Abstract
Objectives
This study examined the dose-dependent actions of hydrogen sulfide donor sodium hydrosulphide (NaHS) on isometric contractions and ion transport in rat aorta smooth muscle cells (SMC).
Methods
Isometric contraction was measured in ring aortas segments from male Wistar rats. Activity of Na+/K+-pump and Na+,K+,2Cl-cotransport was measured in cultured endothelial and smooth muscle cells from the rat aorta as ouabain-sensitive and ouabain-resistant, bumetanide-sensitive components of the 86Rb influx, respectively.
Results
NaHS exhibited the bimodal action on contractions triggered by modest depolarization ([K+]o=30 mM). At 10−4 M, NaHS augmented contractions of intact and endothelium-denuded strips by ~ 15% and 25%, respectively, whereas at concentration of 10−3 M it decreased contractile responses by more than two-fold. Contractions evoked by 10−4 M NaHS were completely abolished by bumetanide, a potent inhibitor of Na+,K+,2Cl-cotransport, whereas the inhibition seen at 10−3 M NaHS was suppressed in the presence of K+ channel blocker TEA. In cultured SMC, 5×10−5 M NaHS increased Na+,K+,2Cl- - cotransport without any effect on the activity of this carrier in endothelial cells. In depolarized SMC, 45Ca influx was enhanced in the presence of 10−4 M NaHS and suppressed under elevation of [NaHS] up to 10−3 M. 45Ca influx triggered by 10−4 M NaHS was abolished by bumetanide and L-type Ca2+ channel blocker nicardipine.
Conclusions
Our results strongly suggest that contractions of rat aortic rings triggered by low doses of NaHS are mediated by activation of Na+,K+,2Cl-cotransport and Ca2+ influx via L-type channels.
Abbreviations: NaHS, sodium hydrosulphide; NO, nitric oxide; CO, carbon monoxide; H2S, hydrogen sulfide; CSE, cystathionine-γ-lyase; NaHS, sodium hydrosulphide; sGC, soluble guanylyl cyclase; cGMP, cyclic guanosine monophosphate; KATP, ATP-sensitive potassium channels; KCa, Ca2+-activated potassium channels; EDHF, endothelium-derived hyperpolarizing factor; SMC, smooth muscle cells; VSMC, vascular smooth muscle cells; PE, phenylephrine; NKCC, Na+,K+,2Cl- cotransport; PSS, physiologically-balanced salt solution; VSMR, vascular smooth muscles from rat; EC, endothelial cells; TEA, tetraethylammonium chloride; RAEC, endothelial cells from rat aorta; RASMC, smooth muscle cells from rat aorta; COX, cyclooxygenase
Keywords: Smooth muscle cells; Rat aorta; Hydrogen sulfide; Na+,K+,2Cl-cotransport; Ca2+ influx; Contraction
Highlights
-
•
NaHS induces rat aorta contractions through activation of Na+,K+,2Cl-cotransport and Ca2+ influx via L-type channels.
-
•
NaHS increased Na+,K+,2Cl--cotransport activity in smooth muscle cells but not in endothelial cells.
-
•
Low doses of NaHS enhanced 45Ca influx in smooth muscle cells, but high doses of NaHS suppressed it.
1. Introduction
Nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S) are gasotransmitters synthesized endogenously from arginine, glycine-derived heme and cysteine, respectively. They freely penetrate across the plasma membrane and trigger cell signaling in a receptor-independent manner. In the cardiovascular system, H2S is mainly produced in vascular smooth muscle cells (SMC), periventitial adipose tissue, and erythrocytes by cystathionine-β-synthetase, cystathionine-γ-lyase (CSE) and 3-mercaptosulfurtransferase [1], [2].
Numerous research teams reported that the application of H2S donor sodium hydrosulphide (NaHS) leads to dilatation in blood vessels of different origin [1], [3], [4]. In contrast to NO and CO the vasorelaxant effect of NaHS is not mediated by the cGMP pathway [5], [6]. H2S activates ATP-sensitive K+ channels (KATP) and/or Ca2+-activated K+ channels (KCa) [1], [7]. Indeed, H2S-induced vasodilatation was mimicked by KATP openers pinacidil diazoxide, and abolished by glibenclamaide and other inhibitors of K+ channels [3], [4]. Vasorelaxative actions of H2S might also be mediated by partial inhibition of phosphodiesterase activity [8].
Al-Magableh and Hart demonstrated that NaHS-induced relaxation of mouse aorta was not affected by the removal of endothelium [9] thus indicating vascular SMC as a major target of H2S. This conclusion, however, contradicts the data obtained in mice with a target deletion of the gene encoding CSE. Yang and co-workers found that CSE-/--mice display pronounced hypertension and diminished endothelium-dependent vasorelaxation [10]. More recently, it was shown that CSE knock-out mice exhibited elevated resting membrane potential in the SMC of mesenteric arteries but not that of the aorta thus fulfiling the role of H2S in peripheral resistant blood vessels as an endothelium-derived hyperpolarizing factor (EDHF) [11].
H2S actions are complex, showing great species and vascular bed differences. Several research groups reported that NaHS at low concentrations exhibited contractile activity in norepinephrine-treated rat pulmonary arteries [12], in rat and mouse aortic rings precontracted with phenylephrine (PE) and elevated [K+]o [13], in PE-treated rat gastric arteries [14] and human internal mammary arteries [15]. In contrast to relaxant actions, elevation of PE-induced contraction by low doses of NaHS was abolished in endothelium-denuded mouse aorta [13] as well as in mouse aorta and rat gastric artery treated with inhibitors of NO synthase, soluble guanylate cyclase (sGC) and cyclooxygenase (COX) [13], [14]. NaHS-induced contraction was also stronger in the acetylcholine-prerelaxed human internal mammary artery suggesting inhibition of NO- and EDHF-induced relaxation [15].
Previously, we found that ubiquitous isoform of Na+,K+,2Cl- -cotransporter (NKCC1) affects vascular SMC (VSMC) contraction via regulation of intracellular Cl- concentration, electrical membrane potential and Ca2+ influx mediated by voltage-gated L-type Ca2+ channels [16], [17]. In preliminary experiments we have also shown that NaHS triggers constriction in endothelium-denuded vascular segments that was diminished by NKCC inhibitor [18]. In this study we expanded examination of the role of ion transporters in H2S-induced signaling by comparing the dose-dependent actions of NaHS on the contractions of intact and endothelium-denuded rat aortic rings and inwardly-directed K+ and Ca2+ transport in cultured SMC and endothelial cells (EC).
