Abstract
Structural and functional changes in the olfactory system are increasingly implicated in the expression of PTSD. Still, very little is known about the neurobiological networks of trauma-related odor sensitivity or how they relate to other objective and subjective measures of olfaction and PTSD. The purpose of this study was to replicate prior findings and further characterize olfactory function in trauma-exposed combat veterans with and without PTSD. We also sought to extend this area of research by exploring the effects of time since the combat-related index trauma (TST) on post-trauma olfactory function, as well as by correlating odor-elicited brain activity to general olfactory ability and odor-elicited PTSD symptoms. Participants included combat veterans with PTSD (CV+PTSD; n = 21) or without any psychiatric disorder (CV-PTSD; n = 27). TST was coded as greater (n = 24) or less (n = 24) than 5 years. There were main effects and/or interaction for PTSD-status and TST across several parameters of olfactory function: odor detection, odor identification, ratings for trauma-related odor intensity and triggered PTSD symptoms, and trauma odor-elicited brain activation. Overall, results suggest olfactory impairment in chronic PTSD, but not necessarily in the earlier stages of the disorder, although some early-stage olfactory findings may be predictive of later olfactory impairment. Results also suggest that trauma-exposed individuals who never develop PTSD may demonstrate olfactory resiliency. Finally, results highlight a potentially unique role of trigeminal odor properties in the olfactory-PTSD relationship.
Keywords: PTSD, Olfaction, Odor-threat, Trigeminal, fMRI
Highlights
-
•
PTSD group showed processing deficits for neutral odors, but increased sensitivity & neural processing for burning odors.
-
•
Olfactory and trigeminal processing of threat-related odors may help to identify course of illness in PTSD.
-
•
Longitudinal studies can determine the progression of olfactory/trigeminal changes after trauma.
1. Introduction
A growing literature indicates structural and functional changes of the olfactory system with fear and threat (Ahs et al., 2013, Jones et al., 2008, Kass et al., 2013), as well as olfactory differences with anxiety (La Buissonniere-Ariza et al., 2013, Takahashi et al., 2015) and fear-related disorders, including posttraumatic stress disorder (PTSD) (Berlin et al., 2017, Buron et al., 2015, Cortese et al., 2015b, Dileo et al., 2008, Vasterling et al., 2000). These observations suggest a linkage between the neurobiology of olfactory function and anxiety-fear systems. Consistent with this notion is the overlapping anatomical organization of these parallel systems, such that the primary olfactory (piriform) cortex and the extended olfactory circuit (i.e., amygdala, hippocampus and surrounding cortex, anterior insular and orbitofrontal cortices) (Savic, 2002, Seubert et al., 2013, Zald and Pardo, 2000, Zatorre et al., 2000) share neuroanatomy with the fear/threat circuit (LeDoux, 2012, Price, 1990), including many of the same limbic/paralimbic structures identified in the pathophysiology of PTSD (Etkin and Wager, 2007). Accordingly, odor processing is tightly linked to emotion and memory, allowing odors to elicit the spontaneous retrieval of very emotional, and sometimes very distant, autobiographical memories (Proustian phenomenon) (Chu and Downes, 2002, Nickell and Uhde, 1994). Given that PTSD is defined by recurrent emotional memories of trauma and that traumatic events are often accompanied by specific odors (e.g. burning odor experienced during military combat) (Cortese et al., 2015b), investigating odor threat cues as part of the extended fear network and how olfactory function changes after trauma and with the development and clinical course of PTSD may provide a new understanding of the brain circuits that mediate the core symptoms of this often chronic condition.
To date, olfactory processing differences, including odor detection and/or identification deficits, have been identified across various child and adolescent (Schecklmann et al., 2013) and adult neuropsychiatric disorders (Atanasova et al., 2008, Martzke et al., 1997). However, indices of olfactory function (e.g. odor detection and/or identification) among anxiety and other fear-related disorders including PTSD have been inconsistent (Vasterling et al., 2000, Dileo et al., 2008, Goldberg et al., 1991, Segalas et al., 2011, Locatelli et al., 1996, Kopala and Good, 1996, Hermesh et al., 1999, Fenger et al., 2005, Croy et al., 2010, Schecklmann et al., 2013, Wintermann et al., 2013). This variability suggests that additional unknown factors may play a role (e.g. moderating effect) in the relationship between fear/threat and general olfactory function. It also highlights the need to extend beyond between-group testing of general odor detection/identification performance, to include additional objective measures of olfactory function in the assessment of the olfactory-fear/threat relationship.
Neuroimaging and the use of odor threat cues and/or trauma-related odorants (e.g. burning-related odors in combat veterans) allow the investigation of central olfactory structure and function of the extended olfactory-fear network. To our knowledge, we are the only research group to report structural deficits of the central olfactory system in PTSD, namely less gray matter volume (GMV) in piriform and olfactory orbitofrontal cortices in combat veterans with PTSD compared to trauma-exposed, but healthy, combat veterans (Cortese et al., 2015a). Interestingly, olfactory GMV in the combined group of veterans was inversely related to their ratings of burning odor-elicited PTSD symptoms (Cortese et al., 2015a). Although we and others have identified specific odors as being primary precipitants of re-experiencing and hyperarousal in both military and civilian trauma-exposed individuals (Cortese et al., 2015b, Hinton et al., 2004, Kline and Rausch, 1985, Vermetten and Bremner, 2003), very little is known regarding the sensory perception of and behavioral responses to trauma-related odors, especially when it comes to the neurobiology underlying changes in trauma-related odor processing. In fact, just one study to date has assessed the role of trauma odors and trauma odor-elicited brain responses in PTSD (Vermetten et al., 2007). In that study, trauma-related and control odors were delivered to war veterans with and without PTSD while undergoing positron emissions tomography (PET). While significantly greater odor-elicited regional cerebral blood flow (rCBF) to a number of limbic/paralimbic and prefrontal regions was found in the veterans with PTSD compared to the healthy combat controls, the findings were not specific to the trauma odor, suggesting more general changes in odor processing in PTSD. In addition, while that study reported regional differences in odor-elicited activation between groups, it did not quantify those differences, or assess potential relationships between those differences and both olfactory psychometric and behavioral measures of PTSD.
Therefore, with the use of functional magnetic resonance imaging (fMRI), we sought to 1) replicate the work of Vermetten and colleagues (Vermetten et al., 2007), and 2) to further characterize olfactory function in trauma-exposed combat veterans with and without PTSD by examining the potential relationship between odor-elicited brain activity and general olfactory ability/performance, as well as odor-elicited PTSD symptoms.
2. Material and methods
2.1. Participants
Combat veterans were recruited from the Ralph H. Johnson Veterans Affairs Medical Center (VAMC), as well as the greater Charleston, South Carolina community via advertisement. To meet eligibility, participants were required to: 1) have served in a combat zone in Iraq or Afghanistan (Operation Enduring Freedom (OEF), Iraqi Freedom (OIF), or New Dawn (OND)); 2) meet a current (past month) or lifetime DSM-IV primary diagnosis of combat-related PTSD (assessed by the Clinician Administered PTSD Scale (CAPS)) (Blake et al., 1995), or have no history of any DSM-IV disorder including alcohol or other substance-use disorder (assessed by the Mini International Neuropsychiatric Interview (MINI)) (Sheehan et al., 1998); 3) have no history of head injury/trauma (e.g., blast exposure), given the association between head trauma and olfactory dysfunction (Frasnelli et al., 2016, Xydakis et al., 2015); 4) be psychiatric medication-free; 5) be able to undergo an MRI exam (contraindications such as shrapnel injuries, pregnancy, and claustrophobia excluded); 6) be right handed; and 7) pass a urine drug screen (CLIAwaived ™, San Diego, CA, USA). All study procedures were approved by the Institutional Review Board at the Medical University of South Carolina and the Research and Development (R&D) Committee at the Charleston VAMC. All participants provided informed consent prior to the start of any study procedures.
2.2. Assessment of odor detection and odor identification abilities
Odor detection/sensitivity was assessed with the Smell Threshold Test™ (STT™, Sensonics, Inc. Haddon Heights, NJ, USA) (Doty, 2009), which comprises a series of sniff bottles containing a serial dilution of phenyl ethyl alcohol (PEA), a neutral “rose-like” odor. Sniff bottles of varying concentrations were systematically presented until the lowest concentration of PEA that could be detected reliably was determined. Odor identification was assessed with the University of Pennsylvania Smell Identification Test™ (UPSIT™, Sensonics, Inc. Haddon Heights, NJ, USA) (Doty et al., 1984), a standardized scratch-n-sniff test widely used to determine the ability to identify 40 common odors.
