Abstract
The objective of the present study is to evaluate the utility of melt-cast, topical, ocular inserts for delivery of drugs with different physicochemical properties. The model drugs tested include indomethacin (IN), ciprofloxacin Hydrochloride (CIP) and prednisolone sodium phosphate (PSP). Melt-cast method was used to fabricate ophthalmic inserts. Poly (ethylene oxide) N10, a semi-crystalline thermoplastic polymer (PE0 N10; Mol.wt: 100 kDa) was used as the matrix forming material. Polymeric insert units (4 × 2 × 0.2 mm) with a 10% w/w drug load were tested for in vitro release, transmembrane permeability and in vivo ocular tissue distribution. Marketed ophthalmic solutions were used as control solutions. Drug content in all the formulations ranged between 93 –102% of the theoretical value. Transmembrane flux of IN, PSP and CIP were enhanced by ~3.5-folds, ~3.6-folds and ~2.9-folds, respectively, from the polymeric inserts when compared to the control formulations, post 3 h. Moreover, ocular inserts generated significantly higher drug levels in all the ocular tissues, including the retina-choroid, when compared to their control formulations. The melt-cast ophthalmic inserts show promise as an effective noninvasive ocular drug delivery platform, which will be highly beneficial in the intervention and treatment of a wide variety of ocular complications.
Keywords: Ocular delivery, Ocular inserts, topical polymeric inserts, anti-inflammatory drugs, ocular bioavailability, ocular penetration
1. Introduction
Prolonged ocular inflammation/infection could precipitate several sight threatening disorders including, but not limited to, keratitis, conjunctivitis, iritis, uveitis, endophthalmitis, cystoid macular edema and choroidal neovascularization (1, 2). A host of different ocular tissues are involved in these complications, and the intricate barriers in the ocular milieu, which are essential and exclusive to the ocular anatomy and physiology, limit the penetration of therapeutic agents. Delivery of drugs to the posterior segment of the eye through oral/parenteral routes is highly challenging because of the expression of blood ocular barriers. Topical administration is promising in terms of safety and patient compliance but the delivery of therapeutic agents to the posterior ocular tissues through this route is also an extremely difficult task (3, 4). Although various technological advances enhanced drug delivery into the front-of-the eye, back-of-the eye delivery through the topical route needs innovative strategies to improve pre-corneal residence and trans-ocular permeation characteristics of drug molecules (5, 6). The complex interplay between static, dynamic, efflux barriers in the eye and their remarkable barrier functionalities, severely impedes the penetration and distribution of drug molecules into the inner ocular tissues. Delivering the drugs to the posterior segment of the eye, in effective concentrations, using conventional formulations like topical solutions, viscous solutions and gels, surfactant based systems, emulsions, suspensions has not been successful so far (7, 8). The topical solution based dosage form typically has the inherent drawback that most of the instilled volume is eliminated from the pre-corneal area, resulting in poor ocular bioavailability, ranging from 1–10%, of the total administered dose (9). Bioavailability in vitreous cavity following topical instillation of solutions range from 0.0001 to 0.0004%. Viscous mucoadhesive solutions and ophthalmic ointments prolongs the ocular residence time, forming drug depot on the ocular surface. Various colloidal nanoparticulate systems have been designed/developed to prolong the ocular residence of drugs for delivery to the back-of-the eye (10).
Topical formulation approaches targeting the retina through the trans-scleral pathway is still in its infancy. Effective strategies will require candidates with ideal physicochemical characteristics and novel strategies capable of increasing the retention and absorption on the ocular surface. Ointments, gels and conjunctival films are dosage forms that provide close proximity to the conjunctiva and sclera and high, localized, concentration gradient generated in the conjunctival cul-de-sac, which favors ocular penetration, probably via the trans-conjunctiva-scleral pathway. Solvent casting method is widely used for the fabrication of ocular inserts (11–13). Di Colo et al extensively studied the feasibility of poly (ethylene) oxide (PEO) based inserts (6 mm diameter; 0.8–0.9 mm thickness and 20 mg weight) for ocular delivery. Inserts were prepared using powder compression method following organic solvent processing facilitating drug adsorption on to the polymeric carrier. In these studies, PEO based gel erodible ocular inserts were fabricated with a range of polymer molecular weights from 200–2000 KDa. The inserts with lower polymer molecular weights namely PEO 200, PEO 400 and PEO 900 exhibited erosion controlled release kinetics, whereas, PEO 2000 based inserts showed diffusion-erosion controlled release kinetics. The results also demonstrated that PEO 2000 is not suitable for ocular applications (14), while PEO 400 or 600 showed promise in enhancement of ocular bioavailability (Cmax increased by 3.5-folds) of drugs in comparison to commercial solutions tested at an equivalent dose (0.3 mg) (15, 16). However, solvent cast or powder compression methods used for the preparation of ocular inserts may contain trace amounts of residual solvents, which could present safety issues (9, 17). Melt-cast or melt-extrusion technique particularly overcomes this disadvantage of solvent cast method/powder compression method while maintaining all the advantages (18, 19). Additionally, the technique can be easily transferred to the manufacturing floor employing melt-extruders (20). The ocular inserts/films offers an attractive approach to prolong the pre-ocular residence time as well as prolong the duration of the ocular absorption phase through the controlled release characteristics of the films - thus increasing drug exposure to the posterior ocular tissues.
The goal of the current research was to develop, characterize and evaluate the drug loaded topical inserts. Indomethacin (IN), prednisolone sodium phosphate (PSP) and ciprofloxacin hydrochloride (CIP) commonly employed in treatment of ocular inflammation and infections, and representing a wide range of physicochemical characteristics, were chosen for investigating ocular disposition from these topical, melt-cast films.
Materials and Methods
1.1. Chemicals
PEO [PolyOx® WSR N-10 (PEO N-10), MW: 100,000 Daltons] was kindly donated by Dow Chemical Company (Midland, MI). IN (Indomethacin), CIP (Ciprofloxacin hydrochloride) and PSP (Prednisolone sodium phosphate) were purchased from Sigma Aldrich (St. Louis, MO). All other chemicals were purchased from Fisher Scientific (St. Louis, MO).
