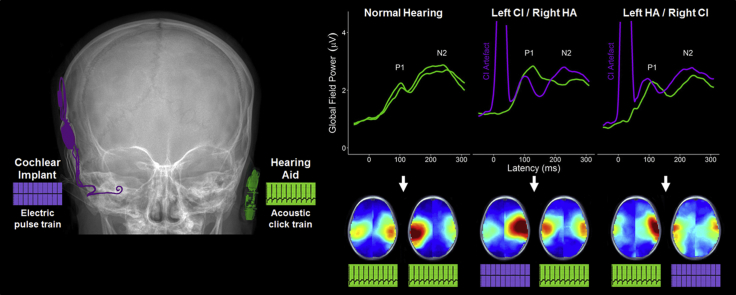
Abstract
Bilateral hearing in early development protects auditory cortices from reorganizing to prefer the better ear. Yet, such protection could be disrupted by mismatched bilateral input in children with asymmetric hearing who require electric stimulation of the auditory nerve from a cochlear implant in their deaf ear and amplified acoustic sound from a hearing aid in their better ear (bimodal hearing). Cortical responses to bimodal stimulation were measured by electroencephalography in 34 bimodal users and 16 age-matched peers with normal hearing, and compared with the same measures previously reported for 28 age-matched bilateral implant users. Both auditory cortices increasingly favoured the better ear with delay to implanting the deaf ear; the time course mirrored that occurring with delay to bilateral implantation in unilateral implant users. Preference for the implanted ear tended to occur with ongoing implant use when hearing was poor in the non-implanted ear. Speech perception deteriorated with longer deprivation and poorer access to high-frequencies. Thus, cortical preference develops in children with asymmetric hearing but can be avoided by early provision of balanced bimodal stimulation. Although electric and acoustic stimulation differ, these inputs can work sympathetically when used bilaterally given sufficient hearing in the non-implanted ear.
Abbreviations: CI, cochlear implant; HA, hearing aid; FDR, false discovery rate; BEM, boundary element model; MNI, Montreal Neurologic Institute; MRI, magnetic resonance imaging; EEG, electroencephalography; SD, standard deviations; SE, standard errors
Keywords: Bimodal, Electro-acoustic stimulation, Development, Hearing loss, Deafness, Evoked related potential, Evoked potential, Electrophysiology, Beamformer, Cortex
Graphical abstract
Highlights
-
•
Children with asymmetric hearing develop cortical preference for the better ear.
-
•
Cortical preference can be avoided by early cochlear implantation of the deaf ear.
-
•
Electrical implant stimulation can work sympathetically with acoustic hearing.
-
•
The implanted ear is preferred with poor acoustic hearing in the unimplanted ear.
1. Introduction
Children who have one deaf ear with better hearing in their other ear are at risk for unilateral listening and abnormal cortical development because they are not candidates for cochlear implantation using standard criteria (Cadieux et al., 2013). Yet, the most effective treatment for each ear should be provided to children with hearing loss (Gordon et al., 2015). Whereas symmetric hearing loss can be treated with similar devices in each ear (two cochlear implant (CIs) for severe/profound deafness or two hearing aids (HAs) for less severe hearing impairments), children with asymmetric hearing loss may require electrical stimulation of the deaf ear with a CI and amplified acoustic sound through a HA in the better ear (Arndt et al., 2015, Cadieux et al., 2013, Ramos Macias et al., 2016). It is not clear, however, that this bimodal input (electrical CI in one ear and acoustic HA in the other) can be combined to limit unilaterally driven reorganization or promote binaural/spatial hearing in children. The concern is that electrical CI hearing completely differs from listening to amplified sound through a HA and thus could provide unbalanced or even conflicting bilateral access to sound. To test this clinical recommendation, we asked: 1) can bilateral cortical development be protected in children with asymmetric hearing loss through bimodal hearing; and 2) what factors prevent expected cortical development in children provided with bimodal hearing? We hypothesized that bimodal stimulation with limited delay restricts cortical reorganization underlying preference of one ear by providing bilateral access to sound.
Young children with asymmetric hearing loss have impaired access to bilateral sound and are at risk of developing poor sound localization and speech detection in noise (Gordon et al., 2014, Litovsky et al., 2010), as well as social, educational and language deficits (Kuppler et al., 2013, Lieu et al., 2010, Lieu et al., 2013). These hearing difficulties and associated challenges likely reflect cortical reorganization with prolonged unilateral hearing. In children with congenital bilateral deafness, early hearing through one CI for > 2 years increases activity in the contralateral auditory cortex (Gordon et al., 2013b, Jiwani et al., 2016) and both left and right auditory cortices develop an abnormal preference for stimulation from the hearing ear (Gordon et al., 2013b). These effects are consistent with abnormal strengthening of uncrossed pathways from the stimulated ear in unilaterally hearing cats with congenital deafness (Kral et al., 2013a, Kral et al., 2013b, Tillein et al., 2016). Importantly, cortical representation of the stimulated ear in children increases with delay to bilateral implantation and persists despite several years of bilateral CI use (Gordon et al., 2013b). Unilateral deprivation also reorganizes cortical networks involved in attention and executive functioning (Tibbetts et al., 2011, Wang et al., 2014, Yang et al., 2014). Given that impairments in these networks correlate with educational outcomes (Rachakonda et al., 2014), and that bilateral hearing is important for social and educational development (Lieu et al., 2013), it makes sense to avoid cortical reorganization resulting from unilateral hearing in children.
Treating asymmetric hearing loss with bimodal devices may restore bilateral access to sound, but it remains unclear how the two very different signals are processed and integrated in the cortex. Contributions from the CI could disrupt information from the better hearing ear. Sound frequencies are more poorly translated by CIs than by HAs to the auditory pathways which impairs CI users' perception of pitch and music (Gfeller et al., 2002, Gfeller et al., 2012, Hopyan et al., 2012, Limb and Rubinstein, 2012, Polonenko et al., 2017), and emotion in speech and music (Giannantonio et al., 2015, Hopyan et al., 2016, Volkova et al., 2013). On the other hand, acoustic stimulation of the non-implanted ear might be limited by deterioration of the cochleae and/or auditory neurons, affecting auditory nerve stimulation (reviewed by Korver et al., 2017). Moreover, HAs often are not capable of providing enough amplification to the basal cochlea (Stelmachowicz et al., 2004) which is the cochlear region often most affected in individuals with hearing loss (Pittman and Stelmachowicz, 2003). In addition, bimodal hearing could also be detrimental for binaural/spatial hearing by introducing large asymmetries in timing of input between the ears (direct CI stimulation of the auditory nerve is ~ 1.5 ms faster than acoustic input) (Polonenko et al., 2015, Zirn et al., 2015) and large mismatches in inter-aural place of stimulation which potentially compromise integration/fusion of bilateral input (Landsberger et al., 2015, Reiss et al., 2014).
To evaluate the potential benefits and limitations of bimodal hearing for bilateral auditory development, we examined cortical activity and functional outcomes in children with asymmetric hearing loss who use bimodal devices. The present findings demonstrate that bimodal stimulation can promote typical cortical activity when: 1) delay to implantation is limited and 2) bilateral access to sound through the HA and CI is balanced. When these conditions are not met, prolonged asymmetric hearing restructures auditory cortices, creating a preference for the better hearing ear. Speech perception skills depended on access to high-frequency information in each ear independently rather than on broadband-evoked aural preference measures.
2. Materials and methods
Parental/guardian written informed consent and child assent were obtained under study protocol #100000294 approved by the Hospital for Sick Children Research Ethics Board.
2.1. Participants
Sample size calculations for sufficient power (1 − β ≥ 0.8, α = 0.05) were completed a priori using G*Power v3.1.7 software (Faul et al., 2007), based on partial eta-squared values estimated from previous work (Gordon et al., 2013b, Gordon et al., 2010). Accordingly, 50 children aged 1.3–12.9 years were recruited: 34 bimodal users (mean ± SD: 6.8 ± 3.2 years old) who wore both devices for > 6 months and 16 peers with normal hearing (6.4 ± 3.5 years old). Audiometric screening confirmed normal hearing (≤ 20 dB HL) thresholds at 250–8000 Hz prior to testing. The implanted ear in the bimodal group was evenly split (17 left: 17 right). Ages between the three groups (normal hearing, left CI/right HA, left HA/right CI) were similar (one-way ANOVA: F(2,47) = 0.03, P = 0.97). Responses were compared to the same measures collected in 28 bilateral implant users (6.7 ± 2.4 years old) who were part of a previously reported cohort (Gordon et al., 2013b). Bilateral implant users received their first (right) implant at 1.8 ± 1.1 years old; 12 received both CIs simultaneously and 16 waited 2.3 ± 1.6 years to receive a left implant. Although mean cortical responses of the bilateral CI group were previously reported, aural preference measures per subject and the analysis of change over time are provided here for the first time.
