Abstract
Usher syndrome, Type 1C (USH1C) is an autosomal recessive inherited disorder in which a mutation in the gene encoding harmonin is associated with multi-sensory deficits (i.e., auditory, vestibular, and visual). USH1C (Usher) mice, engineered with a human USH1C mutation, exhibit these multi-sensory deficits by circling behavior and lack of response to sound. Administration of an antisense oligonucleotide (ASO) therapeutic that corrects expression of the mutated USH1C gene, has been shown to increase harmonin levels, reduce circling behavior, and improve vestibular and auditory function. The current study evaluates the organization of exploratory movements to assess spatial organization in Usher mice and determine the efficacy of ASO therapy in attenuating any such deficits. Usher and heterozygous mice received the therapeutic ASO, ASO-29, or a control, non-specific ASO treatment at postnatal day five. Organization of exploratory movements was assessed under dark and light conditions at two and six-months of age. Disruptions in exploratory movement organization observed in control-treated Usher mice were consistent with impaired use of self-movement and environmental cues. In general, ASO-29 treatment rescued organization of exploratory movements at two and six-month testing points. These observations are consistent with ASO-29 rescuing processing of multiple sources of information and demonstrate the potential of ASO therapies to ameliorate topographical disorientation associated with other genetic disorders.
Keywords: Vestibular system, Visual system, Dead reckoning, Path integration, Spatial orientation, Home base, Pre-mRNA splicing, Antisense oligonucleotides, Usher syndrome, Harmonin, USH1C, Deafness
1. Introduction
Spatial orientation is maintained by information from environmental and self-movement cues [13]. Each source of information has a network of structures in the central nervous system that mediates information processing. Neurological disorders that target either network can impair information processing and produce topographical disorientation [1]. A growing body of literature has provided evidence that support a role for the vestibular system in maintaining spatial orientation. For example, acquired [14, 15, 44] and congenital [38] vestibular pathologies have been shown to disrupt performance on various spatial tasks. Genetic disorders that impact vestibular function would also be expected to influence spatial orientation, though this concept has not been widely explored.
Usher syndrome refers to a group of autosomal recessive inherited disorders with varied multisensory deficits [37]. For example, the most severe form, Usher syndrome, Type 1, is associated with congenital hearing loss and vestibular dysfunction; with visual impairments (i.e., retinitis pigmentosa) developing prior to the onset of puberty. Usher syndrome, Type 1C is caused by mutations in the gene USH1C, which encodes harmonin, a scaffolding protein located near the tips of stereocilia and in the ribbon synapse of cochlear and vestibular hair cells [18, 20]. This form of Usher syndrome has been attributed to the loss of sensory transduction within auditory and vestibular sensory systems [18, 20]. A knock-in mouse model of USH1C, that has a c.216G → A mutation, found most commonly in people of French-Acadian descent [11, 24, 26], has been developed. This Ush1c c.216A (216A, Usher) mouse model captures the congenital auditory (attenuated auditory brainstem response) and vestibular (e.g., head-tossing, circling behaviors, vestibular sensory-evoked potential (VsEP)) pathologies, and there is evidence supporting the development of visual pathologies [22]. The 216G → A mutation causes mis-splicing of USH1C pre-mRNA. Application of an antisense oligonucleotide, ASO-29, targeted to block the aberrant splicing partially corrects the splicing defect, increases harmonin expression, and rescues auditory and vestibular function [23, 32]. As of yet, it remains to be determined if the rescued vestibular function is sufficient to maintain spatial orientation.
The organization of rodent exploratory behavior has been used to assess spatial orientation. Upon exposure to a novel environment, rodents organize their behavior into a sequence of stops and progressions that are typically concentrated around one location, or home base [9, 12, 30, 31]. Both environmental and self-movement cues have been shown to guide exploratory movements. First, environmental cues have been shown to polarize the location of home base establishment [7]. Next, rodents use self-movement cues to maintain a stable home base location under completely dark conditions [17, 33] and without access to olfactory cues [19]. Further, impaired self-movement cue processing associated with otoconia-deficient mice has been observed to disrupt progression path circuity, change in heading during stops, and home base stability during dark exploration [5]. Finally, in otoconia-deficient mice, compensatory use of environmental cues improved progression path circuity and home base stability; however, disruptions in change in heading during stops persisted even under light conditions. These observations demonstrate that exploratory movement organization can dissociate environmental or self-movement cue processing impairments. Characterization of these exploratory movements in Usher mice will provide an important measure of the efficacy of ASO treatment to rescue spatial orientation deficits associated with Usher syndrome-related vestibular pathology.
The current study examines disruptions in the organization of exploratory movements associated with the Ush1c c.216A mouse model and the efficacy of ASO-29 treatment to ameliorate these disruptions in performance. Usher and control mice received either ASO-29 or ASO-C, a non-specific control ASO, at postnatal day five. Exploratory movements under dark and light conditions were examined at two and six-months of age. This work will evaluate the efficacy of ASO-29 to rescue self-movement cue processing deficits associated with a mouse model of Usher syndrome, Type 1C.
2. Materials and methods
2.1. Animals
All experimental procedures were carried out in accordance with the Institutional Animal Care and Use Committees (IACUCs) at Rosalind Franklin University of Medicine and Science (RFUMS) and Northern Illinois University (NIU) following NIH guidelines for The Care and Use of The Laboratory Animals. Ush1c c.216A mice were bred, treated and housed at RFUMS prior to being transported to NIU via ground transportation under ambient temperature. A sufficiently large sample of female mice was not available, therefore an analysis of sex dimorphisms in genotype or treatment was not possible in the current study. Male homozygous Usher (n = 18) and heterozygous control (n = 20) mice received intraperitoneal (300 mg/kg) injections of ASO-C (Usher: n = 10; control: n = 12) or ASO-29 (Usher: n = 8; control: 8) at postembryonic day P5. Prior to the six-month testing, three Usher mice receiving the ASO-C were euthanized related to developing severe ulcerative dermatitis. Representative mice (n = 2/group) were treated at P5 with ASO-C or ASO-29 and euthanized at P30. Inner ear tissue was collected from the animals to confirm ASO-mediated correction of Ush1c c.216A expression.
2.2. Genotyping
DNA was isolated from mouse ear punches at day of weaning (P21) and, for confirmation purposes, also from tail tissue shortly after euthanization. The Ush1c genotype was determined using Redextract-NAmp For Tissue (Sigma, #XNAT) with primers, M216AF: 5′-CCACTTCATCTGTGACTTCCTGGT-3′ and M216AR: 5′-ACAGATCGAGAGAGCAAGAGAGCA-3′.
2.3. Antisense oligonucleotides
2′-O-methoxyethyl-ASOs (ASO-29: 5′-AGCTGATCATATTCTACC-3′; ASO-C: 5′-TTAGTTTAATCACGCTCG-3′) with fully-modified phosphor-othioate backbones were synthesized by Ionis Pharmaceuticals (Carlsbad, CA) as previously described [23]. ASOs were diluted in 0.9% saline sterile solution.
2.4. RNA splicing analysis
Tissue for RNA isolation was rapidly dissected following euthanasia and snap frozen in liquid nitrogen. Tissue was stored at −80 °C. Frozen tissues were homogenized in TRIZOL solution (ThermoFisher Scientific; Waltham, MA) using a PowerGen 1000 Homogenizer (Fisher Scientific) and RNA was purified from TRIZOL following manufacturer recommendations. RNA (1 µg) was reverse transcribed using oligo-dT primers and GoScript reverse transcriptase (Promega; Madison, WI) following manufacturer’s recommendations. 1 µl of cDNA was used in PCR reactions with GoTaq Green (Promega) supplemented with primers and α–32P–dCTP. Primers specific for human Ush1c exon 3 (5′-GAATATGATCAGCTGACC-3′) and mouse exon 5 (5′-TCTCACTTTGATGGACACGGTCTTC-3′) were used to specifically amplify only mRNA generated from the knocked-in allele of the human Ush1c.216A gene, which is only present in correctly spliced mRNA [8]. Mouse Gapdh primers (5′-GTGAGGCCGGTGCTGAGTATG-3′) and (5′-GCCAAAGTTGTCATGGATGAC-3′) were used to detect and measure endogenous murine Gapdh mRNA. Products were separated on a 6% non-denaturing polyacrylamide gel.
2.5. Apparatus
The exploration arena was a wooden circular table (112 cm diameter) painted white. The surface of the table was 34.5 cm above the floor in a large light-proof room. A rectangular piece of thick transparent plastic (20 cm wide × 15 cm high) was attached with two screws to the edge of the table and extended toward the ceiling of the testing room. The plastic tab could serve as a tactile cue and encourage home base establishment. Tab position remained in a consistent location for each mouse across exploration sessions; however, tab position varied among mice. The light-proof room had infrared emitters positioned on the walls facing upward and a night vision bullet camera attached to the ceiling. Fluorescent ceiling light provided illumination during light exploratory sessions and were turned off during dark exploratory sessions. Exploratory sessions were recorded at 30 frames per second and recorded to DVDs for offline analysis.
