Highlights
-
•
3D printing models were produced for anatomical hepatectomy.
-
•
It contributed our understanding of the positional relationships of tumor and vessel.
-
•
It also contributed common understanding between operator and assistant during operation.
Abbreviations: HCC, hepatocellular carcinoma; 3D, 3-dimensional; 2D, 2-dimensional; IOUS, intraoperative ultrasound sonography; CT, computed tomography; STL, stereolithography
Keywords: 3D printing model, Hepatocellular carcinoma, Anatomical resection
Abstract
Introduction
The 3D printing model of the intrahepatic vessels and regional anatomy are often used for navigation surgery. Here, we report the use of the model for anatomical resection of hepatocellular carcinoma.
Presentation of case
Case 1: A tumor, 31 mm in diameter, was located in segment 7 of the liver. Using the 3D model, we identified the regional Glissonian pedicle and performed resection of segment 7. Case 2: The tumor was located in segment 4/8 and involved the middle hepatic vein. Radical resection of segment 4 and of the ventral area of the right anterior section was performed using the 3D model.
Discussion
The positional relationship between the intrahepatic vessels and liver tumors is the most important factor for anatomical resection for hepatocellular carcinoma. Therefore our simplified 3D model of intrahepatic vessels without liver parenchyma is sufficient for effective guidance during surgery and has the advantage of being feasible to use for all HCC surgeries.
Conclusion
Use of 3D printed models might have many merits and contribute to the great improvement of the surgical quality.
1. Introduction
Hepatocellular carcinoma (HCC) is a common malignancy worldwide, with most patients having poor hepatic functional reserve. Although hepatic resection is the radical treatment of choice, it remains associated with a high rate of mortality and morbidity due to the technical difficulty of the anatomical resection of the tumors in most cases [1], [2]. Moreover, the anatomical structure of intrahepatic liver vessels and the location of tumors can vary significantly between cases, which underlines the importance of reliable navigation for this surgery.
Recently, 3-dimensional (3D) imaging has been widely adopted in the preoperative planning of hepatic resection [3]. Although 3D imaging improves understanding of the regional anatomy on a case-by-case basis, ultimately the anatomy of the intrahepatic vessels is projected on a 2-dimensional (2D) monitor during the surgery. Therefore, there is a disconnect between preoperative planning based on 3D recognition of the regional anatomy from 3D image analysis and the actual intraoperative ultrasound (IOUS) images used to guide surgeons during hepatic resection.
With increasing availability of 3D printing technology, several research groups have described the use of 3D printed models of the liver for preoperative simulation of HCC resection [4], [5], [6]. However the use of these models has not generally been translated into practice, principally due to the high cost of 3D printers, the complicated process of printing a 3D model, and the overall associated costs. To address this issue, we have developed a simple 3D printing process to obtain a model of intrahepatic vessels that is feasible to use on a case-by-case basis for anatomical resection of HCC. We have used this modeling approach with success for several years now.
2. Method
In our process, we begin with a 3D reconstruction of the regional anatomy of the liver from dynamic computed tomography (CT) data, and convert the reconstructed image to a stereolithography (STL) file format using the 3D image analysis system, Ziostation 2 (version 2.40.4; Ziosoft Inc., Tokyo, Japan). The STL file provides the input data to the 3D printer (Agilista-3200; Keyence Co., Osaka, Japan) to produce a 3D model of the intrahepatic vessels. The model is created using rigid acryl resin (AR-M2; Keyence Co., Osaka, Japan), with hydrosoluble acryl resin used as the support material (AR-S1, Keyence Co., Osaka, Japan). The supporting material is removed after printing by bathing the model in water for one day. The pitch of the lamination layer is 0.015 mm, with a 1:1 scaling between the 3D reconstructed image and the model. The 3D model includes only the intrahepatic portal veins, the hepatic veins and the liver carcinoma, without the hepatic parenchyma. Inclusion of these structures is sufficient for accurate and speedy anatomical resection.
During anatomical hepatic resection, approach to the regional Glissonian pedicle, or dye puncture, and identification of the resection area are important. However, mobilization and rotation of the liver during the surgery alters the positional relationship between the Glissonian pedicle and the surface of the liver; this change in positional relationship is difficult to resolve based solely on 2D images under IOUS guidance. Moreover, the intrahepatic Glisson and hepatic vein are intricately crossed, with confirmation of each structure being difficult under IOUS guidance to intrinsic noise with this 2D imaging technique. Therefore, the positional relationship between vessels and tumors is commonly difficult to effectively resolve during the surgery itself using conventional navigation methods. Moreover, communication regarding the complex regional anatomy of the liver between the surgeons and surgical assistants to confirm the correct anatomical resection is also difficult. Our 3D model of the intrahepatic vessels correctly presents the positions of the intrahepatic vessels and liver tumors, improving surgeons’ understanding of these positional relations during each step of the anatomical resection.