2. Material and methods
2.1. Preparation of aortic rings
Endothelium-denuded aortic rings were obtained from the thoracic aorta of 11- to 13-week-old Wistar rats euthanized under deep intraperitoneal anaesthesia with sodium pentobarbital (Nembutal, 70 mg/kg) in accordance with institutional animal care guidelines. The isolated aorta was placed in physiologically-balanced salt solution (PSS) containing 120.4 mM NaCl, 5.9 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 5.5 mM glucose and 15 mM tris-HCl (pH 7.4). Connective tissue and fat were taken out with scissors whereas the endothelium was removed by careful rotation of a wooden manipulator inside the VSMR lumen just before the experiments. The 2- to 3-mm aortic rings were either used immediately or stored at 4 єC for up to 24 h. In preliminary experiments, we documented that 24-h storage did not affect aortic ring contractile responses.
2.2. Measurement of aortic ring tension
Isometric contraction of aortic rings was measured using four-channel Tissue Bath System Myobath II. Aortic rings were mounted in 10-ml baths with stainless steel hooks inserted into the vascular ring orifice. One hook was fastened to a mechanical force transducer. The tissues were bathed in PSS at 37 °C and bubbled with room air at a volumetric speed of ~1 ml/min. To control the contractile response, the rat vascular smooth muscles (VSMR) were equilibrated for 1 h at tension of 0.5–1 g and exposed to K+o-induced depolarization caused by isosmotic substitution of NaCl with KCl. The responses to NaHS were expressed in percentage of the contraction obtained with 30 mM KCl, which was taken as 100%. In part of experiments aortic rings were treated with 10 μM bumetanide or10 mM tetraethylammonium chloride (TEA).
2.3. Cultured cells
The precise measurement of cell volume and inward ion fluxes in VSMR are complicated by a relatively large extracellular space, the presence of fibroblasts, and VSMC heterogeneity. On the other hand, long-term cultured VSMC rapidly down regulate the expression of several specific genes that define their contractile phenotype in vivo. Keeping this in mind, we employed VSMC isolated from rat aorta and maintained in culture in accordance with previously published protocol up to passages 3–8 [19]. Endothelial cells from rat aorta (EC) were kindly provided by Dr. Thorin-Trescases (Institute of Cardiology, University of Montreal, Canada). These cells were isolated and passaged 3–4 times as described elsewhere [20], [21]. To establish quiescence, VSMC were incubated before experiments during 24 h in the presence of 0.2% calf serum.
2.4. Measurement of 86Rb and 45Ca influx
Activity of Na+/K+-pump and Na+,K+,2Cl- – cotransporter was measured as an ouabain-sensitive and ouabain-resistant, bumetanide-sensitive components of the 86Rb influx, respectively. The cells seeded in 24-well plates were washed twice with 2-ml aliquots of medium A containing 150 mM NaCl and 10 mM HEPES-tris buffer (pH 7.4). Then, the medium was aspirated and 0.25 ml of medium B containing 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5 mM glucose, 20 mM HEPES-tris (pH 7.4) and tested compounds were added. It was followed by 10 min incubation at 37 °C, 0.25 ml of medium B containing 1–2 μC/ml 86Rb ± ouabain and bumetanide at concentrations of 3 mM and 20 μM, respectively. To measure 45Ca influx, washed cells were incubated during 30 min in 1 ml medium B and 10 min in medium B containing 0.2 mM CaCl2 ± 2 μM nicardipine and other compounds indicated in the Table 1 legends. Thereafter, each well was supplemented with 0.25 ml of prewarmed medium containing 4 μCi/ml 45Ca and NaCl and KCl at total concentration of 150 mM. In preliminary experiments, we found that 86Rb and 45Ca uptake by VSMC from rat aorta and EC from rat aorta is linear up to 25 and 10 min, respectively. Considering this, 86Rb and 45Ca uptake was terminated in 10 and 5 min, respectively, by the addition of 2 ml of ice-cold medium W containing 100 mM MgCl2 and 10 mM HEPES-tris buffer (pH 7.4). The cells were washed 3 times with ice-cold medium W and radioactivity of the incubation medium and cell lysate was measured with a liquid scintillation analyzer. The rate of ion influx (V) was calculated as V=A/am, where A was the radioactivity of the samples (cpm), a was the specific radioactivity of K+ (86Rb) or 45Ca (cpm/nmol), and m was protein content measured with the modified Lowry method. For more details, see [22], [23].
Table 1.
Effect of NaHS, bumetanide, TEA and nicardipine on the rate of 45Ca influx in cultured smooth muscle cells.
| Additions, μM |
45Ca influx, pmol/mg protein/5 min |
|
|---|---|---|
| [K+]o=5 mM | [K+]o=30 mM | |
| 1. None (control) | 100 | 278±23* |
| 2. Nicardipine, 2 | 121±15 | 96±11# |
| 3. Bumetanide, 10 | 106±13 | 186±28*, # |
| 4. TEA, 104 | 87±9 | 268±18* |
| 5. NaHS, 100 | 110±20 | 387±25*, # |
| 9. NaHS, 1000 | 106±15 | 177±18*, # |
Means ± S.E. obtained in 5 independent experiments performed in quadruplicates are shown. The rate of Ca2+ influx at [K+]o = 5 mM and in the absence of any additions listed in the left column varied from 389 and 425 nmol / mg protein / 5 min was taken as 100%.
p<0.05 compared to 45Ca influx at [K+]o = 5 mM.
- p < 0.05 compared to control at [K+]o = 30 mM (at [K+]o = 5 mM, there was no significant differences of Ca2+ influx in the presence of additions in comparison with control).
2.5. Chemicals and statistics
86RbCl and 45CaCl was from Perkin Elmer (Waltman, MA, USA). The rest of chemicals were obtained from Sigma (St. Louis, MO, USA) and Serva (Heidelberg, Germany). The stock solution of bumetanide (20 mM) was prepared in DMSO. The stock solution of NaHS was prepared in distalled water immediately before use. The maximal concentrations of H2S in the solutions of NaHS may be calculated according to the formula: [H2S] = [NaHS]/(1 + 10−pK/10−pH) [24], [25], [26], where the pK at 37 °C is 6.755 [26] and the pH of the solution is 7.4. Earlier studies [27], [28], [29], [30] demonstrated that at pH of 7.4 and 37 °C about 20% of sulfide is present as H2S H2S. According to previous data, solutions prepared by bubbling H2S gas and by dissolving NaHS produce the similar effects on smooth muscle contraction [31], [32], [33].
The data, presented as means ± SE, were analyzed by Student's t-test or the t-test for dependent samples, as appropriate. Significance was defined as p < 0.05.
