Abstract
Previous research in patients with obsessive-compulsive disorder (OCD) has indicated performance decrements in working memory (WM) and response inhibition. However, underlying neural mechanisms of WM deficits are not well understood to date, and empirical evidence for a proposed conceptual link to inhibition deficits is missing.
We investigated WM performance in a numeric n-back task with four WM load conditions during functional Magnetic Resonance Imaging (fMRI) in 51 patients with OCD and 49 healthy control participants who were matched for age, sex, and education. Additionally, a stop signal task was performed outside the MRI scanner in a subsample.
On the behavioral level, a significant WM load by group interaction was found for both accuracy (p < 0.02) and reaction time measures (p < 0.03), indicating increased reaction times as well as reduced accuracy specifically at high WM load (3-back) in patients with OCD. Whole-brain analyses of fMRI-data identified neural correlates of a load-dependent WM decrement in OCD in the supplementary motor area (SMA) and the inferior parietal lobule (IPL). Within the OCD sample, SMA-activity as well as n-back performance were correlated with stop signal task performance.
Results from behavioral and fMRI-analyses indicate a reduced WM load-dependent modulation of neural activity in OCD and suggest a common neural mechanism for inhibitory dysfunction and WM decrements in OCD.
Keywords: Obsessive-compulsive disorder, Working memory, fMRI, Neuroimaging, Stop signal task, Inhibition
Highlights
-
•
Numeric working memory was tested in 51 OCD patients during fMRI for the first time.
-
•
OCD show increased neural activity at low and decreased activity at high load.
-
•
BOLD signal in supplementary motor area predicts symptoms and response inhibition.
-
•
Study shows first evidence for neural alterations in numeric working memory in OCD.
-
•
Study suggests common neural mechanism for inhibitory and working memory dysfunction.
1. Introduction
Obsessive-compulsive disorder (OCD) affects approximately 2–3% of the population (Ruscio et al., 2010) and is associated with highly unpleasant obsessive thoughts and compulsive behaviors in the majority of patients (Mendlowicz and Stein, 2000) that strongly impair their everyday lives. Empirical evidence suggests that besides these clinical symptoms, OCD is related to cognitive dysfunctions (Abramovitch et al., 2013, Shin et al., 2014, Snyder et al., 2014) mainly in executive functions (e.g., inhibition and shifting) and complex memory tasks such as working memory (WM) updating (de Vries et al., 2014, Harkin and Kessler, 2011, Koch et al., 2012, Purcell et al., 1998, van der Wee et al., 2003). Studies on the neural underpinnings of cognitive deficits in OCD have repeatedly reported dysregulations in fronto-striatal networks (Casale et al., 2011, Pauls et al., 2014). In OCD, a reduced inhibition of projections from the striatum to the thalamus and further to prefrontal cortex is thought to play a role in imbalanced fronto-striatal circuits and was found to relate to inhibition deficits in OCD (Chamberlain et al., 2005, Chamberlain et al., 2006). In the context of WM, this dysfunction could be associated with a deficient updating of information in prefrontal cortex (Chatham et al., 2011, Frank et al., 2001). At the same time, alterations in the functioning of a fronto-parietal network that is specifically relevant for WM processing, have been proposed in OCD (Melloni et al., 2012, Menzies et al., 2008). Despite relatively strong evidence for deficits in executively demanding WM tasks (e.g., WM updating), their underlying neural mechanisms are not well understood to date, and previous results have been heterogeneous showing both increased and decreased activations in fronto-parietal WM-related areas (de Vries et al., 2014, Henseler et al., 2008, Koch et al., 2012, Nakao et al., 2009, van der Wee et al., 2003). A recent study by Koch et al. (2012) suggested that inconsistencies could be reduced by taking differences in task demand into account, indicating that patients with OCD may show increased prefrontal activations at low task demand (low WM load) and decreased activations at high task demand (high WM load) when tested against healthy controls (HC). Such a pattern has been described in terms of a reduced WM load-dependent modulation of neural activity (Heinzel et al., 2014, Park and Reuter-Lorenz, 2009, Reuter-Lorenz and Cappell, 2008).
Recent hypotheses explaining OCD-related alterations in brain activations and performance during WM have suggested that impaired WM in OCD may relate to difficulties in focusing on the relevant information and failures to inhibit irrelevant stimuli (de Vries et al., 2014). While such a mechanism has been demonstrated in healthy older subjects (Gazzaley et al., 2005), and appears plausible in the context of OCD, it has not been specifically investigated in OCD, to date. Key regions within a fronto-parietal network that were found to be commonly activated during both WM updating and response inhibition in healthy participants include the (pre-) supplementary area (SMA), the lateral inferior frontal gyrus (IFG), and the inferior parietal lobule (IPL); see (Nee et al., 2013) for a meta-analysis for executive components of WM and (Boehler et al., 2010) for a conjunction analysis in response inhibition. Most importantly, SMA has been reported to show aberrant activations in OCD during WM (de Vries et al., 2014) as well as inhibitory control tasks (de Wit et al., 2012, Grützmann et al., 2016).
Current investigations of OCD-related alterations in functional connectivity have proposed that dysregulations of fronto-parietal neural activations may be associated with altered limbic-frontal connectivity (de Vries et al., 2014). More specifically, the authors found an increased functional connectivity between Amygdala and SMA in low-performing patients with OCD that was interpreted in terms of an increased uncertainty of their task performance (Stern et al., 2013).
Since previous fMRI research in OCD has mainly focused on visuospatial WM tasks (de Vries et al., 2014), one aim of our study was to test if similar alterations in neural activation and connectivity can be found during a numeric n-back task as well, indicating a more general underlying dysfunction of WM deficits in OCD. It is investigated if altered frontal activity, as reported in previous visuospatial n-back studies, is also found in this numeric n-back study. This finding would support the notion of a dysfunctional involvement of content-unspecific executive components of WM (Baddeley, 2003) as a possible underlying neural mechanism of WM decrements in OCD.
Thus, for the first time, we adopted a numeric n-back paradigm with four different WM load conditions (Heinzel et al., 2014) during fMRI in a relatively large sample of OCD and HC participants, in the current study. Since a subsample also participated in a stop signal task, we were able to explore the relationship between neural activation patterns during WM performance and inhibitory performance in the stop signal task for the first time in OCD. The following hypotheses were tested:
-
1)
Patients with OCD would show both lower accuracy and higher reaction times in an n-back task, specifically at high WM load.
-
2)
Patients with OCD would show increased activation at low and decreased activation at high WM load in fronto-parietal WM regions, indicating reduced WM load-dependent modulation of neural activity.
-
3)
Patients with OCD would show increased connectivity between Amygdala and frontal WM regions.
-
4)
Analyses within the OCD group would show a negative correlation between fronto-parietal WM load-dependent modulation of neural activity and OCD symptoms.
-
5)Exploratory analyses in a subsample of participants that performed a stop signal task would show a
-
a.positive relationship between n-back and stop signal performance in the OCD sample.
