Abstract
Background
Cocaine use disorder remains a significant public health issue for which there are no FDA-approved pharmacotherapies. Amphetamine maintenance reduces cocaine use in preclinical and clinical studies, but the mechanism of this effect is unknown. Previous studies indicate a role for endogenous opioid release and subsequent opioid receptor activation in some amphetamine effects; therefore, the current study examined the role of mu-opioid receptor activation in d-amphetamine treatment effects in an assay of cocaine-vs-food choice.
Methods
Adult male rhesus monkeys with double-lumen intravenous catheters responded for concurrently available food pellets and cocaine injections (0–0.1 mg/kg/injection) during daily sessions. Cocaine choice and overall reinforcement rates were evaluated during 7-day treatments with saline or test drugs.
Results
During saline treatment, cocaine maintained a dose-dependent increase in cocaine-vs.-food choice. The mu-opioid receptor agonist morphine (0.032–0.32 mg/kg/h) dose-dependently increased cocaine choice and decreased rates of reinforcement. A dose of the mu-selective opioid receptor antagonist naltrexone (0.0032 mg/kg/h) that completely blocked morphine effects had no effect on cocaine choice when it was administered alone, but it enhanced the effectiveness of a threshold dose of 0.032 mg/kg/h amphetamine to decrease cocaine choice without also enhancing nonselective behavioral disruption by this dose of amphetamine. Conversely, the kappa-selective opioid antagonist norbinalorphimine did not enhance amphetamine effects on cocaine choice.
Conclusions
These results suggest that amphetamine maintenance produces mu opioid-receptor mediated effects that oppose its anti-cocaine effects. Co-administration of naltrexone may selectively enhance amphetamine potency to decrease cocaine choice without increasing amphetamine potency to produce general behavioral disruption.
Keywords: cocaine, choice, amphetamine, naltrexone, rhesus monkey, morphine
1. Introduction
There are currently no pharmacotherapies approved by the United States Food and Drug Administration for treatment of cocaine use disorder; however, maintenance on the dopamine and norepinephrine releaser amphetamine has been shown repeatedly and across a range of conditions to selectively decrease cocaine self-administration in rats (Chiodo et al., 2008; Thomsen et al., 2012), monkeys (Banks et al., 2013a; Czoty et al., 2011; Negus, 2003; Negus and Mello, 2003), and humans (Greenwald et al., 2010; Rush et al., 2010). Furthermore, clinical trials have demonstrated the effectiveness of amphetamine maintenance to decrease metrics of cocaine use in cocaine-dependent patients (Grabowski et al., 2001; Mariani et al., 2012; Nuijten et al., 2016; Perez-Mana et al., 2011). However, numerous obstacles exist to the clinical deployment of amphetamine as a maintenance medication for treatment of cocaine use disorder (see Negus and Henningfield, 2015).
The mechanism by which amphetamine produces its therapeutic reductions in cocaine-maintained behavior is unknown, but elucidating this mechanism could reveal novel treatment strategies. Although the primary targets of amphetamine are the dopamine and norepinephrine transporters (Rothman et al., 2001), amphetamine also promotes the release of endogenous opioids (Mick et al., 2014). Furthermore, opioid antagonist doses that do not alter abuse-related cocaine effects have been shown to reduce amphetamine effects. For example, naloxone in rodents (Andrews and Holtzman, 1987; Dettmar et al., 1978; Hitzemann et al., 1982; Holtzman, 1974) or naltrexone in monkeys (Winslow and Miczek, 1988) attenuated increases in locomotor activity produced by amphetamine. Furthermore, naloxone blunted the abuse-related effects of amphetamine on intracranial self-stimulation in rats (Esposito et al., 1980; Holtzman, 1976), and naltrexone decreased amphetamine self-administration in monkeys (Jimenez-Gomez et al., 2011), suggesting a role for opioid receptors in mediating at least some behavioral effects of amphetamine. The role of opioid systems in the therapeutic effectiveness of amphetamine maintenance to decrease cocaine use has not been investigated.
The aim of the current study was to evaluate the role of mu-opioid receptors in mediating amphetamine treatment effects on cocaine self-administration using a cocaine vs. food choice procedure in rhesus monkeys (Negus, 2003). The choice procedure was chosen for three primary reasons (Banks and Negus, 2012, 2017; Czoty et al., 2016; Heyman, 2009; Jones and Comer, 2013). First, drug choice procedures provide a dependent measure of drug reinforcement (i.e., percent drug choice) that is relatively independent of treatment effects on overall rates of responding or reinforcement. Second, drug choice procedures provide a simplified preclinical model of natural environments that contain both drug and non-drug reinforcers, and these procedures can be used to study determinants of behavioral allocation toward or away from drug use. Specifically, clinical drug abuse treatments seek not only to reduce drug use but also to promote more adaptive behaviors maintained by non-drug reinforcers, and preclinical choice procedures provide a useful tool to examine effects of candidate treatments on behavioral allocation. Finally, drug choice procedures are also widely used in human laboratory studies, so their use in preclinical research enhances preclinical-to-clinical translation. To examine the role of mu-opioid receptor activation in the therapeutic effects of amphetamine, we first tested the hypothesis that mu receptor activation would be sufficient to mimic amphetamine-induced decreases in cocaine choice by treating monkeys with the mu agonist morphine (Bowen et al., 2002; Emmerson et al., 1994). Morphine maintenance increased rather than decreased cocaine choice, suggesting that any mu receptor-activating effects of amphetamine might oppose rather than contribute to amphetamine-induced decreases in cocaine choice. Accordingly, follow-up studies evaluated the effects of co-administering amphetamine and the opioid antagonist naltrexone to test the hypothesis that blockade of mu receptors would enhance amphetamine-induced decreases in cocaine choice. Naltrexone was selected as the opioid antagonist for two reasons. First, it is a moderately selective for mu vs. other opioid receptors, its relative potency and selectivity as a mu antagonist in monkeys are well established, and more selective reversible and systemically active mu antagonists are not currently available (Bidlack and Matthews, 2009; Bowen et al., 2002; Emmerson et al., 1994; Ko et al., 1998). Second, naltrexone is approved as a maintenance medication for treatment of opioid and ethanol abuse, and it showed some effectiveness as a candidate medication for treatment of amphetamine abuse (Anton, 2009; Jayaram-Lindstrom et al., 2008; Sevarino and Kosten, 2009). Nonetheless, because naltrexone has only modest (≤10-fold) selectivity for mu vs. kappa opioid receptors (Emmerson et al., 1994; Ko et al., 1998), the effects of amphetamine were also examined in combination with the kappa-selective antagonist norbinaltorphimine (Butelman et al., 1998; Butelman et al., 1993).