2.6. Procedure
The timing of exploratory sessions was selected to parallel previous work investigating the harmonin levels and circling behavior after P5 treatment with ASO-29 [23]. The first set of exploratory sessions (two-month assessment) was conducted eight weeks after P5. Mice received three dark exploratory sessions, each separated by 24 h. The following day, mice received three light exploratory sessions separated by 24 h. The last set of exploratory sessions (six-month assessment) was conducted 24 weeks after P5. Mice received two dark exploratory sessions separated by 24 h. The following day mice received two light exploratory sessions separated by 24 h.
During an exploratory session, a mouse was individually transported to the testing room in an opaque container that was rotated several times, thereby reducing the possibility of using the colony room as an anchor for spatial representations. Upon entering the room, the cage was placed on a pedestal next to the table, the mouse was removed from the cage and placed in the center of the table, and the experimenter left the room. Exploration sessions lasted for 50 min. At the end of the session, the mouse was transported to the colony room in an opaque container that was rotated several times. The table was wiped clean with ammonia based cleaner prior to running the next mouse.
2.7. Data analysis
The position of the mouse during exploration sessions was captured using the Ethovision 3.0 (Noldus, NL) software. Five five-minute samples were taken after two minutes into the exploratory session. Previous work has shown that grooming occurs within two minutes after placement on the exploratory table [5] and is a marker of home base establishment [12]. Movements during each sample were segmented into progressions (moment-to-moment speeds greater than or equal to 3.0 cm/s for two or more frames) and stops (moment-to-moment speeds less than 3.0 cm/s for two or more frames).
Multiple measures were used to characterize the organization of exploratory movements. First, the total distance traveled was calculated for each sample and provide a general measure of locomotor function. Next, several measures were developed to describe behavior during progressions. Peak speed was recorded for all progressions and provides an additional measure of locomotor function. Path circuity was calculated by dividing the Euclidean distance (distance between start and end points of a progressions) by the actual distance traveled for each progression. Path circuity values range from 0.0 (circuitous paths) to 1.0 (direct paths). Previous work with rats [33] and mice [5] has demonstrated that progressions are typically non-circuitous paths through the environment, independent of the access to visual cues. Both peak speed and path circuity were averaged across all progressions within a sample.
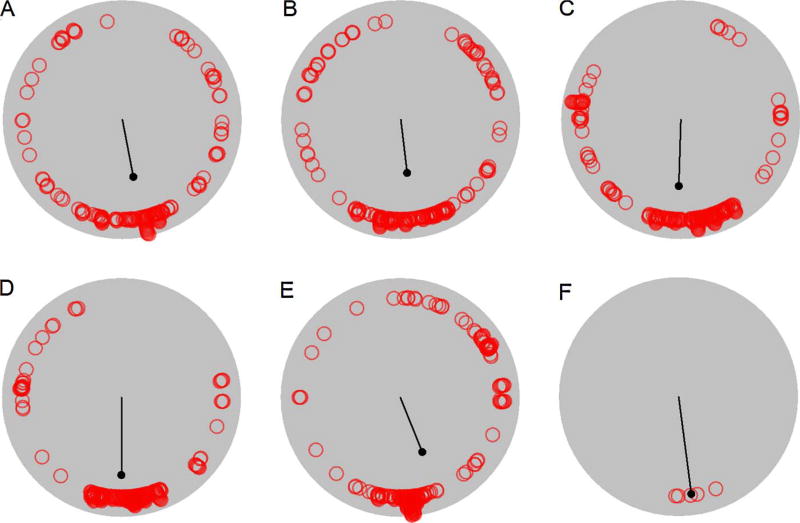
Several measures were developed to characterize stopping behavior. First, the average stop duration was calculated for each sample. Previous work has demonstrated that shorter stop durations during open field behavior contribute to the increase in hyperactivity associated with damage to the hippocampal formation [35]. Next, most of the changes in heading along a path occur during stops [33]. The changes in heading between progressions can be quantified by calculating the angle subtended by the following points: preceding progression peak speed location, average stop location, and subsequent progression peak speed location. The resulting set of angles for a rodent can be averaged for each sample. Previous work has shown that otoconia-deficient tilted mice exhibit significantly larger between progression angles relative to control mice [5]. Finally, home base establishment has been associated with the distribution of stopping behavior [5, 7, 12, 16]. The mean vector length (i.e., r) is a circular statistic [3] that was used to quantify the concentration and stability of stops across samples. Specifically, the Cartesian coordinates (x, y) associated with each stop were converted into polar coordinates (theta, r). The duration of each stop was converted into a set of theta heading frequencies (i.e., five second stop at 90° =five observations at 90°). First order circular statistics (parameter of concentration and average heading) were applied to each sample’s set of theta heading frequencies. The resulting parameter of concentration provided a measure of within sample stop density. Second order circular statistics (parameter of concentration) were applied to each mouse’s average heading from the five samples and provided a measure of stability of stops across samples.
Between-subject ANOVAs were used to evaluate the effects of mutation, ASO treatment, and mutation by ASO treatment interactions. Separate analyses were conducted for dark and light exploratory sessions at two and six-month time points. Partial eta squared values ( ) were reported for each main effect and interaction as a measure of effect size.
3. Results
3.1. Antisense oligonucleotide correction of Ush1c c.216A aberrant splicing
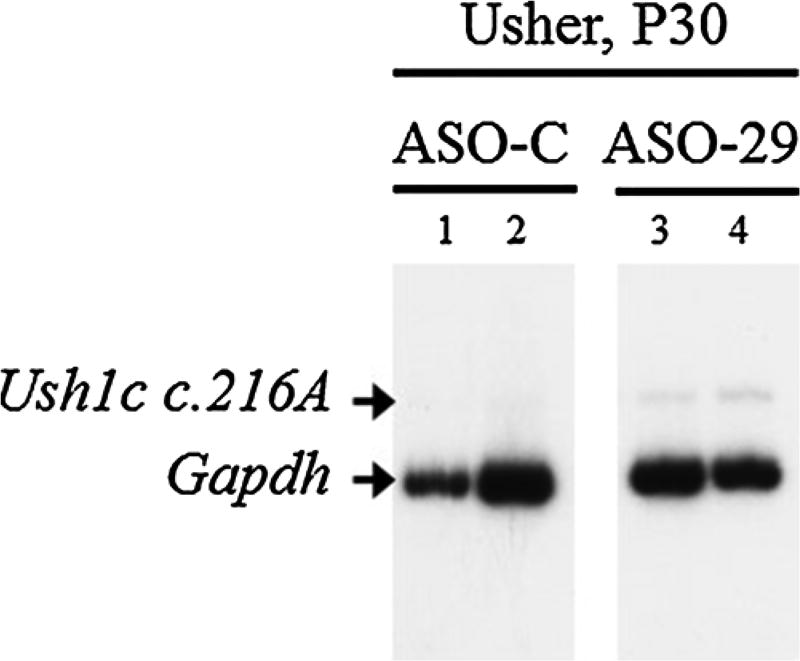
Correctly spliced Ush1c c.216A mRNA was not detected in samples from the inner ear of Usher mice treated with ASO-C (Fig. 1). Tissue from Usher mice treated with ASO-29 at P5 had detectable amounts of correctly spliced mRNA from the Ush1c c.216A gene allele. These results are consistent with previous reports on the efficacy of ASO-29 in Usher mice and demonstrate the partial correction of Ush1c c.216A gene expression in the Usher mice [8, 23, 32].
Fig. 1.
RT-PCR analysis of cochlear RNA isolated from one month-old mice treated with 300 mg/kg of ASO-29 at postnatal day five. Gel image shows PCR products from representative mouse samples (1–4) separated on a polyacrylamide gel (panel A). The correctly spliced Ush1c c.216A product is labeled. Mouse endogenous Gapdh is shown as a control.
3.2. Exploratory movement organization at two-months of age
Mice actively moved throughout the arena across all samples. Preliminary analyses did not reveal significant changes in performance across samples. Therefore, subsequent analyses were collapsed across the five samples.
No significant group differences were observed in the distance traveled under dark conditions at two-months of age (Table 1). The ANOVA conducted on distance traveled under dark conditions failed to reveal a significant effect of mutation [F(1,34) = 0.004, p = 0.949, < 0.001], treatment [F(1,34) = 0.927, p = 0.342, = 0.027], and Mutation by Treatment interaction [F(1,34) = 0.023, p = 0.880, = 0.001]. Similarly, no significant group differences were observed in distance traveled under light conditions (Table 1). The ANOVA conducted on distance traveled under light conditions failed to reveal a significant effect of mutation [F(1,34) = 3.161, p = 0.084, = 0.085], treatment [F(1,34) = 2.120, p = 0.155, = 0.059], and Mutation by Treatment interaction [F(1,34) = 2.571, p = 0.118, = 0.070]. Distance traveled did not vary among groups under either dark or light testing conditions.
Table 1.