1.2. Animal tissues
Whole eye globes of New Zealand albino rabbits were purchased from Pel-Freez Biologicals® (Rogers, AK), shipped overnight in Hanks Balanced Salt Solution (HBSS) over wet ice. Corneas and whole eye globes were used on the day of receipt.
1.3. Animals
Male New Zealand albino rabbits (2–2.5 kg) procured from Harlan laboratories® (Indianapolis, IN) were used for all the studies. All animal experiments conformed to the tenets of the Association for Research in Vision and Ophthalmology statement on the Use of Animals in Ophthalmic and Vision Research and followed the University of Mississippi Institutional Animal Care and Use Committee approved protocols (UM protocol no: 14-022).
1.4. Formulations
Preparation of ocular inserts / films
Melt-cast technique was utilized for the preparation of the polymeric ocular inserts. PEO N10, a thermoplastic polymer was selected as the matrix forming material because of its excellent pliability and thermogelation characteristics. Physical mixtures of IN or CIP or PSP and PEO N10 were prepared by geometric dilution. Drug load in all the inserts was maintained at 10% w/w. Polymeric films were cast using 13 mm die placed over a brass plate heated to 70°C using a hot plate. The physica l mixture of drug and polymer was introduced into the center of the die and compressed with the 13 mm punch to form a flat solid pre-mix. The mixture was further heated for 2–3 min. After cooling, 4 mm × 2 × 0.2 mm rectangular inserts weighing approximately 8 mg, providing a drug load of 0.8 mg, were cut and used for further studies.
IN control solution (IN-CS-SOL)
IN control formulation was prepared by dissolving IN in an aqueous solution containing 1% w/v Tween 80® and 29.3% w/v propylene glycol. Cationic polymer namely chitosan chloride (Mol wt < 200kDa) (0.25% w/v) was added into the formulation and pH was adjusted to 6.8.
PSP and CIP control solutions
Marketed PSP 1% w/v ophthalmic solution (Mfg. By: Bausch and Lomb®) and CIP 0.3% w/v ophthalmic solution (0.35 mg of CIP equivalent to 0.3 mg ciprofloxacin (base)) (Mfg. By: Hi-Tech Pharmacal) were used as control formulations to compare with PSP and CIP inserts. CIP control solution (0.2% w/v) prepared by dissolving CIP in phosphate buffer (pH 6.0) was used for in vitro studies. CIP marketed formulation (0.3% w/v) served as the control for the in vivo studies.
1.5. Analytical procedure for in vitro samples
Analysis of drug molecules was carried out using Waters HPLC system with 600 E pump controller, 717 plus auto sampler and 2487 UV detector. Data handling was carried out using an Agilent 3395 integrator. IN stock solution was prepared in methanol. A 71:29.5:0.5 mixture of methanol, water and o-phosphoric acid was used as mobile phase with Phenomenex Luna® 5 µm C18 100 Å, 250 × 4.6 mm column at a flow rate of 1 mL/min and 270 nm (21).
Analysis of CIP was carried out using mobile phase mixture of Acetonitrile and water (50:50), pH was adjusted to 3.0 with o-phosphoric acid. Phenomenex Luna® 5 µm C18 100 Å, 250 × 4.6 mm column used at a flow rate of 1 mL/min and 299 nm (22).
PSP was analyzed using a mixture of 18 mM phosphate buffer: methanol (38:62) and 0.1% w/v trimethylamine (pH 2.5) as the mobile phase. Phenomenex Luna® 5 µm C8 100 Å, 250 × 4.6 mm column was used at a flow rate of 1 mL/min and detection wavelength 218 nm. The reported analytical method was modified and validated prior to sample analysis (23). The linearity regression correlation coefficient in the constructed calibration curves was within the limit (≥0.99). Percentage relative standard deviation (RSD) values for the precision were found to be less than 2%. The percentage RSD for peak area response and retention were found within the prescribed limits. The linearity, accuracy and precision were observed to be acceptable over the working standard ranges in the all of the analytical methods.
1.6. Assay and content uniformity
IN content in polymeric inserts was determined using methanol and dimethyl sulfoxide (DMSO) 50:50 mixture as an extraction solvent. CIP and PSP was extracted from the films using 50:50 water and methanol. Content uniformity was determined from four, randomly cut, units from a single 13 mm insert, and analyzed as described under analytical procedure using a high performance liquid chromatography – UV (HPLC-UV) method.
1.7. Differential scanning calorimetry (DSC) and Fourier transform infrared (FT-IR) Spectroscopy
DSC thermograms for pure IN or CIP, PEO N10 and the 10% w/w inserts were collected using a Diamond Differential Scanning Calorimeter (Perkin-Elmer Life and Analytical Sciences) (24).The samples were weighed and sealed in aluminum pans and were heated from 0°C to 270°C at a heating rate of 10°C/min under nitrogen purge (20 mL/min). Infrared spectra (IR) for PEO N10, drug/polymer physical mixture, IN / CIP, and melt cast inserts (10% w/w) were obtained using a Cary 660 series FTIR (Agilent technologies) and MIRacle ATR (Attenuated Total reflectance).
1.8. In vitro release and transcorneal permeability studies
In vitro release of CIP, IN and PSP from the ocular inserts was tested across standard US 100 mesh sieve in 20 mL scintillation vials. The drugs showed higher solubility in 5% w/v RMβCD (randomly methylated beta cyclodextrin) in IPBS and therefore was used in the media to maintain the sink condition during the release studies. The insert was placed in the vial and the sieve was placed over the films and 10 mL of 5% w/v RMβCD in IPBS (pH 7.4) was added as the release/dissolution media. A stir bar was placed on the sieve and the whole assembly was placed over a hot plate maintained at 34°C under stirring (spin bar) for 2 h. Aliquots (600 µL) were collected at specific intervals and replaced with fresh medium. Samples taken were analyzed using HPLC-UV.