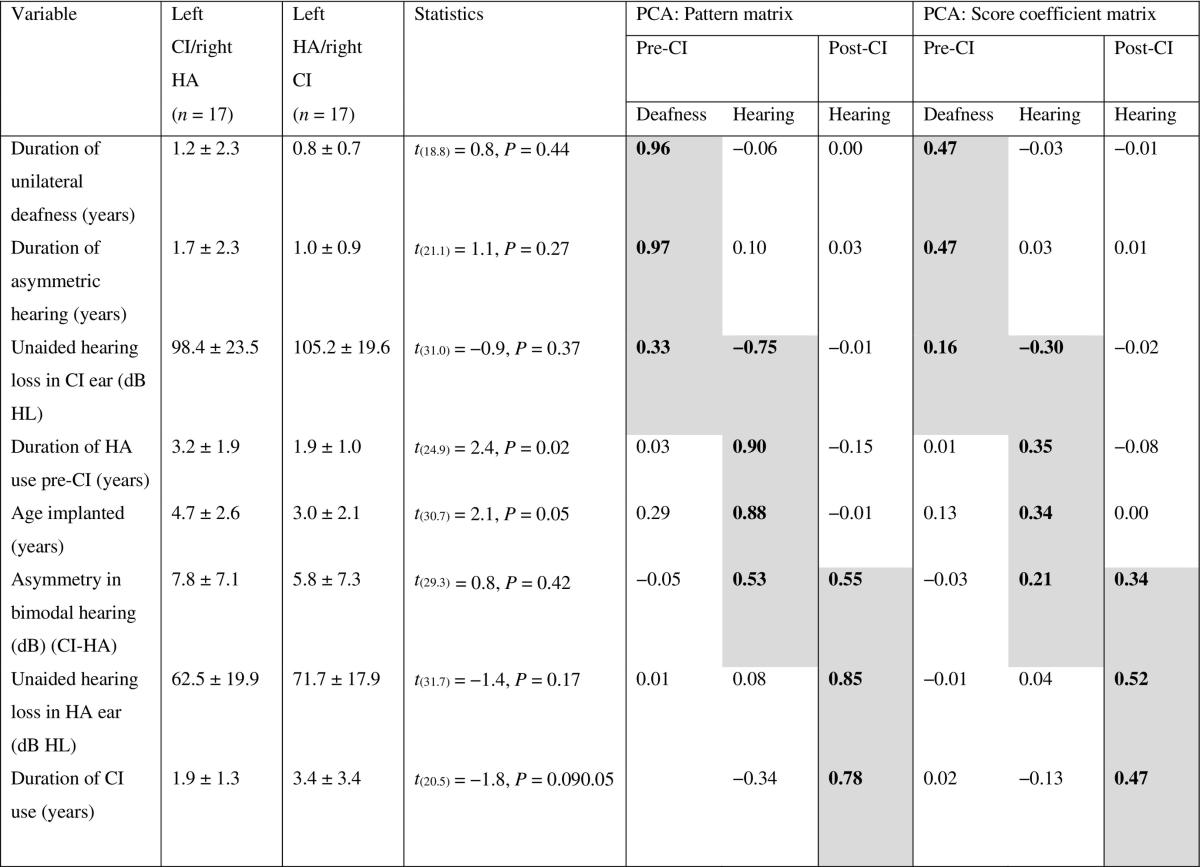
Details of several demographic variables are provided in Table 1. Most variables were similar between the two bimodal groups (P > 0.05). Both groups were implanted over a similar range of ages, but the left implanted group was implanted later and had longer bilateral HA use on average than the right implanted group (P < 0.05).
Table 1.
Bimodal group mean ± SD demographic information and categorization by principal component analysis (PCA). Shaded regions and bolded text denote which variables significantly load to the component (factor loading > 0.3).
CI = cochlear implant; HA = hearing aid.
2.2. Hearing history categorizes into pre- and post-CI hearing experience components
Candidacy for bimodal implantation continues to evolve, resulting in a cohort with more varied hearing histories than bilateral CI users. Some of this variability can be explained by etiology of deafness (Fig. 1). Bimodal users tended to have etiologies associated with progressive and asymmetric deafness (i.e., inner ear malformations; 11 (32%) bimodal: 4 (14%) bilateral CI; OR = 2.8, P = 0.14), whereas more bilateral CI users had genetic mutations causing congenital bilateral deafness (e.g., GJB2/6; 5 (15%) bimodal: 11 (39%) bilateral CI; OR = 0.3, P = 0.04). All other etiologies were similar between the groups (OR ~ 1, P > 0.05). To better characterize these complex histories, an oblique principal component analysis (PCA) using promax rotation with Kaiser normalization was completed. Only components with eigenvalues > 1 were included. PCA analysis identified three components related to hearing experience (Table 1). Variables included in two pre-CI components related to deafness or hearing asymmetry while waiting to receive an implant. Variables included in the post-CI component related to hearing asymmetry during the period of bimodal hearing. Because components did not correlate with each other (R < 0.01), only the pattern matrix was shown along with the component score coefficient matrix used to create component scores for each child. Component scores were used for regression analyses of cortical activity and correlational analyses with speech perception scores.
Fig. 1.
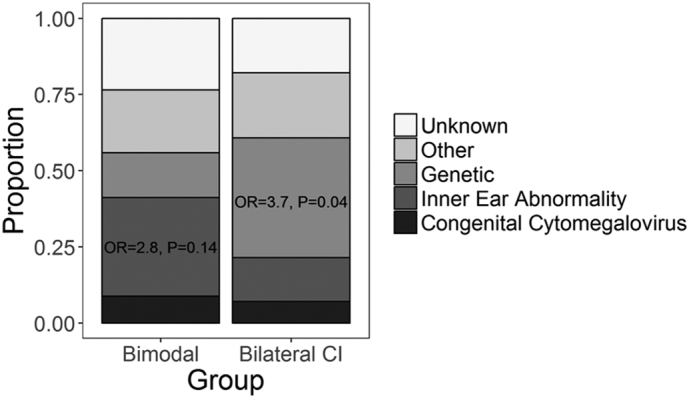
Distribution of hearing loss etiology. Proportions of bimodal and bilateral cochlear implant (CI) users with each known and unknown etiology are shown. The odds of an inner ear abnormality were greater for bimodal users whereas the odds of a genetic etiology were greater for bilateral CI users. OR = odds ratio.
2.3. Cortical recording
EEG measures of evoked cortical activity were recorded across 64-cephalic electrodes using a standard 10–20 configuration referenced to the right earlobe. Responses were measured using the NeuroScan-4.5 system and Synamps-II amplifier (Compumedics USA, Inc., Charlotte, NC), sampled at 1000 Hz and online band-pass filtered between 0.15 and 100 Hz. Responses were common referenced and filtered offline from 1 to 30 Hz for source analysis. A minimum of 220 sweeps with at least two visually replicable responses were collected. Rejected epochs included eye blinks in frontal electrodes or activity in a midline cephalic electrode (CZ) greater than ± 100 μV.
Matlab (MathWorks) and Nucleus Implant Communicator (NIC; Cochlear Ltd., Melbourne, Australia) programs were created to deliver monaurally presented stimuli while children sat in a soundproof booth watching a captioned movie without sound or reading a book. Stimuli were presented at 1 Hz and included 36 ms trains of 250 Hz biphasic electric pulses (57 μs) delivered through an L34 research processor to an apical electrode (#20) of the internal device in the implanted ear, and 250 Hz acoustic clicks (100 μs) delivered to non-implanted ears through an Etymonic Research (ER-3A) insert earphone. Loud but comfortable levels were confirmed using maximum auditory brainstem response wave eV/V amplitude, thereby ensuring similar activation of both ears at the upper part of the dynamic range (Gordon et al., 2013b, Polonenko et al., 2015). Brainstem responses were recorded from CZ referenced to the ipsilateral earlobe using 11 Hz single pulses/clicks, averaged across trials, and filtered from 10 to 3000 Hz. Rejected epochs contained responses over ± 30–40 μV. Levels determined from single pulses/click evoked brainstem responses were reduced by 10 current units (CU) (20.96 μA) or dB on each side for pulse train evoked cortical responses to account for temporal integration and ensure comfortable stimulation.
2.4. Source localization
The time-restricted, artefact and coherent source suppression (TRACS) linearly constrained minimum variance type beamformer (Wong and Gordon, 2009) was used to estimate source dipole activity underlying latency windows encompassing the first visually identified positive (P1) and negative (N2) peaks of the immature cortical waveform (Fig. 2), as previously described (Easwar et al., 2017a, Gordon et al., 2013b, Gordon et al., 2010). Briefly, application of the time-restricted artefact suppression algorithm maintained evoked responses while suppressing ~ 97% of the CI artefact corresponding to the four largest singular vector values between − 80 to 10 ms (Wong and Gordon, 2009). Because an internally implanted magnet precludes MRI testing for implanted children, age-appropriate Montreal Neurologic Institute (MNI) head model templates created using the Template-O-matic toolbox (Wilke et al., 2008) were used to construct a 3-layer boundary element model (BEM) mesh. This mesh accounted for age-dependent head geometry and tissue conductivities through the brain, skull and scalp when calculating lead potentials for dipoles in ~ 64,000 3 × 3 × 3 mm voxels. Source activity in each hemisphere was evaluated by suppressing the other hemisphere (Dalal et al., 2006).
Fig. 2.
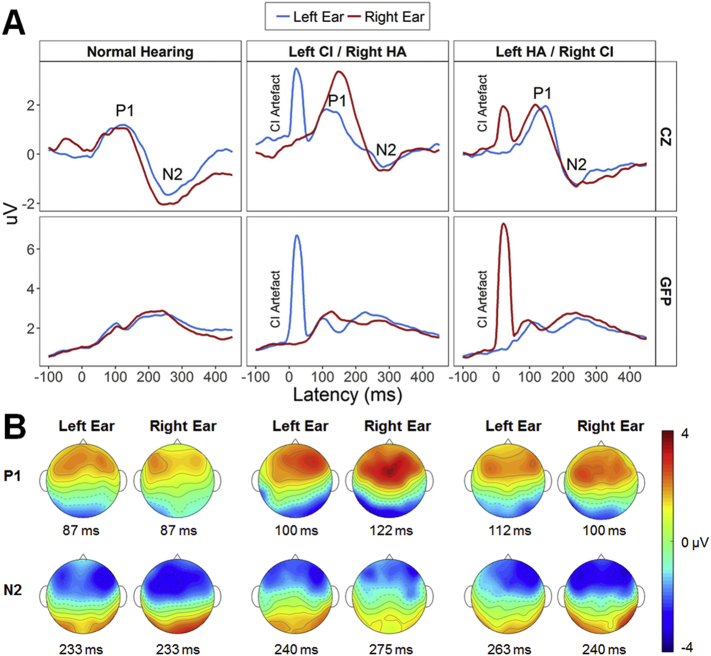
Evoked surface activity. (A) Mean common-reference cortical responses at a cephalic electrode (CZ; top row) and mean global field power (GFP; bottom row) in response to stimulation of the left ear (blue) and right ear (red) for children with normal hearing (n = 16), and bimodal hearing (n = 34; n = 17 each for left or right CI). The first positive (P1) and negative (N2) peaks are labelled for responses recorded at Cz. In general, responses were similar in amplitude across groups but slower in the bimodal groups. (B) Group average common-reference head topographic maps display the distribution of EEG activity across the surface of the head at GFP peak latencies for P1 and N2 peaks. CI = cochlear implant; HA = hearing aid.