Total distance (cm) traveled across five-minute samples.
| ASO-C
|
ASO-29
|
|||
|---|---|---|---|---|
| HET (M, SD) | Usher (M, SD) | HET (M, SD) | Usher (M, SD) | |
| Two-month | ||||
| Dark | 1816.2, 727.5 | 1880.4, 1475.6 | 1575.7, 502.2 | 1549.3, 255.49 |
| Light | 840.2, 408.3 | 2520.4, 2860.4 | 913.5, 270.8 | 1000.2, 462.8 |
| Six-month | ||||
| Dark | 1266.7, 555.9 | 1256.3, 1010.0 | 1499.5, 437.5 | 1307.9, 401.3 |
| Light | 834.9, 580.2 | 1002.8, 893.6 | 863.6, 139.5 | 614.2, 343.8 |
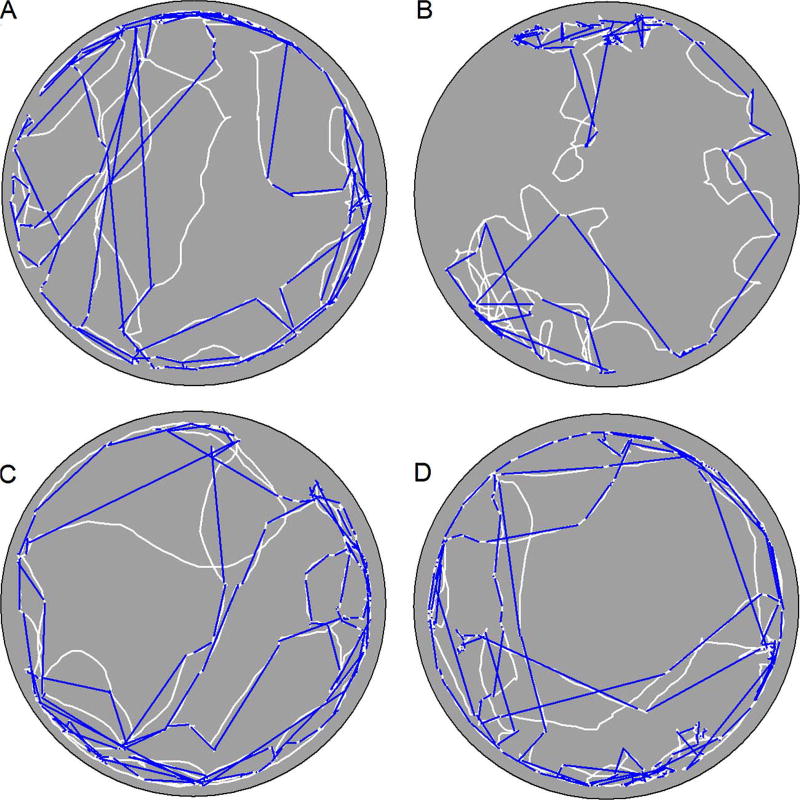
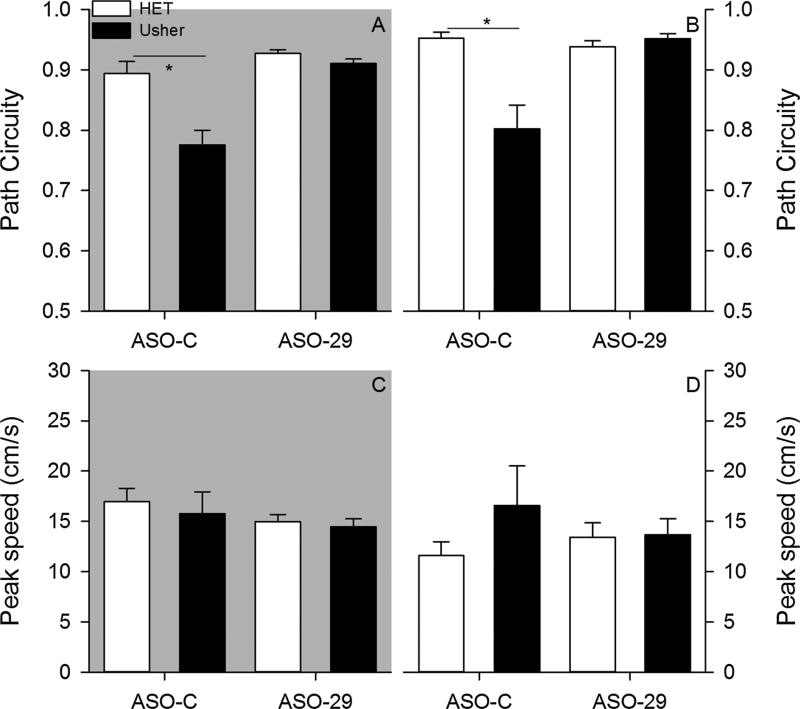
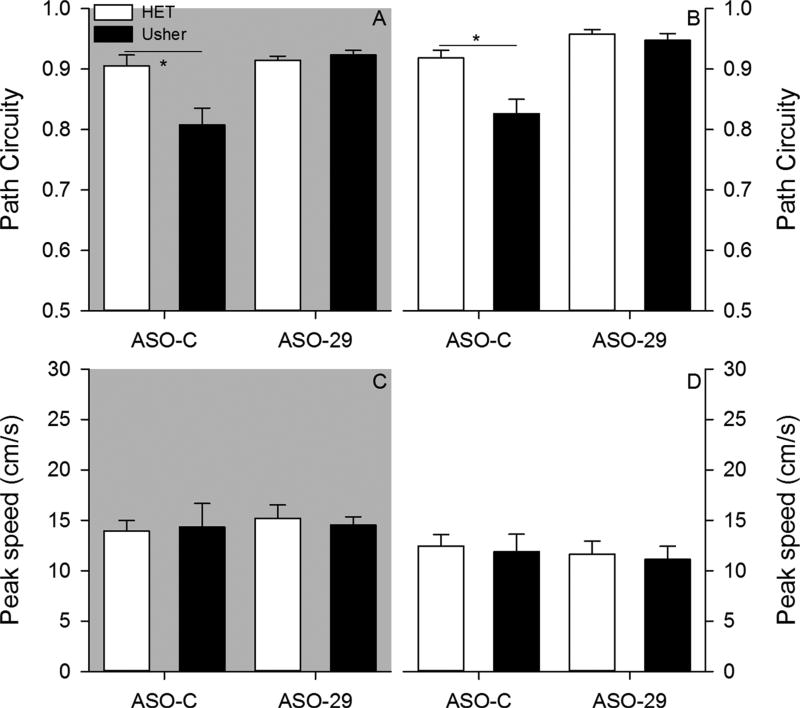
Within a sample, mice organized their exploratory behavior into a sequence of progressions (Fig. 2). In general, mice exhibited progressions that were non-circuitous paths under both testing conditions, with the exception of ASO-C treated Usher mice (Fig. 3). The ANOVA conducted on average progression path circuity under dark conditions revealed a significant effect of mutation [F(1,34) = 12.826, p = 0.001, = 0.274], treatment [F(1,34) = 19.955, p < 0.001, = 0.370], and Mutation by Treatment interaction [F(1,34) = 7.215, p = 0.011, = 0.175]. Post hoc analysis revealed that heterozygous mice treated with the ASO-C exhibited significantly more direct progressions relative to the Usher mice treated with the ASO-C (Tukey HSD, p < 0.05). In contrast, no group differences were observed in mice treated with the ASO-29. The ANOVA conducted on average progression path circuity under light conditions revealed a significant effect of mutation [F(1,34) = 9.047, p = 0.005, = 0.210], treatment [F(1,34) = 8.927, p = 0.005, = 0.208], and Mutation by Treatment interaction [F1,34) = 12.792, p = 0.001, = 0.273]. Post hoc analysis revealed that heterozygous mice treated with the ASO-C exhibited significantly more direct progressions relative to the Usher mice treated with the ASO-C; however, no group differences were observed among mice treated with ASO-29. The Usher mutation was observed to disrupt progression topography. ASO-29 treatment was observed to improve these disruptions associated with the Usher mutation.
Fig. 2.
Representative paths (white lines) from one sample under dark conditions are plotted for a heterozygous mouse treated with ASO-C (panel A), an Usher mouse treated with ASO-C (panel B), a heterozygous mouse treated with ASO-29 (panel C), and an Usher mouse treated with ASO-29 (panel D). Euclidean distance or the shortest path between the start and end point of a progression (blue lines) is plotted for all progressions. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
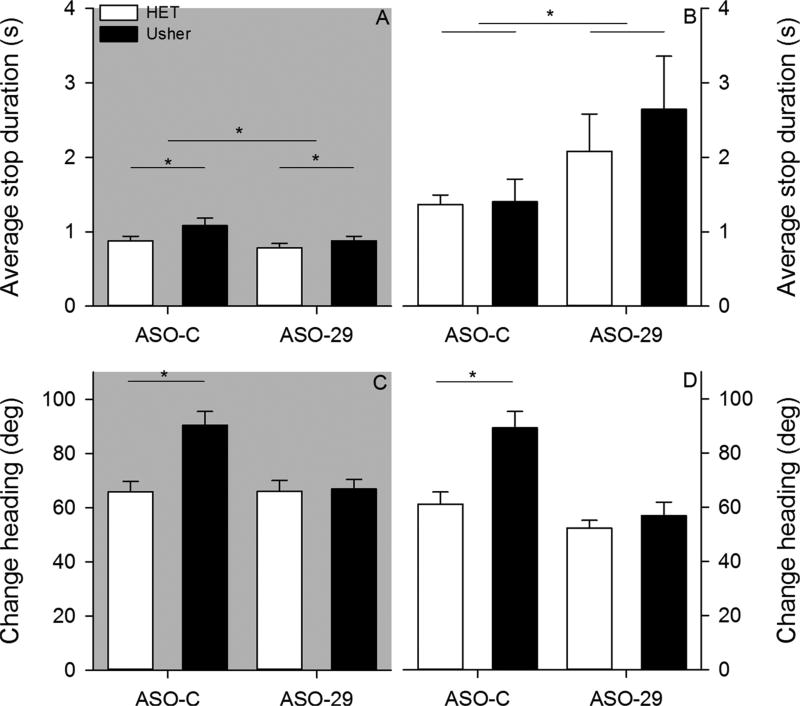
Two-month – Average progression path circuity is plotted for each group under dark (panel A) and light (panel B) conditions. Average progression peak speed is plotted for each group under dark (panel C) and light (panel D) conditions. (*p < 0.05).