Corneas excised from whole rabbit eyes were used for the determination of in vitro transcorneal flux of the compounds from the various formulations. Whole eyes were shipped overnight in HBSS, over wet ice, and were used immediately upon receipt. The corneas were excised with some scleral portion adhering to help secure the membrane between the donor and receptor compartments during the course of a transport study. The temperature was maintained at 34°C with the help of a circulating water bath. In vitro permeability of IN, PSP, CIP across the corneal membrane was investigated using the Valia-Chien cells (PermeGear, Inc., Hellertown, PA). The in vitro permeability studies were carried out for a period of 3h. All the formulations were tested for transcorneal permeability at equivalent doses. Four hundred microliters of IN Control solution and IN Insert (5% w/w) (Dose: 400 µL (400 µg); 400 µg), PSP Control marketed solution (1% w/v) and PSP Insert (10% w/w) (Dose: 80 µL (800 µg); 800 µg), CIP Control (0.2% w/v) and CIP Insert (10% w/w) (Dose: 400 µL (800 µg); 800 µg) formulations were placed in the donor compartment. The inserts were slightly wetted, by adding 50 µL of IPBS in the donor compartment, at the start of the experiment. Aliquots were withdrawn at specific time intervals and analyzed for CIP, PSP or IN content using HPLC-UV method.
1.9. Data Analysis
The transcorneal flux was obtained by dividing the rate of drug transport by the surface area exposed to permeant and rate of transport was obtained from slope of linear regression analysis plot between cumulative amounts of drug transported versus time. Transcorneal flux was calculated using the equation below.
Where M is cumulative amount of drug and A is the surface area of the corneal membrane (0.636 cm2) exposed to the permeant (drug).
1.10. In vivo bioavailability studies
In vivo ocular bioavailability of IN, PSP and CIP was determined in conscious Male New Zealand albino rabbits weighing between 2.0–2.5 kgs. In these studies, IN-CS-SOL, PSP and CIP solutions (control; 100 µL) were instilled as instilled as two doses (50 µL each dose) at two different time points, −30min and 0 min, to reduce pre-corneal loss. 10% w/w loaded IN insert (Dose: 0.8 mg) was placed in the cul-de-sac of the rabbit eyes. Ocular bioavailability of PSP and CIP was investigated following the instillation of 1% w/v PSP marketed topical ophthalmic formulation (control; 100 µL: 1 mg) (Bausch & Lomb), 0.3% w/v CIP marketed solution (Control; 100 µL: 300 µg) and administration of PSP insert (Dose: 0.8 mg), CIP insert (Dose: 0.8 mg) in conjunctival sacs of the rabbit eyes. At the end of 2 h, following topical application of the aforementioned formulations, the rabbits were anesthetized using a combination of ketamine (35 mg/kg) and xylazine (3.5 mg/kg) injected intramuscularly and then euthanized with an overdose of pentobarbital injected through the marginal ear vein. The eyes were washed with ice cold IPBS and immediately enucleated and washed again. Ocular tissues were separated, weighed and preserved at −80°C until further analysis. All experiments were carried out in triplicate.
1.11. Bio analytical method and samples preparation
The in vitro analytical HPLC-UV methods described above was employed for bio-sample analysis following method validation. Protein precipitation technique was used for determining drug concentrations in the ocular tissue homogenates. Briefly, tissues such as cornea, sclera, iris-ciliary (IC), retina-choroid (RC) were cut into very small pieces and added into Eppendorf® tubes. A mixture of ice-cold acetonitrile and 0.1% w/v formic acid (1 mL) was then added to precipitate proteins from each individual tissue. The supernatant was then collected after centrifugation for 1 h at 13,000 rpm prior to analysis. Aqueous humor (AH) (200 µL) and Vitreous humor (VH) (500 µL) were precipitated by adding an ice-cold mixture of 200 µL for AH and 500 µL for VH in the ratio (1:1). Quantification was carried out using calibration curves constructed from respective ocular tissues such as cornea (20–500 ng), sclera (20–500 ng), AH (10–200 ng), VH (10–200 ng), IC (10–200 ng), RPE (10–200 ng). All the standard curves had a coefficient of determination r2 ≥ 0.96. Recovery of the drugs was quantitated by spiking drug in blank AH, VH, and comparing the expected drug concentration with standard concentration. Recovery values were determined in AH (93.1–94.7) and VH (91.5–93.2). Interference was not observed from co-eluted protein residues with respect to drug peaks in all the tissues. The precision and accuracies obtained with analytical methods are within the prescribed limits of method validation guidelines.
1.12. Statistical analysis
All the data presented in these experiments was reported as an average of triplicate for the same time points. One way ANOVA was carried out to test and compare treatment groups at different levels of significance and Student t-test was used to compare differences within two groups. A ‘p’ value less than 0.05 was considered to express statistically significant difference.
2. Results
2.1. Assay and content uniformity
Predicted physico-chemical characteristics (ACD/ I lab 2.0 software) of the drugs studied are presented in the Table 1. IN / CIP / PSP content in all the inserts was approximately between 90% and 95% of the theoretical value. The compounds were observed to be uniformly distributed within the polymeric framework (RSD < 2.3%) and the films exhibited good pliability and tensile strength.
Table 1.