3. Results
3.1. Effect of NaHS on aortic ring contractions
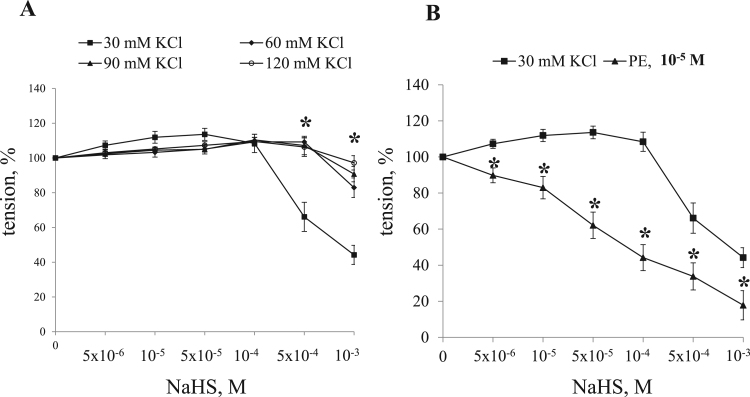
We did not detect any significant impact of NaHS in the range from 5×10−6 till 10−3 M on the baseline tension of endothelium-denuded aortic rings (data not shown). Fig. 1 displays that under partial depolarization triggered by elevation of KCl up to 30 mM, NaHS exhibit the bimodal action: in the range from 5×10−6 to 10−4 M this compound dose-dependently increases contractions by ~ 25% (127.5 ± 5.7% (n = 6; p < 0.05) of KCl-induced contraction) whereas further elevation of its concentration up to 10−3 M decreased the maximal tension by ~ 2-fold (48.3 ± 5.0% (n = 6; p < 0.05) of KCl-induced contraction). Stronger depolarization in the presence of 60 mM, 90 mM and 120 mM KCl attenuated both contractile and inhibitory responses of NaHS (Fig. 1A). The inhibitory action of NaHS was also revealed in endothelium-denuded aortic strips precontracted by α1-adrenomimetic phenylephrine (Fig. 1B). Previously we reported that high-ceiling diuretic bumetanide suppresses contractions of rat aorta and mice mesenteric arteries triggered by phenylephrine and modest depolarization in the presence of 30 mM KCl but does not affect contractions triggered by higher depolarization in the presence of 60 mM KCl. Ubiquitous Na+,K+,2Cl- - cotransporter NKCC1 is the only isoform of this carrier expressed in smooth muscle and endothelial cells [34]. Data obtained in genetically engineering mice demonstrated that bumetanide affect smooth muscle contraction by inhibition of NKCC1, attenuation of [Cl-]i, smooth muscle hyperpolarization and inhibition of Ca2+-influx via L-type Ca2+ channels [16], [17], [35]. Keeping this observation in mind, in the rest of experiments we investigated the role of Na+,K+,2Cl- cotransporter in the bimodal action of NaHS on aortic strips precontracted by 30 mM KCl.
Fig. 1.
Dose-dependent actions of NaHS on contractions of endothelium-denuded aortic rings at extracellular KCl concentration of 30, 60, 90 and 120 mM. and in the presence of 10 µM phenylephrine. Maximal contractions in the absence of NaHS were taken as 100%. Means and standard errors obtained in 6 independent experiments are shown. * - p <0.05.
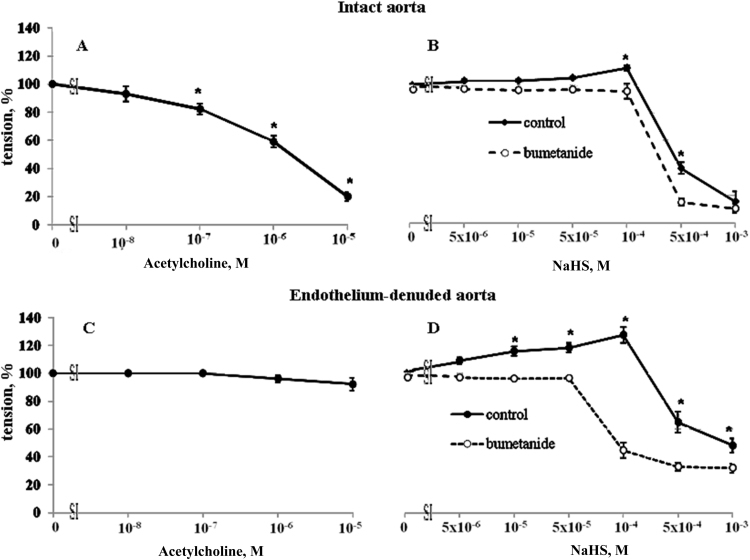
Acetylcholine suppressed baseline contraction of intact aorta (Fig. 2A) without any significant impact on this parameter in endothelium-denuded aortic rings (Fig. 2C). Unlike in endothelium-denuded aorta (Fig. 2D), NaHS at concentration of 10−5, 5×10−5 and 10-4 M did not significantly affect contraction of intact aortic rings subjected to depolarization in the presence of 30 mM KCl whereas inhibitory actions of 5×10−4 and 10−3 M NaHS were preserved (39.7 ± 4.3 % and 15.5 ± 7.5%, respectively, of KCl-induced contraction (n = 6; p < 0.05)) (Fig. 2B).
Fig. 2.
Dose-dependent actions of acetylcholine (A and C) and NaHS (B and D) on contractions of intact (A and B) and endothelium-denuded (C and D) rat aortic rings in the presence and absence of 10−5 M bumetanide. Maximal contractions in the absence of acetylcholine and NaHS were taken as 100%. Means and standard errors obtained in 6 independent experiments are shown. * p < 0.05 compared to bumetanide-treated rings.
Considering the involvement of the NKCC1 in the contractile responses of SMC subjected to partial depolarization [16], we examined the action of a potent inhibitor of NKCC1 bumetanide on the bimodal modulation of aortic ring contraction by NaHS. Both in intact and endothelium-denuded aorta 10 μM bumetanide completely blocked contractile responses triggered by low doses of NaHS, whereas inhibitory actions of high doses of NaHS were preserved (Figs. 2B and 2D). We also observed that in endothelium denuded aorta bumetanide reversed contractile responses of 10−4 M NaHS (33.8 ± 5.8% (n = 6; p < 0.05)) and augmented inhibitory actions on contractile responses of higher doses of NaHS (19.0 ± 4.0% (n = 5, p < 0.05) of KCl-induced contraction) (Fig. 2D).