Progressions are also associated with changes in moment-to-moment speeds. Peak speeds were not observed to significantly differ among groups under either testing condition (Fig. 3). The ANOVA conducted on average progression peak speed under dark conditions failed to reveal a significant effect of mutation [F(1,34) = 0.314, p = 0.579, = 0.009], treatment [F(1,34) = 1.216, p = 0.278, = 0.035], and Mutation by Treatment interaction [F(1,34) = 0.050, p = 0.824, = 0.001]. The ANOVA conducted on average progression peak speed under light conditions failed to reveal a significant effect of mutation [F(1,34) = 1.103, p = 0.301, = 0.031], treatment [F(1,34) = 0.048, p = 0.828, = 0.001], and Mutation by Treatment interaction [F(1,34) = 0.929, p = 0.342, = 0.027]. Neither the Usher mutation nor the ASO-29 therapy was observed to influence progression peak speeds.
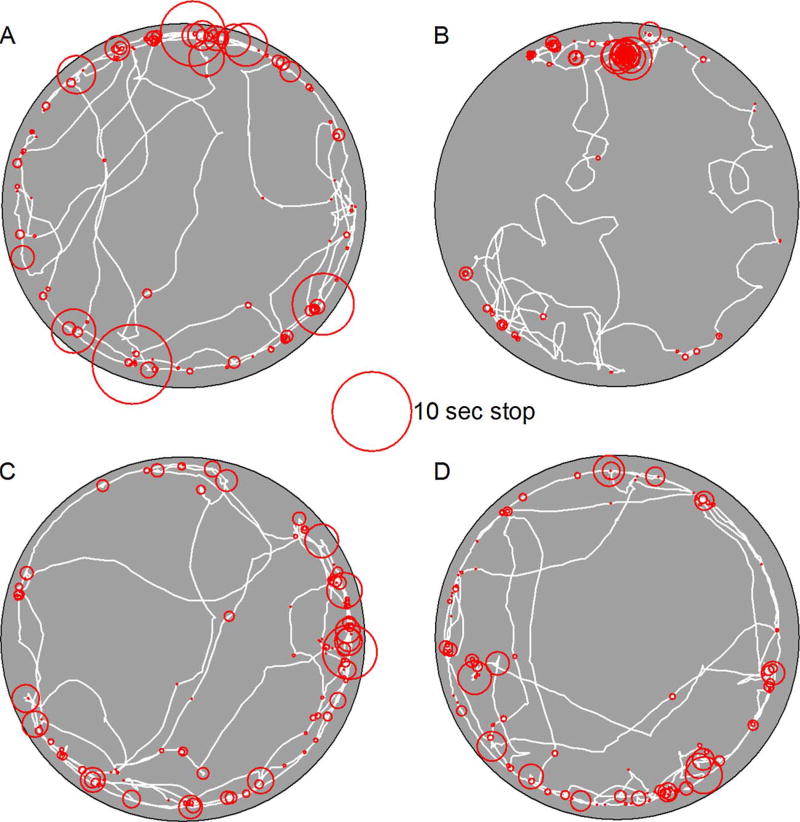
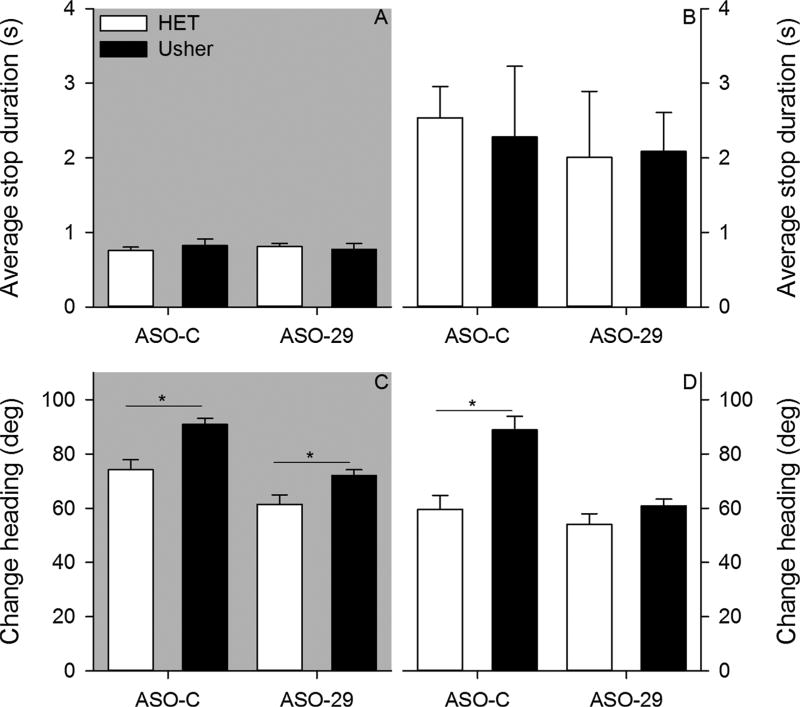
Stops represent another dimension of exploratory behavior organization (Fig. 4). The average stop duration did not vary among groups (Fig. 5). The ANOVA conducted on average stop duration under dark conditions failed to reveal a significant effect of mutation [F(1,34) = 0.056, p = 0.814, = 0.002], treatment [F(1,34) = 0.001, p = 0.984, = 0.001], and Mutation by Treatment interaction [F (1,34) = 0.603, p = 0.443, = 0.017]. The ANOVA conducted on average stop duration under light conditions failed to reveal a significant effect of mutation [F(1,34) = 0.014, p = 0.905, = 0.001], treatment [F(1,34) = 0.256, p = 0.616, = 0.007], and Mutation by Treatment interaction [F(1,34) = 0.054, p = 0.817, = 0.002]. Neither mutation nor treatment was observed to influence stop duration under either testing condition.
Fig. 4.
Representative paths (white lines) from one sample are plotted for a heterozygous mouse treated with ASO-C (panel A), an Usher mouse treated with ASO-C (panel B), a heterozygous mouse treated with ASO-29 (panel C), and an Usher mouse treated with ASO-29 (panel D). Position and duration (diameter) are plotted for a single sample’s set of stops (red circles). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
Two-month – Average stop duration is plotted for all groups under dark (panel A) and light (panel B) conditions. Average change in heading is plotted for all groups under dark (panel C) and light (panel D) conditions. (*p < 0.05).
The average change in heading was observed to vary among groups (Fig. 5). The ANOVA conducted on average change in heading under dark conditions revealed significant effects of mutation [F(1,34) = 18.047, p < 0.001, = 0.347] and treatment [F(1,34) = 24.041, p < 0.001, = 0.414]; however, the Mutation by Treatment interaction [F(1,34) = 0.893, p = 0.351, = 0.026] was not significant. Usher mice exhibited significantly larger changes in heading relative to control mice. In addition, ASO-29 significantly reduced the change in heading relative to the ASO-C treatment. The ANOVA conducted on average change in heading under light conditions revealed significant effects of mutation [F(1,34) = 14.977, p < 0.001, = 0.306], treatment [F(1,34) = 12.828, p = 0.001, = 0.274], and Mutation by Treatment interaction [F(1,34) = 5.771, p = 0.022, = 0.145]. Post hoc analysis revealed that Usher mice treated with the control ASO exhibited significantly larger changes in heading relative to the control mice treated with the ASO-C (Tukey HSD, p < 0.05). In contrast, no group differences were observed in mice treated with the ASO-29.
