Abstract
Recent neuroimaging studies have provided converging evidence of structural and functional abnormalities of the thalamus in patients with juvenile myoclonic epilepsy (JME). There has also been limited evidence indicating involvement of the subcortical grey matter structures other than thalamus in JME, but with inconsistent findings across the studies. In the present study, we combined volumetric MRI and diffusion tensor imaging analyses to investigate macrostructural and microstructural alterations of the subcortical grey matter in 64 JME patients compared to 58 matched control subjects. Raw volume, fractional anisotropy (FA), and mean diffusivity (MD) of 6 subcortical grey matter structures (amygdala, hippocampus, caudate, pallidum, putamen, thalamus) were measured in both hemispheres. Between-group (controls versus patients) comparisons of normalized volume, FA, and MD, as well as within-group (patients) correlation analyses between structural changes and clinical variables were carried out. Compared to controls, JME patients exhibited significant volume reductions in left pallidum and bilateral putamen and thalamus. Duration of epilepsy negatively correlated with bilateral putamen volumes. Patients and controls did not differ in FA values of all structures. Compared to controls, JME patients showed significant MD increases in left pallidum and bilateral hippocampus, putamen, and thalamus. Significant positive correlations were found between duration of epilepsy and MD values of bilateral hippocampus and thalamus. We have provided evidence that macrostructural and microstructural abnormalities may not only be confined to the thalamus but also affect basal ganglia and hippocampus in JME. Our findings could further support the pathophysiological hypothesis of striato-thalamo-frontal network abnormality underlying JME, and may implicate disease progression.
Keywords: Juvenile myoclonic epilepsy, Thalamus, Basal ganglia, Hippocampus, Volumetry, Fractional anisotropy, Mean diffusivity
Highlights
-
•
Reduced volumes of left pallidum and bilateral putamen and thalamus in JME patients
-
•
Negative correlation between disease duration and putamen volumes
-
•
Increased MD of left pallidum and bilateral hippocampus, putamen, and thalamus in JME patients
-
•
Positive correlation between disease duration and MD of bilateral hippocampus and thalamus
-
•
Structural changes may not only be confined to the thalamus but also affect basal ganglia and hippocampus in JME.
1. Introduction
Juvenile myoclonic epilepsy (JME) represents a common subsyndrome of idiopathic generalized epilepsy (IGE) with a strong genetic basis, accounting for approximately 4%–10% of all epilepsies (Camfield et al., 2013). It usually begins in the age at puberty and is clinically characterized by myoclonic jerks of the upper extremities preferentially occurring early in the morning, generalized tonic-clonic seizures (GTCS), and, less frequently, absence seizures (Janz, 1985). Typical interictal electroencephalography (EEG) features of JME consist of 3–6 Hz generalized spike-wave or polyspike-wave discharges on a normal background, dominantly with frontocentral accentuation.
The fundamental pathogenesis of JME remains elusive; however, cumulative evidence over the decades has suggested that the thalamus along with aberrant thalamocortical circuit plays a pivotal role in the generation of generalized spike-wave discharges (Blumenfeld, 2005). In support of this finding from experimental studies, a number of neuroimaging studies have provided converging evidence of both structural and functional abnormalities of the thalamus in patients with JME (Anderson and Hamandi, 2011, Seneviratne et al., 2014). These abnormalities included thalamic volume reduction (Kim et al., 2013, Kim et al., 2007, Mory et al., 2011, Pulsipher et al., 2009), metabolic dysfunction (Bernasconi et al., 2003, Hattingen et al., 2014, Helms et al., 2006, Lin et al., 2009a), altered microstructural integrity of the thalamocortical network (Deppe et al., 2008, Keller et al., 2011, Kim et al., 2012, von Podewils et al., 2015), increased thalamic blood oxygenation level-dependent activity in relation to generalized spike-wave discharges (Gotman et al., 2005, Pugnaghi et al., 2014, Tyvaert et al., 2009), and thalamocortical functional dysconnectivity (Ji et al., 2015, Kim et al., 2014, McGill et al., 2014, O'Muircheartaigh et al., 2012).
The other subcortical grey matter (GM) structures have currently received less attention than thalamus due to a robust finding of thalamic involvement in JME. A few MRI studies showed a reduction in volumes of putamen (Ciumas et al., 2008, Keller et al., 2011, Seeck et al., 2005) and hippocampus (Kim et al., 2015, Lin et al., 2013) in JME patients in comparison with healthy controls. However, the majority of above-mentioned studies used relatively a small number of subjects, and the main findings were inconsistent across the studies and not replicated in others (Goldberg et al., 2014, Saini et al., 2013). To our knowledge, there is no currently available study that comprehensively examined microstructural integrity of the subcortical GM structures using diffusion tensor imaging (DTI) in JME. DTI is an advanced and noninvasive MRI technique that can detect the magnitude and directionality of water diffusion in vivo. The most widely used parameters derived from DTI are fractional anisotropy (FA) and mean diffusivity (MD), both of which can provide complementary information on microstructural integrity of the white matter tracts. Although DTI has been primarily developed to evaluate white matter integrity, it is now increasingly used to investigate microstructural changes of GM, particularly the subcortical GM (Cherubini et al., 2010, Luo et al., 2011). In the present study, we combined volumetry and DTI analysis to investigate macrostructural and microstructural alterations of the subcortical GM in a large cohort of JME patients compared with matched healthy controls. We predicted that structural alterations may not only be restricted to the thalamus but also affect other subcortical GM in patients with JME. These in turn would be reflected in the disease status of the patients, as predicted by their clinical variables (i.e., disease duration, seizure frequency).
2. Methods
2.1. Participants
We prospectively recruited 67 patients with JME who were followed up in the outpatient epilepsy clinic of Korea University Guro Hospital. Thirty-three patients were identified from our previous studies (Kim et al., 2012, Kim et al., 2013, Kim et al., 2014). The diagnosis of JME was based on electroclinical criteria according to the ILAE (International League Against Epilepsy) classification. Inclusion criteria we used were as follows: (1) myoclonic seizure preferentially occurring early in the morning, with or without GTCS or absence seizure; (2) seizure beginning from the teens or early twenties; (3) normal neurological examination; (4) normal cognitive functions as briefly assessed by a Mini-Mental State Examination score of 28 or higher (Crum et al., 1993); (5) at least one EEG disclosing typical 3–6 Hz generalized spike-wave discharges on a normal background; and (6) neither abnormal nor unusual findings on clinical MR images. Patients with co-morbid neurological, psychiatric, or chronic systemic disorders were excluded. Demographic data and clinical information such as seizure semiology, age of seizure onset, duration of epilepsy, total number of GTCS, and current antiepileptic drugs were obtained through interviews with the patients and their parents and reviews of medical records.
For group comparison, 60 healthy volunteers matched for age, gender, and education years were prospectively recruited to serve as controls. All control subjects underwent neurological examination and a detailed interview to ensure that they had (1) no neurological abnormality and global cognitive impairment (Mini-Mental State Examination score ≥ 28/30) (Crum et al., 1993); (2) no history of neurological, psychiatric, or systemic disorders; (3) no family history of epilepsy; and (4) no history of alcohol or drug abuse. Control subjects with abnormal MRI findings were also excluded. The local ethics committee approved the study protocol, and all participants gave written informed consent prior to study inclusion.