Physico-chemical characteristics of drugs (predicted using ACD/ I Lab 2.0 software)
| Drugs studied | Molecular Weight (Da) |
Log P | Log D7.4 | Pka | PSA | HBA | HBD |
|---|---|---|---|---|---|---|---|
| Prednisolone Sodium Phosphate (PSP) | 484.4 | 1.27 | −5.32 | 1.2, 6.1, 14.4 | 147 | 8 | 4 |
| Indomethacin (IN) | 357.7 | 4.27 | −0.16 | 4.5 | 68.53 | 4 | 1 |
| Ciprofloxacin Hydrochloride (CIP) | 331.34 | −0.81 | −2.36 | 6.1, 8.0 | 72.88 | 6 | 2 |
2.2. DSC and FT-IR studies
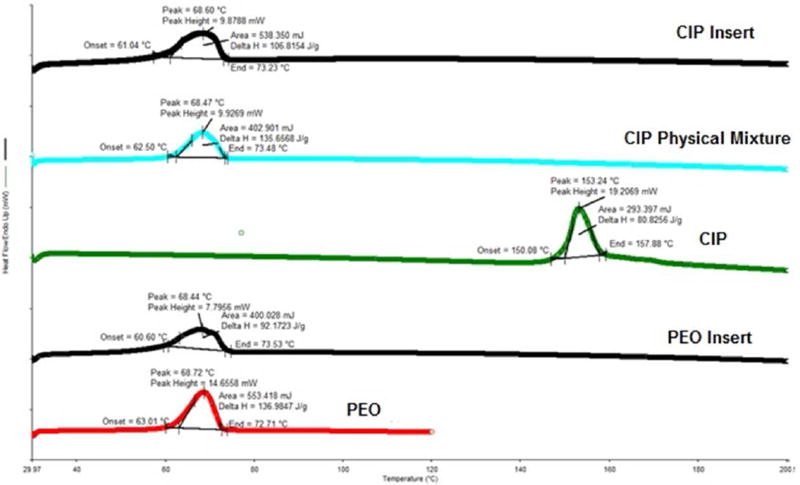
The polymeric inserts were studied for thermal characteristics and excipient compatibility using DSC. DSC thermograms of pure PEO N10, PEO N10 film, pure drug, physical mixture and polymeric insert are presented in Figures 1–3. IN (Fig. 1), CIP (Fig. 2) and PSP (Fig. 3) exhibited an endothermic peak at 162°C, 153°C, 212. 8°C corresponding to their melting points respectively. PSP exhibited characteristic peak at ~ 130°C owing to the bonded water molecule (corresponding to FTIR band between 3400 cm−1 – 3000 cm−1). PEO N10 and PEO N10 film exhibited a melting point temperature of 68°C. The characteristic peaks of IN, CIP and PSP were absent in the polymeric inserts indicating that there was significant reduction in crystallinity.
Figure 1.
DSC thermograms of IN Insert, IN physical Mixture, pure IN, PEO Insert and pure PEO.
Figure 3.
DSC thermograms of PSP Insert, PSP physical Mixture, pure PSP, PEO Insert and pure PEO.
Figure 2.
DSC thermograms of CIP Insert, CIP physical Mixture, pure CIP, PEO Insert and pure PEO.
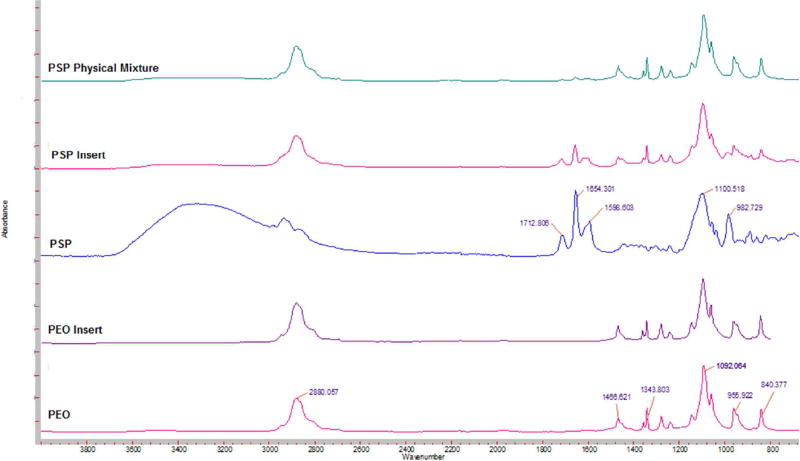
FTIR spectra of pure IN exhibited characteristic bands of secondary carbonyl groups (C=O) at 1710 cm−1, (C=O amide) at 1687 cm−1, phenyl groups (C=C stretch vibration) at 1593 cm−1 and (O-H stretch vibration) at 2964 cm−1. Pure PEO revealed –CH stretching at 2880 cm−1 (Fig. 4). The FTIR spectra of CIP showed characteristic peak at 3500 and 3450 cm−1 corresponding to OH stretching vibration. The band at 1701 cm−1 indicated carbonyl C = O stretching, while the peak at 1617 cm−1 belongs to quinolone. The band at the 1445 cm−1 represented carbonyl group and the peak at 1250 cm−1 suggested bending vibration of O-H group which indicated the presence of carboxylic acid (Fig. 5). FTIR spectra of PSP showed two carbonyl stretching peaks at 1708 cm−1 and 1654 cm−1 while peaks at 1596 cm−1 and 1100 cm−1 correspond to the NH and OH bending (Fig. 6). The FTIR spectra of physical mixtures were similar to those of respective drugs and PEON10 individual spectra, which suggest that there was no chemical interaction between drug and PEON10 in physical mixtures. However, characteristic bands of the IN (carbonyl, amide and phenyl functional groups), CIP (quinolone group) and PSP (carbonyl group) displayed lesser degree of intensities / disappeared in the insert formulations indicating chemical interaction between drug and the carrier occurred during the formulation processing steps.
Figure 4.
FT-IR spectra of IN Insert, IN Physical Mixture, pure IN, PEO Insert and pure PEO N10.
Figure 5.
FT-IR spectra of CIP Physical Mixture, CIP Insert, PEO N10 Insert, pure CIP and PEO N10.
Figure 6.
FT-IR spectra of PSP Physical Mixture, PSP Insert, pure PSP, PEO Insert and pure PEO N10.
2.3. In vitro release
Percentage release of IN, PSP, CIP from the polymeric films was determined to be 100.2 ± 6.9, 92.7 ± 4.8 and 85.3 ± 9.4 %, respectively, at the end of 2 h.