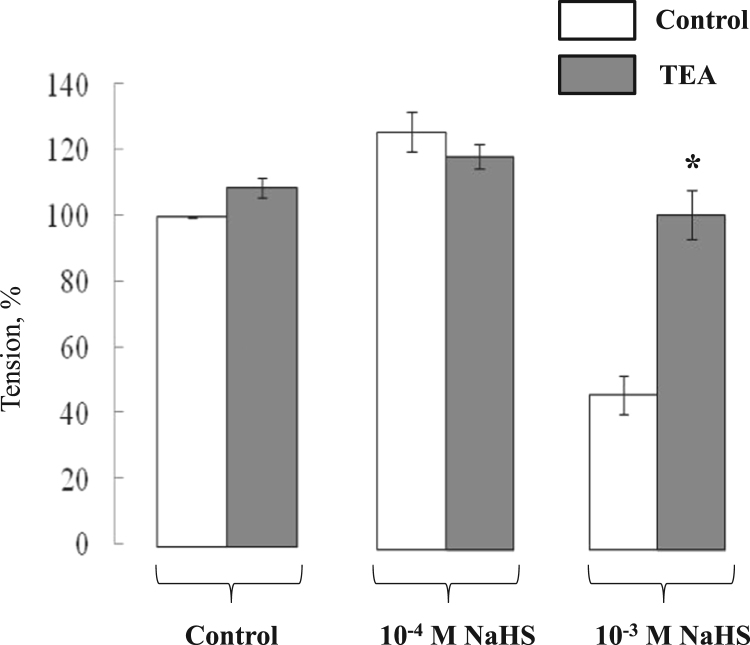
Compelling evidence indicates that H2S-induced dilatation of distinct vascular beds is mediated by activation of KATP- and/or KCa-channels (for review, see [1], [7]). Considering this, we employed TEA as non selective inhibitor of all K+ channels studied so far. Fig. 3 shows that the inhibitory action of 10−3 M NaHS on aortic rings contractions was completely abolished (95.4 ± 7.1% (n = 6; p < 0.05 compared to TEA-untreated rings) in the presence of 10 mM TEA whereas contractions evoked by 10−4 M NaHS were preserved (116.7 ± 3.7% (n = 6; p > 0.05 compared to TEA untreated rings)).
Fig. 3.
Effect of TEA on contractions of rat aortic rings in the presence and absence of 10−4 M and 10−3 M NaHS. Contractions were evoked by elevation of [K+]o up to 30 mM. Maximal contractions in the absence of NaHS were taken as 100%. Means and standard errors obtained in 6 independent experiments are shown. * - p < 0.05 compared to TEA-untreated rings.
3.2. Effect of NaHS on K+ influx
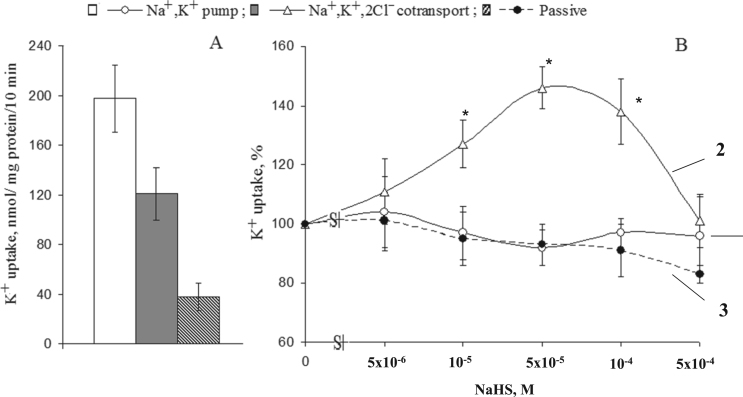
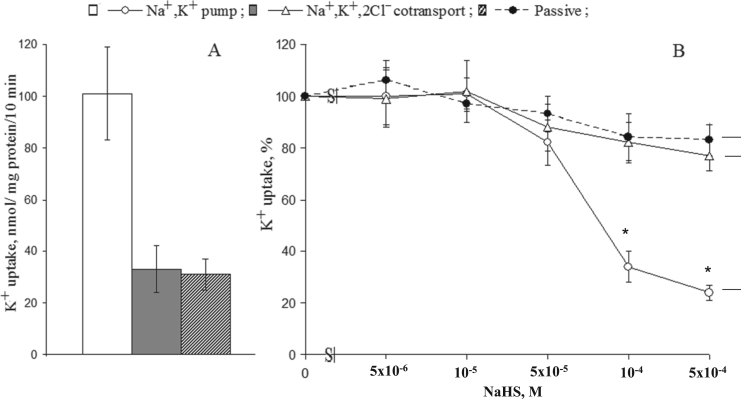
Fig. 4A shows that in SMC, Na+,K+-pump and NKCC measured as a rate of ouabain- and bumetanide-sensitive 86Rb influx, respectively, contributed to 55% and 34% of net K+ uptake. These numbers are consistent with previously reported data [22], [36]. Elevation of NaHS up to 5×10−5 − 10−4 M dose-dependently increased NKCC (Fig. 4B). We did not observe any significant actions of NaHS on the activity of Na+/K+-pump as well on the passive permeability of SMC for K+ estimated as (ouabain+bumetanide)-resistant component of the rate of 86Rb influx.
Fig. 4.
K+ (86Rb) influx in smooth muscle cells from the rat aorta. A. Absolute values of the activity of Na+,K+-pump (ouabain-sensitive component of the rate of 86Rb-influx), NKCC (ouabain-resistant, bumetanide-sensitive component of the rate of 86Rb-influx) and passive permeability for K+ (ouabain+bumetanide)-resistant 86Rb influx). B. Dose-dependent actions of NaHS on Na+/K+-pump (1), NKCC (2) and the rate (ouabain+bumetanide)-resistant K+ (86Rb) influx (3). The values obtained in the absence of NaHS were taken as 100%. Means and standard errors obtained in 5 independent experiments are shown. * p < 0.05 compared to controls.
Fig. 5A shows that in endothelial cells, Na+,K+-pump contributes to ~65% of the net K+ (86Rb) uptake. Elevation of NaHS from 5×10−5 M to 5×10−4 M resulted in ~80%- inhibition of the Na+,K+-pump (Fig. 5B) (Fig. 5A). Unlike SMC, we did not observe any activation of NKCC by low doses of NaHS in cultured endothelial cells from the rat aorta.
Fig. 5.
K+ (86Rb) influx in cultured endothelial cells from the rat aorta. A. Absolute values of the activity of Na+,K+-pump (ouabain-sensitive component of the rate of 86Rb-influx), NKCC (ouabain-resistant, bumetanide-sensitive component of the rate of 86Rb-influx) and passive permeability for K+ (ouabain+bumetanide)-resistant 86Rb influx). B. Dose-dependent actions of NaHS on Na+/K+-pump (1), NKCC (2) and the rate (ouabain+bumetanide)-resistant K+ (86Rb) influx (3). The values obtained in the absence of NaHS were taken as 100%. Means and standard errors obtained in 5 independent experiments are shown. * - p < 0.05 compared to controls.
3.3. Effect of NaHS on Ca2+ influx
Consistently with previous results [22], [23], depolarization of SMC in high-K+ medium resulted in elevation of 45Ca influx by 2–3-fold that was completely abolished by inhibition of voltage-gated L-type Ca2+ channel with nicardipine (Table 1). Addition of 10 μM bumetanide decreased depolarization-induced 45Ca uptake by ~60% without significant impact on the baseline 45Ca uptake. Unlike SMC, neither elevation of [K+]o nor nicardipine and bumetanide affected the rate of 45Ca influx in endothelial cells from the rat aorta (data not shown).
