Abstract
Background
Previous work suggests that major depressive disorder (MDD) is associated with disturbances in global connectivity among brain regions, as well as local connectivity within regions. However, the relative importance of these global versus local changes for successful antidepressant treatment is unknown. We used multiscale entropy (MSE), a measure of brain signal variability, to examine how the propensity for local (fine scale MSE) versus global (coarse scale MSE) neural processing measured prior to antidepressant treatment is related to subsequent treatment response.
Methods
We collected resting-state EEG activity during eyes-open and closed conditions from unmedicated individuals with MDD prior to antidepressant pharmacotherapy (N = 36) as well as from non-depressed controls (N = 36). Treatment response was assessed after 12 weeks of treatment using the Montgomery-Åsberg Depression Rating Scale (MADRS), at which time participants with MDD were characterized as either responders (≥ 50% MADRS decrease) or non-responders. MSE was calculated from baseline EEG, and compared between controls, future treatment responders and non-responders. Putative interactions with the well-documented age effect on signal variability (increased reliance on local neural communication with increasing age, indexed by greater finer-scale variability) were assessed.
Results
Only in responders, we found that reduced MSE at fine temporal scales (especially fronto-centrally) and increased MSE diffusely at coarser temporal scales was related to the magnitude of the antidepressant response. In controls and MDD non-responders, but not MDD responders, there was an increase in MSE with age at fine temporal scales and a decrease in MSE with age at coarse temporal scales.
Conclusion
Our results suggest that an increased propensity toward global processing, indexed by greater MSE at coarser timescales, at baseline appears to facilitate eventual antidepressant treatment response.
Keywords: Depression, Treatment, Response, Multi-scale entropy (MSE), Electroencephalography (EEG), Signal variability, Spectral power density (SPD)
Highlights
-
•
We measured resting-state EEG prior to antidepressant pharmacotherapy.
-
•
We examined global vs. local processing in relation to antidepressant response.
-
•
Greater response was linked with increased global processing.
-
•
Age-related decreases in global communication were absent in future responders.
-
•
Baseline brain dynamics in those who are/are not responsive to antidepressants differ.
1. Introduction
Major depressive disorder (MDD) affects > 300 million people globally and is the leading cause of disability worldwide (World Health Organization, 2017). As such, the long-term personal and societal consequences of untreated and persistent MDD are substantial (Patten et al., 2012). From a neuropsychiatric perspective, there is a growing consensus that MDD is characterized by disturbances in connectivity among cortical and subcortical brain regions. Connectivity can be measured using resting-state functional magnetic resonance imaging (fMRI) as well as electroencephalography (EEG)-indexed coherence. Recent work using these approaches suggests that both global connectivity, within and among distributed networks, as well as local connectivity within regions are altered in the context of MDD.
Studies employing fMRI have identified global connectivity disturbances between and within networks comprised of regions supporting emotional processing (including the amygdala, subgenual anterior cingulate [sgACC] and pallidum; Disner et al., 2011, Sheline et al., 2010), in the default mode network (DMN, comprised of the posterior cingulate, precuneus and medial prefrontal cortex [PFC]; Sheline et al., 2010, Kuhn and Gallinat, 2013, Kaiser et al., 2015, Mulders et al., 2015), in networks sub-serving attention (including dorso-/ventro-lateral frontal and posterior parietal cortices; Hamilton et al., 2015, Kaiser et al., 2015), as well as executive function (encompassing the dorsolateral/dorsomedial PFC [DLPFC/dmPFC] and posterior parietal cortex (Mulders et al., 2015, Wang et al., 2016). In addition to global connectivity disturbances, local intra-regional connectivity alterations have also been observed in MDD (Mulders et al., 2015, Wang et al., 2016). A meta-analysis of resting-state fMRI connectivity data found that MDD was associated with increased regional homogeneity (ReHo) - a measure of local connectivity - in anterior regions (e.g., medial PFC) and decreased ReHo in more posterior areas (e.g. para-central gyrus; Iwabuchi et al., 2015).
Similarly, EEG studies point to both distributed and local connectivity disturbances in MDD. Global disturbances have been demonstrated by several groups, with reports of decreased inter-hemispheric coherence in MDD patients compared to controls (Knott et al., 2001, Sun et al., 2008, Lee et al., 2011). Others reported that MDD patients exhibit lower synchronization likelihood (Montez et al., 2006) and a relative loss of small-world network characteristics (i.e., lower path length) in lower (delta/theta) EEG frequency bands during sleep (Leistedt et al., 2009). However, yet other groups have shown increased connectivity in MDD, particularly in the theta and alpha bands both within and among brain regions (e.g., via structural synchrony between EEG pairs) (Fingelkurts et al., 2007), longer-range coherence assessments (Leuchter et al., 2012) and lagged phase synchronization of alpha in frontal areas (Olbrich et al., 2014). Finally, local connectivity disturbances were highlighted in a review by Fingelkurts and Fingelkurts (2015), which suggests that disturbances within the anterior and posterior cortices play an important role in both the pathogenesis and severity of MDD. Thus, both the fMRI- and EEG-indexed connectivity literature supports the idea that MDD is associated with both global and local connectivity disturbances.
These somewhat diverse findings likely reflect, at least to a certain extent, heterogeneity in analytic methods as well as clinical factors (i.e., MDD is characterized by a constellation of symptom profiles which may differ between individuals). Nevertheless, certain neural profiles prior to intervention, including network dynamics and connectivity measures, may be associated with the course of antidepressant response. In this context, the relative importance of global and local processing changes in relation to successful antidepressant treatment response has not been extensively examined. Multiscale entropy (MSE) is a measure of brain signal variability (i.e., transient temporal fluctuations in brain signal) that describes the way signals behave over a range of temporal scales from fine (e.g., across 2 ms intervals) to coarse (e.g., across 40 ms intervals) timeframes (Costa et al., 2005). Studies using MSE with EEG and magnetoencephalography (MEG) suggest that increased MSE at fine temporal scales, typically reflecting high frequencies, is associated with an increased reliance on local neuronal processing (Mizuno et al., 2010, Vakorin et al., 2011, McIntosh et al., 2014). On the other hand, increased variability at coarse temporal scales, typically linked with lower frequencies, is associated with increased reliance on global/larger-scale processing. This relationship is further supported by the presence of correlations (or anti-correlations) between MSE values at short and long temporal scales with measures of local and distributed entropy or functional connectivity, respectively (McDonough and Nashiro, 2014, McIntosh et al., 2014, Vakorin et al., 2011).
Although studies have used signal variability measures to enhance our understanding of the neural underpinnings of MDD (Linkenkaer-Hansen et al., 2005, Lee et al., 2007, Li et al., 2008, Tang et al., 2009, Mendez et al., 2012, Demirtas et al., 2016), to our knowledge, only one study to date has used MSE to measure the effect of antidepressant treatment on local (fine scale MSE) versus global (coarse scale MSE) neural signals. Specifically, Okazaki et al. (2013) examined how within-subject MSE profiles changed from before to after electroconvulsive therapy (ECT) in three depressed patients. They found that depression symptom improvement after ECT was associated with decreases in local processing. MSE decreased at fine temporal scales, reflecting primarily gamma band activity, from pre- to post-treatment in the three patients who were assessed. However, this paper did not directly examine whether MSE profiles measured prior to treatment were predictive or associated with eventual treatment response (Okazaki et al., 2013).
There is another area of MSE research that is relevant to the investigation of the relative importance of local versus global communication for successful treatment response in MDD. Work in healthy aging has demonstrated that increasing age is associated with a decrease in coarse-scale MSE and a concomitant increase in fine scale MSE, suggesting a shift from global toward more local neural processing (Vakorin et al., 2011, McIntosh et al., 2014, Sleimen-Malkoun et al., 2015). It is therefore important to consider whether age might alter the MSE profile associated with positive treatment response. Of note, Mendez et al. (2012) examined brain changes associated with successful antidepressant pharmacotherapy using a measure of brain signal variability (Lempel-Ziv complexity), but did not examine coarse versus fine scale dependencies in their data. Nevertheless, they showed that MDD patients displayed higher pre-treatment MEG signal variability than controls, particularly anteriorly, which then decreased with treatment. Importantly, this effect was significant only in younger patients, suggesting an interaction between age and treatment response in the context of brain signal variability assessments (Mendez et al., 2012).