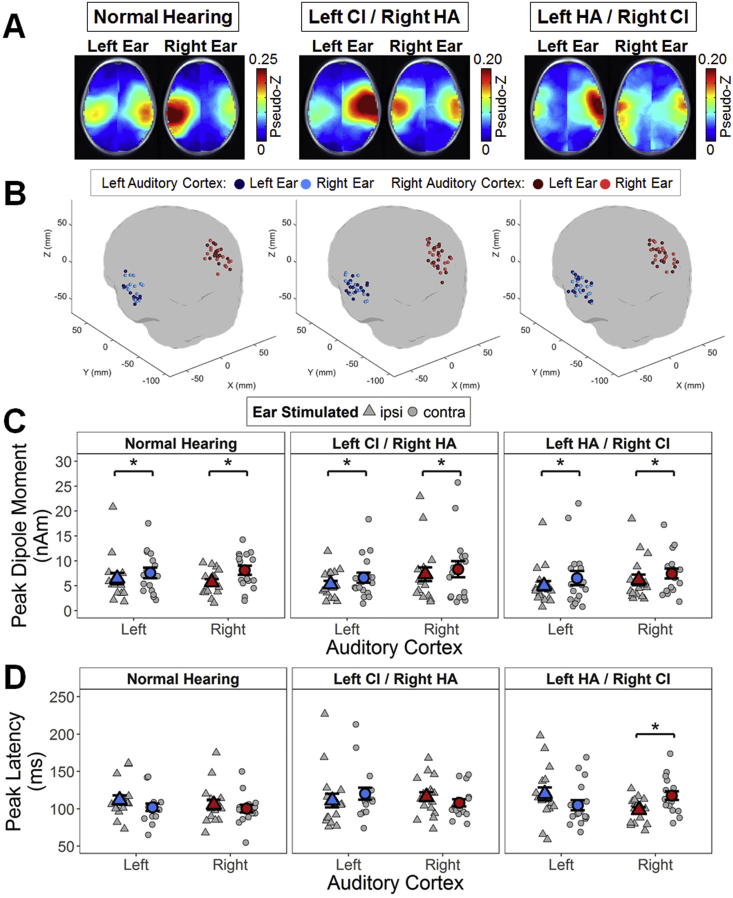
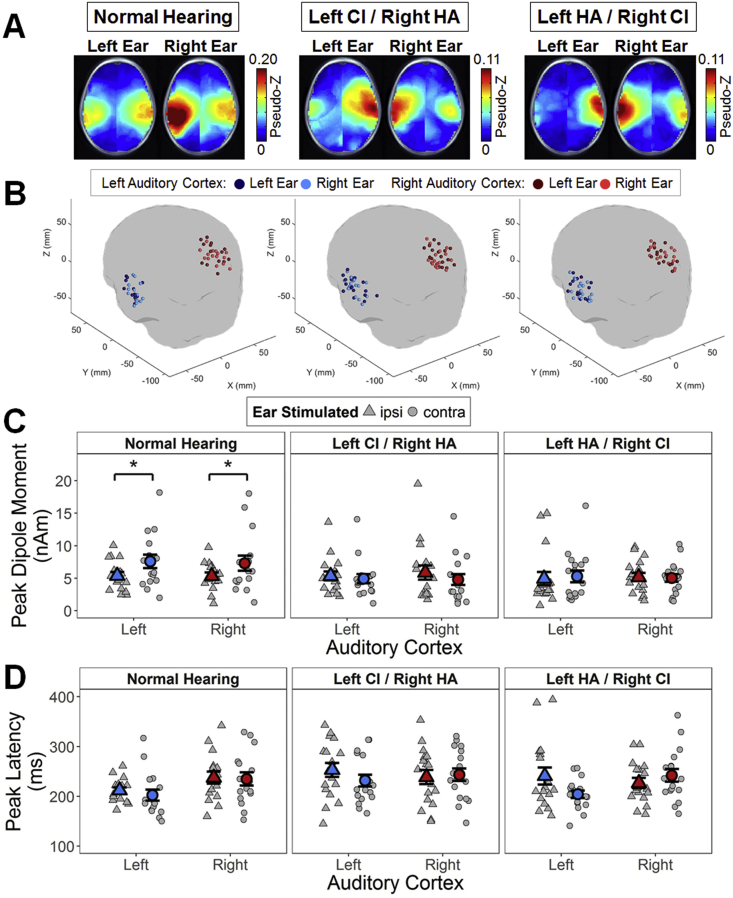
The signal-to-noise ratio of evoked source activity relative to baseline activity in the pre-stimulus interval (− 200 to − 80 ms) was normalized using a pseudo-Z statistic (Vrba and Robinson, 2001). A one-tailed omnibus-noise t-test (Petersson et al., 1999) calculated a statistical threshold pseudo-Z value (P ≤ 0.0005) reflecting baseline activity (omnibus value). Pseudo-Z values were corrected relative to this omnibus value. Group average corrected pseudo-Z values for each voxel were plotted on the average age-appropriate head model for each condition (Fig. 3A), in order to identify consistently activated cortical areas. Maximum dipole strength (nAm) and latency were extracted for all voxels, and the voxel with the largest corrected pseudo-Z value in both the left (MNI coordinates X ≤ − 55, − 35 ≤ Y ≤ 5, − 10 ≤ Z ≤ 20) and right (MNI coordinates X ≥ 55, − 35 ≤ Y ≤ 5, − 10 ≤ Z ≤ 20) auditory cortices was chosen for each condition and child (Easwar et al., 2017a, Jiwani et al., 2016). For children in which stimulation of one ear evoked activity with pseudo-Z values above omnibus in only one auditory cortex, the peak dipole moment associated with the highest pseudo-Z value (although below omnibus) was chosen in the less-activated hemisphere to calculate aural preference. Aural (stimulation) preference (%) of each auditory cortex was calculated as: 100 × (dipole magnitude evoked by contralateral stimulation − dipole magnitude evoked by ipsilateral stimulation) / (ipsilateral + contralateral evoked dipole magnitudes). Positive aural preference scores indicated preferential stimulation by the contralateral ear while negative scores indicated preferential stimulation by the ipsilateral ear.
Fig. 3.
Evoked source activity for P1. (A) Axial views of each group mean omnibus-corrected pseudo-Z (higher signal-to-noise-ratio in red) maps show regions of activation during P1 upon stimulation of the left and right ears. Activity localized to both auditory cortices in all conditions and groups. (B) Peak dipoles were located at similar locations in the left (blue) and right (red) auditory cortices upon stimulation of the left (dark colour) and right (light colour) ear. For each group, the mean ± SE (coloured) and individual (gray) maximum peak (C) dipole moments (nAm) and (D) latencies extracted from chosen voxels are shown for the left (blue) and right (red) auditory cortices upon stimulating the ipsilateral (ipsi; triangles) and contralateral (contra; circles) ear in each group. CI = cochlear implant; HA = hearing aid; *P < 0.05.
2.5. Speech perception to assess functional outcomes
Functional outcomes with bimodal devices were evaluated using age- and language-appropriate speech perception tests. Words were presented at 0° azimuth in quiet to each device separately. Speech scores were available for both devices in all but five bimodal users. Scores were obtained in a limited number of conditions in four of the five children with missing data, reflecting the young age of these five participants (5.2 ± 2.8 years). The following tests were used for the remaining 29 children: Early Speech Perception test (ESP; n = 1), Word Identification by Picture Identification (WIPI; n = 1), Glendonald Auditory Screening Procedure (GASP; n = 7), Multisyllabic Lexical Neighbourhood Test (MLNT; n = 5), and Phonemic Balanced Kindergarten test (PBK; n = 16). Children responded by either pointing to a picture best representing the heard word from a group of pictures (closed-set: ESP, WIPI) or repeating the heard word (open-set: GASP, MLNT, PBK). Because number of words varied across tests (12 to 25 words), percent correct scores were transformed to rationalized arsine units (RAU) and then corrected for guessing on closed-set tests (Sherbecoe and Studebaker, 2004). Speech perception tests were often not completed on the same day of cortical recording due to length of testing, but the two tests were within 5.0 ± 1.9 months of one another.
2.6. Statistical analysis
To evaluate differences in evoked surface and source activity for each peak time window (P1 and N2), mixed repeated measures ANOVA were used with hearing group (normal, left CI/right HA, left HA/right CI) as a between-subject factor. One within-subject factor was included for comparing surface peak amplitudes and latencies (ear) as well as cortical aural preference (cortex). Two within-subject factors (ear and cortex) were included for confirming similar voxel locations were chosen for further analyses and for comparing peak dipole moments and latencies. Greenhouse-Geisser corrections for lack of sphericity were used when indicated. Post-hoc two-tailed paired t-test tests were completed using false discovery rate (FDR) corrections (Benjamini and Hochberg, 1995) for multiple comparisons. Multiple linear regressions were used to assess changes in aural preference with implanted ear (i.e., bimodal group), PCA component scores, and demographic variables. Pearson correlations were used to assess associations between speech perception scores and PCA component scores, demographic variables, and aural preference. Partial and bivariate R2 and FDR-corrected P-values were provided. PCA was completed using SPSS Statistics v.23 (IBM Corp, Somers, NY, USA); all other analyses and graphics were completed using R v3.3.1 (R Core Team, 2016).
