Abstract
Environmental factors have been shown to contribute to the incidence of Parkinson’s disease (PD). Pesticides, which represent one of the primary classes of environmental agents associated with PD, share the common feature of being intentionally released into the environment to control or eliminate pests. Pesticides consist of multiple classes and subclasses of insecticides, herbicides, rodenticides, fungicides, fumigants and others and exhibit a vast array of chemically diverse structures. In this review we examine the evidence regarding the ability of each of the major pesticide subclasses to increase the incidence of PD. We propose that, from a toxicological perspective, it would be beneficial to identify specific subclasses, common structural features and the propensity for widespread human exposure when considering the potential role in PD, rather than using the overly broad term of ‘pesticides’ to describe this diverse group of chemicals. Furthermore, these chemicals and their environmentally relevant combinations should be evaluated for their ability to promote or accelerate PD and not merely for being singular causative agents.
Introduction
Parkinson’s disease (PD), the most common neurodegenerative movement disorder, affects greater than 1% of the worldwide population over the age of 65 [1]. Pathologically, PD is characterized by a progressive loss of dopamine neurons in the substantia nigra pars compacta (SNpc), a loss of dopamine input to the striatum, the presence of ubiquitin- and α-synuclein-positive cytoplasmic inclusions known as Lewy bodies [2], depigmentation of the locus ceruleus and autonomic dysfunction including sympathetic denervation of the heart [3]. The loss of dopamine is responsible for the majority of the motor symptoms of PD. Although 5%–10% of classical PD cases result from monogenetic mutations, the remaining cases have no known etiology [4]. The suggestion that environmental factors play a role in the etiology of PD quickly gained popularity after the discovery of a group of intravenous drug users who unwittingly injected a synthetic analog of demerol that was contaminated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) causing an acute, permanent parkinsonian state that was levodopa (L-dopa) responsive [5]. Once inside the brain, MPTP enters astrocytes and is converted into its active metabolite, MPP+ (1-methyl-4-phenylpyrdinium), which then can enter dopamine neurons and exert its toxicity. Although not typically found in the environment itself, the ability of MPTP to reproduce so many of the features of PD intensified the search for potential environmental toxicants that might contribute to the development of PD.
This suspicion that factors other than genetics play a major role in the etiology of PD was further strengthened by a study that showed that the incidence of PD was virtually identical in monozygotic and dizygotic twins [6]. A more recent study of PD using the Swedish Twin Registry found that only three twin pairs out of a total of 49 814 participants were concordant for PD and that the prevalence of PD was slightly lower in monozygotic twins than in dizygotic twins (OR = 0.79, 95% CI 0.46, 1.22), suggesting that environmental factors played a substantial role in PD [7].
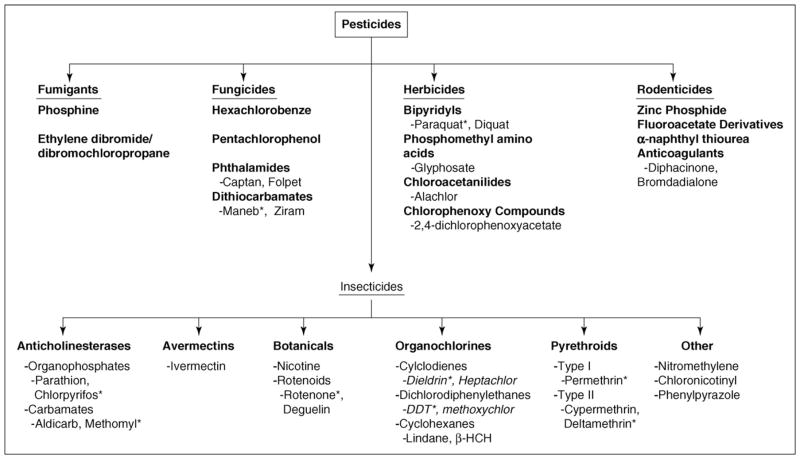
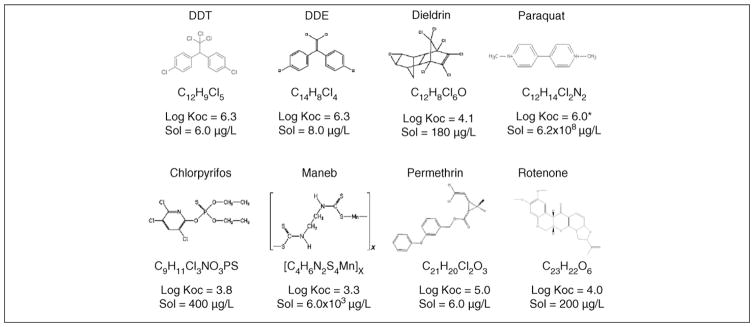
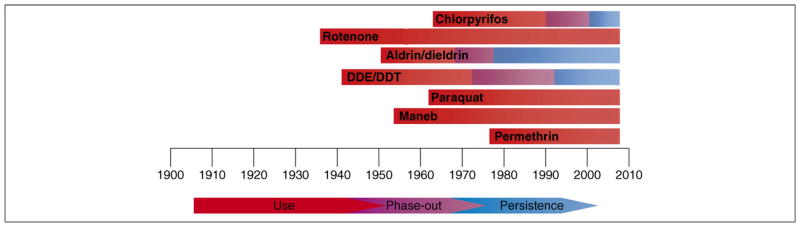
Pesticides encompass an array of compounds designed to deter or kill insects (insecticides), rodents (rodenticides), plants (herbicides) and fungi (fungicides). In 2001 over five billion pounds of pesticides were used worldwide. Of that amount, 1 944 000 000 lbs were herbicides, 1 355 000 000 lbs were insecticides, 516 000 000 lbs were fungicides and 1 536 000 000 were other compounds in various classes [2000–2001 Pesticide Market Estimates: Usage – Pesticides - US EPA (www.epa.gov/oppbead1/pestsales/01pestsales/usage2001.htm)]. Figure 1 shows a common pesticide-classification system that is based on both chemical structure and function[8], and Figure 2 shows chemical structures and key properties of representative pesticides. Although numerous compounds are listed in Figure 1, substantial epidemiological [9–12] and laboratory evidence implicates only a subset of these pesticide exposures in the pathogenesis of PD. However, specific environmentally and physiologically relevant compounds that explain this association have yet to be identified. As shown in Figure 3, numerous classes of pesticides were introduced during the twentieth century. Although PD existed long before the introduction of these pesticides, the thought is that pesticide exposure has contributed to the increased incidence of the disease. The goal of this review is to examine the literature to identify those subclasses of pesticides that are associated with PD and to evaluate the strength of that association with respect to environmental exposure and mechanistic plausibility.