2.2. MRI data acquisition
All participants were scanned on a Siemens Trio 3T scanner (Erlangen, Germany) with a 12-channel phased array head coil. For identification of structural abnormalities, the following clinical MR images were acquired: axial T2-weighted and fluid-attenuated inversion recovery images (4 mm thickness), and oblique coronal T2-weighted and fluid-attenuated inversion recovery images perpendicular to the long axis of hippocampus (3 mm thickness). The MR images were reviewed by a board-certified neuroradiologist (S.I.S.) for any structural abnormalities and reported as normal in all participants.
For volumetric analysis, a high-resolution 3D magnetization-prepared rapid gradient-echo sequence was acquired using the following parameters: TR = 1780 ms, TE = 2.34 ms, matrix = 256 × 256, FOV = 256 × 256 mm, voxel size = 1 mm3. For DTI analysis, a single-shot spin-echo echoplanar imaging sequence was acquired with the following parameters: 30 noncollinear diffusion directions (b-value = 1000 s/mm2) with two nondiffusion gradient (b-value = 0 s/mm2), TR = 6500 ms, TE = 89 ms, matrix = 128 × 128, FOV = 230 × 230 mm, voxel size = 1.8 × 1.8 × 3 mm3. The acquisitions were repeated two times to improve the signal-to-noise ratio and to reproduce more diffusion directionalities. Particular attention was taken to center the subject in the head coil and to restrain head movements with cushions and adhesive medical tape. Resting-state functional MRI data were acquired simultaneously but not included in the current analysis. All patients reported no seizure during the scanning.
2.3. Volumetric analysis
Image preprocessing and volumetric measurement were performed using FMRIB's Software Library (FSL 5.0.9, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Automated segmentation of the subcortical GM was carried out using FIRST (FMRIB's Integrated Registration and Segmentation) that uses Bayesian probabilistic approach, as described in detail elsewhere (Patenaude et al., 2011). Briefly, registration in FIRST comprises an affine transformation (12 degrees of freedom) of the raw, volumetric T1-weighted images to MNI 152 standard space. After subcortical registration, subcortical masks were applied in order to locate the different subcortical structures, followed by segmentation based on shape models and voxel intensities. All segmentations were visually inspected for accuracy prior to inclusion in the analysis. Absolute volumes of bilateral amygdala, hippocampus, caudate, pallidum, putamen, and thalamus were measured in cubic millimeters. To reduce the effects of inter-individual variability in head size, volumetric scaling factor was obtained for each subject by using SIENAX tool (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/SIENA) from the corresponding volumetric T1-weighted image. Thus, normalized volume for each GM was obtained by multiplying the measured volume from FIRST by the volumetric scaling factor from SIENAX.
Data were first tested for normality of distribution and homogeneity of variance assumption using Kolmogorov-Smirnov test and Levene test, respectively. Differences in normalized volumes of 12 GM structures between patients and controls were assessed by analysis of covariance with age, gender, and education years as nuisance covariates. Given the number of multiple comparisons, all p values were adjusted for multiple significance testing using the false discovery rate (FDR) adjustment by Benjamini and Hochberg (Benjamini and Hochberg, 1995). This adjustment avoids the inflated rate of false negatives arising from Bonferroni adjustments while still controlling for false positives, and has been recommended for use in health studies (Glickman et al., 2014). Statistical significance was thresholded at FDR-corrected p < 0.05 in all between-group comparisons. Possible relationships were explored between normalized volumes of significant between-group differences and duration of epilepsy and total number of GTCS using Pearson or Spearman correlation analysis, where appropriate. In addition, partial correlation coefficients were computed between volumes and duration of epilepsy while controlling for the effect of age at seizure onset. Correlations between volumes and duration of epilepsy could not be corrected for age due to the multicollinearity between age and duration of epilepsy (Pearson correlation coefficient, r = 0.901, p < 0.000001, variance inflation factor = 5.300). All p values for correlations were further corrected for multiple comparisons using FDR (corrected p < 0.05). Statistical analyses were performed with the Statistical Package for Social Sciences (Version 21.0; IBM, Armonk, New York).
2.4. Diffusion tensor imaging analysis
DTI data were preprocessed using the FMRIB's Diffusion Toolbox (FDT), part of FSL. First, DTI data were visually inspected for image quality, and then corrected for eddy current and head motion by registering each subject's 30 diffusion weighted images to their own nondiffusion-weighted image using FLIRT (FMRIB Linear Image Registration Tool). Brain extraction tool (BET) implemented in FSL was used to remove nonbrain tissues and background noise by applying a fractional intensity threshold of 0.35. Next, a diffusion tensor model was fitted at each voxel using DTIFIT to generate FA and MD maps.
In order to retrieve FA and MD values in native DTI space, each subject's volumetric T1-weighted image was coregistered to the FA and MD images using a three-dimensional rigid body registration with FLIRT. The calculated transformation matrix was then applied to the subcortical GM segmented from FIRST. Binary masks were created for the bilateral amygdala, hippocampus, caudate, pallidum, putamen, and thalamus. FA and MD values were then retrieved from these 12 GM structures and fed into statistical analysis. Differences in FA and MD values between patients and controls were assessed by analysis of covariance adjusting for the effects of age, gender, and education years. Possible relationships were explored between FA and MD values of significant between-group differences and duration of epilepsy and total number of GTCS by using Pearson or Spearman correlation analysis, where appropriate. In addition, partial correlation coefficients were computed between FA or MD values and duration of epilepsy while controlling for the effect of age at seizure onset. Correlations between FA or MD values and duration of epilepsy could not be corrected for age due to the multicollinearity between age and duration of epilepsy. Statistical significance for group comparisons and correlations was set at FDR-corrected p < 0.05.
3. Results
3.1. Clinical characteristics
Three patients and two control subjects were excluded due to poor image quality from excessive head motion or DTI distortion. A total of 64 patients with JME (32 women, mean age = 25.7 years) and 58 control subjects (30 women, mean age = 26.8 years) were finally included for statistical analysis (Table 1). Patients and controls did not differ in age, gender, education years, and Mini-Mental State Examination score. Mean age of seizure onset was 16.2 ± 3.5 years, and mean duration of epilepsy was 9.5 ± 7.2 years. Total number of GTCS was 5.7 ± 9.6. Semiological features included myoclonic seizure in 64 patients (100%), GTCS in 58 (91%), and absence seizure in 34 (53%). Family history of epilepsy was reported in 21 patients (33%). The antiepileptic drugs that patients received at the time of study consisted of valproate monotherapy in 25 patients (39%), levetiracetam monotherapy in 12 (19%), topiramate monotherapy in 5 (8%), lamotrigine monotherapy in 2 (3%), zonisamide monotherapy in 2 (3%), polytherapy with valproate and one of levetiracetam, topiramate, or lamotrigine in 16 (25%), and none in 2 (3%).
Table 1.