-
b.positive relationship between SMA activity during n-back and stop signal performance in the OCD sample.
-
a.
2. Materials and methods
2.1. Participants
Fifty-four patients with OCD were recruited from the OCD out-patient clinic at Humboldt-University Berlin and 56 healthy control (HC) participants were recruited via online advertisements. The OCD group was interviewed with the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) by licensed clinical psychologists and diagnosed using the German version of the Structured Clinical Interview for DSM-IV (SCID). Four participants in the HC and two in the OCD sample had to be excluded from data analyses due to technical failures during fMRI scanning. Furthermore, three participants in the HC and one in the OCD sample showed performance at chance level (performance below 30% hitrate or above 30% false alarm rate) in the WM task, and thus, had to be excluded from data analyses as well. Therefore, the final analysis sample consisted of 51 patients with OCD and 49 HC (see Table 1 for demographic data). All participants had normal or corrected-to-normal vision, and no history of any neurological diseases or brain injuries. The study was approved by the local Ethics Committee of the Humboldt-Universität zu Berlin and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants after the procedures had been fully explained. As this study was part of a larger project, a subsample of 41 patients with OCD and 29 HC also had participated in a stop-signal paradigm outside the MRI scanner. Stop signal reaction time (SSRT) could not be reliably estimated in one OCD patient (performance below 2.5 standard deviations from the mean), and therefore, this participant was excluded from analyses that included SSRT.
Table 1.
Demographics of healthy control (HC) and obsessive-compulsive patient (OCD) samples. Means and standard deviations (in parentheses) are shown. Units: Age [years]; Verbal test score [sum score]; Y-BOCS [sum score]; Performance [% correct]; Reaction time [ms].
| Measure | HC (N = 49) | OCD (N = 51) | p = |
|---|---|---|---|
| Age | 30.92 (7.31) | 33.00 (9.73) | 0.231 |
| Sex | 20 m/29 f | 26 m/25 f | 0.324 |
| Verbal test score | 32.16 (3.72) | 31.20 (4.78) | 0.263 |
| Y-BOCS severity scale (sum)a | n. a. | 23.25 (5.21) | n. a. |
| Y-BOCS subdimension taboo | n. a. | 3.10 (2.77) | n. a. |
| Y-BOCS subdimension contamination | n. a. | 4.33 (3.37) | n. a. |
| Y-BOCS subdimension rituals | n. a. | 2.41 (2.48) | n. a. |
| Y-BOCS subdimension hoarding | n. a. | 4.47 (2.85) | n. a. |
| Y-BOCS subdimension doubt | n. a. | 4.12 (2.85) | n. a. |
| Comorbid axis I disorderb | n. a. | 43 | n. a. |
| Current medicationc | n. a. | 22 | n. a. |
| Performance 0-back | 99.62 (1.22) | 97.89 (5.07) | 0.022 |
| Performance 1-back | 96.87 (5.79) | 96.62 (5.41) | 0.823 |
| Performance 2-back | 83.6 (14.58) | 81.18 (15.22) | 0.426 |
| Performance 3-back | 82.44 (18.75) | 72.33 (23.82) | 0.021 |
| Reaction time 0-back | 380 (45) | 391 (55) | 0.297 |
| Reaction time 1-back | 439 (62) | 471 (86) | 0.033 |
| Reaction time 2-back | 545 (85) | 582 (93) | 0.039 |
| Reaction time 3-back | 531 (101) | 598 (119) | 0.003 |
Bold p-values indicate significance at p < 0.05.
Subdimensions of Y-BOCS according to Katerberg et al. (2010).
Comorbid mental disorders: 44 mood disorders, 15 anxiety disorders, 3 eating disorders, 2 somatoform disorder, 1 tic disorder, 1 cannabis abuse. 16 OCD patients had more than one comorbid disorder.
19 SSRIs, 4 SSNRIs, 5 tricyclic antidepressants, 2 neuroleptics, 1 benzodiazepine.
2.2. N-back paradigm during fMRI
A modified version of the n-back paradigm with numerical stimuli as described in the study of Heinzel et al. (2014) was used in this study. Sixteen blocks (4 blocks of each 0-, 1-, 2-, and 3-back) were presented in three different pseudo-randomized orders counterbalanced across subjects. The total duration of the task was 9 min. Please refer to the Supplementary Methods in the Supplementary material and Heinzel et al. (2014) for details on the task design. N-back performance (defined as hitrate minus false alarm rate) and reaction time during correct responses were used as outcome scores for behavioral analyses of the n-back task.
2.3. MR image acquisition
fMRI data were collected at the Berlin Center for Advanced Neuroimaging, Charité Campus Mitte, Berlin, Germany with a 3 Tesla Magnetom Trio Tim MR system (Siemens, Erlangen, Germany). In the beginning of each scanning procedure, one T1-weighted 3D pulse sequence was obtained (repetition time (TR) = 2440 ms, echo time (TE) = 4.81 ms, flip angle = 8°, matrix size = 256 × 256, 192 sagittal slices with 0.91 mm thickness, voxel size = 0.91 × 0.91 × 0.91 mm3). Additionally, a T2-weighted 3D pulse sequence was measured (TR = 5000 ms, TE = 499 ms, flip angle = 120°, acquisition matrix = 256 × 258, 192 sagittal slices, with an isotropic voxel size of 0.91 mm). Functional data were obtained using a gradient echo-planar imaging (GE-EPI) pulse sequence (TR = 2000 ms, TE = 30 ms, flip angle = 78°, matrix size = 64 × 64, voxel size = 3.0 × 3.0 × 3.75 mm). 32 slices were acquired descending parallel to the bicommissural plane. MR image processing and analysis are described in detail in the Supplementary Methods in the Supplementary material.
2.4. Estimation of BOLD effects in n-back
The WM experiment was analyzed within the framework of the General Linear Model (GLM). At the single subject level, we created design matrices comprising the experimental conditions of 0-, 1-, 2-, and 3-back as separate regressors of interest and all other experimental conditions (cue, button presses, and the six rigid body realignment parameters) as regressors of no interest. The GLM was fitted voxel-wise into the filtered time series using the restricted maximum likelihood algorithm as implemented in SPM12.
On the second level, a random effects model as implemented in the GLM_Flex_Fast4 toolbox (version August 21st 2015 http://mrtools.mgh.harvard.edu/index.php?title=GLM_Flex) was applied to test a repeated measures ANOVA with the between-subjects factor group (OCD vs. HC) and the within-subjects factor WM load (0-, 1-, 2-, and 3-back). Whole brain analyses of the group by WM interaction effects as well as post hoc main effects of group were thresholded at p < 0.05, FWE cluster-level. Analyses were restricted to a WM mask (including 26.298 voxels) derived from http://neurosynth.org (Yarkoni et al., 2011) based on an automated meta-analysis of 901 WM experiments. We used a Monte Carlo simulation correction (10,000 iterations) with an initial voxel-wise threshold of p < 0.001 (http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html; revision December 2015). Clusters with a minimum cluster size of 37 voxels yielded a cluster-level FWE threshold of p < 0.05 and are described in the Results section and in Table 2.
