Abstract
Triglyceride (TG) accumulation causes macrophage cell death, which affects the development of atherosclerosis. Here, we examined whether caspase-2 is implicated in TG-induced macrophage cell death. We found that caspase-2 activity is increased in TG-treated THP-1 macrophages, and that inhibition of caspase-2 activity drastically inhibits TG-induced cell death. We previously reported that TG-induced macrophage cell death is triggered by caspase-1, and thus investigated the relationship between caspase-2 and caspase-1 in TG-induced macrophage cell death. Inhibition of caspase-2 activity decreased caspase-1 activity in TG-treated macrophages. However, caspase-1 inhibition did not affect caspase-2 activity, suggesting that caspase-2 is upstream of caspase-1. Furthermore, we found that TG induces activation of caspase-3, -7, -8, and -9, as well as cleavage of PARP. Inhibition of caspase-2 and -1 decreased TG-induced caspase-3, -7, -8, and -9 activation and PARP cleavage. Taken together, these results suggest that TG-induced macrophage cell death is mediated via the caspase-2/caspase-1/apoptotic caspases/PARP pathways.
Keywords: Apoptotic caspases, Caspase-1, Caspase-2, THP-1 macrophages, Triglyceride (TG)
INTRODUCTION
Triglycerides (TGs) are a type of lipid that can cause inflammation and lead to chronic vascular disease (1). The uptake of TGs by macrophages is an important contributing factor during the progression of inflammatory vascular diseases (2). TGs have been proposed to induce foam cell formation, resulting in increased programmed cell death (PCD) of macrophages, and inflammatory reactions in vascular diseases (3, 4). The process of PCD, which includes apoptosis and pyroptosis, is generally characterized by distinct morphological characteristics and various biochemical mechanisms.
Apoptosis involves the activation of a group of cysteine proteases, known as apoptotic caspases, that mediate DNA cleavage. Apoptotic cells are targeted for phagocytosis and removal, resulting in an absence of inflammation and lack of cytokine secretion (5, 6). Pyroptosis is mechanistically distinct from apoptosis, causing inflammatory cell death commonly mediated by the activation of caspase-1 (6, 7). Caspase-1 induces the release of proinflammatory cytokines (IL-1β and IL-18) and rapid plasma-membrane rupture, leading to cell lysis. Several reports have shown that caspases mediating DNA damage during apoptosis also participate in DNA damage during pyroptosis (8, 9).
Classical apoptotic caspases are categorized as either initiator caspases (caspase-8 and caspase-9) or effector caspases (caspase-3 and caspase-7) (10, 11). Effector caspases are cleaved and activated by activated initiator caspases. Activated effector caspases also increase the cleavage of poly ADP ribose polymerase (PARP), a hallmark of apoptotic cell death (10–12). In addition, apoptosis occurs through two main apoptotic pathways: the extrinsic pathway, in which caspase-8 is mainly involved, and the intrinsic (mitochondrial) pathway, in which caspase-9 is mainly activated (5, 12).
Previous studies have shown that intracellular TG accumulation influences mitochondria in order to activate intrinsic pathway-associated apoptotic caspases in macrophages (3, 13). In addition, loading macrophages with TGs has been reported to induce caspase-1-dependent pyroptotic cell death (14). Although caspase-1 has been reported to play a role in TG-triggered macrophage cell death, neither its upstream molecules nor its downstream mediators have been identified in detail. Caspase-1 is involved in the intrinsic and extrinsic apoptotic pathways in a variety of stimuli-induced cell death (12, 15, 16). However, it remains unclear whether caspase-1 is associated with the apoptotic pathway as well as the pyroptotic pathway in TG-induced macrophage cell death.
Caspase-2 is a highly conserved, but poorly understood member of the caspase family (17, 18). Caspase-2 has been implicated in PCD induced by multiple intrinsic and extrinsic stimuli, including DNA damage, reactive oxygen species (ROS), and cytoskeletal disruption (17). However, it is unknown whether caspase-2 is involved in TG-stimulated macrophage cell death. According to recent studies, caspase-2 is associated with activation of caspase-1, as well as interleukin-1β (IL-1β) secretion (6), and the chemical inhibition of caspase-2 decreases the activation of caspase-1 and cell death in Salmonella-infected macrophages (9). Meanwhile, several studies have reported that activated caspase-2 mediates the activation of both caspase-3 and -8, as well as the secretion of tumor necrosis factor-α (TNF-α) in macrophages (6, 19).