In the current study, we expand on previous work by investigating how brain characteristics associated with global and local processing, using MSE, relate to antidepressant treatment response. We measured eyes-open (EO) and eyes-closed (EC) resting-state EEG data prior to pharmacological treatment (three regimens: escitalopram, bupropion, escitalopram + bupropion) in adults with MDD and healthy controls. For MDD patients, we evaluated depression symptoms at baseline and at 12 weeks post-treatment using the Montgomery-Åsberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979). Given that previous work suggests that the propensity for global versus local processing changes with age, we examined whether we could replicate previously identified age effects. Importantly, we examined whether there were differences among eventual treatment responders versus non-responders in their baseline MSE profiles, and how these differences related to age and depression score (MADRS) changes from baseline to week 12 of treatment. Although our primary interest was to assess responder/non-responder differences at baseline, we included healthy controls in our analyses to examine putative MSE differences between patients and controls in this study. Finally, as supplementary analyses, we assessed spectral power density (SPD) measures. These analyses allowed us to better interpret our MSE results (Courtiol et al., 2016), as they enabled us to compare results based on MSE profiles to results based on the power content of EEG data (which has been extensively investigated in the context of depression, and in relation to antidepressant response prediction). To our knowledge, this work is the first to assess the interplay between age, and the relative importance of global and local processing changes in relation to successful antidepressant treatment response.
2. Methods
2.1. Participants
Similar to what has previously been reported (Jaworska et al., 2012), resting EEG activity was assessed in 53 adults with a primary diagnosis of psychiatrist-assessed major depressive disorder (MDD – Structured Clinical Interview for DSM-IV-TR [SCID]-assessed; Table 1). However, due to strict criteria regarding EEG data quality and missing clinical data (details in Section 2.4), 36 MDD patients were included in the current study and are discussed herein. Four patients (out of 36) also had a co-morbid anxiety disorder. The 17-item Hamilton Rating Scale for Depression (HAMD17; Hamilton, 1960; scores are included in tables but are not discussed further) and MADRS were used to assess symptom severity; all patients had MADRS scores ≥ 22 at enrolment. Patient exclusion criteria included: other Axis I disorder (apart from anxiety), current (< 6 months) substance abuse/dependence, seizure history, unstable medical condition and significant suicide risk. Participants were also excluded if they had previously obtained adequate treatment for their current depressive episode with the study medications (outlined below). At the time of testing, patients were not taking any psychoactive drugs; appropriate drug washout periods were applied in previously medicated patients (> 5 wk. for fluoxetine, 1 wk. for all others).
Table 1.
Major depressive disorder (MDD) & control group characteristics (means ± S.D.).
| MDD group (N = 36) |
Control group (N = 36) |
Significance | |
|---|---|---|---|
| Sex (M/F) | 15/21 | 15/21 |
p = 1.0 (Chi-square test) |
| Age | 40.03 ± 12.8 (range: 19–63) |
36.9 ± 9.3 (range: 25–60) |
p = 0.16 |
| Education (yrs.) | 15.7 ± 2.4 | 16.4 ± 2.0 | p = 0.18 |
| Ethnicity | 1 African; 2 Asian; 1 South Asian; 32 Caucasian | 1 African; 1 Asian; 2 South Asian; 32 Caucasian | N.A. |
| Pre-treatment HAMD17 | 20.8 ± 5.1 | N.A. | N.A. |
| Pre-treatment MADRS | 29.9 ± 5.1 | N.A. | N.A. |
| Pre-treatment BDI-II | N.A. | 2.7 ± 4.6 | N.A. |
BDI-II: Beck Depression Inventory-II; HAMD17: 17-Item Hamilton Rating Scale for Depression; MADRS: Montgomery-Åsberg Depression Rating Scale; N.A.: not available.
Note: P values index one-way analyses of variance (ANOVAs), unless stated otherwise.
Thirty-six controls, with no psychiatric or neurological history, were included in the analyses (N = 43 controls were recruited and tested, but data from N = 36 was used in the analyses due to strict EEG data inclusion criteria (Section 2.4; Table 1). Psychiatric history absence was established using SCID-IV-TR screening questions; if a question was endorsed, the appropriate modulate was administered. If a likely psychiatric disorder was suspected, these individuals were excluded (screener/modules administered over-the-phone by well-trained research personnel). To be included, controls also had to score ≤ 13 on the Beck Depression Inventory-II (BDI-II; Beck et al., 1996) and report no psychiatric history in first-degree relatives.
2.2. Antidepressant regimens
MDD patients were randomized (double-blind) to one of three regimens: escitalopram (ESC) + placebo (N = 11), bupropion (BUP) + placebo (N = 14) or ESC + BUP (N = 11; Stewart et al., 2014). Patients were assessed weekly for the first four weeks, and bi-weekly thereafter. Dosing was raised if tolerated and remission not reached (average mg dose at 12 wk. for dual treatment: ESC = 30, BUP = 362.5; for monotherapy: ESC = 34.2, BUP = 407.1). In total, 20 individuals were treatment responders and 16 were non-responders (Table 2).
Table 2.
Characteristics of antidepressant treatment responders & non-responders (means ± S.D.)
| Responder (N = 20) |
Non-responder (N = 16) |
Significance | |
|---|---|---|---|
| Sex (M/F) | 9/11 | 6/10 |
p = 0.65 (Chi-square test) |
| Age | 34.2 ± 11.7 (range: 28–63) |
47.3 ± 10.3 (range: 19–57) |
p < 0.001 |
| Education (yrs.) | 15.4 ± 2.4 | 16.1 ± 2.4 | p = 0.25 |
| Ethnicity | 17 Caucasian; 2 Asian; 1 South Asian | 1 African; 15 Caucasian | N.A. |
| Co-morbid anxiety | N = 2 (panic disorder; specific phobia) | N = 2 (GAD, PTSD) | N.A. |
| Age of MDD onset | 24.9 ± 14.1 | 30.5 ± 12.9 | p = 0.31 |
| Pre-treatment MADRS | 29.7 ± 4.5 | 30.3 ± 5.9 | p = 0.51 |
| Pre-treatment HAMD17 | 20.4 ± 6.0 | 21.3 ± 3.8 | p = 0.49 |
| Week 12 MADRS | 6.0 ± 5.0 | 23.1 ± 6.8 | p < 0.001 |
| Week 12 HAMD17 | 4.3 ± 3.3 | 15.6 ± 4.4 | p < 0.001 |
GAD: Generalized Anxiety Disorder; HAMD17: 17-Item Hamilton Rating Scale for Depression; MADRS: Montgomery-Åsberg Depression Rating Scale; MDD: major depressive disorder; N.A.: not available; PTSD: Post-Traumatic Stress Disorder.
Note: P values index one-way analyses of variance (ANOVAs), unless stated otherwise.
2.3. Session overview
Prior to testing, all participants abstained for > 3 h from caffeine and/or nicotine, and overnight from all other drugs (other than those for a stabilized physical condition). Electrodes were applied, and resting EEG was recorded. All participants were compensated $30.00 CDN. This study was approved by the Royal Ottawa Health Care Group and University of Ottawa Social Sciences & Humanities Research Ethics Boards; written informed consent was obtained from all participants.
2.4. Electrophysiological recordings and data reduction
While participants were seated in a testing chamber, EEG recordings were obtained during 3 min vigilance-controlled eyes-closed (EC) and 3 min eyes-open (EO) conditions (counterbalanced). EEG activity was recorded (500 Hz) using a 32 Ag/AgCl electrode cap (EasyCap, Inning am Ammersee, Germany) positioned according to the 10–10 system (Chatrian et al., 1985); an AFz electrode was the ground and averaged mastoids (TP9/10) were the reference. Electrooculographic (EOG) activity was monitored with 4 additional electrodes; impedance was maintained at ≤ 5 KΩ during EEG recordings (BrainVision Recorder, Gilching, Germany).