Table 2.
Anatomical locations and MNI coordinates for A) the WM load (0-, 1-, 2-, and 3-back) by group (healthy control [HC] vs. obsessive-compulsive patients [OCD]) interaction, and B) WM load-specific group effects; whole-brain results are reported at p < 0.05, family-wise error (FWE) cluster-corrected. (Hem = Hemisphere; L = left; R = right; BA = Brodmann area).
| Contrast/region | Hem | BA | MNI Coordinates |
t-Value | Cluster size | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| A: Group × WM load interaction | |||||||
| (k > 37, p < 0.05 FWE cluster-corr.) | |||||||
| Inferior parietal lobule | R | 40 | 38 | − 34 | 44 | 8.68 | 37 |
| Supplementary motor area | L | 6 | − 4 | 8 | 60 | 8.38 | 40 |
| B: Group effects | |||||||
| 0-Back (k > 37, p < 0.05 FWE cluster-corr.) | |||||||
| HC > OCD | No significant clusters | ||||||
| OCD > HC | |||||||
| Insula/superior temporal gyrus | L | 13/22 | − 44 | 10 | 0 | 4.16 | 76 |
| Precentral lobule | R | 9/6/8 | 52 | 6 | 42 | 4.04 | 58 |
| Inferior parietal lobule/supramarginal gyrus | R | 40 | 52 | − 38 | 44 | 3.49 | 41 |
| 1-Back (k > 37, p < 0.05 FWE cluster-corr.) | |||||||
| HC > OCD | |||||||
| Inferior parietal lobule | R | 40 | 46 | − 48 | 52 | 4.22 | 111 |
| OCD > HC | |||||||
| Precentral gyrus | R | 6/9/8 | 52 | 6 | 42 | 4.54 | 213 |
| Inferior frontal gyrus/insula | L | 47/13/22/38 | − 28 | 26 | − 2 | 3.26 | 274 |
| Inferior parietal lobule | L | 7/40/19 | − 38 | − 50 | 40 | 5.00 | 1067 |
| Inferior parietal lobule | L | − 52 | − 38 | 52 | 4.82 | Included | |
| Superior parietal lobule | L | − 24 | − 68 | 46 | 4.73 | Included | |
| Inferior parietal lobule/supramarginal gyrus | R | 40 | 48 | − 42 | 44 | 4.83 | 343 |
| Precentral gyrus | L | 6/9 | − 42 | − 4 | 38 | 4.52 | 111 |
| Precuneus/gyrus angularis/superior parietal lobule/superior occipital lobule | R | 7/40 | 24 | − 64 | 46 | 4.15 | 181 |
| 2-Back (k > 37, p < 0.05 FWE cluster-corr.) | |||||||
| HC > OCD | |||||||
| Inferior occipital lobule | L | 19/37 | − 42 | − 76 | − 10 | 5.36 | 152 |
| Fusiform gyrus | L | − 38 | − 56 | − 10 | 4.28 | Included | |
| Hippocampus | R | 34 | 28 | − 10 | − 16 | 5.34 | 43 |
| Postcentral gyrus | R | 40 | 38 | − 34 | 46 | 4.95 | 45 |
| Inferior frontal lobule | L | 9 | − 38 | 8 | 22 | 4.71 | 106 |
| Caudate | R | 14 | 14 | 12 | 4.69 | 96 | |
| Cerebellum | R | 30 | − 52 | − 28 | 4.64 | 83 | |
| Mid-cingulate gyrus/limbic lobe | L | 32/9/24 | − 8 | 18 | 36 | 4.33 | 269 |
| Anterior cingulate gyrus | R | 12 | 24 | 28 | 4.07 | Included | |
| Superior frontal gyrus/supplementary motor area | L | 6 | − 4 | 8 | 60 | 4.17 | 41 |
| OCD > HC | |||||||
| Precentral gyrus | R | 6 | 32 | − 4 | 46 | 5.00 | 49 |
| Middle frontal gyrus | L | 9 | − 48 | 22 | 38 | 4.24 | 38 |
| 3-Back (k > 37, p < 0.05 FWE cluster-corr.) | |||||||
| HC > OCD | |||||||
| Parahippocampal gyrus/amygdala/hippocampus | R | 28 | − 10 | − 16 | 6.86 | 44 | |
| Cingulate gyrus/limbic lobe/supplemental motor area | L | 32/6/24/9 | − 4 | 8 | 60 | 6.16 | 799 |
| Superior frontal gyrus/medial frontal gyrus | R | 6 | 20 | 42 | 5.23 | Included | |
| Anterior cingulate gyrus | R | 8 | 34 | 20 | 3.64 | Included | |
| Caudate | R | 14 | 14 | 12 | 5.96 | 293 | |
| Putamen | R | 18 | 6 | − 6 | 4.64 | Included | |
| Caudate | R | 16 | − 6 | 20 | 4.08 | Included | |
| Postcentral gyrus | R | 40/2 | 38 | − 34 | 46 | 5.83 | 132 |
| Inferior occipital lobule | L | 17 | − 26 | − 94 | − 8 | 5.67 | 37 |
| Thalamus | R | 8 | − 8 | 10 | 5.62 | 45 | |
| Inferior occipital lobule | L | 19/37 | − 40 | − 76 | − 12 | 5.46 | 163 |
| Fusiform gyrus | L | − 38 | − 56 | − 10 | 4.66 | Included | |
| Putamen/caudate | L | − 14 | 4 | 18 | 5.36 | 279 | |
| Pallidum | L | − 22 | 0 | 0 | 3.27 | Included | |
| Inferior frontal gyrus | L | 44/9/6 | − 38 | 6 | 22 | 5.02 | 247 |
| Inferior frontal gyrus | L | − 58 | 14 | 12 | 3.50 | Included | |
| Superior temporal gyrus/postcentral gyrus | L | 22/21/41 | − 54 | − 18 | 16 | 5.01 | 96 |
| Middle temporal gyrus | L | − 58 | − 10 | − 6 | 4.63 | Included | |
| Postcentral gyrus/inferior parietal lobule | L | 40/2 | − 38 | − 40 | 56 | 4.89 | 148 |
| Inferior parietal lobule | L | − 50 | − 30 | 40 | 3.74 | Included | |
| Inferior frontal gyrus | L | − 38 | 30 | 16 | 4.12 | 39 | |
| OCD > HC | |||||||
| Supplementary motor area/superior frontal gyrus | L | 6 | − 6 | 6 | 64 | 4.92 | 37 |
| Inferior temporal lobule | L | 19/37 | − 54 | − 50 | − 8 | 4.61 | 49 |
| Cerebellum | R | 30 | − 52 | − 28 | 4.19 | 66 | |
2.5. ROI-analyses
To replicate analyses of (de Vries et al., 2014), testing OCD-related alterations in WM-load dependent BOLD response, we used the same method as described in their paper. Thus, we built 9 ROIs using 10 mm spheres around the coordinates reported in (de Vries et al., 2014), combined them to one WM network ROI (see Table S1 in the Supplementary material for all peaks) and tested a 2 (group) by 3 (1-, 2-, and 3-back) ANOVA.