2. Methods
2.1. Subjects
A total of 11 adult male rhesus monkeys (Macaca mulatta) were housed individually and had extensive behavioral and drug histories, including exposure to a range of monoaminergic compounds (e.g., d-amphetamine, pimavanserin, lorcaserin, and lisdexamphetamine), as well as more limited exposure with opioid and nicotinic compounds. Monkeys weighed 8.3–13.6 kg and were maintained on a diet of primate chow (LabDiet High Fiber Monkey Biscuits; PMI Feeds, St. Louis, MO) and fresh fruit and vegetables. They had continuous access to water in the home chamber and were maintained under controlled temperature on a 12/12-h light-dark cycle (lights on from 0600 to 1800h). Environmental enrichment was provided on a daily basis. The maintenance and experimental use of animals was carried out in accordance with the 2011 Guide for Care and Use of Laboratory Animals (National Research Council Committee for the Update of the Guide for the and Use of Laboratory, 2011). The facility was accredited by the AAALAC international and all experimental protocols were approved by the Institutional Animal Care and Use Committee.
2.2. Surgery
Monkeys were treated with intramuscular (IM) ketoprofen (2 mg/kg/day; Zoetis Inc., Kalamazoo, MI) for a total of 5 days, beginning the day before surgery. On the day of surgery, monkeys were initially anesthetized with ketamine (10 mg/kg IM; Vedco Inc., St. Joseph, MO), followed by injections of cefazolin (30 mg/kg IM; WG Critical Care LLC, Paramus, NJ), xylazine (1 mg/kg IM; Lloyd Laboratories, Shenandoah, IA) and atropine (0.04 mg/kg IM; Med-Pharmex Inc., Pomona, CA); anesthesia was maintained by isoflurane (1–3%; Zoetis Inc.). A double-lumen intravenous (IV) catheter (Tygon 3350 i.d.=.76 mm, o.d.=2.36 mm; STI Components, Roanoke, VA or silicone extruded i.d.=.76 mm, o.d.=2.36 mm; Reiss Manufacturing, Blackstone, VA) was surgically implanted into a femoral or jugular vein and secured to the vessel with sutures (Ethibond Excel 3–0; Ethicon Inc., Somerville, NJ). The catheter extended from the vessel to an exit point at the midscapular region of the back.
2.3. Apparatus
Each housing chamber was equipped with a customized operant response panel, which had two response keys that could be illuminated red or green, and a pellet dispenser (ENV-203–1000; Med Associates Inc., St. Albans, VT) that delivered 1g banana-flavored food pellets (Grain-Based Non-Human Primate Tablet; TestDiet, St. Louis, MO) to a receptacle below the operant panel. The externalized portion of the catheter was routed through a custom jacket and tether system connected to a dual-channel fluid swivel (Lomir Biomedical, Quebec, Canada) on the chamber top and then to two safety syringe pumps (PHM-108; Med Associates Inc.), one for each lumen of the double-lumen catheter. One pump was used to deliver contingent cocaine injections through one lumen of the double-lumen catheter. The second pump was used to deliver noncontingent saline or drug treatments through the second lumen at a programmed rate of 0.1 ml injections every 20 min from 12:00 PM each day until 11:00 AM the next morning.
2.4. Cocaine Vs. Food Choice Procedure
Behavioral sessions were conducted seven days a week from 9:00 am to 11:00 am as described previously (Banks et al., 2013a; Banks et al., 2011, 2013b). Under the terminal schedule, each session consisted of five 20-min components separated by 5 min time-out periods, and a maximum of 10 reinforcers could be earned in each component. During each component, the left key was illuminated red and completion of a fixed-ratio (FR) 100 resulted in delivery of a food pellet. In addition, completion of an FR 10 on the right key resulted in delivery of the unit cocaine dose available during that component (0, 0.0032, 0.01, 0.032, or 0.1 mg/kg per injection for components 1–5, respectively). The cocaine-associated key was not illuminated during the first component, and during subsequent components, it was transilluminated with green stimulus lights that flashed on and off in 3s cycles (i.e., longer flashes associated with higher cocaine doses). Ratio requirement completion on either key initiated a 3-s timeout, during which all stimulus lights were turned off, and responding had no scheduled consequences. Behavior was considered stable when the lowest unit cocaine dose maintaining greater than 80% cocaine vs. food choice did not vary by more than a half-log unit for three consecutive days.
Once behavior was stable, the effects of drug treatments were evaluated in four phases. First, the effects of morphine (0.032–0.32 mg/kg/h) administered alone were examined to evaluate changes in cocaine vs. food choice during activation of mu-opioid receptors. Second, the effects of naltrexone (0.00032–0.0032 mg/kg/h) administered either alone or in combination with 0.32 mg/kg/h morphine were examined to identify a naltrexone dose sufficient to block the effects of morphine. Third, the effects of amphetamine (0.032–0.1 mg/kg/h) were examined alone or in combination with a mu antagonist dose of naltrexone (0.0032 mg/kg/h) to evaluate the degree to which naltrexone might modify effects of amphetamine maintenance. Finally, the effects of 0.032 mg/kg/h amphetamine were also determined alone or in combination with the kappa antagonist norbinaltorphimine (norBNI) (10 mg/kg administered IM once on the first day of amphetamine treatment). The single-dose treatment regimen was based on previous studies to indicate that 10 mg/kg norBNI produces kappa antagonist effects for at least two weeks and does not alter cocaine vs. food choice when administered alone (Hutsell et al., 2016; Ko et al., 1998; Negus, 2004). Only one monkey received all treatments; other monkeys exhausted their catheterization sites, and these monkeys had to be replaced over the course of the study. Ultimately, each treatment was tested in a group of 3–5 monkeys, and supplementary Table 1 shows the monkeys that contributed to each data set. Each treatment was administered for 7 consecutive days, following which treatment drugs were replaced with saline for at least 4 days and until cocaine vs. food choice had returned to pretest levels.
2.5. Data Analyses
The primary-dependent measures for each component were (1) percent cocaine choice, defined as [(number of ratio requirements, or ‘choices’, completed on the cocaine- associated key/total number of choices completed on both the cocaine- and food-associated keys)×100], and (2) reinforcement rate defined as total number of choices completed. Mean data from the 3 days preceding each pharmacological treatment were averaged for each individual monkey and then averaged across monkeys to yield the group mean ‘baseline’ data. Mean data from the last 3 days of each 7-day treatment were averaged for each individual monkey and then averaged across monkeys to yield the group mean test data. Percent cocaine choice and total choices completed per component were then plotted as a function of the unit cocaine dose and analyzed using a generalized linear mixed-model analysis with treatment and unit cocaine dose as the fixed main effects and subject as the random effect to better account for missing data points due to experimental manipulation-induced behavioral suppression [Cnann, 1997; Krueger and Tian, 2004]. A Dunnett’s test was performed to compare treatment effects with baseline within a unit cocaine dose. Additionally, for experiments with amphetamine ± naltrexone, a Tukey HSD test was performed to compare effects of saline treatment, amphetamine alone treatment, and amphetamine + naltrexone treatment within a unit cocaine dose of 0.032 mg/kg/injection cocaine. This unit dose was chosen for these comparisons because previous results from our laboratory have demonstrated that this unit cocaine dose is most sensitive to treatment with amphetamine and other monoamine transporter substrates [Banks, 2011; Banks, 2013b; Banks, 2015]. This statistical approach has also been utilized in other preclinical cocaine vs. food choice studies [Czoty, 2005]. Additional dependent measures collected during each behavioral session included the numbers of food, cocaine, and total choices summed across all components. These data were also analyzed using a linear mixed-effects model with treatment condition as the fixed main effect and subject as a random effect. In the presence of a significant treatment effect, a Dunnett’s post-hoc test was performed comparing treatment effects to baseline. All analyses were performed using JMP Pro software (JMP Pro 12.2; SAS Institute Inc., Cary, NC).