Demographic and clinical data.
| JME patients | Controls | p-Value | |
|---|---|---|---|
| Number of subjects | 64 | 58 | |
| Gender (female:male) | 32:32 | 30:28 | 0.721 |
| Age (years) | 25.7 ± 8.1 (range, 15–44) | 26.8 ± 6.9 (range, 15–44) | 0.390 |
| Handedness (right:left) | 61:3 | 56:2 | > 0.999 |
| Education years | 13.9 ± 1.9 | 14.1 ± 2.0 | 0.581 |
| MMSE score | 29.5 ± 0.7 | 29.6 ± 0.6 | 0.245 |
| Seizure semiology | MS (100%), GTCS (91%), AS (53%) | ||
| Age of seizure onset (years) | 16.2 ± 3.5 (range, 10–25) | ||
| Duration of epilepsy | 9.5 ± 7.2 (range, 1–29) | ||
| Total number of GTCS | 5.7 ± 9.6 (range, 0–53) |
Abbreviations: AS = absence seizure; GTCS = generalized tonic-clonic seizure; JME = juvenile myoclonic epilepsy; MMSE = Mini-Mental State Examination; MS = myoclonic seizure.
Data are presented as mean ± standard deviation.
Group comparisons were made using two-sample t-test, chi-square test, or Fisher's exact test, where appropriate.
3.2. Volumetric analysis
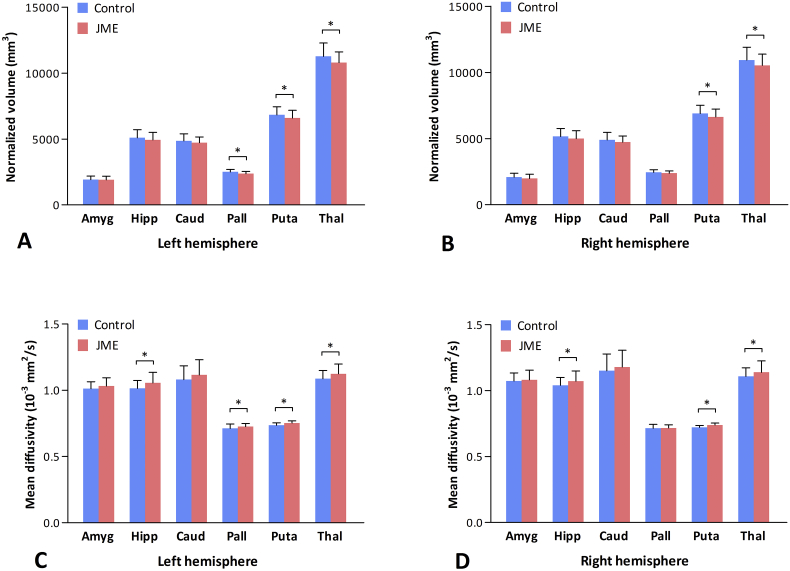
Normalized volumes for the 12 subcortical GM structures and statistical results are detailed in Table 2 and depicted in Fig. 1. Compared to controls, JME patients had significant volume reductions in left pallidum (F1,117 = 7.635, p = 0.007), left putamen (F1,117 = 5.728, p = 0.018), right putamen (F1,117 = 7.136, p = 0.009), left thalamus (F1,117 = 8.857, p = 0.004), and right thalamus (F1,117 = 6.289, p = 0.014). These differences remained significant after correcting for multiple comparisons using FDR. There were no significant differences in normalized volumes of bilateral amygdala, hippocampus, caudate, and right pallidum between controls and patients (all p > 0.05). No differences were found in normalized volumes of all 12 GM structures between JME patients with absence seizures (n = 34) and those without (n = 30) (all p > 0.05).
Table 2.
Normalized volume, fractional anisotropy, and mean diffusivity of the subcortical grey matter structures in controls and patients with juvenile myoclonic epilepsy.
| Subcortical structures | Volume (mm3) |
Fractional anisotropy |
Mean diffusivity (10− 3 mm2/s) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Controls | Patients | p-Value | Controls | Patients | p-Value | Controls | Patients | p-Value | |
| L amygdala | 1916 (283) | 1909 (267) | 0.947 | 0.222 (0.017) | 0.221 (0.021) | 0.609 | 1.012 (0.052) | 1.032 (0.062) | 0.064 |
| R amygdala | 2078 (299) | 1985 (323) | 0.117 | 0.209 (0.017) | 0.210 (0.018) | 0.919 | 1.073 (0.062) | 1.082 (0.074) | 0.486 |
| L hippocampus | 5093 (616) | 4936 (576) | 0.157 | 0.230 (0.017) | 0.227 (0.019) | 0.415 | 1.015 (0.059) | 1.056 (0.080) | 0.001⁎ |
| R hippocampus | 5153 (604) | 4997 (602) | 0.153 | 0.239 (0.016) | 0.236 (0.019) | 0.359 | 1.041 (0.060) | 1.072 (0.077) | 0.010⁎ |
| L caudate | 4855 (541) | 4722 (431) | 0.080 | 0.273 (0.023) | 0.267 (0.027) | 0.123 | 1.081 (0.103) | 1.115 (0.115) | 0.067 |
| R caudate | 4893 (581) | 4726 (471) | 0.058 | 0.267 (0.028) | 0.265 (0.030) | 0.577 | 1.151 (0.127) | 1.179 (0.128) | 0.200 |
| L pallidum | 2456 (199) | 2367 (177) | 0.007⁎ | 0.417 (0.037) | 0.417 (0.029) | 0.878 | 0.712 (0.034) | 0.726 (0.023) | 0.010⁎ |
| R pallidum | 2448 (198) | 2386 (164) | 0.064 | 0.428 (0.036) | 0.433 (0.032) | 0.219 | 0.715 (0.029) | 0.717 (0.023) | 0.821 |
| L putamen | 6837 (611) | 6597 (586) | 0.018⁎ | 0.308 (0.016) | 0.309 (0.020) | 0.604 | 0.735 (0.020) | 0.743 (0.016) | 0.017⁎ |
| R putamen | 6899 (614) | 6630 (602) | 0.009⁎ | 0.313 (0.018) | 0.316 (0.017) | 0.233 | 0.722 (0.014) | 0.729 (0.016) | 0.017⁎ |
| L thalamus | 11,274 (1001) | 10,793 (819) | 0.004⁎ | 0.314 (0.016) | 0.309 (0.019) | 0.159 | 1.087 (0.062) | 1.124 (0.074) | 0.002⁎ |
| R thalamus | 10,937 (967) | 10,534 (848) | 0.014⁎ | 0.313 (0.016) | 0.310 (0.019) | 0.351 | 1.108 (0.065) | 1.140 (0.086) | 0.010⁎ |
Abbreviations: L = left; R = right.
Data are presented as mean (standard deviation).
Group comparisons were made using analysis of covariance adjusting for the effects of age, gender, and education years.
Significant at p < 0.05, corrected for multiple comparisons using false discovery rate.
Fig. 1.
Mean (bars) and standard deviation (whiskers) for the normalized volumes (A, B) and mean diffusivity values (C, D) of the subcortical grey matter structures in controls and patients with juvenile myoclonic epilepsy. The asterisk (*) indicates statistical significance between the groups (p < 0.05, corrected for multiple comparisons using false discovery rate).