2.6. Generalized Psychophysiological Interaction Analysis
To test if results from visuospatial WM on altered connectivity between the Amygdala and fronto-parietal regions in OCD as reported in (de Vries et al., 2014) can be replicated in our current investigation on numeric WM, we replicated their generalized Psychophysiological Interaction (gPPI) Analysis (McLaren et al., 2012). We chose the same MNI coordinates as reported in (de Vries et al., 2014) to build seed regions in left Dorsolateral Prefrontal Cortex (DLPFC), left pre-supplementary motor area (SMA), and left Precuneus. Also, we chose the same anatomical definition of the Amygdala by using the automatic labeling atlas. More information on the procedure is reported in the Supplementary Methods in the Supplementary material.
2.7. Correlational analysis between n-back BOLD effects and stop-signal reaction time
Because we hypothesized an association of stop-signal reaction time with activity of the SMA region, a SMA region of interest (ROI) was used for parameter extraction. The boundaries of this SMA ROI were derived from the Automated Anatomical Labeling (AAL) toolbox for SPM (Tzourio-Mazoyer et al., 2002). We extracted parameter estimates from significant voxels within this a priori determined SMA region (for the high load n-back conditions relative to implicit baseline), and carried out correlational analysis with estimates of stop-signal-reaction times from a behavioral stop signal task.
2.8. Stop signal task
The stop signal task used in this study is an adapted version of a task used in previous OCD research (Chamberlain et al., 2006), where subjects respond rapidly to left- or right-facing arrows with corresponding motor responses, but attempt to inhibit responses when an auditory signal appears in temporal proximity (stop signal). It is then possible to estimate the time taken to internally suppress motor responses (stop-signal reaction time, SSRT). SSRTs were estimated by subtracting the mean stop-signal delay from the observed mean reaction time (RT) during “go trials” (trials without an auditory stop signal) (Verbruggen and Logan, 2009).
3. Results
3.1. Behavioral results n-back
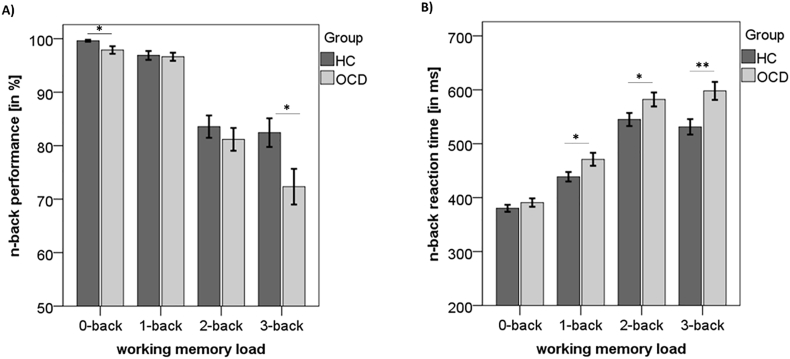
A two (group) by four (WM load) ANOVA of the n-back performance revealed a significant interaction (F(3, 294) = 3.74, p = 0.012, partial η2 = 0.037) as well as a significant main effect of WM load (F(3, 294) = 85.29, p < 0.001, partial η2 = 0.465). There was a trend for a main effect of group (F(1, 98) = 3.88, p = 0.052, partial η2 = 0.038). Follow-up two-sample t-tests indicated that compared to patients with OCD, healthy control participants showed better performance at 0-back (t(98) = 2.33, p = 0.022) and 3-back (t(98) = 2.35, p = 0.021), but not at 1- and 2-back (both p > 0.42; see Fig. 1, panel A).
Fig. 1.
Behavioral n-back data in healthy controls (HC) and obsessive-compulsive patients (OCD). Means and standard errors of the mean are reported for A) performance and B) reaction time. *p < 0.05; **p < 0.01.
Similarly to the results of n-back performance, RT analyses revealed a significant two (group) by four (WM load) interaction (F(3, 294) = 3.25, p = 0.022, partial η2 = 0.032) as well as a significant main effect of WM load (F(3, 294) = 187.92, p < 0.001, partial η2 = 0.657) and a main effect of group (F(1, 98) = 8.45, p = 0.005, partial η2 = 0.079). Follow-up two-sample t-tests indicated that compared to patients with OCD, healthy control participants had faster RT at 1-back (t(98) = 2.16, p = 0.033), 2-back (t(98) = 2.09, p = 0.039), and 3-back (t(98) = 3.02, p = 0.003), but not at 0-back (p > 0.29; see Fig. 1, panel B).
3.2. fMRI results during n-back
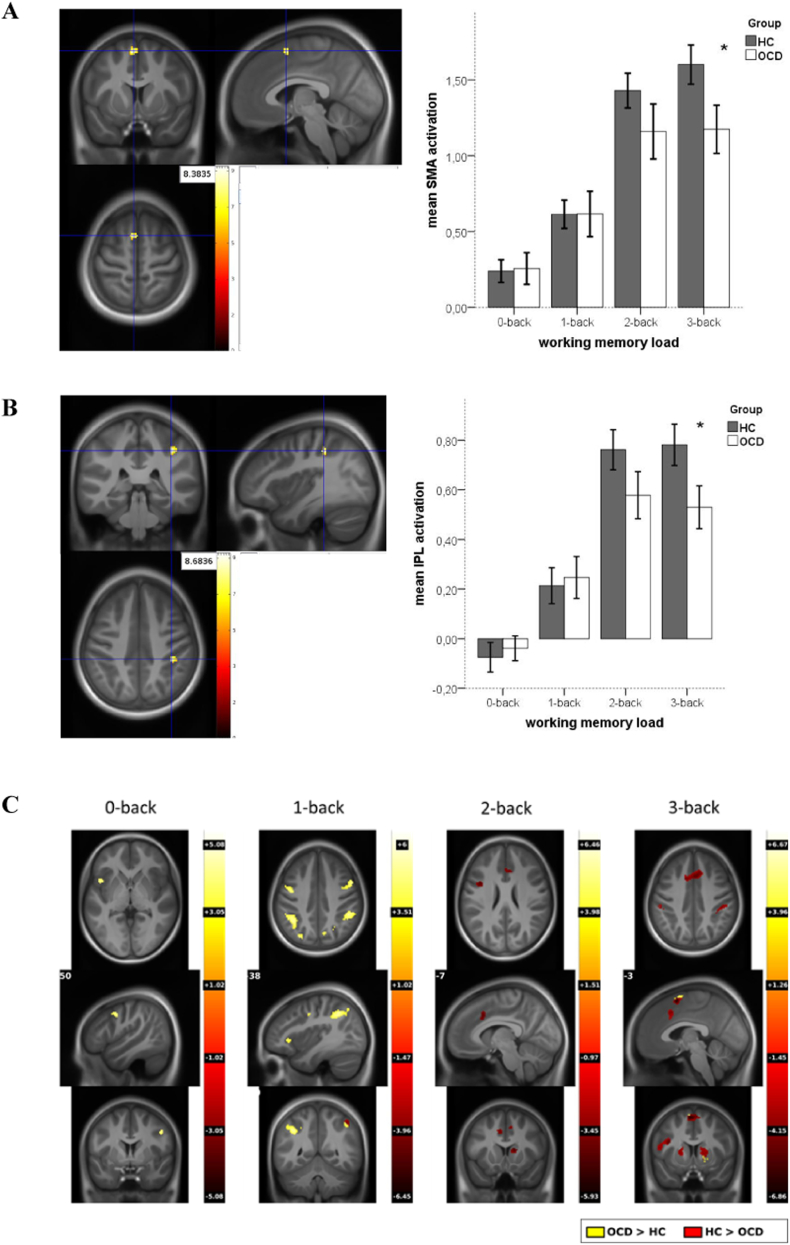
As shown in Fig. 2, panels A and B, whole-brain analyses (p < 0.05, FWE cluster-level corrected) of the two (group) by four (WM load) interaction revealed a significant interaction effect in the left supplementary motor area (SMA, t = 8.38) and in the right inferior parietal lobule (IPL, t = 8.68), indicating reduced activation in the OCD group compared to the HC group at high WM load (3-back). To obtain a score for WM load-dependent modulation of neural activity, difference scores of parameter estimates of BOLD response at high (3-back) minus low (1-back) WM load were computed (see Heinzel et al., 2014, Nagel et al., 2011 for details of the procedure) in left SMA and right IPL. Group comparisons between OCD and HC revealed higher neural modulation scores in HC in both SMA (t(98) = 3.89, p < 0.001) and IPL (t(98) = 3.69, p < 0.001).