Figure 1.
Classification of Pesticides. Pesticides are classified by their target species and can be further subdivided based upon chemical structure. Of those compounds associated with Parkinson’s disease, all are classified as insecticides, herbicides or fungicides. Compounds with asterisks are used as example compounds in Figures 2, 3 and 4.
Figure 2.
Structures of representative pesticides. Log organic carbon–water partition coefficient (Koc) and aqueous solubility (Sol) of selected pesticides at 25 °C. *Estimated Koc value because paraquat sorption is primarily due to cation exchange rather than partitioning into soil organic matter.
Figure 3.
Introduction and use of various pesticides in the U.S. The commercial introduction of several widely used pesticides are shown for the past century. Whereas many of these compounds have had their use restricted, some continue to persist in the environment. Aldrin and dieldrin, as well as DDT and DDE, are grouped together given their similarities in structure. Similar trends of use and restriction have been observed in Europe. Naturally occurring rotenoids have been used since the 1800s. Other organophosphates were developed and utilized starting in the 1940s.
Fumigants
The fumigant class encompasses a variety of agents most commonly used to control insects or fungi in grains, soil or other various consumables. Fumigants, such as ethylene dibromide, are highly toxic to humans but most adverse actions are non-neuronal [8]. There is some evidence that carbon disulfide-based fumigants can induce parkinsonian-like neurotoxicity [13], but there are no published reports suggesting that fumigants are involved in the pathogenesis of PD.
Fungicides
Fungicides are systemic or contact agents that are often sulfur- or metal-based and include a wide variety of chemical structures [14]. Metal cores found in fungicides include manganese, zinc and mercury, which is no longer used [15]. They can be used to both prevent and treat the presence of fungi and molds on crops. Although most fungicides are minimally toxic to humans, some nonselective toxic effects have been reported. General central nervous system (CNS) effects, such as generalized depression of activity, parasthesias and tremor, have been noted [8,16]. Maneb, or manganese ethylene bis-dithiocarbamate, is a contact pesticide that is used to treat seeds or for direct application to crops that have emerged from the soil [ATSDR - CERCLA Priority List of Hazardous Substances (http://www.atsdr.cdc.gov/cercla)]. It is possible that the parkinsonian symptoms are secondary to exposure to the manganese metal core and lead to symptoms similar to those seen in manganism [17]. Maneb has been shown to exacerbate toxicity of other agents in mouse models, such as paraquat (see below) [18]. Additionally, other dithiocarbamates have been shown to disrupt function of the ubiquitin proteasome system, a system thought to be involved in both genetic and idiopathic forms of PD [19]. It would be prudent to further examine the effects of these compounds for their ability to induce PD-like neurotoxicity.
Herbicides
The class of herbicides represents chemicals that target undesirable plant species but often lack specificity (Figure 1). These include compounds such as glyphosate (e.g. Roundup), alachlor, the bipyridyl paraquat and atrazine. Paraquat is the only herbicide substantially investigated in relation to PD, although there have been studies on the ability of atrazine to disrupt dopamine homeostasis at high concentrations [20]. A recent case-control study reported that herbicide exposure increased the risk of PD [21].
Paraquat
Paraquat is a nonselective bipyridyl contact pesticide that was first produced in 1961. It gained considerable attention because of its extreme toxicity in cases of human exposure, its use in eradicating marijuana crops and because paraquat has a structure similar to that of MPP+. This led to speculation that paraquat also might be a potential dopamine neurotoxin [22,23]. Several epidemiological studies have reported a positive association between paraquat exposure and the development of PD [24–26], although these findings have not been replicated in a large cohort study. Laboratory studies have provided conflicting results. Early studies suggested that the pharmacokinetic properties of paraquat made it an unlikely candidate due to its low partition coefficient, limited absorption and poor CNS penetration [27]. In addition paraquat showed little penetration into the brain structures of rats with an intact blood–brain barrier [28,29]. More recent studies, however, have reported the ability of paraquat to cross the blood–brain barrier, possibly via the neutral amino acid transporter [30], and to accumulate in certain brain regions of the mouse [31].