In control medium containing 5 mM K+, neither 10−4 nor 10−3 M NaHS changed the rate of 45Ca influx in SMC (Table 1). In depolarized SMC, nicardipine-sensitive 45Ca influx was further potentiated by 10−4 M NaHS whereas at a concentration of 10−3 M the H2S donor decreased this component of 45Ca influx by ~2-fold. These results are in accordance with increment of nifedipine-sensitive Ca2+ influx detected in Fura-3-loaded rat coronary artery treated with modest doses of NaHS [37]. We also observed that side-by side with nicardipine, the increment of depolarization-induced 45Ca influx seen in the presence of 10−4 M NaHS was completely abolished by bumetanide but was insensitive to TEA (Table 2).
Table 2.
Effect of 100 µM NaHS, bumetanide, TEA and nicardipine on the rate of 45Ca influx in cultured smooth muscle cells.
| Additions, μM |
45Ca influx, pmol/mg protein/5 min |
|
|---|---|---|
| [K+]o=5 mM | [K+]o=30 mM | |
| 1. None (control) | 100 | 278±23* |
| 2. NaHS, 100 | 110±20 | 387±25* |
| 3. NaHS, 100 + bumetanide, 10 | 101±17 | 289±22*, # |
| 4. NaHS, 100 + TEA, 104 | 108±19 | 367±30* |
| 5. NaHS, 100 + nicardipine, 2 | 125±21 | 104±10# |
Means±S.E. obtained in 5 independent experiments performed in quadruplicates are shown. The rate of Ca2+ influx at [K+]o = 5 mM and in the absence of any additions listed in the left column varied from 389 and 425 nmol / mg protein / 5 min was taken as 100%.
- p < 0.05 compared to 45Ca influx at [K+]o = 5 mM.
- p<0.05 compared to control at [K+]o = 30 mM in the presence of 100 μM NaHS ( there was no significant differences of Ca2+ influx in the presence of additions at [K+]o = 5 mM).
In contrast, the inhibitory action of high doses of NaHS on depolarization-induced 45Ca influx was insensitive to bumetanide but was suppressed by TEA (Table 3).
Table 3.
Effect of 1000 µM NaHS, bumetanide, TEA and nicardipine on the rate of 45Ca influx in cultured smooth muscle cells.
| Additions, μM |
45Ca influx, pmol/mg protein/5 min |
|
|---|---|---|
| [K+]o=5 mM | [K+]o=30 mM | |
| 1. None (control) | 100 | 278±23* |
| 2. NaHS, 1000 | 106±15 | 177±18* |
| 3. NaHS, 1000 + bumetanide, 10 | 113±21 | 170±25* |
| 4. NaHS, 1000 + TEA, 104 | 112±16 | 277±18*, # |
| 5. NaHS, 1000 + nicardipine, 2 | 88±14 | 100±12# |
Means ± S.E. obtained in 5 independent experiments performed in quadruplicates are shown. The rate of Ca2+ influx at [K+]o = 5 mM and in the absence of any additions listed in the left column varied from 389 and 425 nmol / mg protein / 5 min was taken as 100%.
- p < 0.05 compared to 45Ca influx at [K+]o = 5 mM.
p<0.05 compared to control at [K+]o = 30 mM, in the presence of 1000 μM NaHS ( there was no significant differences of Ca2+ influx in the presence of additions at [K+]o = 5 mM).
4. Discussion
Our results show the excitatory and inhibitory actions of modest (< 10−4 M) and high (10−3 M) doses of NaHS on the contractions of rat aortic rings triggered by K+o-derived depolarization. These results are consistent with the bimodal action of NaHS on the baseline tension of rat aorta and portal vein [38], human internal mammary arteries [13], [15], rat and mouse aortic rings precontracted with PE and elevated [K+]o [13], PE-treated rat gastric [14] and rat pulmonary artery precontracted with norepinephrine [12]. Most studies reported blood plasma H2S concentration at levels up to 5×10−5 M [13], [31]. We found that aortic ring relaxation triggered by high doses of NaHS is abolished in the presence of TEA, a potent inhibitor of K+ channels, thus indicating activation of these channels. This conclusion is also consistent with data obtained with specific inhibitors of KATP and KCa channels [1], [15], [39], [40], [41], [42], [43]. It is well-documented that activation of K+ channels leads to SMC hyperpolarization and partial inactivation of voltage-gated Ca2+ channels [44]. Indeed, treatment with 10−3 M NaHS resulted in suppression of 45Ca influx evoked by modest depolarization (Table 1) that is consistent with dose-dependent inhibition by NaHS of L-type Ca2+ channels obtained by patch clamp [45].
In contrast to inhibitory effects of NaHS, the mechanisms of activation of contractile responses by low doses of this compound remains poorly understood. Telezhkin and co- workers demonstrated the inhibitory effects of H2S on the α-subunit of big-conductance calcium-activated potassium (BKCa) channels in heterologously transfected HEK293 cells [24]. Likewise, H2S inhibited native BKCa channels in type 1 glomus cells from the isolated mouse carotid body [46] and colonic longitudinal muscle and circular muscle strips [47]. H2S donor directly activated BKCa currents in mouse coronary smooth muscle cells [48], piglet cerebral arterioles [49], [50] and in endothelial cells from rat mesenteric arteries [51], [52]. Guo and co-workers reported that BKCa blockers inhibits H2S-induced relaxation [49]. Our previous results didn’t reveal any modulation of H2S-induced contractions in the presence of BKCa blocker charybdotoxin [53].
Our results demonstrate for the first time that contractile actions of low doses of NaHS is at least partially mediated by activation of Na+,K+,2Cl- - cotransport that, in turn, leads to elevation of intracellular chloride concentration, SMC depolarization and activation of voltage-gated Ca2+ channels. This conclusion is supported by several observations listed below. First, contractions of aortic rings triggered by NaHS at concentrations less than 10−4 M were completely abolished in the presence of bumetanide (Fig. 2), a potent and selective inhibitor of Na+,K+,2Cl- cotransport. It might be also assumed that the lower increment of contraction by modest doses of NaHS seen in intact aorta is partially caused by diffusional problems of this compound to Na+,K+,2Cl- cotransporter localized in SMC sarcolemma. Second, low doses of NaHS increased activity of Na+,K+,2Cl- - cotransport in cultured SMC without any significant impact on other K+ (86Rb) transport systems (Fig. 4). Third, the contractile action of low doses of NaHS was increased in aortic rings subjected to modest depolarization the presence of 30 mM KCl as compared to depolarization at 60 mM KCl (Fig. 1). Previously, we reported that bumetanide inhibits contractions evoked by partial depolarization via elevation of intracellular concentration of Cl- and plasma membrane depolarization [17]. Fourth, the treatment with 10−4 M NaHS resulted in ~1.5-fold elevation of depolarization-induced 45Ca influx that was suppressed by bumetanide and completely abolished in the presence of inhibitor of voltage-gated L-type Ca2+ channel nicardipine (Table 1).