Fig. 2.
fMRI group by working memory (WM) load interaction, (p < 0.05 FWE cluster-corrected) in panel A) SMA, and panel B) IPL. Yellow: high T-value of interaction, red: low T-value of interaction. Panel C: Post-hoc t-tests for 0-, 1-, 2-, and 3-back (p < 0.05 FWE cluster-corr.). Yellow: higher activations in OCD, red: higher activations in HC.
Post hoc group effects (p < 0.05, FWE cluster-level corrected) testing group differences for each WM load separately, are depicted in Fig. 2, panel C, showing higher activations in OCD patients in 1-back and lower activations in 2- and 3-back mainly in fronto-parietal regions (see Table 2 for MNI coordinates, t-values, and cluster extents). Taken together, these results indicate that fronto-parietal BOLD response in OCD patients seem to be less adaptive to increasing WM load.
3.3. ROI analyses
ROI analyses using the same WM network ROI as reported in (de Vries et al., 2014) showed a significant WM load by group interaction (F(2, 196) = 3.87, p = 0.027) in extracted parameter estimates of the WM network ROI (see Fig. S1 in the Supplementary material).
3.4. Generalized Psychophysiological Interaction (gPPI) Analysis
As shown in the Supplementary Table S2, the group comparisons showed no significant results for any of the performed connectivity analyses (left SMA, left DLPFC, and left Precuneus seed ROIs), even when using a relatively liberal statistical threshold (p < 0.001 uncorrected). Thus, in our study we could not replicate the previously reported altered connectivity between frontal and parietal seeds and the left or right Amygdala. However, as displayed in the Supplementary Fig. S2 in the Supplementary material, we found a similar pattern of connectivity parameters as reported in (de Vries et al., 2014) when comparing high vs. low performing patients with OCD vs. HC, indicating a (non-significant) tendency towards an increased connectivity between Amygdala and SMA specifically in low-performing patients with OCD.
3.5. Relationship between fMRI results and Y-BOCS
The Y-BOCS sum score was negatively correlated with WM load-dependent modulation of neural activity in SMA (r = − 0.281, p = 0.046) but not in IPL (r = − 0.227, p = 0.109), indicating that higher modulation in SMA was related to lower symptom severity in patients.
3.6. Relationship between SSRT and n-back performance and BOLD response
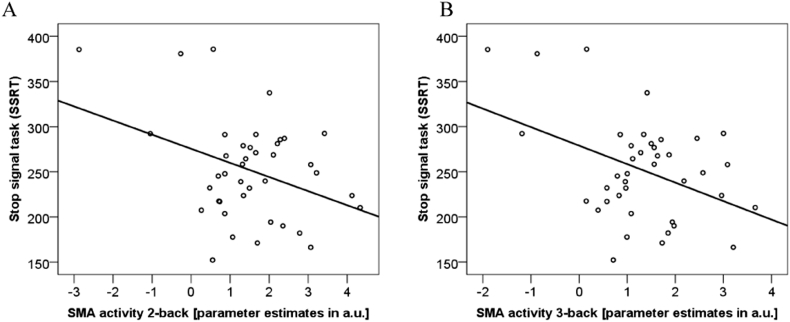
In the subgroup of 40 patients with OCD, where Stop Signal Task data were available, SSRT correlated significantly with the mean n-back RT (r = 0.346, p = 0.029), but not with the mean n-back performance (r = − 0.108, p = 0.506). Thus, in the OCD subsample, faster responses in n-back are related to lower SSRTs (indicating a higher inhibition performance). Most importantly, there was a significant relationship between SSRT performance and SMA-activity during 0-back (r = − 0.321, p = 0.044), 1-back (r = − 0.407, p = 0.009), 2-back (r = − 0.368, p = 0.020), and 3-back (r = − 0.415, p = 0.008), indicating that higher SMA-activity during n-back is related to lower SSRT values and therefore better inhibition performance (see Fig. 3, panel A and B) in OCD. In the SSRT subgroup of HC subjects, SSRT did neither correlate with mean n-back performance (r = − 0.017, p = 0.930) nor with RT (r = 0.157, p = 0.416). Also, BOLD response in SMA during n-back was not related to SSRT in HC (all p > 0.21).
Fig. 3.
Stop signal task and SMA-activity in n-back in the OCD sample: (panel A) 2-back, R2 linear = 0.135; (panel B) 3-back, R2 linear = 0.173.
4. Discussion
Results reflect a WM load-dependent performance decrement in patients with OCD that is specifically visible at high WM load (3-back). At the same time, a whole-brain analysis of the group by WM load interaction revealed reduced BOLD responses in SMA and IPL at 3-back in patients with OCD. Group effects for the separate WM load levels indicate a reduced WM load-dependent modulation of neural activity in a fronto-parietal network in patients with OCD, reflected by hyperactivations at low WM load and hypoactivations at high WM load. These results from voxel-wise analyses were confirmed by ROI analyses testing a WM network ROI derived from an independent study (de Vries et al., 2014). Within the OCD sample, higher symptom severity (Y-BOCS sum score) was related to reduced WM load-dependent modulation of neural activity in SMA. Results in a subsample that also participated in a stop signal task revealed that higher inhibition performance, indicated by low SSRT values, corresponds to shorter n-back RT as well as higher BOLD responses in SMA during n-back.