Off-line, signals were filtered (0.5–55 Hz; notch filter: 60 Hz; slope: 24 dB/octave), ocular-corrected (Gratton et al., 1983) and segmented into 2 s epochs. This was followed by an automatic artifact rejection procedure, which excluded epochs with EEG activity exceeding ± 50 μV (BrainVision Analyzer, Gilching, Germany). Data were also visually inspected to ensure that no faulty channels were included/no channel drift existed (this resulted in the exclusion of data from 14 MDD and 7 controls from our original sample). Subsequently, a minimum of 50 s of artifact-free EEG data (MDD: 146.5/141.2 s of EO/EC EEG data; controls: 159.2/157.6 s of EO/EC EEG data) from 28 electrodes (Fp1/2; F3/4; F7/8; FC1/2; FC5/6; C3/4; CP1/2; CP5/6; P3/4; P7/8; T7/8; O1/2; Fz/Cz/Pz/Oz for each of the EO and EC conditions) were exported in ASCII format.
2.5. Multi-scale entropy (MSE) estimation of temporal signal complexity
Full details of multi-scale entropy (MSE) and its relevance for the analyses of signal complexity are provided in Costa et al. (2005). The MSE method calculates entropy as a measure of regularity (predictability) of the EEG signal at different temporal scales. The calculation of MSE involves two steps. First, data are resampled to create several temporal scales. For each scale, data points within non-overlapping windows are averaged. For example, scale 1 is the raw time series (i.e., 2 ms windows in the context of a 500 Hz sampling rate), scale 2 averages over 2 time points (i.e., 4 ms windows), scale 20 averages over 20 time points (i.e., 40 ms windows), etc. Second, sample entropy is calculated for each epoch, measuring predictability by evaluating the appearance of repetitive patterns. We calculated MSE for each epoch using the algorithm available at www.physionet.org/physiotools/mse/, with parameter values m (pattern length) = 2 and r (tolerance) = 0.5. The length of the time series was 1000 data points (corresponding to 2 s epochs at a sampling rate of 500 Hz). For each participant, electrode- and condition-specific (EO/EC) MSE estimates were obtained as a mean across within-epoch entropy measures for scales 1–20 (or 2 ms to 40 ms windows), where lower values represent fine temporal scales, and higher values represent coarse temporal scales. Given that there are no known published data on whether differences exist in MSE profiles between EO and EC conditions, both were included. It is also feasible that the sensitivity for detecting group differences may have differed between conditions, thus, assessing both was a prudent approach.
2.6. Spectral power density (SPD)
In addition to MSE, we calculated spectral power density (SPD). A comparison of MSE and SPD results allows us to examine how MSE measures relate to the frequency content of the EEG signal, and to evaluate if treatment response and age-related changes depend more on linear (assessed by both MSE and SPD) or nonlinear (assessed only by MSE) dependencies in the data (Courtiol et al., 2016, Wang et al., 2016). SPD was evaluated using a Fast Fourier Transform (FFT) for each epoch. Power was first normalized (M = 0, SD = 1), then, the relative contribution of different frequencies to total spectral power was calculated, with a frequency resolution of 0.5 Hz. Single epoch estimates were averaged to obtain mean SPD for each condition (EO/EC) per subject (calculated using in-house code ran on Matlab; The MathWorks, Inc., Natick, Massachusetts).
2.7. Partial least squares (PLS) analyses
Statistical assessments of MSE and SPD measures were carried out using partial least squares analyses (PLS; McIntosh and Lobaugh, 2004, Krishnan et al., 2011). We addressed the following questions in our MSE and SPD analyses: 1. Whether there were group differences (MDD responders, non-responders, controls) in condition-dependent MSE and SPD measures (conditions: EO, EC) at baseline. 2. Whether there were group-dependent (MDD responders, non-responders, controls) differences in baseline correlations between MSE and age, and SPD and age. 3. We also examined whether there were responder/non-responder differences in brain-behaviour correlations between MSE with MADRS change scores (MADRSPre-treatment-MADRSWeek12; a greater positive change score indicates greater depression symptom improvement from baseline/pre-treatment) and with age, and between SPD with MADRS change scores and with age. We kept responders and non-responders in separate groups as we wanted to allow for the assessment of potential group differences in MADRS change scores and age effects. Controls were not included in this analysis as they lacked MADRS change scores (controls were tested once).
For the sake of simplicity, the implementation of PLS analyses is described in the context of MSE, but this process was the same for SPD.
2.7.1. Task PLS
Task PLS identifies latent variables (LVs; i.e., the expression of the PLS results) that highlight similarities or differences between groups and/or conditions. The LVs contain three vectors. The first vector indicates the strength of the effect. The remaining two vectors relate experimental design and brain signal. The experimental design vector contains task saliences, which indicate the degree to which each condition in each group is related to the brain signal pattern identified in the LV. These task saliences can be interpreted as the contrast that codes the effect depicted in the LV. The brain signal vector contains MSE temporal scale saliences. These are numeric electrode weights which identify the electrodes at temporal scales that are most related to the effects expressed in the LV. For each LV, there is one salience per electrode timescale that applies to all groups and all conditions.
2.7.2. Behaviour PLS
We used behaviour PLS to examine: (a) group- and condition-dependent correlations between age and MSE/SPD in all three groups (responder, non-responder, controls), and (b) group- and condition-dependent correlations between age and MADRS change scores in MSE/SPD in the MDD groups (responder, non-responder).
We performed PLS on a correlation matrix comprised of the covariance between MSE measures and age/MADRS change scores across participants in each group. Behaviour PLS results are very similar to those of task PLS, except that LVs show similarities or differences between groups/conditions in terms of brain-‘behaviour’ (age/MADRS) correlations.
2.7.3. PLS statistical assessment
Statistical assessment in PLS is carried out across two levels. First, the overall significance of each LV is assessed with permutation testing (Good, 2000). An LV was considered significant if the observed singular value exceeded the permuted singular value in > 95% of the permutations (p < 0.05). Second, bootstrap resampling is used to estimate confidence intervals around electrode weights in each LV, allowing for an assessment of the relative contribution of particular electrodes/timescales, and the stability of the relation with age, MADRS change scores and group/condition (Efron and Tibshirani, 1986). No corrections for multiple comparisons are necessary because the electrode timescale weights are calculated in a single mathematical step on the whole brain. For the MSE and SPD measures, we plotted bootstrap ratios (ratio of individual weights over estimated standard error) as a proxy for z-scores. Confidence intervals were plotted for group effects. A minimum threshold of a stable 95% confidence interval was used for all analyses.
3. Results
3.1. Participants
Participant characteristics (MDD vs. controls; treatment responders vs. non-responders) are presented in Table 1, Table 2. A one-way analysis of variance (ANOVA) yielded a main effect of group (MDD responders, non-responders, controls) on age [F(2,69) = 8.08, p = 0.001]; follow-up comparisons indicated that non-responders were older than responders and controls (p < 0.001). Though problematic from a statistical perspective, the composition of responder and non-responder groups was beyond our control (i.e., these groups could not be matched on age ahead of time). Importantly, this age difference was not problematic with respect to the interpretation of our results as atypical age effects appear only in the responder group (outlined in subsequent sections), who did not differ in age from the control group.
3.2. MSE
3.2.1. Group (MDD responders, non-responders, controls) & condition (EO/EC) effects
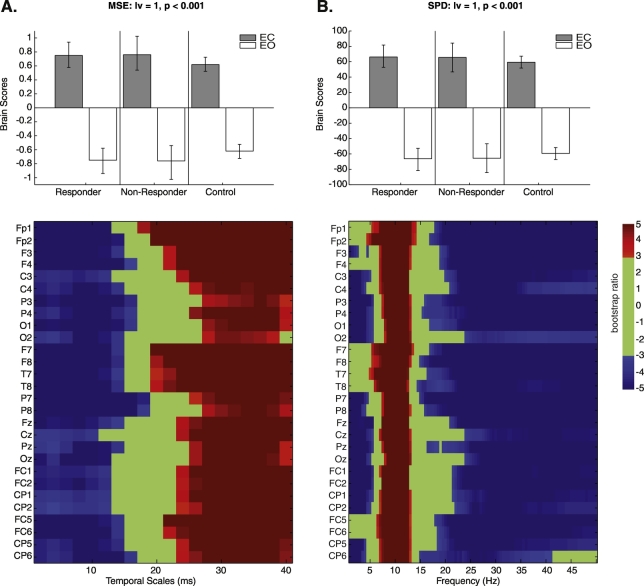
Task PLS examining groups at baseline (MDD responders, non-responders, controls) and conditions (EO/EC) identified one significant LV, indicating an MSE difference between EC and EO conditions that was common across groups (p < 0.001; Fig. 1A). Increased sample entropy was observed at fine temporal scales in the EO condition at 2–14 ms, with a cross-over at coarser scales, with greater sample entropy for the EC condition starting at 22 ms. These differences were homogeneous across all electrodes.