2.6. Drugs
(−)-Cocaine HCl, (−)-morphine sulfate, and (−)-naltrexone HCl (National Institute on Drug Abuse Drug Supply Program, Rockville, MD) and d-amphetamine hemisulfate (Sigma Aldrich, St. Louis, MO) were dissolved in sterile saline and passed through a sterile 0.22-μm filter (Millex GV; Merck Millipore Corp., Billerica, MA) prior to IV administration via chronic indwelling catheters as described above. Norbinaltorphimine 2HCl was synthesized in the Drug Design and Synthesis Section and provided by Dr. Kenner Rice and was dissolved in 1% lactic acid in sterile water for IM injection. Drug doses are expressed as the weight, in mg/kg, of the forms listed above.
3. Results
3.1. Baseline Cocaine Vs. Food Choice
During saline treatment (“Baseline” data in top panels of Figures 1–4), monkeys responded primarily for food at lower cocaine doses (i.e., 0, 0.0032, and 0.01 mg/kg/inj), but responded primarily for cocaine at higher cocaine doses (i.e., 0.032 and 0.1 mg/kg/inj). Monkeys usually earned the maximum of 10 reinforcers during each component (“Baseline” data in middle panels of Figures 1–4), thus earning the maximum 50 reinforcers for the entire session, allocated as approximately 30 food choices and 20 cocaine choices (“Baseline” data in bottom panels of Figures 1–4).
Figure 1.
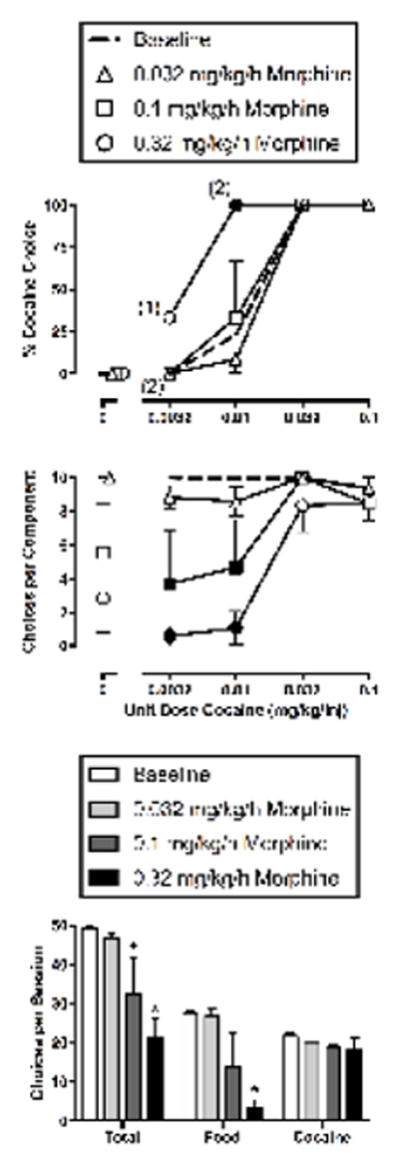
Effects of 7-day treatment with morphine (0.032–0.32 mg/kg/h, IV) on cocaine vs. food choice in rhesus monkeys (n=3–4). Abscissae: unit dose cocaine in mg/kg/injection (top and middle). Ordinates: percentage of FR requirements (i.e., choices) completed on the cocaine key (top) and the sum of cocaine and food choices completed per component (middle). Summary results for morphine effects on total choices, food choices and cocaine choices summed across all cocaine doses are depicted in the bottom panel. All points and bars represent mean (± S.E.M.) data obtained for days 5–7 from three (0.1 mg/kg/h morphine) or four (0.032 and 0.32 mg/kg/h morphine) monkeys as detailed in Table 1. Filled symbols (top and middle) and asterisks (bottom) indicate statistical significance compared to baseline conditions (saline; p<0.05). Numbers in parentheses in the top panel indicate the number of subjects contributing to that data point if less than the total number of subjects tested (i.e., if one or more monkeys failed to complete at least one choice during a component and did not contribute to mean data graphed in the top panel).
Figure 4.
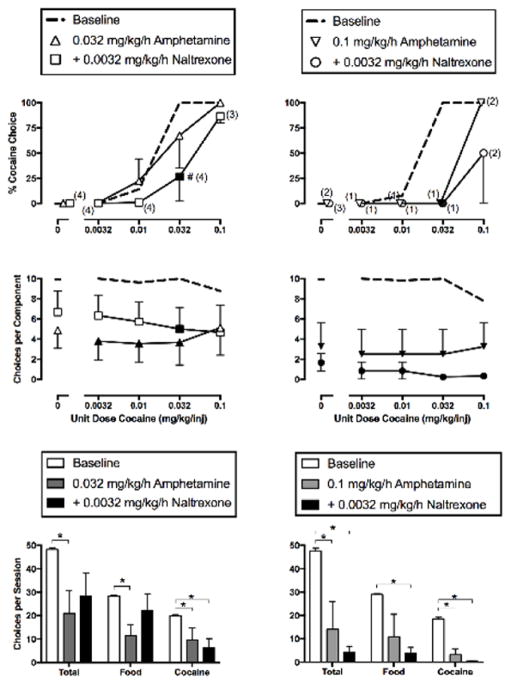
Effects of 7-day treatment with amphetamine (0.032 or 0.1 mg/kg/h, IV) alone or in combination with 0.0032 mg/kg/h IV naltrexone on cocaine vs. food choice in rhesus monkeys (n=4–5). Abscissae: unit dose cocaine in mg/kg/injection (top and middle panels). Ordinates: percentage of choices completed on the cocaine key (top panels) and the sum of total cocaine and food choices completed per component (middle panels). Summary results for treatment effects on total choices, food choices and cocaine choices summed across all cocaine doses are depicted in the bottom panels. All points and bars represent mean (± S.E.M.) data obtained for days 5–7 from five (left panels) or four (right panels) monkeys. Filled symbols (top and middle panels) indicate statistical significance compared to baseline conditions (saline; p<0.05). Pound sign indicates statistical significance compared to amphetamine alone conditions (p<0.05). Asterisks (bottom panels) indicate statistical significance compared to baseline conditions (saline or 0.032 mg/kg/h amphetamine alone; p<0.05). Numbers in parentheses indicate the number of subjects contributing to that data point if less than the total number of subjects tested (i.e., if one or more monkeys failed to complete at least one choice during a component and did not contribute to mean data graphed in the top panel).