2.4. Transcorneal permeability studies
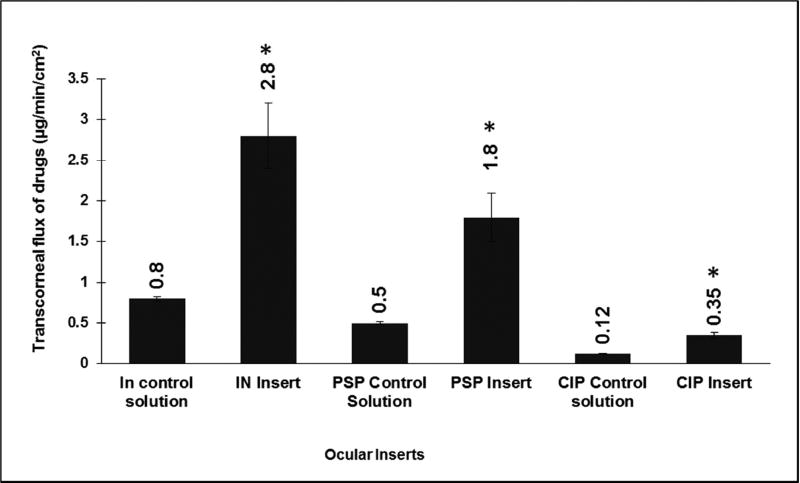
In vitro transcorneal flux profiles obtained from different topical insert formulations, compared to their respective control solutions at an equivalent dose, is presented in (Fig. 7). Transcorneal flux of IN, PSP, CIP were enhanced by ~3.5, ~3.6 and ~2.9-folds, respectively, from the polymeric inserts when compared to their control formulations.
Figure 7.
In vitro transcorneal flux obtained from different topical formulations at equivalent doses using valia-chien cells at 34°C. Formulation tested include IN Control (0.1% w/v), IN Insert (5% w/w) (Dose: 400 µL (400 µg); 400 µg). PSP Control solution (1% w/v), PSP Insert (10% w/w) (Dose: 80 µL (800 µg); 800 µg). CIP Control (0.2% w/v), CIP Insert (10% w/w) (Dose: 400 µL (800 µg); 800 µg). * symbol represented on ocular tissues indicate statistically significant difference of flux from test formulations in comparison to control solutions (p<0.05).
2.5. In vivo bioavailability studies
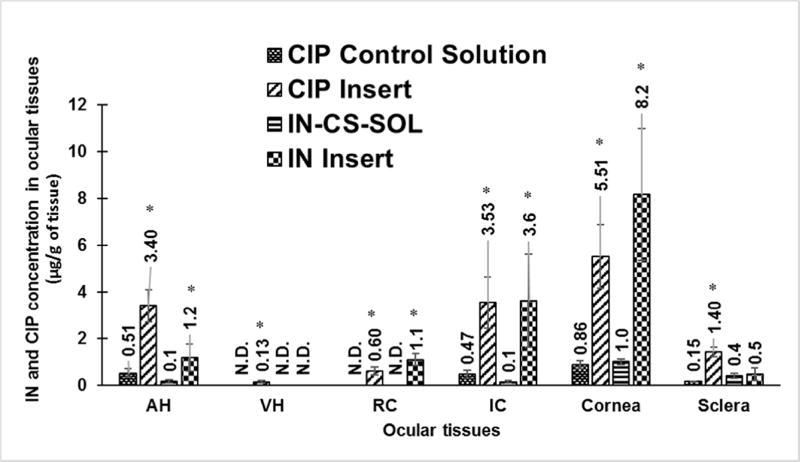
Ocular tissue concentrations obtained with drug loaded polymeric inserts were evaluated in conscious rabbit model, 2 h post topical application. IN-CS-SOL did not deliver drug into RC but delivered into the cornea (0.9 ± 0.09 µg/g), sclera (0.4 ± 0.19 µg/g), IC (0.12 ± 0.04 µg/g) and AH (0.15 ± 0.03 µg/g) whereas IN insert delivered drug into RC (1.08 ± 0.26 µg/g). Furthermore, IN insert enhanced IN levels by ~10 folds (AH), ~27-folds (IC) and ~8.5-folds (cornea) when compared to IN-CS-SOL formulation (fig.8.).
Figure 8.
In vivo IN and CIP concentrations obtained from 0.1% w/v IN-CS-SOL (Dose: 0.1 mg), 10% w/w IN Insert (Dose: 0.8 mg); 0.3% w/v CIP control solution (Dose: 100 µL; 350 µg) and 10% w/w CIP Insert (Dose: 0.8 mg), 2 h post topical application in conscious rabbit model. All experiments were performed in triplicate. AH: Aqueous Humor; RC: Retina- Choroid; IC: Iris-Ciliary. No levels were detected in VH. ND-not detectable. Symbol (*) represented on ocular tissues indicate statistically significant difference of IN and CIP formulations from respective controls (p<0.05).
Similarly, CIP concentrations obtained with the control solution in the AH (0.51± 0.2 µg/mL), IC (0.47 ± 0.17 µg/g), cornea (0.86 ± 0.16 µg/g) and sclera (0.15 ± 0.01 µg/g) were significantly lower than that obtained with the inserts. The control solution formulations did not deliver CIP to the VH and RC, whereas the insert successfully delivered CIP into the VH (0.13 ± 0.05 µg/mL) and RC (0.6 ± 0.17 µg/g) (fig.8.).
PSP control solution delivered drug into ocular tissues namely AH (0.3 ± 0.04 µg/mL), IC (1.07 ± 0.5 µg/g), cornea (1.55 ± 0.85 µg/g) and sclera (0.77 ± 0.41 µg/g). While the PSP control solution couldn’t deliver drug into the RC and VH, the insert - even at a lower dose compared to the PSP control - delivered PSP into the VH (0.09 ± 0.008 µg/mL) and RC (1.28 ± 0.52 µg/g). Moreover, the insert generated significantly greater PSP in all the other ocular tissues tested (fig.9.).