2.3. Assessment of odor threat sensitivity
Odor cues were selected based on survey data which identified specific burning odors to be highly related to combat experiences in Iraq or Afghanistan and to trigger significant PTSD-related distress (Cortese et al., 2015b), or to be unrelated to combat trauma or PTSD. Odor cues were prepared from a library of odorants obtained from ScentAir™ (Charlotte, NC, USA) and included burning rubber (BR), a trauma-related “burning” odor cue, lavender (LAV), a relatively pleasant non-trauma-related control odor cue, and cigarette smoke (SMK), a non-trauma-related “burning” odor cue. Propylene glycol (PG) (Thermo Fisher Scientific, Inc., Waltham, MA, USA) served as the odorless control as well as the base oil for preparing the other odor cues. Similar to previously published methods (Khan et al., 2007), the odor cues were prepared and pilot tested in healthy, normosmic, adults to be an average perceived intensity of 50 mm on a 100 mm visual analog scale (VAS) that ranged from “not at all” to “extremely” intense.
One week prior to the MRI, participants sampled each odor cue. Using the same VAS and anchor points, ratings were acquired for odor intensity and odor-elicited re-experiencing (i.e., “the odor triggered memories of my trauma”), avoidance/numbing (i.e., “the odor made me feel numb”), and hyperarousal (i.e., “the odor made me feel anxious”). A composite score for odor-elicited PTSD symptoms was derived for each participant as the sum of their individual ratings for re-experiencing, avoidance/numbing, and hyperarousal.
2.4. MRI data acquisition
A 6-chamber, MRI-compatible olfactometer (Emerging Tech Trans, LLC, Hershey, PA, USA) delivered all odor cues through humidified, room temperature, air that was maintained at a constant rate of 8 l/min, providing consistent airflow to the nose throughout the entire scan and a stimulus rise time of < 150 ms. Breathing instructions were provided with picture cues, stating “breathe in” or “breathe out”, serially delivered throughout the scan to 1) promote consistent breathing (6-s breathing cycle) and limit sniffing, and 2) so that odorants, when delivered, were available at the beginning of a 3-s inhalation for all participants. The odor cues (BR, LAV, SMK, and PG) were each delivered 4 times, for 8-s durations, interspersed throughout the 12-min scan. A pseudorandom odor delivery schedule was utilized so that the same odor was never presented consecutively.
Neuroimaging data were acquired on a 3 T TIM Trio scanner (Siemens Medical, Erlangen, Germany) at the MUSC's Center for Biomedical Imaging. High-resolution, T1-weighted structural images for subsequent registration were acquired with a ~ 6-min, magnetization-prepared rapid gradient-echo (MP-RAGE) sequence. Image acquisition parameters were: repetition/echo time = 2250/4 ms; flip angle = 9°; matrix = 256 × 256; voxel size = (1.0 mm)3, which yielded 176 contiguous, sagittal slices. Functional images were acquired with a gradient echo-planar imaging (EPI) sequence, with the following image acquisition parameters: repetition/echo time = 2200/35 ms; flip angle = 90°; field of view = 192 mm; matrix = 64 × 64; voxel size = (3 mm)3; and 36 contiguous, transverse slices, that yielded 329 volumes during the 12-min scan.
2.5. fMRI data processing and analyses
Structural (T1-weighted) and functional MRI data were preprocessed using FSL 5.09 (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki) created and maintained by the FMRIB Analysis Group at University of Oxford, U.K. (https://www.ndcn.ox.ac.uk/divisions/fmrib/fmrib-analysis-group). T1-weighted structural scans were skull-stripped using FSL's BET tool and aligned to the 6th generation non-linear MNI152 standard-space brain atlas at (2 mm)3 resolution (http://nist.mni.mcgill.ca/?page_id=714) using a 12°-of-freedom linear alignment model via FSL's FLIRT. fMRI data was motion-corrected, high-pass filtered (cutoff freq. 60 Hz), spatially smoothed (3D-Gaussian Kernel FWHM 5 mm), and skull-stripped (FSL BET) before a boundary-based coregistration (BBR) to the corresponding skull-stripped structural images using FAST/FLIRT. Applying both EPI-to-T1 and T1-to-MNI152 transformations all functional scans were automatically aligned to each other in standard MNI152 space and ready for further between-subject level analysis.
For the whole brain statistical fMRI analysis, we used a 2-step approach. First, we modeled each within-subject odor-specific BOLD-response for BR, LAV, and PG, as well as odor contrasts of BR > PG, LAV > PG, and BR > LAV, assuming a generalized linear model (GLM) and using a double gamma hemodynamic response function (HRF) convolved with our experiment's design matrix (FSL FEAT). Next, we obtained both within-group and between-group averages, as well as group by time since combat-related index trauma (TST < 5 years and TST > 5 years) for all odors and contrasted odor combinations. We used FILM pre-whitening in step one and a mixed-effects model via FLAME 1 and automatic outlier de-weighting in step two. To further investigate how TST influenced neural activation patterns, TST (in months) was entered as a regressor in whole brain analyses for CV+PTSD and CV-PTSD separately. Post-modeling statistics employed a cluster-thresholding approach of Z > 2.3 and a corrected cluster threshold of p < 0.05 to mitigate the risk of misinterpretation of our results due to random and spatially very localized but statistically significant activations of either a single voxel or a small cluster of voxels.
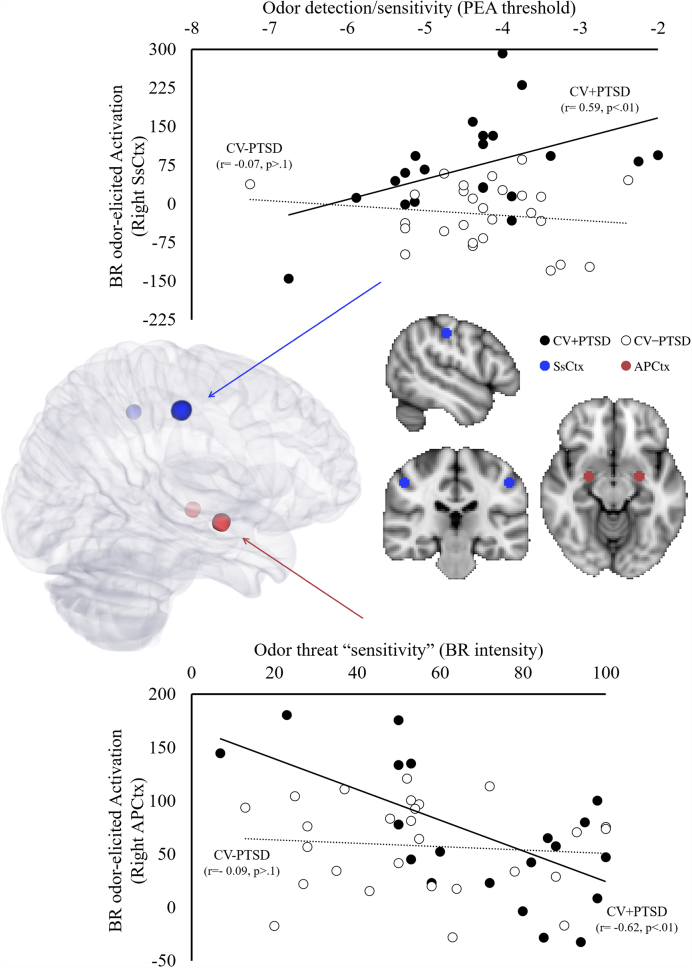
Using FEATquery, post-hoc, region of interest (ROI) parameter estimates were extracted from 5-mm-radius spheres centered around bilateral anterior piriform (1° olfactory) cortex (MNI = ± 22, − 2, − 14) (Cortese et al., 2015a, Seubert et al., 2013) and bilateral 1° somatosensory cortex provided by the Juelich Histological Atlas in FSL (see Fig. 4).
Fig. 4.