Previously, it was shown that hydralazine, a potent stimulator of K+,Cl- cotransport, i.e. another carrier playing a key role in [Cl-]i regulation, reduced tension in precontracted porcine aortic strips [54]. In mammalian erythrocytes K+,Cl- cotransport is completely inhibited by 100 μM DIOA [55]. It should be noted that at this concentration DIOA exerts diverse side-effects on cultured SMC [56] and cannot be used to analyze the role of this carrier in contraction regulation.
Data obtained by Lim and co-workers suggest that vasoconstriction of NaHS-treated aortic rings involves inhibition of adenylyl cyclase / cAMP pathway [57]. We found that contractile actions of low doses of NaHS are increased in endothelium-denuded aortic strips as compared to intact ones (Fig. 2) thus suggesting that endothelium dysfunction promotes the H2S-induced contraction. This conclusion is consistent with augmented contractile actions of low doses of NaHS after inhibition of nitric oxide-mediated signaling in intact human internal mammary artery pretreated with acetylcholine [15]. On the other hand, the NaHS-induced contraction of rat coronary artery was elevated following the removal of endothelium or the use of the nitric oxide synthase inhibitor L-NAME [37]. Considering these results, it should be noted that molecular mechanisms underlying the regulation of contractile responses of evoked by NaHS are species- and vascular bed-specific [1], [7], [58], [59]. It is also important to underline that unlike the above-cited studies our experiments were performed in the absence of bicarbonate anions. Previously we reported that addition of 25 mM NaHCO3 inhibits NKCC and attenuates the inhibitory action of bumetanide on contractions of mouse mesenteric artery evoked by 30 mM KCl and PE [35].
In contrast to SMC, we did not find any activation of NKCC in NaHS-treated endothelial cells (Fig. 5) expressing the same NKCC1 isoform of this carrier [60]. Thus, it might be assumed that H2S-induced regulation of NKCC targets unknown intermediates of SMC-specific signaling rather than NKCC1 per se. We also revealed that NaHS sharply inhibits Na+,K+-pump in endothelial but not in SMC. Recently, we demonstrated that elevation of the [Na+]i/[K+]i ratio in endothelial cells evoked by sustained Na+,K+-pump inhibition is accompanied by sharp elevation of cyclooxygenase COX-2 expression [61]. It is known that side-by-side with elevation of cGMP in SMC triggered by NO release, endothelium-dependent vasorelaxation is initiated by COX-mediated synthesis of prostacyclin PGI2 [59], [62], [63]. The role of recently discovered Na+i,K+i-sensitive mechanism of excitation-transcription coupling [64] in vasorelaxation triggered by Na+,K+-pump inhibition and COX-2 transcription in endothelial cells treated with high doses of H2S remains unknown.
In conclusion, our results strongly suggest that excitatory actions of NaHS (5x10−6 to 10−4 M) on vascular smooth muscle contraction are mediated via activation of Na+,K+,2Cl--cotransport and Ca2+ influx through voltage-gated L-type Ca2+ channels. As well, additional experiments should be performed to clarify the relative impact of Na+,K+-pump and other anion transporters in bimodal involvement of H2S in vascular tone regulation.
Funding
This work was supported by the Federal Target Program "Scientific and Scientific-Pedagogical Personnel of Russia" (№ 8487, 23.10.2012) and grants from the Russian Foundation for Fundamental Research (15-04-00101, 16–34-00262) and the Russian Scientific Foundation (14–15-00006).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.09.010.
Contributor Information
Sergei N. Orlov, Email: sergeinorlov@ya.ru.
Svetlana V. Gusakova, Email: gusacova@yandex.ru.
Appendix A. Transparency document
Supplementary material
References
- 1.Wang R. Signaling pathways for the vascular effects of hydrogen sulfide. Curr. Opin. Nephrol. Hypertens. 2011;20:107–112. doi: 10.1097/MNH.0b013e3283430651. [DOI] [PubMed] [Google Scholar]
- 2.Fang L., Zhao J., Chen Y. Hydrogen sulfide derived from periadventitial adipose tissue is a vasodilator. J. Hypertens. 2009;27:2174–2185. doi: 10.1097/HJH.0b013e328330a900. [DOI] [PubMed] [Google Scholar]
- 3.Leffler C.W., Parfenova H., Jaggar J.H., Wang R. Carbon monoxide and hydrogen sulfide: gaseos messengers in cerebrovascular circulation. J. Appl. Phys. 2006;100:1065–1076. doi: 10.1152/japplphysiol.00793.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowicka E., Beltowski J. Hydrogen sulfide (H2S) - the third gas of interest for pharmacologists. Pharmacol. Rep. 2007;59:4–24. [PubMed] [Google Scholar]
- 5.Mancardi D., Penna C., Merlino A. Physiological and pharmacological features of the novel gasotransmitter: hydrogen sulfide. Biochim. Biophys. Acta. 2009;1787:864–872. doi: 10.1016/j.bbabio.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheang W.S., Wong W.T., Shen B. 4-Aminopyridine-sensitive K+ channels contributes to NaHS-induced membrane hyperpolarization and relaxation in the rat coronary artery. Vascul. Pharmacol. 2010;53:94–98. doi: 10.1016/j.vph.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Holwerda K.M., Karumanchi S.A., Lely A.T. Hydrogen sulfide: role in vascular physiology and pathology. Curr. Opin. Nephrol. Hypertens. 2015;24:170–176. doi: 10.1097/MNH.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 8.Bucci M., Papapetropoulos A., Vellecco V. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler. Thromb. Vasc. Biol. 2010;30:1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- 9.Al-Magableh M.R., Hart J.L. Mechanism of vasorelaxation and role of endogenous hydrogen sulfide production in mouse aorta. Naunyn-Schmied. Arch. Pharmacol. 2011;383:403–413. doi: 10.1007/s00210-011-0608-z. [DOI] [PubMed] [Google Scholar]
- 10.Yang G., Wu L., Jiang B. H2S as a physiological vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322(5901):587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang G., Yang G., Jiang B., Ju Y., Wu L., Wang R. H2S is an endothelium-derived hyperpolarizing factor. Antioxid. Redox Signal. 2013;19:1634–1646. doi: 10.1089/ars.2012.4805. [DOI] [PubMed] [Google Scholar]