Fig. 1.
Task partial-least squares (PLS) examining group (antidepressant treatment responders, non-responders, controls) and condition (eyes-open [EO]/eyes-closed [EC]) effects in multiscale entropy (MSE) (A) and spectral power density (SPD) at baseline (B). Bar graphs depict the contrast between EO/EC conditions across groups that was significantly expressed across the entire dataset as determined by permutation tests. The statistical image plots (figures below) represent bootstrap ratio maps. Each row represents electrodes and timescales/frequencies at which the contrast displayed in the bar graphs was most stable as determined by bootstrapping. Values represent the ratio of the parameter estimate for the electrode source divided by the bootstrap-derived standard error (roughly z-scores). Positive values indicate timescales and electrode sources showing higher MSE/SPD during the EC condition, while negative values depict timescales and electrode sources showing higher MSE/SPD during the EO condition.
3.2.2. Group (MDD responders, non-responders, controls), condition (EO/EC) & age effects
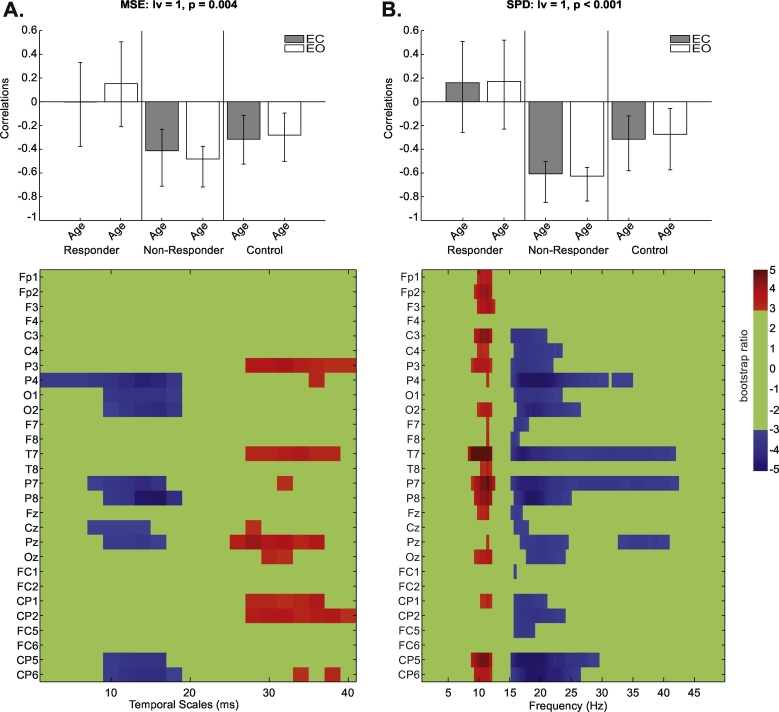
Behaviour PLS examining group- (responders, non-responders, controls) and condition- (EO/EC) dependent changes in MSE associated with age identified one significant LV (p = 0.004; Fig. 2A). It revealed an age effect across both conditions that was specific to controls and MDD non-responders, but was absent in responders. An increase in MSE (both EO/EC) with age was noted at fine temporal scales (2–18 ms) at P4, O1/2, P7/8, Cz, Pz and CP5/6. There was a crossover to a decrease in MSE for EO/EC conditions with age at coarse temporal scales (26–40 ms). These effects were strongest at electrodes P3, T7, Pz, and CP1/2.
Fig. 2.
Behaviour partial-least squares (PLS) results for the correlation of multiscale entropy (MSE) and age (A) as well as spectral power density (SPD) and age (B) by condition (eyes-open [EO]/eyes-closed [EC]) and group (antidepressant responders, non-responders and healthy controls) at baseline. Bar graphs depict the contrast between groups across EO/EC conditions that were significantly expressed across the entire dataset as determined by permutation tests. The statistical image plots (figures below) represent bootstrap ratio maps. Each row represents electrodes and timescales/frequencies at which the correlation between MSE/SPD and age was most stable as determined by bootstrapping. Values represent the ratio of the parameter estimate for the electrode divided by the bootstrap-derived standard error (roughly z-scores). Positive values indicate timescales and electrode sources showing decreases in MSE/SPD in EC/EO conditions with increasing age, while negative values show increases in MSE/SPD in EC/EO conditions with increasing age.
3.2.3. MDD group (responders/non-responders), condition (EO/EC), age & MADRS change score effects
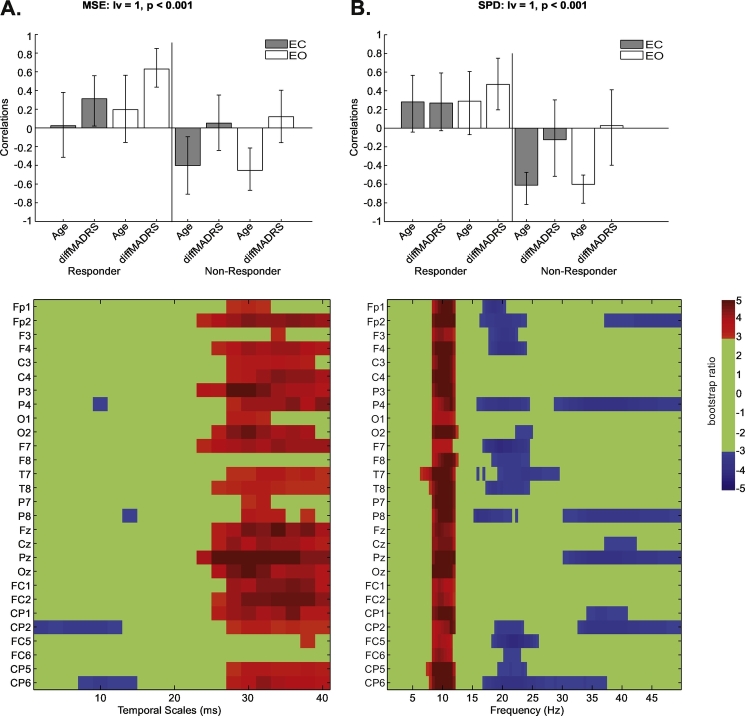
For our behaviour PLS examining MDD group- and condition-dependent changes in MSE associated with age and MADRS change scores, we kept responders and non-responders in separate groups because age-related differences (Section 3.2.1) could interact with MADRS-related effects. Our analysis identified one significant LV (p < 0.001; Fig. 3A). This LV identified a relation between MSE and MADRS change scores in MDD responders that was not associated with age. A decrease in EO/EC MSE with greater positive MADRS change scores was observed at fine temporal scales (2–16 ms), which was strongest at CP2 and CP6. There was a crossover to an increase in MSE with greater positive MADRS change scores at coarse temporal scales (24–40 ms). These effects were widespread, across almost all electrodes, and strongest at P3, O2, Fz, Pz, Oz, and FC1/2. For MDD non-responders, this LV identified an age effect that was not associated with MADRS score changes. In non-responders, there was increase in EO/EC MSE with age at fine temporal scales (2–16 ms), with a crossover to a decrease in EO/EC MSE with age at coarse emporal scales (26–40 ms). The spatial configuration of these effects matched that of the responder MADRS effect described above. Supplementary Fig. 1 illustrates MSE-MADRS change score correlations for responders and non-responders at electrode Pz at 30 ms per treatment regimen.
Fig. 3.
Behaviour partial-least squares (PLS) results for the correlation of multiscale entropy (MSE) with age and Montgomery-Åsberg Depression Rating (MADRS) changes (A) and spectral power density (SPD) with age and MADRS changes (B) by condition (eyes-open [EO]/eyes-closed [EC]) and group (antidepressant responders, non-responders) at baseline. Bar graphs depict the contrast between groups across the EO/EC conditions that were significantly expressed across the entire dataset as determined by permutation tests. The statistical image plots (figures below) represent bootstrap ratio maps. Each row represents electrodes and timescales/frequencies at which the correlation between MSE/SPD with age and MADRS change scores was most stable as determined by bootstrapping. Values represent the ratio of the parameter estimate for the electrode divided by the bootstrap-derived standard error (roughly z-scores). In responders, positive values indicate timescales and electrodes showing increases in MSE/SPD in EC/EO conditions with increasing MADRS change score, while negative values show decreases with increasing MADRS change score; the correlation with age is not stable. In non-responders, positive values indicate timescales and electrodes showing decreases in MSE/SPD in EC/EO conditions with increasing age, while negative values show increases with age; the correlation with MADRS change scores is not stable.