3. Results
3.1. Children show immature P1-N2 cortical responses to both electric and acoustic stimulation
Children in all three groups exhibited immature cortical responses at Cz characterized by a positive (P1) then negative (N2) peak (Fig. 2A, top), which resembled cortical responses recorded in other studies with young children (Easwar et al., 2017a, Gordon et al., 2013b, Ponton et al., 2002, Ponton et al., 2000). Corresponding peaks were evident in the global field power (GFP; Fig. 2A, bottom) and were analysed. Mean ± SD peak amplitudes and latencies are provided in Supplemental Table 1. Despite having similar peak amplitudes irrespective of ear (left/right) or stimulation mode (acoustic (HA)/electric (CI)) (all P > 0.05), groups differed by early peak latencies (P1: F(2,46) = 4.0, P = 0.02; N2: F(2,46) = 0.5, P = 0.60). Specifically, P1 latencies were faster for children with normal hearing than bimodal users (left CI: t(28.4) = − 2.6, P = 0.04; right CI: t(29.8) = − 2.3, P = 0.04), but similar for both bimodal groups (t(31.2) = 0.1, P = 0.90). For all three groups, average-referenced topographical maps of surface EEG activity at group-specific peak latencies (Fig. 2B) indicated positive and negative bilateral activation at fronto-temporal electrodes for P1 and N2 respectively.
3.2. Auditory cortices respond more quickly to electrical than acoustic stimulation
Axial views of mean omnibus-corrected pseudo-Z maps showed similar activated regions (higher pseudo-Z in red) underlying P1 upon stimulation of each ear for each group (Fig. 3A). Due to similarity of outcomes between P1 and N2, N2 results are provided in Supplementary Fig. 1, Supplementary Fig. 2 and Supplemental Table 2, as well as described in the Supplementary Material. Voxels chosen for further analyses had similar coordinates (group: F(2,97) = 0.05, P = 0.95; ear: F(1,97) = 0.02, P = 0.87) within auditory cortical areas (Fig. 3B). Furthermore, the centroid location of these peak voxels were within 3 voxel spaces of one another (6.1 ± 1.4 and 6.3 ± 3.9 mm between the normal hearing group and the left CI/right HA and left HA/right CI groups respectively, and by 4.5 ± 1.0 mm between bimodal groups), and variation around these centroids were similar (group: F(2,47) = 0.2, P = 0.83; ear: F(1,47) = 0.01, P = 0.91; cortex: F(1,47) = 0.5, P = 0.48).
Supplementary Fig. 1.
Evoked source activity for N2. (A) Axial views of each group mean omnibus-corrected pseudo-Z (higher signal-to-noise-ratio in red) maps show regions of activation during N2 upon stimulation of the left and right ears. Activity localized to both auditory cortices in all conditions and groups. (B) Peak dipoles in the auditory cortices were located at similar locations in the left (blue) and right (red) auditory cortices upon stimulation of the left (dark colour) and right (light colour) ear. For each group, the mean ± SE (coloured) and individual (gray) maximum peak (C) dipole moments (nAm) and (D) latencies extracted from chosen voxels are shown for the left (blue) and right (red) auditory cortices upon stimulating the ipsilateral (ipsi; triangles) and contralateral (contra; circles) ear in each group. CI = cochlear implant; HA = hearing aid; *P < 0.05.
Supplementary Fig. 2.
Aural preference for N2. (A) Aural preference (%) for each auditory cortex is plotted for each child with normal hearing (white), a left cochlear implant (CI)/right hearing aid (HA) (gray), and a left HA/right CI (dark gray). For bimodal users, aural preference was calculated for cortices ipsilateral (Ipsi) to each type of device to evaluate whether some children prefer one type of stimulation (e.g., Ipsi to CI is the left cortex for children with a left CI). Aural preference in bimodal users is then shown for the cortices ipsi to the HA and ipsi to the CI as a function of the pre- (B) and post- (C) implant hearing experience components identified by Principal Component Analyses (PCA), and as a function of (D) duration of HA use before implantation, and (E) duration of CI use. Positive values indicate preference for stimulation of the contralateral ear. Solid regression line: P < 0.05; dashed regression line: P < 0.1.
Peak dipole moments (Fig. 3C) from voxels in each cortex were stronger for contralateral versus ipsilateral stimulation (ear: F(1,47) = 14.0, P < 0.001) in all groups (ear ∗ group: F(2,47) = 0.2, P = 0.81; group: F(2,47) = 0.2, P = 0.84). By contrast, peak dipole latencies (Fig. 3D) did not follow the same pattern in each group (3-way interaction: F(2,47) = 5.4, P = 0.007). Children with normal hearing tended to have faster peak latencies in each cortex to contralateral stimulation (left cortex: t(15) = 2.1, P = 0.21; right cortex: t(15) = 0.80, P = 0.52) whereas peak dipole latencies in bimodal users tended to be slower in response to HA than CI stimulation; this was only significant in the right auditory cortex in children with left HA/right CI (t(16) = − 3.5, P = 0.04).
3.3. Some bimodal users have abnormal preference for input from one ear
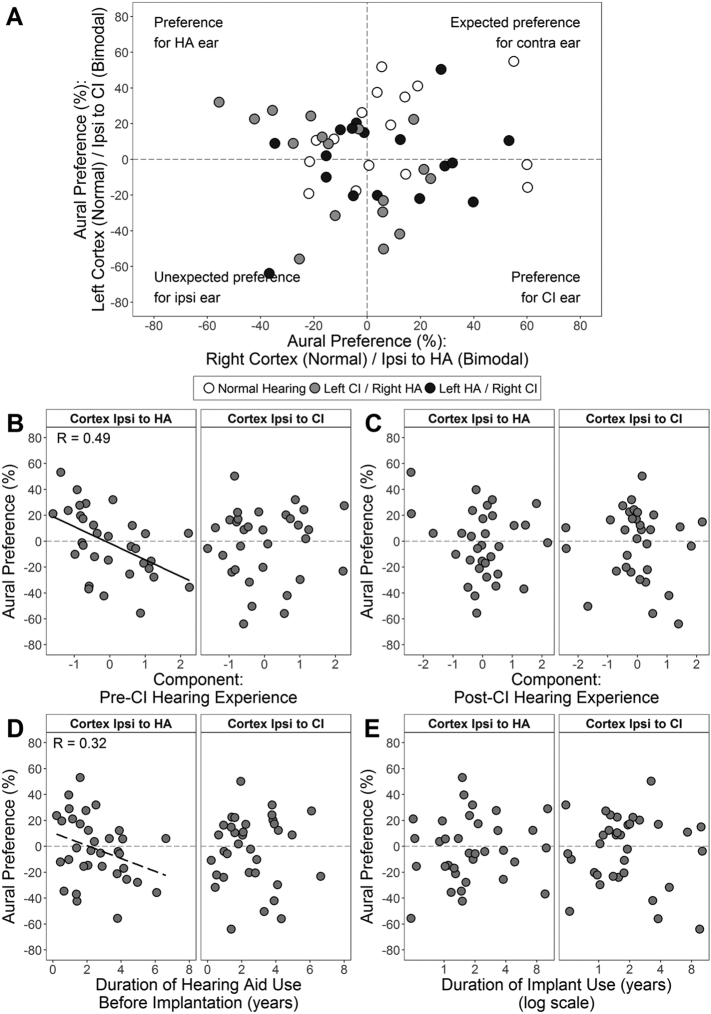
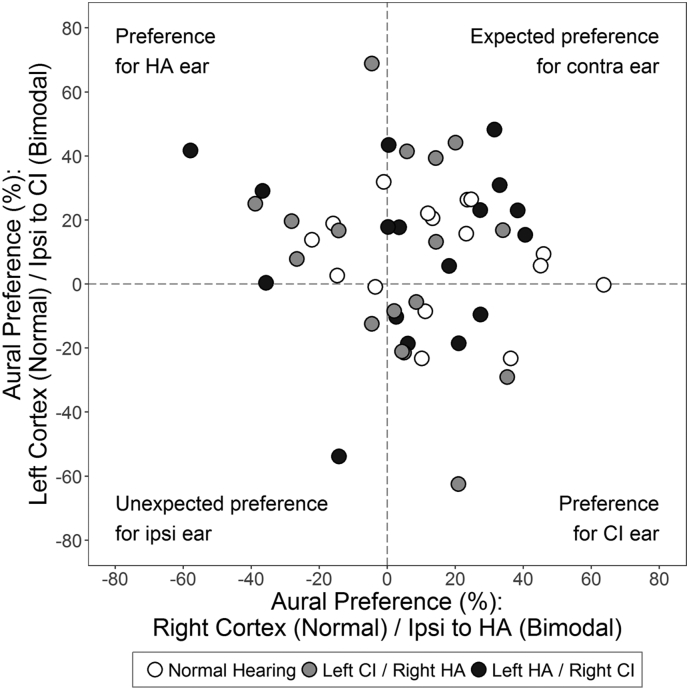
Aural preference was calculated for each cortex to evaluate the relative cortical representations of each ear. On average, all three groups (F(2,47) = 0.68, P = 0.51) developed expected contralateral aural preference in both cortices (F(1,47) = 0.18, P = 0.67). Aural preference for cortices ipsilateral to each type of device (e.g., ipsilateral to CI is the left cortex for left implanted children) are plotted for each child in Fig. 4. Many bimodal users (filled circles) developed primarily contralateral aural preference in both cortices, like their peers with normal hearing (open circles) (Fig. 4) but 9 (26.5%) bimodal users developed abnormal preference in both cortices for stimulation from one ear (n = 6 for the HA ear; n = 3 for the ear with CI).
Fig. 4.
Aural preference for P1. Aural preference (%) for each auditory cortex is plotted for each child with normal hearing (white), a left cochlear implant (CI)/right hearing aid (HA) (gray), and a left HA/right CI (dark gray). For bimodal users, aural preference was calculated for cortices ipsilateral (Ipsi) to each type of device to evaluate whether some children preferred stimulation from one ear (e.g., Ipsi to CI is the left cortex for children with a left CI). Positive values indicate preference for stimulation of the contralateral ear.