- 12.Dombkowski R.A., Russell M.J., Schulman A.A., Doellman M.M., Olson K.R. Vertebrate phylogeny of hydrogen sulfide vasoactivity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;88:R243–R252. doi: 10.1152/ajpregu.00324.2004. [DOI] [PubMed] [Google Scholar]
- 13.Kubo S., Doe I., Kurokawa Y., Nishikawa H., Kawabata A. Direct inhibition of endothelial nitric oxide synthase by hydrogen sulfide: contribution to dual modulation of vascular tension. Toxicology. 2007;232:138–146. doi: 10.1016/j.tox.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Kubo S., Kajiwara M., Kawabata A. Dual modulation of the tension of isolated gastric artery and gastric mucosal circulation by hydrogen sulfide in rats. Inflammopharmacology. 2007;15:288–292. doi: 10.1007/s10787-007-1590-4. [DOI] [PubMed] [Google Scholar]
- 15.Webb G.D., Lim L.H., Oh V.M. Contractile and vasorelaxant effects of hydrogen sulfide and its biosynthesis in the human internal mammary artery. J. Pharmacol. Exp. Ther. 2008;324:876–882. doi: 10.1124/jpet.107.133538. [DOI] [PubMed] [Google Scholar]
- 16.Koltsova S.V., Kotelevtsev S.V., Tremblay J., Hamet P., Orlov S.N. Excitation-contraction coupling in resistant mesenteric arteries: evidence for NKCC1-mediated pathway. Biochem. Biophys. Res. Commun. 2009;379:1080–1083. doi: 10.1016/j.bbrc.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Anfinogenova Y.J., Baskakov M.B., Kovalev I.V. Cell-volume-dependent vascular smooth muscle contraction: role of Na+,K+,2Cl-cotransport, intracellular Cl- and L-type Ca2+ channels. Pflugers Archiv. 2004;449:42–55. doi: 10.1007/s00424-004-1316-z. [DOI] [PubMed] [Google Scholar]
- 18.Smagliy L.V., Gusakova S.V., Birulina Y.G., Kovalev I.V., Orlov S.N. The role of hydrogen sulfide in volume-dependent mechanisns of regulation of vascular smooth muscle cells contractile activity. Ross Fiziol Zh Im I M Sechenova. 2015;101(4):441–450. [PubMed] [Google Scholar]
- 19.Hadrava V., Tremblay J., Hamet P. Abnormalities in growth characteristics of aortic smooth muscle cells in spontaneously hypertensive rats. Hypertension. 1989;13:589–597. doi: 10.1161/01.hyp.13.6.589. [DOI] [PubMed] [Google Scholar]
- 20.Akimova O.A., Mongin A.A., Hamet P., Orlov S.N. The rapid decline of MTT reduction is not a marker of death signaling in ouabain-treated cells. Cell. Mol. Biol. 2006;52(8):71–77. [PubMed] [Google Scholar]
- 21.Voghel G., Thorin-Trescases N., Farhat N. Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress asociated with cardiovascular risk factors. Mech. Ageing Dev. 2007;128:662–671. doi: 10.1016/j.mad.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Orlov S.N., Tremblay J., Hamet P. Cell volume in vascular smooth muscle is regulated by bumetanide-sensitive ion transport. Am. J. Physiol. 1996;270:C1388–C1397. doi: 10.1152/ajpcell.1996.270.5.C1388. [DOI] [PubMed] [Google Scholar]
- 23.Orlov S.N., Tremblay J., Hamet P. cAMP signaling inhibits dihydropyridine-sensitive Ca2+ influx in vascular smooth muscle cells. Hypertension. 1996;27:774–780. doi: 10.1161/01.hyp.27.3.774. [DOI] [PubMed] [Google Scholar]
- 24.Telezhkin V., Brazier S.P., Cayzac S. Hydrogen sulfide inhibits human BKCa channels. Adv. Exp. Med. Biol. 2009;648:65–72. doi: 10.1007/978-90-481-2259-2_7. [DOI] [PubMed] [Google Scholar]
- 25.Sitdikova G.F., Fuchs R., Kainz V. Phosphorylation of BK channels modulates the sensitivity to hydrogen sulfide (H2S) Front. Physiol. 2014;5:431. doi: 10.3389/fphys.2014.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dombkowski R.A., Russell M.J., Olson K.R. Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286(4):R678–R685. doi: 10.1152/ajpregu.00419.2003. [DOI] [PubMed] [Google Scholar]
- 27.Hughes M.N., Centelles M.N., Moore K.P. Making and working with hydrogen sulfide: the chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic. Biol. Med. 2009;47:1346–1353. doi: 10.1016/j.freeradbiomed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Kolluru G.K., Shen X., Bir Sh.C., Kevil Ch.G. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen K.Y., Morris J.C. Kinetics of oxidation of aqueous sulfide by oxygen. Environ. Sci. Technol. 1972;6:529–537. [Google Scholar]
- 30.Nielsen A.H., Vollertsen J., Hvitved-Jacobsen T. Kinetics and stoichiometry of aerobic sulfide oxidation in wastewater from sewers-effects of pH and temperature. Water Environ. Res. 2006;78:275–283. doi: 10.2175/106143005x94367. [DOI] [PubMed] [Google Scholar]
- 31.Hosoki R., Matsuki N., Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 32.Abe K., Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16(3):1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao W., Wang R. H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2002;283(2):H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- 34.Orlov S.N., Koltsova S.V., Kapilevich L.V. NKCC1 and NKCC2: the pathogenetic role of cation-chloride cotransporters in hypertension. Genes Dis. 2015;2(2):186–196. doi: 10.1016/j.gendis.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koltsova S.V., Luneva O.G., Lavoie J.L. HCO3-dependent impact of Na+,K+,2Cl-cotransport in vascular smooth muscle excitation-contraction coupling. Cell. Physiol. Biochem. 2009;23:407–414. doi: 10.1159/000218187. [DOI] [PubMed] [Google Scholar]
- 36.Orlov S.N., Resink T.J., Bernhardt J., Buhler F.R. Na+-K+-pump and Na+-K+-co-transport in cultured vascular smooth muscle cells from spontaneously hypertensive rats: baseline activity and regulation. J. Hypertens. 1992;10:733–740. [PubMed] [Google Scholar]
- 37.Ping N.N., Li S., Mi Y.N., Cao L., Cao Y.X. Hydrogen sulfide induces vasoconstriction of rat coronary artery via activation of Ca2+ influx. Acta Physiol. (Oxf) 2015;214:88–96. doi: 10.1111/apha.12475. [DOI] [PubMed] [Google Scholar]
- 38.Semenykhina O.M., Baziliuk O.V., Korkach Iu.P., Sahach V.F. The effect of hydrogen sulfide on contractile activity of the vascular smooth muscles in rats. Fiziol. Zh. 2011;57:3–11. [PubMed] [Google Scholar]