3.2. Effects of Morphine Treatment
Figure 1 shows that morphine shifted the cocaine choice dose-response curve to the left and decreased rates of reinforcement, especially during early components of the choice sessions when food was usually preferred to low cocaine doses. Specifically, morphine treatment (0.32 mg/kg/h) significantly increased percent cocaine choice during availability of 0.01 mg/kg/inj cocaine from baseline (cocaine dose: F3,35.3=52.33, p<0.0001; cocaine dose x morphine dose: F9,35.8=4.83, p=0.0003) (Figure 1, top panel). Morphine treatment (0.1 and 0.32 mg/kg/h) significantly decreased the number of choices completed during availability of 0.0032 and 0.01 mg/kg/injection cocaine (morphine dose: F3,41.2=23.32, p<0.0001; cocaine dose x morphine dose: F9,41.1=5.44, p<0.0001) (Figure 1, middle panel). Summary data indicated that both 0.1 and 0.32 mg/kg/h morphine significantly decreased total choices per session (F3,8.3=12.81, p=0.0018), and 0.32 mg/kg/h morphine significantly decreased total food choices (F3,8.4=12.61, p=0.0018); however, the total number of cocaine choices was not significantly altered (Figure 1, bottom panel).
3.3. Effects of Naltrexone Treatment
Figure 2 shows that naltrexone administered alone had no effect on cocaine vs. food choice. Specifically, neither 0.001 nor 0.0032 mg/kg/h naltrexone altered the cocaine choice dose-response curve (cocaine dose: F3,29.5=451.17, p<0.0001) (Figure 2, top panel). Furthermore, there was no effect of naltrexone on the number of choices per component (Figure 2, middle panel), and summary data revealed no change in total choices, food choices, or cocaine choices (Figure 2, bottom panel).
Figure 2.
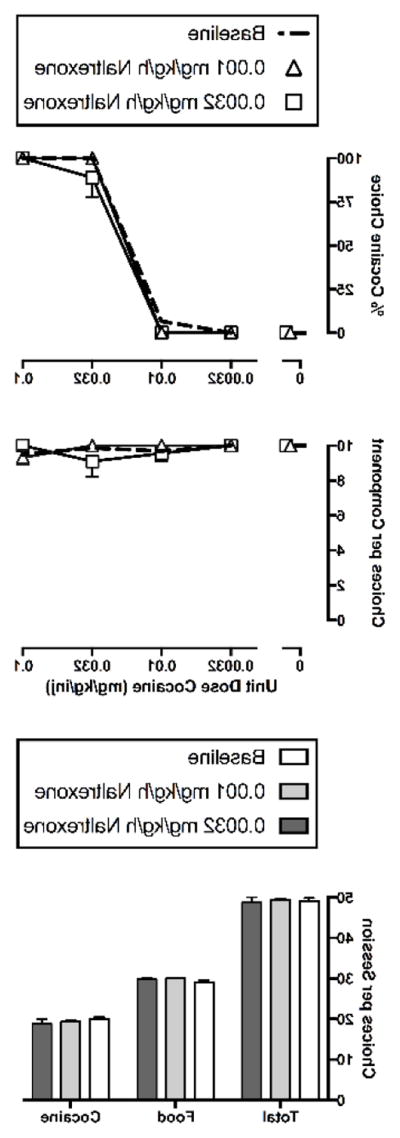
Effects of 7-day treatment with naltrexone (0.001–0.0032 mg/kg/h, IV) on cocaine vs. food choice in rhesus monkeys (n=3–4). Abscissae: unit dose cocaine in mg/kg/injection (top and middle). Ordinates: percentage of choices completed on the cocaine key (top) and the sum of cocaine and food choices completed per component (middle). Summary results for naltrexone effects on total choices, food choices and cocaine choices summed across all cocaine doses are depicted in the bottom panel. All points and bars represent mean (± S.E.M.) data obtained for days 5–7 from three (0.0032 mg/kg/h naltrexone) or four (0.001 mg/kg/h naltrexone) monkeys as detailed in Table 1.
3.4. Effects of Morphine Treatment Alone and in Combination with Naltrexone
Figure 3 demonstrates that naltrexone dose-dependently blocked the effects of morphine on cocaine-vs.-food choice. When 0.32 mg/kg/h morphine was administered alone in this group of monkeys, results were qualitatively similar to the first determination of the effects of 0.32 mg/kg/h morphine, although in this second study, increases in cocaine choice were more pronounced and decreases in reinforcement were less pronounced than in the first study. Naltrexone blocked both morphine-induced increases in percent cocaine choice (cocaine dose: F3,55.8=14.30, p<0.0001; cocaine dose x treatment: F12,55.9=3.71, p=0.0004) (Figure 3, top panel) and morphine-induced decreases in the number of choices per component (cocaine dose: F3,57=2.87, p=0.0444; treatment: F4,57=4.23, p=0.0045) (Figure 3, middle panel). Across the entire session, there was a significant treatment effect on food choices (F4,12=5.19, p=0.012), but no treatment effect on total or cocaine choices. Specifically, treatment with 0.32 mg/kg/h morphine alone significantly decreased total food choices, and this morphine effect was significantly blocked by 0.0032 mg/kg/h naltrexone (Figure 3, bottom panel).
Figure 3.
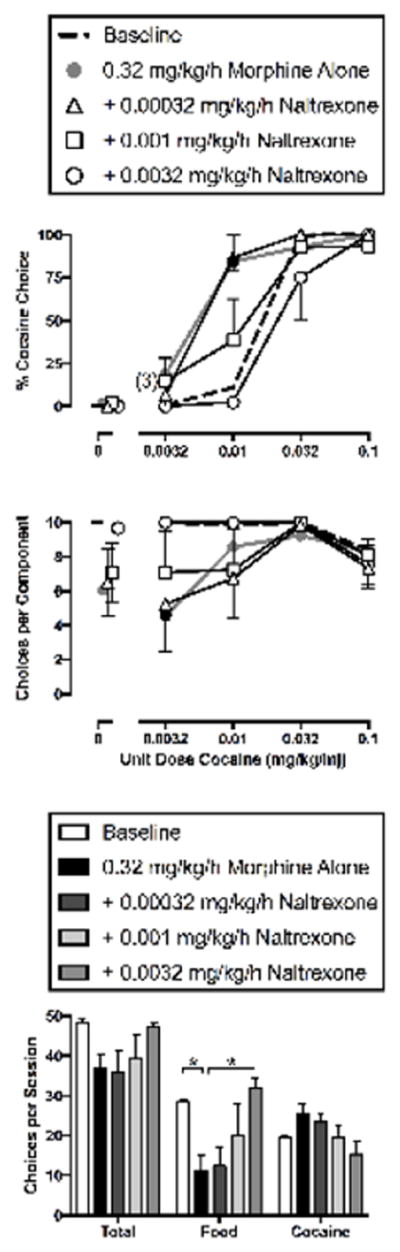
Effects of 7-day treatment with 0.32 mg/kg/h IV morphine alone or in combination with naltrexone (0.00032–0.0032 mg/kg/h, IV) on cocaine vs. food choice in rhesus monkeys (n=4). Abscissae: unit dose cocaine in mg/kg/injection (top and middle). Ordinates: percentage of choices completed on the cocaine key (top) and the sum of cocaine and food choices completed per component (middle). Summary results for treatment effects on total choices, food choices and cocaine choices summed across all cocaine doses are depicted in the bottom panel. All points and bars represent mean (± S.E.M.) data obtained for days 5–7 from four monkeys. Filled symbols (top and middle) indicate statistical significance compared to saline baseline conditions (saline; p<0.05). Asterisks (bottom) indicate statistical significance compared to either saline baseline or 0.32 mg/kg/h morphine alone baseline. Numbers in parentheses indicate the number of subjects contributing to that data point if less than the total number of subjects tested (i.e., if one or more monkeys failed to complete at least one choice during a component and did not contribute to mean data graphed in the top panel).