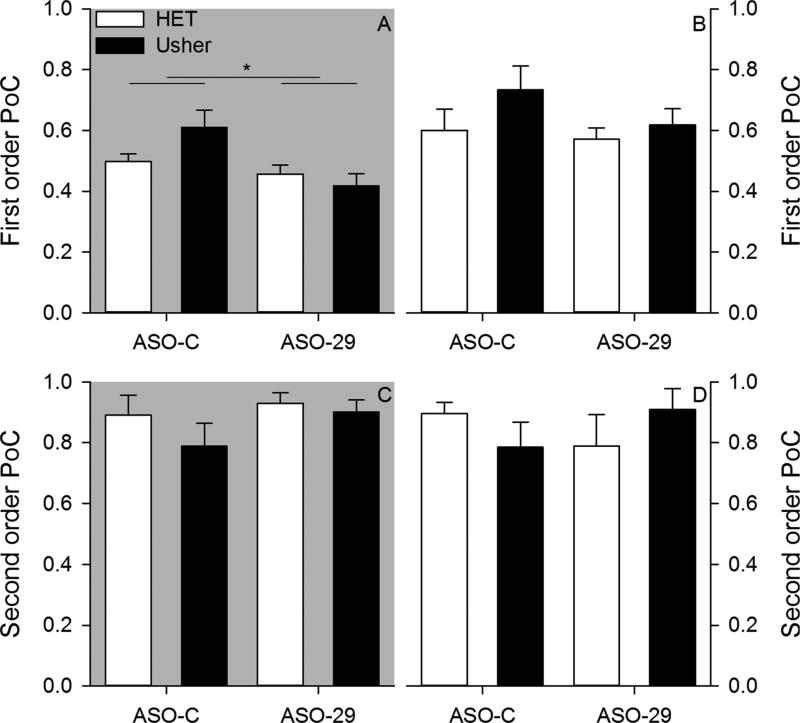
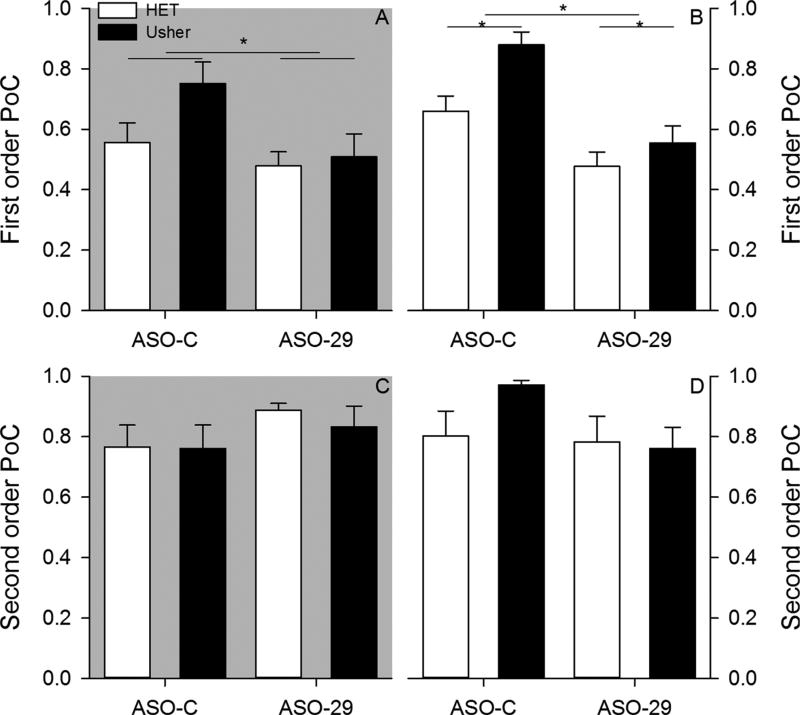
Mice cluster their stops in consistent locations within and between samples (Fig. 6). Only the ASO treatment factor was observed to influence first order (within sample) stop clustering (Fig. 7). The ANOVA applied to first order parameter of concentration under dark conditions revealed a significant effect of treatment [F(1,34) = 8.364, p = 0.007, = 0.197]; however, neither mutation [F(1,34) = 0.844,p = 0.365, = 0.024] nor Mutation by Treatment interaction [F(1,34) = 3.517, p = 0.069, = 0.094] were significant. ASO-29 treated mice had less dense stop clustering relative to ASO-C treated mice. The ANOVA applied to first order parameter of concentration under light conditions failed to reveal a significant effect of mutation [F(1,34) = 1.756, p = 0.194, = 0.049], treatment [F(1,34) = 1.165, p = 0.288, = 0.033], and Mutation by Treatment interaction [F(1,34) = 0.411, p = 0.526, = 0.012]. ASO-29 was observed to decrease within sample stop clustering only under dark conditions.
Fig. 6.
Stops (red circles) and first order circular statistics (black line) are plotted for a representative heterozygous mouse from the first (panel A), second (panel B), third (panel C), fourth (panel D), and fifth (panel E) samples. Average heading (red circles) and second order circular statistics (black line) are plotted for the five samples (panel F). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 7.
Two-month – Average first order parameter of concentration is plotted for each group under dark (panel A) and light (panel B) conditions. Average second order parameter of concentration is plotted for each group under dark (panel C) and light (panel D) conditions.
No group differences were observed in second order (between samples) stop clustering (Fig. 7). The ANOVA conducted on second order parameter of concentration under dark conditions failed to reveal significant effects of mutation [F(1,34) = 1.067, p = 0.309, = 0.030], treatment [F(1,34) = 1.454, p = 0.236, = 0.041], Mutation by Treatment interaction [F(1,34) = 0.349, p = 0.558, = 0.010].The ANOVA conducted on second order parameter of concentration under light conditions failed to reveal significant effects of mutation [F(1,34) = 0.004, p = 0.952, < 0.001], treatment [F (1,34) = 0.014, p = 0.907, < 0.001], Mutation by Treatment interaction [F(1,34) = 2.495, p = 0.123, = 0.068]. Independent of testing condition, mice consistently clustered stops in a similar location across samples.
3.3. Exploratory movement organization at six-months of age
No significant group differences were observed in the distance traveled under dark conditions (see Table 1). The ANOVA conducted on average distance traveled under dark conditions failed to reveal a significant effect of mutation [F(1,31) = 0.222, p = 0.641, = 0.007], treatment [F(1,31) = 0.439, p = 0.512, = 0.014], and Mutation by Treatment interaction [F(1,31) = 0.178, p = 0.676, = 0.006]. Similarly, no significant differences were observed in distance traveled under light conditions (see Table 1). The ANOVA conducted on average distance traveled under light conditions failed to reveal a significant effect of mutation [F(1,31) = 0.021, p = 0.887, = 0.001], treatment [F(1,31) = 0.763, p = 0.389, = 0.024], and Mutation by Treatment interaction [F(1,31) = 1.049, p = 0.314, = 0.033]. Distance traveled did not vary among groups under either testing condition.
During six-month testing, mice exhibited progressions that were non-circuitous paths under both conditions, with the exception of ASOC treated Usher mice (Fig. 8). The ANOVA conducted on average progression path circuity under dark conditions revealed a significant effect of mutation [F(1,31) = 6.452, p = 0.016, = 0.172], treatment [F(1,31) = 12.635, p = 0.001, = 0.290], and Mutation by Treatment interaction [F(1,31) = 9.171, p = 0.005, = 0.228]. Post hoc analysis revealed that heterozygous mice treated with the ASO-C exhibited significantly more direct progressions relative to the Usher mice treated with the ASO-C (Tukey HSD, p < 0.05). In contrast, no group differences were observed in mice treated with the ASO-29. The ANOVA conducted on average progression path circuity under light conditions revealed a significant effect of mutation [F(1,31) = 12.273, p = 0.001, = 0.284], treatment [F(1,31) = 30.404, p < 0.001, = 0.495], and Mutation by Treatment interaction [F(1,31) = 7.741, p = 0.009, = 0.200]. Post hoc analysis revealed that heterozygous mice treated with the ASO-C exhibited significantly more direct progressions relative to the Usher mice treated with the ASO-C; however, no group differences were observed in mice treated with ASO-29. The Usher mutation continued to disrupt progression topography at six-months. ASO-29 treatment continued to improve disruptions in progression topography associated with the Usher mutation at six-months.
Fig. 8.
Six-month – Average progression path circuity is plotted for each group under dark (panel A) and light (panel B) conditions. Average progression peak speed is plotted for each group under dark (panel C) and light (panel D) conditions. (p < 0.05).
Progression peak speeds were not observed to significantly differ among groups under either testing condition at six-months (Fig. 8). The ANOVA conducted on average progression peak speed under dark conditions failed to reveal a significant effect of mutation [F(1,31) = 0.010, p = 0.920, < 0.001], treatment [F(1,31) = 0.282, p = 0.599, = 0.009], and Mutation by Treatment interaction [F (1,31) = 0.149, p = 0.702, = 0.005]. The ANOVA conducted on average progression peak speed under light conditions failed to reveal a significant effect of mutation [F(1,31) = 0.151, p = 0.700, = 0.005], treatment [F(1,31) = 0.341, p = 0.563, = 0.011], and Mutation by Treatment interaction [F(1,31) = 0.001, p = 0.991, < 0.001]. Neither the Usher mutation nor the ASO-29 treatment were observed to influence progression peak speed at six-month testing.
The average stop duration was observed to vary among groups at six-months (Fig. 9). The ANOVA conducted on average stop duration under dark conditions revealed a significant effect of mutation [F(1,31) = 4.547, p = 0.041, = 0.128] and treatment [F(1,31) = 4.432, p = 0.043, = 0.125]; however, the Mutation by Treatment interaction [F(1,31) = 0.543, p = 0.467, = 0.017] was not significant. Usher mice stopped for a longer time than control mice. ASO-C treated mice stopped for longer durations than ASO-29 treated mice. The ANOVA conducted on average stop duration under light conditions revealed a significant effect of treatment [F(1,31) = 5.120, p = 0.031, = 0.142]; however, neither the effect of mutation [F(1,31) = 0.486, p = 0.491, = 0.015] nor the Mutation by Treatment interaction [F (1,31) = 0.366, p = 0.550, = 0.012] were significant. ASO-29 treated mice stopped for longer durations relative to mice treated with ASO-C. At six-months, mutation and treatment influenced stop duration under dark conditions; however, only treatment influenced stop duration under light conditions.
