Abstract
Background and Objectives:
There is a lack of consensus on the optimal repair technique and the definition of good outcomes in paraesophageal hernia (PEH) repair. We reviewed long-term patient-reported outcomes of open and laparoscopic PEH repair to assist with our future surgical consent process.
Methods:
This was a retrospective case–control study including all patients with PEH repair performed from 2000 through 2012 at a single center without the use of mesh. We mailed questionnaires to patients to assess reoperation, symptom control, and satisfaction.
Results:
Chart review identified 217 patients who underwent PEH repair. Nineteen died during the follow-up period. Of the 106 returning the questionnaire, 87 underwent laparoscopic repair, and 19 had open repair, with follow-up of 6.6 (SD 3.9) years and 7.0 (SD 4.1) years, respectively. Reoperation rates were 9.9% and 5.3%, respectively (P = .720). Dysphagia, heartburn, and regurgitation improved in 95.4% of patients after laparoscopic repair and 89.5% after open repair (P = .318). Medication for symptom control was necessary in 54.0% of patients after laparoscopic repair and 26.3% after open repair (P = .029). In each group, 90% stated that they would still choose to have the operation (P = .713).
Conclusions:
Long-term patient-specific outcomes showed comparable, encouraging results between open and laparoscopic repair of PEH without mesh reinforcement. However, half of those undergoing laparoscopic repair required the use of medication for symptom control. This study adds to the literature describing long-term patient-specific outcomes and can be useful when counseling patients about PEH repair.
Keywords: Cruroplasty, Hiatal hernia, Laparoscopic repair, Mesh repair, Paraesophageal hernia
INTRODUCTION
Even though recurrence after PEH repair is common, reoperation is much less common. When obtaining consent from patients for surgery, it is important to describe long-term patient-specific outcomes, such as medication use, symptoms, and reoperation rate. Several reasons account for the paucity of long-term patient-specific outcomes in the literature. There are controversies regarding the optimal technique, as well as a lack of a standardized definition of a good outcome. Historically, repair has been performed via a left thoracotomy or a laparotomy to allow for direct visualization of the esophagus and hernia, with mobilization of the esophagus, repair of the hiatal defect, and an antireflux procedure (either a Belsey repair or Nissen fundoplication).1 Occasionally, a Collis gastroplasty was performed if shortening of the esophagus was encountered.2 The development of minimally invasive techniques to repair PEH resulted in a decrease in postoperative length of stay, a shorter recovery time, and fewer short-term complications, leading to a widespread adoption of this approach.3 In some studies, long-term outcomes of laparoscopic repair are comparable to those of open repair, but recurrence rates as high as 30% after laparoscopic repair continue to be a vexing problem.1,4–11 In a meta-analysis, Müller-Stich et al12 found a pooled rate of 20.5%, based on radiographic, endoscopic, and clinically significant recurrence requiring medication or reoperation. One could argue, however, that medication use and reoperation are significantly different outcomes from a patient's perspective, especially considering that asymptomatic or minimally symptomatic recurrences do not require any treatment. To decrease recurrence rates, many surgeons have adopted mesh reinforcement of the crura. Some studies report a decreased recurrence rate with mesh repair, but others find no difference at long-term follow-up.13–17 Some investigators use diaphragm-relaxing incisions, whereas others do not find it necessary. Some surgeons perform partial wraps versus 360° wraps, depending on manometry findings, whereas others do not.18,19 Finally, several investigators report the need for esophagus-lengthening procedures, but others feel that adequate hiatal mobilization obviates this need.20,21
Because of the controversy regarding the ideal technique for PEH repair and the complexity of evaluating outcomes, long-term patient-specific data are needed. The purpose of this study was to evaluate long-term outcomes, including control of symptoms, patient satisfaction, and the incidence of reoperation for both laparoscopic and open PEH repair at a single tertiary referral center using a standardized approach. We routinely perform a 360° wrap without the use of mesh or esophageal lengthening.