In this study, we investigated both the involvement of caspase-2 in TG-induced macrophage cell death, and the association of caspase-2 with caspase-1 and classical apoptotic caspases in TG-treated THP-1 macrophage cells. We report here that caspase-2 mediates TG induced-macrophage cell death by activating caspase-1 and classical apoptotic caspases.
RESULTS
Caspase-2 is implicated in TG-induced THP-1 macrophage cell death
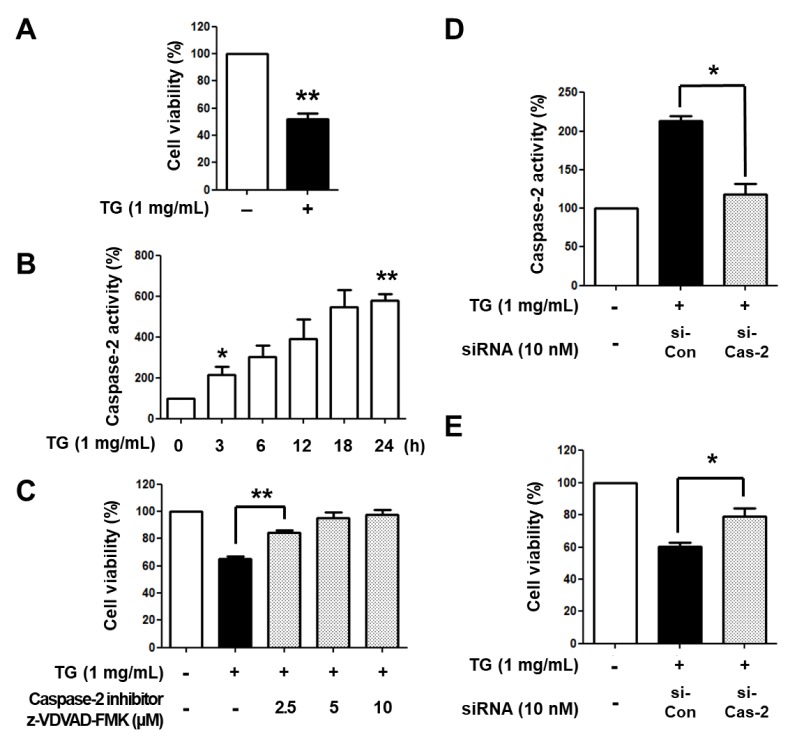
Previous studies have reported that caspase-2 is associated with a variety of stimuli-induced cell death (5, 6, 9), but its involvement in TG-induced macrophage cell death remains unclear. To determine whether caspase-2 is involved in TG-stimulated macrophage cell death, we reconfirmed that TG induces decreased cell viability in THP-1 macrophages. As shown in Fig. 1A, treatment with TG for 24 h reduced cell viability in THP-1 macrophages. Next, we examined the activity of caspase-2 in TG-treated THP-1 macrophages. PMA-differentiated THP-1 cells were treated with TG for different times (0, 3, 6, 12, 18, 24 h) and caspase-2 activity was analyzed. Caspase-2 activity was increased in THP-1 macrophages exposed to TG in a time-dependent manner (Fig. 1B). To elucidate whether the increased caspase-2 activity was responsible for macrophage cell death, THP-1 macrophages were treated with TG in the presence or absence of z-VDVAD-FMK, a caspase-2 specific inhibitor, for 24 h and viable cells were enumerated. Treatment with z-VDVAD-FMK inhibited TG-induced macrophage cell death in an inhibitor dose-dependent manner (Fig. 1C). In addition, when caspase-2 was knocked down by siRNA in THP-1 macrophages, TG-induced macrophage cell death was decreased (Fig. 1D, E). These results suggest that caspase-2 is involved in TG-stimulated macrophage cell death.
Fig. 1.
Caspase-2 is involved in TG-induced THP-1 macrophage cell death. (A) THP-1 cells were differentiated with 200 nM PMA for 48 h and incubated with or without TG for an additional 24 h. Viable cells were enumerated by trypan blue dye exclusion assay. The number of viable cells in THP-1 macrophages without TG treatment was set as 100%. (B) THP-1 macrophages were incubated with TG for the indicated times, after which caspase-2 activity was assessed. The absorbance of THP-1 macrophages without TG treatment was set as 100%. (C) THP-1 macrophages were incubated with TG in the presence of the caspase-2 inhibitor z-VDVAD-FMK for 24 h. Viable cells were enumerated. The number of viable cells in THP-1 macrophages without TG treatment was set as 100%. THP-1 macrophages were transiently transfected with the control siRNA (si-Con) or caspase-2 siRNA (si-Cas-2) for 24 h and incubated with TG for an additional 24 h. (D) Caspase-2 activity was assessed. The absorbance of THP-1 macrophage without siRNA transfection and TG treatment was set as 100%. (E) Viable cells were enumerated. The number of viable cells in THP-1 macrophages without siRNA transfection and TG treatment was set as 100%. Data are expressed as the mean ± SEM of three independent experiments. The P values were determined with Student’s t-test. *P < 0.05, **P < 0.01.