Fig. 9.
Six-month – Average stop duration is plotted for all groups under dark (panel A) and light (panel B) conditions. Average change in heading is plotted for all groups under dark (panel C) and light (panel D) conditions. (p < 0.05).
Group differences in average change in heading were observed under both conditions at six-months (Fig. 9). The ANOVA conducted on average change in heading under dark conditions revealed a significant effect of mutation [F(1,31) = 9.201, p = 0.005, = 0.229], treatment [F(1,31) = 7.808, p = 0.009, = 0.201], and Mutation by Treatment interaction [F(1,31) = 7.971, p = 0.008, = 0.205]. Post hoc analysis revealed that Usher mice treated with ASO-C exhibited significantly larger changes in heading relative to heterozygous mice treated with the ASO-C (Tukey HSD, p < 0.05). No group differences were observed between groups treated with ASO-29. The ANOVA conducted on average change in heading under light conditions revealed a significant effect of mutation [F(1,31) = 11.566, p = 0.002, = 0.272], treatment [F(1,31) = 18.413, p < 0.001, = 0.373], and Mutation by Treatment interaction [F(1,31) = 6.023, p = 0.020, = 0.163]. Post hoc analysis revealed that Usher mice treated with ASO-C exhibited significantly larger changes in heading relative to heterozygous mice treated with ASO-C (Tukey HSD, p < 0.05). No group differences were observed between groups treated with ASO-29. At six-months, ASO-29 significantly attenuated the Usher related increased change in heading under both testing conditions.
At six-months, differences in within sample (first order) parameter of concentration stop clustering were observed among groups (Fig. 10). The ANOVA conducted on first order parameter of concentration under dark conditions revealed a significant effect of treatment [F(1,31) = 5.447, p = 0.026, = 0.149]; however, neither mutation [F(1,31) = 2.720, p = 0.109, = 0.081] nor Mutation by Treatment interaction [F(1,31) = 1.445, p = 0.238, = 0.045] were significant. ASO-C treated mice exhibited higher within sample stop density relative to ASO-29 treated mice. The ANOVA conducted on first order parameter concentration under light conditions revealed significant main effects of mutation [F(1,31) = 8.209, p = 0.007, = 0.209] and treatment [F (1,31) = 23.921, p < 0.001, = 0.436]; however, the Mutation by Treatment interaction [F(1,31) = 1.861, p = 0.182, = 0.057] was not significant. Usher mice exhibited higher density stop clustering relative to heterozygous mice. In addition, control ASO-C treated mice exhibited higher within sample stop density, relative to ASO-29 treated mice.
Fig. 10.
Six-month – Average first order parameter of concentration is plotted for each group under dark (panel A) and light (panel B) conditions. Average second order parameter of concentration is plotted for each group under dark (panel C) and light (panel D) conditions.
No group differences were observed in second order (between samples) stop clustering at six-month testing (Fig. 10). The ANOVA conducted on second order parameter of concentration under dark conditions failed to reveal significant effects of mutation [F(1,34) = 0.185, p = 0.670, = 0.006], treatment [F(1,34) = 1.963, p = 0.171, = 0.060], and Mutation by Treatment interaction [F (1,34) = 0.126, p = 0.725, = 0.004].The ANOVA conducted on second order parameter of concentration under light conditions failed to reveal significant effects of mutation [F(1,34) = 0.887, p = 0.354, = 0.028], treatment [F(1,34) = 2.159, p = 0.152, = 0.065], and Mutation by Treatment interaction [F(1,34) = 1.485, p = 0.232, = 0.046]. Mice consistently clustered stops in a similar location across samples independent of testing condition at six-months.
4. Discussion
The current study examined the organization of exploratory movements in Usher and heterozygous control mice subsequent to receiving either the control, non-targeting ASO-C, or the therapeutic ASO-29. Disruptions in exploratory movement organization were observed in ASO-C treated Usher mice. In contrast, ASO-29 treated Usher mice did not exhibit any disruptions in organization of exploratory movements. In general, this pattern of results was observed independent of access to environmental cues and across both (two and six-month) testing sessions. These observations are consistent with the efficacy of ASO-29 in rescuing impaired spatial orientation associated with the USH1C mouse model of Usher syndrome. The following sections will discuss Usher-associated information processing deficits and the effectiveness of ASO-29 treatment to ameliorate these deficits.
4.1. Spatial orientation deficits associated with a mouse model of Usher syndrome
Several differences in exploratory movement organization were observed between heterozygous and Usher mice treated with the control, ASO-C. First, Usher mice treated with ASO-C exhibited more circuitous progressions and larger changes in heading during stops, relative to heterozygous mice. Unlike previous work with tilted mice, group differences were not attenuated by access to environmental cues under light testing. Several factors may have contributed to these group differences. A deficit in basic locomotion is one factor that may have contributed to these disruptions in exploratory movement organization. For example, previous work has revealed significant circling behavior in Usher mice treated with ASO-C [23, 24]. If the disruption in the organization of exploratory movements was exclusively driven by locomotor deficits, group differences would have been expected to be less specific and span across other measures. However, Usher and heterozygous ASO-C treated mice did not differ in total distance traveled, progression peak speed, or stop clustering. Therefore, it is possible that other factors contribute to these more selective disruptions in exploratory movement organization.
Impaired self-movement cue processing may be another factor contributing to the disruption in exploratory movement organization observed in Usher mice. Impaired self-movement cue processing has been posited as a contributing factor in disruptions of exploratory behavior observed in headbanger mice [2], Tristram’s jirds (Meriones tristami) [42], and tilted mice [5]. Interestingly, tilted mice typically do not engage in circling behavior, despite showing disruptions in exploratory movement organization similar to Usher mice. Therefore, the circling behavior observed in the control-treated Usher mice in the current study may be sufficient, but is likely not necessary, to impair self-movement cue processing. For example, ‘knot-sketching’ behavior or topographically focused paths with many twists and turns has been posited to enhance self-movement cue processing [10]. The circling behavior of control-treated Usher mice would prevent mice from engaging in ‘knot-sketching’ behavior and potentially attenuate self-movement cue processing. These Usher-related deficits might be associated with a gradual accumulation of directional error. An association between vestibular dysfunction and directional error is supported by work demonstrating that head direction signals in tilted mice degrade over time [41]. Accumulating directional error in Usher mice would be expected to: impair online estimates of current position, produce more circuitous progressions, influence the magnitude of heading change during stops, and reduce home base stability. Both of the former disruptions were observed in Usher mice treated with ASO-C; however, Usher and heterozygous ASO-C mice did not differ in first (within sample) or second (between sample) order density of stop clustering. Both groups exhibited highly concentrated stop clustering that did not vary across samples, which indicates similar levels of home base establishment. It is possible that the Usher mice have an intermediate impairment in self-movement cue processing that spared the ability to establish stable home bases. Another possibility is that the circling behavior restricted the range of movement; however, total distance traveled did not differ among the groups. Further, spared home base establishment in the Usher mice may reflect recruitment of compensatory strategies. For example, it is possible that control-treated Usher mice compensated for impaired self-movement cue processing by using olfactory cues to maintain home base stability [14]. However, this possibility is unlikely considering that several studies discounted the role of olfactory cues in maintaining home base stability [19, 36]. Alternatively, tactile cues from the table may have anchored the home base. This is unlikely considering the plastic tab attached to the edge of the table failed to polarize home base establishment in the current study and subtle tactile cues from the surface of the table were not sufficient to anchor home base behavior in previous work with tilted mice [5]. Results of the current study are consistent with Usher mice exhibiting impaired processing of self-movement cues; however, this may not be the only information processing deficit mediating disruption in exploratory movement organization.
Finally, deficits in using environmental cues may have contributed to disruptions in exploratory movement organization. Previous work has demonstrated that rats [21] and mice [6] encode the position of salient environmental cues and use them to organize exploratory movements. Under light conditions, control-treated Usher mice continued to exhibit more circuitous progressions and larger changes in heading, relative to control-treated heterozygous mice. This finding is in contrast to attenuated disruptions in exploratory movement organization observed in the tilted mouse when tested under light conditions [5]. One possible explanation for significant group differences observed in the current study under light conditions is loss of visual acuity. Individuals suffering from Type 1 Usher syndrome develop visual impairments (i.e., retinitis pigmentosa) with the onset of puberty [37]. Further, recent work suggests that the USH1C model of Usher syndrome may also express similar developmental visual impairments [22]. Additional work is needed to characterize the visual acuity of these USH1C mice. In addition to visual impairment, it is possible that control treated mice were impaired in encoding the position or relationships among environmental cues. For example, bilateral vestibular pathology has been shown to produce an enduring loss of location-specific hippocampal place cell activity [27, 29] and impairs encoding relationships among environmental cues [4, 25]. In contrast, stable place cell activity [40] and spared use of environmental cues [38, 39] has been observed in tilted mice. Given the conflicting observations from varied types of vestibular pathology, additional behavioral studies are needed to characterize the nature of the deficits preventing improved exploratory movement organization under light conditions. Future work examining cue control of home base behavior [31] for review see [31] and food hoarding behavior [38] in Usher mice may provide further insights to the nature of the deficit mediating the disruptions in exploratory movement organization.