Using the rigid acryl resin, the model is resistant to heat up to 77–80 °C, allowing for sterilization of the model with ethylene oxide gas for use in the operative field. The physical 3D model can be manipulated during hepatic resection to confirm positional relationships of the tumor and vessels and plan the cutting line into the liver parenchyma relative to the intrahepatic vessels. Therefore, our 3D model might have merit in improving the quality of anatomical hepatic resection. The applicability of our model to surgical practice is demonstrated in the two anatomical hepatic resection cases described below.
3. Case 1
A tumor, 31 mm in diameter, was located in segment 7 of the liver. As the patient had a poor hepatic functional reserve, resection of segment 7 was required. Using the intrahepatic 3D model, we identified the regional Glissonian pedicle and performed resection of segment 7 without difficulty (Fig. 1).
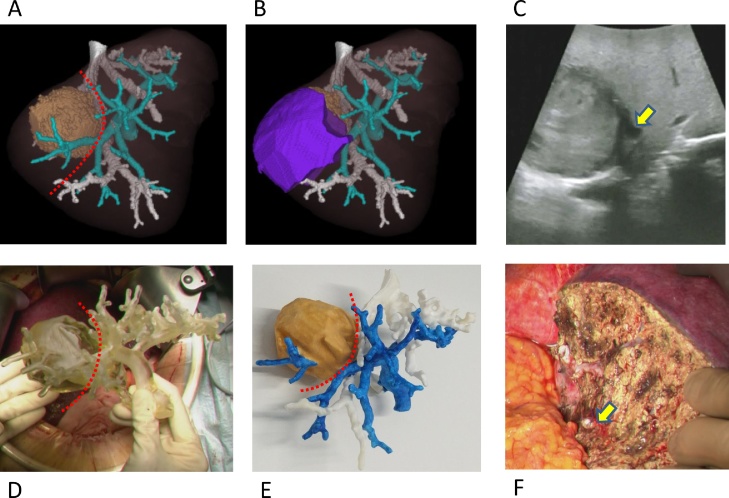
Fig. 1.
A: 3D reconstructed image of the intrahepatic vessels and tumor from dynamic CT images. Blue, portal vein; White, hepatic vein; Brown; the tumor; Red broken line, cut line for resection of segment 7. B: Simulation of the anatomical resection of segment 7, shown as the purple area. C: ultrasound guidance (IOUS) image, with the yellow arrow showing the regional Glissonian pedicle of segment 7. D: The 3D printed model of the intrahepatic vessels being used in the operative field. E: The 3D printed model of the intrahepatic vessels after painting specifically for the figure. F: Transected surface of the liver, with the yellow arrow showing the regional Glissonian pedicle of segment 7 after cutting.
4. Case 2
The tumor was located in segment 4/8 and involved the middle hepatic vein. Radical resection required resection of segment 4 and of the ventral area of the right anterior section. The positional relationship and diverging pattern of the ventral and dorsal branches of the intrahepatic vessels in the anterior section was confirmed using the 3D model (Fig. 2).
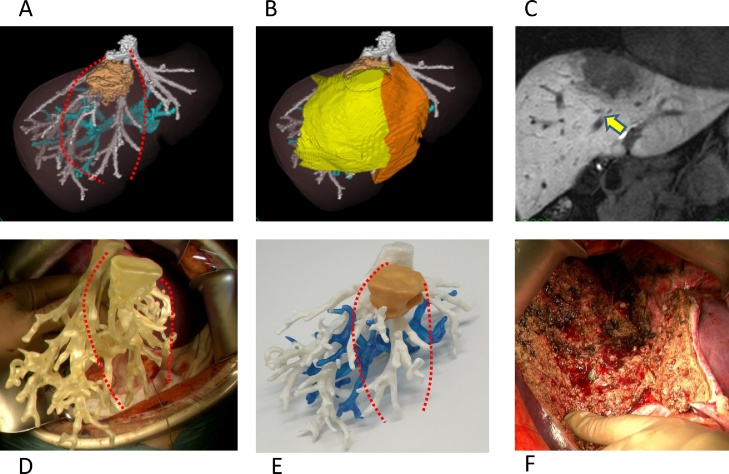
Fig. 2.
A: 3D reconstructed image of the intrahepatic vessels and tumor from dynamic CT images. The tumor, with a 65 mm diameter, was located in segment 4/8 and involved middle hepatic vein. Blue, portal vein; White, hepatic vein; Brown, tumor; Red broken line, cut line of resection of segment 4 and ventral area of the right anterior section. B: Simulation of the resection Orange area, segment 4; Yellow area, ventral area of the right anterior section. C: Magnetic resonance image, with the yellow arrow showing the middle hepatic vein. D: The 3D printed model of intrahepatic vessels model used in the operative field. E: The 3D printed model of the intrahepatic vessels after painting specifically for the figure. F: Transected surface of the liver.