Overall, these results suggest that decreased fine scale MSE at centro-parietal electrodes, and increased mid- to coarse scale MSE throughout the brain at baseline is associated with an increased antidepressant response specifically in responders. To ensure that these results hold independent of age, we performed a supplementary analysis without age. This behaviour PLS, examining changes in MSE associated with MADRS change scores, identified one LV (p = 0.004), which replicated the MADRS change score effect described above. Again, in responders, an EO/EC MSE decrease existed with greater positive MADRS change scores (i.e., greater antidepressant response) at fine temporal scales (2–12 ms; strongest at T8, CP2 and FC5). There was a crossover to an increase in EO/EC MSE with greater positive MADRS change scores at coarse temporal scales (24–40 ms; effects were widespread, and strongest at C4, P4, F7, Pz, FC1/2 and FC5).
3.3. SPD
3.3.1. Group (MDD responders, non-responders, controls) & condition (EO/EC) effects
The task PLS examining group (MDD responders, non-responders, controls) and condition (EO/EC) identified one significant LV, indicating an SPD difference between EC and EO conditions that was common across all groups (p < 0.001; Fig. 1B). Increased power in delta (< 4 Hz) and beta/gamma (16–50 Hz) was observed in EO across all electrodes. For EC, increased alpha power (8–13 Hz) was noted across all electrodes, and in theta (4–8 Hz) at Fp2, F7 and T7.
3.3.2. Group (MDD responders, non-responders, controls), condition (EO/EC), & age effects
The behaviour PLS examining group- and condition-dependent changes in SPD associated with age revealed an age effect specific to MDD non-responders and controls (absent in responders; p < 0.001; Fig. 2B). SPD decreased with age during EO/EC conditions in alpha (8–13 Hz) throughout the brain (maximal at Fp2, C3, T7, P7/8 and CP5). Additionally, SPD increased with age in the beta (16–30 Hz) and gamma bands (30–44 Hz) throughout the brain (maximal at P4, T7, T5, P8 and CP5).
3.3.3. MDD group (responders/non-responders), condition (EO/EC), age & MADRS change score effects
The behaviour PLS examining group- and condition-dependent changes in SPD associated with age and MADRS change scores identified one significant LV (p < 0.001; Fig. 3B). This LV indicated a MADRS change score effect for MDD responders in the EO condition that was not associated with age. EO SPD increased with greater positive MADRS change scores in high theta (6–7 Hz) and alpha (8–13 Hz); effects were homogeneous across electrodes. EO SPD decreased with increased MADRS change scores in beta (16–30 Hz) and gamma (30–50 Hz). These effects were strongest at Fp2, P4, F7, T7/8, P8, FC5 and CP6 for beta (17–25 Hz), and Fp2, P4, P8, Pz and CP2 for gamma (37–50 Hz). For MDD non-responders, this LV identified an age effect in EO/EC conditions that was not associated with MADRS change scores. EO/EC SPD decreased with age and in high theta (6–7 Hz) and alpha (8–13 Hz); effects were homogeneous across all electrodes. EO/EC SPD increased with age in the beta (16–30 Hz) and gamma bands (30–50 Hz). Effects were strongest at Fp2, P4, F7, T7/8, P8, FC5, and CP6 for beta (17–25 Hz), and Fp2, P4, P8, Pz, and CP2 for gamma (37–50 Hz).
4. Discussion
4.1. Overview
Using resting-state EEG collected prior to antidepressant pharmacotherapy, our study is the first to investigate the relative importance of global and local processing for successful treatment response. Additionally, because previous work suggests that the propensity for more local versus global processing changes with age, we examined if such age-related changes interact with the EEG signal variability profile associated with treatment outcome. We found that normal aging MSE profiles were not evident in future responders. Instead, in future responders, an increased propensity toward large-scale network processing prior to treatment was associated with a greater antidepressant response.
4.2. MSE & SPD differences between eyes-open and closed conditions
Our task PLS identified only one significant LV, a main effect of condition, differentiating EO from EC resting-state profiles across all groups (responders, non-responders and controls). Contrary to our expectations, this analysis did not identify a main effect of treatment response group. Treatment effects do exist on our data, and are discussed in Section 4.4.
Our results suggest that the EO condition was associated with greater MSE than the EC condition at fine temporal scales, whereas the EC resting-state condition was associated with increased MSE at mid- to coarse-temporal scales. Although there are no known published MSE comparisons between EO and EC resting-state conditions, the MSE data are complementary to our SPD findings, which are consistent with previous work. We found that higher frequencies (16–50 Hz) were more dominant during EO, whereas alpha was more dominant during EC. Alpha is thought to be inversely associated with cortical and physiological arousal (Barry et al., 2007); it is prominent when the eyes are closed and is maximal in occipital-parietal regions, though it can extend to frontal/thalamic regions (O'Gorman et al., 2013). Given suggestions that lower EEG frequencies modulate activity over larger spatial regions in longer temporal windows (von Stein and Sarnthein, 2000, Vakorin et al., 2011, McIntosh et al., 2014), increased alpha may reflect greater longer-range variability associated with more diffuse oscillations during EC. Conversely, increased SPD in beta/gamma during EO may reflect relatively increased activity in more local networks, though this is speculative.
4.3. Age-related changes in MSE & SPD
Our behaviour PLS examining the relationship between resting-state MSE and age replicated the age effect reported in previous studies (Hogan et al., 2012, McIntosh et al., 2014, Sleimen-Malkoun et al., 2015, Wang et al., 2016). However, this effect was stable only for controls and eventual antidepressant non-responders, and did not exist in future responders. In controls and non-responders, MSE increased with age at fine temporal scales (2–18 ms) bilaterally in non-frontal regions (with strongest effects parietally), and decreased with increasing age at coarser temporal scales (26–40 ms) in the same regions. Consistent with previous literature, this supports the idea that healthy aging is characterized by increased local information processing (higher MSE at fine temporal scales) and decreased long-range interactions with other neural populations (lower MSE at coarse temporal scales) in controls and non-responders (Hogan et al., 2012, McIntosh et al., 2014, Sleimen-Malkoun et al., 2015, Wang et al., 2016). That is, aging tends to shift the integration between distributed neural populations toward more local neural processing, particularly in more posterior brain regions (Vakorin et al., 2011). Given that this aging pattern is ubiquitous in the MSE literature, it is interesting that it does not hold in future treatment responders. As such, our data suggests that the EEG signal variability profile associated with treatment outcome might interact with age-related changes.
Our SPD results were complementary to our MSE results, where the age effect was stable only for controls and future antidepressant non-responders, but not for future responders. For controls and non-responders, increasing age was associated with greater power in beta and gamma bands, and decreased alpha power throughout the brain. Previous work indicates that healthy aging (i.e., from young to older adulthood) tends to be accompanied by a decrease in resting-state activity in low frequencies (0.5–6.5 Hz), with increases in beta power (Vlahou et al., 2014; though profiles can change in the very elderly). These SPD results indicate that with increasing age, there is a relative increase in the contribution of local communication (likely supported by higher frequencies) and a relative decrease in the contribution of long-range communication (supported by low frequencies; von Stein and Sarnthein, 2000).
Previous literature is mixed about the degree of complementarity between MSE and power spectrum/SPD analyses. For instance, while some studies show similar effects between the two approaches (e.g., Lippe et al., 2009, McIntosh et al., 2008, McIntosh et al., 2014, Mizuno et al., 2010, Misic et al., 2011, Sleimen-Malkoun et al., 2015, Szostakiwskyj et al., 2017), others identified differences (e.g., Catarino et al., 2011, Heisz et al., 2012, Takahashi et al., 2009, Ueno et al., 2015, Wang et al., 2016). Potential inconsistencies between MSE and SPD results have been interpreted to reflect differences in the relative contribution of linear versus nonlinear dependencies in the data; changes in linear dependencies are evident in both MSE and SPD, whereas changes in nonlinear dependencies are evident only in MSE (Courtiol et al., 2016, Wang et al., 2016). In the context of the current work, a comparison of MSE and SPD results suggests that our age-related effects are associated with alterations in the linear dependencies that are evident in both MSE and SPD, rather than nonlinear dependencies that would be evident only in MSE.