- 39.Kubo S., Doe I., Kurokawa Y., Kawabata A. Hydrogen sulfide causes relaxation in mouse bronchial smooth muscle. J. Pharmacol. Sci. 2007;104(4):392–396. doi: 10.1254/jphs.sc0070199. [DOI] [PubMed] [Google Scholar]
- 40.Tang G., Wu L., Liang W., Wang R. Direct stimulation of KATP channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol. Pharmacol. 2005;68:1757–1764. doi: 10.1124/mol.105.017467. [DOI] [PubMed] [Google Scholar]
- 41.Zhao P., Huang X., Wang Z. Dual effect of exogenous hydrogene sulfide on the spontaneous contraction of gastric smooth muscle in guinea-pig. Eur. J. Pharmacol. 2009;616:223–228. doi: 10.1016/j.ejphar.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 42.Zhao W., Zhang J., Lu Y., Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20(21):6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang J., Luo Y.L., Hao Y. Cellular mechanism underlying hydrogen sulfide induced mouse tracheal smooth muscle relaxation: role of BKCa. Eur. J. Pharmacol. 2014;741:55–63. doi: 10.1016/j.ejphar.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Michelakis E.D., Reeve H.L., Huang J.M. Potassium channel diversity in vascular smooth muscle cells. Can. J. Physiol. Pharamacol. 1997;75:889–897. [PubMed] [Google Scholar]
- 45.Tian X.Y., Wong W.T., Sayed N. NaHS relaxes rat cerebral artery in vitro via inhibition of L-type voltage-sensitive Ca2+ channels. Pharmacol. Res. 2012;65(2):239–246. doi: 10.1016/j.phrs.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Li Q., Sun B., Wang X. A crucial role for hydrogen sulfide in oxygen sensing via modulating large conductance calcium-activated potassium channels. Antioxid. Redox Signal. 2010;12:1179–1189. doi: 10.1089/ars.2009.2926. [DOI] [PubMed] [Google Scholar]
- 47.Quan X., Luo H., Liu Y. Hydrogen sulfide regulates the colonic motility by inhibiting both L-type calcium channels and BKCa channels in smooth muscle cells of rat colon. PLoS One. 2015;10(3):e0121331. doi: 10.1371/journal.pone.0121331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chai Q., Lu T., Wang X.L. Hydrogen sulfide impairs shear stress-induced vasodilation in mouse coronary arteries. Pflugers Arch. -Eur. J. Physiol. 2015;467(2):329–340. doi: 10.1007/s00424-014-1526-y. [DOI] [PubMed] [Google Scholar]
- 49.Liang G.H., Xi Q., Leffler C.W., Jaggar J.H. Hydrogen sulfide activates Ca2+ sparks to induce cerebral arteriole dilatation. J. Physiol. 2012;590(11):2709–2720. doi: 10.1113/jphysiol.2011.225128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishijima Y., Beyer A.M. H2S in the vasculature: controversy of mechanisms in physiology. Pathology and beyond. Cardiol. Pharmacol. 2015;4(2) [Google Scholar]
- 51.Cheng Y., Ndisang J.F., Tang G. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am. J. Physiol. Heart Circ. Physiol. 2004;287(5):H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 52.Jackson-Weaver O., Osmond J.M., Riddle M.A. Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca2+-activated K+ channels and smooth muscle Ca2+ sparks. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H1446–H1454. doi: 10.1152/ajpheart.00506.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smagliy L.V., Zheludeva A.S., Gusakova S.V. Hydrogen sulfide vasorelaxing action: role of potassium conductance of membrane. Mod. Prob. Sci. Educ. 2012:5. [Google Scholar]
- 54.Adragna N.C., White R.E., Orlov S.N., Lauf P.K. K-Cl cotransport in vascular smooth muscle and erythrocytes: possible implication in vasodilation. Am. J. Physiol. Cell. Physiol. 2000;278(2):C381–C390. doi: 10.1152/ajpcell.2000.278.2.C381. [DOI] [PubMed] [Google Scholar]
- 55.Garay R.P., Nazaret C., Hannaert P.A., Cragoe E.J., Jr Demonstration of a [K+,Cl-]-cotransport system in human red cells by its sensitivity to [(dihydroindenyl)oxy]alkanoic acids: regulation of cell swelling and distinction from the bumetanide-sensitive [Na+,K+,Cl-]-cotransport system. Mol. Pharmacol. 1988;33(6):696–701. [PubMed] [Google Scholar]
- 56.Anfinogenova Y.J., Rodriguez X., Grygorczyk R. Swelling-induced K(+) fluxes in vascular smooth muscle cells are mediated by charybdotoxin-sensitive K(+) channels. Cell. Physiol. Biochem. 2001;11(6):295–310. doi: 10.1159/000047816. [DOI] [PubMed] [Google Scholar]
- 57.Lim J.J., Liu Y.-H., Khin E.S.W., Bian J.-S. Vasoconstrictive effect of hydrogen sulfide involves downregulation of cAMP in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2008;295:C1261–C1270. doi: 10.1152/ajpcell.00195.2008. [DOI] [PubMed] [Google Scholar]
- 58.Musafa A.K., Sikka G., Gazi S.K. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ. Res. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beltowski J., Jamroz-Wisniewska A. Hydrogen sulfide and endothelium-dependent vasorelaxation. Molecules. 2014;19:21183–21199. doi: 10.3390/molecules191221183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yerby T.R., Vibat R.T., Sun D. Molecular characterization of the Na-K-Cl cotransporter of bovine aortic endothelila cells. Am. J. Physiol. 1997;273:C188–C197. doi: 10.1152/ajpcell.1997.273.1.C188. [DOI] [PubMed] [Google Scholar]
- 61.Koltsova S.V., Trushina Y., Haloui M. Ubiquitous [Na+]i/[K+]i-sensitive transcriptome in mammalian cells: evidence for Ca2+i-independent excitation-transcription coupling. PLoS One. 2012;7:e38032. doi: 10.1371/journal.pone.0038032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen R.A. The endothelium-derived hyperpolarizing factor puzzle. A mechanism without a mediator? Circulation. 2005;111:724–727. doi: 10.1161/01.CIR.0000156405.75257.62. [DOI] [PubMed] [Google Scholar]
- 63.Feletou M., Vanhoutte P.M. Endothelium-dependent hyperpolarization: past beliefs and present facts. Ann. Med. 2007;39:495–516. doi: 10.1080/07853890701491000. [DOI] [PubMed] [Google Scholar]
- 64.Orlov S.N., Hamet P. Salt and gene expression: evidence for Na+i,K+i-mediated signaling pathways. Pflugers Arch. Eur. J. Physiol. 2015;467:489–498. doi: 10.1007/s00424-014-1650-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material