3.5. Effects of Amphetamine Treatment Alone and in Combination with Naltrexone
Figure 4 shows the effects of amphetamine administered alone or in combination with a mu-antagonist dose of naltrexone (0.0032 mg/kg/h). A dose of 0.032 mg/kg/h amphetamine alone failed to alter cocaine choice; however, treatment with this amphetamine dose in combination with naltrexone decreased cocaine choice during availability of 0.032 mg/kg/inj cocaine compared to both baseline and 0.032 mg/kg/h amphetamine alone treatment conditions (cocaine dose: F3,31.7=50.37, p<0.0001; cocaine dose x treatment: F6,31.9=4.26, p=0.0029) (Figure 4, top left panel). Additionally, treatment with 0.032 mg/kg/h amphetamine alone significantly decreased the number of choices per component during availability of 0.0032, 0.01, and 0.032 mg/kg/inj cocaine, and addition of naltrexone attenuated this effect (treatment: F2,44=7.70, p=0.0014) (Figure 4, middle left panel). Naltrexone also attenuated the effects of 0.032 mg/kg/h amphetamine on both total choices (F2,8=6.46, p=0.0214) and food choices (F2,8=5.64, p=0.0296) for the entire session; however, naltrexone did not attenuate the amphetamine-induced decrease in cocaine choices (F2,8=7.14, p=0.0166) (Figure 4, bottom left panel).
Treatment with 0.1 mg/kg/h amphetamine alone significantly decreased cocaine choice during availability of 0.032 mg/kg/inj cocaine and this effect was not significantly changed by addition of 0.0032 mg/kg/h naltrexone (cocaine dose: F3,12.8=8.29, p=0.0025; cocaine dose x treatment: F6,12.5=4.00, p=0.0184) (Figure 4, top right panel). Amphetamine alone also significantly decreased the number of choices completed per component, and this effect was also not significantly altered by addition of 0.0032 mg/kg/h naltrexone (treatment: F2,33=14.47, p<0.0001) (Figure 4, middle right panel). Results for summary measures revealed significant effects of treatment on total choices (F2,6=11.40, p=0.009), choices made for food (F2,6=5.16, p=0.0496) as well as choices made for cocaine (F2,6=67.44, p<0.0001) (Figure 4, bottom right panel). In particular, treatment with 0.1 mg/kg/h amphetamine and 0.0032 mg/kg/h naltrexone nearly eliminated responding for both cocaine and food.
3.6. Effects of Amphetamine Treatment Alone and in Combination with norBNI
To evaluate the possibility that naltrexone effects might involve antagonism of kappa rather than mu receptors, the effects of 0.032 mg/kg/h amphetamine were also redetermined alone and in combination with the kappa antagonist norBNI (10 mg/kg) in a group of three monkeys. Relative to the saline-treatment baseline, the cocaine vs. food choice dose-effect curve was not altered by treatment with either amphetamine alone or amphetamine in combination with norBNI (cocaine dose: F3,22=42.77, p<0.0001, but no significant main effect of treatment: F2,22<0.01, p=1.0, and no significant interaction: F6,22=0.53, p=0.78).
4. Discussion
The present study examined the role of mu-opioid receptor activation in mediating amphetamine maintenance effects on cocaine vs. food choice in rhesus monkeys. There were two main findings. First, maintenance on the mu-opioid receptor agonist morphine increased cocaine choice and reduced overall rates of reinforcement. Second, the mu antagonist naltrexone alone had no effect on cocaine vs. food choice, but it enhanced the effectiveness of a threshold amphetamine dose (0.032 mg/kg/h) to reduce cocaine choice without also increasing the general behavioral disruptive effects of amphetamine. These findings are consistent with the hypothesis that amphetamine maintenance produces mu receptor activation that opposes and limits its potency and/or effectiveness to reduce cocaine reinforcement by other, yet to be discovered mechanisms. Additionally, insofar as naltrexone maintenance may also reduce the abuse-related effects of amphetamine (see below), these findings suggest that amphetamine-naltrexone combinations may be safer than amphetamine alone to reduce cocaine use.
4.1 Effects of Morphine Maintenance on Cocaine Choice
Among its other mechanisms of action, amphetamine increases endogenous opioid release to activate mu opioid receptors (Mick et al., 2014). The goal of the present study was to examine the role of these opioid effects in amphetamine maintenance-induced decreases in cocaine vs. food choice. As a first step, we evaluated cocaine vs. food choice during maintenance on the mu agonist morphine to test the hypothesis that mu receptor activation might be sufficient to mimic amphetamine effects. Contrary to the working hypothesis, morphine increased cocaine choice while also reducing overall rates of reinforcement. This finding suggests that any mu agonist effects of amphetamine might contribute to the nonselective rate-decreasing effects of amphetamine maintenance, but oppose amphetamine-induced decreases in cocaine choice. These results confirm and extend previous studies that examined cocaine self-administration by nonhuman primates during chronic treatment with morphine and other mu agonists. In particular, we found previously that maintenance on the high-efficacy mu agonist methadone also dose-dependently decreased overall rates of reinforcement in rhesus monkeys responding under the same cocaine vs. food choice procedure (Negus and Mello, 2004). In that study, methadone produced small increases in mean levels of cocaine choice maintained by low cocaine doses, but unlike morphine in the present study, methadone effects on cocaine choice did not achieve criteria for statistical significance. This difference between methadone and morphine may be related to their different efficacies as mu agonists. Specifically, morphine has lower efficacy than methadone as a mu agonist (Selley et al., 1998), and this lower efficacy results in both shallower dose-effect curves and greater potency differences for production of different effects (e.g., Banks et al., 2010). Consequently, use of morphine may have facilitated detection of mu agonist-induced increases in cocaine choice at doses that did not eliminate responding or produce other dose-limiting side effects.
Previous studies have also examined effects of morphine maintenance on cocaine self-administration under non-choice schedules of reinforcement in which cocaine was available either alone (Winger and Woods, 2001) or in alternating sessions with food availability (Negus and Mello, 2002). Under these conditions, morphine generally decreased rates of cocaine self-administration, consistent with the morphine-induced decrease in overall reinforcement rate observed here. Choice procedures are distinguished from these other types of procedures by their evaluation of behavioral allocation as well as behavioral rate, and choice measures of behavioral allocation provide a relatively rate-independent measure of drug reinforcement (Banks and Negus, 2012, 2017). In the present study, morphine maintenance both increased cocaine vs. food choice and decreased overall rates of reinforcement, suggesting that mu receptor activation both increases the relative reinforcing effects of cocaine in comparison to food while also decreasing overall motor competence to engage in operant responding.