METHODS
This is a retrospective review of hospital records of all patients who underwent PEH repair from 2000 through 2012. There were 227 patients who had a primary surgical repair of a PEH during this period performed by 10 surgeons. Only 10 patients had mesh placed for an attenuated and capacious hiatus at the discretion of the surgeon, and 217 had primary repair of the crura without mesh reinforcement; the latter group constitutes our study group, and this primary repair method is our preferred way of repairing the hiatus. Because the number of patients who received mesh was small, those patients were excluded from the study. All patients had undergone a standard preoperative workup, including a detailed medical and surgical history, physical examination, and relevant laboratory or imaging tests on a case-by-case basis. The patients received primary crura repair, via either a laparoscopic or open approach as part of their procedure, both of which routinely included a 360° wrap. All laparoscopic surgeons used the following approach: the laparoscopic procedure is performed with the patient positioned supine, with modified lithotomy with the surgeon working between the legs. After the hernia sac is reduced, upper short gastric vessels are divided, and the esophagus is retracted with a Penrose drain. We take care to dissect up to the right pulmonary artery if possible, to allow for true 3-cm intra-abdominal esophagus length without tension. We do not find it necessary to use an esophagus-lengthening procedure with adequate hiatal mobilization. To accurately identify the GE junction we remove the left side of the hernia sack while avoiding the anterior Vagus nerve on the patient's right side. We then close the crura primarily with interrupted, braided, nonabsorbable sutures. We size the closure and wrap over a 54–60-French bougie. We rarely use mesh to bridge the defect, and those few patients in whom mesh was used were excluded from this analysis. We do not cover the repair with mesh. We perform a 3-stitch, 360° wrap of the uppermost fundus measuring 2.5 cm total, taking esophageal bites with the upper 2 stitches.
For comparison, a brief description of the open procedure at our center is as follows. The procedure is performed through an upper midline laparotomy incision. The stomach and contents of the hernia are reduced into the abdomen, and the hernia sac is excised. The esophagus is mobilized through the hiatus, to reduce a minimum of 4 cm of esophagus below the diaphragm without tension. The crura are reapproximated with horizontal mattress sutures reinforced with felt pledgets behind the esophagus with a no. 50 Maloney dilator in the esophagus to avoid too tight a closure. A Nissen fundoplication is performed with construction of a 3-cm wrap.
The choice of laparoscopic versus open repair was made in accordance with the surgeon's preference. At our center, 1 surgeon routinely performed open repair while the remaining 9 surgeons performed laparoscopic repair with very few exceptions in patients with anatomic or physiologic contraindications to laparoscopy.
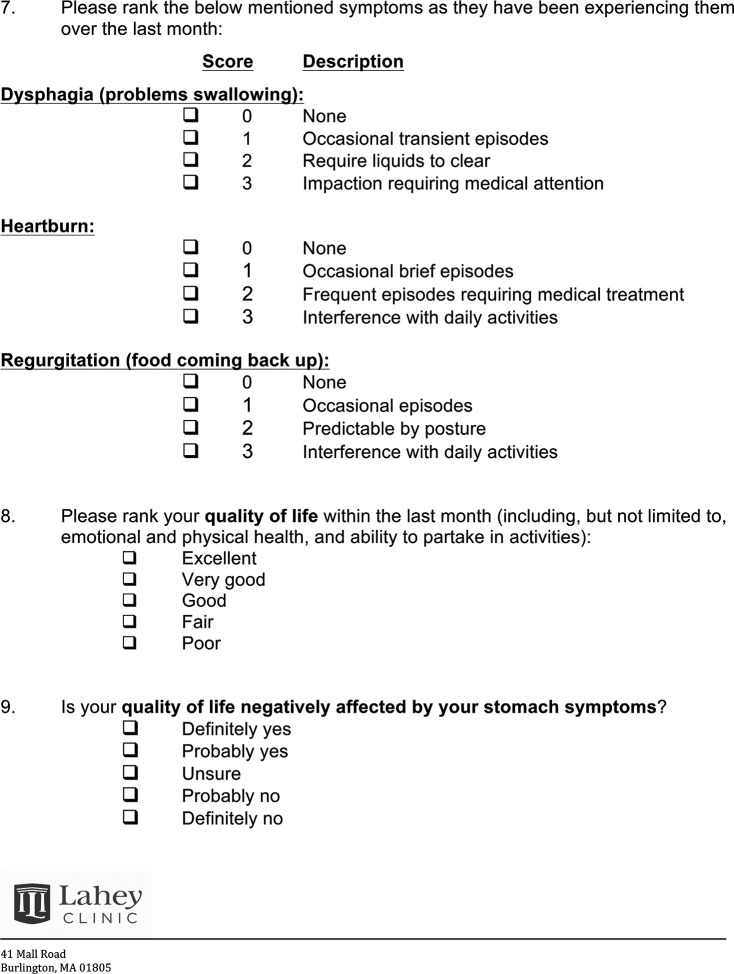
