To the Editor
Diabetes mellitus increases the risk of dependence in Activities of Daily Living (ADLs) in older adults.1,2 However, stringent glycemic control in frail older adults may not improve physical function3 and can lead to adverse effects,4 especially in frail older adults. Thus, we sought to determine the relationship between Hemoglobin A1c (HbA1c) levels, persistent functional decline and death in a national sample of nursing home (NH) residents.
Methods
Study subjects were residents in one of 114 Veterans Affairs (VA) NHs age 65 years or older who were admitted between 2005 and 2011 with a diagnosis of diabetes mellitus by ICD-9 codes or HbA1c >6.5%. In addition, we excluded patients not receiving glucose lowering medications.
Our outcomes were death and persistent functional decline. Functional decline was determined using the Minimum Data Set (MDS)-ADL score, which accounts for seven ADLs. Each ADL was scored from 0–4 (0= independence and 4= total dependence) resulting in a summary score ranging from 0–28, with 28 indicating total dependence. Since functional status may fluctuate, we chose persistent functional decline as our outcome, defined as an increase in MDS-ADL score of 2 points on 2 consecutive assessments compared to the baseline score.5
Our primary predictor was Hemoglobin A1c (HbA1c) level on the day of the MDS-ADL assessment, determined through linear interpolation of HbA1c measurements before and after the MDS-ADL assessment. HbA1c was categorized into 4 levels: 6.0–6.9%, 7.0–7.9%, 8.0–8.9%, ≥9.0%.
We accounted for potential confounders, including sex, age, baseline HbA1c level, baseline MDS-ADL score, and comorbidities (congestive heart failure, cardiac arrhythmias, valvular heart disease, hypertension, peripheral vascular disease, chronic pulmonary diseases, cancer, hypothyroidism, liver disease and paralysis.) The unit of analysis was each resident’s first admission. We stratified results by type of glucose lowering treatment: 1) Insulin use, 2) Sulfonylurea use (but no insulin), or 3) other glucose lowering medications (but no insulin or sulfonylureas).
We performed a Kaplan-Meier survival analysis, censoring for death or discharge of more than 30 days. We used Cox proportional hazards regression to estimate the hazard for functional decline considering death as a competing risk using the Fine and Gray method.6 We performed a sensitivity analysis, restricting our analysis to those with a NH stay of 6 months or more.
Results
In our cohort of 7459 residents, average age was 76 (±7.0), 98% were male, 49% had a baseline HbA1c of 6.0–6.9% and 17% had a baseline ADL score ≥17, suggesting the need for extensive assistance with most ADLs, and 68% of residents stayed for <6 months. There was no statistically significant difference in percentage with functional decline or death over 24 months between the 4 levels of baseline HbA1c. (Figure 1) Compared to the reference group (HbA1c 7–7.9%), the adjusted hazard ratio (HR) for persistent functional decline considering death as a competing risk among those with baseline HBA1c of 6–6.9% was 0.94 (95% CI = 0.80–1.10), while the adjusted HR for HbA1c ≥9.0% was 0.88 (95% CI = 0.65–1.18). Stratified results were similar, with no level of HbA1c associated with persistent functional decline among residents on insulin, sulfonylureas or other glucose lowering medications. Results were unchanged when restricting analysis to residents who stayed for 6 months or more.
Figure 1. Survival Curves for Functional Decline or Death by baseline A1C Value.
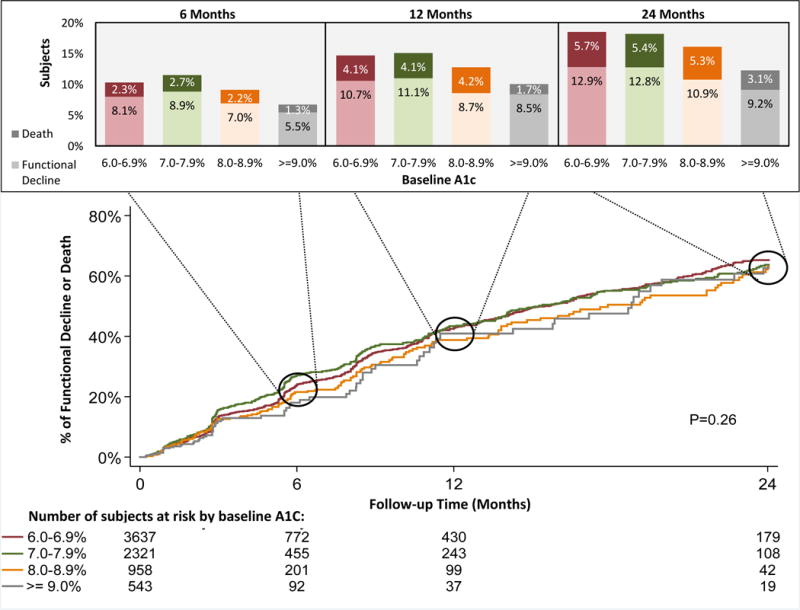
*Kaplan-Meier figure accounts for censoring while the bar graphs do not, so the percentage with outcome differs between the Kaplan-Meier figure and bar graphs.
Discussion
In a national sample of VA NH residents with diabetes mellitus from 2005 to 2011, the degree of glycemic control was not associated with persistent functional decline or death over 2 years. Our results suggest that even HbA1c level greater than 9% may not increase the risk of persistent functional decline in NH residents with diabetes mellitus, suggesting stringent glycemic control is not beneficial for these patients.
Table 1.