Our first hypothesis on a WM load-dependent deficit in WM was supported by the current data and confirms previous findings (de Vries et al., 2014, Harkin and Kessler, 2011, Koch et al., 2012, Purcell et al., 1998, van der Wee et al., 2003). These performance decrements are possibly due to inefficient strategies that fail at high task demand (Harkin and Kessler, 2011). Furthermore, higher indecisiveness in OCD may add to the generally increased reaction times found in this study (Sarig et al., 2012).
Results from the whole-brain analysis, testing a group by WM load interaction, suggest a neuronal correlate in SMA and IPL for the performance decrements in OCD at high WM load. These results are in line with the concept of a reduced WM load-dependent modulation of neural activity in fronto-parietal WM areas in OCD because both SMA and IPL are involved in WM updating (Owen et al., 2005). IPL was found to play an important role in selective attention, WM rehearsal, and capacity (Ravizza et al., 2004, Rottschy et al., 2012, Smith and Jonides, 1999), whereas SMA is a key region in response selection and preparation (Nee et al., 2013, Petit et al., 1998, Rottschy et al., 2012), as well as distractor susceptibility (Nee et al., 2013), and response inhibition (Boehler et al., 2010, Chikazoe, 2010). Alterations in SMA activation were reported in previous WM studies in OCD (de Vries et al., 2014, Koch et al., 2012, van der Wee et al., 2003) and interpreted as a neural substrate of an OCD-related difficulty to inhibit irrelevant information and suppress responses to distractors, an ability that is most relevant for high WM load in the n-back task. This interpretation is supported by fMRI findings from inhibitory control tasks (e.g. stop signal task) in OCD. A study by de Wit et al. (2012) found increased SMA activity in OCD in a stop signal task, indicating compensatory activation for an inhibition deficit. Our finding of decreased SMA activity accompanied by a marked performance decrement in 3-back suggests that a SMA-driven compensatory attempt seems to fail at high WM load as pointed out by current models of an inverse U-shaped relationship between WM load and associated BOLD responses (Lustig et al., 2009, Manoach, 2003, Nyberg et al., 2009, Reuter-Lorenz and Cappell, 2008). Thus, as expected, WM load-dependent modulation of neural activity in SMA, as indicated by the difference between 3- and 1-back activity (Heinzel et al., 2014, Nagel et al., 2011), was reduced in patients with OCD. A negative correlation with OCD symptoms suggests that the ability to flexibly increase SMA activation with increasing WM load is negatively related to the amount of OCD symptoms. This finding is in line with cross-sectional research (de Vries et al., 2014, de Wit et al., 2012) and a longitudinal study on psychotherapy-induced modulations of frontal activity in OCD (van der Wee et al., 2007).
Following up on the concept of reduced fronto-parietal adaptability to increasing WM load as supported by a recent study in OCD (Koch et al., 2012), we had expected an increased SMA-activity at low WM load (1-back) in OCD, which was not found in the current whole-brain group by WM load interaction analysis. However, post hoc group effects at each WM load separately showed increased activity at 1-back in OCD mainly in lateral fronto-parietal areas (see Fig. 2, panel C and Table 2, panel B). These voxel-wise results are supported by ROI analyses using a WM network ROI as reported in (de Vries et al., 2014), indicating a significant group by WM load interaction.
It seems that compensatory hyperactivations may not occur in the same brain areas that show activity decrease at high WM load. Thus, OCD-related hyperactivations at low WM load were located more laterally than hypoactivations at high WM load. This notion may explain seeming discrepancies to the study by de Vries et al. (2014), as their finding of increased activity in OCD was found in a lateral SMA cluster including parts of premotor cortex. No WM load-specific voxel-wise analyses were reported in their study, but ROI-analyses suggest that OCD-related increases in activity are mainly driven by increased 1-back activity, possibly related to an inefficiently elevated involvement of cognitive control functions (de Vries et al., 2014, Henseler et al., 2008). However, differences in sample characteristics (e.g. number of patients with comorbid mental disorders and medication), task design, and analyses need to be considered when comparing our study with the study by de Vries et al. (2014).
Taken together, results of the current study reporting behavioral and neural alterations during the performance of a numeric n-back task, partially replicate results from a previous study in a visuospatial n-back task (de Vries et al., 2014). Even though previous research suggests that visually presented number stimuli are represented predominantly verbally in WM (Reuter-Lorenz et al., 2000, Smith and Jonides, 1999), we do not know whether participants used a verbal or a visual representation of the stimuli during WM rehearsal in our study. Thus, conclusions on specific neural underpinnings of WM decrements in OCD are still speculative. Nevertheless, keeping this limitation in mind, our findings may suggest that OCD-related alterations in WM processing during n-back are not content specific (Harkin and Kessler, 2011) and tap into executive aspects of the WM system that are thought to process information relatively independent from the type of stimulus representations (Baddeley, 2003).
Our gPPI connectivity analyses between three frontal and parietal regions and the left and right Amygdala did not reveal significant group comparisons. Thus, we could not replicate the findings reported in (de Vries et al., 2014), even though, viewing the connectivity parameters might suggest a similar pattern in the SMA-Amygdala connectivity (see Supplementary Fig. S2 in the Supplementary material). Possibly, different characteristics of the task and/or the samples used in our study and the study by de Vries et al. (2014) may explain these differences. It seems that performance rates in OCD were higher in our compared to their task. Therefore, OCD participants may have been less uncertain about their responses, indicating lower connectivity between Amygdala and fronto-parietal regions. Furthermore, to follow the idea of a replication analysis, we used a more conservative approach in selecting the seed regions. We built the same seed ROIs as reported in (de Vries et al., 2014), using the peak coordinates of group differences from their study (i.e. not from our study) to create the spheres for parameter extraction.
Importantly, significant correlations between SMA-activity during n-back and SSRT provide a direct link between WM-related alterations in medial frontal brain activation in OCD (de Vries et al., 2014, Koch et al., 2012, van der Wee et al., 2003) and deficits in inhibition performance (Chamberlain et al., 2006, de Wit et al., 2012, Menzies et al., 2008). To our knowledge, this direct relationship was investigated for the first time in the current study and is first evidence in support of the hypothesis of a common underlying mechanism for both inhibition and WM performance decrements in OCD.