Some groups have reported no neurotoxic changes after paraquat exposure [28,32], whereas others have shown varying degrees of toxicity. For example, systemic paraquat exposure has been shown to cause a 20%–30% selective dopamine neuronal loss in the SNpc [33] and increased expression and aggregation of α-synuclein [33]. Furthermore, overexpression of either wild-type or mutant α-synuclein is protective against paraquat toxicity [34]. Others report that paraquat causes loss of nigral dopamine neurons and degeneration of striatal terminals, as well as decreased locomotor activity [35,36]. These studies support the ability of paraquat exposure to result in a reproducible loss of dopamine neurons. Although paraquat is structurally similar to MPP+, it does not appear to exert its toxicity in a similar manner. For example, paraquat differs from MPP+ in its ability to enter the dopamine neuron, inhibit complex I, activate particular cell-death pathways and oxidize cellular redox couples [37,38].
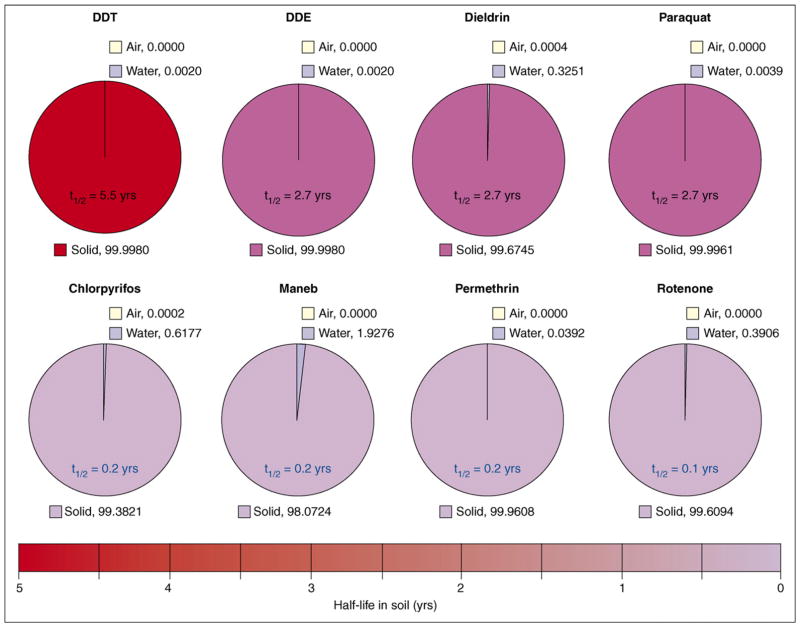
In addition to studying the effects of paraquat exposure alone, other groups have suggested that studying exposures to combinations of pesticides is a more appropriate strategy. One such combination is that of paraquat and the fungicide maneb. The overlap in geographic usage of these compounds was used as a rationale for animal studies that showed that coexposure caused decreased motor activity, altered striatal dopamine and dopamine metabolites, decreased striatal tyrosine hydroxylase and dopamine transporter (DAT) expression and increased accumulation of dopamine in synaptosomes [18,39,40]. However, the compounds are not used simultaneously [ATSDR - CERCLA Priority List of Hazardous Substances (http://www.atsdr.cdc.gov/cercla)] and, although paraquat does persist in the environment (Figure 4), due to its high affinity for soil it is not biologically available, making such simultaneous exposures unlikely in the human population. The relevance of human exposure to mixtures of compounds is well noted, but the design of combination exposures should account carefully for both temporal and geographic differences in pesticide use and persistence.
Figure 4.
Environmental fate of various pesticides. The vast majority of pesticides are found in the solid (soil) phase and exhibit a wide range of environmental half-lives. Equilibrium phase distributions of selected pesticides are shown for a system containing equal volumes of water, air and soil (1% organic carbon) at 25 °C, based on recommended or average Henry’s law constant, aqueous solubility, sorption coefficient and soil half-life data [75,76]. Although paraquat has a high water solubility, its charged nature results in strong surface adsorption, whereas the other compounds are lipophilic and primarily partition into soil organic matter.
Insecticides
There are several subclasses of insecticides, each with their own subdivisions (Figure 1). Many of the compounds in this class are, by design, neurotoxic. Similarities between the insect and human nervous systems can lead to crosstoxicity of these compounds. There has been no evidence to suggest that avermectins or other pesticides, such as nitromethylene, phenylpyrazole or neonicotinoids, are associated with PD. However, the neurotoxicity of other subclasses, such as organophosphates, rotenoids (rotenone), organochlorines and pyrethroids, has been studied extensively.
Organophosphates
Organophosphates (OPs) are a group of acetylcholinesterase inhibitors that is the largest group of insecticides sold worldwide [16], although their use recently was restricted. Although evidence supporting a role for OPs in PD pathogenesis is scant, there are case reports of people with severe parkinsonism after OP exposure [41]; however, the reversibility of the symptoms and lack of responsiveness to L-dopa are not consistent with PD. A recent family-based case-control study did implicate organophoshates and other insecticides in PD [42]. In mice exposure to 100 mg/kg chlorpyrifos decreased open-field activity and rearing and decreased DAT-mediated 3H-dopamine uptake [43]. It might be that the effects of OP exposure probably involve disturbance of the balance between the dopamine and acetylcholine systems and not specific pathological changes in the dopamine system.