Caspase-2 induces activation of caspase-1 in TG-stimulated THP-1 macrophages cell death
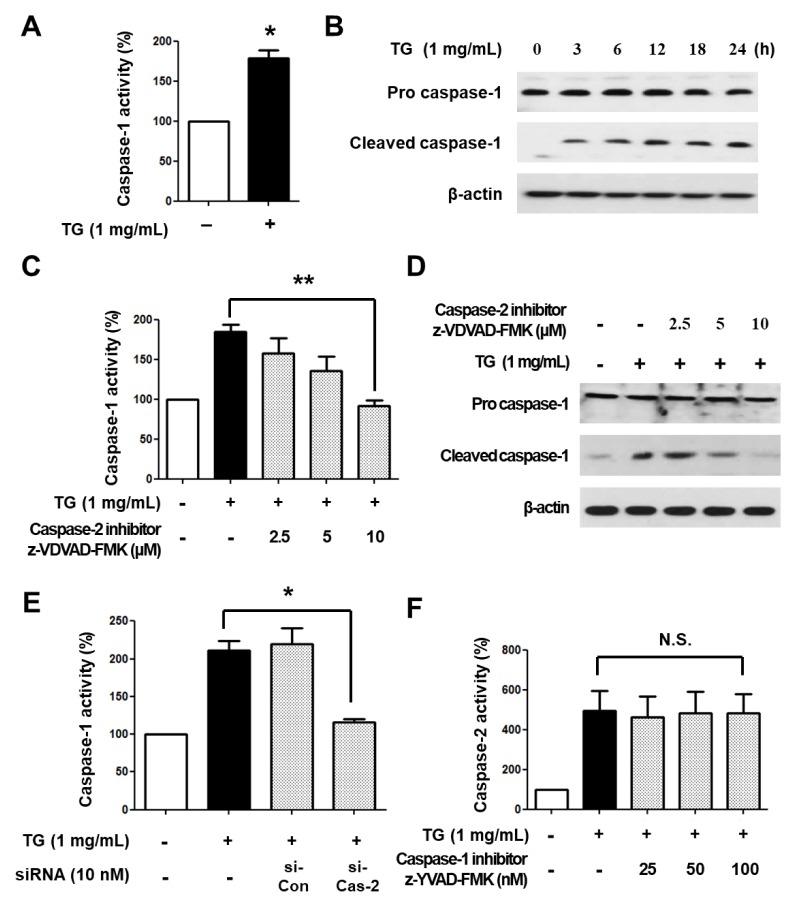
We recently showed that TG-induced macrophage cell death is mediated by caspase-1 (14). We confirmed the involvement of caspase-1 in TG-stimulated macrophage cell death by treating THP-1 macrophages with TG for 24 h. As expected, the activity of caspase-1 was increased in TG-treated THP-1 macrophages (Fig. 2A) and cleaved caspase-1 (i.e., activated caspase-1) was also increased in a time-dependent manner (Fig. 2B).
Fig. 2.
Caspase-2 is an upstream molecule of caspase-1 in TG-stimulated cell death of THP-1 macrophages. (A) THP-1 macrophages were treated with or without TG for 24 h and caspase-1 activity was assessed. The absorbance of THP-1 macrophages without TG treatment was set as 100%. (B) THP-1 macrophages were treated with TG for the indicated times. Cleavage of caspase-1 was detected by Western blotting. β-actin was used as an internal control. (C) THP-1 macrophages were treated with TG in the presence of the caspase-2 specific inhibitor z-VDVAD-FMK for 24 h, after which caspase-1 activity was assessed. The absorbance of THP-1 macrophages without TG treatment was set to 100%. (D) Cleaved caspase-1 was detected by Western blotting. (E) THP-1 macrophages were transfected with control siRNA or caspase-2 siRNA for 24 h and incubated with or without TG for an additional 24 h. Caspase-1 activity was assessed. The absorbance of THP-1 macrophage without TG treatment was set as 100%. (F) THP-1 macrophages were treated with TG in the presence of the caspase-1 specific inhibitor z-YVAD-FMK for 24 h and the caspase-2 activity was assessed. All data are expressed as the mean ± SEM of three independent experiments. P values were determined with Student’s t-test. *P < 0.05, **P < 0.01.