4.1. Limitations and future perspectives
There are a few limitations that need to be considered when interpreting the results of this study. First, no assessment of subject's indecisiveness or individual use of strategies during task performance was done. Since features of the presented stimulus material may be represented differently in WM according to individual strategies, it remains unclear whether participants encoded the presented material verbally using verbal rehearsal strategies or visuospatially or used another strategy. Second, only a subgroup of participants performed the stop signal task and correlations to SMA activity during n-back are relatively weak and need to be replicated in a larger sample, ideally also testing neural activity during stop signal task performance. Third, the n-back task involves several different WM subprocesses, thus, n-back does not allow to capture specific deficient subprocesses, and it would be interesting to apply different types of WM tasks (e.g. Sternberg tasks) to compare OCD-related alterations in neural activity between different WM subprocesses (e.g. maintenance vs. updating) in future studies. As indicated by previous WM training studies (Dahlin et al., 2008, Heinzel et al., 2016, Klingberg, 2010), inefficient WM processing may be altered through a cognitive training program both on a behavioral and a neural level. These findings encourage application in patients with OCD in future research (Buhlmann et al., 2006).
5. Conclusions
In the current study, patients with OCD showed WM performance decrements specifically at high WM load that were accompanied by WM load-dependent alterations in neural activity in SMA and IPL as well as reduced response inhibition. Importantly, our behavioral and neuroimaging findings in a numeric n-back task partially replicate results from a previous study in a visuospatial n-back task. Thus, WM deficits and associated neural alterations in OCD are most likely not content specific and a common neural mechanism for WM deficits and inhibitory dysfunction in OCD is suggested. Findings may support the development and investigation of cognitive training programs specifically targeting these cognitive functions.
Acknowledgments
Acknowledgements
This work was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft) grants KA815/6-1 and KA815/7-1 (Norbert Kathmann), WA 731/10-1 (Michael Wagner), HE 7464/4-1 (Stephan Heinzel), and GR 4901/2-1 (Rosa Grützmann).
Disclosure statement
The authors declare no competing financial interests and no other conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2017.10.039.
Appendix A. Supplementary data
Supplementary material
References
- Abramovitch A., Abramowitz J.S., Mittelman A. The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clin. Psychol. Rev. 2013;33:1163–1171. doi: 10.1016/j.cpr.2013.09.004. (S0272-7358(13)00130-X [pii]) [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat. Rev. Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Boehler C.N., Appelbaum L.G., Krebs R.M., Hopf J.-M., Woldorff M.G. Pinning down response inhibition in the brain—conjunction analyses of the stop-signal task. NeuroImage. 2010;52:1621–1632. doi: 10.1016/j.neuroimage.2010.04.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhlmann U., Deckersbach T., Engelhard I., Cook L.M., Rauch S.L., Kathmann N., Wilhelm S., Savage C.R. Cognitive retraining for organizational impairment in obsessive-compulsive disorder. Psychiatry Res. 2006;144:109–116. doi: 10.1016/j.psychres.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Casale A.D., Kotzalidis G.D., Rapinesi C., Serata D., Ambrosi E., Simonetti A., Pompili M., Ferracuti S., Tatarelli R., Girardi P. Functional neuroimaging in obsessive-compulsive disorder. Neuropsychobiology. 2011;64:61–85. doi: 10.1159/000325223. [DOI] [PubMed] [Google Scholar]
- Chamberlain S.R., Blackwell A.D., Fineberg N.A., Robbins T.W., Sahakian B.J. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci. Biobehav. Rev. 2005;29:399–419. doi: 10.1016/j.neubiorev.2004.11.006. (S0149-7634(04)00166-6 [pii]) [DOI] [PubMed] [Google Scholar]
- Chamberlain S.R., Fineberg N.A., Blackwell A.D., Robbins T.W., Sahakian B.J. Motor inhibition and cognitive flexibility in obsessive-compulsive disorder and trichotillomania. Am. J. Psychiatry. 2006;163:1282–1284. doi: 10.1176/ajp.2006.163.7.1282. (163/7/1282 [pii]) [DOI] [PubMed] [Google Scholar]
- Chatham C.H., Herd S.A., Brant A.M., Hazy T.E., Miyake A., O'Reilly R., Friedman N.P. From an executive network to executive control: a computational model of the n-back task. J. Cogn. Neurosci. 2011;23:3598–3619. doi: 10.1162/jocn_a_00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J. Localizing performance of go/no-go tasks to prefrontal cortical subregions. Curr. Opin. Psychiatry. 2010;23:267–272. doi: 10.1097/YCO.0b013e3283387a9f. [DOI] [PubMed] [Google Scholar]
- Dahlin E., Neely A.S., Larsson A., Bäckman L., Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;320:1510–1512. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- Frank M.J., Loughry B., O'Reilly R.C. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn. Affect. Behav. Neurosci. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Gazzaley A., Cooney J.W., Rissman J., D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat. Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Grützmann R., Endrass T., Kaufmann C., Allen E., Eichele T., Kathmann N. Presupplementary motor area contributes to altered error monitoring in obsessive-compulsive disorder. Biol. Psychiatry. 2016;80:562–571. doi: 10.1016/j.biopsych.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Harkin B., Kessler K. The role of working memory in compulsive checking and OCD: a systematic classification of 58 experimental findings. Clin. Psychol. Rev. 2011;31:1004–1021. doi: 10.1016/j.cpr.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Heinzel S., Lorenz R.C., Brockhaus W.-R., Wüstenberg T., Kathmann N., Heinz A., Rapp M.A. Working memory load-dependent brain response predicts behavioral training gains in older adults. J. Neurosci. 2014;34:1224–1233. doi: 10.1523/JNEUROSCI.2463-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel S., Lorenz R.C., Pelz P., Heinz A., Walter H., Kathmann N., Rapp M.A., Stelzel C. Neural correlates of training and transfer effects in working memory in older adults. NeuroImage. 2016;134:236–249. doi: 10.1016/j.neuroimage.2016.03.068. [DOI] [PubMed] [Google Scholar]
- Henseler I., Gruber O., Kraft S., Krick C., Reith W., Falkai P. Compensatory hyperactivations as markers of latent working memory dysfunctions in patients with obsessive-compulsive disorder: an fMRI study. J. Psychiatry Neurosci. 2008;33:209–215. [PMC free article] [PubMed] [Google Scholar]