Paradoxical effects of the olfactory and intranasal trigeminal systems in chronic PTSD. Greater odor detection impairment was associated with increased burned rubber (BR) odor-elicited activation in right 1° somatosensory cortex (SsCtx) in combat veterans with PTSD (CV+PTSD), but not healthy combat veterans (CV-PTSD) (top scatter plot). In contrast, greater odor threat “sensitivity” (BR intensity ratings) in CV+PTSD, but not CV-PTSD, was associated with less BR odor-elicited activity in right primary olfactory (anterior piriform) cortex (APCtx) (bottom scatter plot).
2.6. Statistical analyses
Analyses were conducted using IBM's SPSS 23.0. Demographic and clinical characteristics were assessed with Chi-square and Independent t-tests. Main effects as well as interactions of Group (combat veterans with PTSD (CV+PTSD) and without PTSD (CV-PTSD)) and TST (TST produced by an overall median split, which resulted in TST < 5 years and TST > 5 years) were determined with ANCOVA, using age as a covariate given its high correlation with TST, for each dependent measure: odor detection, odor identification, odor intensity and odor-elicited PTSD symptoms for trauma- and non-trauma-related odor cues. Pearson's correlation was utilized to demonstrate the relationship between olfactory ability, odor cue ratings, and neural activation. Based on our previous work suggesting BR as the primary PTSD-related odor cue (Cortese et al., 2015b, Cortese et al., 2015a), analyses for subjective ratings and odor-elicited brain activity in response to burning odor utilized data for BR only.
3. Results
3.1. Participant characteristics
Twenty-one combat veterans with PTSD (CV+PTSD) and twenty-seven combat veterans without PTSD (CV-PTSD) completed the full psychiatric evaluation, odor testing, and olfactory imaging procedures. Forty-five veterans in the present sample (CV+PTSD: N = 20, CV-PTSD: N = 25) were included in our previous report of a PTSD-related decrease of GMV in primary and secondary olfactory cortices (Cortese et al., 2015a).
CV+PTSD were comprised of 17 veterans that met criteria for current combat-related PTSD and 4 that met current sub-clinical PTSD (i.e., met criterion A and 2/3 symptom clusters) and met criteria for lifetime PTSD related to their combat-related index trauma. Within this group, six met diagnostic criteria for secondary depression, 2 had comorbid panic disorder and 1 had comorbid generalized anxiety disorder.
CV-PTSD had no history of any DSM-IV disorder. Table 1 shows that CV+PTSD and CV-PTSD differed on CAPS-assessed PTSD (t46 = 8.8, p < 0.001), but were well-matched on age, sex, race, education, employment, combat exposure plus additional lifetime traumatic experiences (Keane et al., 1989, Resnick et al., 1996), and time since combat-related index trauma (TST) (all ps > 0.05).
Table 1.
Demographic and clinical characteristics of combat veterans.
| CV+PTSD (n = 21) | CV-PTSD (n = 27) | χ2 or t | p | |
|---|---|---|---|---|
| Sex - n (%) male | 20 (95.2) | 26 (96.3) | 0.03 | ns |
| Race - n (%) minority | 7 (33.3) | 3 (11.1) | 3.54 | ns |
| Employment - n (%) employed | 13 (61.9) | 17 (63.0) | 0.01 | ns |
| Age in years (mean ± SD) | 31.4 ± 9.4 | 32.2 ± 8.3 | 0.30 | ns |
| Education in years (mean ± SD) | 14.0 ± 1.2 | 14.8 ± 2.3 | 1.50 | ns |
| Cumulative traumaa (mean ± SD) | 25.5 ± 9.2 | 22.8 ± 10.2 | 0.95 | ns |
| Time Since Traumab (mean ± SD) | 59.5 ± 33.0 | 71.6 ± 34.4 | 1.22 | ns |
| CAPS total score (mean ± SD) | 59.3 ± 22.5 | 14.4 ± 12.3 | 8.83 | < 0.001 |
CV+PTSD = combat veterans with PTSD, CV-PTSD = combat veterans without PTSD.
CAPS = Clinician Administered PTSD Scale for DSM-IV (Blake et al., 1995).
ns = p-value > 0.05.
Sum of Combat Exposure Scale & Trauma Assessment for Adults (Keane et al., 1989, Resnick et al., 1996).
Number of months since combat-related index trauma
There were no PTSD group differences in the number of months since trauma once TST was dichotomized via an overall median split by more recent trauma (TST < 5 years: CV+PTSD (N = 11, M = 33.6, SD = 14.3), CV-PTSD (N = 13, M = 46.2, SD = 18.9)) and more distant trauma (TST > 5 years: CV+PTSD (N = 10, M = 87.0, SD = 23.1), CV-PTSD (N = 14, M = 95.7, SD = 24.0)) (ps > 0.05). In addition, subgrouping by TST did not expose any differences in sex, race, education, or combat exposure plus additional lifetime traumatic experiences (all ps > 0.05). However, differences in age and employment were revealed, as subgroups with TST < 5 years were significantly younger and less employed than subgroups with TST > 5 years, regardless of PTSD diagnosis (ps < 0.05).
3.2. Odor detection
Odor detection results (see Fig. 1a) revealed that both groups of combat veterans had similar sensitivity to the neutral “rose-like” odor of PEA (CV+PTSD: M = − 4.4, SD = 1.1; CV-PTSD: M = − 4.3, SD = 0.9; F (1, 43) = 0.18, p > 0.1; groups collapsed across TST). While a main effect of TST approached significance (F (1, 43) = 3.59, p = 0.06; collapsed across PTSD group), there was a significant Group x TST interaction (F (1, 43) = 4.76, p < 0.05) in odor detection that was driven by TST-related difference in CV+PTSD. In other words, detection ability was most sensitive in the veterans with PTSD who had more recent trauma and least sensitive in the veterans with PTSD who had more distant trauma, even when controlling for the significant age difference between these PTSD subgroups (F (1, 18) = 6.74, p < 0.05). However, there was no impact of TST on odor detection in CV-PTSD, regardless of the age difference between the subgroups of healthy veterans (see Fig. 1a).
Fig. 1.
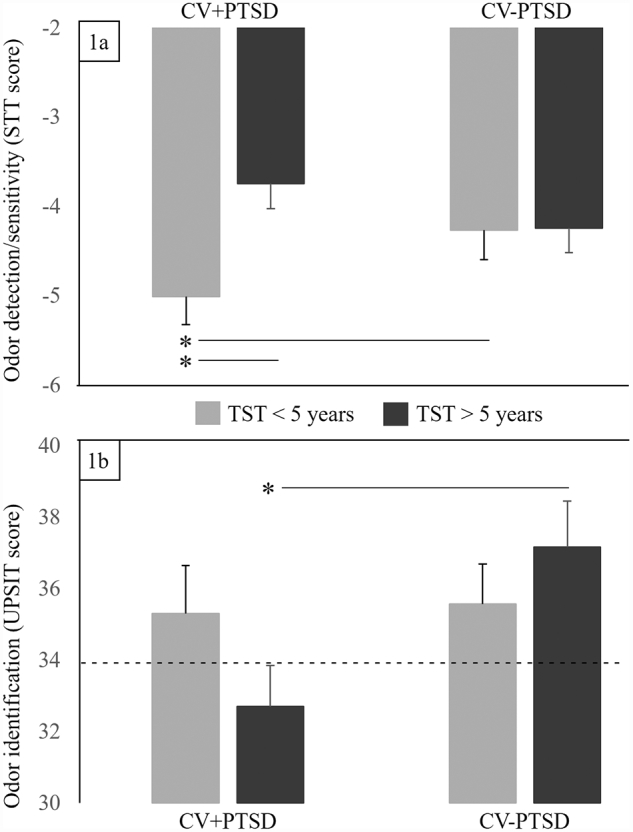
The influence of posttraumatic stress disorder (PTSD) and time since trauma (TST) on general olfactory function. 1a. odor detection was determined with the Smell Threshold Test (STT™), wherein a higher number (less negative) indicates a greater concentration of odorant and reduced detection/sensitivity. 1b. odor identification, with the University of Pennsylvania Smell Identification Test (UPSIT™), determined identification of 40 common odorants (---- indicates cut-off between normosmia and microsmia). Combat veterans with PTSD and more recent trauma (CV+PTSD with TST < 5 years) demonstrated increased detection/sensitivity (i.e. reliably detected the lowest concentration of phenyl ethyl alcohol (PEA) compared to the other groups), while the PTSD group with more distant traumas demonstrated the most reduced detection/sensitivity. Only combat veterans with more distant trauma (CV+PTSD with TST > 5 years) performed in the microsmia range for odor identification. Healthy combat veterans = CV-PTSD. * = group contrast with p < 0.05.