5. Discussion
With regards to the resolution of the 3D model, we can print small branches of vessels, 2 or 3 mm in diameter, as long as quality contrast CT images are available. However, representation of such small branches is not necessary in the operative field. Additionally, although we use a very rigid and strong material to construct the model, very small branches might still be broken during the operation. Therefore, we have opted not to include these small vessels and, have thereby simplified our model.
A previously published study on the development and use of a 3D printed model of the liver used several materials and different color for each vessel, as well as including the clear liver parenchyma [6]. Although this more detailed 3D liver model, including the parenchyma, might be of benefit to guide anatomical resection, ultimately it is the positional relationship between the intrahepatic vessels and liver tumors, which is the most important factor. In fact, including of the liver parenchyma, even if it is clear, can prevent correct recognition of positional relationships in the deep parenchyma. Therefore, our simplified 3D model of intrahepatic vessels is sufficient for effective guidance during surgery and has the advantage of being feasible to use for all HCC surgeries.
This model is also widely applicable. We think this model has a merit for all patients with borderline liver function who will undergo hepatic resection, especially for the donor of living liver transplantation or hilar cholangio carcinoma. In right or left hepatic lobectomy, even the minor deviation of the cutting line continues to the major difference of the resection liver volume. Moreover, we can add the biliary tree to the portal and hepatic vein model. When hepatic tumor locates behind or above hilar plate, the relation of tumor and biliary tree is very important, because the risk of biliary leakage after operation is high in such cases.
The general use of 3D printed models in surgery is still limited by the time needed to create the model and the financial costs associated with the approximate 30 min of data processing needed and the 40–50 h of printing, the 1 day of removal of the support material, and the $500 to $800 cost for materials. Printing time and cost are ultimately determined by the size of the liver. Although these cost and time constraints do limit the use of 3D printed models in all cases of HCC resection, 3D models should be used for cases with complicated positional relationship between intrahepatic vessels and tumors.
6. Conclusion
As 3D printing technology continues to evolve, it is possible that the costs of creating 3D models for surgery will be reasonable and, therefore, that use of 3D printed models in the operative field will be common practice. The work in this case has been reported in line with the SCARE criteria [7].
Conflict of interest
All the authors declare that they have no conflict of interest.
Funding
This article received no funding.
Ethical approval
Manuscript involves a case report for which approval was taken from “Ethics Committee for Academic Research Purpose, Hiroshima University” for its publication.
Approval to publish case report is waived by the institution.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chiefof this journal on request.
Author contribution
SK drafted the manuscript and carried out the acquisition of data. TK and HO revised the manuscript and has given final approval of the version to be published. All authors read and approved the final manuscript.
Guarantor
Shintaro Kuroda.
References
- 1.Kenjo A., Miyata H., Gotoh M. Risk stratification of 7,732 hepatectomy cases in 2011 from the National Clinical Database for Japan. J. Am. Coll. Surg. 2014;218:412–422. doi: 10.1016/j.jamcollsurg.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Eguchi S., Kanematsu T., Arii S. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery. 2008;143:469–475. doi: 10.1016/j.surg.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Ohshima S. Volume analyzer SYNAPSE VINCENT for liver analysis. J. Hepatobiliary Pancreat. Sci. 2014;21:235–238. doi: 10.1002/jhbp.81. [DOI] [PubMed] [Google Scholar]
- 4.Igami T., Nakamura Y., Hirose T. Application of a three-dimensional print of a liver in hepatectomy for small tumors invisible by intraoperative ultrasonography: preliminary experience. World J. Surg. 2014;38:3163–3166. doi: 10.1007/s00268-014-2740-7. [DOI] [PubMed] [Google Scholar]
- 5.Xiang N., Fang C., Fan Y. Application of liver three-dimensional printing in hepatectomy for complex massive hepatocarcinoma with rare variations of portal vein: preliminary experience. Int. J. Clin. Exp. Med. 2015;8:18873–18878. [PMC free article] [PubMed] [Google Scholar]
- 6.Takagi K., Nanashima A., Abo T. Three-dimensional printing model of liver for operative simulation in perihilar cholangiocarcinoma. Hepatogastroenterology. 2014;61:2315–2316. [PubMed] [Google Scholar]
- 7.Agha R.A., Fowler A.J., Saetta A. The SCARE statement: Consensus-based surgical case report guidelines. Int. J. Surg. 2016 doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]