Rotenone
Rotenone and other rotenoids are highly toxic, naturally occurring botanical pesticides that are primarily used in the management of nuisance fish populations and organic farming rather than widespread commercial agriculture. Rotenone is very hydrophobic and, thus, easily can cross biological membranes without the need for a transporter. It has poor bioavailability and has a short half-life in the environment (Figure 4). Because of the known systemic inhibition of mitochondrial complex I (NADH dehydrogenase) in PD patients [44], the possibility of rotenone, also a complex I inhibitor, being an environmental contributor to PD has been extensively examined. Some early studies with rotenone exposure found minimal nigrostriatal damage [45] or found damage to striatal dopamine fibers but not to nigral dopamine neuronal bodies [46]. Later studies showed that chronic and subcutaneous administration of rotenone could result in a parkinsonian syndrome with selective dopamine neuron degeneration, oxidative damage and cytoplasmic inclusions reminiscent of early Lewy bodies [47]. The damage reported in these studies was seen in the striatum first, followed by the SNpc (this is similar to the ‘dying-back’ phenomenon in PD); however, these changes were seen only in a subset of exposed animals. Additionally, increased oxidative stress, ubiquitin accumulation, proteasomal inhibition and inflammation all have been observed in response to rotenone exposure [19,48–50]. Despite this evidence of a role for rotenone in PD pathogenesis, it is unlikely the rotenone is a major contributor because of its limited commercial use, short half-life in the environment and low bioavailability. However, numerous other complex-I-inhibitor pesticides currently in use, such as fenpyroximate and pyridaben, might contribute to damage of the dopamine system in PD [51].
Organochlorines
Organochlorine pesticides are a class of chlorinated pesticides that were heavily used during the 1950s–1970s in food and non-food crops such as corn, wheat and tobacco. These compounds are known to be acutely toxic at high concentrations. However, most human exposure is not acute but, rather, chronic and at much lower doses, primarily through contaminated food. They have low volatility, exhibit chemical stability and are highly lipophilic and, therefore, persist in the environment and demonstrate a strong tendency to bioaccumulate and biomagnify (Figure 4). The Agency for Toxic Substances and Disease Registry (ATSDR) lists several organochlorine pesticides in the list of the top 25 most hazardous substances, including dichlorodiphenyltrichloroethane (DDT), dichlorodiphenyldichloroethylene (DDE), dichlorodiphenyldichloroethane (DDD), dieldrin, aldrin and chlordane, based upon their toxicity, widespread distribution and potential for human exposure [ATSDR - CERCLA Priority List of Hazardous Substances (http://www.atsdr.cdc.gov/cercla)]. Although the use of most organochlorines has been banned in the U.S. for decades, many of these compounds are still in use in other countries. Additionally, because organochlorines are resistant to degradation and are still present in our environment, these compounds continue to be of great concern even decades after their use has been banned.
The three main classes of organochlorine pesticides (Figure 1) differ in their propensity to damage the dopamine system. Although there are some reports of higher levels of DDT or its main metabolite DDE in the postmortem brain tissue of PD patients [52,53], there has been no experimental evidence to support the role of DDT or DDE in PD pathogenesis [54], suggesting that these compounds might be markers of exposure to other, more toxic compounds. Although concerns over hexachlorocyclohexanes (HCHs) have arisen, including several groups lobbying to have lindane added to the list of 12 persistent organic pollutants banned by the Stockholm Convention and reporting higher levels of lindane in the postmortem PD brain tissue [55], there is little experimental evidence to support a role in PD.
The correlation between organochlorine pesticides and the incidence of PD is largely driven by the cyclodienes [56]. Production of the organochlorine aldrin and the bioactivated and more persistent form dieldrin, ranked second among agricultural chemicals used in the U.S. with a peak in the mid-1960s at nearly 20 million pounds per year [57]. Several studies in dopamine cell lines have demonstrated the ability of dieldrin to generate reactive oxygen species, induce ubiquitin–proteasome system dysfunction, promote α-synuclein aggregation, release and deplete intracellular dopamine, alter mitochondrial membrane potential and activate caspases [58,59]. In vitro dieldrin has been shown to promote fibrillization of α-synuclein [60] and cause synaptosomal release of dopamine by a mechanism independent of direct binding to the picrotoxin-binding site on the gamma-aminobutyric acid (GABAA) receptor [61]. In mice dieldrin exposure has been shown to recapitulate many of the neuropathological changes seen in PD, such as increased α-synuclein expression, alterations in dopamine homeostasis and increased markers of oxidative stress including the formation of cysteinylcatechols [62]. Additionally, developmental exposure to dieldrin results in alterations in DAT and vesicular monoamine transporter 2 levels and increases the susceptibility to the neurotoxic effects of MPTP [63]. Additional studies have shown that dopamine neurons appear to be more vulnerable to the neurotoxic effects of dieldrin than other neuronal populations [64,65]. It should be noted, however, that dieldrin does not cause dopamine neuron cell loss or motor deficits that are characteristic of PD. Also, many of these in vitro studies used concentrations much higher than one would expect to observe in human tissue. Finally, several studies have reported increased levels of dieldrin in the postmortem brain tissue of PD patients versus age-matched controls [53,55]. Heptachlor, which contaminated the milk supply in Oahu, Hawaii in the 1980s, also exhibits some neurotoxic effects on the dopamine system [66–69], but it has not been associated with PD in any human studies.