3.3. Odor identification
Odor identification results (see Fig. 1b) revealed significantly reduced ability in CV+PTSD (M = 34.0, SD = 5.2) compared to healthy CV-PTSD (M = 36.4, SD = 3.0; F (1, 43) = 4.12, p < 0.05). Although both groups fell within the diagnostic category of normosmia, i.e. normal sense of smell (UPSIT score = 34–40), CV+PTSD as a group performed at the 18th percentile, just short of the cut-off for microsmia (i.e. diminished sense of smell), while performance of CV-PTSD as a group approached the 50th percentile, mid-range within that diagnostic category. A Group x TST interaction that approached significance (F (1, 43) = 3.65, p = 0.06) revealed that only the CV+PTSD who were > 5 years beyond their index trauma performed in the microsmia range. Pairwise comparisons revealed that while CV+PTSD and CV-PTSD who had more recent trauma (TST < 5 years) performed equally well, there was a significant PTSD-related difference in the veterans with more distant trauma (TST > 5 years) (t22 = 2.29, p < 0.05) (see Fig. 1b).
3.4. Trauma odor intensity
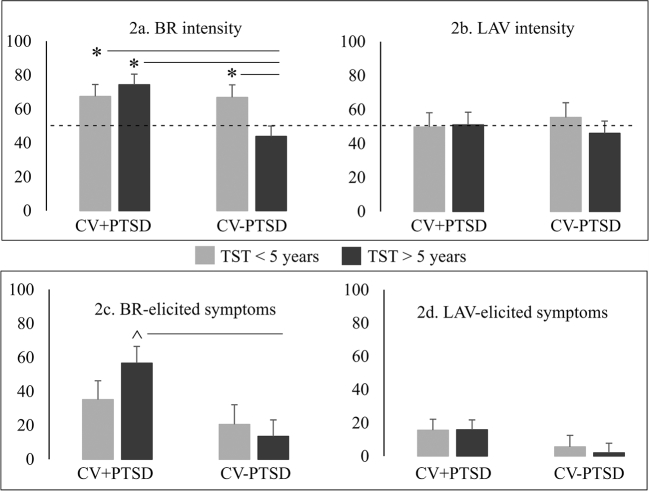
Intensity ratings for BR (see Fig. 2a) revealed a main effect of Group (F (1, 43) = 6.01, p < 0.05) and a Group × TST interaction (F (1, 43) = 5.55, p < 0.05). Overall, the perception of BR was more intense for CV+PTSD (M = 70.76, SD = 20.6) than for healthy CV-PTSD (M = 54.9, SD = 24.7), but TST impacted this effect. For veterans who were < 5 years from their index trauma, intensity ratings were approximately equal and well above the piloted intensity of 50 mm on a 100 mm VAS for the CV+PTSD and CV-PTSD groups. For veterans who were beyond 5 years from their index trauma, CV+PTSD provided significantly higher intensity ratings (well above piloted intensity) than the healthy CV-PTSD (at approximately the piloted intensity rating). In fact, healthy CV-PTSD who were beyond 5 years from their index trauma rated BR significantly less intense than the other 3 groups (see Fig. 2a). Intensity ratings for LAV did not differ by Group or TST (all ps > 0.1; see Fig. 2b).
Fig. 2.
The influence of posttraumatic stress disorder (PTSD) and time since trauma (TST) on ratings of trauma odor intensity and odor-elicited PTSD symptoms. Ratings for burned rubber (BR) and lavender (LAV) were acquired on 100 mm visual analog scales (anchor points: 0 = not at all, 100 = extremely) for odor intensity and odor-elicited PTSD symptoms. ---- indicates mean perceived intensity piloted prior to the current study. Combat veterans with PTSD and more distant trauma (CV+PTSD with TST > 5 years) rated BR the most intense compared to the ratings of the other 3 groups. A trend also existed for CV+PTSD with TST > 5 years to rate more BR odor-elicited PTSD symptoms than CV-PTSD with TST > 5 years. Healthy combat veterans = CV-PTSD. * = group contrast with p < 0.05. ^ = group contrast with p < 0.1.
3.5. Trauma odor-elicited PTSD symptoms
A significant Group difference was revealed for BR-elicited PTSD symptoms (F (1, 43) = 8.39 p < 0.01), such that CV+PTSD (M = 45.1, SD = 44.3) reported greater BR-elicited symptoms compared to CV-PTSD (M = 17.2, SD = 25.7) (see Fig. 2c). Similar to the findings for BR odor intensity, there was no PTSD-related difference in BR-elicited symptoms in veterans who were < 5 years from their index trauma (p > 0.1). However, there was a trend for CV+PTSD who were beyond 5 years from their index trauma to endorse greater BR-elicited PTSD symptoms than CV-PTSD (t22 = 1.78, p = 0.09) (see Fig. 2c). There was no notable TST difference in BR odor-elicited symptoms for CV-PTSD, as well as no significant findings for LAV-elicited PTSD symptoms (all ps > 0.1; see Fig. 2d).
3.6. Trauma odor-elicited brain activation
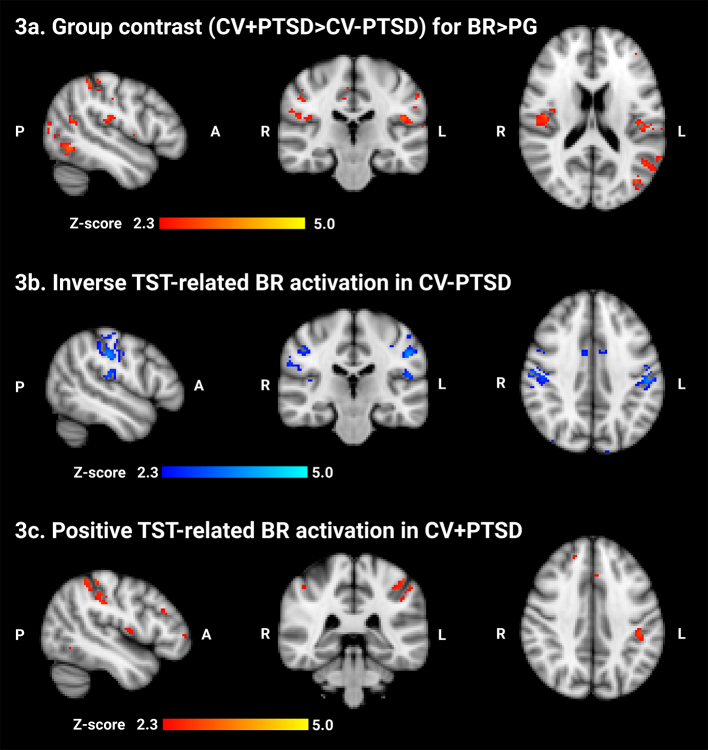
Whole brain analysis uncovered PTSD-related differences in BR-elicited, but not LAV-elicited, brain activity. A main effect of Group for the contrast BR > PG revealed that CV+PTSD, compared to CV-PTSD, demonstrated greater BR-elicited activation in bilateral primary and secondary somatosensory cortices (SsCtx) as well as bilateral precuneous and lateral occipital cortex (see Table 2, Fig. 3a, and supplementary image file ‘zstat_BR_group_contrast.nii’). A significant Group × TST interaction for BR > PG (see Table 3 top) and separate group correlational analyses for TST (in months) revealed that activation in bilateral SsCtx and surrounding multimodal sensory association areas (i.e. angular gyrus, precuneous, lateral occipital cortex) decreased as a function of greater TST in the healthy CV-PTSD (see Table 3 bottom, Fig. 3b, and supplementary image file ‘zstat_CV-PTSD_TST_correlation.nii’), yet increased as a function of greater TST in CV+PTSD (see Table 3 middle, Fig. 3c, and supplementary image file ‘zstat_CV+PTSD_TST_correlation.nii’). No significant Group or Group × TST effects were found for LAV > PG or BR > LAV.