Between May 2013 and December 2015, patients were sent a questionnaire designed to assess symptoms, the need for medication for symptom control, occurrence of reoperation, whether patients would choose to have the operation again in retrospect, and quality-of-life scores on a 5-point Likert scale along with the impact of symptoms on quality of life (see the Appendix). The questionnaire was adopted from examples in the literature and modified by the authors to address the research question in the shortest amount of time, to increase the response rate. This survey contained questions in the 4 primary domains defined by Bolier et al22 in their systematic review of surveys assessing gastrointestinal esophageal reflux disease (GERD) treatment—namely, symptoms, response to treatment, diagnosis, and burden on the quality of life of patients with GERD.22 The questionnaires were mailed to patients via the U.S. Postal Service at each patient's last known address, along with a return-addressed, prepaid envelope. Patients and questionnaires were assigned a study number, and responses were codified and subjected to statistical analysis.
Frequency analysis, Student's t test, Pearson's χ2 test, and nonparametric Kruskal-Wallis test were performed; α was set a priori at P < .05. Analyses were performed with SAS 9.4 with SAS/STAT 13.2 (SAS Institute Inc., Cary, North Carolina, USA).
This is a retrospective review of records from a single center. The Institutional Review Board approved the study, with waiver of individual patient consent.
RESULTS
The authors identified 217 patients who met the criteria, with a mean age of 64.9 (SD 14.8) years (range, 20.1–90.1) at the time of surgery. Of the 217 patients, 19 were identified as deceased. Of the remaining 198 patients, 106 (54%) returned completed questionnaires. Respondents' mean age at the time of surgery was 66.6 (SD 12.0) years (range, 24.3–90.1). Eighty-seven of the respondents had laparoscopic repairs and 19 had open repairs. Mean follow-up was 6.6 years for the patients having laparoscopic repair and 7.0 years for those having an open repair. Indications for surgery were postprandial epigastric pain, heartburn, regurgitation, or dysphagia affecting quality of life. Preoperative symptoms of patients in each repair group can be found in Table 1. There was comparable improvement of dysphagia, heartburn, and regurgitation in 85 of 87 patients (95.4%) in the laparoscopic group and in 17 of 19 (89.5%) in the open group (Table 2). However, more than half of the patients having a laparoscopic repair still required medication for symptom control. There was no statistically significant difference in rate of reoperation. Eight patients (9.9%) of those who had a laparoscopic repair and 1 patient (5.3%) of those who had an open repair required reoperation for symptoms.
Table 1.
Preoperative Symptoms by Repair Group
| Laparoscopic Repair, % (n = 87) | Open Repair, % (n = 19) | |
|---|---|---|
| Epigastric pain | 57.5 (n = 50) | 47.4 (n = 9) |
| Heartburn | 74.7 (n = 65) | 57.9 (n = 11) |
| Regurgitation | 49.4 (n = 43) | 57.9 (n = 11) |
| Dysphagia | 43.7 (n = 38) | 47.4 (n = 9) |
Table 2.