4.4. MDD treatment response and its relation to age and pre-treatment MSE and SPD
Our task PLS identified no significant main effect of treatment response group. Given that in this analysis eventual treatment responders and non-responders were categorized based on a specific cutoff score (i.e., responders exhibited ≥ 50% reduction in MADRS scores from pre-treatment to week 12), it was feasible that variation in response magnitude within each group could mask potential differences between groups. That is, there was a broad distribution of response magnitudes in both samples. Thus, we felt that characterizing the relationship between response magnitude and MSE patterns within each group was worthwhile. Additionally, non-responders showed the typical age-related MSE profile as controls, but responders did not. This differential age effect might influence potential MSE differences between the magnitude of treatment response in future responders and non-responders. As such, we examined whether the magnitude of response by week 12 of treatment (i.e., MADRSPre-treatment -MADRSWeek12) and age were related to baseline brain signal variability per group. Our behaviour PLS identified a significant association between MSE and treatment response specifically in the responder group. In responders, greater depression symptom alleviation (i.e., greater change in MADRS scores) was associated with increased mid- to coarse-scale MSE (24–40 ms) in diffuse (frontal, central and parietal) brain regions and decreased fine-scale MSE (2–12 ms) in fronto-central regions. This relation was not stable/not significant in non-responders. As with our age analysis described above, the canonical MSE age effect was not present in future responders. This suggests that greater propensity for mid-to-long-range communication among neuronal populations, coupled with decreased reliance on local communication before treatment, is important for good treatment response. Lastly, as a supplementary analysis, we assessed whether there were responder/non-responder differences in MSE measures that correlated with MADRS change scores without age included in the analysis, and confirmed previous assessments. Namely, that in responders, increased mid-to-long-range communication, and decreased reliance on local communication, was coupled not with age, but with a good treatment response.
Antidepressant treatments (pharmacological and otherwise) most consistently affect large-scale connectivity normalization within the DMN (Sheline et al., 2010, Kuhn and Gallinat, 2013, Li et al., 2013, Kaiser et al., 2015, Mulders et al., 2015, Martino et al., 2016) and connectivity changes between cortico-limbic regions (Gudayol-Ferre et al., 2015, Dichter et al., 2015). Thus, our results suggest that an increased propensity toward distal communication (perhaps involving the DMN or cortical-limbic regions) is what may facilitate large-scale network normalization in treatment responders.
Our SPD results are complementary to our MSE results, suggesting that linear (rather than nonlinear) dependencies are driving our effects. For SPD, greater treatment response was associated with increased pre-treatment power in the theta and alpha bands, and decreased power in beta and gamma bands in responders. This relationship was not stable in non-responders. Decreased MSE at fine temporal scales appears to reflect the negative association between treatment response (i.e., MADRS score changes) and SPD at higher frequencies (beta/gamma). On the other hand, increased MSE at coarse temporal scales appears to reflect the positive association between treatment response and SPD at lower frequencies (alpha/theta band). Elevated alpha power/amplitude at baseline has been quite consistently observed in eventual responders (vs. non-responders) to various antidepressant pharmacotherapies (Knott et al., 1996, Tenke et al., 2011, Jaworska et al., 2014). One interpretation is that this may reflect a hypo-aroused state that benefits from the arousing electrocortical effects of some antidepressants. Again, our all-encompassing analysis examining whether there were responder/non-responder differences in SPD measures that correlated with both age and MADRS change scores confirmed the results from the individual age- and MARDS change score analyses.
To our knowledge, very few studies have assessed the relationship between pre-treatment EEG signal variability and antidepressant treatment response, and as such, our work addresses this literature gap. Arns et al. (2014) compared pre-treatment EEG variability using Lempel-Ziv complexity [LZC] in depressed responders and non-responders to repetitive transcranial magnetic stimulation (rTMS). Although they found no prognostic value in averaged LZC measures, they showed that LZC changes during the measuring session were informative. Eventual treatment responders (categorized based on depression score changes from baseline) exhibited an increase in signal complexity in the alpha band from the first to the second minute of recording at baseline, while non-responders showed a decrease (Arns et al., 2014). Although a direct comparison of their results with the current study is not possible, both studies suggest that variability metrics associated with longer-range neural communication at pre-treatment appears pertinent for a positive treatment response.
4.5. Conclusions & limitations
Our paper is not without limitations. Notably, our eventual non-responder group was older than our responders and controls. However, the effect of age on treatment response is quite weak (De Carlo et al., 2016). Additionally, responders (not non-responders) were the ones who exhibited atypical age-related MSE and SPD profiles. Second, although our sample size was adequate for this study (and comparable to other published research), larger sample sizes grouped by age may have revealed more subtle effects, and possibly allowed for improved characterization of the interplay between treatment response and age. Third, future research would also benefit from the addition of fMRI-connectivity based measures, as spatial assessments of connectivity profiles between and within networks, would complement the current work. Finally, ideally, the HC and MDD groups would have been assessed with the same depression symptom scales (however, the use of the BDI in the HC group was to ascertain the absence of significant depression symptoms).
In sum, our study aimed to assess how the propensity for local versus global communication, as measured with pre-treatment EEG signal variability, relates to antidepressant pharmacotherapy response. We found that in eventual responders, decreased MSE at fine temporal scales (especially fronto-centrally) and increased MSE at coarser temporal scales (especially fronto-centrally and parietally) at baseline was associated with greater antidepressant response following 12 weeks of antidepressant pharmacotherapy. Complimentary SPD analyses indicated that greater MADRS score decreases were associated with greater pre-treatment theta/alpha and lower fronto-central beta/gamma in responders. Interestingly, when we examined how this relationship is modulated by age, we found that the canonical age-related shift in neural profiles, from more global to more local neural processing with increasing age, was not evident in future responders. Complimentary SPD analyses showed a similar effect, wherein age-associated changes, including decreased theta/alpha and increased beta/gamma with increased age, was evident only in controls and non-responders, but not in future responders. Thus, our aging analyses underscore the idea that an increased propensity toward distal communication supports greater antidepressant response.
Overall, this work presents preliminary, yet compelling, evidence that the brain dynamics of individuals who are sensitive/responsive to antidepressant treatment is different from those who do not adequately respond. Increased treatment response, regardless of age, is characterized by an increased propensity for long-range interactions among neural populations (lower MSE at coarse temporal scales). This contributes to the literature regarding the utility of objective electrocortical markers in predicting clinical response. Future examination of variability changes throughout the course of treatment would be especially worthwhile to deepen our characterization of the pathophysiology of MDD (Okazaki et al., 2013, Arns et al., 2014), particularly with respect to comparing variability profiles in eventual treatment responders compared with non-responders.
The following is the supplementary data related to this article.
Multiscale entropy (MSE) - Montgomery-Åsberg Depression Rating (MADRS) change score correlations with linear fits. MSE-MADRS change score scatterplots for responders (A) and non-responders (B) at electrode Pz at 30 ms (i.e., coarse temporal scale with highest bootstrap ratio). We identify participants in different antidepressant regimens by colour: escitalopram + bupropion (BUP + ESC) is indicated with blue, bupropion (BUP) is indicated with green, and escitalopram (ESC) is indicated with red circles.
Funding sources
Patients from this study were recruited from an NIH-funded clinical trial (5R01MH077285). The research was supported by an Alberta Innovates Health Solutions Fellowship to N.J., a Canadian Foundation for Innovation Leaders Opportunity Fund grant to A.B.P. [grant number 30320], and Alberta Enterprise and Advanced Education Research Capacity Program, Alberta Alignment Grant to A.B.P. [grant number RCP-13-38-SEG].
Acknowledgments
Acknowledgments
We would like to thank Dr. Signe Bray for her constructive feedback on our manuscript. Additionally, we would like to thank Drs. Claude Blondeau, Pierre Tessier and Sandhaya Norris for their help with clinical assessments and diagnoses.