Table 2.
Significant group difference in whole brain activation results in response to odor cues.
| Contrast | Cluster | Z-max | p-value | Voxel | MNI | Anatomy |
|---|---|---|---|---|---|---|
| CV+PTSD > CV-PTSD | ||||||
| BR > PG | 4 | 3.47 | 5.00E-05 | 803 | 40, − 30, 22 | R parietal operculum (2° somatosensory ctx) |
| 54, − 18, 26 | R postcentral gyrus (1° somatosensory ctx) | |||||
| 50, − 28, 22 | R parietal operculum (2° somatosensory ctx) | |||||
| 44, − 14, 18 | R central operculum (2° somatosensory ctx) | |||||
| 36, − 30, 48 | R postcentral gyrus (1° somatosensory ctx) | |||||
| 44, − 32, 50 | ||||||
| 3 | 3.27 | 0.000499 | 620 | − 28, − 70, 26 | L lateral occipital (superior division) | |
| − 36, − 72, 26 | ||||||
| − 22, − 62, 28 | L precuneous | |||||
| − 38, − 78, 32 | L lateral occipital (superior division) | |||||
| − 40, − 74, 18 | ||||||
| − 18, − 68, 28 | L precuneous | |||||
| 2 | 3.34 | 0.000547 | 613 | − 48, − 36, 54 | L postcentral gyrus (1° somatosensory ctx) | |
| − 42, − 26, 30 | L cerebral white matter | |||||
| − 46, − 24, 20 | L parietal operculum (2° somatosensory ctx) | |||||
| − 58, − 28, 22 | ||||||
| − 52, − 30, 52 | L postcentral gyrus (1° somatosensory ctx) | |||||
| − 58, − 26, 30 | L supramarginal gyrus | |||||
| 1 | 3.23 | 0.0132 | 388 | 0, − 46, 56 | Precuneous | |
| 12, − 36, 56 | R postcentral gyrus (1° somatosensory ctx) | |||||
| 6, − 46, 58 | R precuneous | |||||
| 10, − 46, 56 | ||||||
| 10, − 50, 62 | ||||||
| − 14, − 60, 56 | L lateral occipital (superior division) | |||||
| LAV > PG | no clusters survived thresholding | |||||
| BR > LAV | no clusters survived thresholding | |||||
| CV-PTSD > CV+PTSD | ||||||
| BR > PG | no clusters survived thresholding | |||||
| LAV > PG | no clusters survived thresholding | |||||
| BR > LAV | no clusters survived thresholding | |||||
All analyses completed using cluster thresholding (Z > 2.3 and corrected cluster threshold of p < 0.05) at individual and group levels.
Z-MAX is the local maximum Z value.
Voxel is the number of activated voxel within each cluster.
MNI (x, y, z) are the MNI coordinates for the local maximum.
Anatomy is the Harvard-Oxford Cortical and Subcortical Structural Atlases for the local maximum (or closet label to maximum).
Fig. 3.
Burned rubber (BR) odor-elicited brain activation in combat veterans with and without PTSD (CV+PTSD, CV-PTSD respectively). PG = propylene glycol, the odorless control. 3a. Increased BR odor-elicited brain activity was revealed in multimodal association areas of the parietal lobe that extended into somatosensory cortex in CV+PTSD compared to CV-PTSD. As time since trauma (TST) increased, BR odor-elicited activity decreased in somatosensory cortex of CV-PTSD (3b) and increased in CV+PTSD (3c). Z > 2.3, corrected cluster threshold of p < 0.05. Gray matter mask applied for visualization.
Table 3.
Whole brain analysis results for the interaction of group x time since trauma (TST) for BR > PG.
| Contrast | Cluster | Z-MAX | p-value | Voxel | MNI | Anatomy |
|---|---|---|---|---|---|---|
| CV+PTSD slope > CV-PTSD slope | ||||||
| 2 | 3.96 | 1.04E-05 | 2371 | − 38, − 52, 22 | L angular gyrus | |
| − 18, − 54, 22 | L precuneous | |||||
| − 38, − 64, 28 | L lateral occipital (superior division) | |||||
| − 34, − 60, 24 | L angular gyrus | |||||
| − 56, − 70, 26 | L lateral occipital (superior division) | |||||
| 14, − 52, 36 | R precuneous | |||||
| 1 | 4.01 | 0.00749 | 998 | 60, − 30, 28 | R supramarginal gyrus (anterior) | |
| 60, − 30, 18 | R planum temporale | |||||
| 36, − 42, 32 | R supramarginal gyrus (posterior) | |||||
| 42, − 44, 22 | ||||||
| 52, − 40, 28 | ||||||
| 32, − 42, 34 | ||||||
| CV+PTSD slope < CV-PTSD slope | ||||||
| No clusters survived thresholding | ||||||
| CV+PTSD only | ||||||
| TST + | 1 | 3.33 | 0.00061 | 1000 | − 28, − 54, 62 | L superior parietal lobe |
| − 56, − 26, 30 | L supramarginal gyrus (anterior) | |||||
| − 36, − 50, 60 | L superior parietal lobe | |||||
| − 42, − 30, 34 | L supramarginal gyrus (anterior) | |||||
| − 48, − 40, 50 | ||||||
| − 44, − 44, 56 | L superior parietal lobe | |||||
| CV-PTSD only | ||||||
| TST- | 1 | 3.85 | 0.0167 | 914 | − 50, − 24, 36 | L postcentral gyrus (1° somatosensory ctx) |
| − 46, − 26, 42 | ||||||
| − 46, − 18, 54 | ||||||
| − 52, − 16, 26 | ||||||
| − 40, − 28, 32 | L supramarginal gyrus (anterior) | |||||
| − 52, − 22, 18 | L central operculum (2° somatosensory ctx) | |||||
All analyses completed using cluster thresholding (z > 2.3 and corrected cluster threshold of p < 0.05) at individual and group levels.
Z-MAX is the local maximum z value.
Voxel is the number of activated voxel within each cluster.
MNI (x, y, z) are the MNI coordinates for the local maximum.
Anatomy is the Harvard-Oxford Cortical and Subcortical Structural Atlases for the local maximum (or closet label to maximum).
Consistent with whole brain analysis of the influence of TST on BR odor-elicited brain activation, and with the findings from odor testing which revealed greater odor detection impairment and increased BR odor intensity ratings in CV+PTSD with more distant traumatic experiences (TST > 5 years), correlational analyses of ROI parameter estimates demonstrated that greater odor detection impairment related to greater BR odor-elicited activation in SsCtx in CV+PTSD (r = 0.59, p < 0.01), but not CV-PTSD (r = − 0.07, p > 0.1) (see Fig. 4). Furthermore, greater BR odor intensity ratings (trauma odor “sensitivity”) were associated with reduced BR odor-elicited activity in primary olfactory (anterior piriform) cortex (APCtx) in CV+PTSD (r = − 0.62, p < 0.01), but not CV-PTSD (r = − 0.09, p > 0.1) (see Fig. 4). Additionally, a positive relationship between BR odor-elicited activation in right APCtx and SsCtx was revealed in CV-PTSD (r = 0.41, p < 0.05), but not CV+PTSD (r = 0.18, p > 0.1).
4. Discussion
The present cross-sectional study assessed the influence of combat-related PTSD and time since trauma on olfactory function utilizing a variety of objective and subjective assessment tools. General odor detection testing with a neutral “rose-like” odorant revealed that odor sensitivity was increased (i.e. lower threshold score) in the PTSD group with more recent trauma, and decreased (i.e. higher threshold score) in the PTSD group with more distant trauma. On the other hand, time since trauma was not a factor for odor detection ability in veterans who did not develop PTSD, as both groups of healthy veterans (TST < 5 years versus TST > 5 years) had comparable threshold scores. Odor identification performance followed a similar pattern. That is, the most impaired identification performance occurred in the PTSD group with greater time since trauma. Overall, these results (see Fig. 1a and b) suggest olfactory impairment in chronic PTSD, but not necessarily in the earlier stages of the disorder, although early-stage olfactory functioning (i.e. increased odor sensitivity) may be predictive of later olfactory impairment (i.e. decreased odor sensitivity). Results also suggest that trauma-exposed individuals who never develop PTSD may demonstrate olfactory resiliency. While it may be that olfactory function represents a risk factor for the development of PTSD and/or reflects a marker of PTSD-related symptom severity and chronicity, future longitudinal studies are necessary to determine true differences and/or changes in olfactory function before and after trauma and with the development and clinical course of PTSD.