4.2 Effects of Naltrexone Maintenance on Cocaine Choice
Up to a naltrexone dose that was effective in blocking 0.32 mg/kg/h morphine effects on cocaine choice, treatment with naltrexone alone had no effect on cocaine choice. These results are consistent with the literature examining both acute and chronic effects of naltrexone and other opioid antagonists on cocaine self-administration. For example, chronic naltrexone treatment up to 3.2 mg/kg/day produced a partial decrease in cocaine vs. food-maintained responding by monkeys responding under a second-order schedule in monkeys, but these effects were not selective, and tolerance developed initial naltrexone treatment effects on cocaine self-administration (Mello et al., 1990). Furthermore, acute naltrexone pretreatment did not alter cocaine self-administration in either rats (Ettenberg et al., 1982; Hemby et al., 1996) or monkeys (Rowlett et al., 1998), and naltrexone maintenance has generally failed to reduce metrics of cocaine use in clinical trials with co-dependent cocaine-alcohol users (Schmitz et al., 2009; Pettinati et al., 2014). Other opioid antagonists, including naloxone (Killian et al., 1978) and nalorphine (Goldberg et al., 1971), have also failed to acutely decrease cocaine self-administration in monkeys. Altogether, the present naltrexone results are consistent with the literature suggesting that mu-opioid receptors are not necessary for cocaine reinforcement.
4.3 Effects of Amphetamine Alone and in Combination with Naltrexone and norBNI on Cocaine Choice
Consistent with previous results in our laboratory, amphetamine maintenance produced dose-dependent decreases in both cocaine vs. food choice and overall reinforcement rates (Banks et al., 2013b; Banks et al., 2015; Negus, 2003). The lowest dose used in the present study, 0.032 mg/kg/day, is generally at the threshold for reducing cocaine choice and reinforcement rates, and for the monkeys used in this study, this amphetamine dose did not significantly alter cocaine choice but did significantly decrease reinforcement rates. The higher amphetamine maintenance dose of 0.1 mg/kg/h significantly reduced cocaine choice but also eliminated reinforcement for most monkeys during most components of daily choice sessions. Thus, for the cohort of monkeys used in this study, the rate-decreasing effects of amphetamine maintenance were more pronounced than its effectiveness to reduce cocaine choice. The reason for this profile of amphetamine maintenance effects in these monkeys is not known. Nonetheless, it is notable that while amphetamine and morphine maintenance both dose-dependently reduced reinforcement rates, they had opposite effects on cocaine vs. food choice. These results provide one example of the potential for dissociable effects of test drugs on measures of behavioral allocation and behavioral rates.
Results described above with morphine maintenance suggested the possibility that any mu receptor activation by amphetamine might oppose amphetamine-induced decreases in cocaine choice. Results obtained with the amphetamine plus naltrexone combinations provide some support for this hypothesis. In particular, 0.032 mg/kg/h amphetamine in combination with a mu-antagonist dose of 0.0032 mg/kg/h naltrexone produced greater decreases in cocaine choice than amphetamine alone. There was also a trend for naltrexone to enhance the cocaine choice-decreasing effects of the higher dose of 0.1 mg/kg/h amphetamine, but this effect did not achieve criteria for statistical significance. Insofar as treatment-induced decreases in cocaine vs. food choice are predictive of therapeutic efficacy to reduce cocaine use clinically (Banks and Negus, 2017; Czoty et al., 2016), these results suggest that addition of naltrexone may increase potency of amphetamine as a maintenance medication for cocaine use disorder.
The failure of naltrexone to reliably attenuate amphetamine-induced rate-decreasing effects suggests that mu receptor activation does not play a major role in this amphetamine effect. Insofar as nonselective decreases in reinforcement rates are undesirable, the present results therefore provide at best only weak evidence to suggest that combinations of amphetamine and naltrexone might be safer than amphetamine alone. However, naltrexone did not enhance non-selective rate-decreasing effects of amphetamine. Moreover, amphetamine’s own abuse liability is another undesirable amphetamine effect, and previous studies have suggested that naltrexone may attenuate abuse-related effects of amphetamine. For example, naltrexone decreased amphetamine self-administration in rhesus monkeys (Jimenez-Gomez et al., 2011), methamphetamine self-administration in a human laboratory study (Marks et al., 2016) and amphetamine use by patients meeting criteria for amphetamine dependence (Jayaram-Lindstrom et al., 2008). These findings suggest that combinations of amphetamine and naltrexone may have lower abuse liability than amphetamine alone. However, it should be noted that a recent clinical trial found no effect of extended-release naltrexone on metrics of amphetamine use in amphetamine-dependent patients (Runnarsdottir et al., 2017).
In contrast to the effects of naltrexone, the kappa antagonist norBNI did not enhance the effectiveness of amphetamine to reduce cocaine choice. We have shown previously that norBNI alone does not alter cocaine vs. food choice (Hutsell et al., 2016; Negus, 2004), and the dose of norBNI used in the present study (10 mg/kg) has been shown previously to produce selective kappa antagonist effects for up to two weeks (Ko et al., 1998). Taken together, these results suggest that kappa receptor antagonism is not sufficient to enhance amphetamine-induced reductions in cocaine vs. food choice, and provide further support for the conclusion that effects of naltrexone were mediated by its antagonism of mu rather than kappa receptors.
In summary, the present study supports the hypothesis that amphetamine maintenance produces mu receptor activation that opposes its potency and/or effectiveness to decrease cocaine vs. food choice in rhesus monkeys. Blockade of mu receptors by concurrent naltrexone maintenance selectively enhanced amphetamine-induced decreases in cocaine choice produced by a threshold amphetamine dose without enhancing the non-selective rate-decreasing effects of amphetamine. Overall, these results support consideration of amphetamine-naltrexone combinations as candidate medications for treatment of cocaine use disorder.
Table 1.
Identification of monkeys contributing to specific data sets.
| Treatment | ||||||
|---|---|---|---|---|---|---|
| Monkey ID | Morphine Alone | Naltrexone Alone | Morphine + Naltrexone | .032 Amphetamine +/− Naltrexone | 0.1 Amphetamine +/− Naltrexone | .032 Amphetamine +/− norBNI |
| 1475 | X | X | X | X | X | |
| 1504 | X | |||||
| 1473 | X* | |||||
| 1474 | X | X | X | |||
| 1523 | X | X | X | X | ||
| 1489 | X** | X | ||||
| 1478 | X | |||||
| 1519 | X | |||||
| 1510 | X | |||||
| 1511 | X | X | X | |||
| 1528 | X | X | ||||
Received only 0.032 and 0.32 mg/kg/h morphine
Received only 0.001 mg/kg/h naltrexone
Highlights.
Morphine increased cocaine choice and decreased rates of reinforcement
Naltrexone alone had no effect on cocaine choice
Naltrexone enhanced the potency of amphetamine to reduce cocaine choice
Acknowledgments
Role of Funding Source This work was supported by the National Institutes of Health under R01DA026946 and T32DA007027. A portion of this work was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse (NIDA) and the National Institute of Alcohol Abuse and Alcoholism (NIAAA).
Footnotes
Contributors
MJM, MLB, and SSN were responsible for study concept, design, and execution. MJM performed the data analysis. MJM, MLB, and SSN drafted the manuscript. KC and KCR synthesized the norBNI. All authors critically reviewed the manuscript for content and approved the final manuscript version submitted for publication.