References
- Arns M., Cerquera A., Gutierrez R.M., Hasselman F., Freund J.A. Non-linear EEG analyses predict non-response to rTMS treatment in major depressive disorder. Clin. Neurophysiol. 2014;125(7):1392–1399. doi: 10.1016/j.clinph.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Barry R.J., Clarke A.R., Johnstone S.J., Magee C.A., Rushby J.A. EEG differences between eyes-closed and eyes-open resting conditions. Clin. Neurophysiol. 2007;118(12):2765–2773. doi: 10.1016/j.clinph.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. Psychological Corporation; San Antonio, TX, USA: 1996. Manual for the Beck Depression Inventory-II. [Google Scholar]
- Catarino A., Churches O., Baron-Cohen S., Andrade A., Ring H. Atypical EEG complexity in autism spectrum conditions: a multiscale entropy analysis. Clin. Neurophysiol. 2011;122:2375–2383. doi: 10.1016/j.clinph.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Chatrian G.E., Lettich E., Nelson P.L. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activity. Am. J. EEG Technol. 1985;25:83–92. [Google Scholar]
- Costa M., Goldberger A.L., Peng C.K. Multiscale entropy analysis of biological signals. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2005;71(2 Pt 1):021906. doi: 10.1103/PhysRevE.71.021906. [DOI] [PubMed] [Google Scholar]
- Courtiol J., Perdikis D., Petkoski S., Muller V., Huys R., Sleimen-Malkoun R., Jirsa V.K. The multiscale entropy: guidelines for use and interpretation in brain signal analysis. J. Neurosci. Methods. 2016;273:175–190. doi: 10.1016/j.jneumeth.2016.09.004. [DOI] [PubMed] [Google Scholar]
- De Carlo V., Calati R., Serretti A. Socio-demographic and clinical predictors of non-response/non-remission in treatment resistant depressed patients: a systematic review. Psychiatry Res. 2016;240:421–430. doi: 10.1016/j.psychres.2016.04.034. [DOI] [PubMed] [Google Scholar]
- Demirtas M., Tornador C., Falcon C., Lopez-Sola M., Hernandez-Ribas R., Pujol J., Menchon J.M., Ritter P., Cardoner N., Soriano-Mas C., Deco G. Dynamic functional connectivity reveals altered variability in functional connectivity among patients with major depressive disorder. Hum. Brain Mapp. 2016;37(8):2918–2930. doi: 10.1002/hbm.23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter G.S., Gibbs D., Smoski M.J. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J. Affect. Disord. 2015;172:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner S.G., Beevers C.G., Haigh E.A., Beck A.T. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 2011;12(8):467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Efron B., Tibshirani R. Bootstrap methods for standard errors, confidence intervals and other measures of statistical accuracy. Stat. Sci. 1986;1:54–77. [Google Scholar]
- Fingelkurts A.A., Fingelkurts A.A. Altered structure of dynamic electroencephalogram oscillatory pattern in major depression. Biol. Psychiatry. 2015;77(12):1050–1060. doi: 10.1016/j.biopsych.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Fingelkurts A.A., Fingelkurts A.A., Rytsala H., Suominen K., Isometsa E., Kahkonen S. Impaired functional connectivity at EEG alpha and theta frequency bands in major depression. Hum. Brain Mapp. 2007;28(3):247–261. doi: 10.1002/hbm.20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good P. 2nd edition. Springer; New York, NS, USA: 2000. Permutation Tests: A Practical Guide to Resampling Methods for Testing Hypotheses. [Google Scholar]
- Gratton G., Coles M.G., Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gudayol-Ferre E., Pero-Cebollero M., Gonzalez-Garrido A.A., Guardia-Olmos J. Changes in brain connectivity related to the treatment of depression measured through fMRI: a systematic review. Front. Hum. Neurosci. 2015;9:582. doi: 10.3389/fnhum.2015.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Farmer M., Fogelman P., Gotlib I.H. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol. Psychiatry. 2015;78(4):224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisz J.J., Shedden J.M., McIntosh A.R. Relating brain signal variability to knowledge representation. NeuroImage. 2012;63:1384–1392. doi: 10.1016/j.neuroimage.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Hogan M.J., Kilmartin L., Keane M., Collins P., Staff R.T., Kaiser J., Lai R., Upton N. Electrophysiological entropy in younger adults, older controls and older cognitively declined adults. Brain Res. 2012;1445:1–10. doi: 10.1016/j.brainres.2012.01.027. [DOI] [PubMed] [Google Scholar]
- Iwabuchi S.J., Krishnadas R., Li C., Auer D.P., Radua J., Palaniyappan L. Localized connectivity in depression: a meta-analysis of resting state functional imaging studies. Neurosci. Biobehav. Rev. 2015;51:77–86. doi: 10.1016/j.neubiorev.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Jaworska N., Blier P., Fusee W., Knott V. Alpha power, alpha asymmetry and anterior cingulate cortex activity in depressed males and females. J. Psychiatr. Res. 2012;46(11):1483–1491. doi: 10.1016/j.jpsychires.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworska N., Blondeau C., Tessier P., Norris S., Fusee W., Blier P., Knott V. Examining relations between alpha power as well as anterior cingulate cortex-localized theta activity and response to single or dual antidepressant pharmacotherapies. J. Psychopharmacol. 2014;28(6):587–595. doi: 10.1177/0269881114523862. [DOI] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiat. 2015;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott V.J., Telner J.I., Lapierre Y.D., Browne M., Horn E.R. Quantitative EEG in the prediction of antidepressant response to imipramine. J. Affect. Disord. 1996;39(3):175–184. doi: 10.1016/0165-0327(96)00003-1. [DOI] [PubMed] [Google Scholar]
- Knott V., Mahoney C., Kennedy S., Evans K. EEG power, frequency, asymmetry and coherence in male depression. Psychiatry Res. 2001;106(2):123–140. doi: 10.1016/s0925-4927(00)00080-9. [DOI] [PubMed] [Google Scholar]
- Krishnan A., Williams L.J., McIntosh A.R., Abdi H. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. NeuroImage. 2011;56(2):455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Kuhn S., Gallinat J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr. Bull. 2013;39(2):358–365. doi: 10.1093/schbul/sbr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.S., Yang B.H., Lee J.H., Choi J.H., Choi I.G., Kim S.B. Detrended fluctuation analysis of resting EEG in depressed outpatients and healthy controls. Clin. Neurophysiol. 2007;118(11):2489–2496. doi: 10.1016/j.clinph.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Lee T.W., Yu Y.W., Chen M.C., Chen T.J. Cortical mechanisms of the symptomatology in major depressive disorder: a resting EEG study. J. Affect. Disord. 2011;131(1–3):243–250. doi: 10.1016/j.jad.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Leistedt S.J., Coumans N., Dumont M., Lanquart J.P., Stam C.J., Linkowski P. Altered sleep brain functional connectivity in acutely depressed patients. Hum. Brain Mapp. 2009;30(7):2207–2219. doi: 10.1002/hbm.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchter A.F., Cook I.A., Hunter A.M., Cai C., Horvath S. Resting-state quantitative electroencephalography reveals increased neurophysiologic connectivity in depression. PLoS One. 2012;7(2):e32508. doi: 10.1371/journal.pone.0032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Tong S., Liu D., Gai Y., Wang X., Wang J., Qiu Y., Zhu Y. Abnormal EEG complexity in patients with schizophrenia and depression. Clin. Neurophysiol. 2008;119(6):1232–1241. doi: 10.1016/j.clinph.2008.01.104. [DOI] [PubMed] [Google Scholar]
