Email: stokek1@mmc.org.
Jonathan B. Zuckerman, Email: JZuckerman@cmamaine.com.
David P. Nicolau, Email: David.Nicolau@hhchealth.org.
Minkey Wungwattana, Email: MWungwatta@mmc.org.
Edmund H. Sears, Email: tsears@cmamaine.com.
References
- 1.Registry C.F.F.P. 2015. 2015 Patient Registry Annual Data Report. [Google Scholar]
- 2.Murano K. Structural requirements for the stability of novel cephalosporins to AmpC beta-lactamase based on 3D-structure. Bioorg. Med. Chem. 2008;16(5):2261–2275. doi: 10.1016/j.bmc.2007.11.074. [DOI] [PubMed] [Google Scholar]
- 3.Sutherland C.A., Nicolau D.P. Susceptibility profile of ceftolozane/tazobactam and other parenteral antimicrobials against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa from US hospitals. Clin. Ther. 2015;37(7):1564–1571. doi: 10.1016/j.clinthera.2015.05.501. [DOI] [PubMed] [Google Scholar]
- 4.Kuti J.L. Microbiological activity of ceftolozane/tazobactam, ceftazidime, meropenem, and piperacillin/tazobactam against Pseudomonas aeruginosa isolated from children with cystic fibrosis. Diagn. Microbiol. Infect. Dis. 2015;83(1):53–55. doi: 10.1016/j.diagmicrobio.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Kraynack N.C., McBride J.T. Improving care at cystic fibrosis centers through quality improvement. Semin. Respir. Crit. Care Med. 2009;30(5):547–558. doi: 10.1055/s-0029-1238913. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland C.A., Nicolau D.P. Development of an HPLC method for the determination of ceftolozane/tazobactam in biological and aqueous matrixes. J. Chromatogr. Sci. 2016;54(6):1037–1040. doi: 10.1093/chromsci/bmw047. [DOI] [PubMed] [Google Scholar]
- 7.Vickery S.B., McClain D., Wargo K.A. Successful use of ceftolozane-tazobactam to treat a pulmonary exacerbation of cystic fibrosis caused by multidrug-resistant Pseudomonas aeruginosa. Pharmacotherapy. 2016;36(10):e154–e159. doi: 10.1002/phar.1825. [DOI] [PubMed] [Google Scholar]
- 8.Monogue M.L. Population pharmacokinetics and safety of ceftolozane-tazobactam in adult cystic fibrosis patients admitted with acute pulmonary exacerbation. Antimicrob. Agents Chemother. 2016;60(11):6578–6584. doi: 10.1128/AAC.01566-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller B. Pharmacokinetics and safety of intravenous ceftolozane-tazobactam in healthy adult subjects following single and multiple ascending doses. Antimicrob. Agents Chemother. 2012;56(6):3086–3091. doi: 10.1128/AAC.06349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandorkar G. Population pharmacokinetics of ceftolozane/tazobactam in healthy volunteers, subjects with varying degrees of renal function and patients with bacterial infections. J. Clin. Pharmacol. 2014;55(2):230–239. doi: 10.1002/jcph.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandorkar G. Intrapulmonary penetration of ceftolozane/tazobactam and piperacillin/tazobactam in healthy adult subjects. J. Antimicrob. Chemother. 2012;67(10):2463–2469. doi: 10.1093/jac/dks246. [DOI] [PubMed] [Google Scholar]