The data supporting a role for organochlorines in increasing the risk of PD continue to grow, including a recent family-based case-control study that demonstrated such an association [42]. These compounds exhibit chemical properties, toxicokinetic features and temporal and geographic-use patterns that make them reasonable candidates to contribute to the incidence of PD. Further studies investigating the specific mechanisms by which these compounds increase the vulnerability of the nigrostriatal dopamine system to degeneration are warranted.
Pyrethrins/Pyrethroids
Pyrethrins are naturally occurring compounds with insecticidal properties. These compounds are often sold as mixtures for commercial and household use and are found in many household insecticides, flea sprays, animal shampoos, treatments for head lice and mosquito repellents [ATSDR - Toxicological Profile: Pyrethrins and Pyrethroids (http://www.atsdr.cdc.gov/toxprofiles/tp155.html)]. Pyrethroid intoxication has long been known to cause neurological symptoms including salivation, tremor, choreoathetosis or coma, depending on the specific exposure. Several studies have reported the ability of pyrethroids, such as permethrin and deltamethrin, to increase DAT-mediated 3H-dopamine uptake or cause neurotransmitter release [70,71]. Given the importance of DAT function to the dopamine neuron [72], this is an area of research that deserves more attention. Although pyrethroids are a relatively new class of insecticide and, thus, are unlikely to be involved in PD cases diagnosed before 1970s–1980s, the potential for widespread human exposure and ability to alter dopamine homeostasis warrants further study.
Rodenticides
Rodenticides are used for the eradication and deterrence of rats, mice and other nuisance rodents. Compounds within this class have various mechanisms of action and do not usually target the CNS, with the exception of strychnine, which is no longer used in the U.S. or the U.K.. There are no published reports supporting a role of rodenticides in PD pathogenesis.
Discussion
Based on several comprehensive epidemiological studies [9–11], pesticide exposure does appear to be a risk factor for PD. However, the specific compounds or classes responsible for this association have yet to be identified [12]. By examining the various classes of compounds from a toxicological perspective, viz., taking into consideration their environmental persistence, likelihood of human exposure and potential mechanisms of action, it might be possible to narrow down the candidate compounds for further study. Because PD has existed for centuries, pesticides are obviously not the sole cause of the disease. However, if one considers these compounds as accelerators or promoters of PD pathogenesis, we can begin to explain why the association with PD might arise continually and why it might not always be consistent. Exposure at some point during the pathogenesis, which has been suggested to occur over the course of decades, could accelerate the underlying neurodegenerative process. However, such compounds might not initiate the disease process, such that without other genetic or metabolic risk factors they might be innocuous. Several of the compounds associated with PD have long half-lives in the environment and in the body (Figure 4). This allows them to bioaccumulate and to exert adverse effects over extended periods of time. Coupled with plausible mechanistic risks to the nigrostriatal dopamine system, such compounds could produce subtle toxic effects, but when these occur over the course of decades their cumulative effects could lead to an accelerated course of a progressive disease. These compounds might individually and collectively increase the likelihood of dopamine cell death and increase the risk of reaching the critical pathophysiological threshold at which PD symptoms become clinically visible. Alternatively, acute exposures to highly toxic compounds that damage the mitochondria or induce significant oxidative damage could result in an injury that initiates self-perpetuating damage, such as neuroinflammatory processes, that could persist for many years. For example, the initial insult could specifically damage a cellular component, which sets off an inflammatory cascade that leads to more cellular damage, as seen with humans exposed to MPTP [73].
Of the compounds discussed, rotenone, paraquat and maneb all appear to exhibit a level of mechanistic plausibility and reproduce many of the features of PD in animal models. However, none of these compounds possess the physical-chemical properties and use patterns that would lead to widespread human exposure due to either short environmental half-lives or high-affinity binding to soil. For example, studies with rotenone have been very helpful to our understanding of complex I inhibition and mitochondrial dysfunction in PD pathogenesis, whereas paraquat has provided insight into the role of oxidative stress in the disease. Given their ability to reproduce features of PD in animals, these compounds have been especially beneficial in understanding the events that lead to dopamine neuron cell death. However, the numerous studies in animal models suggesting an association do not override the properties of use, persistence and bioaccumulation that would preclude them from being significant contributors to the disease. These studies might help identify similar, but more environmentally relevant, compounds in the disease process.
Organophosphates and pyrethroids, although quite neurotoxic, do not appear to contribute to the incidence of PD. This might be due to their inability to selectively damage dopamine neurons or their short half-lives. The cyclodienes have been found to be elevated in postmortem PD brains in multiple studies. Dieldrin, which was widely used for several decades and persists in the environment, has been shown to damage various components of the dopamine system. However, these compounds do not reproduce dopamine neuron loss of behavioral deficits in the animal model. Thus, although the cyclodienes are not overt dopamine toxicants, they do demonstrate potential biological plausibility and environmental relevance.
The diversity in chemical structure among the various classes of pesticides leads one to wonder if there are any common structural features that are associated with PD-related neurotoxicity. Unfortunately, if one looks at the model toxicants used to produce certain features of PD (rotenone, paraquat, dieldrin and maneb), what is most striking is their absence of similar chemical structure. One interpretation of this could be that there are multiple molecular targets than can either kill or increase the vulnerability of dopamine neurons. If one considers a suite of molecular targets (mitochondrial components, dopamine neuron constituents, oxidative mechanisms, α-synuclein and the proteasome), we might gain insight from structure–activity relationships at one particular site of action, but when looking at the ultimate outcome of dopamine neuron cell death, there probably will be no identifiable structural feature that unites compounds associated with the incidence of PD. However, structural features that lead to environmental persistence and biological uptake (for example, highly halogenated, lipophilic), along with use patterns that allow widespread human exposure, are useful in identifying the classes of compounds most likely to be associated with human exposure, uptake into the brain and potential neurtoxic effects. In addition, because there appears to be multiple molecular targets that can promote neurodegeneration, it is especially important to consider how mixtures of compounds that exert actions at different sites might work in concert to damage neurons [74].