Death or functional decline at 2 years by hemoglobin A1c level among NH residents treated with glucose lowering agents
| Group | HbA1c,% | N | Total deaths, n (row %) | Total with persistent functional decline, n (row %) | Total with death or persistent functional decline, n (row %) | Unadjusted HR for death or persistent functional decline (95% CI) | Adjusted HR for death or persistent functional decline (95% CI) | Adjusted HR for persistent functional decline, with death as competing risk (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Overall | 6–6.9 | 3637 | 287 (7.9) | 469 (12.9) | 676 (18.6) | 0.96 (0.85 – 1.08) | 0.94 (0.83 – 1.06) | 0.94 (0.80 – 1.10) |
| 7–7.9 | 2321 | 200 (8.6) | 298 (12.8) | 424 (18.3) | Referencea | Referencea | Referencea | |
| 8–8.9 | 958 | 75 (7.8) | 104 (10.9) | 155 (16.2) | 0.87 (0.72 – 1.04) | 0.87 (0.73 – 1.03) | 0.81 (0.65 – 1.01) | |
| ≥ 9 | 543 | 25 (4.6) | 50 (9.2) | 67 (12.3) | 0.82 (0.63 – 1.05) | 0.85 (0.66 – 1.08) | 0.88 (0.65 – 1.18) | |
| Insulin | 6–6.9 | 921 | 63 (6.8) | 111 (12.1) | 162 (17.6) | 1.03 (0.82 – 1.29) | 1.00 (0.82 – 1.24) | 1.01 (0.76 – 1.35) |
| 7–7.9 | 853 | 55 (6.5) | 94 (11.0) | 137 (16.1) | Referencea | Referencea | Referencea | |
| 8–8.9 | 437 | 35 (8.0) | 50 (11.4) | 73 (16.7) | 0.99 (0.74 – 1.31) | 1.00 (0.75 – 1.34) | 0.97 (0.67 – 1.40) | |
| ≥ 9 | 281 | 13 (4.6) | 24 (8.5) | 33 (11.7) | 0.81 (0.55 – 1.19) | 0.89 (0.63 – 1.26) | 0.89 (0.58 – 1.36) | |
| Sulfonyl urea | 6–6.9 | 1586 | 155 (9.8) | 216 (13.6) | 329 (20.7) | 0.89 (0.75 – 1.07) | 0.88 (0.72 – 1.07) | 0.84 (0.67 – 1.06) |
| 7–7.9 | 854 | 103 (12.1) | 132 (15.5) | 187 (21.9) | Referencea | Referencea | Referencea | |
| 8–8.9 | 290 | 22 (7.6) | 36 (12.4) | 50 (17.2) | 0.78 (0.57 – 1.06) | 0.77 (0.60 – 0.98) | 0.82 (0.61 – 1.09) | |
| ≥ 9 | 143 | 9 (6.3) | 20 (14.0) | 26 (18.2) | 1.11 (0.74 – 1.67) | 1.19 (0.80 – 1.77) | 1.21 (0.77 – 1.90) | |
| Other Oral | 6–6.9 | 1130 | 69 (6.1) | 142 (12.6) | 185 (16.4) | 0.98 (0.77 – 1.25) | 0.92 (0.72 – 1.17) | 1.03 (0.77 – 1.37) |
| 7–7.9 | 614 | 42 (6.8) | 72 (11.7) | 100 (16.3) | Referencea | Referencea | Referencea | |
| 8–8.9 | 231 | 18 (7.8) | 18 (7.8) | 32 (13.9) | 0.83 (0.55 – 1.23) | 0.85 (0.58 – 1.23) | 0.63 (0.38 – 1.07) | |
| ≥ 9 | 119 | 3 (2.5) | 6 (5.0) | 8 (6.7) | 0.48 (0.23 – 0.99) | 0.44 (0.22 – 0.87) | 0.47 (0.22 – 1.02) |
A HbA1c level of 7–7.9% was chosen as reference because the American Geriatrics Society recommends 7–8% in older adults as appropriate glycemic target.
Acknowledgments
This work was conducted with support from the Paul Beeson Career Development Award from the National Institute on Aging (K23AG040779) and the American Federation on Aging Research (SJL). Additionally, this work was made possible with the support and facilities from the San Francisco VA Medical Center.
References
- 1.Wong E, Backholer K, Gearon E, et al. Diabetes and risk of physical disability in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinology. 2013;1:106–14. doi: 10.1016/S2213-8587(13)70046-9. 10.1016. [DOI] [PubMed] [Google Scholar]
- 2.Volapto S, Maraldi C, Rellin R. Type 2 diabetes and risk for functional decline and disability in older person. Current Diabetes Reviews. 2010;6(3):134–143. doi: 10.2174/157339910791162961. [DOI] [PubMed] [Google Scholar]
- 3.Yau CK, Eng C, Cenzer-Stijacic I, Boscardin WJ, Rice-Trumble K, Lee SJ. Glycosylated hemoglybin and functional decline in community-dwelling nursing home-eligible elderly adults with diabetes mellitus. Journal of the American Geriatrics Society. 2012;60(7):1215–1221. doi: 10.1111/j.1532-5415.2012.04041.x. 10.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, et al. Effectss of intensive glucose lowering in type 2 diabetes. NEJM. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. 10.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finlayson E, Zhao S, Boscardin WJ, Fries BE, Landefeld CS, Dudley RA. Functional status after colon cancer surgery in elderly nursing home residents. Journal of the American Geriatrics Society. 2012;60(5):967–973. doi: 10.1111/j.1532-5415.2012.03915.x. 10.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. 10.2307. [Google Scholar]