Abbreviations: Amyg = amygdala; Hipp = hippocampus; Caud = caudate; Pall = pallidum; Puta = putamen; Thal = thalamus.
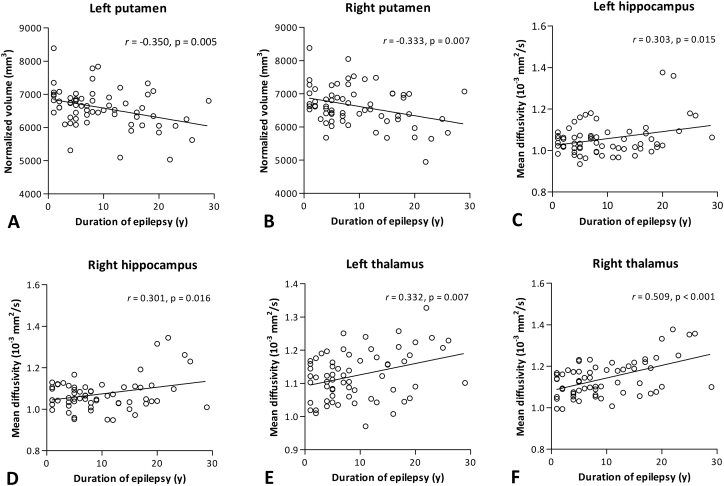
In controls, no significant correlations were found between age and volumes of left putamen (r = − 0.073, p = 0.586) and right putamen (r = − 0.106, p = 0.428). In patients with JME, duration of epilepsy negatively correlated with volumes of left putamen (r = − 0.350, p = 0.005) and right putamen (r = − 0.333, p = 0.007) (Fig. 2). Partial correlation analysis with duration of epilepsy controlling for age at seizure onset also revealed significant negative correlations with volumes of left putamen (r = − 0.349, p = 0.005) and right putamen (r = − 0.332, p = 0.008). These correlations remained significant after FDR correction. No significant correlations were found between duration of epilepsy and volumes of left pallidum and bilateral thalamus (all p > 0.05). There were no significant correlations between normalized volumes of these 5 subcortical GM and total number of GTCS (all p > 0.05).
Fig. 2.
Correlations between duration of epilepsy and bilateral putamen volumes (A, B) and mean diffusivity values of bilateral hippocampus (C, D) and thalamus (E, F). All correlations presented in the figure remained significant after false discovery rate correction.
3.3. FA and MD analysis
FA and MD values for the 12 subcortical GM structures and statistical results are presented in Table 2 and depicted in Fig. 1. Patients and controls did not differ in FA values of the 12 GM structures (all p > 0.05). Compared to controls, JME patients exhibited significant MD increases in left pallidum (F1,117 = 6.927, p = 0.010), left hippocampus (F1,117 = 10.750, p = 0.001), right hippocampus (F1,117 = 6.949, p = 0.010), left putamen (F1,117 = 5.837, p = 0.017), right putamen (F1,117 = 5.880, p = 0.017), left thalamus (F1,117 = 9.686, p = 0.002), and right thalamus (F1,117 = 6.797, p = 0.010). These differences remained significant after correcting for multiple comparisons using FDR. MD values of the other GM structures were not different between patients and controls (all p > 0.05). No differences were found in FA and MD values of all 12 GM structures between JME patients with absence seizures (n = 34) and those without (n = 30) (all p > 0.05). In addition, Pearson correlation coefficients were computed between normalized volume and MD values of significantly altered subcortical GM structures to delineate relationships between macrostructural and microstructural changes in patient group. Significant correlations were found between volume and MD value in left thalamus (r = − 0.503, p < 0.001) and right thalamus (r = − 0.363, p = 0.003). The other structures (left pallidum, bilateral hippocampus, bilateral putamen) showed no significant correlations (all p > 0.05).
In controls, there were negative correlations between age and MD values of left hippocampus (r = − 0.395, p = 0.002) and right hippocampus (r = − 0.310, p = 0.018), while no significant correlations were observed between age and MD values of left thalamus (r = − 0.088, p = 0.513) and right thalamus (r = − 0.054, p = 0.685). In patients with JME, significant positive correlations were found between duration of epilepsy and MD values of left hippocampus (r = 0.303, p = 0.015), right hippocampus (r = 0.301, p = 0.016), left thalamus (r = 0.332, p = 0.007), and right thalamus (r = 0.509, p < 0.001) (Fig. 2). Partial correlation analysis with duration of epilepsy controlling for age at seizure onset also revealed significant positive correlations with MD values of left hippocampus (r = 0.302, p = 0.016), right hippocampus (r = 0.303, p = 0.016), left thalamus (r = 0.332, p = 0.008), and right thalamus (r = 0.508, p < 0.001). These correlations remained significant after FDR correction. MD values of bilateral putamen did not correlate with duration of epilepsy (both p > 0.05). No significant correlations were found between MD values of these 7 GM and total number of GTCS (all p > 0.05).
4. Discussion
This study attempted to explore structural changes of the subcortical GM in patients with JME using a multimodal MRI approach that took into account volumetric and microstructural integrity changes. The main findings are macrostructural or microstructural alterations of the thalamus, basal ganglia, and hippocampus in JME patients, which may extend our understanding of the neuroanatomical changes underlying JME.
4.1. Thalamus
Our finding of thalamic volume reduction is in good accordance with recent MRI studies that employed voxel-based morphometry or manual volumetry (Kim et al., 2013, Kim et al., 2007, Kim et al., 2015, Lin et al., 2009b, Mory et al., 2011, Pulsipher et al., 2009), strongly suggesting a specific macrostructural alteration of the thalamus in JME. In addition, we found evidence for the microstructural alteration of increased thalamic MD in JME patients. MD, a sensitive DTI parameter that provides information about microstructural integrity, increases with microscopic barrier disruption and extracellular fluid accumulation (Assaf, 2008). Concurrent macrostructural and microstructural changes of the thalamus observed in our JME patients are in line with previous studies revealing both reduced volume and increased MD in the thalamus in patients with absence epilepsy (Luo et al., 2011) and temporal lobe epilepsy with hippocampal sclerosis (Gong et al., 2008), implicating structural disorganization and expansion of extracellular space in the thalamus in the epileptogenesis underlying JME. Our additional finding of increasing thalamic MD in relation to increasing duration of epilepsy suggests that thalamic microstructural changes may result from chronic, accumulating effect of epileptic spikes and be the consequence of the long-standing burden of the disease. Taken together, compelling evidence from these neuroimaging studies could provide a robust basis to the hypothesis of thalamic dysfunction in the fundamental pathogenesis of JME.