Caspase-2 is involved in the activation of caspase-1 in bacteria-induced cell death (6, 9). Therefore, we examined the association of caspase-2 with caspase-1 activation in TG-induced macrophage cell death. To this end, THP-1 macrophages were treated with TG in the presence of the caspase-2 inhibitor z-VDVAD-FMK for 24 h and caspase-1 activity was determined. Caspase-1 activity and cleaved caspase-1 levels were decreased by the inhibitor in a dose-dependent manner (Fig. 2C, D). In addition, when caspase-2 was knocked down by siRNA in THP-1 macrophages, TG-induced increase of caspase-1 activity was inhibited (Fig. 2E). On the other hand, when THP-1 macrophages were treated with TG in the presence of the caspase-1 inhibitor z-YVAD-FMK, we observed no significant changes in caspase-2 activity in cells in response to TGs (Fig. 2F). Taken together, these results indicate that caspase-2 is upstream of caspase-1, and is associated with its activation in TG-induced THP-1 macrophage cell death.
The cleavage of apoptotic caspases and PARP is induced in TG-stimulated macrophage cell death
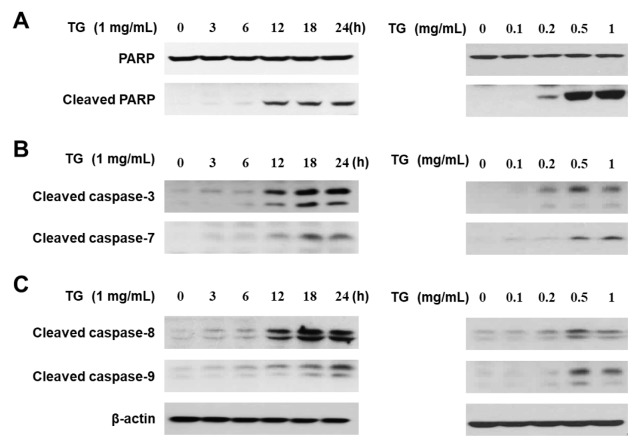
It has been reported that TG accumulation induces dysfunction of mitochondria and activation of intrinsic pathway-associated caspases (13). In addition, PARP is cleaved and inactivated during caspase-dependent cell death (12). Thus, we investigated whether PARP and apoptotic caspases are modulated in response to TG in THP-1 macrophages. THP-1 macrophages were treated with concentrations of TG (0, 0.1, 0.2, 0.5, and 1 mg/ml) for the indicated times (0, 3, 6, 12, 18, 24 h). We first examined cleavage of PARP in TG-stimulated THP-1 macrophages. As shown in Fig. 3A, PARP cleavage was increased in a TG dose- and time-dependent manner. Next, we investigated activation of caspase-3 and caspase-7, which are known to cleave PARP (12). TG treatment induced the cleavage of both caspase-3 and caspase-7 in a TG dose- and time-dependent manner (Fig. 3B). Caspase-8 and caspase-9 are upstream molecules of caspase-3 and -7, therefore we also examined the activation of caspase-8 and -9, and found that both caspase-8 and -9 are cleaved in TG-treated THP-1 macrophages (Fig. 3C). Taken together, these results suggest that both the intrinsic and extrinsic apoptotic pathway are activated in TG-stimulated macrophage cell death.
Fig. 3.
TG treatment induces the cleavage of apoptotic caspases and PARP in THP-1 macrophages. THP-1 macrophages were treated with the indicated concentration of TG for the indicated times. (A) The cleaved form of PARP, (B) cleavage of caspase-3 and -7, and (C) cleavage of caspase-8 and -9 was detected by Western blotting.