Odor ratings in response to the trauma-related odor (burned rubber – BR) revealed a somewhat different pattern in the veterans. BR odor intensity ratings, but not ratings for the control odor (lavender – LAV), were similarly elevated in both veteran groups (PTSD and healthy) with more recent trauma. In contrast to this, a significant difference in BR odor intensity ratings and a trend-level difference in BR odor-elicited symptoms was revealed between the PTSD and healthy groups with greater time since trauma (see Figs. 2a-d). These results suggest that even healthy veterans may have post-trauma changes in the intensity processing of specific threat-related odors, but that those changes may be time-limited, i.e. BR intensity ratings for healthy veterans with more distant trauma were significantly lower than all other groups. These data align with studies in laboratory animals that reported a post-conditioned increase in synaptic output of sensory neurons coding for a shock-predictive odor (Kass et al., 2013), as well as structural changes in primary olfactory cortex that correlated with enhanced detection and discrimination of a fear-conditioned odor (Jones et al., 2008). They also align with human studies reporting that odor-shock pairing resulted in an odor-specific increase in detection sensitivity (Ahs et al., 2013) and discrimination of previously indiscriminable odor cues (Li et al., 2008) in healthy adults.
Our finding of elevated BR intensity ratings in both PTSD groups, regardless of time since trauma, is consistent with a growing literature showing enhanced odor threat processing with increased state/trait anxiety (Krusemark and Li, 2012, La Buissonniere-Ariza et al., 2013) and across a variety of fear-related disorders including panic disorder, social anxiety, as well as PTSD (Croy et al., 2010, Pause et al., 2009, Wintermann et al., 2013). However, elevated BR intensity ratings in the PTSD group with more distant trauma conflicts with this group's level of general olfactory functioning. That is, despite their poor olfactory function (i.e. impaired odor detection and identification performance), the PTSD group with more distant trauma rated BR well-above the piloted intensity of 50 mm and equally intense as the PTSD group with more recent trauma who had significantly better odor detection ability. Although odor detection of subthreshold stimuli and intensity measurements of suprathreshold stimuli are not equivalent measures, and determining true sensitivity to BR would require odor threshold testing of that specific odorant, the lack of correspondence between general olfactory function and BR odor “sensitivity” in the PTSD group with more distant trauma is notable. In fact, these results are consistent with a chronicity-related paradox between self-report and objective physiological measures of threat in PTSD and other fear-related disorders. For example, despite the endorsement of increased arousal and distress, adults with more chronic forms of stress and anxiety disorders showed blunted defensive reactivity to threat (e.g. reduced startle reflex) (McTeague and Lang, 2012, McTeague et al., 2011, McTeague et al., 2010).
Disparity between self-reported odor “sensitivity” and objective odor sensitivity (PEA detection threshold) has not been well studied in psychiatry, though it has been described in patients with chemical sensitivity/intolerance (Azuma et al., 2016, Doty, 1994, Hummel et al., 1996), a condition with high psychiatric comorbidity (Bell et al., 1995, Bornschein et al., 2002, Katerndahl et al., 2012). Even less is known regarding the brain mechanisms that might support a disparity in self-reported versus objectively-measured odor sensitivity. One potential mechanism is based on the neural circuitry involved in odor processing. That is, the brain processes most odors through two separate, but interacting, neural pathways, i.e. the olfactory and the trigeminal pathways. Stimulation to the olfactory nerve (olfactory pathway) activates a number of central regions including, but not limited to, piriform and orbitofrontal cortices (Seubert et al., 2013), and provides information about odor quality. In addition to activating olfactory regions, intranasal trigeminal nerve stimulation also activates brain regions associated with nociception including superior parietal lobe, precentral gyrus, precuneus, and both primary and secondary somatosensory cortex (Albrecht et al., 2010, Tobia et al., 2016). Intranasal trigeminal stimuli (e.g. odors with trigeminal properties) produce the “feel” of the odor, and if the odor is highly (or purely) trigeminal (e.g. CO2, ammonia, etc.) an irritating or even painful sensation in the nose. Although PEA has trigeminal properties (Doty et al., 1978, Kobal and Hummel, 1992), the subthreshold concentrations used to determine odor detection/sensitivity would likely produce less trigeminal activation than BR, the trauma-related odor cue prepared at a much higher perceived intensity (Hummel and Livermore, 2002). Given this potential difference in trigeminal activation between PEA and BR, we might therefore speculate that the paradoxical finding of PEA detection impairment (reduced general odor sensitivity) and increased BR intensity ratings (increased odor threat “sensitivity”) demonstrated in the PTSD group with more distant trauma may be related to separate, and opposite, effects to both systems. That is to say, chronic PTSD may be associated with blunted olfactory function and augmented trigeminal function.
Our imaging results are consistent with this notion. While whole brain analysis of odor-elicited brain activation was consistent with previous findings (Vermetten et al., 2007) and revealed no differences between BR or LAV in primary or secondary olfactory cortices, a PTSD-related increased response to BR, but not LAV, was exposed in primary and secondary somatosensory cortices (see Table 2 and Fig. 3a), regions known to activate in response to trigeminal stimulation (Albrecht et al., 2010). Additionally, time since trauma influenced the BR-elicited regional activity in somatosensory cortex differently depending on PTSD-status, as BR-elicited somatosensory activation increased with increasing time since trauma in the veterans with PTSD and decreased with increasing time since trauma in the healthy veterans (see Table 3 and Fig. 3b-c). Given that time since trauma also related positively to general odor detection impairment in the veterans with PTSD, the positive relationship between general odor detection impairment and BR odor-elicited activation in right somatosensory cortex in the PTSD group (see Fig. 4) also supports the notion that PTSD is characterized by a differential disturbance in the olfactory versus trigeminal sensory systems that process threat-related odor cues.
Evidence indicates interactive effects of the olfactory and intranasal trigeminal systems, such that olfactory perception can be potentiated (Bensafi et al., 2007, Hummel and Livermore, 2002, Jacquot et al., 2004, Moessnang et al., 2013), as well as dampened by trigeminal activation (Cain, 1976, Hummel et al., 1992, Kobal and Hummel, 1988). Interestingly, results in the healthy veterans, but not those with PTSD, revealed a positive association between odor-elicited activation in primary olfactory and somatosensory cortices. We might speculate, therefore, that increased somatosensory cortex activation in PTSD could be a compensatory mechanism for loss of general olfactory function and/or a loss of functional connectivity or communication between these separate but routinely interacting systems. Interestingly, LAV also possesses trigeminal properties, but failed to differentially activate the trigeminal system between veteran groups, suggesting that PTSD-related increased trigeminal activation may be specific to threat-related odors.
5. Conclusions
In conclusion, this cross-sectional assessment of olfaction in combat trauma-exposed, veterans revealed interesting PTSD-related differences that interacted with time since trauma across several quantitative parameters of olfactory function—odor detection, odor identification, ratings for trauma odor intensity and triggered PTSD symptoms, as well as trauma odor-elicited brain activation. These results are consistent with the compelling notion of an evolution of olfactory-trigeminal changes after trauma and with the development of chronic PTSD. However, limitations of this preliminary study include small group size, the cross-sectional design and inability to measure true changes in post-trauma olfactory function over time, as well as variables that were not assessed including 1) potential PTSD-related differences in olfactory habituation or sniffing patterns (although we implemented breathing instructions to help control sniffing during fMRI), and 2) data regarding the trigeminal properties for each of the odor cues (e.g. degree of trigeminal activation). Despite of these study limitations, our results warrant larger, longitudinal, investigations aimed to determine the progression of olfactory/trigeminal changes, including potential effects such as olfactory habituation, after trauma and with the development and clinical course of PTSD.
Footnotes
Funding for this study was provided by NIMH Grant K01 MH090548 (BMC). The study sponsor had no role in the study design, collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
All authors declare that they have no conflicts of interest.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2017.09.018.
Appendix A. Supplementary data
Group difference in BR-elicited brain activation.
Inverse relationship between TST and BR-elicited brain activation in healthy veterans.