Laparoscopic Versus Open Repair Groups
| Laparoscopic Repair (n = 87) | Open Repair (n = 19) | P | |
|---|---|---|---|
| Mean follow-up time, y | 6.6 y (SD 3.9) | 7.0 (SD 4.1) | |
| Rate of reoperation, % | 9.9 (n = 8) | 5.3 (n = 1) | 0.720 |
| Improvement of dysphagia, heartburn, and regurgitation, % | 95.4 (n = 85) | 89.5 (n = 17) | 0.318 |
| Still requiring medications for symptom control, % | 54.0 (n = 47) | 26.3 (n = 5) | 0.029 |
| Would choose the operation again, % | 90.6 (n = 80) | 89.5 (n = 17) | 0.713 |
Control of specific symptoms of dysphagia, heartburn, and regurgitation was comparable between the 2 groups in the month before they completed the survey, as documented in Table 3. Quality of life in the last month before completion of the questionnaire was reported to be excellent, very good, or good by 87.5% (n = 77) and 100% (n = 19) of respondents in the laparoscopic and open repair groups, respectively. In addition, 65.9% (n = 56) of patients in the laparoscopic repair group and 77.8% (n = 14) of respondents in the open group reported that their quality of life was not affected by symptoms at the time of the survey.
Table 3.
Symptom Control
| Laparoscopic Repair, % (n = 87) | Open Repair, % (n = 19) | |
|---|---|---|
| No or only occasional, transient dysphagia in the last month | 91.7 (n = 77) | 94.7 (n = 18) |
| No or only occasional, transient heartburn in the last month | 90.7 (n = 78) | 94.5 (n = 17) |
| No or only occasional regurgitation in the last month | 97.6 (n = 84) | 100 (n = 18) |
DISCUSSION
In this single-center study, we demonstrated acceptable long-term outcomes of primary PEH repair without the use of mesh. We do not know the indications for the 9 instances of reoperation in our patient base, because 6 of the 9 revision operations were performed at other medical centers, but our reoperation rates of 9.9% for laparoscopic repair and 5.3% for open repair are equivalent to both the 8.0% estimated reoperation rate for laparoscopic repair without mesh reported by Müller-Stich et al12 and the reoperation rate range of 1–7% of open repairs reported by Low and Unger.23 There is a wide range of recurrence rates for surgical repair of PEH in the literature. Although it is possible that the open repair group saw fewer reoperations because patients with a prior open repair are less likely to undergo reoperation, even when they experience symptoms due to the likely increased complexity of a revisional procedure, given that a smaller proportion of patients in the open repair group required continued medication for symptom control, it is unlikely that a higher rate of undocumented hernia recurrence occurred in the open repair group. In addition, we demonstrated good long-term patient satisfaction in both groups, with about 90% of patients being satisfied with their surgery to the point of recommending it to others. However, as previously mentioned, in the laparoscopic group, about half the patients still take medication to control symptoms. Although this is a higher proportion than expected, it represents an improvement for >95% of patients compared to their preoperative symptoms. One could argue that the increased use of medications in this group represents a recurrence of the hernia. However, because their symptoms were improved relative to those experienced before surgery and are well controlled with medication, it is unlikely that these patients would need a second operation, and, more important, they are satisfied with their care.
Repair of PEH is associated with a high anatomical recurrence rate, and it has been thought that the use of mesh to reinforce repair of the crura would reduce this complication.14,24–26 A large survey of >1000 members of the Society of Gastrointestinal and Endoscopic Surgeons conducted by Frantzides et al13 regarding failure of hiatal hernia repair found a retrospectively reported recurrence rate of 3.17%. This rate is considerably lower than that historically reported for primary repair without mesh. A prospective randomized trial by Frantzides et al14 of 72 patients found that those treated with mesh repair had no recurrences compared with a 22% recurrence rate in those repaired without mesh at a mean follow-up of 3 years. Recurrences were identified via esophagogastroduodenoscopy and esophagogram at 3 and 6 months after surgery and followed by esophagograms at 6-month intervals thereafter. A multicenter trial conducted by Oelschlager et al15 corroborated the findings by Frantzides et al at 6-month follow-up.