4.2. Basal ganglia
In addition to the well-known thalamic involvement, a growing body of evidence suggests a possible role of basal ganglia in the modulation of generalized spike-waves or seizures in IGE (Deransart et al., 2000, Deransart et al., 1998). Specifically, an electrophysiological study using an animal genetic model of absence epilepsy demonstrated aberrant electrical events in the striatal output neurons in the corticostriatal pathway during spontaneous generalized spike-wave discharges, implicating the basal ganglia in the promotion or termination of absence seizures (Slaght et al., 2004). Simultaneous EEG-functional MRI studies have shown a reduction in blood oxygenation level-dependent activity of the basal ganglia in association with generalized spike-wave discharges in patients with IGE (Hamandi et al., 2006, Li et al., 2009, Moeller et al., 2008). A resting-state functional MRI study also found enhanced functional connectivity within the basal ganglia network in IGE patients compared with controls, pointing to a modulatory role of basal ganglia in IGE (Luo et al., 2012). It is generally accepted that the striatum modulates the activity of the output nuclei of the basal ganglia, which tonically inhibit their target nuclei in the thalamus and brainstem. Reduced activity in these output nuclei may cause a disinhibition of the thalamocortical projections, leading to a subsequent enhancement in cortical excitability (Alexander and Crutcher, 1990). It is therefore conceivable that functional impairment in the striatum (e.g., putamen) may exaggerate thalamocortical activation and result in promotion of generalized spike-wave discharges or seizures in IGE, although no robust evidence is currently available in human IGE.
In the current study, we observed that volumes of bilateral putamen and left pallidum were significantly reduced in JME patients relative to controls, and that bilateral putamen volumes negatively correlated with duration of epilepsy. Our findings are consistent with those of prior volumetric or morphometric studies on various IGE syndromes (Ciumas and Savic, 2006, Ciumas et al., 2008, Du et al., 2011, Keller et al., 2011, Luo et al., 2011, Seeck et al., 2005), further providing evidence for the macrostructural changes of the putamen and pallidum in IGE. Moreover, we observed significant MD increases in bilateral putamen and left pallidum in JME patients compared to controls, according well with a DTI study performed on patients with absence epilepsy (Luo et al., 2011). Functional impairment of the putamen has also been suggested by a recent positron emission tomography study that demonstrated a reduction in dopamine receptor binding restricted to the posterior putamen in patients with JME (Landvogt et al., 2010). Given the strong connections between the basal ganglia and frontal lobe, our findings of concomitant macrostructural and microstructural abnormalities of the putamen and pallidum are congruent with previous neurophysiologic studies suggesting preferential involvement of striato-thalamo-frontal networks in JME or IGE (Ciumas et al., 2008, Lin et al., 2009a, Moeller et al., 2008, Yang et al., 2014, Zhang et al., 2016).
4.3. Hippocampus
Several lines of evidence suggest that the hippocampus is involved in the epileptogenic network of JME. A limited number of MRI revealed hippocampal volume reduction (Kim et al., 2015, Lin et al., 2013) and metabolic dysfunction (Ristic et al., 2011) in JME patients relative to controls. In an electrophysiological study using source analysis of dense array scalp EEG, epileptiform discharges of JME were localized not only to the orbitofrontal/medial frontopolar cortex but also to the basal-medial temporal cortex (Holmes et al., 2010). However, such a finding of hippocampal volume reduction was not replicated in our study and others (Goldberg et al., 2014, Saini et al., 2013, Seneviratne et al., 2014). This discrepancy between the studies could not be properly explained but may be attributed in part to genetic heterogeneity and differences in sample size, methods for volumetric measurement, and magnetic field strength.
An unexpected but intriguing finding of our results is increased MD of the hippocampus in JME patients. This is a new finding and therefore requires careful interpretation. Since it is widely believed that an increase in MD indicates an enlargement of the extracellular space due to altered cytoarchitecture, our finding of increased hippocampal MD might reflect either primary pathologic change or secondary degeneration due to disruption of white matter tracts connecting the hippocampus to other structures, possibly leading to cortical dysfunction. In support of the latter hypothesis, recent studies have shown subtle microstructural abnormalities of the temporal lobe white matter and other regions anatomically connected to the hippocampus in patients with JME or IGE (Kim et al., 2015, O'Muircheartaigh et al., 2011, Xue et al., 2014, Yang et al., 2012). Based on the finding that microstructural change of the hippocampus is a more sensitive indicator of memory impairment than hippocampal volumetry, as demonstrated in healthy elderly individuals (Carlesimo et al., 2010) and in patients with neurodegenerative diseases (Carlesimo et al., 2012, Cherubini et al., 2010), future study using combined DTI and a comprehensive cognitive assessment should elucidate relationships between microstructural alterations of the hippocampus and memory dysfunctions in this patient group (Lin et al., 2013, Pascalicchio et al., 2007, Sonmez et al., 2004).
4.4. Limitations and future directions
Several limitations of the present study should be addressed. First, our study is cross-sectional, and thus, our results with respect to causal relation are limited. Given our findings of significant correlations between disease duration and volumetric or DTI data, we speculated that structural changes of the subcortical GM might be the consequence of cumulative epileptic spikes and repeated seizures, and that these alterations could have a potential role as a biomarker for the disease progression in JME. Future prospective studies incorporating a longitudinal design would provide a hint to disentangle causal relations between structural changes and disease progression. Second, only JME patients were included in our study. Although IGE subsyndromes are considered to share a common pathogenetic mechanism (Nordli, 2005), a possible difference in neuroanatomical substrate between the subsyndromes has been suggested in several neuroimaging studies (Ciumas et al., 2010, Liu et al., 2011, Savic et al., 2004). Indeed, our results have some similarities as well as inconsistencies with previous investigations studying patients with absence epilepsy, IGE with GTCS, or heterogenous IGE syndromes. For instance, Luo et al. (2011) observed that patients with absence epilepsy had reduced volume and increased MD in the thalamus and basal ganglia structures as compared to controls. However, we failed to find any differences in volume, FA, and MD of all 12 subcortical grey matter structures between JME patients with absence seizure and those without. To our knowledge, there is no well-designed study available to date investigating structural differences in the subcortical GM between the IGE subsyndromes. Therefore, future studies using homogenous patient groups of subsyndromes should determine whether the structural alterations observed in our study are specific to JME or common to IGE. Third, the possible effects of antiepileptic drugs on subcortical GM could not be entirely ruled out. The majority (64%) of our patients were taking valproate, a drug of choice for the treatment of JME, at the time of study inclusion. Although anecdotal case reports showed that valproate could cause pseudoatrophy of the brain (Papazian et al., 1995), there has been no evidence that the chronic use of valproate affected subcortical GM volumes. No difference in thalamic volume between IGE patients with and without valproate exposure was found in a longitudinal volumetric study (Pulsipher et al., 2011). With regard to DTI, there is also currently little evidence for the influence of valproate on FA or MD of the subcortical GM (Hafeman et al., 2012). In our study, we failed to find significant differences in either volumes or FA/MD values of all subcortical GM between patients treated with valproate (n = 41) and patients not treated with valproate (n = 23). Moreover, no significant correlations were found between MRI data (putamen volume, hippocampal MD, thalamic MD) and dose and exposure duration of valproate in 41 patients. Thus, our results seem to be independent of the use of antiepileptic drugs. Lastly, nonisotropic voxels (1.8 × 1.8 × 3 mm3) of our DTI data may introduce bias or underestimation of the quantitative assessment of DTI metrics, and cause geometric distortions of the brain structures, particularly the frontal base and medial temporal lobe (Fujiwara et al., 2007, Oouchi et al., 2007).