Caspase-2 and -1 mediate activation of apoptotic caspases in TG-stimulated THP-1 macrophage cell death
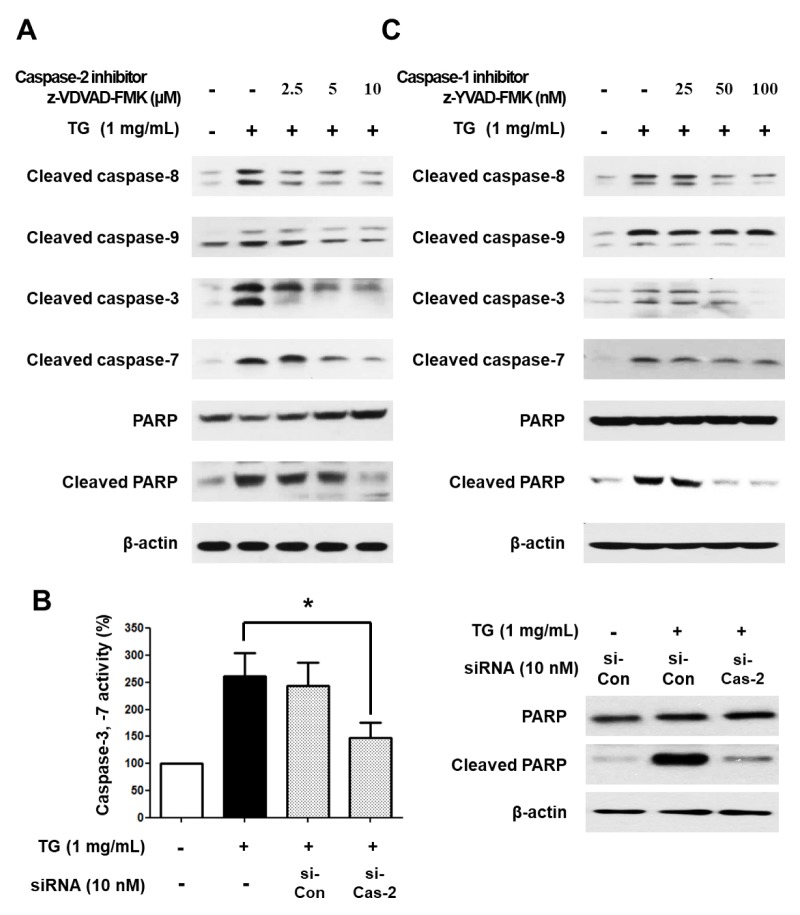
It has been reported that caspase-1, originally known for its involvement in pyroptotic cell death, induces the activation of caspase-7, which is mainly associated with apoptotic cell death (16). Furthermore, recent studies have shown that caspase-2 modulates the activation of apoptotic caspase-9 and -8 (6, 17). Therefore, we investigated whether caspase-2 and -1 are involved in the activation of caspase-3, -7, -8, and -9 in TG-induced macrophage cell death. Specifically, THP-1 macrophages were treated with TG in the absence or presence of caspase-1 or -2 specific inhibitors for 24 h. As shown in Fig. 4A, treatment with a caspase-2 specific inhibitor decreased cleavage of caspase-8/9, -3/7, and PARP in a dose-dependent manner. In addition, when caspase-2 was knocked down in THP-1 macrophages using siRNA, caspase-3/7 activity and cleaved PARP levels were decreased (Fig. 4B). Inhibition of caspase-1 also reduced cleavage of caspase-8/9, -3/7, and PARP (Fig. 4C). Taken together, these results suggest that caspase-2 and caspase-1 are upstream of the apoptotic caspases, thereby implicating them in the activation of apoptotic caspases in TG-stimulated THP-1 macrophage cell death.
Fig. 4.
Activation of apoptotic caspases is mediated via caspase-1 and caspase-2 in TG-treated THP-1 macrophages. (A) THP-1 macrophages were treated with TG in the presence of the caspase-2 inhibitor z-VDVAD-FMK for 24 h. Cleavage of caspase-8, -9, -3, -7, and PARP was detected by Western blotting. THP-1 macrophages were transfected with control siRNA or caspase-2 siRNA for 24 h and incubated with or without TG for an additional 24 h. (B) THP-1 macrophages were transiently transfected with the control siRNA or caspase-2 siRNA for 24 h and incubated with TG for an additional 24 h. Caspase-3/7 activity was assessed (left) and cleavage of PARP was detected by Western blotting (right). (C) THP-1 macrophages were treated with TG in the presence of the caspase-1-specific inhibitor z-YVAD-FMK for 24 h. Cleavage of caspase-8, -9, -3, -7, and PARP was detected by Western blotting. Data are expressed as the mean ± SEM of three independent experiments. The P values were determined with Student’s t-test. *P < 0.05.
DISCUSSION
Plaque formation is an important step in the development of atherosclerosis. One of the key events in plaque formation and instability is macrophage cell death. It has been demonstrated that uptake of TG into macrophages contributes to the formation of a necrotic core that is conducive to the inflammatory process, as well as plaque development and vessel rupture (3, 20, 21). However, the mechanisms by which TG induces macrophage cell death has yet to be fully revealed. We previously reported that caspase-1 is involved in TG-induced macrophage cell death (14). Herein, we investigated the role of caspase-2 in TG-stimulated cell death of macrophages. We found that i) caspase-2 is involved in TG-induced macrophage cell death, ii) TG activates both intrinsic and extrinsic apoptotic caspases, and iii) caspase-2 is an upstream molecule of caspase-1 that induces activation of apoptotic caspases in TG-stimulated macrophage cell death.