Investigators should continue to search for compounds that display the biological and mechanistic plausibility to promote PD, but, just as importantly, we must not overlook the plausibility of true human exposure. Because it is unlikely that individuals are exposed to only one specific compound, we also must start to develop strategies to evaluate combinations of compounds that are based on realistic environmental exposures, including appropriate routes of entry and use patterns. Such studies should involve experts in toxicology, epidemiology, environmental science and engineering, exposure assessment and neurology. Identifying improved methods of measuring exposure, such as biomarkers, and earlier detection of PD could greatly facilitate progress in the field. In closing, the environmental relevance of putative pesticide candidates for PD must be taken into consideration when evaluating their potential to contribute to the disease.
Acknowledgments
This work was supported by the Emory Collaborative Center for Parkinson’s Disease Environmental Research (NIH U54 ES012068 to G.W.M.), the Woodruff Health Sciences Center Fund (G.W.M.) and National Institutes of Health grants NIH F31 ES014141 to J.M.H, NIH R21 ES012315 to G.W.M and NIH K25 ES014659 to K.D.P.
References
- 1.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 2.Bennett MC, et al. Degradation of alpha-synuclein by proteasome. J Biol Chem. 1999;274:33855–33858. doi: 10.1074/jbc.274.48.33855. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein DS. Cardiac denervation in patients with Parkinson disease. Cleve Clin J Med. 2007;74:S91–S94. doi: 10.3949/ccjm.74.suppl_1.s91. [DOI] [PubMed] [Google Scholar]
- 4.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 5.Langston JW, et al. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 6.Tanner CM, et al. Parkinson disease in twins: an etiologic study. JAMA. 1999;281:341–346. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- 7.Wirdefeldt K, et al. Complete ascertainment of Parkinson disease in the Swedish Twin Registry. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2007.04.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klaassen CD, editor. Casarett & Doull’s Toxicology: The Basic Science of Poisons. McGraw-Hill; 2001. [Google Scholar]
- 9.Ascherio A, et al. Pesticide exposure and risk for Parkinson’s disease. Ann Neurol. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- 10.Frigerio R, et al. Education and occupations preceding Parkinson disease: a population-based case-control study. Neurology. 2005;65:1575–1583. doi: 10.1212/01.wnl.0000184520.21744.a2. [DOI] [PubMed] [Google Scholar]
- 11.Priyadarshi A, et al. A meta-analysis of Parkinson’s disease and exposure to pesticides. Neurotoxicology. 2000;21:435–440. [PubMed] [Google Scholar]
- 12.Brown TP, et al. Pesticides and Parkinson’s disease–is there a link? Environ Health Perspect. 2006;114:156–164. doi: 10.1289/ehp.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman LJ, et al. Finger tremor after carbon disulfide-based pesticide exposures. Arch Neurol. 1991;48:866–870. doi: 10.1001/archneur.1991.00530200108029. [DOI] [PubMed] [Google Scholar]
- 14.Costa LG, et al. Neurotoxicity of pesticides: a brief review. Front Biosci. 2008;13:1240–1249. doi: 10.2741/2758. [DOI] [PubMed] [Google Scholar]
- 15.Krieger R, editor. Handbook of Pesticide Toxicology. Academic Press; 2001. [Google Scholar]
- 16.Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Meco G, et al. Parkinsonism after chronic exposure to the fungicide maneb (manganese ethylene-bis-dithiocarbamate) Scand J Work Environ Health. 1994;20:301–305. doi: 10.5271/sjweh.1394. [DOI] [PubMed] [Google Scholar]
- 18.Thiruchelvam M, et al. Potentiated and preferential effects of combined paraquat and maneb on nigrostriatal dopamine systems: environmental risk factors for Parkinson’s disease? Brain Res. 2000;873:225–234. doi: 10.1016/s0006-8993(00)02496-3. [DOI] [PubMed] [Google Scholar]
- 19.Wang XF, et al. Inhibitory effects of pesticides on proteasome activity: implication in Parkinson’s disease. Neurobiol Dis. 2006;23:198–205. doi: 10.1016/j.nbd.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez VM, et al. Sustained exposure to the widely used herbicide atrazine: altered function and loss of neurons in brain monoamine systems. Environ Health Perspect. 2005;113:708–715. doi: 10.1289/ehp.7783. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Brighina L, et al. {alpha}-Synuclein, pesticides, and Parkinson disease. A case-control study. Neurology. doi: 10.1212/01.wnl.0000304049.31377.f2. in press. [DOI] [PubMed] [Google Scholar]
- 22.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 23.Snyder SH, D’Amato RJ. Predicting Parkinson’s disease. Nature. 1985;317:198–199. doi: 10.1038/317198a0. [DOI] [PubMed] [Google Scholar]
- 24.Hertzman C, et al. Parkinson’s disease: a case-control study of occupational and environmental risk factors. Am J Ind Med. 1990;17:349–355. doi: 10.1002/ajim.4700170307. [DOI] [PubMed] [Google Scholar]