Figure 9.
In vivo PSP concentrations (µg/gm of tissue) obtained from 10% w/w PSP Insert and PSP control solution (1% w/v) (Dose: 0.8 mg, 1 mg respectively), 2 h post application in conscious rabbit model. All experiments were in triplicates. AH: Aqueous Humor; RC: Retina-Choroid; IC: Iris-Ciliary; VH: Vitreous Humor. Low levels were detectable in VH. (N.D - Not detectable). Symbol representation on ocular tissues indicate the statistically significant difference of PSP concentrations obtained from the insert formulation in comparison to control (p<0.05).
3. Discussion
The overall objective of the current research is to develop a standard prototypical, noninvasive, ocular drug delivery platform, effective for molecules representing a wide spectrum of physico-chemical properties, for back-of-the eye drug delivery. The melt-cast / melt-extrusion technology is a simple, solvent free, continuous process, for preparing unit dosage forms, which undergo gelation upon contact with tear fluid, prolonging the release and retention on the ocular surface. The compound to be delivered can possess a range of hydrophilicity/lipophilicity, as demonstrated in the present project, and could be formulated at significantly higher loading. Compounds embedded in the carrier matrix will be transformed into an amorphous state or get entrapped as a molecular dispersion after fabrication using thermal technique. Moreover, preservatives and solubilizers are not required, eliminating unnecessary excipients and processing steps. Furthermore, modified release platforms could be designed by varying the thermoplastic polymers or molecular weights and combinations.
The solubility of CIP, a zwitterion, is greatly reduced at the pH 7.4, which is close to its isoelectric point. This may cause CIP to crystalize when the ophthalmic solutions (formulated in the acidic pH range) come into contact with the tear fluid. In the melt-cast inserts, for all three drugs, absence of detectable crystalline domains in the DSC thermograms demonstrate that the drug was completely dispersed or solubilized in the carrier polymer, at this 10% w/w drug loading.
An interaction between drug and polymer could be reflected by a change in the position of C=O vibration and disappearance of O-H stretching in the FT-IR spectra depending on the extent of the interaction. The FT-IR spectra of the insert formulations exhibited lower peak intensities / were masked indicating slight interaction with the polymer PEO N10. The release studies, however, indicated that 100% of the drug was released within 2 hours. Moreover, in vitro transcorneal flux of the three actives from the inserts was increased by ~ 2.5 to 3.5-folds when compared to the respective control solutions. This could be due to enhanced drug retention and accumulation in the corneal and conjunctival epithelial layers from the transformed gel (in vitro).
Penetration of drugs through the conjunctival scleral pathway is reported in the literature (25, 26). Ocular tissue concentrations attained with the polymeric inserts in AH, IC and cornea was increased by ~6–8 folds in comparison to the control formulations. Cationic mucoadhesive polymer (CS) was used at a concentration of 0.1% w/v to improve corneal penetration characteristics and thereby intraocular bioavailability of IN from control solution. However, IC levels obtained with IN control solution was ~27-fold lower at 1/8th dose of insert formulation. IN control Retinal concentrations were obtained in vivo with the IN insert (1.08 ± 0.27 µg/g), PSP insert (1.28 ± 0.52 µg/g) and CIP insert (0.60 ± 0.17 µg/g) formulations, whereas control solutions, couldn’t deliver drug into RC. The inserts form localized drug depots, in close proximity to the conjunctival-scleral tissues, and thus helps drive the molecules to the retinal tissue, through the conjunctival-scleral route. Thus, advantage of inserts, as a platform providing access to the trans-scleral pathway, could be exploited to overcome the physico-chemical limitations of molecules. Additionally, feasibility of higher drug loading and use of penetration enhancers, if needed, provides even greater flexibility to the dosage form. The erodible inserts fabricated using PEON10 (bio-degradable and bio-compatible polymer) does not need removal after the administration. The melt-cast technique can be easily translated or scaled up for manufacturing using melt-extrusion technology, which also minimizes the exposure time to higher temperatures (2–3 minutes) allowing processing of some thermolabile compounds also.
There have been a few reports attempting back-of-the eye delivery of therapeutic agents through the topical route. In an earlier study, IN was loaded into nanostructured lipid carrier (NLC) at 0.8% w/v and was evaluated in conscious rabbits, 2 h post-topical administration of 100 µL (Dose: 800 µg). Retinal levels (0.89 ± 0.31 µg/g) were also observed with the NLC formulation (27). In another report, an IN (0.1% w/v) ophthalmic solution made up of poloxamer® 407 (10% w/w) enhanced AH concentrations by ~2-fold when compared to Indocollyre® (marketed ophthalmic solution), 2 h post-topical administration of 150 µL formulation (Dose: 150 µg) (28). Schultz et al impregnated prednisolone into hydrogel contact lenses by placing them in diluted 5 mg/mL prednisolone suspension. These contact lenses were evaluated for posterior segment (retina, choroid, macula) concentrations in anesthetized rabbits following 4 h treatments/application on days 1, 2, 5, 8 and 10. On day 11 retinal concentrations obtained were in range of 26–166 ng/g (29). Taha et al evaluated CIP loaded liposomes (0.3% w/v) in conscious rabbits following topical application of 50 µL formulation (Dose: 150 µg) (30). The authors report that AH concentrations was increased by ~0.3-folds when compared to CIP marketed ophthalmic formulation. The polymeric noninvasive inserts developed in this study, delivered significantly higher drug levels to all the ocular tissues tested, in comparison to the control and other literature reports.