- Katerberg H., Delucchi K.L., Stewart S.E., Lochner C., Denys D.A., Stack D.E., Andresen J.M., Grant J.E., Kim S.W., Williams K.A., den Boer J.A., van Balkom A.J., Smit J.H., van Oppen P., Polman A., Jenike M.A., Stein D.J., Mathews C.A., Cath D.C. Symptom dimensions in OCD: item-level factor analysis and heritability estimates. Behav. Genet. 2010;40:505–517. doi: 10.1007/s10519-010-9339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T. Training and plasticity of working memory. Trends Cogn. Sci. 2010;14:317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Koch K., Wagner G., Schachtzabel C., Peikert G., Schultz C.C., Sauer H., Schlösser R.G. Aberrant anterior cingulate activation in obsessive-compulsive disorder is related to task complexity. Neuropsychologia. 2012;50:958–964. doi: 10.1016/j.neuropsychologia.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Lustig C., Shah P., Seidler R., Reuter-Lorenz P.A. Aging, training, and the brain: a review and future directions. Neuropsychol. Rev. 2009;19:504–522. doi: 10.1007/s11065-009-9119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach D.S. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr. Res. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni M., Urbistondo C., Sedeno L., Gelormini C., Kichic R., Ibanez A. The extended fronto-striatal model of obsessive compulsive disorder: convergence from event-related potentials, neuropsychology and neuroimaging. Front. Hum. Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendlowicz M.V., Stein M.B. Quality of life in individuals with anxiety disorders. Am. J. Psychiatry. 2000;157:669–682. doi: 10.1176/appi.ajp.157.5.669. [DOI] [PubMed] [Google Scholar]
- Menzies L., Chamberlain S.R., Laird A.R., Thelen S.M., Sahakian B.J., Bullmore E.T. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci. Biobehav. Rev. 2008;32:525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel I.E., Preuschhof C., Li S.-C., Nyberg L., Bäckman L., Lindenberger U., Heekeren H.R. Load modulation of BOLD response and connectivity predicts working memory performance in younger and older adults. J. Cogn. Neurosci. 2011;23:2030–2045. doi: 10.1162/jocn.2010.21560. [DOI] [PubMed] [Google Scholar]
- Nakao T., Nakagawa A., Nakatani E., Nabeyama M., Sanematsu H., Yoshiura T., Togao O., Tomita M., Masuda Y., Yoshioka K., Kuroki T., Kanba S. Working memory dysfunction in obsessive-compulsive disorder: a neuropsychological and functional MRI study. J. Psychiatr. Res. 2009;43:784–791. doi: 10.1016/j.jpsychires.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Nee D.E., Brown J.W., Askren M.K., Berman M.G., Demiralp E., Krawitz A., Jonides J. A meta-analysis of executive components of working memory. Cereb. Cortex N. Y. N. 2013;1991(23):264–282. doi: 10.1093/cercor/bhs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L., Dahlin E., Stigsdotter Neely A., Bäckman L. Neural correlates of variable working memory load across adult age and skill: dissociative patterns within the fronto-parietal network. Scand. J. Psychol. 2009;50:41–46. doi: 10.1111/j.1467-9450.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- Owen A.M., McMillan K.M., Laird A.R., Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D.C., Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu. Rev. Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls D.L., Abramovitch A., Rauch S.L., Geller D.A. Obsessive-compulsive disorder: an integrative genetic and neurobiological perspective. Nat. Rev. Neurosci. 2014;15:410–424. doi: 10.1038/nrn3746. [DOI] [PubMed] [Google Scholar]
- Petit L., Courtney S.M., Ungerleider L.G., Haxby J.V. Sustained activity in the medial wall during working memory delays. J. Neurosci. 1998;18:9429–9437. doi: 10.1523/JNEUROSCI.18-22-09429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell R., Maruff P., Kyrios M., Pantelis C. Neuropsychological deficits in obsessive-compulsive disorder: a comparison with unipolar depression, panic disorder, and normal controls. Arch. Gen. Psychiatry. 1998;55:415–423. doi: 10.1001/archpsyc.55.5.415. [DOI] [PubMed] [Google Scholar]
- Ravizza S.M., Delgado M.R., Chein J.M., Becker J.T., Fiez J.A. Functional dissociations within the inferior parietal cortex in verbal working memory. NeuroImage. 2004;22:562–573. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P.A., Cappell K.A. Neurocognitive aging and the compensation hypothesis. Curr. Dir. Psychol. Sci. 2008;17:177–182. [Google Scholar]
- Reuter-Lorenz P.A., Jonides J., Smith E.E., Hartley A., Miller A., Marshuetz C., Koeppe R.A. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J. Cogn. Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rottschy C., Langner R., Dogan I., Reetz K., Laird A.R., Schulz J.B., Fox P.T., Eickhoff S.B. Modelling neural correlates of working memory: a coordinate-based meta-analysis. NeuroImage. 2012;60:830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio A.M., Stein D.J., Chiu W.T., Kessler R.C. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol. Psychiatry. 2010;15:53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarig S., Dar R., Liberman N. Obsessive-compulsive tendencies are related to indecisiveness and reliance on feedback in a neutral color judgment task. J. Behav. Ther. Exp. Psychiatry. 2012;43:692–697. doi: 10.1016/j.jbtep.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Shin N.Y., Lee T.Y., Kim E., Kwon J.S. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol. Med. 2014;44:1121–1130. doi: 10.1017/S0033291713001803. (S0033291713001803 [pii]) [DOI] [PubMed] [Google Scholar]
- Smith E.E., Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Snyder H.R., Kaiser R.H., Warren S.L., Heller W. Obsessive-compulsive disorder is associated with broad impairments in executive function: a meta-analysis. Clin. Psychol. Sci. 2014;3:301–330. doi: 10.1177/2167702614534210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern E.R., Welsh R.C., Gonzalez R., Fitzgerald K.D., Abelson J.L., Taylor S.F. Subjective uncertainty and limbic hyperactivation in obsessive-compulsive disorder. Hum. Brain Mapp. 2013;34:1956–1970. doi: 10.1002/hbm.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Verbruggen F., Logan G.D. Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci. Biobehav. Rev. 2009;33:647–661. doi: 10.1016/j.neubiorev.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries F.E., de Wit S.J., Cath D.C., van der Werf Y.D., van der Borden V., van Rossum T.B., van Balkom A.J.L.M., van der Wee N.J.A., Veltman D.J., van den Heuvel O.A. Compensatory frontoparietal activity during working memory: an endophenotype of obsessive-compulsive disorder. Biol. Psychiatry. 2014;76:878–887. doi: 10.1016/j.biopsych.2013.11.021. [DOI] [PubMed] [Google Scholar]
- van der Wee N.J.A., Ramsey N.F., Jansma J.M., Denys D.A., van Megen H.J.G.M., Westenberg H.M.G., Kahn R.S. Spatial working memory deficits in obsessive compulsive disorder are associated with excessive engagement of the medial frontal cortex. NeuroImage. 2003;20:2271–2280. doi: 10.1016/j.neuroimage.2003.05.001. [DOI] [PubMed] [Google Scholar]
- van der Wee N.J.A., Ramsey N.F., van Megen H.J.G.M., Denys D., Westenberg H.G.M., Kahn R.S. Spatial working memory in obsessive-compulsive disorder improves with clinical response: a functional MRI study. Eur. Neuropsychopharmacol. 2007;17:16–23. doi: 10.1016/j.euroneuro.2006.04.012. [DOI] [PubMed] [Google Scholar]
- de Wit S.J., de Vries F.E., van der Werf Y.D., Cath D.C., Heslenfeld D.J., Veltman E.M., van Balkom A.J.L.M., Veltman D.J., van den Heuvel O.A. Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. Am. J. Psychiatry. 2012;169:1100–1108. doi: 10.1176/appi.ajp.2012.12010073. [DOI] [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material