Conflict of Interest The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews JS, Holtzman SG. Effects of naloxone and diprenorphine on amphetamine-stimulated behavior in guinea pigs and rats. Neuropharmacology. 1987;26:1115–1120. doi: 10.1016/0028-3908(87)90256-5. [DOI] [PubMed] [Google Scholar]
- Anton RF. Clinical use of opioid antagonists in the treatment of alcohol dependence. In: Dean R, Bilsky EJ, Negus SS, editors. Opiate Receptors and Antagonists: From Bench to Clinic. Humana Press; N.Y: 2009. pp. 371–386. [Google Scholar]
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS. Effects of phendimetrazine treatment on cocaine vs food choice and extended-access cocaine consumption in rhesus monkeys. Neuropsychopharmacology. 2013a;38:2698–2707. doi: 10.1038/npp.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Effects of monoamine releasers with varying selectivity for releasing dopamine/norepinephrine versus serotonin on choice between cocaine and food in rhesus monkeys. Behav Pharmacol. 2011;22:824–836. doi: 10.1097/FBP.0b013e32834d63ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Effects of 14-day treatment with the schedule III anorectic phendimetrazine on choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend. 2013b;131:204–213. doi: 10.1016/j.drugalcdep.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Hutsell BA, Blough BE, Poklis JL, Negus SS. Preclinical assessment of lisdexamfetamine as an agonist medication candidate for cocaine addiction: Effects in rhesus monkeys trained to discriminate cocaine or to self-administer cocaine in a cocaine versus food choice procedure. Int J Neuropsychopharmacol. 2015;18:8. doi: 10.1093/ijnp/pyv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv Pharmacol Sci. 2012;2012:281768. doi: 10.1155/2012/281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Insights from preclinical choice models on treating drug addiction. Trends Pharmacol Sci. 2017;38:181–194. doi: 10.1016/j.tips.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Rice KC, Negus SS. Antinociceptive interactions between Mu-opioid receptor agonists and the serotonin uptake inhibitor clomipramine in rhesus monkeys: Role of Mu agonist efficacy. J Pharmacol Exp Ther. 2010;335:497–505. doi: 10.1124/jpet.110.169276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlack JM, Matthews JL. The chemistry and pharmacology of μ-opioid antagnists. In: Dean R, Bilsky EJ, Negus SS, editors. Opiate Receptors and Antagonists: From Bench to Clinic. Humana Press; N.Y: 2009. pp. 83–97. [Google Scholar]
- Bowen CA, Fischer BD, Mello NK, Negus SS. Antagonism of the antinociceptive and discriminative stimulus effects of heroin and morphine by 3-methoxynaltrexone and naltrexone in rhesus monkeys. J Pharmacol Exp Ther. 2002;302:264–273. doi: 10.1124/jpet.302.1.264. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Ko MC, Sobczyk-Kojiro K, Mosberg HI, Van Bemmel B, Zernig G, Woods JH. kappa-Opioid receptor binding populations in rhesus monkey brain: Relationship to an assay of thermal antinociception. J Pharmacol Exp Ther. 1998;285:595–601. [PubMed] [Google Scholar]
- Butelman ER, Negus SS, Ai Y, de Costa BR, Woods JH. Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther. 1993;267:1269–1276. [PubMed] [Google Scholar]
- Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16:2349–2380. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Chiodo KA, Lack CM, Roberts DC. Cocaine self-administration reinforced on a progressive ratio schedule decreases with continuous D-amphetamine treatment in rats. Psychopharmacology (Berl) 2008;200:465–473. doi: 10.1007/s00213-008-1222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Martelle JL, Nader MA. Prolonged attenuation of the reinforcing strength of cocaine by chronic d-amphetamine in rhesus monkeys. Neuropsychopharmacology. 2011;36:539–547. doi: 10.1038/npp.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. Assessment of the relative reinforcing strength of cocaine in socially housed monkeys using a choice procedure. J Pharmacol Exp Ther. 2005;312:96–102. doi: 10.1124/jpet.104.073411. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Stoops WW, Rush CR. Evaluation of the "pipeline" for development of medications for cocaine use disorder: A review of translational preclinical, human laboratory, and clinical trial research. Pharmacol Rev. 2016;68:533–562. doi: 10.1124/pr.115.011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmar PW, Cowan A, Walter DS. Naloxone antagonizes behavioural effects of d-amphetamine in mice and rats. Neuropharmacology. 1978;17:1041–1044. doi: 10.1016/0028-3908(78)90031-x. [DOI] [PubMed] [Google Scholar]
- Emmerson PJ, Liu MR, Woods JH, Medzihradsky F. Binding affinity and selectivity of opioids at mu, delta and kappa receptors in monkey brain membranes. J Pharmacol Exp Ther. 1994;271:1630–1637. [PubMed] [Google Scholar]
- Esposito RU, Perry W, Kornetsky C. Effects of d-amphetamine and naloxone on brain stimulation reward. Psychopharmacology (Berl) 1980;69:187–191. doi: 10.1007/BF00427648. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: Mediation by separate neural systems. Psychopharmacology (Berl) 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Woods JH, Schuster CR. Nalorphine-induced changes in morphine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 1971;176:464–471. [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: A double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Lundahl LH, Steinmiller CL. Sustained release d-amphetamine reduces cocaine but not 'speedball'-seeking in buprenorphine-maintained volunteers: A test of dual-agonist pharmacotherapy for cocaine/heroin polydrug abusers. Neuropsychopharmacology. 2010;35:2624–2637. doi: 10.1038/npp.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Smith JE, Dworkin SI. The effects of eticlopride and naltrexone on responding maintained by food, cocaine, heroin and cocaine/heroin combinations in rats. J Pharmacol Exp Ther. 1996;277:1247–1258. [PubMed] [Google Scholar]
- Heyman GM. Addiction: A disorder of Choice. Harvard University Press; Cambridge, MA: 2009. [Google Scholar]
- Hitzemann R, Curell J, Hom D, Loh H. Effects of naloxone on d-amphetamine- and apomorphine-induced behavior. Neuropharmacology. 1982;21:1005–1011. doi: 10.1016/0028-3908(82)90114-9. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Behavioral effects of separate and combined administration of naloxone and d-amphetamine. J Pharmacol Exp Ther. 1974;189:51–60. [PubMed] [Google Scholar]
- Holtzman SG. Comparison of the effects of morphine, pentazocine, cyclazocine and amphetamine on intracranial self-stimulation in the rat. Psychopharmacologia. 1976;46:223–227. doi: 10.1007/BF00421106. [DOI] [PubMed] [Google Scholar]
- Hutsell BA, Cheng K, Rice KC, Negus SS, Banks ML. Effects of the kappa opioid receptor antagonist nor-binaltorphimine (nor-BNI) on cocaine versus food choice and extended-access cocaine intake in rhesus monkeys. Addict Biol. 2016;21:360–373. doi: 10.1111/adb.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram-Lindstrom N, Hammarberg A, Beck O, Franck J. Naltrexone for the treatment of amphetamine dependence: A randomized, placebo-controlled trial. Am J Psychiatry. 2008;165:1442–1448. doi: 10.1176/appi.ajp.2008.08020304. [DOI] [PubMed] [Google Scholar]