5. Conclusion
Combining volumetric and DTI analyses, we have provided evidence for concomitant macrostructural and microstructural alterations of the subcortical GM in patients with JME, suggesting that structural abnormalities may not only be confined to the thalamus but also affect basal ganglia and hippocampus in JME. Our findings of decreasing putamen volume and increasing MD of thalamus and hippocampus in relation to increasing disease duration could support the pathophysiological hypothesis of striato-thalamo-frontal network abnormality underlying JME, and may implicate disease progression.
Acknowledgments
Acknowledgements
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (Grant No. 20100004827, 20110005418) and a Korea University Grant. The authors are very grateful to the participants for taking part in the present study.
Conflict of interest statement
None of the authors have potential conflicts of interest to be disclosed.
Financial disclosures
None of the authors have any financial disclosures.
References
- Alexander G.E., Crutcher M.D. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Anderson J., Hamandi K. Understanding juvenile myoclonic epilepsy: contributions from neuroimaging. Epilepsy Res. 2011;94:127–137. doi: 10.1016/j.eplepsyres.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Assaf Y. Can we use diffusion MRI as a bio-marker of neurodegenerative processes? BioEssays. 2008;30:1235–1245. doi: 10.1002/bies.20851. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. [Google Scholar]
- Bernasconi A., Bernasconi N., Natsume J., Antel S.B., Andermann F., Arnold D.L. Magnetic resonance spectroscopy and imaging of the thalamus in idiopathic generalized epilepsy. Brain. 2003;126:2447–2454. doi: 10.1093/brain/awg249. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia. 2005;46(Suppl. 9):S21–S33. doi: 10.1111/j.1528-1167.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- Camfield C.S., Striano P., Camfield P.R. Epidemiology of juvenile myoclonic epilepsy. Epilepsy Behav. 2013;28(Suppl. 1):S15–S17. doi: 10.1016/j.yebeh.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Carlesimo G.A., Cherubini A., Caltagirone C., Spalletta G. Hippocampal mean diffusivity and memory in healthy elderly individuals: a cross-sectional study. Neurology. 2010;74:194–200. doi: 10.1212/WNL.0b013e3181cb3e39. [DOI] [PubMed] [Google Scholar]
- Carlesimo G.A., Piras F., Assogna F., Pontieri F.E., Caltagirone C., Spalletta G. Hippocampal abnormalities and memory deficits in Parkinson disease: a multimodal imaging study. Neurology. 2012;78:1939–1945. doi: 10.1212/WNL.0b013e318259e1c5. [DOI] [PubMed] [Google Scholar]
- Cherubini A., Peran P., Spoletini I., Di Paola M., Di Iulio F., Hagberg G.E., Sancesario G., Gianni W., Bossu P., Caltagirone C., Sabatini U., Spalletta G. Combined volumetry and DTI in subcortical structures of mild cognitive impairment and Alzheimer's disease patients. J. Alzheimers Dis. 2010;19:1273–1282. doi: 10.3233/JAD-2010-091186. [DOI] [PubMed] [Google Scholar]
- Ciumas C., Savic I. Structural changes in patients with primary generalized tonic and clonic seizures. Neurology. 2006;67:683–686. doi: 10.1212/01.wnl.0000230171.23913.cf. [DOI] [PubMed] [Google Scholar]
- Ciumas C., Wahlin T.B., Jucaite A., Lindstrom P., Halldin C., Savic I. Reduced dopamine transporter binding in patients with juvenile myoclonic epilepsy. Neurology. 2008;71:788–794. doi: 10.1212/01.wnl.0000316120.70504.d5. [DOI] [PubMed] [Google Scholar]
- Ciumas C., Wahlin T.B., Espino C., Savic I. The dopamine system in idiopathic generalized epilepsies: identification of syndrome-related changes. NeuroImage. 2010;51:606–615. doi: 10.1016/j.neuroimage.2010.02.051. [DOI] [PubMed] [Google Scholar]
- Crum R.M., Anthony J.C., Bassett S.S., Folstein M.F. Population-based norms for the mini-mental state examination by age and educational level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- Deppe M., Kellinghaus C., Duning T., Moddel G., Mohammadi S., Deppe K., Schiffbauer H., Kugel H., Keller S.S., Ringelstein E.B., Knecht S. Nerve fiber impairment of anterior thalamocortical circuitry in juvenile myoclonic epilepsy. Neurology. 2008;71:1981–1985. doi: 10.1212/01.wnl.0000336969.98241.17. [DOI] [PubMed] [Google Scholar]
- Deransart C., Vercueil L., Marescaux C., Depaulis A. The role of basal ganglia in the control of generalized absence seizures. Epilepsy Res. 1998;32:213–223. doi: 10.1016/s0920-1211(98)00053-9. [DOI] [PubMed] [Google Scholar]
- Deransart C., Riban V., Le B., Marescaux C., Depaulis A. Dopamine in the striatum modulates seizures in a genetic model of absence epilepsy in the rat. Neuroscience. 2000;100:335–344. doi: 10.1016/s0306-4522(00)00266-9. [DOI] [PubMed] [Google Scholar]
- Du H., Zhang Y., Xie B., Wu N., Wu G., Wang J., Jiang T., Feng H. Regional atrophy of the basal ganglia and thalamus in idiopathic generalized epilepsy. J. Magn. Reson. Imaging. 2011;33:817–821. doi: 10.1002/jmri.22416. [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Sasaki M., Kanbara Y., Matsumura Y., Shibata E., Inoue T., Nishimoto H., Ogawa A. Improved geometric distortion in coronal diffusion-weighted and diffusion tensor imaging using a whole-brain isotropic-voxel acquisition technique at 3 Tesla. Magn. Reson. Med. Sci. 2007;6:127–132. doi: 10.2463/mrms.6.127. [DOI] [PubMed] [Google Scholar]
- Glickman M.E., Rao S.R., Schultz M.R. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J. Clin. Epidemiol. 2014;67:850–857. doi: 10.1016/j.jclinepi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Goldberg H., Weinstock A., Bergsland N., Dwyer M.G., Farooq O., Sazgar M., Poloni G., Treu C., Weinstock-Guttman B., Ramanathan M., Zivadinov R. MRI segmentation analysis in temporal lobe and idiopathic generalized epilepsy. BMC Neurol. 2014;14:131. doi: 10.1186/1471-2377-14-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G., Concha L., Beaulieu C., Gross D.W. Thalamic diffusion and volumetry in temporal lobe epilepsy with and without mesial temporal sclerosis. Epilepsy Res. 2008;80:184–193. doi: 10.1016/j.eplepsyres.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Gotman J., Grova C., Bagshaw A., Kobayashi E., Aghakhani Y., Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc. Natl. Acad. Sci. U. S. A. 2005;102:15236–15240. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman D.M., Chang K.D., Garrett A.S., Sanders E.M., Phillips M.L. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord. 2012;14:375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- Hamandi K., Salek-Haddadi A., Laufs H., Liston A., Friston K., Fish D.R., Duncan J.S., Lemieux L. EEG-fMRI of idiopathic and secondarily generalized epilepsies. NeuroImage. 2006;31:1700–1710. doi: 10.1016/j.neuroimage.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Hattingen E., Luckerath C., Pellikan S., Vronski D., Roth C., Knake S., Kieslich M., Pilatus U. Frontal and thalamic changes of GABA concentration indicate dysfunction of thalamofrontal networks in juvenile myoclonic epilepsy. Epilepsia. 2014;55:1030–1037. doi: 10.1111/epi.12656. [DOI] [PubMed] [Google Scholar]