- 25.Liou HH, et al. Environmental risk factors and Parkinson’s disease: a case-control study in Taiwan. Neurology. 1997;48:1583–1588. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- 26.Semchuk KM, et al. Parkinson’s disease and exposure to agricultural work and pesticide chemicals. Neurology. 1992;42:1328–1335. doi: 10.1212/wnl.42.7.1328. [DOI] [PubMed] [Google Scholar]
- 27.Koller WC. Paraquat and Parkinson’s disease. Neurology. 1986;36:1147. doi: 10.1212/wnl.36.8.1147-b. [DOI] [PubMed] [Google Scholar]
- 28.Naylor JL, et al. Further evidence that the blood/brain barrier impedes paraquat entry into the brain. Hum Exp Toxicol. 1995;14:587–594. doi: 10.1177/096032719501400706. [DOI] [PubMed] [Google Scholar]
- 29.Widdowson PS, et al. Influence of age on the passage of paraquat through the blood-brain barrier in rats: a distribution and pathological examination. Hum Exp Toxicol. 1996;15:231–236. doi: 10.1177/096032719601500308. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu K, et al. Carrier-mediated processes in blood–brain barrier penetration and neural uptake of paraquat. Brain Res. 2001;906:135–142. doi: 10.1016/s0006-8993(01)02577-x. [DOI] [PubMed] [Google Scholar]
- 31.Prasad K, et al. Prolonged toxicokinetics and toxicodynamics of paraquat in mouse brain. Environ Health Perspect. 2007;115:1448–1453. doi: 10.1289/ehp.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woolley DE, et al. Does paraquat (PQ) mimic MPP+ toxicity? Proc West Pharmacol Soc. 1989;32:191–193. [PubMed] [Google Scholar]
- 33.Manning-Bog AB, et al. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem. 2002;277:1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- 34.Manning-Bog AB, et al. Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J Neurosci. 2003;23:3095–3099. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brooks AI, et al. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res. 1999;823:1–10. doi: 10.1016/s0006-8993(98)01192-5. [DOI] [PubMed] [Google Scholar]
- 36.Ossowska K, et al. A slowly developing dysfunction of dopaminergic nigrostriatal neurons induced by long-term paraquat administration in rats: an animal model of preclinical stages of Parkinson’s disease? Eur J Neurosci. 2005;22:1294–1304. doi: 10.1111/j.1460-9568.2005.04301.x. [DOI] [PubMed] [Google Scholar]
- 37.Ramachandiran S, et al. Divergent mechanisms of paraquat, MPP+, and rotenone toxicity: oxidation of thioredoxin and caspase-3 activation. Toxicol Sci. 2007;95:163–171. doi: 10.1093/toxsci/kfl125. [DOI] [PubMed] [Google Scholar]
- 38.Richardson JR, et al. Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol Sci. 2005;88:193–201. doi: 10.1093/toxsci/kfi304. [DOI] [PubMed] [Google Scholar]
- 39.Cory-Slechta DA. Studying toxicants as single chemicals: does this strategy adequately identify neurotoxic risk? Neurotoxicology. 2005;26:491–510. doi: 10.1016/j.neuro.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Barlow BK, et al. Increased synaptosomal dopamine content and brain concentration of paraquat produced by selective dithiocarbamates. J Neurochem. 2003;85:1075–1086. doi: 10.1046/j.1471-4159.2003.01773.x. [DOI] [PubMed] [Google Scholar]
- 41.Bhatt MH, et al. Acute and reversible parkinsonism due to organophosphate pesticide intoxication: five cases. Neurology. 1999;52:1467–1471. doi: 10.1212/wnl.52.7.1467. [DOI] [PubMed] [Google Scholar]
- 42.Hancock DB, et al. Pesticide exposure and risk of Parkinson’s disease: a family-based case-control study. BMC Neurology. 2008;8:6. doi: 10.1186/1471-2377-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karen DJ, et al. Striatal dopaminergic pathways as a target for the insecticides permethrin and chlorpyrifos. Neurotoxicology. 2001;22:811–817. doi: 10.1016/s0161-813x(01)00063-8. [DOI] [PubMed] [Google Scholar]
- 44.Schapira AH, et al. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- 45.Thiffault C, et al. Increased striatal dopamine turnover following acute administration of rotenone to mice. Brain Res. 2000;885:283–288. doi: 10.1016/s0006-8993(00)02960-7. [DOI] [PubMed] [Google Scholar]
- 46.Ferrante RJ, et al. Systemic administration of rotenone produces selective damage in the striatum and globus pallidus, but not in the substantia nigra. Brain Res. 1997;753:157–162. doi: 10.1016/s0006-8993(97)00008-5. [DOI] [PubMed] [Google Scholar]
- 47.Betarbet R, et al. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 48.Sherer TB, et al. An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22:7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med. 2006;38:333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- 50.Liu B, et al. Parkinson’s disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environ Health Perspect. 2003;111:1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherer TB, et al. Mechanism of toxicity of pesticides acting at complex I: relevance to environmental etiologies of Parkinson’s disease. J Neurochem. 2007;100:1469–1479. doi: 10.1111/j.1471-4159.2006.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corrigan FM, et al. Organochlorine insecticides in substantia nigra in Parkinson’s disease. J Toxicol Environ Health Part A. 2000;59:229–234. doi: 10.1080/009841000156907. [DOI] [PubMed] [Google Scholar]