Positive relationship between TST and BR-elicited brain activation in veterans with PTSD.
References
- Ahs F., Miller S.S., Gordon A.R., Lundstrom J.N. Aversive learning increases sensory detection sensitivity. Biol. Psychol. 2013;92(2):135–141. doi: 10.1016/j.biopsycho.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Albrecht J., Kopietz R., Frasnelli J., Wiesmann M., Hummel T., Lundstrom J.N. The neuronal correlates of intranasal trigeminal function-an ALE meta-analysis of human functional brain imaging data. Brain Res. Rev. 2010;62(2):183–196. doi: 10.1016/j.brainresrev.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasova B., Graux J., El Hage W., Hommet C., Camus V., Belzung C. Olfaction: a potential cognitive marker of psychiatric disorders. Neurosci. Biobehav. Rev. 2008;32(7):1315–1325. doi: 10.1016/j.neubiorev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Azuma K., Uchiyama I., Tanigawa M., Bamba I., Azuma M., Takano H., Sakabe K. Association of odor thresholds and responses in cerebral blood flow of the prefrontal area during olfactory stimulation in patients with multiple chemical sensitivity. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0168006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell I.R., Peterson J.M., Schwartz G.E. Medical histories and psychological profiles of middle-aged women with and without self-reported illness from environmental chemicals. J. Clin. Psychiatry. 1995;56(4):151–160. [PubMed] [Google Scholar]
- Bensafi M., Frasnelli J., Reden J., Hummel T. The neural representation of odor is modulated by the presence of a trigeminal stimulus during odor encoding. Clin. Neurophysiol. 2007;118(3):696–701. doi: 10.1016/j.clinph.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Berlin H.A., Stern E.R., Ng J., Zhang S., Rosenthal D., Turetzky R.…Goodman W. Altered olfactory processing and increased insula activity in patients with obsessive-compulsive disorder: an fMRI study. Psychiatry Res. 2017;262:15–24. doi: 10.1016/j.pscychresns.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S., Keane T.M. The development of a clinician-administered PTSD scale. J. Trauma. Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bornschein S., Hausteiner C., Zilker T., Forstl H. Psychiatric and somatic disorders and multiple chemical sensitivity (MCS) in 264 ‘environmental patients’. Psychol. Med. 2002;32(8):1387–1394. doi: 10.1017/s0033291702006554. [DOI] [PubMed] [Google Scholar]
- Buron E., Bulbena A., Bulbena-Cabre A. Olfactory functioning in panic disorder. J. Affect. Disord. 2015;175:292–298. doi: 10.1016/j.jad.2015.01.049. [DOI] [PubMed] [Google Scholar]
- Cain W.S. Olfaction and the common chemical sense: some psychophysical contrasts. Sens Processes. 1976;1(1):57–67. [PubMed] [Google Scholar]
- Chu S., Downes J.J. Proust nose best: odors are better cues of autobiographical memory. Mem. Cogn. 2002;30(4):511–518. doi: 10.3758/bf03194952. [DOI] [PubMed] [Google Scholar]
- Cortese B.M., McConnell P.A., Froeliger B., Leslie K., Uhde T.W. Burning odor-elicited anxiety in OEF/OIF combat veterans: inverse relationship to gray matter volume in olfactory cortex. J. Psychiatr. Res. 2015;70:58–66. doi: 10.1016/j.jpsychires.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese B.M., Leslie K., Uhde T.W. Differential odor sensitivity in PTSD: implications for treatment and future research. J. Affect. Disord. 2015;179:23–30. doi: 10.1016/j.jad.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I., Schellong J., Joraschky P., Hummel T. PTSD, but not childhood maltreatment, modifies responses to unpleasant odors. Int. J. Psychophysiol. 2010;75(3):326–331. doi: 10.1016/j.ijpsycho.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Dileo J.F., Brewer W.J., Hopwood M., Anderson V., Creamer M. Olfactory identification dysfunction, aggression and impulsivity in war veterans with post-traumatic stress disorder. Psychol. Med. 2008;38(4):523–531. doi: 10.1017/S0033291707001456. [DOI] [PubMed] [Google Scholar]
- Doty R.L. Olfaction and multiple chemical sensitivity. Toxicol. Ind. Health. 1994;10(4–5):359–368. [PubMed] [Google Scholar]
- Doty R.L. 2nd Edition. Sensonics, Inc.; Haddon Heights, NJ: 2009. The Smell Threshold Test Administration Manual. [Google Scholar]
- Doty R.L., Brugger W.E., Jurs P.C., Orndorff M.A., Snyder P.J., Lowry L.D. Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol. Behav. 1978;20(2):175–185. doi: 10.1016/0031-9384(78)90070-7. [DOI] [PubMed] [Google Scholar]
- Doty R.L., Shaman P., Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol. Behav. 1984;32(3):489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenger M.M. Cognitive deficits in obsessive-compulsive disorder on tests of frontal lobe functions. Nord. J. Psychiatry. 2005;59(1):39–44. doi: 10.1080/08039480510018814. [DOI] [PubMed] [Google Scholar]
- Frasnelli J., Lague-Beauvais M., LeBlanc J., Alturki A.Y., Champoux M.C., Couturier C.…de Guise E. Olfactory function in acute traumatic brain injury. Clin. Neurol. Neurosurg. 2016;140:68–72. doi: 10.1016/j.clineuro.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Goldberg E.D., Goldberg R.J., Vannoppen B. Sense of smell and obsessional behavior. Am. J. Psychiatry. 1991;148(12):1757. doi: 10.1176/ajp.148.12.1757a. [DOI] [PubMed] [Google Scholar]
- Hermesh H. Orbitofrontal cortex dysfunction in obsessive-compulsive disorder? II. Olfactory quality discrimination in obsessive-compulsive disorder. Eur. Neuropsychopharmacol. 1999;9(5):415–420. doi: 10.1016/s0924-977x(99)00018-8. [DOI] [PubMed] [Google Scholar]
- Hinton D.E., Pich V., Chhean D., Pollack M.H., Barlow D.H. Olfactory-triggered panic attacks among Cambodian refugees attending a psychiatric clinic. Gen. Hosp. Psychiatry. 2004;26(5):390–397. doi: 10.1016/j.genhosppsych.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Hummel T., Livermore A. Intranasal chemosensory function of the trigeminal nerve and aspects of its relation to olfaction. Int. Arch. Occup. Environ. Health. 2002;75(5):305–313. doi: 10.1007/s00420-002-0315-7. [DOI] [PubMed] [Google Scholar]
- Hummel T., Livermore A., Hummel C., Kobal G. Chemosensory event-related potentials in man: relation to olfactory and painful sensations elicited by nicotine. Electroencephalogr. Clin. Neurophysiol. 1992;84(2):192–195. doi: 10.1016/0168-5597(92)90025-7. [DOI] [PubMed] [Google Scholar]
- Hummel T., Roscher S., Jaumann M.P., Kobal G. Intranasal chemoreception in patients with multiple chemical sensitivities: a double-blind investigation. Regul. Toxicol. Pharmacol. 1996;24(1 Pt 2):S79–86. doi: 10.1006/rtph.1996.0082. [DOI] [PubMed] [Google Scholar]
- Jacquot L., Monnin J., Brand G. Influence of nasal trigeminal stimuli on olfactory sensitivity. C. R. Biol. 2004;327(4):305–311. doi: 10.1016/j.crvi.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Jones S.V., Choi D.C., Davis M., Ressler K.J. Learning-dependent structural plasticity in the adult olfactory pathway. J. Neurosci. 2008;28(49):13106–13111. doi: 10.1523/JNEUROSCI.4465-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass M.D., Rosenthal M.C., Pottackal J., McGann J.P. Fear learning enhances neural responses to threat-predictive sensory stimuli. Science. 2013;342(6164):1389–1392. doi: 10.1126/science.1244916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katerndahl D.A., Bell I.R., Palmer R.F., Miller C.S. Chemical intolerance in primary care settings: prevalence, comorbidity, and outcomes. Ann. Fam. Med. 2012;10(4):357–365. doi: 10.1370/afm.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane T., Fairbank J., Caddell J., Zimering R., Taylor K., Mora C. Clinical evaluation of a measure to assess combat exposure. Psychol. Assess. 1989;1:53–55. [Google Scholar]