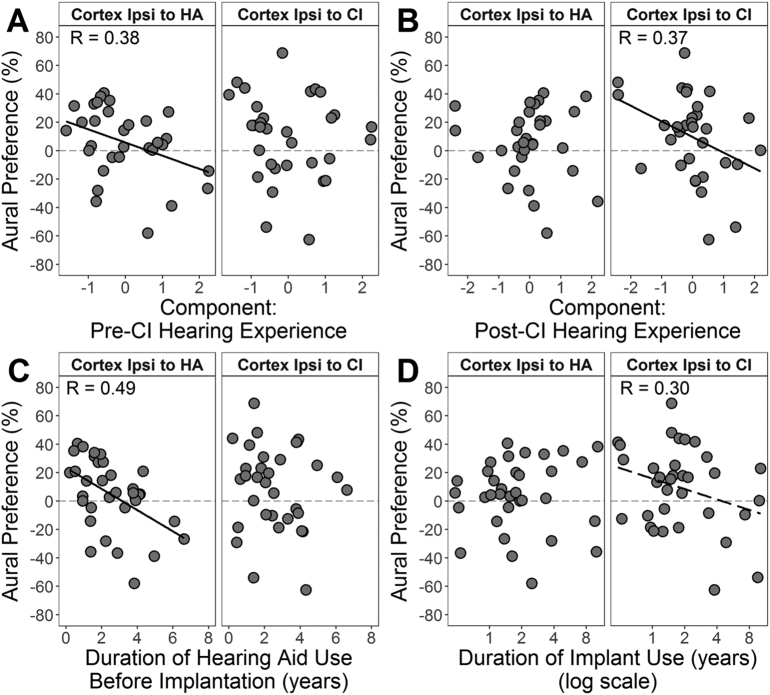
Multiple linear regression analyses were completed to identify relationships between the aural preference measures and the pre- and post-implant PCA components. Aural preference of the cortex ipsilateral to the HA reversed to abnormally prefer this better hearing ear as the pre-CI hearing experience component increased (Fig. 5A). The cortex ipsilateral to the CI abnormally preferred the implanted ear as the post-CI experience component increased (Fig. 5B). These findings were independent of implanted ear (unstandardized β P > 0.05). Bivariate and partial R2 values and corresponding P-values are provided in Table 2.
Fig. 5.
Changes in aural preference for P1. (A) Aural preference in bimodal users is shown for the cortices ipsi to the HA and ipsi to the CI as a function of the pre- (A) and post- (B) implant hearing experience components identified by Principal Component Analyses (PCA), and as a function of (C) duration of HA use before implantation, and (D) duration of CI use (log scale). Positive values indicate preference for stimulation of the contralateral ear. Solid regression line: P < 0.05; dashed regression line: P < 0.1.
Table 2.
Multiple regression parameters for significant predictors of aural preference.
| Cortex | Predictor | Bivariate |
Partial |
|||
|---|---|---|---|---|---|---|
| R2 | P-value | R2 | P-value | Adjusted P-value | ||
| Ipsilateral to HA | Component: Pre-CI hearing experience | 0.15 | 0.03 | 0.13 | 0.04 | 0.08 |
| Duration of hearing aid use before implantation | 0.24 | < 0.01 | 0.26 | < 0.01 | 0.02 | |
| Ipsilateral to CI | Component: Post-CI hearing experience | 0.14 | 0.04 | 0.17 | 0.02 | 0.06 |
| Duration of implant use | 0.10 | 0.07 | 0.12 | 0.05 | 0.08 | |
Effects of individual variables comprising the pre- and post-CI components on aural preference measures were then assessed. Two significant variables were identified in multiple regression analyses; both were based on experience with auditory prostheses (Fig. 5C, D). In the auditory cortex ipsilateral to the HA, aural preference abnormally reversed to favour this better hearing ear at a rate of − 7.5 ± 2.3%/year of asymmetric hearing prior to implantation (delay to implant) (Fig. 5C). In the auditory cortex ipsilateral to the CI, most children had normal aural preference for the contralateral HA ear. Those children with abnormal aural preference for the implanted ear tended to have longer implant experience (Fig. 5D). The rate of change was − 24.4 ± 13.8%/log10(year of CI use) which corresponds to a change of − 7.4% per doubling of time of CI use. Bivariate and partial R2 values and corresponding P-values are provided in Table 2. Pre-implant unaided hearing loss in the CI and HA ears did not significantly predict abnormal cortical reorganization (P > 0.05), although children with abnormal aural preference tended to have a severe/profound hearing loss (n = 3; deaf ear: 102.1 ± 6.4 dB HL; better ear: 77.5 ± 9.8 dB HL).
3.4. Reorganization follows a similar time course for unilateral deafness and asymmetric hearing
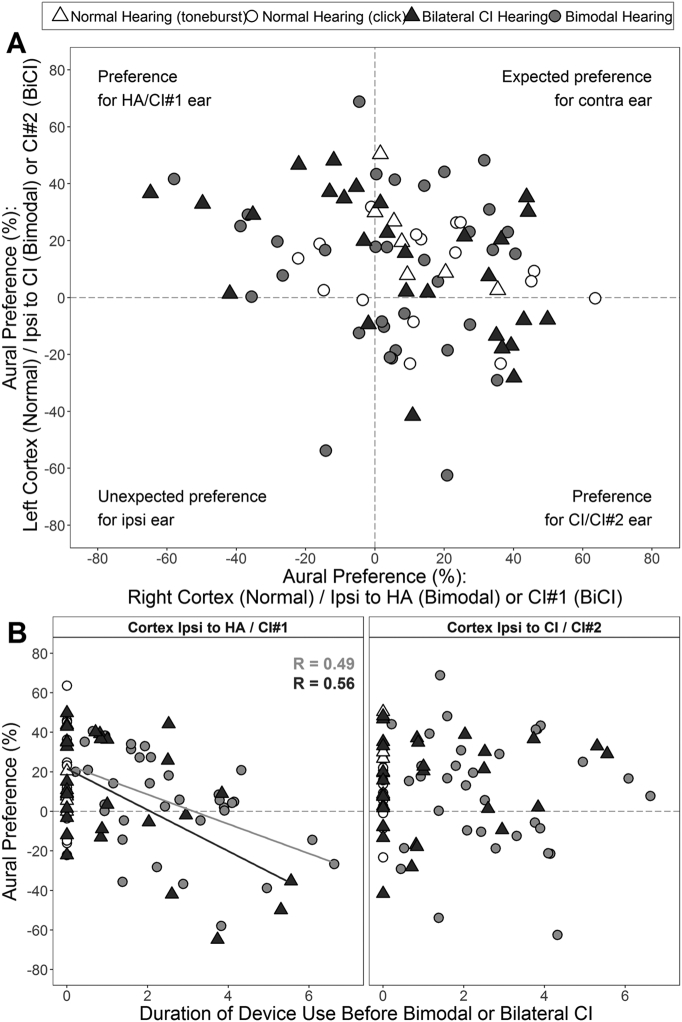
Cortical development in bimodal users was compared to responses from a cohort of children with bilateral implants to determine if effects were similar for asymmetric and unilateral CI hearing. Aural preference for each child in the bimodal and bilateral CI groups are plotted for the left versus right cortex (ipsilateral to CI versus HA for bimodal users) in Fig. 6A. Some children using bilateral implants (n = 4) and bimodal devices (n = 6) had similar abnormal preference for the first (i.e., first CI) or better hearing (i.e., HA) ear respectively (Fig. 6A, top left quadrant). Multiple linear regressions were used to compare changes in aural preference with duration of device use prior to bimodal/bilateral CI hearing (aural preference ~ time ∗ group). The rate of change in aural preference for the cortex ipsilateral to HA (bimodal users) or first implanted (bilateral CI users) ear was similar (multivariate regression: F(3,58) = 7.7, R2 = 0.28, P < 0.001; time: P = 0.004; group: P = 0.84, time ∗ group: P = 0.44). Prior to bilateral input, the rate of change toward abnormal preference for the better hearing ear was 7.4 ± 2.3%/year in the bimodal group (bivariate regression: F(1,32) = 10.2, P = 0.003, R2 = 0.24; light gray line in Fig. 6B) and 10.4 ± 3.0%/year in the bilateral CI group (bivariate regression: F(1,26) = 11.8, P = 0.002, R2 = 0.31; dark gray line in Fig. 6B). The x-intercepts calculated from the full model for bimodal and bilateral CI users differed by 1.1 years (3.2 versus 2.1 years respectively).
Fig. 6.
Aural preference (%) in children with normal, bimodal and bilateral implant hearing. (A) Aural preference for P1 is plotted for left versus right auditory cortex (ipsilateral to CI versus HA for bimodal users and CI#2 versus CI#1 for bilateral implant users) for each child with normal hearing (white), bilateral implants (dark gray) and bimodal devices (gray) in the current study (circles) and a previous study (triangles) (Gordon et al., 2013b). (B) Relationship between aural preference and duration of device use before receiving bimodal devices or bilateral CIs. Significant regression lines (P < 0.05) are shown. HA = hearing aid; CI = cochlear implant; Ipsi = ipsilateral.