- Helms G., Ciumas C., Kyaga S., Savic I. Increased thalamus levels of glutamate and glutamine (Glx) in patients with idiopathic generalised epilepsy. J. Neurol. Neurosurg. Psychiatry. 2006;77:489–494. doi: 10.1136/jnnp.2005.074682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M.D., Quiring J., Tucker D.M. Evidence that juvenile myoclonic epilepsy is a disorder of frontotemporal corticothalamic networks. NeuroImage. 2010;49:80–93. doi: 10.1016/j.neuroimage.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Janz D. Epilepsy with impulsive petit mal (juvenile myoclonic epilepsy) Acta Neurol. Scand. 1985;72:449–459. doi: 10.1111/j.1600-0404.1985.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Ji G.J., Zhang Z., Xu Q., Wang Z., Wang J., Jiao Q., Yang F., Tan Q., Chen G., Zang Y.F., Liao W., Lu G. Identifying corticothalamic network epicenters in patients with idiopathic generalized epilepsy. Am. J. Neuroradiol. 2015;36:1494–1500. doi: 10.3174/ajnr.A4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S.S., Ahrens T., Mohammadi S., Moddel G., Kugel H., Ringelstein E.B., Deppe M. Microstructural and volumetric abnormalities of the putamen in juvenile myoclonic epilepsy. Epilepsia. 2011;52:1715–1724. doi: 10.1111/j.1528-1167.2011.03117.x. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Lee J.K., Koh S.B., Lee S.A., Lee J.M., Kim S.I., Kang J.K. Regional grey matter abnormalities in juvenile myoclonic epilepsy: a voxel-based morphometry study. NeuroImage. 2007;37:1132–1137. doi: 10.1016/j.neuroimage.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Suh S.I., Park S.Y., Seo W.K., Koh I., Koh S.B., Seol H.Y. Microstructural white matter abnormality and frontal cognitive dysfunctions in juvenile myoclonic epilepsy. Epilepsia. 2012;53:1371–1378. doi: 10.1111/j.1528-1167.2012.03544.x. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Kim J.B., Seo W.K., Suh S.I., Koh S.B. Volumetric and shape analysis of thalamus in idiopathic generalized epilepsy. J. Neurol. 2013;260:1846–1854. doi: 10.1007/s00415-013-6891-5. [DOI] [PubMed] [Google Scholar]
- Kim J.B., Suh S.I., Seo W.K., Oh K., Koh S.B., Kim J.H. Altered thalamocortical functional connectivity in idiopathic generalized epilepsy. Epilepsia. 2014;55:592–600. doi: 10.1111/epi.12580. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Lim S.C., Kim W., Kwon O.H., Jeon S., Lee J.M., Shon Y.M. Extrafrontal structural changes in juvenile myoclonic epilepsy: a topographic analysis of combined structural and microstructural brain imaging. Seizure. 2015;30:124–131. doi: 10.1016/j.seizure.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Landvogt C., Buchholz H.G., Bernedo V., Schreckenberger M., Werhahn K.J. Alteration of dopamine D2/D3 receptor binding in patients with juvenile myoclonic epilepsy. Epilepsia. 2010;51:1699–1706. doi: 10.1111/j.1528-1167.2010.02569.x. [DOI] [PubMed] [Google Scholar]
- Li Q., Luo C., Yang T., Yao Z., He L., Liu L., Xu H., Gong Q., Yao D., Zhou D. EEG-fMRI study on the interictal and ictal generalized spike-wave discharges in patients with childhood absence epilepsy. Epilepsy Res. 2009;87:160–168. doi: 10.1016/j.eplepsyres.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Lin K., Carrete H., Jr., Lin J., Peruchi M.M., de Araujo Filho G.M., Guaranha M.S., Guilhoto L.M., Sakamoto A.C., Yacubian E.M. Magnetic resonance spectroscopy reveals an epileptic network in juvenile myoclonic epilepsy. Epilepsia. 2009;50:1191–1200. doi: 10.1111/j.1528-1167.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- Lin K., Jackowski A.P., Carrete H., Jr., de Araujo Filho G.M., Silva H.H., Guaranha M.S., Guilhoto L.M., Bressan R.A., Yacubian E.M. Voxel-based morphometry evaluation of patients with photosensitive juvenile myoclonic epilepsy. Epilepsy Res. 2009;86:138–145. doi: 10.1016/j.eplepsyres.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Lin K., de Araujo Filho G.M., Pascalicchio T.F., Silva I., Tudesco I.S., Guaranha M.S., Carrete Junior H., Jackowski A.P., Yacubian E.M. Hippocampal atrophy and memory dysfunction in patients with juvenile myoclonic epilepsy. Epilepsy Behav. 2013;29:247–251. doi: 10.1016/j.yebeh.2013.06.034. [DOI] [PubMed] [Google Scholar]
- Liu M., Concha L., Beaulieu C., Gross D.W. Distinct white matter abnormalities in different idiopathic generalized epilepsy syndromes. Epilepsia. 2011;52:2267–2275. doi: 10.1111/j.1528-1167.2011.03313.x. [DOI] [PubMed] [Google Scholar]
- Luo C., Xia Y., Li Q., Xue K., Lai Y., Gong Q., Zhou D., Yao D. Diffusion and volumetry abnormalities in subcortical nuclei of patients with absence seizures. Epilepsia. 2011;52:1092–1099. doi: 10.1111/j.1528-1167.2011.03045.x. [DOI] [PubMed] [Google Scholar]
- Luo C., Li Q., Xia Y., Lei X., Xue K., Yao Z., Lai Y., Martinez-Montes E., Liao W., Zhou D., Valdes-Sosa P.A., Gong Q., Yao D. Resting state basal ganglia network in idiopathic generalized epilepsy. Hum. Brain Mapp. 2012;33:1279–1294. doi: 10.1002/hbm.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill M.L., Devinsky O., Wang X., Quinn B.T., Pardoe H., Carlson C., Butler T., Kuzniecky R., Thesen T. Functional neuroimaging abnormalities in idiopathic generalized epilepsy. NeuroImage Clin. 2014;6:455–462. doi: 10.1016/j.nicl.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller F., Siebner H.R., Wolff S., Muhle H., Boor R., Granert O., Jansen O., Stephani U., Siniatchkin M. Changes in activity of striato-thalamo-cortical network precede generalized spike wave discharges. NeuroImage. 2008;39:1839–1849. doi: 10.1016/j.neuroimage.2007.10.058. [DOI] [PubMed] [Google Scholar]