4.2. ASO-29 treatment rescues impaired spatial orientation
Several changes in exploratory movement organization were observed with the postnatal day five administration of ASO-29. First, in contrast to ASO-C treated mice, no group differences in progression path circuity were observed between Usher and heterozygous mice treated with ASO-29. Both groups followed non-circuitous progressions under dark and light conditions. Next, under dark conditions at two and six-months of age, the ASO-29 treatment significantly reduced changes in heading during stops relative to the ASO-C treatment; however, Usher mice treated with ASO-29 continued to exhibit significantly larger changes in heading relative to heterozygous mice at two-months of age but not at six-months. In contrast, no differences in change in heading were observed between Usher and heterozygous mice treated with ASO-29 under light conditions. Finally, ASO-29 treatment reduced within sample stop density under dark conditions. ASO-29 related changes in exploratory movement organization may reflect the rescuing of basic locomotor function. For example, previous work has shown that ASO-29 treated Usher mice do not engage in circling behavior [23, 24]. In the current study, ASO-29 administration was observed to decrease first order (within sample) stop clustering. This may reflect a release or breaking away from the more focused exploratory movements associated with circling behavior. However, administration of ASO-29 was not observed to influence total travel distance or progression peak speeds. Both measures would be expected to be sensitive to disruptions in general locomotor function. Although these observations do not exclude the possibility that the ASO-29 amelioration of exploratory movement organization is mediated by general locomotor function, it is possible that ASO-29 may be rescuing information processing related to maintenance of spatial orientation.
Results of the current study and previous work [2, 5] demonstrate a role for the vestibular system in organizing exploratory movements. Considering that ASO-29 administration has been shown to increase USH1C c.216G > A correct splicing and expression of the harmonin protein [23], it is possible that improved vestibular function is mediating the changes in exploratory movement organization. For example, improvements in vestibular function would be expected to enhance processing of self-movement cues. Spatial orientation under dark conditions depends on using self-movement cues to update a representation of the current position to guide subsequent movements. Administration of ASO-29 may have rescued vestibular function sufficient enough to improve both progression path circuity and change in heading under dark conditions. However, a complete rescuing of vestibular function is unlikely considering that significant group differences in change of heading were observed between two-month old Usher and heterozygous mice receiving ASO-29. The possibility of residual vestibular deficits is supported by recent work investigating vestibular evoked potentials [32]. Specifically, Usher mice treated with ASO-29 at postnatal day five exhibited higher vestibular evoked potential thresholds; however, traditional behavioral assessments (i.e., circling and swimming testing) of vestibular function failed to reveal group differences. Therefore, the residual disruption in exploratory movement organization under dark conditions may reflect a subtle impairment in processing self-movement cues. These observations demonstrate the power of exploratory movement organization to investigate varied levels of vestibular pathology and efficacy of therapies given at different times during development.
Finally, administration of ASO-29 may have also rescued the use of environmental cues. Recall that disruptions in exploratory movement organization associated with the Usher mouse were not selective to testing condition. The failure to observe improved performance in Usher mice under light conditions was attributed to impaired use of vision, possibly involving developmental degeneration of the retina [22]. Similarly, group differences were not observed in Usher and heterozygous mice treated with ASO-29 under light conditions. Therefore, it is possible that ASO-29 treated Usher mice were able to use environmental cues to compensate for residual self-movement cue processing deficits and organize their exploratory movements similar to heterozygous mice. Further work is needed to investigate whether ASO-29 efficacy under light conditions reflected improved visual function or sufficiently restored vestibular function to mediate encoding relationships among environmental cues.
4.3. Persistent deficits and ASO-29 efficacy
Exploratory movement organization was assessed under conditions with varied access to visual cues at two-months and six-months of age. In general, the pattern of results observed at two-months was also observed at six-months. For example, group differences in progression path circuity were observed between Usher and heterozygous mice treated with ASO-C under dark and light conditions. This group difference is consistent with Usher mice maintaining a persistent impairment in self-movement cue processing without compensatory use of environmental cues. In addition, no group differences were observed in progression path circuity between Usher and heterozygous mice treated with ASO-29. This observation is consistent with ASO-29 therapy producing an enduring rescue of vestibular and visual system function sufficient to mediate exploratory movement organization. These observations replicate the two-month results, providing additional support for Usher mice exhibiting spatial orientation deficits that are rescued by postnatal day five administration of ASO-29.
Several differences in the pattern of results were also observed during the six-month testing. For example, no group differences were observed in change in heading under dark conditions in Usher and heterozygous mice treated with ASO-29. The group differences at two-month testing were attributed to a residual self-movement cue deficit. The lack of group differences at the six-month testing may reflect compensatory mechanisms related to plasticity within the vestibular system. Specifically, previous work has shown recovery of homing accuracy sixmonths after unilateral, but not bilateral, vestibular lesions [43]. Further work is needed to characterize vestibular system plasticity in Usher mice with residual vestibular pathology.
Average stop duration was another measure that revealed differences in the pattern of results observed between the testing sessions. Neither mutation nor ASO treatment were observed to influence average stop duration at the two-month testing. In contrast, at six-month testing, both factors influenced average stop duration under dark conditions and only the ASO treatment significantly influenced stop duration under light conditions. Specifically, under dark conditions, both Usher mice and ASO-C treated mice were observed to stop for longer durations; whereas, under light conditions, ASO-29 treated mice were observed to stop for longer durations. Although these effects were significant, the effect sizes were relatively small and no differences were observed in total distance traveled or stop clustering. It is unlikely that these observations are attributed to only one factor. For example, changes in anxiety have been a factor suggested to mediate changes in open field behavior observed in the headbanger mouse [2]. In the current study, the observed increase in stopping behavior in Usher mice under dark conditions could be attributed to increased anxiety. The influence of anxiety on stopping behavior was expected to increase under light conditions; however, there was no effect of mutation observed under light conditions. In addition, rescued vestibular function associated with ASO-29 would be expected to attenuate anxiety and increase exploration. In contrast, under light conditions ASO-29 treated mice stopped for longer durations. It is clear further work is needed to characterize the set of factors that influence stop duration.
5. Conclusion
The current study used the sequential organization of exploratory movements to characterize spatial orientation deficits related to Usher syndrome and evaluate the efficacy of ASO-29 treatment on improving function of multiple sensory systems. Several observations provide novel insight to the information processing deficits associated with Usher syndrome. First, Usher mice exhibited specific disruptions in exploratory movements, which are consistent with impaired use of self-movement and environmental cues. Next, postnatal day five ASO-29 treatment improved Usher related disruptions of exploratory movement organization, demonstrating the potential of ASO-29 as a therapy that rescues use of self-movement and environmental cues. Finally, therapeutic effectiveness of the ASO-29 was observed to be maintained across both two-month and six-month testing. These results add to a growing literature demonstrating the potential of exploratory movement organization to characterize spatial orientation deficits and establish the efficacy of ASO therapies in rescuing information processing deficits.
Acknowledgments
Amber Ballard and Paul John for assistance with animals. We gratefully acknowledge support from the National Institutes of Health (1R01DC012596) (MLH) and NIU and RFUMS.