3.5. Speech perception accuracy increases with early access to high frequencies
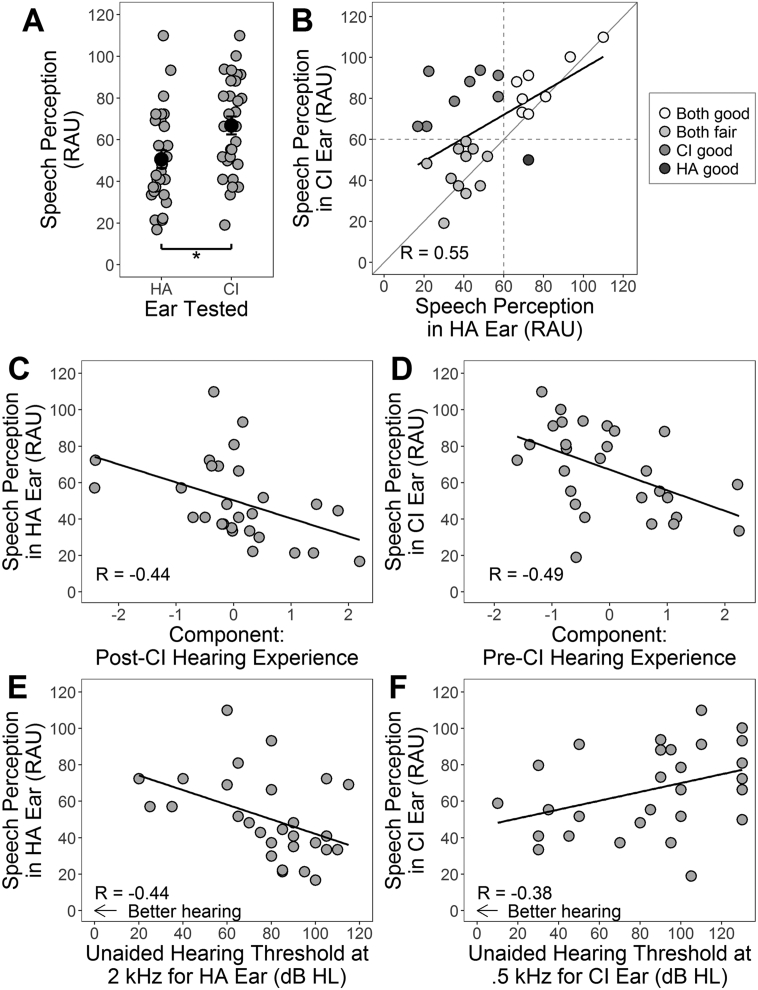
Fig. 7 plots speech perception accuracy when listening through each device in 29/34 bimodal users. Results varied substantially (Fig. 7A) but on average, bimodal users perceived speech 16.1 ± 0.5 RAU more accurately with their CI than HA (t(28) = − 4.0, P < 0.001). Many children (n = 20, 69%) exhibited similarly good (> 60 RAU; n = 8) or similarly fair (< 60 RAU, n = 12) accuracy in each ear (Fig. 7B) and scores with each device alone were correlated (R = 0.55, P = 0.002; Fig. 7B). Thus, consistent with cortical preference data, most bimodal users developed symmetric/balanced function from each of their two ears. Asymmetry in speech perception favoured the implanted ear: of nine (31%) children with asymmetric speech perception, eight had better CI scores and only one had better HA scores. These data did not reflect cortical findings; no significant association was found between unilateral speech perception scores and aural preference in either auditory cortex (ipsi to HA: HA scores: R = 0.31, P = 0.12; CI scores: R = 0.07, P = 0.71; ipsi to CI: HA scores: R = 0.35, P = 0.06; CI scores: R = 0.19, P = 0.31).
Fig. 7.
Speech perception of bimodal users. (A) Speech perception in rationalized arsine units (RAU) is plotted for individuals (gray) and the group mean (black) upon monaural testing of the hearing aid (HA) ear and cochlear implant (CI) ear. (B) Speech perception for the CI ear is plotted in relation to speech perception for the non-implanted HA ear. Dashed lines denote the cut-off (60 RAU) of how speech perception compares in each ear; whether both ears have good scores (white) or poor scores (light gray), or whether only one ear has a good score (medium gray: CI better; dark gray: HA better). Speech perception for each ear correlated with principal component analysis (PCA) demographic components (C, D) and with individual predictors (E, F). *P < 0.05.
Like cortical aural preference, speech perception was predicted by hearing experience, as characterized by the PCA post-CI (R = − 0.44, P = 0.03; Fig. 7C) and pre-CI (R = − 0.49, P = 0.03; Fig. 7D) components. The major difference was that time-based factors relating to asymmetric hearing in the PCA components predicted reversal of contralateral aural preference (Fig. 5C, D) whereas unilateral speech perception depended on factors involving access to high-frequency sound in each ear independently (Fig. 7E, F). In particular, accuracy with the HA increased with better hearing in that ear as measured by unaided hearing thresholds at 2 kHz (R = − 0.44, P = 0.03; Fig. 7E) and 4 kHz (R = − 0.43, P = 0.03), and aided thresholds at 4 kHz (R = − 0.43, P = 0.01). Scores when using the CI decreased as low-frequency access in that ear pre-implantation increased (0.5 kHz: R = 0.38, P = 0.04; Fig. 7F). This may reflect later implantation of children with better low-frequency hearing (R = − 0.56, P = 0.007), as speech perception with the CI also worsened with older age at implantation (R = − 0.52, P = 0.02). Given that all children had profound hearing loss in the deaf ear at high frequencies (2 kHz: 112.5 ± 15.4 dB HL; 4 kHz: 112.8 ± 16.7 dB HL), children implanted with minimal delay consequently received access to high-frequency hearing with the CI at earlier ages.
4. Discussion
This is the first study to address cortical development driven by bimodal stimulation in children with asymmetric hearing loss. Although electric and acoustic inputs each have unique and distinct effects on auditory stimulation in each ear, expected preference for contralateral stimulation in both auditory cortices was achieved in many children. On the other hand, an increasing cortical preference for stimulation from the better hearing ear occurred with ongoing asymmetric hearing loss either with delays to implantation of the deaf ear (preference for better hearing ear) or with CI use if acoustic input in the non-implanted ear was relatively weak (preference for implanted ear). Speech perception in bimodal users strongly depended on access to high frequency sound on each side independently rather than on the bilaterally sensitive measure of aural preference evoked by broadband stimuli.
4.1. Bimodal use can protect symmetric cortical activity and speech perception
Bimodal hearing preserved cortical responses with similar surface waveform morphology to peers with normal hearing (Fig. 2A) and symmetric source activity in most children (25/39, 64.1%) despite lagging response latencies of 10.5–14.1 ms from the acoustic relative to implanted ear (Figs. 2A, 3D). These latency delays exceed previously characterized latency differences between contralateral/ipsilateral stimuli and between ears in young children (Easwar et al., 2017a) and kittens (Kral et al., 2013b). Causes for latency differences within this study and across studies include rapid electrical stimulation of the auditory nerve, which occurs at least 1 ms before acoustic stimulation (Gordon et al., 2006, Polonenko et al., 2015), and greater synchrony across broad populations of electrically evoked neurons (Hartmann et al., 1984, Kral et al., 2009). Compounding the problem, neural synchrony from the acoustic hearing ear is compromised by deterioration of cochlear structures and/or auditory neurons with hearing loss, particularly in basal cochlear regions which respond most quickly (Polonenko et al., 2015, Rosenhamer et al., 1981). Yet, these factors did not affect response magnitude; source dipole activity did not significantly differ between the implanted and normal hearing groups and, in both groups, was larger in response to contralateral than ipsilateral stimulation (Fig. 3C). Moreover, many bimodal users (25/34, 64.1%) showed expected contralateral aural preference in both auditory cortices (Fig. 4) and similar speech perception abilities when using each device separately (20/29, 69.0%; Fig. 7B). Symmetrical activity measured from both ears in bimodal users thus provides the first evidence that bilateral input with limited delay does not need to be restricted to a single modality to protect against development of a cortical or functional preference for one ear.
4.2. Delays to bimodal hearing alter auditory development and compromise speech perception
Some children showed abnormal aural preference for the better non-implanted ear (6/34, 17.4%) or CI ear (3/34, 8.8%). The diverse hearing histories of bimodal users, as characterized by PCA (Table 1), predicted changes in aural preference toward the non-implanted ear (Fig. 5A); main contributors were delayed implantation and longer HA use in the deaf ear (Fig. 5C). Insufficient access in the poorer ear prior to implantation (despite HA use) promoted reorganization over 2–3 years of asymmetric hearing which persisted despite subsequent bimodal stimulation. These findings are consistent with animal models demonstrating increasing cortical activity from the better hearing ear with earlier and longer asymmetric hearing loss in cats with congenital deafness (Kral et al., 2013a, Kral et al., 2013b, Tillein et al., 2016) and in rats and ferrets with temporary asymmetric hearing loss (Keating and King, 2013, Polley et al., 2013, Popescu and Polley, 2010), as explained by strengthening of crossed and uncrossed pathways from the hearing ear and reduction of ipsilateral inhibition or contralateral excitation from the deaf ear (Kral et al., 2013a, Kral et al., 2013b, Tillein et al., 2016). A risk of aural preference for the CI ear was found with ongoing CI use (post-CI component, Fig. 5B) in bimodal users with poor residual hearing in the non-implanted ear (Fig. 5D), suggesting that the HA could not provide sufficient sound for bilateral cortical representation of both ears. Taken together, measures of cortical aural preference reveal limitations of bimodal stimulation for treating asymmetric hearing loss when it is delayed (pre-implant) and/or unbalanced (post-implant).
Speech perception testing also supported the importance of early hearing experience (Fig. 7C, D). Whereas cortical aural preference was sensitive to the timing of symmetric bilateral sound (Fig. 5C, D), speech perception depended on access to high frequencies in each ear independently (Fig. 7E, F). These two measures were not significantly correlated with one another, reflecting a vulnerability of speech perception to high-frequency hearing loss which is not captured by cortical dipoles evoked by high level broadband clicks. Thus, residual low-frequency acoustic function in the non-implanted ear maintained expected input to the auditory cortices but was not translated into benefits for understanding speech through a HA without sufficient hearing in high frequencies.
4.3. Aural preference occurs rapidly with asymmetric hearing in development
There appears to be a rapid time course of cortical plasticity in response to asymmetric hearing loss in children. Bimodal users experienced a change of 7.4%/year of CI delay away from expected contralateral aural preference (Fig. 4, Fig. 5, Fig. 6). A remarkably similar time course of 10%/year of inter-implant delay was shown by children who experienced the most extreme form of asymmetric hearing loss due to unilateral CI use after early onset bilateral deafness (Fig. 6). Consequently, expected aural preference reversed in the ipsilateral cortex of the better stimulated ear by 3.2 years in bimodal users and 2.2 years in bilateral CI users, which indicates that the developing auditory brain rapidly responds to an imbalance in bilateral input. Importantly, the changes in both groups persisted despite chronic bilateral input through bimodal (2.4 ± 2.5 years) or bilateral CI (3.6 ± 0.8 years) use. These results indicate that timing of balanced input, regardless of modality, is important to maintaining bilateral auditory function in children.