However, with a longer follow-up at 58 months, Oelschlager et al16 found no statistically significant difference in hernia recurrence, complication rates, side effects, or quality of life between patients undergoing a primary or mesh repair. Hernia recurrence was documented by an upper gastrointestinal series read by 2 radiologists, while blinded to patients' reported clinical symptoms and quality of life scores. In addition, Asti et al17 conducted a retrospective cohort study that found no statistically significant difference in recurrence rates between patients undergoing a mesh or primary repair at the 5-year follow-up. Recurrence in their series was determined by an annual upper endoscopy and defined as at least 2 vertical centimeters of stomach above the level of the diaphragm. In another prospective randomized clinical trial, Watson et al27 found no significant difference in recurrence or clinical outcomes between the 2 groups at 12-month follow-up, as determined by both barium swallow and upper endoscopy. In a recent systematic review, Tam et al28 concluded that there was no significant difference in rate of reoperation, whether mesh was used or not. They concluded that the quality of evidence supporting routine use of mesh cruroplasty is low and that mesh should be used at the surgeon's discretion until additional studies evaluating symptomatic outcomes, quality of life, and long-term recurrence are available. A decision analysis study performed by Obeid and Velanovich29 found the difference between the 2 approaches to be small and clinically inconsequential. They concluded that, as there is no compelling clinical evidence to support one approach over the other, cost may be a significant driver of the decision.
The strengths of our study include the relatively large number of patients, long-term follow up, the comparison of long-term outcomes between open and laparoscopic PEH repair, and that we used a standardized technique. This study is not without its limitations, however.
The self-reported outcomes create many opportunities for response bias, which could have influenced our findings in several ways. For example, it is possible that the results were affected by positive-response bias, in which respondents who had favorable outcomes were more likely to respond to the survey. However, as it is equally likely that respondents with poor outcomes chose to respond to the study, there is no way to know in which direction the results would be affected other than to acknowledge uncertainty in our findings. There is also the possibility that our results reflected survivorship bias, in that only patients who were still alive could respond to the survey. Given the relatively long mean follow-up time and the fact that many of the subjects were in their 7th and 8th decades at the time of surgery, it is possible that a significant portion of respondents were deceased upon follow-up, particularly those who experienced severe complications from surgery or comorbid conditions. Overall, our results could have been affected in a significant way by response bias.
In addition, the response rate of 55% of living respondents at first glance appears low. However, it must be noted that the expected response rate for a mailed survey in clinical research has been reported at 60 to 61%.30 In light of this information, our response rate should be viewed as only slightly below the anticipated rate. We hypothesize that this is, in large part, because of the long-term follow-up; many of the subjects had not had contact with our health system in years, may have been deceased, or may simply have had outdated mailing addresses in our records.
Furthermore, unlike other studies, this study did not include preoperative manometric tests or use endoscopic or barium investigations to document hernia recurrence at predetermined postoperative intervals. It should be noted that manometric studies were not routinely performed before surgery, because Toupet fundoplication was not performed. Because it has not been definitively demonstrated that a Toupet fundoplication leads to decreased dysphagia or better postoperative outcomes, compared with a Nissen fundoplication,22 we do not routinely perform Toupet fundoplication, and manometric studies do not change our repair approach in PEH. We certainly perform manometry when evaluating patients for antireflux surgery. Regarding the lack of radiographic follow-up, it must be noted that patients were enrolled in this studied retrospectively and were not observed out to 1 year to determine radiographic or endoscopic recurrence outcomes. Instead, we chose to focus on the patient-oriented quality-of-life outcomes reported by the patients at long-term follow-up so as to glean practical information useful in discussion with future patients regarding outcomes they might experience. Therefore, the last direct postoperative follow-up before the study questionnaire was unique to each case and varied by surgeon and patient preference.
In addition, because of the retrospective nature of the follow-up in this case, we do not have information regarding why patients were taking acid-suppression medication at the time of follow-up. We hypothesize that the statistically significant increase in patients who required acid-suppression medication from the laparoscopic repair group might be because of hernia recurrence, though this has not been radiologically or endoscopically confirmed, as mentioned earlier. However, regardless of the reason for the continued acid-suppression therapy, we have chosen to focus on how patients perceive their lives and symptoms. To this end, we can point out that the group of patients who still required acid suppression showed no statistically significant difference in how symptoms affected their current quality of life or in their preference to choose the operation again in retrospect as compared with the group that was not taking acid-suppression medication at follow-up.
Last, we did not report on the size or type of PEH, which likely affects recurrence, but we found that, in nearly all (96%) of our patients, we were able to bring the crura together primarily without the use of mesh.