- Li B., Liu L., Friston K.J., Shen H., Wang L., Zeng L.L., Hu D. A treatment-resistant default mode subnetwork in major depression. Biol. Psychiatry. 2013;74(1):48–54. doi: 10.1016/j.biopsych.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K., Monto S., Rytsala H., Suominen K., Isometsa E., Kahkonen S. Breakdown of long-range temporal correlations in theta oscillations in patients with major depressive disorder. J. Neurosci. 2005;25(44):10131–10137. doi: 10.1523/JNEUROSCI.3244-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe S., Kovacevic N., McIntosh A.R. Differential maturation of brain signal complexity in the human auditory and visual system. Front. Hum. Neurosci. 2009;3 doi: 10.3389/neuro.09.048.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino M., Magioncalda P., Huang Z., Conio B., Piaggio N., Duncan N.W., Rocchi G., Escelsior A., Marozzi V., Wolff A., Inglese M., Amore M., Northoff G. Contrasting variability patterns in the default mode and sensorimotor networks balance in bipolar depression and mania. Proc. Natl. Acad. Sci. U. S. A. 2016;113(17):4824–4829. doi: 10.1073/pnas.1517558113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough I.M., Nashiro K. Network complexity as a measure of information processing across resting-state networks: evidence from the human connectome project. Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A.R., Lobaugh N.J. Partial least squares analysis of neuroimaging data: applications and advances. NeuroImage. 2004;23(Suppl. 1):S250–63. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- McIntosh A.R., Kovacevic N., Itier R.J. Increased brain signal variability accompanies lower behavioral variability in development. PLoS Comput. Biol. 2008;4 doi: 10.1371/journal.pcbi.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A.R., Vakorin V., Kovacevic N., Wang H., Diaconescu A., Protzner A.B. Spatiotemporal dependency of age-related changes in brain signal variability. Cereb. Cortex. 2014;24(7):1806–1817. doi: 10.1093/cercor/bht030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez M.A., Zuluaga P., Hornero R., Gomez C., Escudero J., Rodriguez-Palancas A., Ortiz T., Fernandez A. Complexity analysis of spontaneous brain activity: effects of depression and antidepressant treatment. J. Psychopharmacol. 2012;26(5):636–643. doi: 10.1177/0269881111408966. [DOI] [PubMed] [Google Scholar]
- Misic B., Vakorin V.A., Paus T., McIntosh A.R. Functional embedding predicts the variability of neural activity. Front. Syst. Neurosci. 2011;5 doi: 10.3389/fnsys.2011.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Takahashi T., Cho R.Y., Kikuchi M., Murata T., Takahashi K., Wada Y. Assessment of EEG dynamical complexity in Alzheimer's disease using multiscale entropy. Clin. Neurophysiol. 2010;121(9):1438–1446. doi: 10.1016/j.clinph.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montez T., Linkenkaer-Hansen K., van Dijk B.W., Stam C.J. Synchronization likelihood with explicit time-frequency priors. NeuroImage. 2006;33(4):1117–1125. doi: 10.1016/j.neuroimage.2006.06.066. [DOI] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry J. Ment. Sci. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Mulders P.C., van Eijndhoven P.F., Schene A.H., Beckmann C.F., Tendolkar I. Resting-state functional connectivity in major depressive disorder: a review. Neurosci. Biobehav. Rev. 2015;56:330–344. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- O'Gorman R.L., Poil S.S., Brandeis D., Klaver P., Bollmann S., Ghisleni C., Luchinger R., Martin E., Shankaranarayanan A., Alsop D.C., Michels L. Coupling between resting cerebral perfusion and EEG. Brain Topogr. 2013;26(3):442–457. doi: 10.1007/s10548-012-0265-7. [DOI] [PubMed] [Google Scholar]
- Okazaki R., Takahashi T., Ueno K., Takahashi K., Higashima M., Wada Y. Effects of electroconvulsive therapy on neural complexity in patients with depression: report of three cases. J. Affect. Disord. 2013;150(2):389–392. doi: 10.1016/j.jad.2013.04.029. [DOI] [PubMed] [Google Scholar]
- Olbrich S., Trankner A., Chittka T., Hegerl U., Schonknecht P. Functional connectivity in major depression: increased phase synchronization between frontal cortical EEG-source estimates. Psychiatry Res. 2014;222(1–2):91–99. doi: 10.1016/j.pscychresns.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Patten S.B., Williams J.V., Lavorato D.H., Bulloch A.G., MacQueen G. Depressive episode characteristics and subsequent recurrence risk. J. Affect. Disord. 2012;140(3):277–284. doi: 10.1016/j.jad.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Price J.L., Yan Z., Mintun M.A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. U. S. A. 2010;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleimen-Malkoun R., Perdikis D., Muller V., Blanc J.L., Huys R., Temprado J.J., Jirsa V.K. Brain dynamics of aging: multiscale variability of EEG signals at rest and during an auditory oddball task. eNeuro. 2015;2(3) doi: 10.1523/ENEURO.0067-14.2015. (eCollection 2015 May-Jun) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.W., McGrath P.J., Blondeau C., Deliyannides D.A., Hellerstein D., Norris S., Amat J., Pilowsky D.J., Tessier P., Laberge L., O'Shea D., Chen Y., Withers A., Bergeron R., Blier P. Combination antidepressant therapy for major depressive disorder: speed and probability of remission. J. Psychiatr. Res. 2014;52:7–14. doi: 10.1016/j.jpsychires.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Sun Y., Li Y., Zhu Y., Chen X., Tong S. Electroencephalographic differences between depressed and control subjects: an aspect of interdependence analysis. Brain Res. Bull. 2008;76(6):559–564. doi: 10.1016/j.brainresbull.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Szostakiwskyj J.M.H., Willatt S.E., Cortese F., Protzner A.B. The modulation of EEG variability between internally- and externally-driven cognitive states varies with maturation and task performance. PLoS ONE. 2017 doi: 10.1371/journal.pone.0181894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Cho R.Y., Murata T., Mizuno T., Kikuchi M., Mizukami K., Kosaka H., Takahashi K., Wada Y. Age-related variation in EEG complexity to photic stimulation: a multiscale entropy analysis. Clin. Neurophysiol. 2009;120:476–483. doi: 10.1016/j.clinph.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Li Y., Tong S., Li Y., Zhu Y. Entropy analysis of the EEG alpha activity in depression patients. J. Biomed. Eng. 2009;26(4):739–742. [PubMed] [Google Scholar]
- Tenke C.E., Kayser J., Manna C.G., Fekri S., Kroppmann C.J., Schaller J.D., Alschuler D.M., Stewart J.W., McGrath P.J., Bruder G.E. Current source density measures of electroencephalographic alpha predict antidepressant treatment response. Biol. Psychiatry. 2011;70(4):388–394. doi: 10.1016/j.biopsych.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K., Takahashi T., Takahashi K., Mizukami K., Tanaka Y., Wada Y. Neurophysiological basis of creativity in healthy elderly people: a multiscale entropy approach. Clin. Neurophysiol. 2015;126:524–531. doi: 10.1016/j.clinph.2014.06.032. [DOI] [PubMed] [Google Scholar]
- Vakorin V.A., Lippe S., McIntosh A.R. Variability of brain signals processed locally transforms into higher connectivity with brain development. J. Neurosci. 2011;31(17):6405–6413. doi: 10.1523/JNEUROSCI.3153-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahou E.L., Thurm F., Kolassa I.T., Schlee W. Resting-state slow wave power, healthy aging and cognitive performance. Sci Rep. 2014;4:5101. doi: 10.1038/srep05101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stein A., Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int. J. Psychophysiol. 2000;38(3):301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Wang H., McIntosh A.R., Kovacevic N., Karachalios M., Protzner A.B. Age-related multiscale changes in brain signal variability in pre-task versus post-task resting-state EEG. J. Cogn. Neurosci. 2016;28(7):971–984. doi: 10.1162/jocn_a_00947. [DOI] [PubMed] [Google Scholar]
- Wang Y.L., Yang S.Z., Sun W.L., Shi Y.Z., Duan H.F. Altered functional interaction hub between affective network and cognitive control network in patients with major depressive disorder. Behav. Brain Res. 2016;298(Pt B):301–309. doi: 10.1016/j.bbr.2015.10.040. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Depression Fact Sheet No 369 February 2017. 2017. http://www.who.int/mediacentre/factsheets/fs369/en/ Available: (2017, May)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiscale entropy (MSE) - Montgomery-Åsberg Depression Rating (MADRS) change score correlations with linear fits. MSE-MADRS change score scatterplots for responders (A) and non-responders (B) at electrode Pz at 30 ms (i.e., coarse temporal scale with highest bootstrap ratio). We identify participants in different antidepressant regimens by colour: escitalopram + bupropion (BUP + ESC) is indicated with blue, bupropion (BUP) is indicated with green, and escitalopram (ESC) is indicated with red circles.