Caspase-1 is the central regulator of inflammation and pyroptotic cell death (10, 15). Caspase-1 activation has been detected in inflammatory vascular lesions in patients suffering from acute coronary events (5, 22). Previously, we showed that caspase-1 is associated with TG-induced macrophage cell death, an important event in chronic vascular diseases (14). Previous studies also suggest that pyroptotic cell death may be associated with chronic vascular disease. In the present study, we found that caspase-1 activates apoptotic caspases that are associated with apoptotic cell death in TG-induced macrophage cell death. Recently, caspase-1 was found to be important for apoptotic cell death in response to several stimuli (15, 16, 23, 24). Several studies demonstrated that caspase-1 deficiency reduces processing of caspase-9 and -3, cleavage of Bid, and release of cytochrome c in mouse models (15, 23). Another report showed that caspase-7 is a substrate of caspase-1 (16, 24). Taken together, these results suggest that TG-induced macrophage cell death is processed both through pyroptotic and apoptotic pathways, and that caspase-1 plays a role in both pyroptotic and apoptotic macrophage cell death in response to TGs.
Caspase-2 is associated with cell death induced by a variety of stressors such as DNA damage, ROS, and secreted TNF-α (17). A recent report showed that activation of caspase-1 is driven by the activation of caspase-2 in Brucella abortus RB51-induced hybrid cell death (6). Other studies have shown that caspase-2 induces the truncation of Bid (tBid), which plays a role in the release of cytochrome c, resulting in activation of caspase-9 and caspases-3/7 (6, 9). In addition, studies have shown that the activation of caspase-8 is dependent on caspase-2 (6, 17, 25). Meanwhile, other reports indicate that caspase-2 is able to cleave PARP directly, resulting in disintegration of DNA-repair processing and initiation of apoptosis (18, 26). Based on these studies, we examined whether caspase-2 is also involved in TG-induced macrophage cell death. Our results showed that caspase-2 is associated with TG-stimulated cell death and induces activation of caspase-1, which in turn activates apoptotic caspases.
Caspase-9 and -8 have been linked to intrinsic (mitochondrial) and extrinsic apoptotic pathways, respectively (5, 6). In this study, we found that cleavage of caspase-9 was almost completely recovered by inhibiting caspase-2 in TG-treated THP-1 macrophages. However, inhibition of caspase-1 did not completely prevent cleavage of caspase-9 (Fig. 4). On the other hand, cleavage of caspase-8 was recovered in response to treatment with both caspase-1 and -2 inhibitors (Fig. 4). Taken together, these results suggest that caspase-2 is involved in both the intrinsic and extrinsic apoptotic pathway, while, caspase-1 is primarily involved in the extrinsic apoptotic pathway in TG-treated THP-1 macrophage cell death.
In conclusion, we report the novel finding that caspase-2 is implicated in TG-induced macrophage cell death. Based on our results, we suggest that both the pyroptotic and apoptotic pathways are associated with TG-stimulated macrophage cell death. Although further studies are needed to investigate other possible pathways of TG-induced macrophage cell death, we expect that the results of this study will contribute to a better understanding of the role of caspase-2 in cell death and provide a basis for future studies on the role of caspase-2 in the development of chronic vascular diseases such as atherosclerosis.
MATERIALS AND METHODS
Materials
TG emulsion (Lipofundin® MCT/LCT 20%) was purchased from B. Braun Melsungen AG (Melsungen, Germany). Lipofundin® MCT/LCT 20% was used to deliver TG into cells as described previously (27). Hereafter, Lipofundin® MCT/LCT 20% is referred to as TG for convenience. The caspase-1 substrate Ac-YVAD-p-nitroanilide (Ac-YVAD-pNA) was purchased from Biomal (Plymouth Meeting, PA, USA). The caspase-2 substrate Ac-VAVAD-pNA was purchased from Sigma-Aldrich (St. Louis, MO, USA). The caspase-3/7 substrate Ac-DEVD-pNA was purchased from Enzo Life Sciences (Farmingdale, NY, USA). The caspase-1 specific inhibitor z-YVAD-FMK and caspase-2 specific inhibitor z-VDVAD-FMK were purchased from BioVision (Mountain View, CA, USA). Antibodies specific for caspases-1, -3, -7, -8, and -9, as well as PARP, were purchased from Cell Signaling Technology (Danvers, MA, USA).