4.4. Cortical response to asymmetric hearing loss is particular to early development
The auditory system is most vulnerable to preference for one ear during early development. Effects were greatest in unilateral congenital deaf cats and decreased as the age of unilateral CI stimulation increased in bilaterally deaf cats (Kral et al., 2013a, Kral et al., 2013b). This is consistent with evidence of increasingly abnormal aural preference despite several years of bilateral input in both bimodal and bilateral CI users in this study who had early onset asymmetric hearing that continued for years before receiving bilateral input (Fig. 6, Table 1). Once preference for one ear occurs, restoring expected cortical organization may be challenging if unilaterally driven maturation occurs in the auditory system and more sensitive periods of synaptic plasticity are missed during development (Gordon et al., 2013a; reviewed by Kral and Sharma, 2012). It is possible, however, that some degree of cortical change occurred during bimodal use given that some children developed cortical preference for the implanted ear and expected auditory cortical organization was achieved through implantation in many bimodal users (Fig. 6). Longitudinal measures recently collected in a unique group of young children with single-sided deafness (one normal hearing and one deaf ear) confirms that abnormal aural preference can be reversed within the first 6 months of CI use following initial cortical abnormalities (Polonenko et al., in review). Thus, there is growing evidence that early access to bimodal input can prevent or reverse abnormal cortical aural preference for the first/better hearing ear.
4.5. Implications for management of children with asymmetric hearing loss
Findings in this study support early implantation of children with asymmetric hearing loss and provide evidence for changing standard candidacy criteria. Yet, it is still not clear what combination of auditory prostheses is best for these children. It is possible, for example, that some children using bimodal hearing would fare better with bilateral CIs. For children who have sufficient residual hearing in their non-implanted ear, there may be several advantages of bimodal hearing. First, bimodal hearing protects against unilaterally driven changes by promoting expected cortical organization (Fig. 4, Fig. 5, Fig. 6) which could support specialization of the right versus left auditory cortices (Jiwani et al., 2016) and cortical integration of bilateral input (Easwar et al., 2017b). Second, bimodal hearing improves detection and understanding of speech in noise by providing access to sound from both sides of the head (Arndt et al., 2015, Arndt et al., 2017, Ching et al., 2007, Polonenko et al., 2015, Thomas et al., 2017). Third, bimodal hearing preserves low-frequency acoustic hearing which works better than CI stimulation for pitch perception in music and speech (Bartov and Most, 2014, Giannantonio et al., 2015, Polonenko et al., 2017, Shirvani et al., 2016). Alternatively, when the non-implanted ear has poor residual hearing, findings from this study suggest that bimodal hearing cannot prevent preference for the implanted ear (Fig. 4, Fig. 5) and is insufficient for symmetric speech perception (Fig. 7). Furthermore, bimodal hearing may hinder/disrupt bilateral integration/fusion of sound and binaural/spatial hearing by introducing large mismatches in cochlear place of stimulation between ears (Landsberger et al., 2015, Reiss et al., 2014) and neural conduction in bilateral brainstem pathways (Polonenko et al., 2015, Zirn et al., 2015). These changes could distort how subtle inter-aural differences in sound are detected and integrated in the auditory system, compromising spatial hearing (Grothe et al., 2010). Distortions to these binaural cues by signal processing strategies (Brown et al., 2016) are likely exacerbated by independent hearing devices that differ in modality and processing algorithms. In addition to the benefit of having the same type of device on both sides, bilateral CIs may be easier to match than bimodal devices for level and timing of stimulation. In sum, bimodal hearing may be most effective for children with sufficient hearing in both low and high frequencies.
5. Conclusions
Bimodal hearing promotes expected cortical processing when balanced input is provided during early development. Prolonged asymmetric hearing drives cortical reorganization to prefer stimulation from the better hearing ear. This development favours the ear with more residual hearing prior to cochlear implantation and the implanted ear after implantation if hearing in the non-implanted ear is poor. Evoked by broadband click stimuli, these cortical measures do not correlate well with speech perception scores which reflect access to high frequencies in each ear independently. Thus, delays to implantation of the poorer ear should be avoided in children who could benefit from bimodal use with ongoing monitoring of the non-implanted ear. Bilateral implantation may be warranted to prevent preference for the implanted ear and/or to support speech perception in the non-implanted ear. This decision must take into account the potential loss of residual acoustic hearing.
The following are the supplementary data related to this article.
Supplementary material
Supplementary Table 1
Supplementary Table 2
Acknowledgments
Acknowledgements
We gratefully acknowledge the time and help of the children and their families who participated in this study. We would also like to thank Alexander Andrews for stimulation coding support, Salima Jiwani for initial source imaging support, and Stephanie Jewell and Carmen McKnight for data collection support.
Funding
Funding for this project was provided by the Canadian Institutes of Health Research (MOP-97924 to KAG and BCP, MFE-1748241 to MJP), The Hospital for Sick Children Research Institute (Clinician-Scientist Training Program Studentship and Research Training Competition Award to MJP), and the Ontario Ministry of Ministry of Advanced Education and Skills Development with The University of Toronto (Ontario Graduate Doctoral Scholarship for MJP), and the University of Toronto (Studentship Funding for MJP).
Conflicts of interest
None to declare.
References
- Arndt S., Prosse S., Laszig R., Wesarg T., Aschendorff A., Hassepass F. Cochlear implantation in children with single-sided deafness: does aetiology and duration of deafness matter? Audiol. Neurootol. 2015;20(Suppl. 1):21–30. doi: 10.1159/000380744. [DOI] [PubMed] [Google Scholar]
- Arndt S., Laszig R., Aschendorff A., Hassepass F., Beck R., Wesarg T. Cochlear implant treatment of patients with single-sided deafness or asymmetric hearing loss. HNO. 2017:1–11. doi: 10.1007/s00106-016-0294-8. [DOI] [PubMed] [Google Scholar]
- Bartov T., Most T. Song recognition by young children with cochlear implants: comparison between unilateral, bilateral, and bimodal users. J. Speech Lang. Hear. Res. 2014;57:1929–1941. doi: 10.1044/2014_JSLHR-H-13-0190. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- Brown A.D., Rodriguez F.A., Portnuff C.D.F., Goupell M.J., Tollin D.J. Time-varying distortions of binaural information by bilateral hearing aids. Trends Hear. 2016;20 doi: 10.1177/2331216516668303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadieux J.H., Firszt J.B., Reeder R.M. Cochlear implantation in nontraditional candidates: preliminary results in adolescents with asymmetric hearing loss. Otol. Neurotol. 2013;34:408–415. doi: 10.1097/MAO.0b013e31827850b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T.Y.C., van Wanrooy E., Dillon H. Binaural-bimodal fitting or bilateral implantation for managing severe to profound deafness: a review. Trends Amplif. 2007;11:161–192. doi: 10.1177/1084713807304357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal S.S., Sekihara K., Nagarajan S.S. Modified beamformers for coherent source region suppression. IEEE Trans. Biomed. Eng. 2006;53:1357–1363. doi: 10.1109/TBME.2006.873752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easwar V., Yamazaki H., Deighton M., Papsin B., Gordon K. Simultaneous bilateral cochlear implants: developmental advances do not yet achieve normal cortical processing. Brain Behav. 2017;7:e00638. doi: 10.1002/brb3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easwar V., Yamazaki H., Deighton M., Papsin B., Gordon K. Cortical representation of interaural time difference is impaired by deafness in development: evidence from children with early long-term access to sound through bilateral cochlear implants provided simultaneously. J. Neurosci. 2017;37:2349–2361. doi: 10.1523/JNEUROSCI.2538-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Gfeller K., Witt S., Woodworth G., Mehr M.A., Knutson J. Effects of frequency, instrumental family, and cochlear implant type on timbre recognition and appraisal. Ann. Otol. Rhinol. Laryngol. 2002;111:349–356. doi: 10.1177/000348940211100412. [DOI] [PubMed] [Google Scholar]
- Gfeller K., Jiang D., Oleson J.J., Driscoll V., Olszewski C., Knutson J.F., Turner C., Gantz B. The effects of musical and linguistic components in recognition of real-world musical excerpts by cochlear implant recipients and normal-hearing adults. J. Music. Ther. 2012;49:68–101. doi: 10.1093/jmt/49.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannantonio S., Polonenko M.J., Papsin B.C., Paludetti G., Gordon K.A. Experience changes how emotion in music is judged: evidence from children listening with bilateral cochlear implants, bimodal devices, and normal hearing. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon K.A., Papsin B.C., Harrison R.V. An evoked potential study of the developmental time course of the auditory nerve and brainstem in children using cochlear implants. Audiol. Neurootol. 2006;11:7–23. doi: 10.1159/000088851. [DOI] [PubMed] [Google Scholar]
- Gordon K.A., Wong D.D.E., Papsin B.C. Cortical function in children receiving bilateral cochlear implants simultaneously or after a period of interimplant delay. Otol. Neurotol. 2010;31:1293–1299. doi: 10.1097/MAO.0b013e3181e8f965. [DOI] [PubMed] [Google Scholar]
- Gordon K.A., Jiwani S., Papsin B.C. Benefits and detriments of unilateral cochlear implant use on bilateral auditory development in children who are deaf. Front. Psychol. 2013;4:1–14. doi: 10.3389/fpsyg.2013.00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon K.A., Wong D.D.E., Papsin B.C. Bilateral input protects the cortex from unilaterally-driven reorganization in children who are deaf. Brain J. Neurol. 2013;136:1609–1625. doi: 10.1093/brain/awt052. [DOI] [PubMed] [Google Scholar]