References
- 1.Aguirre GK, D’Esposito M. Topographical disorientation: a synthesis and taxonomy. Brain. 1999;122(9):1613–1628. doi: 10.1093/brain/122.9.1613. [DOI] [PubMed] [Google Scholar]
- 2.Avni R, Elkan T, Dror AA, Shefer S, Eilam D, Avraham KB, Mintz M. Mice with vestibular deficiency display hyperactivity, disorientation, and signs of anxiety. Behav. Brain Res. 2009;202(2):210–217. doi: 10.1016/j.bbr.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 3.Batschelet E. Circular Statistics in Biology. Academic Press; New York: 1981. [Google Scholar]
- 4.Besnard S, Machado ML, Vignaux G, Boulouard M, Coquerel A, Bouet V, et al. Influence of vestibular input on spatial and nonspatial memory and on hippocampal NMDA receptors. Hippocampus. 2012;22(4):814–826. doi: 10.1002/hipo.20942. [DOI] [PubMed] [Google Scholar]
- 5.Blankenship PA, Cherep LA, Donaldson TN, Brockman SN, Trainer AD, Yoder RM, Wallace DG. Otolith dysfunction alters exploratory movement in mice. Behav. Brain Res. 2017;325:1–11. doi: 10.1016/j.bbr.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark BJ, Hamilton DA, Whishaw IQ. Motor activity (exploration) and formation of home bases in mice (C57BL/6) influenced by visual and tactile cues: modification of movement distribution, distance, location, and speed. Physiol. Behav. 2006;87(4):805–816. doi: 10.1016/j.physbeh.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 7.Clark BJ, Hines DJ, Hamilton DA, Whishaw IQ. Movements of exploration intact in rats with hippocampal lesions. Behav. Brain Res. 2005;163(1):91–99. doi: 10.1016/j.bbr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Depreux FF, Wang L, Jiang H, Jodelka FM, Rosencrans RF, Rigo F, et al. Antisense oligonucleotides delivered to the amniotic cavity in utero modulate gene expression in the postnatal mouse. Nucleic Acids Res. 2016;44(20):9519–9529. doi: 10.1093/nar/gkw867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drai D, Kafkafi N, Benjamini Y, Elmer G, Golani I. Rats and mice share common ethologically relevant parameters of exploratory behavior. Behav. Brain Res. 2001;125(1):133–140. doi: 10.1016/s0166-4328(01)00290-x. [DOI] [PubMed] [Google Scholar]
- 10.Dvorkin A, Szechtman H, Golani I. Knots: attractive places with high path tortuosity in mouse open field exploration. PLoS Comput. Biol. 2010;6(1):e1000638. doi: 10.1371/journal.pcbi.1000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebermann I, Lopez I, Bitner-Glindzicz M, Brown C, Koenekoop RK, Bolz HJ. Deafblindness in French Canadians from Quebec: a predominant founder mutation in the USH1C gene provides the first genetic link with the Acadian population. Genome Biol. 2007;8(4):R47. doi: 10.1186/gb-2007-8-4-r47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eilam D, Golani I. Home base behavior of rats (Rattus norvegicus) exploring a novel environment. Behav. Brain Res. 1989;34(3):199–211. doi: 10.1016/s0166-4328(89)80102-0. [DOI] [PubMed] [Google Scholar]
- 13.Gallistel CR. The Organization of Learning. The MIT Press; 1990. [Google Scholar]
- 14.Gire DH, Kapoor V, Arrighi-Allisan A, Seminara A, Murthy VN. Mice develop efficient strategies for foraging and navigation using complex natural stimuli. Curr. Biol. 2016;26(10):1261–1273. doi: 10.1016/j.cub.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goddard M, Zheng Y, Darlington CL, Smith PF. Locomotor and exploratory behavior in the rat following bilateral vestibular deafferentation. Behav. Neurosci. 2008;122(2):448. doi: 10.1037/0735-7044.122.2.448. [DOI] [PubMed] [Google Scholar]
- 16.Golani I, Benjamini Y, Eilam D. Stopping behavior: constraints on exploration in rats (Rattus norvegicus) Behav. Brain Res. 1993;53(1):21–33. doi: 10.1016/s0166-4328(05)80263-3. [DOI] [PubMed] [Google Scholar]
- 17.Gorny JH, Gorny B, Wallace DG, Whishaw IQ. Fimbria-fornix lesions disrupt the dead reckoning (homing) component of exploratory behavior in mice. Learn. Mem. 2002;9(6):387–394. doi: 10.1101/lm.53002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grillet N, Xiong W, Reynolds A, Kazmierczak P, Sato T, Lillo C, et al. Harmonin mutations cause mechanotransduction defects in cochlear hair cells. Neuron. 2009;62(3):375–387. doi: 10.1016/j.neuron.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hines DJ, Whishaw IQ. Home bases formed to visual cues but not to self-movement (dead reckoning) cues in exploring hippocampectomized rats. Eur. J. Neurosci. 2005;22(9):2363–2375. doi: 10.1111/j.1460-9568.2005.04412.x. [DOI] [PubMed] [Google Scholar]
- 20.Lefèvre G, Michel V, Weil D, Lepelletier L, Bizard E, Wolfrum U, et al. A core cochlear phenotype in USH1 mouse mutants implicates fibrous links of the hair bundle in its cohesion, orientation and differential growth. Development. 2008;135(8):1427–1437. doi: 10.1242/dev.012922. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann H, Clark BJ, Whishaw IQ. Similar development of cued and learned home bases in control and hippocampal-damaged rats in an open field exploratory task. Hippocampus. 2007;17(5):370–380. doi: 10.1002/hipo.20274. [DOI] [PubMed] [Google Scholar]
- 22.Lentz JJ, Gordon WC, Farris HE, MacDonald GH, Cunningham DE, Robbins CA, et al. Deafness and retinal degeneration in a novel USH1C knock-in mouse model. Dev. Neurobiol. 2010;70(4):253–267. doi: 10.1002/dneu.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lentz JJ, Jodelka FM, Hinrich AJ, McCaffrey KE, Farris HE, Spalitta MJ, et al. Rescue of hearing and vestibular function by antisense oligonucleotides in a mouse model of human deafness. Nat. Med. 2013;19(3):345–350. doi: 10.1038/nm.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lentz J, Pan F, Deininger P, Keats B. Ush1c216A knock-in mouse survives Katrina. Mutat. Res./Fundam. Mol. Mech. Mutage. 2007;616(1):139–144. doi: 10.1016/j.mrfmmm.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Ossenkopp KP, Hargreaves EL. Spatial learning in an enclosed eight-arm radial maze in rats with sodium arsanilate-induced labyrinthectomies. Behav. Neural Biol. 1993;59(3):253–257. doi: 10.1016/0163-1047(93)91034-k. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang XM, Hejtmancik JF, Jacobson SG, Xia XJ, Li A, Du LL, et al. USH1C: a rare cause of USH1 in a non-Acadian population and a founder effect of the Acadian allele. Clin. Genet. 2003;63(2):150–153. doi: 10.1046/j.0009-9163.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 27.Russell NA, Horii A, Smith PF, Darlington CL, Bilkey DK. Long-term effects of permanent vestibular lesions on hippocampal spatial firing. J. Neurosci. 2003;23(16):6490–6498. doi: 10.1523/JNEUROSCI.23-16-06490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stackman RW, Clark AS, Taube JS. Hippocampal spatial representations require vestibular input. Hippocampus. 2002;12(3):291–303. doi: 10.1002/hipo.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tchernichovski O, Golani I. A phase plane representation of rat exploratory behavior. J. Neurosci. Methods. 1995;62(1):21–27. doi: 10.1016/0165-0270(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 31.Thompson SM, Berkowitz LE, Clark BJ. Behavioral and neural subsystems of rodent exploration. Learn. Motiv. 2017 doi: 10.1016/j.lmot.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vijayakumar S, Depreux FF, Jodelka FM, Lentz JJ, Rigo F, Jones TA, Hastings ML. Rescue of peripheral vestibular function in Usher syndrome mice using a splice-switching antisense oligonucleotide. Hum. Mol. Genet. 2017;26(18):3482–3494. doi: 10.1093/hmg/ddx234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallace DG, Hamilton DA, Whishaw IQ. Movement characteristics support a role for dead reckoning in organizing exploratory behavior. Anim. Cogn. 2006;9(3):219–228. doi: 10.1007/s10071-006-0023-x. [DOI] [PubMed] [Google Scholar]
- 35.Whishaw IQ, Cassel JC, Majchrzak M, Cassel S, Will B. Short-stops in rats with fimbria-fornix lesions: evidence for change in the mobility gradient. Hippocampus. 1994;4(5):577–582. doi: 10.1002/hipo.450040507. [DOI] [PubMed] [Google Scholar]
- 36.Whishaw IQ, Hines DJ, Wallace DG. Dead reckoning (path integration) requires the hippocampal formation: evidence from spontaneous exploration and spatial learning tasks in light (allothetic) and dark (idiothetic) tests. Behav. Brain Res. 2001;127(1):49–69. doi: 10.1016/s0166-4328(01)00359-x. [DOI] [PubMed] [Google Scholar]
- 37.Yan D, Liu XZ. Genetics and pathological mechanisms of usher syndrome. J. Hum. Genet. 2010;55(6):327–335. doi: 10.1038/jhg.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoder RM, Goebel EA, Köppen JR, Blankenship PA, Blackwell AA, Wallace DG. Otolithic information is required for homing in the mouse. Hippocampus. 2015;25(8):890–899. doi: 10.1002/hipo.22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoder RM, Kirby SL. Otoconia-deficient mice show selective spatial deficits. Hippocampus. 2014;24(10):1169–1177. doi: 10.1002/hipo.22300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoder RM, Rutan SA, Siegel JJ. Stable place cell activity in otoconia-deficient tilted mice; Paper Presented at the Annual Meeting of the Midwestern Psychological Association; Chicago. 2014. [Google Scholar]
- 41.Yoder RM, Taube JS. Head direction cell activity in mice: robust directional signal depends on intact otolith organs. J. Neurosci. 2009;29(4):1061–1076. doi: 10.1523/JNEUROSCI.1679-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zadicario P, Avni R, Zadicario E, Eilam D. ‘Looping’—an exploration mechanism in a dark open field. Behav. Brain Res. 2005;159(1):27–36. doi: 10.1016/j.bbr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 43.Zheng Y, Darlington CL, Smith PF. Impairment and recovery on a food foraging task following unilateral vestibular deafferentation in rats. Hippocampus. 2006;16(4):368–378. doi: 10.1002/hipo.20149. [DOI] [PubMed] [Google Scholar]
- 44.Zheng Y, Goddard M, Darlington CL, Smith PF. Long-term deficits on a foraging task after bilateral vestibular deafferentation in rats. Hippocampus. 2009;19(5):480–486. doi: 10.1002/hipo.20533. [DOI] [PubMed] [Google Scholar]