It is widely acknowledged that IN acts as an anti-inflammatory agent through the inhibition of COX-2 enzyme, abundantly present in ocular tissues namely cornea, iris, ciliary body and retina (31). Mitchell et al reported half-maximal inhibitory concentration (IC50) of IN required for COX-2 activity in endotoxin activated J774.2 macrophages to be 0.6 µg/mL (32). MIC90 of ciprofloxacin is reported to be 0.5 µg/mL (33). Therapeutic concentration of prednisolone required to inhibit inflammatory mediated processes in AH (humans) is reported to be 25 ng/mL (34). Drugs levels for optimal activity are reported in terms of solution concentrations, which reflects the therapeutic levels needed in AH and VH tissues. Ocular tissues namely IC and RC are in dynamic equilibrium with AH and VH, so the therapeutic levels required in AH and VH could be interpreted to be good therapeutic response indicators in the ocular tissues. Drug levels obtained in all the ocular tissues, post 2 h, with inserts were several folds higher than the reported minimal concentrations, demonstrating that inserts could maintain therapeutic drug levels for prolonged durations following topical application (35, 36).
4. Conclusion
In conclusion, the results from these studies suggest that the development of solvent free, melt-cast or melt extruded ocular inserts is a highly feasible, noninvasive approach for the delivery of a wide range of drugs, with different physico-chemical properties, to the ocular tissues. The high localized drug loads, intimate contact with the conjunctiva, increased retention time and quick transformation into a gel form make this efficient for delivering to the back-of-the eye tissues also. These formulations would be marketed as unit dosage forms and do not need any preservatives or solubilizers. Penetration enhancers may be incorporated to further increase ocular bioavailability. Thus, the melt-cast / melt-extruded films could shift the paradigm for drug delivery to the back-of-the eye.
Acknowledgments
This project was supported by grants 1R01EY022120-01A1 from the National Eye Institute and P20GM104932 from the National Institute of General Medical Sciences, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations used
- CIP
Ciprofloxacin
- IN
Indomethacin
- PSP
Prednisolone sodium phosphate
- PEO N10
Poly ethylene oxide N10
- IPBS
Isotonic phosphate buffer saline
- RMβCD
Randomly methylated beta-Cyclodextrin
- HPLC
High performance liquid chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
The authors report no declarations of interest.
Bibliography
- 1.Bodh SA, Kumar V, Raina UK, Ghosh B, Thakar M. Inflammatory glaucoma. Oman J Ophthalmol. 2011;4(1):3–9. doi: 10.4103/0974-620X.77655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan J, Kapur M, McCallum R. Noninfectious Immune-Mediated Uveitis and Ocular Inflammation. Current Allergy and Asthma Reports. 2013;14(1):409. doi: 10.1007/s11882-013-0409-1. [DOI] [PubMed] [Google Scholar]
- 3.Schopf LR, Popov AM, Enlow EM, Bourassa JL, Ong WZ, Nowak P, et al. Topical Ocular Drug Delivery to the Back of the Eye by Mucus-Penetrating Particles. Transl Vis Sci Technol. 2015;4(3):11. doi: 10.1167/tvst.4.3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boddu SH, Gupta H, Patel S. Drug delivery to the back of the eye following topical administration: an update on research and patenting activity. Recent Pat Drug Deliv Formul. 2014;8(1):27–36. doi: 10.2174/1872211308666140130093301. [DOI] [PubMed] [Google Scholar]
- 5.Patel A, Cholkar K, Agrahari V, Mitra AK. Ocular drug delivery systems: An overview. World J Pharmacol. 2013;2(2):47–64. doi: 10.5497/wjp.v2.i2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah SS, Denham LV, Elison JR, Bhattacharjee PS, Clement C, Huq T, et al. Drug delivery to the posterior segment of the eye for pharmacologic therapy. Expert Rev Ophthalmol. 2010;5(1):75–93. doi: 10.1586/eop.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cholkar K, Patel SP, Vadlapudi AD, Mitra AK. Novel strategies for anterior segment ocular drug delivery. J Ocul Pharmacol Ther. 2013;29(2):106–23. doi: 10.1089/jop.2012.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shafaie S, Hutter V, Cook MT, Brown MB, Chau DY. In Vitro Cell Models for Ophthalmic Drug Development Applications. Biores Open Access. 2016;5(1):94–108. doi: 10.1089/biores.2016.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumari A, Sharma PK, Garg VK, Garg G. Ocular inserts - Advancement in therapy of eye diseases. J Adv Pharm Technol Res. 2010;1(3):291–6. doi: 10.4103/0110-5558.72419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou HY, Hao JL, Wang S, Zheng Y, Zhang WS. Nanoparticles in the ocular drug delivery. Int J Ophthalmol. 2013;6(3):390–6. doi: 10.3980/j.issn.2222-3959.2013.03.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sreenivas S, Hiremath S, Godbole A. Ofloxacin ocular inserts: Design, formulation and evaluation. Iranian Journal of Pharmacology & Therapeutics (IJPT) 2006;5(2):159–62. [Google Scholar]
- 12.Mundada A, Shrikhande B. Design and evaluation of soluble ocular drug insert for controlled release of ciprofloxacin hydrochloride. Drug development and industrial pharmacy. 2006;32(4):443–8. doi: 10.1080/03639040500534101. [DOI] [PubMed] [Google Scholar]
- 13.Manvi F, Patil M, Mastiholimath V, Rathod R. Development and evaluation of ocular films of cromolyn sodium. Indian journal of pharmaceutical sciences. 2004;66(3):309. [Google Scholar]
- 14.Di Colo G, Burgalassi S, Chetoni P, Fiaschi M, Zambito Y, Saettone M. Relevance of polymer molecular weight to the in vitro/in vivo performances of ocular inserts based on poly (ethylene oxide) International journal of pharmaceutics. 2001;220(1):169–77. doi: 10.1016/s0378-5173(01)00668-8. [DOI] [PubMed] [Google Scholar]