- Gordon K.A., Deighton M.R., Abbasalipour P., Papsin B.C. Perception of binaural cues develops in children who are deaf through bilateral cochlear implantation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0114841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon K., Henkin Y., Kral A. Asymmetric hearing during development: the aural preference syndrome and treatment options. Pediatrics. 2015;136:141–153. doi: 10.1542/peds.2014-3520. [DOI] [PubMed] [Google Scholar]
- Grothe B., Pecka M., McAlpine D. Mechanisms of sound localization in mammals. Physiol. Rev. 2010;90:983–1012. doi: 10.1152/physrev.00026.2009. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Topp G., Klinke R. Discharge patterns of cat primary auditory fibers with electrical stimulation of the cochlea. Hear. Res. 1984;13:47–62. doi: 10.1016/0378-5955(84)90094-7. [DOI] [PubMed] [Google Scholar]
- Hopyan T., Peretz I., Chan L.P., Papsin B.C., Gordon K.A. Children using cochlear implants capitalize on acoustical hearing for music perception. Front. Psychol. 2012;3 doi: 10.3389/fpsyg.2012.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopyan T., Manno F.A.M., 3rd, Papsin B.C., Gordon K.A. Sad and happy emotion discrimination in music by children with cochlear implants. Child Neuropsychol. 2016;22:366–380. doi: 10.1080/09297049.2014.992400. [DOI] [PubMed] [Google Scholar]
- Jiwani S., Papsin B.C., Gordon K.A. Early unilateral cochlear implantation promotes mature cortical asymmetries in adolescents who are deaf. Hum. Brain Mapp. 2016;37:135–152. doi: 10.1002/hbm.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating P., King A.J. Developmental plasticity of spatial hearing following asymmetric hearing loss: context-dependent cue integration and its clinical implications. Front. Syst. Neurosci. 2013;7 doi: 10.3389/fnsys.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korver A.M.H., Smith R.J.H., Van Camp G., Schleiss M.R., Bitner-Glindzicz M.A.K., Lustig L.R., Usami S.-I., Boudewyns A.N. Congenital hearing loss. Nat. Rev. Dis. Primer. 2017;3:16094. doi: 10.1038/nrdp.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A., Sharma A. Developmental neuroplasticity after cochlear implantation. Trends Neurosci. 2012;35:111–122. doi: 10.1016/j.tins.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A., Tillein J., Hubka P., Schiemann D., Heid S., Hartmann R., Engel A.K. Spatiotemporal patterns of cortical activity with bilateral cochlear implants in congenital deafness. J. Neurosci. 2009;29:811–827. doi: 10.1523/JNEUROSCI.2424-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A., Heid S., Hubka P., Tillein J. Unilateral hearing during development: hemispheric specificity in plastic reorganizations. Front. Syst. Neurosci. 2013;7 doi: 10.3389/fnsys.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A., Hubka P., Heid S., Tillein J. Single-sided deafness leads to unilateral aural preference within an early sensitive period. Brain. 2013;136:180–193. doi: 10.1093/brain/aws305. [DOI] [PubMed] [Google Scholar]
- Kuppler K., Lewis M., Evans A.K. A review of unilateral hearing loss and academic performance: is it time to reassess traditional dogmata? Int. J. Pediatr. Otorhinolaryngol. 2013;77:617–622. doi: 10.1016/j.ijporl.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Landsberger D.M., Svrakic M., Roland J.T.J., Svirsky M. The relationship between insertion angles, default frequency allocations, and spiral ganglion place pitch in cochlear implants. Ear Hear. 2015;36:e207–213. doi: 10.1097/AUD.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu J.E.C., Tye-Murray N., Karzon R.K., Piccirillo J.F. Unilateral hearing loss is associated with worse speech-language scores in children. Pediatrics. 2010;125:e1348–1355. doi: 10.1542/peds.2009-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu J.E.C., Karzon R.K., Ead B., Tye-Murray N. Do audiologic characteristics predict outcomes in children with unilateral hearing loss? Otol. Neurotol. 2013;34:1703–1710. doi: 10.1097/MAO.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limb C.J., Rubinstein J.T. Current research on music perception in cochlear implant users. Otolaryngol. Clin. N. Am. 2012;45:129–140. doi: 10.1016/j.otc.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Litovsky R.Y., Jones G.L., Agrawal S., van Hoesel R. Effect of age at onset of deafness on binaural sensitivity in electric hearing in humans. J. Acoust. Soc. Am. 2010;127:400–414. doi: 10.1121/1.3257546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson K.M., Nichols T.E., Poline J.-B., Holmes A.P. Statistical limitations in functional neuroimaging II. Signal detection and statistical inference. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1999;354:1261–1281. doi: 10.1098/rstb.1999.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman A.L., Stelmachowicz P.G. Hearing loss in children and adults: audiometric configuration, asymmetry, and progression. Ear Hear. 2003;24:198–205. doi: 10.1097/01.AUD.0000069226.22983.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley D.B., Thompson J.H., Guo W. Brief hearing loss disrupts binaural integration during two early critical periods of auditory cortex development. Nat. Commun. 2013;4 doi: 10.1038/ncomms3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonenko M.J., Papsin B.C., Gordon K.A. The effects of asymmetric hearing on bilateral brainstem function: findings in children with bimodal (electric and acoustic) hearing. Audiol. Neurootol. 2015;20(Suppl. 1):13–20. doi: 10.1159/000380743. [DOI] [PubMed] [Google Scholar]
- Polonenko M.J., Gordon K.A., Cushing S.L., Papsin B.C. Cortical organization restored by cochlear implantation in young children with single sided deafness. Sci. Rep. 2017 doi: 10.1038/s41598-017-17129-z. (in review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonenko M.J., Giannantonio S., Papsin B.C., Marsella P., Gordon K.A. Music perception improves in children with bilateral cochlear implants or bimodal devices. J. Acoust. Soc. Am. 2017;141:4494–4507. doi: 10.1121/1.4985123. [DOI] [PubMed] [Google Scholar]
- Ponton C.W., Eggermont J.J., Kwong B., Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin. Neurophysiol. 2000;111:220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Ponton C., Eggermont J.J., Khosla D., Kwong B., Don M. Maturation of human central auditory system activity: separating auditory evoked potentials by dipole source modeling. Clin. Neurophysiol. 2002;113:407–420. doi: 10.1016/s1388-2457(01)00733-7. [DOI] [PubMed] [Google Scholar]
- Popescu M.V., Polley D.B. Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron. 2010;65:718–731. doi: 10.1016/j.neuron.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2016. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Rachakonda T., Shimony J.S., Coalson R.S., Lieu J.E.C. Diffusion tensor imaging in children with unilateral hearing loss: a pilot study. Front. Syst. Neurosci. 2014;8 doi: 10.3389/fnsys.2014.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos Macias A., Borkoski-Barreiro S.A., Falcon Gonzalez J.C., Ramos de Miguel A. AHL, SSD and bimodal CI results in children. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016;133(Suppl. 1):S15–20. doi: 10.1016/j.anorl.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Reiss L.A.J., Ito R.A., Eggleston J.L., Wozny D.R. Abnormal binaural spectral integration in cochlear implant users. J. Assoc. Res. Otolaryngol. 2014;15:235–248. doi: 10.1007/s10162-013-0434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenhamer H.J., Lindstrom B., Lundborg T. On the use of click-evoked electric brainstem responses in audiological diagnosis. III. Latencies in cochlear hearing loss. Scand. Audiol. 1981;10:3–11. doi: 10.3109/01050398109076156. [DOI] [PubMed] [Google Scholar]
- Sherbecoe R.L., Studebaker G.A. Supplementary formulas and tables for calculating and interconverting speech recognition scores in transformed arcsine units. Int. J. Audiol. 2004;43:442–448. doi: 10.1080/14992020400050056. [DOI] [PubMed] [Google Scholar]
- Shirvani S., Jafari Z., Motasaddi Zarandi M., Jalaie S., Mohagheghi H., Tale M.R. Emotional perception of music in children with bimodal fitting and unilateral cochlear implant. Ann. Otol. Rhinol. Laryngol. 2016;125:470–477. doi: 10.1177/0003489415619943. [DOI] [PubMed] [Google Scholar]
- Stelmachowicz P.G., Pittman A.L., Hoover B.M., Lewis D.E., Moeller M.P. The importance of high-frequency audibility in the speech and language development of children with hearing loss. Arch. Otolaryngol. Head Neck Surg. 2004;130:556–562. doi: 10.1001/archotol.130.5.556. [DOI] [PubMed] [Google Scholar]
- Thomas J.P., Neumann K., Dazert S., Voelter C. Cochlear implantation in children with congenital single-sided deafness. Otol. Neurotol. 2017;38:496–503. doi: 10.1097/MAO.0000000000001343. [DOI] [PubMed] [Google Scholar]
- Tibbetts K., Ead B., Umansky A., Coalson R., Schlaggar B.L., Firszt J.B., Lieu J.E.C. Interregional brain interactions in children with unilateral hearing loss. Otolaryngol. Head Neck Surg. 2011;144:602–611. doi: 10.1177/0194599810394954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillein J., Hubka P., Kral A. Monaural congenital deafness affects aural dominance and degrades binaural processing. Cereb. Cortex. 2016;1991(26):1762–1777. doi: 10.1093/cercor/bhv351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkova A., Trehub S.E., Schellenberg E.G., Papsin B.C., Gordon K.A. Children with bilateral cochlear implants identify emotion in speech and music. Cochlear Implants Int. 2013;14:80–91. doi: 10.1179/1754762812Y.0000000004. [DOI] [PubMed] [Google Scholar]
- Vrba J., Robinson S.E. Signal processing in magnetoencephalography. Methods. 2001;25:249–271. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- Wang X., Fan Y., Zhao F., Wang Z., Ge J., Zhang K., Gao Z., Gao J.-H., Yang Y., Fan J., Zou Q., Liu P. Altered regional and circuit resting-state activity associated with unilateral hearing loss. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M., Holland S.K., Altaye M., Gaser C. Template-O-Matic: a toolbox for creating customized pediatric templates. NeuroImage. 2008;41:903–913. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]
- Wong D.D.E., Gordon K.A. Beamformer suppression of cochlear implant artifacts in an electroencephalography dataset. IEEE Trans. Biomed. Eng. 2009;56:2851–2857. doi: 10.1109/TBME.2009.2029239. [DOI] [PubMed] [Google Scholar]
- Yang M., Chen H.-J., Liu B., Huang Z.-C., Feng Y., Li J., Chen J.-Y., Zhang L.-L., Ji H., Feng X., Zhu X., Teng G.-J. Brain structural and functional alterations in patients with unilateral hearing loss. Hear. Res. 2014;316:37–43. doi: 10.1016/j.heares.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Zirn S., Arndt S., Aschendorff A., Wesarg T. Interaural stimulation timing in single sided deaf cochlear implant users. Hear. Res. 2015;328:148–156. doi: 10.1016/j.heares.2015.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary Table 1
Supplementary Table 2