- Khan R.M., Luk C.H., Flinker A., Aggarwal A., Lapid H., Haddad R., Sobel N. Predicting odor pleasantness from odorant structure: pleasantness as a reflection of the physical world. J. Neurosci. 2007;27(37):10015–10023. doi: 10.1523/JNEUROSCI.1158-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline N.A., Rausch J.L. Olfactory precipitants of flashbacks in posttraumatic stress disorder: case reports. J. Clin. Psychiatry. 1985;46(9):383–384. [PubMed] [Google Scholar]
- Kobal G., Hummel C. Cerebral chemosensory evoked potentials elicited by chemical stimulation of the human olfactory and respiratory nasal mucosa. Electroencephalogr. Clin. Neurophysiol. 1988;71(4):241–250. doi: 10.1016/0168-5597(88)90023-8. [DOI] [PubMed] [Google Scholar]
- Kobal G., Hummel T. Olfactory evoked potential activity and hedonics. In: van Toller S., Dodd G.H., editors. Fragrance—The Psychology and Biology of Perfume. Elsevier Applied Science; London: 1992. [Google Scholar]
- Kopala L.C., Good K.P. Olfactory identification ability in patients with panic disorder. J. Psychiatry Neurosci. 1996;21(5):340–342. [PMC free article] [PubMed] [Google Scholar]
- Krusemark E.A., Li W. Enhanced olfactory sensory perception of threat in anxiety: an event-related fMRI study. Chemosens. Percept. 2012;5(1):37–45. doi: 10.1007/s12078-011-9111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Buissonniere-Ariza V., Lepore F., Kojok K.M., Frasnelli J. Increased odor detection speed in highly anxious healthy adults. Chem. Senses. 2013;38(7):577–584. doi: 10.1093/chemse/bjt028. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Rethinking the Emotional Brain. Neuron. 2012;73(4):653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Howard J.D., Parrish T.B., Gottfried J.A. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science. 2008;319(5871):1842–1845. doi: 10.1126/science.1152837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locatelli M. EEG power modifications in obsessive-compulsive disorder during olfactory stimulation. Biol. Psychiatry. 1996;39(5):326–331. doi: 10.1016/0006-3223(95)00172-7. [DOI] [PubMed] [Google Scholar]
- Martzke J.S., Kopala L.C., Good K.P. Olfactory dysfunction in neuropsychiatric disorders: review and methodological considerations. Biol. Psychiatry. 1997;42(8):721–732. doi: 10.1016/s0006-3223(96)00442-8. [DOI] [PubMed] [Google Scholar]
- McTeague L.M., Lang P.J. The anxiety spectrum and the reflex physiology of defense: from circumscribed fear to broad distress. Depress Anxiety. 2012;29(4):264–281. doi: 10.1002/da.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague L.M., Lang P.J., Laplante M.C., Cuthbert B.N., Shumen J.R., Bradley M.M. Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity. Biol. Psychiatry. 2010;67(4):346–356. doi: 10.1016/j.biopsych.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague L.M., Lang P.J., Laplante M.C., Bradley M.M. Aversive imagery in panic disorder: agoraphobia severity, comorbidity, and defensive physiology. Biol. Psychiatry. 2011;70(5):415–424. doi: 10.1016/j.biopsych.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moessnang C., Pauly K., Kellermann T., Kramer J., Finkelmeyer A., Hummel T.…Habel U. The scent of salience—is there olfactory-trigeminal conditioning in humans? NeuroImage. 2013;77:93–104. doi: 10.1016/j.neuroimage.2013.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell P.V., Uhde T.W. Dose-response effects of intravenous caffeine in normal volunteers. Anxiety. 1994;1(4):161–168. doi: 10.1002/anxi.3070010403. [DOI] [PubMed] [Google Scholar]
- Pause B.M., Adolph D., Prehn-Kristensen A., Ferstl R. Startle response potentiation to chemosensory anxiety signals in socially anxious individuals. Int. J. Psychophysiol. 2009;74(2):88–92. doi: 10.1016/j.ijpsycho.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Price J.L. Olfactory system. In: P. G, editor. The Human Nervous System. Academic Press; San Diego, CA: 1990. pp. 979–998. [Google Scholar]
- Resnick H.S., Falsetti S.A., Kilpatrick D.G., Freedy J.R. Assessment of Rape and Other Civilian Trauma-related Post-traumatic Stress Disorder: Emphasis on Assessment of Potentially Traumatic Events. In: Miller T.W., editor. Stressful Life Events. International Universities Press; Madison: 1996. pp. 231–266. [Google Scholar]
- Savic I. Imaging of brain activation by odorants in humans. Curr. Opin. Neurobiol. 2002;12(4):455–461. doi: 10.1016/s0959-4388(02)00346-x. [DOI] [PubMed] [Google Scholar]
- Schecklmann M., Schwenck C., Taurines R., Freitag C., Warnke A., Gerlach M., Romanos M. A systematic review on olfaction in child and adolescent psychiatric disorders. J Neural Transm (Vienna) 2013;120(1):121–130. doi: 10.1007/s00702-012-0855-2. [DOI] [PubMed] [Google Scholar]
- Segalas C. Olfactory identification and discrimination in obsessive-compulsive disorder. Depress. Anxiety. 2011;28(10):932–940. doi: 10.1002/da.20836. [DOI] [PubMed] [Google Scholar]
- Seubert J., Freiherr J., Djordjevic J., Lundstrom J.N. Statistical localization of human olfactory cortex. NeuroImage. 2013;66:333–342. doi: 10.1016/j.neuroimage.2012.10.030. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl. 20):22–33. (quiz 34-57) [PubMed] [Google Scholar]
- Takahashi T., Itoh H., Nishikawa Y., Higuchi Y., Nakamura M., Sasabayashi D.…Suzuki M. Possible relation between olfaction and anxiety in healthy subjects. Psychiatry Clin. Neurosci. 2015;69(7):431–438. doi: 10.1111/pcn.12277. [DOI] [PubMed] [Google Scholar]
- Tobia M.J., Yang Q.X., Karunanayaka P. Intrinsic intranasal chemosensory brain networks shown by resting-state functional MRI. Neuroreport. 2016;27(7):527–531. doi: 10.1097/WNR.0000000000000579. [DOI] [PubMed] [Google Scholar]
- Vasterling J.J., Brailey K., Sutker P.B. Olfactory identification in combat-related posttraumatic stress disorder. J. Trauma. Stress. 2000;13(2):241–253. doi: 10.1023/A:1007754611030. [DOI] [PubMed] [Google Scholar]
- Vermetten E., Bremner J.D. Olfaction as a traumatic reminder in posttraumatic stress disorder: case reports and review. J. Clin. Psychiatry. 2003;64(2):202–207. doi: 10.4088/jcp.v64n0214. [DOI] [PubMed] [Google Scholar]
- Vermetten E., Schmahl C., Southwick S.M., Bremner J.D. Positron tomographic emission study of olfactory induced emotional recall in veterans with and without combat-related posttraumatic stress disorder. Psychopharmacol. Bull. 2007;40(1):8–30. [PMC free article] [PubMed] [Google Scholar]
- Wintermann G.B., Donix M., Joraschky P., Gerber J., Petrowski K. Altered olfactory processing of stress-related body odors and artificial odors in patients with panic disorder. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0074655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xydakis M.S., Mulligan L.P., Smith A.B., Olsen C.H., Lyon D.M., Belluscio L. Olfactory impairment and traumatic brain injury in blast-injured combat troops: a cohort study. Neurology. 2015;84(15):1559–1567. doi: 10.1212/WNL.0000000000001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald D.H., Pardo J.V. Functional neuroimaging of the olfactory system in humans. Int. J. Psychophysiol. 2000;36(2):165–181. doi: 10.1016/s0167-8760(99)00110-5. [DOI] [PubMed] [Google Scholar]
- Zatorre R.J., Jones-Gotman M., Rouby C. Neural mechanisms involved in odor pleasantness and intensity judgments. Neuroreport. 2000;11(12):2711–2716. doi: 10.1097/00001756-200008210-00021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Group difference in BR-elicited brain activation.
Inverse relationship between TST and BR-elicited brain activation in healthy veterans.
Positive relationship between TST and BR-elicited brain activation in veterans with PTSD.