- Mory S.B., Betting L.E., Fernandes P.T., Lopes-Cendes I., Guerreiro M.M., Guerreiro C.A., Cendes F., Li L.M. Structural abnormalities of the thalamus in juvenile myoclonic epilepsy. Epilepsy Behav. 2011;21:407–411. doi: 10.1016/j.yebeh.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Nordli D.R., Jr. Idiopathic generalized epilepsies recognized by the International League Against Epilepsy. Epilepsia. 2005;46(Suppl. 9):S48–S56. doi: 10.1111/j.1528-1167.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- O'Muircheartaigh J., Vollmar C., Barker G.J., Kumari V., Symms M.R., Thompson P., Duncan J.S., Koepp M.J., Richardson M.P. Focal structural changes and cognitive dysfunction in juvenile myoclonic epilepsy. Neurology. 2011;76:34–40. doi: 10.1212/WNL.0b013e318203e93d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Muircheartaigh J., Vollmar C., Barker G.J., Kumari V., Symms M.R., Thompson P., Duncan J.S., Koepp M.J., Richardson M.P. Abnormal thalamocortical structural and functional connectivity in juvenile myoclonic epilepsy. Brain. 2012;135:3635–3644. doi: 10.1093/brain/aws296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oouchi H., Yamada K., Sakai K., Kizu O., Kubota T., Ito H., Nishimura T. Diffusion anisotropy measurement of brain white matter is affected by voxel size: underestimation occurs in areas with crossing fibers. Am. J. Neuroradiol. 2007;28:1102–1106. doi: 10.3174/ajnr.A0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian O., Canizales E., Alfonso I., Archila R., Duchowny M., Aicardi J. Reversible dementia and apparent brain atrophy during valproate therapy. Ann. Neurol. 1995;38:687–691. doi: 10.1002/ana.410380423. [DOI] [PubMed] [Google Scholar]
- Pascalicchio T.F., de Araujo Filho G.M., da Silva Noffs M.H., Lin K., Caboclo L.O., Vidal-Dourado M., Ferreira Guilhoto L.M., Yacubian E.M. Neuropsychological profile of patients with juvenile myoclonic epilepsy: a controlled study of 50 patients. Epilepsy Behav. 2007;10:263–267. doi: 10.1016/j.yebeh.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Patenaude B., Smith S.M., Kennedy D.N., Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Podewils F., Runge U., Kruger S., Geithner J., Wang Z.I., Khaw A.V., Angermaier A., Gaida B., Domin M., Kessler C., Langner S. Diffusion tensor imaging abnormalities in photosensitive juvenile myoclonic epilepsy. Eur. J. Neurol. 2015;22:1192–1200. doi: 10.1111/ene.12725. [DOI] [PubMed] [Google Scholar]
- Pugnaghi M., Carmichael D.W., Vaudano A.E., Chaudhary U.J., Benuzzi F., Di Bonaventura C., Giallonardo A.T., Rodionov R., Walker M.C., Duncan J.S., Meletti S., Lemieux L. Generalized spike and waves: effect of discharge duration on brain networks as revealed by BOLD fMRI. Brain Topogr. 2014;27:123–137. doi: 10.1007/s10548-013-0311-0. [DOI] [PubMed] [Google Scholar]
- Pulsipher D.T., Seidenberg M., Guidotti L., Tuchscherer V.N., Morton J., Sheth R.D., Hermann B. Thalamofrontal circuitry and executive dysfunction in recent-onset juvenile myoclonic epilepsy. Epilepsia. 2009;50:1210–1219. doi: 10.1111/j.1528-1167.2008.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsipher D.T., Dabbs K., Tuchsherer V., Sheth R.D., Koehn M.A., Hermann B.P., Seidenberg M. Thalamofrontal neurodevelopment in new-onset pediatric idiopathic generalized epilepsy. Neurology. 2011;76:28–33. doi: 10.1212/WNL.0b013e318203e8f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic A.J., Ostojic J., Kozic D., Vojvodic N.M., Popovic L.M., Jankovic S., Bascarevic V., Sokic D.V. Hippocampal metabolic dysfunction in juvenile myoclonic epilepsy: 3D multivoxel spectroscopy study. J. Neurol. Sci. 2011;305:139–142. doi: 10.1016/j.jns.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Saini J., Sinha S., Bagepally B.S., Ramchandraiah C.T., Thennarasu K., Prasad C., Taly A.B., Satishchandra P. Subcortical structural abnormalities in juvenile myoclonic epilepsy (JME): MR volumetry and vertex based analysis. Seizure. 2013;22:230–235. doi: 10.1016/j.seizure.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Savic I., Osterman Y., Helms G. MRS shows syndrome differentiated metabolite changes in human-generalized epilepsies. NeuroImage. 2004;21:163–172. doi: 10.1016/j.neuroimage.2003.08.034. [DOI] [PubMed] [Google Scholar]
- Seeck M., Dreifuss S., Lantz G., Jallon P., Foletti G., Despland P.A., Delavelle J., Lazeyras F. Subcortical nuclei volumetry in idiopathic generalized epilepsy. Epilepsia. 2005;46:1642–1645. doi: 10.1111/j.1528-1167.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Seneviratne U., Cook M., D'Souza W. Focal abnormalities in idiopathic generalized epilepsy: a critical review of the literature. Epilepsia. 2014;55:1157–1169. doi: 10.1111/epi.12688. [DOI] [PubMed] [Google Scholar]
- Slaght S.J., Paz T., Chavez M., Deniau J.M., Mahon S., Charpier S. On the activity of the corticostriatal networks during spike-and-wave discharges in a genetic model of absence epilepsy. J. Neurosci. 2004;24:6816–6825. doi: 10.1523/JNEUROSCI.1449-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonmez F., Atakli D., Sari H., Atay T., Arpaci B. Cognitive function in juvenile myoclonic epilepsy. Epilepsy Behav. 2004;5:329–336. doi: 10.1016/j.yebeh.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Tyvaert L., Chassagnon S., Sadikot A., LeVan P., Dubeau F., Gotman J. Thalamic nuclei activity in idiopathic generalized epilepsy: an EEG-fMRI study. Neurology. 2009;73:2018–2022. doi: 10.1212/WNL.0b013e3181c55d02. [DOI] [PubMed] [Google Scholar]
- Xue K., Luo C., Zhang D., Yang T., Li J., Gong D., Chen L., Medina Y.I., Gotman J., Zhou D., Yao D. Diffusion tensor tractography reveals disrupted structural connectivity in childhood absence epilepsy. Epilepsy Res. 2014;108:125–138. doi: 10.1016/j.eplepsyres.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Yang T., Guo Z., Luo C., Li Q., Yan B., Liu L., Gong Q., Yao D., Zhou D. White matter impairment in the basal ganglia-thalamocortical circuit of drug-naive childhood absence epilepsy. Epilepsy Res. 2012;99:267–273. doi: 10.1016/j.eplepsyres.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Yang T., Fang Z., Ren J., Xiao F., Li Q., Liu L., Lei D., Gong Q., Zhou D. Altered spontaneous activity in treatment-naive childhood absence epilepsy revealed by regional homogeneity. J. Neurol. Sci. 2014;340:58–62. doi: 10.1016/j.jns.2014.02.025. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li H., Hong P., Zou X. Proton magnetic resonance spectroscopy in juvenile myoclonic epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2016;121:33–38. doi: 10.1016/j.eplepsyres.2016.01.004. [DOI] [PubMed] [Google Scholar]