- 15.Di Colo G, Zambito Y. A study of release mechanisms of different ophthalmic drugs from erodible ocular inserts based on poly(ethylene oxide) European Journal of Pharmaceutics and Biopharmaceutics. 2002;54(2):193–9. doi: 10.1016/s0939-6411(02)00086-3. [DOI] [PubMed] [Google Scholar]
- 16.Di Colo G, Burgalassi S, Chetoni P, Fiaschi MP, Zambito Y, Saettone MF. Gel-forming erodible inserts for ocular controlled delivery of ofloxacin. International Journal of Pharmaceutics. 2001;215(1):101–11. doi: 10.1016/s0378-5173(00)00671-2. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita T, Ozaki S, Kushida I. Solvent shift method for anti-precipitant screening of poorly soluble drugs using biorelevant medium and dimethyl sulfoxide. Int J Pharm. 2011;419(1–2):170–4. doi: 10.1016/j.ijpharm.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Lu M, Guo Z, Huang L, Feng X, Wu C. Improving the chemical stability of amorphous solid dispersion with cocrystal technique by hot melt extrusion. Pharmaceutical research. 2012;29(3):806–17. doi: 10.1007/s11095-011-0605-4. [DOI] [PubMed] [Google Scholar]
- 19.Ashour EA, Majumdar S, Alsheteli A, Alshehri S, Alsulays B, Feng X, et al. Hot melt extrusion as an approach to improve solubility, permeability and oral absorption of a psychoactive natural product, piperine. Journal of Pharmacy and Pharmacology. 2016;68(8):989–98. doi: 10.1111/jphp.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patil H, Feng X, Ye X, Majumdar S, Repka MA. Continuous production of fenofibrate solid lipid nanoparticles by hot-melt extrusion technology: a systematic study based on a quality by design approach. The AAPS journal. 2015;17(1):194–205. doi: 10.1208/s12248-014-9674-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hippalgaonkar K, Adelli GR, Hippalgaonkar K, Repka MA, Majumdar S. Indomethacin-loaded solid lipid nanoparticles for ocular delivery: development, characterization, and in vitro evaluation. J Ocul Pharmacol Ther. 2013;29(2):216–28. doi: 10.1089/jop.2012.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussain AH, Muhammad, Shoaib Muhammad Harris, Yousuf Rabia Ismail, Shafi Nighat. Bioanalytical method development and validation of ciprofloxacin by RP-HPLC method. Asian Journal of Pharmaceutical & Biological Research (AJPBR) 2012 Oct;2(4):219. 2012. [Google Scholar]
- 23.Razzaq SN, Khan IU, Mariam I, Razzaq SS. Stability indicating HPLC method for the simultaneous determination of moxifloxacin and prednisolone in pharmaceutical formulations. Chem Cent J. 2012;6(1):94. doi: 10.1186/1752-153X-6-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fathi M, Varshosaz J, Mohebbi M, Shahidi F. Hesperetin-Loaded Solid Lipid Nanoparticles and Nanostructure Lipid Carriers for Food Fortification: Preparation, Characterization, and Modeling. Food Bioprocess Technol. 2012 [Google Scholar]
- 25.Pescina S, Santi P, Ferrari G, Nicoli S. Trans-scleral delivery of macromolecules. Ther Deliv. 2011;2(10):1331–49. doi: 10.4155/tde.11.104. [DOI] [PubMed] [Google Scholar]
- 26.Ranta V-P, Urtti A. Transscleral drug delivery to the posterior eye: Prospects of pharmacokinetic modeling. Advanced Drug Delivery Reviews. 2006;58(11):1164–81. doi: 10.1016/j.addr.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 27.Sai Prachetan Balguri GRA, Soumyajit Majumdar. Topical ophthalmic lipid nanoparticle formulations (SLN, NLC) of indomethacin for delivery to the posterior segment ocular tissues. European Journal of Pharmaceutics and Biopharmaceutics. 2016 doi: 10.1016/j.ejpb.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chetoni P, Panichi L, Burgalassi S, Benelli U, Saettone MF. Pharmacokinetics and anti-inflammatory activity in rabbits of a novel indomethacin ophthalmic solution. J Ocul Pharmacol Ther. 2000;16(4):363–72. doi: 10.1089/jop.2000.16.363. [DOI] [PubMed] [Google Scholar]
- 29.Schultz C, Breaux J, Schentag J, Morck D. Drug delivery to the posterior segment of the eye through hydrogel contact lenses. Clin Exp Optom. 2011;94(2):212–8. doi: 10.1111/j.1444-0938.2010.00553.x. [DOI] [PubMed] [Google Scholar]
- 30.Taha EI, El-Anazi MH, El-Bagory IM, Bayomi MA. Design of liposomal colloidal systems for ocular delivery of ciprofloxacin. Saudi Pharm J. 2014;22(3):231–9. doi: 10.1016/j.jsps.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Wu Y, Heegaard S, Kolko M. Cyclooxygenase-2 expression in the normal human eye and its expression pattern in selected eye tumours. Acta Ophthalmol. 2011;89(7):681–5. doi: 10.1111/j.1755-3768.2009.01765.x. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993;90(24):11693–7. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kowalski RP, Karenchak LM, Eller AW. The role of ciprofloxacin in endophthalmitis therapy. Am J Ophthalmol. 1993;116(6):695–9. doi: 10.1016/s0002-9394(14)73468-3. [DOI] [PubMed] [Google Scholar]
- 34.Del Sole MJ, Schaiquevich P, Aba MA, Lanusse CE, Moreno L. Plasma and ocular prednisolone disposition after oral treatment in cats. Biomed Res Int. 2013;2013:209439. doi: 10.1155/2013/209439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adelli GR, Balguri SP, Bhagav P, Raman V, Majumdar S. Diclofenac sodium ion exchange resin complex loaded melt cast films for sustained release ocular delivery. Drug Delivery. 2017;24(1):370–9. doi: 10.1080/10717544.2016.1256000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adelli GR, Hingorani T, Punyamurthula N, Balguri SP, Majumdar S. Evaluation of topical hesperetin matrix film for back-of-the-eye delivery. European Journal of Pharmaceutics and Biopharmaceutics. 2015;92:74–82. doi: 10.1016/j.ejpb.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]