- Jimenez-Gomez C, Winger G, Dean RL, Deaver DR, Woods JH. Naltrexone decreases D-amphetamine and ethanol self-administration in rhesus monkeys. Behav Pharmacol. 2011;22:87–90. doi: 10.1097/FBP.0b013e3283423d55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Comer SD. A review of human drug self-administration procedures. Behav Pharmacol. 2013;24:384–395. doi: 10.1097/FBP.0b013e3283641c3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian AK, Bonese K, Schuster CR. The effects of naloxone on behavior maintained by cocaine and heroin injections in the rhesus monkey. Drug Alcohol Depend. 1978;3:243–251. doi: 10.1016/0376-8716(78)90078-9. [DOI] [PubMed] [Google Scholar]
- Ko MC, Butelman ER, Traynor JR, Woods JH. Differentiation of kappa opioid agonist-induced antinociception by naltrexone apparent pA2 analysis in rhesus monkeys. J Pharmacol Exp Ther. 1998;285:518–526. [PMC free article] [PubMed] [Google Scholar]
- Krueger C, Tian L. A comparison of the general linear mixed model and repeated measures ANOVA using a dataset with multiple missing data points. Biol Res Nurs. 2004;6:151–157. doi: 10.1177/1099800404267682. [DOI] [PubMed] [Google Scholar]
- Mariani JJ, Pavlicova M, Bisaga A, Nunes EV, Brooks DJ, Levin FR. Extended-release mixed amphetamine salts and topiramate for cocaine dependence: A randomized controlled trial. Biol Psychiatry. 2012;72:950–956. doi: 10.1016/j.biopsych.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks KR, Lile JA, Stoops WW, Glaser PE, Hays LR, Rush CR. Separate and combined effects of naltrexone and extended-release alprazolam on the reinforcing, subject-rated, and cardiovascular effects of methamphetamine. J Clin Psychopharmacol. 2016;36:213–221. doi: 10.1097/JCP.0000000000000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Bree MP, Lukas SE. Buprenorphine and naltrexone effects on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1990;254:926–939. [PubMed] [Google Scholar]
- Mick I, Myers J, Stokes PR, Erritzoe D, Colasanti A, Bowden-Jones H, Clark L, Gunn RN, Rabiner EA, Searle GE, Waldman AD, Parkin MC, Brailsford AD, Nutt DJ, Lingford-Hughes AR. Amphetamine induced endogenous opioid release in the human brain detected with [(1)(1)C]carfentanil PET: Replication in an independent cohort. Int J Neuropsychopharmacol. 2014;17:2069–2074. doi: 10.1017/S1461145714000704. [DOI] [PubMed] [Google Scholar]
- National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals. The National Academies Collection: Reports funded by National Institutes of Health, Guide for the Care and Use of Laboratory Animals. National Academies Press (US) National Academy of Sciences; Washington (DC): 2011. [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS. Effects of the kappa opioid agonist U50,488 and the kappa opioid antagonist nor-binaltorphimine on choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 2004;176:204–213. doi: 10.1007/s00213-004-1878-7. [DOI] [PubMed] [Google Scholar]
- Negus SS, Henningfield J. Agonist medications for the treatment of cocaine use disorder. Neuropsychopharmacology. 2015;40:1815–1825. doi: 10.1038/npp.2014.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of mu-opioid agonists on cocaine- and food-maintained responding and cocaine discrimination in rhesus monkeys: Role of mu-agonist efficacy. J Pharmacol Exp Ther. 2002;300:1111–1121. doi: 10.1124/jpet.300.3.1111. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend. 2003;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic methadone treatment on cocaine- and food-maintained responding under second-order, progressive-ratio and concurrent-choice schedules in rhesus monkeys. Drug Alcohol Depend. 2004;74:297–309. doi: 10.1016/j.drugalcdep.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Nuijten M, Blanken P, van de Wetering B, Nuijen B, van den Brink W, Hendriks VM. Sustained-release dexamfetamine in the treatment of chronic cocaine-dependent patients on heroin-assisted treatment: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;387:2226–2234. doi: 10.1016/S0140-6736(16)00205-1. [DOI] [PubMed] [Google Scholar]
- Perez-Mana C, Castells X, Vidal X, Casas M, Capella D. Efficacy of indirect dopamine agonists for psychostimulant dependence: A systematic review and meta-analysis of randomized controlled trials. J Subst Abuse Treat. 2011;40:109–122. doi: 10.1016/j.jsat.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Kampman KM, Lynch KG, Dundon WD, Mahoney EM, Wierzbicki MR, O'Brien CP. A pilot trial of injectable, extended-release naltrexone for the treatment of co-occurring cocaine and alcohol dependence. Am J Addict. 2014;23:591–597. doi: 10.1111/j.1521-0391.2014.12146.x. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Wilcox KM, Woolverton WL. Self-administration of cocaine-heroin combinations by rhesus monkeys: Antagonism by naltrexone. J Pharmacol Exp Ther. 1998;286:61–69. [PubMed] [Google Scholar]
- Runarsdottir V, Hansdottir I, Tyrfingsson T, Einarsson M, Dugosh K, Royer-Malvestuto C, Pettinati H, Khalsa J, Woody GE. Extended-release injectable naltrexone (XR-NTX) with intensive psychosocial therapy for amphetamine-dependent persons seeking treatment: A placebo-controlled trial. J Addict Med. 2017;11:197–204. doi: 10.1097/ADM.0000000000000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Sevak RJ, Hays LR. Cocaine choice in humans during D-amphetamine maintenance. J Clin Psychopharmacol. 2010;30:152–159. doi: 10.1097/JCP.0b013e3181d21967. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Lindsay JA, Green CE, Herin DV, Stotts AL, Moeller FG. High-dose naltrexone therapy for cocaine-alcohol dependence. Am J Addict. 2009;18:356–362. doi: 10.3109/10550490903077929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley DE, Liu Q, Childers SR. Signal transduction correlates of mu opioid agonist intrinsic efficacy: Receptor-stimulated [35S]GTP gamma S binding in mMOR-CHO cells and rat thalamus. J Pharmacol Exp Ther. 1998;285:496–505. [PubMed] [Google Scholar]
- Severino KA, Kosten TR. Naltrexone for initiation and maintenance of opiate abstinence. In: Dean R, Bilsky EJ, Negus SS, editors. Opiate Receptors and Antagonists: From Bench to Clinic. Humana Press; N.Y: 2009. pp. 227–245. [Google Scholar]
- Thomsen M, Lindsley CW, Conn PJ, Wessell JE, Fulton BS, Wess J, Caine SB. Contribution of both M1 and M4 receptors to muscarinic agonist-mediated attenuation of the cocaine discriminative stimulus in mice. Psychopharmacology (Berl) 2012;220:673–685. doi: 10.1007/s00213-011-2516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger G, Woods JH. The effects of chronic morphine on behavior reinforced by several opioids or by cocaine in rhesus monkeys. Drug Alcohol Depend. 2001;62:181–189. doi: 10.1016/s0376-8716(00)00166-6. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Miczek KA. Naltrexone blocks amphetamine-induced hyperactivity, but not disruption of social and agonistic behavior in mice and squirrel monkeys. Psychopharmacology (Berl) 1988;96:493–499. doi: 10.1007/BF02180030. [DOI] [PubMed] [Google Scholar]