- 53.Fleming L, et al. Parkinson’s disease and brain levels of organochlorine pesticides. Ann Neurol. 1994;36:100–103. doi: 10.1002/ana.410360119. [DOI] [PubMed] [Google Scholar]
- 54.Hatcher JM, et al. Disruption of dopamine transport by DDT and its metabolites. Neurotoxicology. doi: 10.1016/j.neuro.2008.04.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corrigan FM, et al. Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson’s disease. Exp Neurol. 1998;150:339–342. doi: 10.1006/exnr.1998.6776. [DOI] [PubMed] [Google Scholar]
- 56.Hayes WJJ, editor. Pesticides Studied in Man. Williams & Wilkins; 1982. [Google Scholar]
- 57.Jorgenson JL. Aldrin and dieldrin: a review of research on their production, environmental deposition and fate, bioaccumulation, toxicology, and epidemiology in the United States. Environ Health Perspect. 2001;109(Suppl 1):113–139. doi: 10.1289/ehp.01109s1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanthasamy AG, et al. Dieldrin-induced neurotoxicity: relevance to Parkinson’s disease pathogenesis. Neurotoxicology. 2005;26:701–719. doi: 10.1016/j.neuro.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 59.Kitazawa M, et al. Dieldrin induces apoptosis by promoting caspase-3-dependent proteolytic cleavage of protein kinase Cdelta in dopaminergic cells: relevance to oxidative stress and dopaminergic degeneration. Neuroscience. 2003;119:945–964. doi: 10.1016/s0306-4522(03)00226-4. [DOI] [PubMed] [Google Scholar]
- 60.Uversky VN, et al. Pesticides directly accelerate the rate of alpha-synuclein fibril formation: a possible factor in Parkinson’s disease. FEBS Lett. 2001;500:105–108. doi: 10.1016/s0014-5793(01)02597-2. [DOI] [PubMed] [Google Scholar]
- 61.Kirby ML, et al. Selective effects of cyclodiene insecticides on dopamine release in mammalian synaptosomes. Toxicol Appl Pharmacol. 2002;181:89–92. doi: 10.1006/taap.2002.9405. [DOI] [PubMed] [Google Scholar]
- 62.Hatcher JM, et al. Dieldrin exposure induces oxidative damage in the mouse nigrostriatal dopamine system. Exp Neurol. 2007;204:619–630. doi: 10.1016/j.expneurol.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richardson JR, et al. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. FASEB J. 2006 doi: 10.1096/fj.06-5864fje. ( http://www.fasebj.org/) [DOI] [PubMed]
- 64.Kitazawa M, et al. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free Radic Biol Med. 2001;31:1473–1485. doi: 10.1016/s0891-5849(01)00726-2. [DOI] [PubMed] [Google Scholar]
- 65.Sanchez-Ramos J, et al. Toxicity of dieldrin for dopaminergic neurons in mesencephalic cultures. Exp Neurol. 1998;150:263–271. doi: 10.1006/exnr.1997.6770. [DOI] [PubMed] [Google Scholar]
- 66.Baker DB, et al. Evaluation of human exposure to the heptachlor epoxide contamination of milk in Hawaii. Hawaii Med J. 1991;50:108–112. 118. [PubMed] [Google Scholar]
- 67.Miller GW, et al. Heptachlor alters expression and function of dopamine transporters. Neurotoxicology. 1999;20:631–637. [PubMed] [Google Scholar]
- 68.Purkerson-Parker S, et al. Dopamine transporter binding in the rat striatum is increased by gestational, perinatal, and adolescent exposure to heptachlor. Toxicol Sci. 2001;64:216–223. doi: 10.1093/toxsci/64.2.216. [DOI] [PubMed] [Google Scholar]
- 69.Bloomquist JR, et al. Selective effects of insecticides on nigrostriatal dopaminergic nerve pathways. Neurotoxicology. 2002;23:537–544. doi: 10.1016/s0161-813x(02)00031-1. [DOI] [PubMed] [Google Scholar]
- 70.Gillette JS, Bloomquist JR. Differential up-regulation of striatal dopamine transporter and [alpha]-synuclein by the pyrethroid insecticide permethrin. Toxicol Appl Pharmacol. 2003;192:287–293. doi: 10.1016/s0041-008x(03)00326-0. [DOI] [PubMed] [Google Scholar]
- 71.Elwan MA, et al. Pyrethroid pesticide-induced alterations in dopamine transporter function. Toxicol Appl Pharmacol. 2006;211:188–197. doi: 10.1016/j.taap.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller GW, et al. Dopamine transporters and neuronal injury. Trends Pharmacol Sci. 1999;20:424–429. doi: 10.1016/s0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- 73.Langston JW, et al. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 74.Fei Q, Ethell DW. Maneb potentiates paraquat neurotoxicity by inducing key Bcl-2 family members. J Neurochem. doi: 10.1111/j.1471-4159.2008.05293.x. in press. [DOI] [PubMed] [Google Scholar]
- 75.Hornsby AG, et al., editors. Pesticide Properties in the Environment. Springer-Verlag; 1996. [Google Scholar]
- 76.McCay DS, et al., editors. Environmental Fate of Organic Chemicals. Taylor and Francis; 2006. [Google Scholar]