Despite these limitations, this study represents a significant contribution to understanding how long-term outcomes of PEH repair without the use of mesh are perceived by patients.
In this study, we demonstrated that laparoscopic and open PEH repair without mesh can lead to favorable long-term outcomes in most patients, including low but clinically significant rates of reoperation (5–10%), high satisfaction, and good symptom control, although half of those undergoing laparoscopic repair required medication to achieve it. This study adds to the sparse literature describing long-term patient-specific outcomes that can prove useful when counseling patients regarding PEH repair. Additional reports with long-term follow-up of patient-specific outcomes of other surgical approaches (such as the use of mesh or esophageal lengthening) are warranted to further elucidate and clarify the findings of this study.
Appendix: Questionnaire Sent to Study Participants
Contributor Information
Damien J. Lazar, Tufts University School of Medicine, Boston, Massachusetts..
Desmond H. Birkett, Department of General Surgery.
David M. Brams, Department of General Surgery.
Heather A. Ford, Department of General Surgery.
Christina Williamson, Department of Cardiovascular and Thoracic Surgery, Lahey Hospital and Medical Center, Burlington, Massachusetts..
Dmitry Nepomnayshy, Department of General Surgery.
References:
- 1. Andolfi C, Jalilvand A, Plana A, Fisichella PM. Surgical treatment of paraesophageal hernias: a review. J Laparoendosc Adv Surg Tech. 2016; 26:778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Swanstrom LL, Marcus DR, Galloway GQ. Laparoscopic Collis gastroplasty is the treatment of choice for the shortened esophagus. Am J Surg 1996;171:477–481. [DOI] [PubMed] [Google Scholar]
- 3. Fisichella PM, Patti MG. Laparoscopic repair of paraesophageal hiatal hernias. J Laparoendosc Adv Surg Tech A. 2008;18:629–632. [DOI] [PubMed] [Google Scholar]
- 4. Fullum TM, Oyetunji TA, Ortega G, et al. Open versus laparoscopic hiatal hernia repair. JSLS. 2013;17:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patti MG, Fisichella PM. Laparoscopic paraesophageal hernia repair: how I do it. J Gastrointest Surg. 2009;13:1728–1732. [DOI] [PubMed] [Google Scholar]
- 6. Willekes CL, Edoga JK, Frezza EE. Laparoscopic repair of paraesophageal hernia. Ann Surg. 1997;225:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dallemagne B, Kohnen L, Perretta S, Weerts J, Markiewicz S, Jehaes C. Laparoscopic repair of paraesophageal hernia: long-term follow-up reveals good clinical outcome despite high radiological recurrence rate. Ann Surg. 2011;253:291–296. [DOI] [PubMed] [Google Scholar]
- 8. Latzko M, Borao F, Squillaro A, Mansson J, Barker W, Baker T. Laparoscopic repair of paraesophageal hernias. JSLS 2014;Jul-Sep;18(3):e2014.00009 DOI: 10.4293/JSLS.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zaman JA, Lidor AO. The optimal approach to symptomatic paraesophageal hernia repair: important technical considerations. Curr Gastroenterol Rep. 2016;18:53. [DOI] [PubMed] [Google Scholar]
- 10. Pitcher DE, Curet MJ, Martin DT, Vogt DM, Mason J, Zucker JA. Successful laparoscopic repair of paraesophageal hernia. Arch Surg. 1995;130:590–596. [DOI] [PubMed] [Google Scholar]
- 11. Aly A, Munt J, Jamieson GG, Ludemann R, Devitt PG, Watson DI. Laparoscopic repair of large hiatal hernias. Br J Surg. 2005;92:648–653. [DOI] [PubMed] [Google Scholar]
- 12. Müller-Stich BP, Kenngott HG, Gondan M, et al. Use of mesh in laparoscopic paraesophageal hernia repair: a meta-analysis and risk-benefit analysis. PLoS One. 2015;10:e0139547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frantzides CT, Carlson MA, Loizides S, et al. Hiatal hernia repair with mesh: a survey of SAGES members. Surg Endosc. 2010;24:1017–1024. [DOI] [PubMed] [Google Scholar]