Cell culture
The THP-1 human acute monocytic leukemia cell line (ATCC, Manassas, VA, USA) was grown in RPMI 1640 media supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin and maintained at 37°C in a humidified atmosphere with 5% CO2. To induce differentiation of THP-1 cells into macrophages, cells were seeded in 6-well plates at a density of 1 × 106 cells/well and treated with 200 nM PMA for 48 h.
siRNA transfection
Caspase-2 siRNAs (5′-UGGAAGUAUUUGAGAGAGAdTdT-3′) were synthesized by ST PHARM (Seoul, Korea). Transfections were performed with Lipofectmine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol.
Trypan blue dye exclusion assay
To enumerate viable cells, cells were trypsinized and 10 μl of 0.4% trypan blue stain solution was mixed with 10 μl of the trypsinized cells at a 1:1 ratio. Non-stained cells in the resulting mixture were counted using a hemocytometer.
Measurement of caspase activity
The activity of caspase-1, caspase-2, and caspase-3/7 was determined as previously described (28, 29). Briefly, cells were lysed with PBS buffer containing 1% Triton X-100 and then centrifuged at 19,000 g for 10 min at 4°C. The supernatant was collected and the total protein concentration was quantified. To detect caspase-1 activity, 90 μg of protein sample was combined with 200 μM of the caspase-1 substrate Ac-YVAD-pNA in 150 μl of PBS. To detect caspase-2 activity, protein was combined with the caspase-2 substrate Ac-VAVAD-pNA in PBS. To detect caspase-3/7 activity, protein was combined with the caspase-3/7 substrate Ac-DEVD-pNA in PBS. Reactions were incubated for 3 h at 37°C, and the activity was determined by measuring the absorbance at 405 nm.
Western-blot analysis
Cells were washed with PBS and lysed at 4°C with lysis buffer containing 1% Triton X-100, protease inhibitor cocktail (Sigma-Aldrich), phosphatase inhibitor cocktail (Roche, Mannheim, Germany), and PBS. Lysates were clarified and the supernatants were subjected to Western blotting as described previously (30).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA). The P values were calculated using Student’s t-test. Values are shown as the mean and standard error of the mean (SEM). Each experiment was conducted three times and the data were pooled for analysis. Differences were considered to be statistically significant at *P < 0.05, **P < 0.01, or ***P < 0.001.
ACKNOWLEDGEMENTS
This work was supported by the 2015 Gimcheon University Research Grant.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Kastelein JJ, van der Steeg WA, Holme I, et al. Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation. 2008;117:3002–3009. doi: 10.1161/CIRCULATIONAHA.107.713438. [DOI] [PubMed] [Google Scholar]
- 2.Sofer O, Fainaru M, Schafer Z, Goldman R. Regulation of lipoprotein lipase secretion in murine macrophages during foam cell formation in vitro. Effect of triglyceride-rich lipoproteins. Arterioscler Thromb. 1992;12:1458–1466. doi: 10.1161/01.ATV.12.12.1458. [DOI] [PubMed] [Google Scholar]
- 3.Aronis A, Aharoni-Simon M, Madar Z, Tirosh O. Triacylglycerol-induced impairment in mitochondrial biogenesis and function in J774.2 and mouse peritoneal macrophage foam cells. Arch Biochem Biophys. 2009;492:74–81. doi: 10.1016/j.abb.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Gotto AM., Jr Triglyceride: the forgotten risk factor. Circulation. 1998;97:1027–1028. doi: 10.1161/01.CIR.97.11.1027. [DOI] [PubMed] [Google Scholar]
- 5.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronner DN, O’Riordan MX, He Y. Caspase-2 mediates a Brucella abortus RB51-induced hybrid cell death having features of apoptosis and pyroptosis. Front Cell Infect Microbiol. 2013;3:83. doi: 10.3389/fcimb.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albert ML. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat Rev Immunol. 2004;4:223–231. doi: 10.1038/nri11308. [DOI] [PubMed] [Google Scholar]