- 14. Frantzides CT, Madan AK, Carlson MA, Stavropoulos GP. A prospective, randomized trial of laparoscopic polytetrafluoroethylene (PTFE) patch repair vs simple cruroplasty for large hiatal hernia. Arch Surg. 2002;137:649–652. [DOI] [PubMed] [Google Scholar]
- 15. Oelschlager BK, Pellegrini CA, Hunter J, et al. Biologic prosthesis reduces recurrence after laparoscopic paraesophageal hernia repair: a multicenter, prospective, randomized trial. Ann Surg 2006;244:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oelschlager BK, Pellegrini CA, Hunter JG, et al. Biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg. 2011;213:461–468. [DOI] [PubMed] [Google Scholar]
- 17. Asti E, Lovece A, Bonavina L, et al. Laparoscopic management of large hiatus hernia: five-year cohort study and comparison of mesh-augmented versus standard crura repair. Surg Endosc. 2016;30:5404–5409. [DOI] [PubMed] [Google Scholar]
- 18. Casabella F, Sinanan M, Horgan S, Pellegrini CA. Systematic use of gastric fundoplication in laparoscopic repair of paraesophageal hernias. Am J Surg. 1996;171:485–489. [DOI] [PubMed] [Google Scholar]
- 19. Ellis FH., Jr The Nissen fundoplication. Ann Thorac Surg. 1992;54:1231–1235 [DOI] [PubMed] [Google Scholar]
- 20. O'Rourke RW, Khajanchee YS, Urbach DR, et al. Extended transmediastinal dissection: an alternative to gastroplasty for short esophagus. Arch Surg. 2003;138:735–740. [DOI] [PubMed] [Google Scholar]
- 21. Horvath KD, Swanstrom LL, Jobe BA. The short esophagus: pathophysiology, incidence, presentation, and treatment in the era of laparoscopic antireflux surgery. Ann Surg. 2000;232:630–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bolier EA, Kessing BF, Smout AJ, Bredenoord AJ. Systematic review: questionnaires for assessment of gastroesophageal reflux disease. Dis Esophagus. 2015;28:105–120. [DOI] [PubMed] [Google Scholar]
- 23. Low DE, Unger T. Open repair of paraesophageal hernia: reassessment of subjective and objective outcomes. Ann Thorac Surg. 2005;80:287–294. [DOI] [PubMed] [Google Scholar]
- 24. Carlson MA, Condon RE, Ludwing KA, Schulte WJ. Management of intrathoracic stomach with polypropylene mesh prosthesis reinforced transabdominal hiatus hernia repair. J Am Coll Surg. 1998;187:227–230. [DOI] [PubMed] [Google Scholar]
- 25. Granderath FA, Schweiger UM, Kamolz T, Asche KU, Pointner R. Laparoscopic Nissen fundoplication with prosthetic hiatal closure reduces postoperative intrathoracic wrap herniation: preliminary results of a prospective randomized functional and clinical study. Arch Surg. 2005;140:40–48. [DOI] [PubMed] [Google Scholar]
- 26. Zehetner J, Demeester SR, Ayazi S, et al. Laparoscopic versus open repair of paraesophageal hernia: the second decade. J Am Coll Surg. 2011;212:813–820. [DOI] [PubMed] [Google Scholar]
- 27. Watson DI, Thompson SK, Devitt PG, et al. Laparoscopic repair of very large hiatus hernia with sutures versus absorbable mesh versus nonabsorbable mesh: a randomized controlled trial. Ann Surg 2015;261:282–289. [DOI] [PubMed] [Google Scholar]
- 28. Tam V, Winger DG, Nason KS. A systematic review and meta-analysis of mesh vs suture cruroplasty in laparoscopic large hiatal hernia repair. Am J Surg. 2016;211:226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Obeid NM, Velanovich V. The choice of primary repair or mesh repair for paraesophageal hernia a decision analysis based on utility scores. Ann Surg. 2013;257:655–664. [DOI] [PubMed] [Google Scholar]
- 30. Cummings SM, Savitz LA, Conrad TR. Reported response rates to mailed physician questionnaires. Health Serv Res. 2001;35:1347–1355. [PMC free article] [PubMed] [Google Scholar]