- 8.Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 9.Jesenberger V, Procyk KJ, Yuan J, Reipert S, Baccarini M. Salmonella-induced caspase-2 activation in macrophages: a novel mechanism in pathogen-mediated apoptosis. J Exp Med. 2000;192:1035–1046. doi: 10.1084/jem.192.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thornberry NA, Rano TA, Peterson EP, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 11.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326(Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tewari M, Quan LT, O’Rourke K, et al. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 13.Aflaki E, Radovic B, Chandak PG, et al. Triacylglycerol accumulation activates the mitochondrial apoptosis pathway in macrophages. J Biol Chem. 2011;286:7418–7428. doi: 10.1074/jbc.M110.175703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Son SJ, Rhee KJ, Lim J, Kim TU, Kim TJ, Kim YS. Triglyceride-induced macrophage cell death is triggered by caspase-1. Biol Pharm Bull. 2013;36:108–113. doi: 10.1248/bpb.b12-00571. [DOI] [PubMed] [Google Scholar]
- 15.Zhang WH, Wang X, Narayanan M, et al. Fundamental role of the Rip2/caspase-1 pathway in hypoxia and ischemia-induced neuronal cell death. Proc Natl Acad Sci U S A. 2003;100:16012–16017. doi: 10.1073/pnas.2534856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamkanfi M, Kanneganti TD, Van Damme P, et al. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics. 2008;7:2350–2363. doi: 10.1074/mcp.M800132-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puccini J, Dorstyn L, Kumar S. Caspase-2 as a tumour suppressor. Cell Death Differ. 2013;20:1133–1139. doi: 10.1038/cdd.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fava LL, Bock FJ, Geley S, Villunger A. Caspase-2 at a glance. J Cell Sci. 2012;125:5911–5915. doi: 10.1242/jcs.115105. [DOI] [PubMed] [Google Scholar]
- 19.Bouchier-Hayes L, Green DR. Caspase-2: the orphan caspase. Cell Death Differ. 2012;19:51–57. doi: 10.1038/cdd.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aronis A, Madar Z, Tirosh O. Lipotoxic effects of triacylglycerols in J774.2 macrophages. Nutrition. 2008;24:167–176. doi: 10.1016/j.nut.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Lim J, Kim YS, Kim SH, et al. Triglyceride enhances susceptibility to TNF-α-induced cell death in THP-1 cells. Genes & Genomics. 2013;36:87–93. doi: 10.1007/s13258-013-0144-y. [DOI] [Google Scholar]
- 22.Kolodgie FD, Narula J, Burke AP, et al. Localization of apoptotic macrophages at the site of plaque rupture in sudden coronary death. Am J Pathol. 2000;157:1259–1268. doi: 10.1016/S0002-9440(10)64641-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plesnila N, Zinkel S, Le DA, et al. BID mediates neuronal cell death after oxygen/ glucose deprivation and focal cerebral ischemia. Proc Natl Acad Sci U S A. 2001;98:15318–15323. doi: 10.1073/pnas.261323298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeretssian G, Labbe K, Saleh M. Molecular regulation of inflammation and cell death. Cytokine. 2008;43:380–390. doi: 10.1016/j.cyto.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Huang YT, Huang YH, Hour TC, Pan BS, Liu YC, Pan MH. Apoptosis-inducing active components from Corbicula fluminea through activation of caspase-2 and production of reactive oxygen species in human leukemia HL-60 cells. Food Chem Toxicol. 2006;44:1261–1272. doi: 10.1016/j.fct.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Stefanis L, Troy CM, Qi H, Shelanski ML, Greene LA. Caspase-2 (Nedd-2) processing and death of trophic factor-deprived PC12 cells and sympathetic neurons occur independently of caspase-3 (CPP32)-like activity. J Neurosci. 1998;18:9204–9215. doi: 10.1523/JNEUROSCI.18-22-09204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim J, Kim SH, Kang YW, et al. Triglyceride up-regulates expression of ABCG1 in PMA-induced THP-1 macrophages through activation of JNK and p38 MAPK pathways. Biomed Sci Lett. 2014;20:237–243. doi: 10.15616/BSL.2014.20.4.237. [DOI] [Google Scholar]
- 28.Joo D, Woo JS, Cho KH, et al. Biphasic activation of extracellular signal-regulated kinase (ERK) 1/2 in epidermal growth factor (EGF)-stimulated SW480 colorectal cancer cells. BMB Rep. 2016;49:220–225. doi: 10.5483/BMBRep.2016.49.4.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imre G, Heering J, Takeda AN, et al. Caspase-2 is an initiator caspase responsible for pore-forming toxin-mediated apoptosis. EMBO J. 2012;31:2615–2628. doi: 10.1038/emboj.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jo HS, Kim DS, Ahn EH, et al. Protective effects of Tat-NQO1 against oxidative stress-induced HT-22 cell damage, and ischemic injury in animals. BMB Rep. 2016;49:617–622. doi: 10.5483/BMBRep.2016.49.11.117. [DOI] [PMC free article] [PubMed] [Google Scholar]